Open Access Article
Open Access ArticleOpen-[60]fullerenols with water adsorbed both inside and outside†
Yoshifumi
Hashikawa
 *a,
Shumpei
Sadai
a,
Yuka
Ikemoto
*a,
Shumpei
Sadai
a,
Yuka
Ikemoto
 b and
Yasujiro
Murata
b and
Yasujiro
Murata
 *a
*a
aInstitute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011, Japan
bJapan Synchrotron Radiation Research Institute, Sayo-gun, Hyogo 679-5198, Japan
First published on 5th January 2024
Abstract
The water affinity on [60]fullerenols was found to be governed by surface electrostatic potential while water aggregation is initiated by the hydroxy groups attached on the carbon surface. The molecular water adsorption at the internal sphere caused a significnat inhibition of water adsorption at the external carbon surface.
Water confined between graphene layers is known to adopt a regular ordering on the two-dimensional surface, thus exhibiting a characteristic chemical/physical feature that is otherwise invisible in a bulk environment.1 Likewise, water inside carbon nanotubes2 or fullerenes3 has attracted considerable attention owing to its unique dimensionalities which are offered by the internal curved π-surface of carbon nanomaterials. In contrast, a lack of experimental studies has hampered understanding in depth the interactions and structures of water aggregated on the external π-surface of fullerenes. This is because of very low solubility in an aqueous medium (<10−9 mg L−1) as well as a high degree of aggregation.4 Nevertheless, computational investigations have predicted stable wetting processes of fullerenes.5
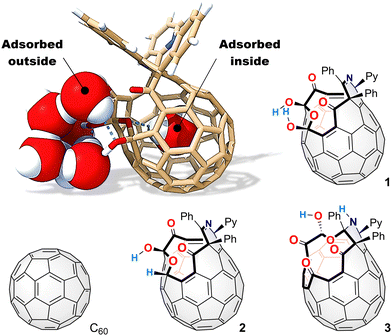
Polydisperse aqueous systems of [60]fullerene, which are colloids rather than true solutions, could be prepared experimentally wherein [60]fullerene aggregates generate fractal clusters with a diameter range of one to several tens of nanometres.6 According to molecular dynamics simulations, water–[60]fullerene interactions are governed by relatively strong dispersive van der Waals attraction7 while hydrogen-bonding (H-bonding) networks contain a mixture of quasi-pentagonal/hexagonal forms with multiple defects induced by dangling hydroxy groups.8 Bovine serum albumin is a biocompatible solubilizer for [60]fullerene in aqueous media, in which unfolding of the albumin exposes its inner hydrophobic residues which enhance interactions with [60]fullerene.9 Even in the albumin, [60]fullerene still maintains its cytotoxicity, implying its potential for biological applications. The introduction of multiple functional groups having high affinity to water is a popular approach to access synthetic water-soluble [60]fullerenes and/or [60]fullerenols.10 At a water–solvent phase boundary, they could arrange in a two-dimensional sheet with a precise thickness at an order of nanometres.11 Furthermore, stable exfoliation of graphene could be achieved by reactions with [60]fullerenols in a water phase since intercalated [60]fullerenols inhibit the restacking of the graphene sheets.12 Thus, it is of particular importance to clarify the adsorptive behaviour/structure of water on the [60]fullerene surface (Fig. 1) for the advanced design of [60]fullerenols for their biological and materials science applications. However, controlled hydroxylation of [60]fullerene is, in general, not feasible, thus producing an isomeric mixture which possesses 15 to 18 hydroxy groups with different dispositions.13 Then, a clear picture on the surface adsorption behaviour of water onto [60]fullerenols is hard to be obtained using an experimental approach so that a computational approach has long been employed, which is the only viable solution to this problem.14 Herein, we studied the adsorption behaviour of water onto three open-[60]fullerenols as well as pristine [60]fullerene using IR microspectroscopy at variable humidity (VH) (Fig. 1). We also disclosed a significant depletion of external water adsorbed on the open-[60]fullerenol surface, being perturbed by internal water trapped within the [60]fullerene cavity.
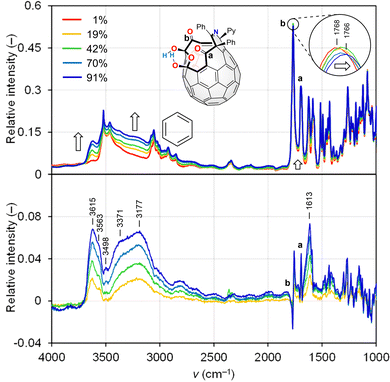
To verify the water adsorptive behaviour on the surface of a powdery [60]fullerene sample, we performed synchrotron IR microspectroscopy15 at 300 K with different relative humidities (RHs). As a result, two sharp bands were observed at ν 1427 and 1180 cm−1 (Fig. S1a, ESI†), which corresponded to cage vibrations of [60]fullerene, whereas any band related to aggregated water could not be detected so that differential spectra are featureless even at RH = 87% (Fig. S1b, ESI†), implying negligible water adsorption on the powdery [60]fullerene surface. This differs from a crystalline [60]fullerene sample, showing an O 1s band found at 533.8 eV attributed to adsorbed water in the X-ray photoelectron spectrum where lower crystallinity causes a significant decrease in the band intensity16 probably due to a larger surface free energy.17
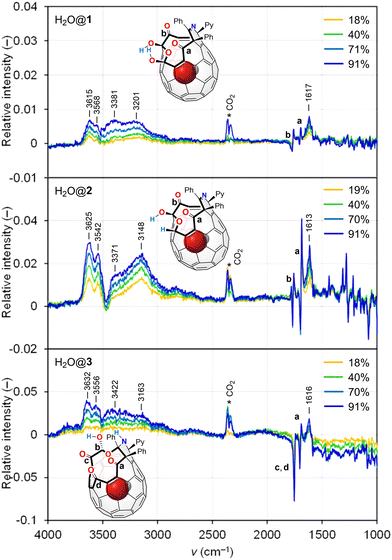
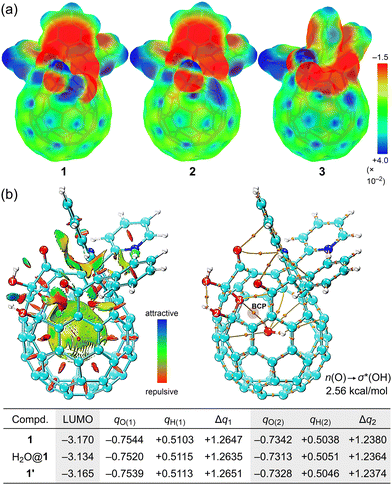
To improve the wettability, we then prepared open-[60]fullerenols, C60(OH)2O3R2 (1, R2 = (CPh)2NCPy), C60(OH)(H)O3R2 (2, R2 = (CPh)2NCPy), and C60(OH)O4R2 (3, R2 = (CPh)2(NH)CPy),18 all of which bear an opening with a ring-atom count of 13 (Fig. 1). The latter two were derived from 1 while they accommodate a single water molecule inside (H2O@2 and H2O@3) due to the synthetic feature. We also synthesized H2O@1 for a reference. The VH IR spectra are shown in Fig. 2 and 3. Contrary to [60]fullerene, 1 showed a significant spectral change attributed to O–H bands (ca. 3800–3000 cm−1) with increasing intensity (Fig. 2). In the differential spectra relative to RH = 1%, there seem to be two bands at ca. 3800–3500 and 3500–3000 cm−1, which are typical bands of free and aggregated water, respectively.19 The bending mode at 1613 cm−1 conclusively indicates the surface water adsorption. From the simulation (B3LYP-D3/6-31G(d,p)), the bands at 1768 and 1697 cm−1 were assigned to the stretching modes of carbonyl groups, b and a, respectively, where the former exhibited a larger red-shift upon exposure to higher RHs. This is indicative of the water adsorption concomitantly assisted by the carbonyl groups, a and b, while the latter is much superior. In the differential spectrum, a complex spectral feature was observed at a fingerprint region (< ca. 1600 cm−1), which was absent for [60]fullerene. Thus, the two hydroxy groups in 1 are considered to facilitate the water adsorption even at the curved-π surface. The differential spectra of H2O@1, H2O@2, and H2O@3 showed a resemblance to that of 1 (Fig. 3). Note that the band of incarcerated H2O could not be observed under the measured conditions.20 As opposed to 1 and H2O@1, a larger red-shift was observed for carbonyl band a of H2O@2 due to the removal of one hydroxy group from H2O@1, whereas water adsorption on H2O@3 occurs with significant assistance from carbonyl group(s), c and/or d. For all open-[60]fullerenols examined, water adsorption/desorption processes were confirmed to be reversible since spectra always came back to the same line at any RH even once RH was increased to ca. 90%.
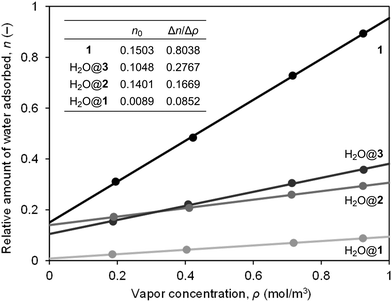
From the area ratio for the OH bands in the differential spectra, relative amounts of water adsorbed were estimated, giving a linear relationship with vapor concentrations in air (Fig. 4). It should be noted that all fitted lines did not pass through the origin, implying the presence of water adsorbed even under dry conditions, whose amounts follow the order of H2O@2 > H2O@3 > H2O@1. From the slope, affinity to water was found to follow the order of H2O@3 > H2O@2 > H2O@1 even though 1 bears two hydroxy groups on its orifice. The replacement of one of the hydroxy groups with a hydrogen atom (H2O@1 to H2O@2) was suggested to induce a local positive potential area on the [60]fullerene cage while the whole caged surface of H2O@3 is positively charged due to the electron-withdrawing character of the three carbonyl groups (Fig. 5a). Thus, the Lewis acidity of carbon cages21 is considered to play a significant role in water affinity while OH and/or NH groups should be a trigger to initiate water adsorption.
To our surprise, placing a water molecule inside 1 considerably decreases water affinity by one tenth (Fig. 4). To clarify the origin of the change in adsorptive character, we performed topological analysis (Fig. 5b), showing weak non-covalent interactions between incarcerated H2O and the hemispherical caged surface where H2O and O(3) were connected via a bond path characterized by a bond critical point (BCP) with positive Laplacian (∇2ρ = 3.46 × 10−2e a0−5) and total energy (H = 8.19 × 10−5 a.u. a0−3) of electron density. Thus, H2O@1 is considered to possess an intramolecular H-bonding which is categorized as a pure closed-shell interaction without covalent nature.22 The interaction energy was estimated to be E(2) 2.56 kcal mol−1 (n(O(3)) → σ*(H2O)). Upon encapsulation of H2O, 1 receives a small distortion (ΔG + 0.365 kcal mol−1) whose geometry itself (1′) does not cause a significant change in the electronic properties. However, interaction with water results in an elevated LUMO level by net +0.031 eV, indicating the reduced Lewis acidity which contributes to lower Δn/Δρ relative to pristine 1. In addition, the polarization of the two hydroxy groups is suggested to become weaker with smaller Δq values. This might be attributed to the diminished amount of water adsorbed under dry conditions (smaller n0).
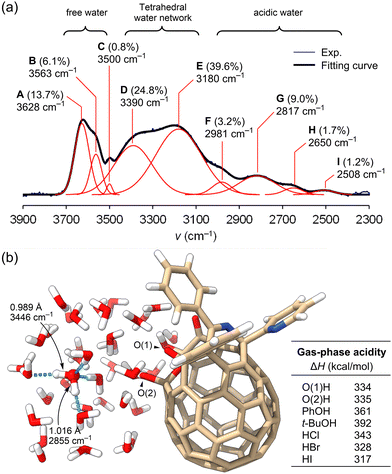
The band deconvolution analysis of 1 at RH = 91% showed the presence of multiple bands (Fig. 6) where A and B are free water probably spread over the [60]fullerene surface while D and E originate from distorted and non-distorted ice-like water networks structured by the two hydroxy groups.19 Though band C is not usually observed for bulk water, it likely arises from unstable/short-lived small water clusters aggregated on the π-surface via defective H-bondings. Importantly, we also found multiple bands below 3000 cm−1 (F–I) which cover 14.1% of the whole OH spectral bands (3750–2410 cm−1). These are typical of flanking water around protons (2500 ± 500 cm−1).23 To our surprise, gas-phase acidity values24 of the two hydroxy groups in 1 were calculated to be ΔH 334 and 335 kcal mol−1 (B3LYP-D3/6-31G(d,p)) which were far smaller than those for PhOH (361 kcal mol−1) and t-BuOH (392 kcal mol−1) but rather similar to those for HCl (343 kcal mol−1) and HBr (328 kcal mol−1), indicating that open-[60]fullerenols are highly acidic (Fig. 6b and Fig. S7, ESI†). This rationally explains the presence of bands, F–I. Thereby, open-[60]fullerenols are considered to enhance the local acidity of water adsorbed owing to their high electron-accepting character. The significantly red-shifted band (ν 2855 cm−1) of adsorbed water with an elongated OH bond (1.016 Å) was also supported by theoretical calculations for a local minimum structure of 1·(H2O)39 (Fig. 6b and Fig. S8–S10, ESI†).
In summary, we examined the adsorptive behaviour of water onto the [60]fullerene surface using synchrotron IR microspectroscopy, showing reversible water adsorption/desorption. The VH IR measurements revealed the presence of water adsorbed on open-[60]fullerenols even under dry conditions while the water affinity is governed by the surface electrostatic potential. To our surprise, molecular water adsorption from the internal sphere resulted in a considerable drop of the water affinity at the external sphere mainly because of lowering the Lewis acidity of the [60]fullerene cage while a high electron-accepting character of [60]fullerenols increases the local acidity of water adsorbed.
Financial support was partially provided by the JSPS KAKENHI Grant Numbers JP23H01784, JP23K17916, JP22H04538, and JP19H05717, The Mazda Foundation, and Advanced Technology Institute Research Grants 2023. IR measurements were carried out at SPring-8 (BL43IR) with the approval of JASRI (2021B1298, 2022A1285, and 2022B1138).
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Garcia, ACS Nano, 2023, 17, 51–69 CrossRef CAS PubMed.
- A. Alexiadis and S. Kassinos, Chem. Rev., 2008, 108, 5014–5034 CrossRef CAS PubMed.
- (a) L. Shi and L. Gan, J. Phys. Org. Chem., 2013, 26, 766–772 CrossRef CAS; (b) Y. Hashikawa and Y. Murata, Bull. Chem. Soc. Jpn., 2023, 96, 943–967 CrossRef CAS.
- A. L. Smith, M. V. Korobov, A. L. Mirakyan, N. V. Avramenko and E. B. Stukalin, J. Phys. Chem. B, 2001, 105, 2499–2506 CrossRef.
- R. Rivelino and F. D. B. Mota, Nano Lett., 2007, 7, 1526–1531 CrossRef CAS PubMed.
- P. Scharff, K. Risch, L. Carta-Abelmann, I. M. Dmytruk, M. M. Bilyi, O. A. Golub, A. V. Khavryuchenko, E. V. Buzaneva, V. L. Aksenov, M. V. Avdeev, Y. I. Prylutskyy and S. S. Durov, Carbon, 2004, 42, 1203–1206 CrossRef CAS.
- L. Li, D. Bedrov and G. D. Smith, J. Chem. Phys., 2005, 123, 204504 CrossRef PubMed.
- S. R. Varanasl, O. A. Guskova, A. John and J.-U. Sommer, J. Chem. Phys., 2015, 142, 224308 CrossRef PubMed.
- H. Wu, L. Lin, P. Wang, S. Jiang, Z. Dai and X. Zou, Chem. Commun., 2011, 47, 10659–10661 RSC.
- K. Harano and E. Nakamura, Acc. Chem. Res., 2019, 52, 2090–2100 CrossRef CAS PubMed.
- P. Ravat, H. Uchida, R. Sekine, K. Kamei, A. Yamamoto, O. Konovalov, M. Tanaka, T. Yamada, K. Harano and E. Nakamura, Adv. Mater., 2022, 34, 2106465 CrossRef CAS PubMed.
- R. Kumar, P. Kumar, S. Naqvi, N. Gupta, N. Saxena, J. Gaur, J. K. Maurya and S. Chand, New J. Chem., 2014, 38, 4922–4930 RSC.
- T. H. Goswami, B. Nandan, S. Alam and G. N. Mathur, Polymer, 2003, 44, 3209–3214 CrossRef CAS.
- A. Dawid, K. Górny and Z. Gburski, J. Phys. Chem. C, 2017, 121, 2303–2315 CrossRef CAS.
- H. Yamagishi, S. Nakajima, J. Yoo, M. Okazaki, Y. Takeda, S. Minakata, K. Albreht, K. Yamamoto, I. Badia-Daminguez, M. M. Oliva, M. C. R. Delgado, Y. Ikemoto, H. Sato, K. Imoto, K. Nakagawa, H. Tokoro, S.-I. Ohkoshi and Y. Yamamoto, Commun. Chem., 2020, 3, 118 CrossRef CAS PubMed.
- D. Erbahar, T. Susi, X. Rocquefelte, C. Bittencourt, M. Scardamaglia, P. Blaha, P. Guttmann, G. Rotas, N. Tagmatarchis, X. Zhu, A. P. Hitchcock and C. P. Ewels, Sci. Rep., 2016, 6, 35605 CrossRef CAS PubMed.
- (a) Y. Tajima, T. Matsuura, Y. Numata, D. Yamazaki, H. Kawamura and H. Osedo, Jpn. J. Appl. Phys., 2008, 47, 5730 CrossRef CAS; (b) X. Ma, B. Wigington and D. Bouchard, Langmuir, 2010, 26, 11886–11893 CrossRef CAS PubMed.
- (a) Y. Hashikawa, K. Kizaki, T. Hirose and Y. Murata, RSC Adv., 2020, 10, 40406–40410 RSC; (b) Y. Hashikawa, S. Hasegawa and Y. Murata, Org. Lett., 2021, 23, 3854–3858 CrossRef CAS PubMed; (c) Y. Hashikawa, K. Kizaki and Y. Murata, Chem. Commun., 2021, 57, 5322–5325 RSC.
- M. Śmiechowski and J. Stangret, J. Mol. Struct., 2008, 878, 104–115 CrossRef.
- A. Shugai, U. Nagel, Y. Murata, Y. Li, S. Mamone, A. Krachmalnicoff, S. Alom, R. J. Whitby, M. H. Levitt and T. Rõõm, J. Chem. Phys., 2021, 154, 124311 CrossRef CAS PubMed.
- J. Iglesias-Sigüenza and M. Alcarazo, Angew. Chem., Int. Ed., 2012, 51, 1523–1524 CrossRef PubMed.
- (a) W. Nakanishi, S. Hayashi and K. Narahara, J. Phys. Chem. A, 2009, 113, 10050–10057 CrossRef CAS PubMed; (b) Y. Hashikawa and Y. Murata, J. Am. Chem. Soc., 2019, 141, 12928–12938 CrossRef CAS PubMed.
- (a) M. Thämer, L. D. Marco, K. Ramasesha, A. Mandal and A. Tokmakoff, Science, 2015, 350, 78–82 CrossRef PubMed; (b) N. Agmon, Nat. Chem., 2016, 8, 206–207 CrossRef CAS PubMed; (c) C. A. Daly Jr, L. M. Streacker, Y. Sun, S. R. Pattenaude, A. A. Hassanali, P. B. Petersen, S. A. Corcelli and D. Ben-Amotz, J. Phys. Chem. Lett., 2017, 8, 5246–5252 CrossRef PubMed; (d) J. A. Fournier, W. B. Carpenter, N. H. C. Lewis and A. Tokmakoff, Nat. Chem., 2018, 10, 932–937 CrossRef CAS PubMed.
- J. E. Bartmess, J. A. Scott and R. T. Mclver Jr, J. Am. Chem. Soc., 1979, 101, 6046–6056 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Optimized geometries. See DOI: https://doi.org/10.1039/d3cc05542f |
| This journal is © The Royal Society of Chemistry 2024 |