Open Access Article
Open Access ArticleEnhancing CO2 electroreduction to ethylene via microenvironment regulation in boron–imidazolate frameworks†
Chen
Lu
ab,
Qin-Long
Hong
a,
Hai-Xia
Zhang
 *a and
Jian
Zhang
*a and
Jian
Zhang
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: zhanghaixia@fjirsm.ac.cn; zhj@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 23rd August 2024
Abstract
Using the structure-induced effect of KBH(mim)3 ligand, four 2-dimensional (2D) boron imidazolate frameworks with identical body framework and different dangling monocarboxylate ligands, have been synthesized. Electrocatalytic results indicate that the surrounding microenvironment regulation could effectively affect the activity and selectivity towards C2H4. BIF-151 showed the highest electrocatalytic performances with the Faraday efficiency (FE) of 25.94% for C2H4 at −1.4 V vs. RHE.
With the rapid development of modern society and the extensive consumption of non-renewable fossil fuels, significant amounts of CO2 gas are being emitted into the atmosphere, resulting in a profound impact on Earth's ecological environment.1–4 Electrochemical CO2 reduction reaction (CO2RR) is a process that utilizes electrical energy to expedite the conversion of CO2 on electrode surfaces into carbon products such as CO, CH4, etc.5–12 This process not only serves as an important approach to address energy issues and achieve carbon-neutral recycling but also plays a crucial role in laboratory investigations regarding CO2 reduction.13–16 Through relentless efforts by scientific researchers, the range of products obtained from CO2RR has gradually expanded from single-carbon compounds like CO and CH4 to multi-carbon compounds including ethylene (C2H4) and ethanol (C2H5OH).17–20 Among these various carbon products, C2H4 possesses high energy density and holds immense economic significance.21–23 However, due to the sluggish kinetics associated with carbon–carbon (C–C) coupling reactions during this process, achieving selective conversion of CO2 into C2H4 remains a major challenge.24–27
Being used as electrocatalysts, crystalline boron imidazolate frameworks (BIFs) have achieved excellent results in the study of electrocatalytic performance–structure relationship.28–30 At present, the reported BIFs catalysts mainly focus on the design of active metal sites (bimetal, monometallic sites) and tandem catalysis by anchoring second active metal sites.31–33 Except for metal sites, the effects of surrounding ligand microenvironment on electrocatalytic activity and selectivity have been poorly studied.34–36 During the process of CO2RR to C2H4, C–C coupling is the indispensable step which require synergistic effect of multiple sites, including metal sites and organinc ligands.37–39 In order to strictly compare the effects of the ligand microenvironment on catalysis, the synthesized catalyst should be constructed from different functional ligands while contain the same metal species, identical metal coordination environment, even exhibit similar topological structures. However, in the synthesis process of crystalline catalysts, small changes in the synthesis conditions (temperature, solvent, pH, etc.) and raw materials will significantly affect the structures of the resulting products. Therefore, to discern the contributions of diverse surrounding ligand microenvironment, it is necessary to construct a stable metal–organic skeleton platform with the same topology, so as to fix the coordination environment of host framework and change the functional ligand systematically.
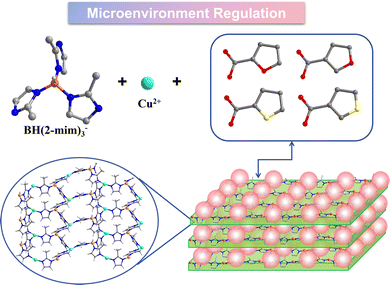
Herein, a series of isostructural 2D BIFs, Cu(II)2[BH(mim)3]2(2-FC) (BIF-151, mim = 2-methylimidazole, 2-FC = furan-2-carboxylate), Cu(II)2[BH(mim)3]2(3-FC) (BIF-152, 3-FC = furan-3-carboxylate), Cu(II)2[BH(mim)3]2(2-TpC) (BIF-153, 2-TpC = thiophene-2-carboxylate) and Cu(II)2[BH(mim)3]2(3-TpC) (BIF-154, 3-TpC = thiophene-3-carboxylate) were successfully constructed under solvothermal condition (Scheme 1). Thanks to the structure-induced effect of KBH(mim)3 ligand, these crystal structures exhibited identical body framework and metal coordination environment. While, four types of monocarboxylate ligands with different substituent elements and substituent positions could be used as dangling ligands to modify these layers. Electrocatalytic results indicate that all four materials demonstrate catalytic activity towards CO2RR, especially with certain selectivity towards C2H4 products. However, the activity and selectivity towards C2H4 were distinctly different. Among them, BIF-151 showed the highest electrocatalytic performances with the FE of 25.94% for C2H4 at −1.4 V vs. RHE, which is about 1.9, 1.7, and 2.8 times higher than that of BIF-152 (13.04%), BIF-153 (15.05%), and BIF-154 (9.26%), respectively.
 | ||
| Scheme 1 Synthesis procedure and the layer structure diagram. The pink sphere denotes the second ligand and the light green lamellar represents CuBH(mim)3+ layer. | ||
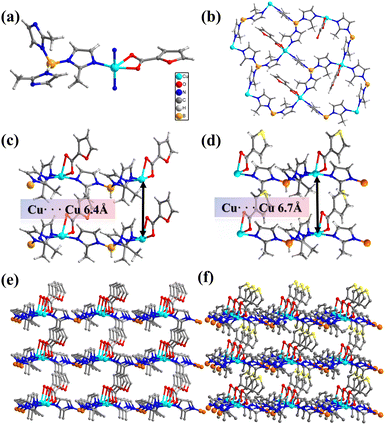
Firstly, tripodal boron imidazolate ligands (KBH(mim)3) were synthesized prior to the solvothermal reaction. The tripodal KBH(mim)3 ligands can induce 3-connected copper sites during the self-assembly process. By adopting a variety of dangling monocarboxylate ligands, four 3-connected layer structures have been obtained (BIF-151 to BIF-154). X-ray single-crystal structure tests revealed that the four structures have almost identical framework, which all crystallized in the monoclinic space group Ia. Since they are isomorphic, BIF-151 is selected to describe their structures. The asymmetric unit of BIF-151 includes one crystallographically independent Cu(II) ion, one BH(mim)3− ligand and one furan-2-carboxylate ligand. As shown in Fig. 1a, each Cu(II) ion adopted a pentadentate triangular dipyramidal coordination pattern, wherein three N atoms originate from three BH(mim)3− ligands and two O atoms derive from carboxyl group of furan-2-carboxylate. Each BH(mim)3− ligand adopted the μ3-bridged mode linking three copper ions. Thus, Cu(II) ions and B atoms are alternately linked via mim ligands to generate a 2D honeycomb layer (Fig. 1b). These 2D layers stack along the c-axis direction through van der Waals interactions (Fig. 1c and e), while maintaining a certain distance between them due to the presence of dangling monocarboxylate ligands on one side of each layer.
The 3-connected layer structures can be constructed precisely due to the structural induction of the tripodal BH(mim)3− ligands. Therefore, we can fix the coordination environment of copper ions and micro-regulate the surrounding environment by changing the dangling monocarboxylate ligands. The position of oxygen on furan can be changed. BIF-152 (CuBH(mim)3(3-FC)) was syntheisized when furan-3-carboxylate ligand is used instead of furan-2-carboxylate ligand (Fig. S1, ESI†). Furtherly, thiophene-2-carboxylate and thiophene-3-carboxylate ligands can be applied in the similar synthetic reaction and two new crystals BIF-153 (CuBH(mim)3(2-TpC)) (Fig. S1, ESI†) and BIF-154 (CuBH(mim)3(3-TpC)) (Fig. 1d and f) were constructed. Specifically, when thiophene carboxylate ligand serves as the second ligand, the spacing between layers was measured at 6.7 Å; whereas when furan carboxylate ligand acts as the second ligand, this spacing reduced to 6.4 Å. The presence of interspaces reduces the weak interactions between layers and may contribute to the intercalation of small molecules.
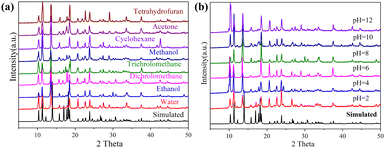
The powder X-ray diffraction (PXRD) patterns were performed to investigate the stability and crystal purity of these crystals. As shown in Fig. 2 and Fig. S2 (ESI†), the synthesized crystal samples have high purity, and the positions of the diffraction peaks of the crystals are basically consistent with the simulated characteristic peaks. By immersing the crystal samples in aqueous solutions with different pH values (using dilute nitric acid or sodium hydroxide to adjust the pH) for 3 days, the diffraction peaks were maintained, demonstrating the chemical stability of these crystals (Fig. S3, ESI†). In addition, the crystal samples were stable and insoluble in common solvents, such as water, dichloromethane, tetrahydrofuran, methanol, ethanol, trichloromethane and acetone (Fig. S4, ESI†). The chemical composition and valence state were studied by X-ray photoelectron spectroscopy (Fig. S6, ESI†). The XPS survey scans displayed the presence of carbon, nitrogen, oxygen, copper and boron in these materials. High-resolution spectra of the Cu 2p region of BIF-151 showed that there are only bivalent copper ions in these crystal structures, and the fitting peaks at 954.2 eV and 934.2 eV correspond to the binding energies of the Cu 2p 1/2 and Cu 2p 3/2 orbitals, respectively. The XPS spectra of crystals BIF-152, BIF-153, and BIF-154 were similar to the above (Fig. S6, ESI†), demonstrating that the peripheral groups have little effect on the valence state of copper.
To study the effect of different organic modifications on catalytic activity, the four BIFs were used as catalysts for electrochemical CO2 reduction reaction. The electrocatalytic activity was measured in 0.05 M CsCO3 and 0.1 M KCl (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
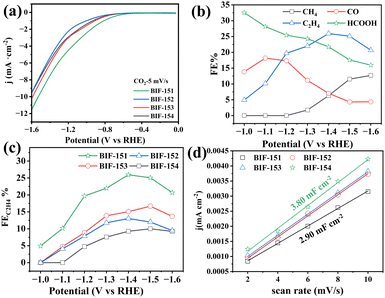
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution saturated with CO2 from 0 to −1.6 V vs. reversible hydrogen electrode (RHE). On the base of linear sweep voltammetry (LSV), BIF-151 displayed the most positive onset potential of −0.37 V vs. RHE and the highest total current density at potential from −0.6 V to −1.6 V vs. RHE when comparing with other crystals (Fig. 3a). The potential gradient and the FE of each product were measured at constant potential. The gas phase products and liquid phase products were detected by gas chromatography (GC) and ion chromatography (IC), respectively. The main gas products included CO, CH4 and C2H4, and the liquid product was HCOOH. As shown in Fig. 3b and Fig. S8 (ESI†), the FEs of each product strictly depended on the working potential. For BIF-151 (Fig. 3b), the highest FE (32.53%) for HCOOH was detected at the lowest potential. With the negative shifting of potential, FEHCOOH decreased gradually while C2H4 became the dominant product of CO2 reduction. The FEs for C2H4 increased with the potential shifted from −1.2 V to −1.4 V and reached the maximum value of 25.94% at −1.4 V, which is much higher than that of CO, CH4. Meanwhile, the high FEC2H4 (about 20%) of BIF-151 can be maintained in a potential range of −1.2 V to −1.6 V, indicating the good selectivity of BIF-151 for C2 products. The other three catalysts (BIF-152, 153 and 154) showed the similar product distributions as BIF-151, but were much less active for C2H4 (Fig. 3c). With the same substituted position (2-substituted) as that of BIF-151, BIF-153 was the second active catalyst with a highest FEC2H4 of 16.67%. In comparison, the 3-substituted two catalysts, BIF-152 and 154 exhibited much lower selectivity for C2H4, with the highest FEsC2H4 of 13.04% and 9.99%, respectively. Correspondingly, electrochemical double-layer capacitances (Cdl) are calculated from cyclic voltammetry (CV) curves to estimate the electrochemical active surface area (Fig. 3d and Fig. S9, ESI†). BIF-151 exhibited the largest Cdl value (3.80 mF cm−2) than those of BIF-152 (3.50 mF cm−2), BIF-153 (3.48 mF cm−2) and BIF-154 (2.90 mF cm−2), basically consistent with the results of electrochemical tests. Given the identical metal coordination environment of the four catalysts, the distinctly different performance between them revealed that the catalytic activity and selectivity are not only strongly dependent on active metal sites, but also influenced by surrounding ligand environment, such as the composition and position of the substituent elements, etc.
1) solution saturated with CO2 from 0 to −1.6 V vs. reversible hydrogen electrode (RHE). On the base of linear sweep voltammetry (LSV), BIF-151 displayed the most positive onset potential of −0.37 V vs. RHE and the highest total current density at potential from −0.6 V to −1.6 V vs. RHE when comparing with other crystals (Fig. 3a). The potential gradient and the FE of each product were measured at constant potential. The gas phase products and liquid phase products were detected by gas chromatography (GC) and ion chromatography (IC), respectively. The main gas products included CO, CH4 and C2H4, and the liquid product was HCOOH. As shown in Fig. 3b and Fig. S8 (ESI†), the FEs of each product strictly depended on the working potential. For BIF-151 (Fig. 3b), the highest FE (32.53%) for HCOOH was detected at the lowest potential. With the negative shifting of potential, FEHCOOH decreased gradually while C2H4 became the dominant product of CO2 reduction. The FEs for C2H4 increased with the potential shifted from −1.2 V to −1.4 V and reached the maximum value of 25.94% at −1.4 V, which is much higher than that of CO, CH4. Meanwhile, the high FEC2H4 (about 20%) of BIF-151 can be maintained in a potential range of −1.2 V to −1.6 V, indicating the good selectivity of BIF-151 for C2 products. The other three catalysts (BIF-152, 153 and 154) showed the similar product distributions as BIF-151, but were much less active for C2H4 (Fig. 3c). With the same substituted position (2-substituted) as that of BIF-151, BIF-153 was the second active catalyst with a highest FEC2H4 of 16.67%. In comparison, the 3-substituted two catalysts, BIF-152 and 154 exhibited much lower selectivity for C2H4, with the highest FEsC2H4 of 13.04% and 9.99%, respectively. Correspondingly, electrochemical double-layer capacitances (Cdl) are calculated from cyclic voltammetry (CV) curves to estimate the electrochemical active surface area (Fig. 3d and Fig. S9, ESI†). BIF-151 exhibited the largest Cdl value (3.80 mF cm−2) than those of BIF-152 (3.50 mF cm−2), BIF-153 (3.48 mF cm−2) and BIF-154 (2.90 mF cm−2), basically consistent with the results of electrochemical tests. Given the identical metal coordination environment of the four catalysts, the distinctly different performance between them revealed that the catalytic activity and selectivity are not only strongly dependent on active metal sites, but also influenced by surrounding ligand environment, such as the composition and position of the substituent elements, etc.
In this work, we successfully constructed a stable metal boron imidazolate skeleton platform with the 2D topology by the structure-induced effect of KBH(mim)3 ligand. Moreover, the surrounding microenvironments of these 2D layers can be adjusted by a series of dangling monocarboxylate ligands with different substituent elements and substituent positions. Electrocatalytic performance results indicate that all these materials demonstrate catalytic activity towards CO2RR, especially with certain selectivity towards C2H4 products. However, the activity and selectivity towards C2H4 were distinctly different. Among them, BIF-151 showed the highest electrocatalytic performances with the FE of 25.94% for C2H4 at −1.4 V vs. RHE, which is about 1.9, 1.7, and 2.8 times higher than that of BIF-152 (13.04%), BIF-153 (15.05%), and BIF-154 (9.26%), respectively.
This work was supported by National Key Research and Development Program of China (2021YFA1501500), National Natural Science Foundation of China (22275192), the STS Project of Fujian-CAS (Grant No. 2023T3054).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Caldeira, A. K. Jain and M. I. Hoffert, Science, 2003, 299, 2052–2054 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- V. Humphrey, J. Zscheischler, P. Ciais, L. Gudmundsson, S. Sitch and S. I. Seneviratne, Nature, 2018, 560, 628–631 CrossRef CAS PubMed.
- S. I. Seneviratne, M. G. Donat, A. J. Pitman, R. Knutti and R. L. Wilby, Nature, 2016, 529, 477–483 CrossRef CAS.
- A. R. Woldu, Z. Huang, P. Zhao, L. Hu and D. Astruc, Coord. Chem. Rev., 2022, 454, 214340 CrossRef CAS.
- C. Cong and H. Ma, Small, 2023, 19, 2207547 CrossRef CAS PubMed.
- C. Zhao, X. Dai, T. Yao, W. Chen, X. Wang, J. Wang, J. Yang, S. Wei, Y. Wu and Y. Li, J. Am. Chem. Soc., 2017, 139, 8078–8081 CrossRef CAS PubMed.
- Y. Guo, H. Yang, X. Zhou, K. Liu, C. Zhang, Z. Zhou, C. Wang and W. Lin, J. Mater. Chem. A, 2017, 5, 24867–24873 RSC.
- Z. Xu, Y. Xie and Y. Wang, Mater. Rep.: Energy, 2023, 3, 100173 CAS.
- J. Jin, G. Cao, Y. Liu, Y. Shu, Z. Deng, W. Sun and X. Yang, Mater. Rep.: Energy, 2023, 3, 100229 CAS.
- B. Xu, I. M. U. Hasan, L. Peng, J. Liu, N. Xu, M. Fan, N. K. Niazi and J. Qiao, Mater. Rep.: Energy, 2022, 2, 100139 CAS.
- Z.-N. Wei, M.-N. Cao and R. Cao, J. Electrochem., 2022, 2215008 Search PubMed.
- Y. Y. Birdja, E. Pérez-Gallent, M. C. Figueiredo, A. J. Göttle, F. Calle-Vallejo and M. T. M. Koper, Nat. Energy, 2019, 4, 732–745 CrossRef CAS.
- N. Kornienko, Y. B. Zhao, C. S. Kiley, C. H. Zhu, D. Kim, S. Lin, C. J. Chang, O. M. Yaghi and P. D. Yang, J. Am. Chem. Soc., 2015, 137, 14129–14135 CrossRef CAS.
- S. Jin, Z. M. Hao, K. Zhang, Z. H. Yan and J. Chen, Angew. Chem., Int. Ed., 2021, 60, 20627–20648 CrossRef CAS PubMed.
- Z.-H. Zhao, K. Zheng, N.-Y. Huang, H.-L. Zhu, J.-R. Huang, P.-Q. Liao and X.-M. Chen, Chem. Commun., 2021, 57, 12764–12767 RSC.
- H. B. Yang, S. F. Hung, S. Liu, K. D. Yuan, S. Miao, L. P. Zhang, X. Huang, H. Y. Wang, W. Z. Cai, R. Chen, J. J. Gao, X. F. Yang, W. Chen, Y. Q. Huang, H. M. Chen, C. M. Li, T. Zhang and B. Liu, Nat. Energy, 2018, 3, 140–147 CrossRef CAS.
- K. P. Kuhl, T. Hatsukade, E. R. Cave, D. N. Abram, J. Kibsgaard and T. F. Jaramillo, J. Am. Chem. Soc., 2014, 136, 14107–14113 CrossRef CAS PubMed.
- H. Song, M. Im, J. T. Song, J. A. Lim, B. S. Kim, Y. Kwon, S. Ryu and J. Oh, Appl. Catal., B, 2018, 232, 391–396 CrossRef CAS.
- Y. Li, S. L. Zhang, W. Cheng, Y. Chen, D. Luan, S. Gao and X. W. Lou, Adv. Mater., 2022, 34, 2105204 CrossRef CAS PubMed.
- C. Kong, G. Jiang, Y. Sheng, Y. Liu, F. Gao, F. Liu and X. Duan, Chem. Eng. J., 2023, 460, 141803 CrossRef CAS.
- C. T. Dinh, T. Burdyny, M. G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. P. G. de Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Q. Zou, R. Quintero-Bermudez, Y. J. Pang, D. Sinton and E. H. Sargent, Science, 2018, 360, 783–787 CrossRef CAS PubMed.
- T. Yan, X. Chen, L. Kumari, J. Lin, M. Li, Q. Fan, H. Chi, T. J. Meyer, S. Zhang and X. Ma, Chem. Rev., 2023, 123, 10530–10583 CrossRef CAS PubMed.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Y. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Norskov, T. F. Jaramillo and I. Chorkendorff, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS.
- Y. Zheng, A. Vasileff, X. L. Zhou, Y. Jiao, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2019, 141, 7646–7659 CrossRef CAS PubMed.
- G. L. De Gregorio, T. Burdyny, A. Loiudice, P. Iyengar, W. A. Smith and R. Buonsanti, ACS Catal., 2020, 10, 4854–4862 CrossRef CAS PubMed.
- F. Chang, M. Xiao, R. Miao, Y. Liu, M. Ren, Z. Jia, D. Han, Y. Yuan, Z. Bai and L. Yang, Electrochem. Energy Rev., 2022, 5, 4 CrossRef CAS.
- H.-X. Zhang, C. Lu and J. Zhang, Acc. Mater. Res., 2023, 4, 995–1007 CrossRef.
- G. Xu, Q.-L. Hong, Y. Sun, M. Liu, H.-X. Zhang and J. Zhang, Chin. Chem. Lett., 2022, 33, 2915–2918 CrossRef CAS.
- Z.-Y. Chen, Q.-L. Hong, H.-X. Zhang and J. Zhang, ACS Appl. Energy Mater., 2022, 5, 1175–1182 CrossRef CAS.
- P. Shao, Y.-M. Wan, L. Yi, S. Chen, H.-X. Zhang and J. Zhang, Small, 2024, 20, 2305199 CrossRef CAS PubMed.
- C. S. Gerke, Y. Xu, Y. Yang, G. D. Foley, B. Zhang, E. Shi, N. M. Bedford, F. Che and V. S. Thoi, J. Am. Chem. Soc., 2023, 145, 26144–26151 CrossRef CAS PubMed.
- P. Shao, W. Zhou, Q.-L. Hong, L. Yi, L. Zheng, W. Wang, H.-X. Zhang, H. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2021, 60, 16687–16692 CrossRef CAS PubMed.
- Y. Zhang, Q. Zhou, Z.-F. Qiu, X.-Y. Zhang, J.-Q. Chen, Y. Zhao, F. Gong and W.-Y. Sun, Adv. Funct. Mater., 2022, 32, 2203677 CrossRef CAS.
- H. Li, J. Zhao, L. Luo, J. Du and J. Zeng, Acc. Chem. Res., 2021, 54, 1454–1464 CrossRef CAS PubMed.
- L. Jiao, J. Wang and H.-L. Jiang, Acc. Mater. Res., 2021, 2, 327–339 CrossRef CAS.
- J. H. Montoya, C. Shi, K. Chan and J. K. Nørskov, J. Phys. Chem. Lett., 2015, 6, 2032–2037 CrossRef CAS.
- D. Cheng, Z.-J. Zhao, G. Zhang, P. Yang, L. Li, H. Gao, S. Liu, X. Chang, S. Chen, T. Wang, G. A. Ozin, Z. Liu and J. Gong, Nat. Commun., 2021, 12, 395 CrossRef CAS PubMed.
- R. Kortlever, J. Shen, K. J. P. Schouten, F. Calle-Vallejo and M. T. M. Koper, J. Phys. Chem. Lett., 2015, 6, 4073–4082 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TGA diagram, powder X-ray diffraction, CD, UV, etc. Crystal data and structure refinement details. CCDC 2362473–2362476. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc02928c |
| This journal is © The Royal Society of Chemistry 2024 |