Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Water detection in organic solvents using a copolymer membrane immobilised with a fluorescent intramolecular charge transfer-type dye: effects of intramolecular hydrogen bonds†
Ami
Morimoto
a,
Kei
Shimizu
a,
Naoya
Suzuki
a,
Shigeyuki
Yagi
a,
Kenji
Sueyoshi
ab,
Tatsuro
Endo
a and
Hideaki
Hisamoto
 *a
*a
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka Metropolitan University, 1-1 Gakuen-cho, Naka-ku, Sakai, Osaka, 599-8531 Japan. E-mail: hisamoto@omu.ac.jp
bCREST, Japan Science and Technology Agency, Japan
First published on 16th February 2024
Abstract
Numerous fluorescent dye-based optical sensors have been developed to detect water in organic solvents. However, only a few such sensors can detect water in polar solvents such as methanol or dimethyl sulfoxide, and their detection range is generally narrow. Therefore, in this study, a copolymer membrane incorporated with a pyridinium betaine dye (denoted PB1), which exhibited intramolecular charge transfer (ICT) characteristics, was developed to realise simple water detection in organic solvents. The pyridinium betaine structure, comprising intramolecular hydrogen bonds between the oxygen in the maleimide moiety and the hydrogen in the pyridinium, was vital for achieving efficient fluorescence emission. The membrane was prepared by copolymerising PB1 with the N,N-dimethyl acrylamide/acrylamide monomer on a glass plate, and the fluorescence in water-mixed organic solvents was investigated (λabs = 490 nm, λfl = 630 nm). The fluorescence intensity of the dye-immobilised membrane decreased with increasing water content of the organic solvents. The detection ranges in tetrahydrofuran, ethanol, methanol, and dimethyl sulfoxide were approximately <40, <40, <40, and <60 vol% water, respectively. In contrast, membranes based on a quaternary pyridinium dye (without intramolecular hydrogen bonds) did not detect water in methanol and dimethyl sulfoxide, although it was more sensitive than PB1 in the narrow region of low water concentration in THF. Theoretical calculations corroborated the importance of the pyridinium betaine structure in detecting water in organic solvents, with the increase in polarity and the formation of intermolecular hydrogen bonds between PB1 and water found to induce molecular rotation and fluorescence quenching.
Introduction
Water detection in organic solvents has garnered significant interest in research and industry fields such as organic synthesis, purity measurement, and quality control. In particular, Karl Fischer (KF) titration is predominantly employed to determine the content of water in liquids, solids, and gases.1–3 Although KF titration offers high accuracy, high precision, and a wide measurement range (water content: 1 ppm to 100%), it is somewhat inconvenient because it requires several instruments such as electrodes and involves the preparation of titration reagents. Additionally, care should be taken to ensure that interferents such as ketones, aldehydes, and redox chemicals are excluded from samples.3–7 Although approaches to preventing the interference reaction have been explored,8,9 simpler water detection methods are required to overcome the aforementioned difficulties.Optical chemical sensors (optodes) have attracted considerable attention because of their simplicity;10 therefore, numerous water-detecting optodes based on colourimetric and/or fluorescent dyes have been developed to date.11–35 Fluorescence-based optodes are typically more sensitive than colourimetry-based optodes. The response mechanism of fluorescence-based optodes containing organic fluorophores can be classified based on the fluorescence mechanism18,19 into photo-induced electron transfer (PET),20–24 intramolecular charge transfer (ICT),21,25–28 twisted ICT (TICT),26,29,30 aggregation-induced emission (AIE),24,29,30,32,33 and excited-state intramolecular proton transfer (ESIPT),31,34,35 and so on. In addition to the organic-fluorophore-based water detectors, other luminescent inorganic and/or organic water detectors – such as graphene quantum dots,36 carbon dots (CDs),37–40 covalent organic frameworks (COFs),41–44 and metal–organic frameworks (MOFs)39,45–47 – have been developed over the past decade. Besides, applications,18 such as inkless writing,32,38 paper strips,25,31–33,35,40 and luminophore-doped films13,15,17,22,23,38 have been developed for simpler water detection as well as traditional solution-based measurement. Although various luminescent materials have been devised for water detection, most are suitable for recognising water in 1,4-dioxane, tetrahydrofuran (THF), and acetonitrile. Sensors for water detection in methanol and dimethyl sulfoxide (DMSO) have also been reported (Table 1); however, their detection range is generally narrow, the choices of fluorescence colour are limited, or the assessment typically involves solution-based measurements or paper-strip-based detection.
| Sensing mechanism | Ref. | Solvent | Detection range | λ fl (nm) | Measurement strategy other than the solution measurement scheme |
|---|---|---|---|---|---|
| AIE/PET | 24 | DMSO | 0–1.18 wt% | 510 | — |
| ICT | 25 | Methanol | 0–16, 60–90% | 450 | Paper strip detection |
| ICT/TICT | 26 | DMSO | <10 vol% | 540 | — |
| ICT/deprotonation | 28 | DMSO | <0.16 vol% | 635–700 | — |
| Methanol | <2.0 vol% | ||||
| AIE | 32 | DMSO | — | 510 | Inkless writing, paper strip detection |
| Methanol | |||||
| AIE | 33 | DMSO | 0–15 vol% | 515 | Paper detection |
| ESIPT | 34 | DMSO | <52 wt% | 480–502 | — |
| ESIPT | 35 | Methanol | 0–80 vol% | 454–506 | Paper detection |
| CDs | 40 | Methanol | 0.5–20.0 vol% | 610 | Paper strip detection |
| COF | 41 | Methanol | <50 vol% | 380 | — |
| MOF/ESIPT | 45 | Methanol | 0–1.3 vol% | 477 | ZnO-supporting hybrid film detection |
| MOF/ESIPT | 47 | DMSO | 0–5.2 vol% | 450–540 | — |
| Methanol | 0–2.2 vol% | ||||
| ICT | Present study | DMSO | 0–60 vol% | 630 | Copolymer membrane detection |
| Methanol | 0–40 vol% |
This study aimed at developing an optode membrane using an ICT-type dye for simple water detection in organic solvents, including polar solvents such as methanol and DMSO. ICT-type dyes are often used as chemical sensors for water detection because they are sensitive to polarity, and their absorption and/or fluorescence spectral changes depend on the solvent polarity. Additionally, they permit adjustment of the absorption and/or fluorescence bands by molecular design and exhibit low background noise owing to a large Stokes shift. However, the fluorescence intensity of ICT-type dyes occasionally diminishes in polar solvents compared to that in non-polar solvents owing to enhanced charge separation and decreased transition probability.27,48 Therefore, ICT-type dyes are suitable for water detection in non-polar solvents but not in polar solvents. Accordingly, to achieve water detection even in polar solvents, a cyclic enolate pyridinium betaine (PB) structure was targeted in the present study (Fig. 1a). In 2021, Suzuki and Yagi et al. reported that PB structure has intramolecular hydrogen bonds between the carbonyl oxygen atoms of the cyclic enolate and the α-hydrogen atoms of the pyridinium ring, which suppresses TICT-induced fluorescence quenching.49 Moreover, the same group reported that π-extension and the introduction of a donor such as an N,N-diphenylamino group to PB structure resulted in exhibiting enhanced fluorescence; additionally, the dyes were found to exhibit solvatochromic behaviour owing to their donor–acceptor (D–A)-type structures.50 By imparting ICT characteristics to the PB structure, the sensitivity to water and detection range would be changed. In anhydrous organic solvents, PB dyes form a planar structure owing to their intramolecular hydrogen bonds, whereas in the presence of water, the intramolecular hydrogen bonds are weakened by intermolecular hydrogen bonds between the dye and water (Fig. 1c). Therefore, the PB structure would transforms into a twisted form, leading to reduced fluorescence.
 | ||
| Fig. 1 (a) The pyridinium betaine (PB) structure, as reported by Suzuki and Yagi et al.49 (b) The molecular design employed in the present study. (c) Concept underlying the fluorescence-based water detection (HB: Hydrogen Bond). (d) The quaternary pyridinium structure QP1, which was used as a reference compound. | ||
In this study, a novel PB dye (PB1) with a terminal olefin moiety (Fig. 1b) was synthesised, and the optical and fluorescence properties in solution were measured using PB-C10 to eliminate the influence of the terminal olefin to optical properties. Subsequently, dye-immobilised copolymer membranes were prepared12,14 using the N,N-dimethylacrylamide (DMAA)/acrylamide (AA) comonomer to develop simple detection devices. Water detection in organic solvents was investigated using the dye-immobilised copolymer membranes. To elucidate the effects of the PB structure, a copolymer membrane immobilised quaternary pyridinium dye (QP1) without hydrogen bonding site was also prepared (Fig. 1d), and its sensing performance was analysed. Finally, the underlying response mechanism was clarified and then verified through theoretical calculations.
Experimental
Materials and instruments
All synthetic reagents and membrane materials were purchased from Tokyo Chemical Industry (Tokyo, Japan), FUJIFILM Wako Pure Chemical Corporation (Tokyo, Japan), or Kanto Chemical (Tokyo, Japan). All super-dehydrated organic solvents were purchased from FUJIFILM Wako Pure Chemical Corporation (Tokyo, Japan). The details of synthesis and characterization of the compounds are described in the ESI document.† UV–vis absorption spectra were acquired using a JASCO V-730 instrument, whereas fluorescence spectra were captured using a JASCO FP-8550 instrument.Preparation of dye-immobilised copolymer membranes
Glass slide plates were cut into segments measuring 11.5 × 26.0 mm2, which were then immersed overnight in a mixture of aqueous NaOH (1 M, 9.4 mL) and ethanol (0.6 mL). The resulting glass plates were washed with water and acetone, dried at 70 °C for 30 min, immersed in a mixture of 3-(trimethoxysilyl)propyl methacrylate (8.0 mL) and aqueous HCl (0.1 M, 2.0 mL), and then sonicated until no suspension was observed (∼10 min). Finally, the methacrylated glass plates were washed with methanol and acetone and then dried at 70 °C for 30 min. Membrane mould spacers were subsequently assembled on the methacrylated glass (Fig. S1†). To that end, two layers of a 0.13 mm-thick fluoroplastic polytetrafluoroethylene film (ASF-110 FR, Chukoh Chemical Industries) with a hollowed out area measuring ∼18 × 8 mm2 were pasted onto the glass, creating a 0.26 mm-thick spacing. Approximately 40 μL of the pre-polymer solution – which comprised DMAA (0.80 mmol), AA (0.20 mmol), N,N-methylenebis(acrylamide) (5.0 μmol), azobis(isobutyronitrile) (3.0 μmol), the functional dye (0.2 μmol), water (150 μL), and 1,4-dioxane (150 μL) – was poured into the created mould, covered with an unmodified glass plates, and then polymerised at 65 °C for 45 min. The resulting membrane was washed with methanol and THF to remove unreacted reagents and then stored in super-dehydrated THF.Preparation of sample solutions
The sample solutions – that is, mixtures of organic solvents and water with different volume ratios – were prepared immediately before the measurements to maximally prevent moisture absorption. The dye-immobilised copolymer membranes on glass plates were immersed in each sample solution for 5 min. Fluorescence spectra were acquired using a freshly prepared solution in a 10 × 10 mm2 quartz cell, with the prepared dye-immobilised copolymer membranes on glass plates fixed diagonally across the quartz cell.Results and discussion
Light absorption and fluorescence in solutions
To elucidate the light absorption and fluorescence properties of the functional dyes, UV–vis absorption and fluorescence spectra were acquired in chloroform (CHCl3), THF, ethyl acetate (AcOEt), and DMSO (5 μM). In addition, fluorescence quantum yield (ΦFL) in each solvent was investigated. The spectra and spectral data are shown in Fig. 2 and Table 2, respectively. In THF, PB-C10 exhibited an absorption maximum (λabs) at 490 nm and a fluorescence maximum (λfl) at 600 nm; these bands can be attributed to ICT transition (vide infra). λabs depended minimally on the solvent polarity, except in the case of chloroform, whereas the λfl for DMSO was red shifted by 41 nm compared to that for THF, indicating that PB-C10 exhibited electrically neutral ICT-type properties. Generally, the solvent polarity influences electrically neutral ICT-type dyes in the excited states to a greater extent than those in the ground states, because the former exhibit a larger dipole moment than that of the latter.48QP1 tended to exhibit superior ICT properties compared with those of PB-C10. For instance, its λabs and λfl values in THF were blue shifted to 460 nm and red shifted to 616 nm, respectively, compared with those of PB-C10. The Stokes shift of QP1 in THF (5505 cm−1; 156 nm) was larger than that of PB-C10 (3741 cm−1, 110 nm). Unfortunately, the photophysical properties of PB-C10 in more polar solvents, such as ethanol and methanol, could not be measured owing to their low solubility.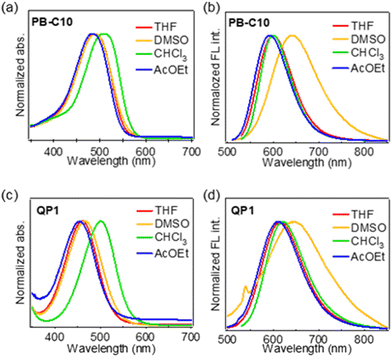 | ||
| Fig. 2 (a and c) UV–vis absorption spectra and (b and d) fluorescence profiles of PB-C10 (a and b) and QP1 (c and d) in THF, DMSO, CHCl3, and AcOEt. | ||
| Solvent | PB-C10 | QP1 | ||||
|---|---|---|---|---|---|---|
| λ abs (nm) | λ fl (nm) | Φ FL | λ abs (nm) | λ fl (nm) | Φ FL | |
| THF | 490 | 600 | 0.33 | 460 | 616 | 0.05 |
| DMSO | 490 | 641 | 0.04 | 466 | 647 | <0.01 |
| CHCl3 | 510 | 600 | 0.49 | 500 | 621 | 0.07 |
| AcOEt | 484 | 593 | 0.26 | 455 | 612 | 0.05 |
Water detection using dye-immobilised copolymer membranes
Water detection using dye solutions is inconvenient because reagent preparation must be performed on each occasion. Additionally, hydrophobic dye molecules cannot be used owing to their low solubility in water-mixed solvents, and the detection sensitivity is significantly lower than that of soluble dyes, except those with AIE luminogens. To overcome these problems, functional dyes were immobilised onto a copolymer membrane at the terminal olefin moiety on a glass plate in the present study, to enable simple detection of water in organic solvents.First, monomer materials were selected from commercially available ones, and DMAA and AA were adopted owing to its amphiphilicity to water and organic solvents. Dye-immobilised polymer membranes with 100% DMAA or 100% AA were prepared at first in above mentioned manner. However, the former was too stiff to swell in water and peeled off from the glass plates after polymerization. The latter was successfully prepared, but peeled off from the glass plates during the experiments because volume change of the membrane by water swelling was large when water content was increased. Therefore, the two monomers, DMAA and AA, were mixed, and an appropriate molar ratio was determined to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which showed the most intense fluorescence (Fig. S15†) and a relatively small degree of water swelling. Similarly, the proportion of the cross-linking reagent, N,N-methylenebis(acrylamide), was also varied from 0 to 2 mol%, and the optimal addition amount was found to be 0.5 mol%. These materials were mixed with the functional dyes, and copolymer membranes were prepared following the method described in the Experimental section.
1 which showed the most intense fluorescence (Fig. S15†) and a relatively small degree of water swelling. Similarly, the proportion of the cross-linking reagent, N,N-methylenebis(acrylamide), was also varied from 0 to 2 mol%, and the optimal addition amount was found to be 0.5 mol%. These materials were mixed with the functional dyes, and copolymer membranes were prepared following the method described in the Experimental section.
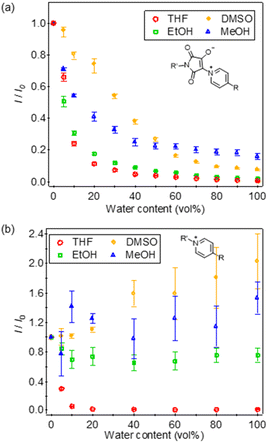
The fluorescence spectral changes of the fabricated membranes were examined in THF/water solvents (Fig. 3). Both dye-immobilised copolymer membranes (designated as PB1-mem and QP1-mem) showed decreases in the fluorescence intensity and small red shifts in λfl with increasing water content; however, the response range was different. For PB1-mem, as the water content increased from 0% to 100%, λfl exhibited red shifts from 591 to 627 nm, whereas the fluorescence intensity (I) decreased from 2560 a.u. (detector setting was low gain) to 11 a.u. Moreover, the fluorescence intensity of PB1-mem was approximately 2.6 times higher than that of QP1-mem (middle gain is ca. 10 times more sensitive than low gain). Additionally, PB1-mem exhibited a wide-range response (<40 vol% water), whereas QP1-mem showed a narrow-range response from 0 vol% (λfl = 577 nm, I = 9054 a.u.) to 10 vol% water (λfl = 613 nm, I = 324 a.u.). In other words, QP1-mem was more sensitive to water in THF than PB1-mem. To confirm the sensitivity to water, the relative fluorescence intensity with respect to the fluorescence intensity at 0 vol% water (I/I0) was plotted against the water content (Fig. 4). At the water content of 5 vol%, the I/I0 ratio of PB1-mem decreased to 0.66 at 5 vol% water content, whereas that of QP1-mem decreased drastically to 0.30. These results highlight the suitability of PB1-mem and QP1-mem for wide-range and low-water-content detection in THF, respectively.
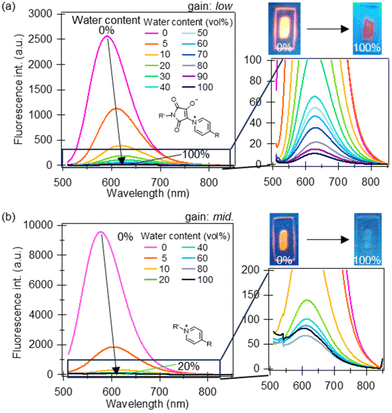 | ||
| Fig. 3 Fluorescence spectra of the dye-immobilised membranes (a) PB1-mem (λex = 490 nm, λfl = 630 nm) and (b) QP1-mem (λex = 460 nm, λfl = 630 nm) in THF/water solvents. | ||
The response and recovery times of PB1-mem were also measured. The prepared PB1-mem stored in THF was soaked in a THF solution with 40 vol% water, and the fluorescence intensity at 630 nm was monitored without replacing the sample mixture (Fig. S2a†). I/I0 reached 0.05 at 3.5 min (95% response); therefore, this value was regarded as the response time. For all other water contents, the response behaviour was almost identical, and all measurements were conducted after immersion for 5 min. The fluorescence of the membranes was recovered in a 100% THF solution, with the intensity ratio plateauing to 0.8 at 32 min without replacement of THF (Fig. S2b†). This slower recovery than response to water in anhydrous THF was presumably due to the slow spontaneous release of water molecules under static conditions. This could be improved by modifying the polymer matrix and membrane thickness and employing a flow system.
The response of the dye-immobilised membranes to water in polar solvents such as DMSO, ethanol, and methanol was examined. Membranes stored in anhydrous THF were soaked in each of the dried organic solvents for 5 min, following which the anhydrous organic solvent was replaced thrice. PB1-mem was excited with the excitation wavelength (λex) of 490 nm, and its fluorescence intensity at 630 nm was monitored. Similarly, QP1-mem was excited at 460 nm, and the fluorescence intensity at 630 nm was investigated. PB1-mem achieved water detection even in methanol and DMSO (Fig. 4a), with a response range in ethanol, methanol, and DMSO of <40, <40, and <60 vol% water, respectively. In contrast, QP1-mem only responded to ethanol/water solvents with <10 vol% water (Fig. 4b). In the methanol/water and DMSO/water solvents, the I/I0 value of QP1-mem varied owing to the considerably low fluorescence intensities. These results underscore the potency of the PB structure in enabling wide range detection of water in polar solvents, including methanol and DMSO.
The prepared membranes showed no signs of decomposition at room temperature, even without special care to oxygen, but discoloration was observed when left for several days without shading. However, when stored in anhydrous THF with shading, the same fluorescence intensity was obtained for several days, so in this study, data was acquired with the membranes stored in anhydrous THF with shading.
Effects of ICT character and PB structure on water detection in organic solvents
To gain insight into the effects of ICT character and the PB structure on its optical properties and water detection sensitivity, density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations were conducted using Gaussian 09 program.51 Optimised structures in the ground and excited states were obtained using the M06 function and 6-31G(d,p) basis sets. An alkyl group at the molecular terminal was omitted for simplification and reducing the calculation cost. Moreover, both dyes were optimised using the solvation model based on density (SMD) in THF or in water to determine the effects of solvent polarity.52Optimised structures in the ground and the first excited states (S1), as well as the distribution and the energy of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), were acquired for both dyes (Fig. S3 and S4†). The HOMO–LUMO transition contributed to the S0–S1 transition in both dyes. The predicted λabs,cal in THF (502 and 480 nm for PB1 and QP1, respectively) roughly corresponded to the experimental results (490 and 460 nm for PB1 and QP1, respectively). The calculated fluorescence maxima, λfl,cal, in THF were 597 and 575 nm for PB1 and QP1, respectively, and trends were different from the experimental results (600 nm and 616 nm for PB1 and QP1, respectively). This would be because ICT character of QP1 was underestimated. The HOMO of QP1 was mainly localised at a (diphenylamino)phenyl group and the ethylene moiety, while the LUMO was distributed over a pyridinium moiety (Fig. S4†). This distribution indicated that QP1 exhibited strong ICT characteristics, as mentioned above. The HOMO and LUMO energy levels of QP1 were −5.45 and −2.31 eV, respectively. However, the PB structure destabilised the HOMO and LUMO, yielding values of −5.29 and −2.22 eV, respectively. The frontier orbitals of PB1 were delocalised over the entire molecule, and the π-conjugation system extended to the maleimide moiety. To confirm the strength of ICT, the difference in dipole moment between the ground and excited states (μe − μg) was estimated from the Lippert–Mataga plot (Fig. S5†); the difference for PB1 (20.96 D) was smaller than that for QP1 (22.00 D). Additionally, the values of oscillator strength of PB1 for the S0–S1 and S1–S0 transition (fS0–S1 = 1.78, fS1–S0 = 2.03) were higher than those of QP1 (fS0–S1 = 1.24, fS1–S0 = 1.16). These results indicate that the ICT characteristic of PB1 was not as prominent as that of QP1, and that the PB structure could be a viable candidate for achieving intense fluorescence.
Furthermore, to clarify the effects of the PB structure on the water detection sensitivity, the intramolecular hydrogen bonds between the carbonyl oxygens of the cyclic enolate and the α-hydrogens of the pyridinium ring were targeted for analysis. As reported previously,49,50 the α-hydrogens of the pyridinium ring exhibited a large chemical shift in proton NMR to 9.73 ppm (in CDCl3), whereas that of QP1 was 9.10 ppm (in CDCl3) (Fig. S14†), because the carbonyl oxygen and α-hydrogens tended to form intramolecular hydrogen bonds. The atomic distance d between the carbonyl oxygen and α-hydrogens in the theoretical optimised structure in THF (Fig. S3†) in the ground state was estimated as 2.06 Å; this value is reasonable for C–H⋯O interactions.53–55 Furthermore, the increase in solvent polarity induced molecular torsion in the ground state, and dg increased to 2.24 Å in water. The theoretical calculations also suggested that water induced molecular torsion of the PB structure, given that the dihedral angle φbet,g between maleimide and the pyridinium ring (C–C–N–C) (Fig. S3†) increased from 1.2° in THF to −23.88° in water, and the oscillator strength decreased to 1.59. Similar trends were observed in the excited-state optimisation; de was increased from 2.08 Å in THF to 2.25 Å in water with the increase of φbet,e from 6.51° in THF to −23.97° in water.
On the other hand, the SMD model could not estimate the effect of an intermolecular hydrogen bond.52 To consider this effect, optimized structures of PB1 with a water molecule in the ground and excited states (M06/6-31G(d,p), SMD/water) were calculated. The values of φbet,g and φbet,e were increased to −34.93° and −32.27°, respectively (Fig. S6†). Furthermore, the total energies in the ground state were stabilized by 4.74 kcal mol−1 compared to sum of the total energy of PB1 and water (Table S1†), which energy was a reasonable value to form C–H⋯O interactions.53–55 Potential energy surface scanning conducted at the M06/6-31G(d,p) level using the SMD model (φbet ≤ 90°) indicated that the PB1–water interactions activated the molecular torsion both in the ground and the excited states (Fig. S7† and Fig. 5). In anhydrous THF, the rotational energy barrier in the excited state (ΔEe) from the most stable state to the most unstable state (φbet, e = 90°) was 6.42 kcal mol−1. In the presence of a water molecule (SMD/water), ΔEe decreased to 1.80 kcal mol−1, and the distance between the oxygen in the maleimide moiety and the hydrogen in pyridinium was longer than that between the oxygen in the maleimide moiety and the hydrogen in water forming intermolecular hydrogen bonds. Similar trends were observed in the ground-state scan calculations. Unfortunately, the rupture of the intramolecular hydrogen bonds in water could not be experimentally ascertained by 1H NMR spectroscopy (PB-C10 in DMSO-d6/water or in CDCl3/methanol-d4). Fourier-transform infrared spectroscopy of PB1-mem was also performed; however, no insight was acquired owing to the presence of numerous C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the copolymer membranes. On the other hand, theoretical calculations of the vibration modes suggested the existence of interactions between the oxygen in the maleimide moiety and the hydrogen in water. The carbonyl group stretching vibrations band (νC
O bonds in the copolymer membranes. On the other hand, theoretical calculations of the vibration modes suggested the existence of interactions between the oxygen in the maleimide moiety and the hydrogen in water. The carbonyl group stretching vibrations band (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) for the water-free maleimide ring appeared at 1700 cm−1, whereas that of the structure with water shifted to 1696 cm−1 (Fig. S8†). This decrease in νC
O) for the water-free maleimide ring appeared at 1700 cm−1, whereas that of the structure with water shifted to 1696 cm−1 (Fig. S8†). This decrease in νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was likely related to the intermolecular hydrogen bonds.
O was likely related to the intermolecular hydrogen bonds.
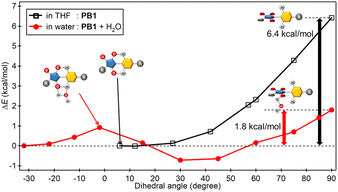 | ||
| Fig. 5 Rotational energy barrier ΔEe plotted against the dihedral angle φbet,e between the maleimide and pyridinium rings (C–C–N–C) in the excited state. | ||
Furthermore, effects of acid and base as impurities on fluorescence was investigated. 0.5 vol% of 1 M HCl aq. or NaOH aq. was added to anhydrous organic solvents such as THF, DMSO, N,N-dimethylformamide (DMF), and fluorescence spectra were measured. As shown in Fig. S16,† fluorescence intensities of PB1-mem in acid or base-added organic solvents were weaker than those in only 0.5 vol% of water-added ones. On the other hand, fluorescence intensities of QP1-mem in these solutions were relatively unaffected. These results would indicate that intramolecular hydrogen bonds were prevented by acid or base, and molecular torsion was promoted. Although strong and enough amount of acid or base is needed to break the intramolecular hydrogen bond at PB structure completely, but small amount is sufficient to promote molecular torsion and fluorescence decrease.
Based on experimental and computational results, the intramolecular hydrogen bonds of PB structure are important for water detection in organic solvents and permit water detection even in polar solvents such as methanol and DMSO. The addition of water, acid, and base causes the PB structure to undergo molecular torsion leading to fluorescence quenching. To further enhance the sensitivity for water detection in organic solvents, optimising the strength of the intramolecular hydrogen bonds by modifying the molecular structure and substituents could be an effective approach.
Conclusions
In this study, simple water detection in organic solvents, which have been difficult to achieve by conventional KF titration, was successfully performed using a copolymer membrane incorporated with a fluorescent pyridinium betaine dye. As the water content of THF/water solvent mixtures increased (0–100 vol%), the fluorescence intensity of the pyridinium betaine dye decreased and water detection was successfully realised. Notably, water detection was achieved in polar solvents such as methanol and DMSO (for <40 and <60 vol% water, respectively). On the other hand, quaternary pyridinium dye, which has only ICT character, showed more sensitive but narrow range response and was not suitable for detection of water in polar solvents compared to pyridinium betaine dye. DFT and TD-DFT calculations revealed that, in the case of pyridinium betaine dye, addition of water to organic solvents would promote molecular torsion due to the formation of intermolecular hydrogen bonds, and fluorescence decreased. To adjust sensitivity and/or response range of water detection devices, the strength of intramolecular hydrogen bonds and electron distribution should be optimised, and support materials with superior attributes should be developed.Author contributions
A. M., K. S., T. E., and H. H. conceptualised, planned, and designed the experiments. A. M., K. S., N. S., S. Y., and H. H. conceptualised the molecular designs. A. M. performed the experiments and analysed the data. A. M., S. Y., K. S., T. E., and H. H. drafted the manuscript. All authors have approved the manuscript and agree with its submission to this journal.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partly supported by the Japan Science and Technology Agency, under the ‘Establishment of University Fellowships towards the Creation of Science Technology Innovation’ program (Grant No. JPMJFS2138), and the Japan Society for the Promotion of Science, through a Grant-in-Aid for Scientific Research (No. 23H01990). Authors greatly acknowledge Prof. Hiroshi Ikeda, Assoc. Prof. Yasunori Matsui, Assist. Prof. Takuya Ogaki, Assist. Prof. Kenichi Michigami, and the Analytical Centre, Graduate School of Science, Osaka Metropolitan University for kind help on characterizing synthesized molecule using mass spectrometry and elemental analysis.References
- K. Fischer, Angew. Chem., 1935, 48, 394–396 CrossRef CAS.
- D. M. Smith, W. M. D. Bryant and J. Mitchell, J. Am. Chem. Soc., 1939, 61, 2407–2412 CrossRef CAS.
- J. Mitchell, Anal. Chem., 1951, 23, 1069–1075 CrossRef CAS.
- F. E. Critchfield and E. T. Bishop, Anal. Chem., 1961, 33, 1034–1035 CrossRef CAS.
- E. Scholz, Anal. Chem., 1985, 57, 2965–2971 CrossRef CAS.
- A. Cedergren and C. Oraedd, Anal. Chem., 1994, 66, 2010–2016 CrossRef CAS.
- J. Hoebus, E. Roets and J. Hoogmartens, J. Pharm. Biomed. Anal., 1996, 15, 359–364 CrossRef CAS PubMed.
- K. Schmitt and H. D. Isengard, Fresenius’ J. Anal. Chem., 1997, 357, 806–811 CrossRef CAS.
- F. R. van de Voort, J. Sedman, R. Cocciardi and S. Juneau, Talanta, 2007, 72, 289–295 CrossRef CAS PubMed.
- P. Bühlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593–1688 CrossRef PubMed.
- M. A. Kessler, J. G. Gailer and O. S. Wolfbeis, Sens. Actuators, B, 1991, 3, 267–272 CrossRef CAS.
- H. Hisamoto, Y. Manabe, H. Yanai, H. Tohma, T. Yamada and K. Suzuki, Anal. Chem., 1998, 70, 1255–1261 CrossRef CAS PubMed.
- H. Hisamoto, H. Tohma, T. Yamada, K.-i. Yamauchi, D. Siswanta, N. Yoshioka and K. Suzuki, Anal. Chim. Acta, 1998, 373, 271–289 CrossRef CAS.
- D. Citterio, K. Minamihashi, Y. Kuniyoshi, H. Hisamoto, S.-I. Sasaki and K. Suzuki, Anal. Chem., 2001, 73, 5339–5345 CrossRef CAS PubMed.
- X. Yang, C.-G. Niu, Z.-J. Shang, G.-L. Shen and R.-Q. Yu, Sens. Actuators, B, 2001, 75, 43–47 CrossRef CAS.
- C. G. Niu, A. L. Guan, G. M. Zeng, Y. G. Liu and Z. W. Li, Anal. Chim. Acta, 2006, 577, 264–270 CrossRef CAS PubMed.
- D. Huang, Y. Bing, H. Yi, W. Hong, C. Lai, Q. Guo and C. Niu, Anal. Methods, 2015, 7, 4621–4628 RSC.
- H. S. Jung, P. Verwilst, W. Y. Kim and J. S. Kim, Chem. Soc. Rev., 2016, 45, 1242–1256 RSC.
- P. Kumar, A. Ghosh and D. A. Jose, ChemistrySelect, 2021, 6, 820–842 CrossRef CAS.
- Y. Ooyama, A. Matsugasako, T. Nagano, K. Oka, K. Kushimoto, K. Komaguchi, I. Imae and Y. Harima, J. Photochem. Photobiol., A, 2011, 222, 52–55 CrossRef CAS.
- N. Khanapurmath, M. D. Prabhu, J. Tonannavar, J. Tonannavar and M. V. Kulkarni, J. Mol. Liq., 2020, 314, 113620 CrossRef CAS.
- S. Miho, K. Imato and Y. Ooyama, RSC Adv., 2022, 12, 25687–25696 RSC.
- N. I. Georgiev, P. V. Krasteva, V. V. Bakov and V. B. Bojinov, Molecules, 2022, 27, 4229 CrossRef CAS PubMed.
- P. P. Dash, P. Mohanty, R. Behura, S. Behera, P. Singla, S. C. Sahoo, S. K. Sahoo and B. R. Jali, J. Photochem. Photobiol., A, 2023, 440, 114650 CrossRef CAS.
- S. A. Yoon, J. H. Oh, S. K. Kim and M. H. Lee, Dyes Pigm., 2019, 165, 421–428 CrossRef CAS.
- T. Sachdeva and M. D. Milton, J. Photochem. Photobiol., A, 2020, 402., 112804 CrossRef.
- F. Hou, X. Liu, X. Hao, G. Li, F. Lu and Q. Deng, Dyes Pigm., 2021, 195, 109667 CrossRef CAS.
- Z. Zhao, Q. Hu, W. Liu, X. Xiong, Z. Wang and H. Wang, Dyes Pigm., 2023, 213, 111186 CrossRef CAS.
- L. Cai, X. Sun, W. He, R. Hu, B. Liu and J. Shen, Talanta, 2020, 206, 120214 CrossRef CAS PubMed.
- Z. Ruan, H. Zheng, C. Deng, X. Cheng, X. Ruan, S. Lv, Y. Chen, S. Liu and J. Lin, Dyes Pigm., 2022, 204, 110476 CrossRef CAS.
- F. Wu, L. Wang, H. Tang and D. Cao, Anal. Chem., 2019, 91, 5261–5269 CrossRef CAS PubMed.
- S. K. Panda and A. K. Singh, J. Mol. Liq., 2023, 375, 121381 CrossRef CAS.
- F. Xin, J. Zhao, X. Wang, H. Wang, H. Wang, M. Xing, Y. Fu, Y. Tian and Y. Tian, Spectrochim. Acta, Part A, 2023, 296, 122621 CrossRef CAS PubMed.
- J. T. Wang, Y. Y. Pei, S. F. Ren, M. Y. Yan, W. Luo, B. Zhang and Q. F. Li, Spectrochim. Acta, Part A, 2020, 229, 117956 CrossRef CAS PubMed.
- G. Xiao, X. Ji, J. Ji, G. Li, G. Yang and Y. Wang, J. Photochem. Photobiol., A, 2023, 441, 114714 CrossRef CAS.
- S. Tang, D. Chen, Y. Yang, C. Wang, X. Li, Y. Wang, C. Gu and Z. Cao, J. Colloid Interface Sci., 2022, 617, 182–192 CrossRef CAS PubMed.
- Q. He, S. Zhuang, Y. Yu, H. Li and Y. Liu, Anal. Chim. Acta, 2021, 1174, 338743 CrossRef CAS PubMed.
- Y. Qin, Y. Bai, P. Huang and F.-Y. Wu, ACS Appl. Nano Mater., 2021, 4, 10674–10681 CrossRef CAS.
- W. Li, X. Yu, Y. Tang, Z. Li, S. J. Shah, Y. Liu, H. Zhao, M. Song, J. Li, G. Wang, L. Zhou, Z. Zhao, S. Liu and Z. Zhao, Sens. Actuators, B, 2023, 390, 139954 Search PubMed.
- X. Mu, X. Song, D. Gao, P. Ma, Q. Wu and D. Song, Spectrochim. Acta, Part A, 2022, 276, 121195 CrossRef CAS PubMed.
- Y. Chen, C. Zhang, J. Xie, H. Li, W. Dai, Q. Deng and S. Wang, Anal. Chim. Acta, 2020, 1109, 114–121 CrossRef CAS PubMed.
- C. Guan, J. Cai, X. Liu and L. Guo, Sens. Actuators, B, 2022, 355, 131323 CrossRef CAS.
- Z. Li, Q. Li, Z. Hu, C. Hu, X. Cui, Y. Fu and Z. Chen, Mikrochim. Acta, 2022, 189, 361 CrossRef CAS PubMed.
- Z. Li, X. Cui, Z. Hu, C. Hu and Z. Chen, Microporous Mesoporous Mater., 2023, 348, 112401 CrossRef CAS.
- L. Chen, J.-W. Ye, H.-P. Wang, M. Pan, S.-Y. Yin, Z.-W. Wei, L.-Y. Zhang, K. Wu, Y.-N. Fan and C.-Y. Su, Nat. Commun., 2017, 8, 15985 CrossRef CAS PubMed.
- J. Dang, R. Zhu, W. Fang, Y. Hu, Y. Wu, S. Xin, M. Li, B. Chen, H. Zhao and Z. Li, Dyes Pigm., 2022, 206, 110602 CrossRef CAS.
- S. Y. Li, X. Yan, J. Lei, W. J. Ji, S. C. Fan, P. Zhang and Q. G. Zhai, ACS Appl. Mater. Interfaces, 2022, 14, 55997–56006 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, NY, 2006 Search PubMed.
- Y. Hayashi, N. Suzuki, T. Maeda, H. Fujiwara and S. Yagi, New J. Chem., 2021, 45, 9770–9779 RSC.
- N. Suzuki, M. Saikusa, Y. Hayashi, T. Maeda and S. Yagi, Dyes Pigm., 2023, 216, 111291 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and a. D. J. Fox, Gaussian 09, revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- D. Ž. Veljković, G. V. Janjić and S. D. Zarić, CrystEngComm, 2011, 13, 5005 RSC.
- T. Steiner and G. R. Desiraju, Chem. Commun., 1998, 891–892 RSC.
- S. J. Grabowski, Chem. Rev., 2011, 111, 2597–2625 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3an02165c |
| This journal is © The Royal Society of Chemistry 2024 |