Open Access Article
Open Access ArticleLayer-wise physicochemical and elemental distribution in an urban river water, Bangladesh: potential pollution, sources, and human health risk assessment†
Tapos
Kormoker
 *ab,
Md. Saiful
Islam
*ab,
Md. Saiful
Islam
 *cd,
Md. Abu Bakar
Siddique
*cd,
Md. Abu Bakar
Siddique
 e,
Sazal
Kumar
f,
Khamphe
Phoungthong
c,
Md Humayun
Kabir
g,
Kazi Farhed
Iqubal
bg,
Rakesh
Kumar
e,
Sazal
Kumar
f,
Khamphe
Phoungthong
c,
Md Humayun
Kabir
g,
Kazi Farhed
Iqubal
bg,
Rakesh
Kumar
 hi,
Mir Mohammad
Ali
j and
Abu Reza Md. Towfiqul
Islam
kl
hi,
Mir Mohammad
Ali
j and
Abu Reza Md. Towfiqul
Islam
kl
aDepartment of Emergency Management, Faculty of Environmental Science and Disaster Management, Patuakhali Science and Technology University, Dumki, Patuakhali-8602, Bangladesh. E-mail: tapos.pstu@gmail.com
bEQMS Consulting Limited, Dhaka, Bangladesh
cEnvironmental Assessment and Technology for Hazardous Waste Management Research Center, Faculty of Environmental Management, Prince of Songkla University, Songkhla 90112, Thailand. E-mail: msaifulpstu@yahoo.com
dDepartment of Soil Science, Patuakhali Science and Technology University, Dumki, Patuakhali, 8602, Bangladesh
eInstitute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhanmondi, Dhaka 1205, Bangladesh
fSchool of Environmental and Life Sciences, The University of Newcastle (UoN), Callaghan, NSW 2308, Australia
gDepartment of Environmental Science and Resource Management, Mawlana Bhashani Science and Technology University, Tangail, Bangladesh
hSchool of Ecology and Environment Studies, Nalanda University, Rajgir 803116, Bihar, India
iDepartment of Biosystems Engineering, Auburn University, Auburn, AL 36849, USA
jDepartment of Aquaculture, Sher-e-Bangla Agricultural University, Dhaka-1207, Bangladesh
kDepartment of Disaster Management, Begum Rokeya University, Rangpur, Bangladesh
lDepartment of Development Studies, Daffodil International University, Dhaka 1216, Bangladesh
First published on 3rd August 2023
Abstract
Buriganga is an economically important river located around the industrialized urban area of Dhaka City, Bangladesh. In this study, 17 water quality parameters (electrical conductivity, pH, total suspended solids, temperature, F−, Cl−, SO42−, Cr, Ni, As, Cd, Hg, Cu, Pb, Fe, Mn, and Zn) of surface and deep waters of the Buriganga River were measured to assess the water quality, pollution level, elemental sources, and their potential ecological and human health risks. Comparing the concentrations of the analyzed parameters with the permissible standards, it was indicated that the water in Buriganga is unsafe for residential and recreational uses. Principal component and correlation analysis revealed that point and diffuse sources, such as the combustion of lubricant oils, fuel additives, exhaust fumes from vehicles, domestic wastewater, and inorganic fertilizers from agricultural fields, control the water quality. Regardless of depth, a higher degree of contamination and ecological risk was observed during the dry season, indicating a higher content of heavy metals in river water, which might impact the ecological balance in the future. Through ingestion, the hazard quotient (HQ) of As, Cd, Pb, and Hg and the hazard index (HI) values were higher than the risk threshold (HQ > 1). Total HI values for children in both residential and recreational water were higher than those for adults (1.04 × 101 and 1.73 × 100 for surface and deep water, respectively), indicating that children are more sensitive to elemental contamination. Total carcinogenic risks of Cr and As due to exposure to water were higher than the standard limit (>1.0 × 10−4), which indicated possible cancer risks to the inhabitants around the river. Therefore, regular monitoring of river water quality and sustainable management could be implemented to recover the polluted river water and keep it pollution-free in the future.
Environmental significanceThe accumulation of toxic metals in the riverine ecosystems can pose serious environmental problems. It is important to determine the intensity of pollution by inventorying the metal concentrations in water and their possible ecological and health risk. Surface and deep waters from one important urban river of Bangladesh have been used for metal analysis and ecological and health risk assessment. The major findings indicated that the river water of Buriganga is unsafe for residential and recreational uses and poses moderate to very high ecological risks and possible cancer risks to the inhabitants around the river. The outcome of the study will create awareness in society about the frequent utilization of polluted river water for various purposes. |
1. Introduction
Researchers around the world are increasingly concerned about the deterioration of water quality and the presence of toxic elements in riverine water bodies.1 Owing to their significant ecological toxicity, availability, extensive persistence, and bioaccumulation propensity, the toxic element contamination of waterways has become a widespread issue in recent years for human existence.2–4 The accessibility of potable, cooking, agricultural, recreational, residential, and manufacturing water is jeopardized by the high levels of toxic elements in river waters. In addition, the intake of fish and other aquatic foods sourced from polluted water environments often poses potential threats to the health of the environment and human beings as well.5–7 Toxic elements are primarily emitted by human activities, such as sewerage draining, the discharge of industrial waste and effluents, hospital waste dumping, unsustainable farming practices, unnecessary traffic activities, municipal waste disposal, and recreational operations. On the other hand, metals occur naturally in trace amounts and may enter aquatic ecosystems through the leaching of rocks, soil erosion, airborne dust, and forest fires.8–11 River pollution has become an increasing threat due to government law enforcement weaknesses and a scarcity of frequent monitoring, particularly in developing nations like Bangladesh, where hundreds of manufacturers discharge waste materials into adjacent waterways.12–14 Nearly every day, a considerable volume of harmful effluents is poured into Bangladesh's lower land and water basins. The wastewater from various industries (located beside the river), such as battery manufacturing, garments, steel mills, and tanneries, is extremely toxic and reduces the oxygen levels in river water.15–17 As a consequence, essential and toxic elements are constantly precipitated and integrated into the water, polluting the aquatic habitat with heavy metals. Consequently, toxic elements in river water may have substantial negative consequences, affecting aquatic life, the natural ecology, water quality, and public health.18,19 Thus, studies have continued to analyze the hazards of toxic elements to public health, as well as their spatial and seasonal fluctuations in freshwater environments.Because toxic elements are highly persistent and also have the capability to be hazardous to biological systems through bioaccumulation in food chains, they are of major concern for the aquatic ecosystem.20–23 Except for disrupting both surface and deep-water quality, trace metal bio-accumulation in the aquatic food chain is a threat to public health; thus, their potential exposure consequences on ecosystem integrity cannot be overlooked.24,25 Moreover, these toxic elements could build up in the body of a human through contact with the skin or ingestion of water with toxic elements and/or freshwater creatures. Long-term exposure to such toxic elements can lead to various diseases such as Parkinson's, sclerosis on the body surface, and prolonged cancer.12,18,26 Pollution of freshwater bodies of water, particularly rivers, has reached dangerous levels for human interaction, and this can be evaluated through heavy metal analysis in water.27 However, regular monitoring of river water quality is critical for maintaining environmental health and the achievement of sustainable development goals (SDGs) such as “Goal 6: Clean water and sanitation” and “Goal 14: Life below water” by dropping pollution and contamination.8,24,28–30 Thus, a thorough assessment of freshwater quality in terms of toxic metal contamination is critical for environmental and human health protection.
The Buriganga River, which flows from the Dhaleshwari near Kalatia, is a tide-influenced river that forms the western and southern borders of Dhaka city, the eco-political capital of Bangladesh.31 The river runs 11 km across Dhaka district and 7 km through Narayanganj district, with a little portion in Munshiganj. Some of the most prominent tourist sites in Dhaka, Bangladesh, such as Sadarghat (Buriganga River port), Ahsan Manzil Museum (architectural buildings), Tara Masjid (Islamic architecture), Lalbagh Fort (historical landmark), and Dhakeshwari Mandir (Bangladesh's National Temple), are situated on the bank or within a short distance of this river. A large number of tourists visit these locations for recreational purposes and approximately one million people live alongside the river. This increases the river's importance to residents and recreational users. The Buriganga River is vital for a wide variety of reasons, including several industrial activities, agricultural irrigation, fisheries, and recreational opportunities for residents and visitors alike. However, the river is severely polluted by a variety of sources, including but not limited to industrial wastewater, commercial waste, municipal wastewater, recreational activities, a variety of farming operations, navigational wastewater, and agricultural runoff.8,23,32
Despite a government injunction and a High Court judgment, the majority of tanneries continue to pollute the Buriganga River. Rivers serve humans and other creatures in a number of ways, especially in impoverished countries like Bangladesh, where water sources are limited.33 However, large volumes of pollutants such as toxic elements including As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn are discharged into the riverine water bodies from various sources such as unplanned urbanization and rapid industrialization, which poses significant threats to the river ecosystems.25,34 Skin contact with river water while bathing and washing, as well as direct use of water for household purposes, is a potential route for toxic elements to enter the bodies of adult and child residents and recreationists.35,36 For years, the surface and deep waters of this river have been used by many restaurants and residences beside the river, resulting in trace element exposure. Therefore, an assessment of possible water quality deterioration factors is needed, and human health risks through ingestion and dermal contact (two common exposure routes) of water must be assessed for judicious management of river water.37,38 However, little is known about the potential health problems that the toxic elements in water may present to the residents of the Buriganga River.
The distribution and contamination of the river water by toxic elements have been depicted in previous studies.8,14,31 A recent study estimated the concentration of 48 metals in the surface water of the Buriganga River, targeting their seasonal variation, and found that the metals' concentration was lower in the rainy season than in the winter.39 Some of the most recent studies on the surface water of Buriganga River evaluated metal concentration, major anions, and cations as well as physicochemical properties.40–43 However, no detailed study has been conducted yet on the layer-wise distribution (surface and deep waters) of toxic elements, major anions, and physical parameters in this urban river water with a focus on seasonal variation. Besides, no studies have highlighted the health risks to the residents who permanently live there or the recreationists who visit the historical places beside the studied Buriganga River. Therefore, the objectives of the current research are to (1) quantify and compare the levels of 17 water quality parameters including concerned elements (Cr, Ni, As, Cd, Hg, Cu, Pb, Fe, Mn, and Zn), anions (F−, Cl−, and SO42−), and some physical parameters (electrical conductivity, pH, total suspended solids, and temperature) in both surface and deep waters of the river during dry and wet seasons, (2) identify the potential sources and factors that regulate the levels of toxic elements in the river, and (3) determine the human health risk of toxic elements in the river water by assessing the non-carcinogenic and carcinogenic risks for both adults and children who are residents and recreationists in the area.
2. Methodology
2.1. Study area
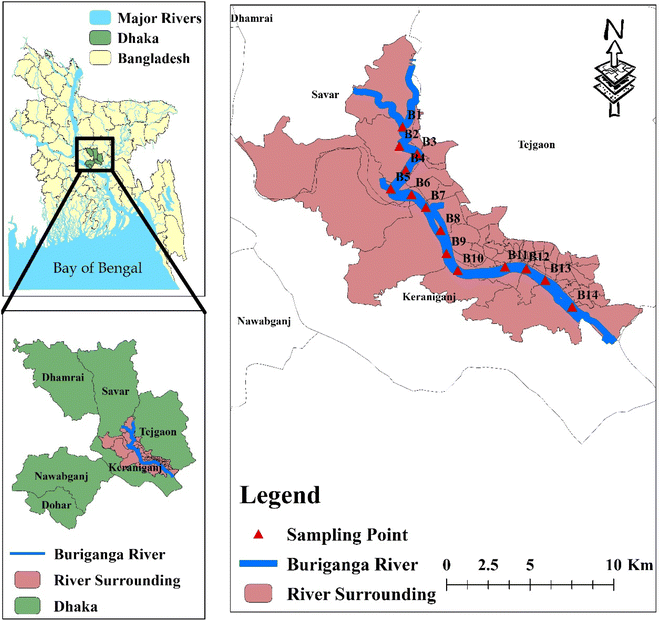
Buriganga River is one of the most polluted rivers in Bangladesh and is located in the central region of the country. The depth of the Buriganga River varies from 7.6 meters up to 18 meters on average.44 The studied river's biological and hydrological health has deteriorated to the point of death due to the undiscerning discarding of residential, municipal, and industrial pollutants and the lack of authorities to maintain the ecological health of the river.45 The river Buriganga has a stream of water running through it during the rainy season (monsoon); however, there is no water flowing through it during the dry season (winter). As a result, the body of water becomes stagnant, and the direct discharge of waste from industries as well as households and medical facilities significantly raises the degree of pollution in it during the dry/winter season. To collect the surface and deep waters of the Buriganga River throughout the summer and winter seasons for this study, a total of 14 sample sites were chosen (Fig. 1). The sampling location was chosen based on the activities of numerous industries such as urban waste dumping and burning, electroplating, city waste sewerage lines, lead smelting factories, leather tanning zones, distilleries, dyeing industries, textile, paper, glass, plastic, chemical, and battery manufacture, etc.2.2. Sample collection and preservation
In this study, in total, 84 surface (0–20 cm) and 84 deep (400–800 cm) water samples were collected from 14 selected stations of the Buriganga River during the wet (July–August, 2021) and dry (February–March, 2022) seasons. The river water samples were collected directly in previously cleaned polypropylene bottles (500 mL), which were washed thoroughly with the sampling water before collecting the samples. For elemental analysis, the collected samples were immediately acidified with concentrated HNO3 (2 mL L−1), while an equal number of non-acidified samples were collected for the analysis of other parameters such as F−, Cl−, SO42−, electrical conductivity (EC), pH, and total suspended solids (TSS). Water samples were collected and put in an ice chest before being transferred to the laboratory and kept in the refrigerator until chemical analysis.2.3. Sample analysis and quality control
All samples were analyzed at an ISO/IEC 17025:2017 accredited laboratory (INARS, BCSIR, Dhaka, Bangladesh). However, the EC and pH of the river water samples were measured in situ during sample collection using a multimeter (Model: Sension™156, HACH, USA), which was calibrated with traceable standards. A calibrated thermometer was used to take water temperature readings on the spot as well. The EC, pH, and temperature of the water samples were measured directly by immerging the probes (for EC and pH) and thermometer (for temperature) into the water sample taken in a small beaker (100 mL) from both surface and deep waters separately. The surface water body was also checked directly for EC, pH, and temperature by immerging the respective probes into the water system to ensure the data quality. The TSS was determined gravimetrically in the laboratory.14 The collected non-acidified samples were vacuum filtered with 0.45 μm filter paper and taken in small sample vials to be used for F−, Cl−, and SO42− analysis using an ion chromatograph (IC, Model: SIC10AVP, Shimadzu, Japan). Acidified water samples were utilized for elemental analysis after filtration with Whatman No. 41 filter paper. The concentrations of As, Cd, Pb, Hg, Cr, Ni, Cu, Fe, Mn, and Zn were measured using an Atomic Absorption Spectrometer (AAS) with different arrangements and models of the instruments ((a) AA240FS, (b) GTA 120-AA240Z, and (c) SpectrAA 220, Varian, Australia). As and Hg were analyzed through VGA (vapor generation accessories)-77 by hydride vapor generation and cold vapor techniques, respectively. The concentrations of Cd, Pb, Cr, and Ni were determined through a graphite furnace, while Cu, Fe, Mn, and Zn were analyzed using an air-acetylene flame. NIST (National Institute of Standards and Technology) traceable certified reference materials (CRM) for individual elements and anions obtained from Fluka Analytical, Sigma-Aldrich, Germany, were employed for the preparation of calibration curves with high linearity (r2 = 0.9999), and the concentration of each element and anion was determined against the respective individual calibration curve. The analysis accuracy and precision were guaranteed by repeated measurements of the CRM and the samples. The analysis of quality control standard, sample blank, and method blank was also performed sequentially. The spike recovery in the analysis of elements and anions using an AAS and IC, respectively, was within 97–102%. The limit of quantification (LOQ), the limit of detection (LOD), calibration range, and measurement uncertainty for the analyzed elements and anions are provided in ESI Table S1.† Further details about the analysis and quality control protocol employed in the analysis are described in our previous works (Fig. 2).14,46–492.4. Environmental and ecological risk indices
The heavy metal evaluation index (HEI), degree of contamination (CD), and potential ecological risk (PER) indices were used to evaluate contamination levels for the studied metals. The details of these indices are described in ESI Table S2.†2.5. Health risk assessment
Although the appearance of the Buriganga river water seems dark in color with a bad odor, some poor local people still use this water for their bathing and washing, which is an important health concern because metals from water systems can enter the human body through ingestion or skin contact, causing a health hazard. In this work, health risks associated with non-carcinogenic and carcinogenic effects of heavy metals through oral intake and dermal/skin contact with river water were calculated for both adult and child inhabitants following the guidelines of the USEPA.36,50,51 Non-carcinogenic risk parameters such as hazard quotient (HQ) and hazard index (HI) were calculated for the possible effects of non-carcinogenic risks of heavy metals via ingestion and skin contact using the following eqn (1)–(4).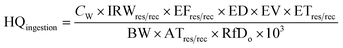 | (1) |
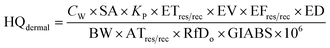 | (2) |
Total HI was calculated by summing the HIs from each of the pathways.
| HI = ∑HQs | (3) |
| THI = HIingestion + HIdermal | (4) |
The value of HQ < 1 represents no adverse health effects, while a value of HI/THI > 1 indicates that there may be non-carcinogenic effects from the close contact of metals.52
Carcinogenic risk (CR) of human health defines the risk of cancer for an individual due to lifetime contact with As, Cr, and Pb and is calculated by eqn (5)–(10).
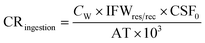 | (5) |
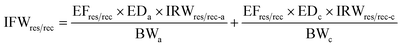 | (6) |
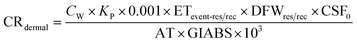 | (7) |
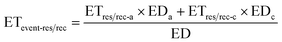 | (8) |
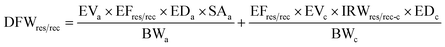 | (9) |
Total cancer risk (TCR) was calculated by the sum of CRs from oral and skin contact.
| TCR = CRingestion + CRdermal | (10) |
Units and values of the parameters used in the above equations associated with the carcinogenic and non-carcinogenic risks, oral reference dose (RfDo), gastrointestinal absorption (GIABS), dermal permeability constant (KP), and oral slope factor (CSF0) are presented in ESI Tables S3 and S4.†
2.6. Statistical analysis
SPSS software (version 28, USA) was used to analyze the estimated water quality parameters. Means, standard deviations, and inferential statistics of measured data of water quality parameters were calculated to evaluate the data dispersion. Additionally, multivariate statistics, namely, principal component analysis (PCA) and Pearson correlation matrix were used to identify the sources of water quality controlling factors.123. Results and discussion
3.1. Physical and chemical properties of the surface and deep waters
The physical and chemical parameters of Buriganga River water during the wet and dry seasons are summarized in Table 1. In both surface and deep waters, F−, Cl−, and SO42− showed higher concentrations in the dry season compared to the wet season. However, in a deep-water sample, a significantly higher concentration of SO42− was observed compared to surface water. Water samples collected during the dry season had a significantly higher EC value than those collected during the wet season, which could be attributed to the dilution effect of river water caused by the wet season's high rainfall. However, in the wet season, higher TSS was reported, which is attributable to the influence of waste materials from agricultural runoff beside the river along with industrial discharge.2,53 Furthermore, the concentrations of EC and TSS in the surface water were found to be higher than in deep water since suspended and ionic matter from anthropogenic sources mostly comes first at the water's surface. The temperature of the Buriganga River water fluctuated with temporal variations. In the dry season, the mean (±SD) pH of surface and deep waters was 5.86 ± 0.49 and 5.80 ± 0.52, respectively; in the wet season, the pH of surface and deep waters was 5.66 ± 0.50 and 5.61 ± 0.50, respectively, indicating slightly acidic water in both seasons. The lower pH levels at some sampling sites could be attributed to the inputs of untreated wastewater from industries and domestic uses.15 Interestingly, the lower pH and higher TSS in river water might be attributed to higher concentrations of anions (F−, Cl−, and SO42−), possible anaerobic conditions in water resulting in acidic substances, particularly from organic wastes, and potential adsorption of cations on the suspended organic and inorganic substances.54,55 Other elements including topography, hydrology, land outflow, rainfall, and industrial emissions (solid or liquid waste) may have had an important impact on the alteration of physicochemical qualities of the surface and deep waters of the Buriganga river.24| Sites | F− (mg L−1) | Cl− (mg L−1) | SO42− (mg L−1) | EC (mS m−1) | pH | TSS (mg L−1) | Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |
| a Indicates statistically significant difference between dry and wet seasons for the parameters (p < 0.05). | ||||||||||||||
| Surface water | ||||||||||||||
| B1 | 0.11 | 0.09 | 6.61 | 6.23 | 3.51 | 3.34 | 40.5 | 18.9 | 5.22 | 6.13 | 326 | 398 | 23.5 | 28.1 |
| B2 | 0.21 | 0.15 | 7.5 | 7.01 | 3.89 | 3.52 | 33.2 | 13.7 | 6.13 | 5.16 | 319 | 333 | 24.2 | 30.3 |
| B3 | 0.23 | 0.17 | 7.31 | 7.21 | 4.33 | 3.84 | 33.7 | 24.8 | 6.05 | 6.03 | 256 | 310 | 25.5 | 30.7 |
| B4 | 0.33 | 0.22 | 5.16 | 5.1 | 4.25 | 3.87 | 33.5 | 26.3 | 6.84 | 6.25 | 261 | 299 | 22.3 | 30.3 |
| B5 | 0.22 | 0.16 | 7.02 | 6.29 | 4.28 | 3.89 | 47.2 | 25.2 | 5.77 | 5.36 | 265 | 302 | 24.1 | 28.7 |
| B6 | 0.32 | 0.31 | 8.01 | 7.12 | 3.69 | 3.36 | 53.3 | 18.8 | 6.27 | 6.27 | 365 | 381 | 26.2 | 29.8 |
| B7 | 0.37 | 0.31 | 7.73 | 6.91 | 4.29 | 3.07 | 52.1 | 27.5 | 6.14 | 6.11 | 289 | 301 | 24.3 | 30.5 |
| B8 | 0.19 | 0.09 | 7.12 | 6.33 | 4.63 | 3.38 | 41.6 | 19.1 | 5.93 | 5.56 | 389 | 395 | 22.1 | 30.4 |
| B9 | 0.13 | 0.07 | 6.18 | 6.01 | 3.42 | 3.13 | 39.3 | 28.2 | 6.18 | 6.13 | 412 | 486 | 23.3 | 30.2 |
| B10 | 0.22 | 0.12 | 7.19 | 6.59 | 4.26 | 4.15 | 48.2 | 33.5 | 5.58 | 5.85 | 403 | 412 | 23.1 | 30.5 |
| B11 | 0.24 | 0.14 | 6.69 | 6.09 | 4.06 | 3.11 | 38.3 | 23.5 | 6.08 | 5.15 | 313 | 312 | 23.5 | 28.5 |
| B12 | 0.26 | 0.16 | 7.15 | 6.22 | 4.22 | 4.14 | 43.2 | 33.4 | 5.38 | 5.05 | 303 | 333 | 23.6 | 29.2 |
| B13 | 0.27 | 0.12 | 7.79 | 6.99 | 4.29 | 3.95 | 41.1 | 35.5 | 5.22 | 5.01 | 411 | 387 | 23.3 | 30.1 |
| B14 | 0.28 | 0.18 | 7.67 | 6.51 | 4.26 | 4.05 | 40.3 | 30.5 | 5.18 | 5.15 | 376 | 402 | 23.9 | 30.1 |
| Mean ± SD | 0.24 ± 0.07a | 0.16 ± 0.07 | 7.08 ± 0.75a | 6.47 ± 0.56 | 4.10 ± 0.35a | 3.63 ± 0.40 | 41.8 ± 6.46a | 25.6 ± 6.42 | 5.86 ± 0.49 | 5.66 ± 0.50 | 335 ± 57.1 | 361 ± 56.2a | 23.8 ± 1.1 | 29.8 ± 0.84a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Deep water | ||||||||||||||
| B1 | 0.21 | 0.18 | 6.13 | 5.75 | 3.11 | 3.15 | 35.2 | 26.2 | 5.65 | 5.45 | 322 | 329 | 21.5 | 26.4 |
| B2 | 0.08 | 0.06 | 6.22 | 5.87 | 3.72 | 3.33 | 31.1 | 19.9 | 5.06 | 5.07 | 353 | 369 | 22.2 | 25.4 |
| B3 | 0.17 | 0.16 | 6.31 | 6.14 | 4.14 | 4.12 | 33.3 | 25.2 | 6.12 | 6.01 | 290 | 368 | 21.4 | 27.6 |
| B4 | 0.17 | 0.13 | 7.02 | 7.03 | 4.71 | 3.52 | 41.3 | 26.7 | 6.05 | 6.02 | 251 | 295 | 22.7 | 25.3 |
| B5 | 0.18 | 0.13 | 6.89 | 6.34 | 3.13 | 3.21 | 30.5 | 23.1 | 6.01 | 5.08 | 321 | 340 | 24.5 | 26.1 |
| B6 | 0.16 | 0.15 | 7.07 | 6.36 | 4.35 | 3.87 | 41.1 | 27.5 | 5.07 | 5.11 | 257 | 253 | 23.7 | 24.4 |
| B7 | 0.22 | 0.21 | 6.69 | 6.29 | 4.19 | 3.69 | 38.4 | 25.5 | 5.35 | 5.03 | 221 | 285 | 24.2 | 25.5 |
| B8 | 0.24 | 0.18 | 5.56 | 5.73 | 3.93 | 3.45 | 39.2 | 29.3 | 6.21 | 6.22 | 310 | 376 | 24.1 | 27.6 |
| B9 | 0.13 | 0.15 | 7.12 | 6.14 | 4.42 | 4.14 | 34.5 | 22.2 | 6.12 | 6.07 | 316 | 350 | 22.1 | 25.5 |
| B10 | 0.09 | 0.07 | 7.84 | 7.31 | 4.64 | 4.12 | 33.4 | 23.2 | 5.55 | 5.56 | 332 | 366 | 22.2 | 26.3 |
| B11 | 0.19 | 0.17 | 7.11 | 6.33 | 4.65 | 3.81 | 36.6 | 13.8 | 6.55 | 6.57 | 302 | 372 | 21.2 | 26.9 |
| B12 | 0.08 | 0.07 | 6.99 | 6.51 | 4.25 | 3.22 | 30.4 | 23.5 | 6.51 | 5.59 | 330 | 365 | 22.5 | 28.3 |
| B13 | 0.15 | 0.17 | 6.49 | 7.01 | 4.04 | 3.38 | 37.7 | 23.7 | 5.85 | 5.58 | 298 | 331 | 22.9 | 27.5 |
| B14 | 0.18 | 0.17 | 6.68 | 7.05 | 4.11 | 3.91 | 38.4 | 25.1 | 5.05 | 5.16 | 312 | 372 | 22.7 | 26.7 |
| Mean ± SD | 0.16 ± 0.05 | 0.14 ± 0.05 | 6.72 ± 0.56 | 6.42 ± 0.51 | 4.10 ± 0.50a | 3.64 ± 0.36 | 35.8 ± 3.73a | 23.9 ± 3.75 | 5.80 ± 0.52 | 5.61 ± 0.50 | 301 ± 35.9 | 341 ± 38.4a | 22.7 ± 1.1 | 26.4 ± 1.1a |
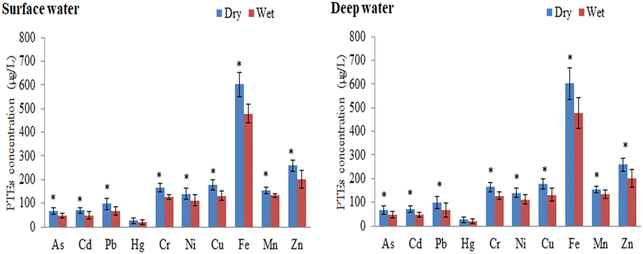
The layer-wise concentrations (μg L−1) of metals in river water for both dry and wet seasons are summarized in Table 2. Table 3 compares the studied metals of the Buriganga River to those of other national and international rivers. Significant variations in metal concentrations were observed among the sampling sites and seasons. The variation of metal concentrations in any riverine ecosystem may have been influenced by the geomorphological setting, land runoff, and domestic-industrial discharges.12,56 The mean concentrations of the studied metals in the Buriganga River followed the descending order of Fe > Zn > Cu > Mn > Cr > Ni > Pb > Cd > As > Hg. Higher levels of metals were observed in the dry season than in the wet season, owing to decreased water flow during the dry season, which may have helped to accumulate metals in the river water.57 In this study, concentrations of studied metals in surface water are clearly higher than in deep water, which indicated that elements after release from the sources quickly mixed with the surface water. Moreover, regardless of the seasons, the examined metals showed higher concentrations in the downstream locations of the studied river (Table 2). Water flow in the river with industrial effluents and wastewater discharge channels with sampling points may be related to variations in metal concentrations along the riverways.58 However, the main reasons for higher metal input at various sampling sites in the Dhaka megacity include industrial discharges, municipal wastewater, domestic garbage, and urban runoff.15Table 3 also reveals the comparison of the minimum, maximum, and mean concentrations of these metals in both seasons (dry and wet) with the Bangladesh drinking water standards59 and60 drinking water guideline values. The results revealed that most of the water quality parameters exceeded the standard guidelines,59,60 indicating severe contamination of the surface and deep waters of the Buriganga River.
| Sites | As | Cd | Pb | Hg | Cr | Ni | Cu | Fe | Mn | Zn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |
| a Indicates statistically significant difference between dry and wet seasons for the parameters (P < 0.05). | ||||||||||||||||||||
| Surface water | ||||||||||||||||||||
| B1 | 70.1 | 55.4 | 71.5 | 57.3 | 91.1 | 60.0 | 16.5 | 15.7 | 177 | 122 | 130 | 95 | 155 | 113 | 550 | 490 | 169 | 152 | 258 | 204 |
| B2 | 77.8 | 30.8 | 75.5 | 45.1 | 99.4 | 39.5 | 16.5 | 13.0 | 156 | 132 | 142 | 107 | 154 | 145 | 573 | 447 | 167 | 156 | 290 | 177 |
| B3 | 66.9 | 44.9 | 77.2 | 33.3 | 83.5 | 41.8 | 13.5 | 12.7 | 149 | 116 | 146 | 117 | 152 | 134 | 588 | 393 | 146 | 139 | 247 | 209 |
| B4 | 55.1 | 41.1 | 65.1 | 25.6 | 61.7 | 47.8 | 25.9 | 22.1 | 174 | 126 | 133 | 120 | 178 | 136 | 566 | 460 | 153 | 148 | 242 | 205 |
| B5 | 86.7 | 63.5 | 78.8 | 55.1 | 79.2 | 44.1 | 25.1 | 13.9 | 185 | 142 | 135 | 83 | 189 | 126 | 594 | 487 | 167 | 152 | 262 | 159 |
| B6 | 51.8 | 44.0 | 65.3 | 48.3 | 79.0 | 56.7 | 17.7 | 15.5 | 148 | 117 | 148 | 122 | 205 | 155 | 601 | 510 | 142 | 132 | 274 | 205 |
| B7 | 82.0 | 47.1 | 55.2 | 44.0 | 57.8 | 59.6 | 17.3 | 14.8 | 169 | 147 | 161 | 139 | 207 | 134 | 554 | 459 | 150 | 135 | 234 | 211 |
| B8 | 78.8 | 44.4 | 75.2 | 41.0 | 74.8 | 56.9 | 28.7 | 15.5 | 144 | 125 | 114 | 82 | 167 | 146 | 670 | 513 | 157 | 136 | 270 | 262 |
| B9 | 77.3 | 32.9 | 88.3 | 44.2 | 108 | 71.1 | 29.6 | 17.3 | 145 | 134 | 123 | 110 | 192 | 176 | 626 | 533 | 167 | 158 | 320 | 293 |
| B10 | 78.1 | 33.6 | 85.1 | 48.9 | 106 | 75.9 | 29.7 | 26.5 | 168 | 141 | 164 | 123 | 188 | 157 | 652 | 544 | 175 | 152 | 290 | 261 |
| B11 | 95.4 | 43.1 | 92.1 | 47.1 | 112 | 88.1 | 35.7 | 22.2 | 188 | 141 | 173 | 132 | 210 | 144 | 656 | 440 | 188 | 152 | 280 | 263 |
| B12 | 88.7 | 59.9 | 90.5 | 59.9 | 122 | 91.3 | 41.2 | 35.6 | 192 | 135 | 172 | 145 | 196 | 136 | 670 | 482 | 179 | 149 | 290 | 261 |
| B13 | 96.3 | 55.7 | 95.6 | 88.0 | 136 | 87.7 | 45.8 | 30.1 | 188 | 133 | 189 | 154 | 200 | 166 | 697 | 500 | 183 | 142 | 278 | 256 |
| B14 | 81.8 | 55.8 | 91.8 | 76.6 | 129 | 89.0 | 44.4 | 34.2 | 192 | 141 | 183 | 144 | 214 | 178 | 689 | 500 | 190 | 137 | 282 | 252 |
| Mean | 77.6 ± 13.2a | 46.6 ± 10.3 | 79.1 ± 12.1a | 51.0 ± 16.1 | 95.6 ± 24.3a | 65.0 ± 18.8 | 27.7 ± 10.8 | 20.7 ± 8.0 | 170 ± 18.3a | 132 ± 9.8 | 151 ± 23.0a | 120 ± 22.5 | 186 ± 21.6a | 146 ± 18.7 | 620 ± 51.4a | 483 ± 40.0 | 167 ± 15.5a | 146 ± 8.7 | 273 ± 22.9a | 230 ± 38.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||||||
| Deep water | ||||||||||||||||||||
| B1 | 51.1 | 33.2 | 56.2 | 39.0 | 73.3 | 43.3 | 13.9 | 11.5 | 133 | 86.6 | 105 | 83.9 | 141 | 104 | 490 | 368 | 128 | 103 | 252 | 143 |
| B2 | 42.0 | 29.9 | 47.0 | 41.5 | 74.0 | 33.3 | 14.9 | 10.0 | 150 | 111 | 120 | 87.0 | 150 | 112 | 534 | 432 | 159 | 121 | 235 | 167 |
| B3 | 55.6 | 33.9 | 59.0 | 47.3 | 76.3 | 38.7 | 13.8 | 9.9 | 142 | 109 | 112 | 83.0 | 149 | 109 | 579 | 446 | 133 | 109 | 242 | 234 |
| B4 | 53.3 | 40.5 | 65.4 | 51.2 | 76.2 | 39.4 | 15.5 | 13.5 | 176 | 126 | 123 | 116 | 172 | 118 | 532 | 399 | 151 | 126 | 241 | 195 |
| B5 | 54.4 | 41.2 | 78.7 | 39.7 | 74.4 | 51.3 | 14.5 | 13.7 | 185 | 135 | 128 | 78.9 | 184 | 123 | 539 | 479 | 161 | 138 | 234 | 176 |
| B6 | 55.2 | 47.8 | 65.1 | 46.7 | 79.9 | 68.4 | 17.2 | 15.9 | 132 | 120 | 124 | 115 | 179 | 138 | 679 | 504 | 140 | 123 | 275 | 163 |
| B7 | 43.9 | 27.1 | 56.2 | 53.3 | 85.8 | 64.9 | 17.0 | 15.6 | 154 | 135 | 154 | 129 | 160 | 109 | 523 | 401 | 149 | 128 | 202 | 199 |
| B8 | 65.6 | 38.9 | 74.1 | 32.2 | 99.9 | 46.0 | 27.8 | 15.4 | 175 | 127 | 147 | 117 | 166 | 116 | 637 | 428 | 152 | 139 | 269 | 160 |
| B9 | 82.4 | 54.3 | 56.1 | 42.3 | 98.8 | 67.7 | 28.8 | 17.8 | 168 | 136 | 137 | 110 | 189 | 127 | 698 | 529 | 162 | 130 | 274 | 206 |
| B10 | 81.1 | 65.6 | 84.3 | 58.9 | 96.6 | 66.1 | 39.2 | 26.6 | 173 | 145 | 157 | 128 | 174 | 116 | 637 | 522 | 151 | 146 | 279 | 193 |
| B11 | 85.4 | 69.7 | 77.8 | 55.5 | 121 | 87.9 | 41.8 | 32.8 | 178 | 131 | 162 | 133 | 186 | 139 | 661 | 552 | 175 | 168 | 279 | 226 |
| B12 | 92.6 | 63.7 | 84.0 | 49.8 | 116 | 99.0 | 45.0 | 31.7 | 187 | 135 | 154 | 121 | 211 | 175 | 632 | 513 | 165 | 152 | 299 | 259 |
| B13 | 77.9 | 55.4 | 88.3 | 61.0 | 138 | 112 | 41.4 | 34.6 | 189 | 142 | 155 | 130 | 204 | 178 | 619 | 563 | 158 | 143 | 258 | 243 |
| B14 | 89.3 | 64.3 | 89.9 | 63.3 | 151 | 123 | 37.8 | 32.1 | 175 | 156 | 169 | 138 | 208 | 188 | 659 | 555 | 176 | 144 | 289 | 253 |
| Mean | 66.4 ± 17.7a | 47.5 ± 14.5 | 70.2 ± 14.0a | 48.7 ± 9.2 | 97.2 ± 25.5a | 67.2 ± 28.6 | 26.3 ± 12.4 | 20.1 ± 9.3 | 165 ± 19.6a | 128 ± 17.4 | 139 ± 20.3a | 112 ± 20.5 | 177 ± 22.1a | 132 ± 28.1 | 601 ± 67.0a | 478 ± 65.0 | 154 ± 14.2a | 134 ± 17.2 | 259 ± 26.3a | 201 ± 37.3 |
| Locations | As | Cd | Pb | Hg | Cr | Ni | Cu | Fe | Mn | Zn | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a NA = data not available, CMC = criterion maximum concentration; CCC = criterion continuous concentration; MCLG = maximum contaminant level goal; MCL = maximum contaminant level. | ||||||||||||
| Buriganga River, Bangladesh (present study) | ||||||||||||
| Surface water (dry season) | Mean | 77.6 | 79.1 | 95.6 | 27.7 | 169.6 | 142.7 | 186.2 | 620 | 166.7 | 272.5 | This study |
| Surface water (wet season) | Mean | 46.6 | 51.0 | 65.0 | 20.7 | 131.4 | 119.6 | 146.2 | 483 | 1086.4 | 229.9 | This study |
| Deep water (dry season) | Mean | 66.4 | 70.2 | 97.2 | 26.3 | 165 | 139 | 176.6 | 601 | 154.2 | 259.0 | This study |
| Deep water (wet season) | Mean | 47.5 | 48.7 | 67.2 | 20.1 | 128.2 | 112.1 | 132.3 | 478 | 133.7 | 201.0 | This study |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Other rivers in Bangladesh and other countries for comparison | ||||||||||||
| Shitalakhya River, Bangladesh | Mean | NA | 2.9 | 4.4 | 3.6 | 4.2 | NA | 20.1 | NA | NA | 632 | 2 |
| Old Brahmaputra River, Bangladesh | Mean | NA | 1.0 | 110 | 1.0 | 10 | 440 | 120 | NA | 1440 | 10 | 71 |
| Turag River, Bangladesh | Range | 11.8–19.3 | 0.21–0.30 | 3.73–4.50 | NA | 11.1–16.0 | 12.9–18.9 | 12.3–41.7 | NA | NA | NA | 66 |
| Karnaphuli River, Bangladesh | Mean | NA | 0.01 | 0.14 | NA | 0.25 | NA | 0.05 | NA | 0.12 | 0.28 | 72 |
| Buriganga River, Bangladesh | Mean | NA | 59 | 112 | NA | 114 | NA | 150 | NA | 157 | 332 | 78 |
| Balu River, Bangladesh | Mean | NA | 13.7 | 1.0 | NA | NA | NA | 6.0 | NA | NA | 10.1 | 73 |
| Bangshi River, Bangladesh | Mean | NA | 1.2 | 13.5 | NA | NA | NA | 70.0 | NA | NA | 210.0 | 74 |
| Subarnarekha River, India | Mean | NA | NA | NA | NA | 0.89 | 25.2 | 16.6 | 134 | 12 | NA | 97 |
| Damodar River, India | Mean | NA | 9 | 10 | NA | 16 | 52 | 18 | NA | 33 | 89 | 75 |
| Ganges River, India | Mean | 0.634 | 0.220 | 0.567 | NA | 1.851 | 0.485 | 0.642 | 0.033 | NA | 2.611 | 69 |
| Meriç-Ergene River, Turkey | Mean | 3.51 | 0.046 | 0.51 | NA | 13.76 | 9.06 | 4.30 | NA | 26.3 | 8.18 | 67 |
| Tigris River, Turkey | Mean | 0.63 | 0.044 | 2.82 | NA | 25.41 | 24.54 | 17.10 | 158.16 | 12.01 | 68 | |
| Catalan River, Spain | Mean | 2.9 | 1.2 | 2.2 | NA | 2.4 | 2.7 | 1.3 | NA | 1.9 | 70 | |
| Hawkesbury-Nepean River, Australia | Mean | NA | 0.045 | 0.111 | NA | NA | 0.26 | 0.81 | 268 | 52 | 0.88 | 76 |
| Trinity River, USA | Mean | NA | 0.008 | 0.026 | NA | NA | 2.07 | 1.15 | 5.8 | 4.15 | NA | 77 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Freshwater quality criteria for protection of aquatic life | ||||||||||||
| USEPA, CMC, acute | 0.34 | 0.0018 | 0.082 | 0.0014 | 0.016 | 470 | NA | NA | NA | 0.12 | 65 | |
| USEPA, CCC, chronic | 0.15 | 0.00072 | 0.0032 | 0.00077 | 0.011 | 52 | NA | NA | NA | 0.12 | 65 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Drinking water quality criteria | ||||||||||||
| Drinking Water Standard Board (DWSB) | 0.05 | 0.005 | 0.05 | 0.001 | 0.05 | 100 | 1 | 0.3–1.0 | 0.1 | 5 | 59 | |
| European Community | 0.01 | 0.005 | 0.01 | 0.001 | 0.05 | 20 | 2 | 0.2 | 0.05 | NA | 63 | |
| WHO | 0.01 | 0.003 | 0.01 | 0.006 | 0.05 | 70 | 2 | NA | 0.5 | 3 | 60 | |
| USEPA | MCLG | 0.0 | 0.005 | 0.0 | 0.002 | 0.1 | NA | 1.3 | NA | NA | NA | 64 |
| USEPA | MCL | 0.01 | 0.005 | 0.015 | 0.002 | 0.1 | NA | NA | NA | NA | NA | 64 |
In surface water, the maximum concentration of As was found at site B13 (96.3 μg L−1) in the dry season, followed by site B5 (63.5 μg L−1) in the wet season. Considering both seasons, the maximum concentrations of As were found at sites B12 (92.6 μg L−1) and B11 (69.7 μg L−1) in deep water (Table 2). The use of As-contaminated pesticides in agricultural areas, the use of chromate copper arsenate to treat wood, the burning of coal for electricity, and the mining of silt from the whole system of the studied river sites might all be contributing factors to the existence of a greater concentration of As.61,62 In comparison to several drinking water quality criteria, the As level in water samples was significantly higher than that recommended by Drinking Water Standard Board (DWSB), European Community (EuC), World Health Organization (WHO), Maximum Contaminant Level Goal (MCLG), and Maximum Contaminant Level (MCL) regardless of season59,60,63,64 (Table 3). The results of the current study were compared with freshwater quality criteria, and the level of As was observed to be several orders of magnitude greater than the water quality permissible standards established by the USEPA.65 Arsenic concentrations in the current study's water samples were compared to those of other relevant studies at the national and international levels, and the As value was clearly higher than those of the rivers of Turag,66 Meriç-Ergene River,67 the Tigris River,68 the Ganges River,69 and the Catalan River70 (Table 3).
The highest concentration of Cd was found in surface water at the B13 site (95.6 and 88.0 μg L−1 in the dry and wet seasons, respectively), followed by deep water at the B14 site (89.9 and 63.3 μg L−1 in dry and wet seasons, respectively) (Table 2). Higher levels of Cd in the Buriganga River's water could be linked to industrial activities, air emissions, and Cd-plated substances.56 Furthermore, greater Cd levels could be attributed to variations in river water availability, where water intake to the river is primarily urban and there is a scarcity of regular water from natural sources, preventing pollutants from mixing with natural water.2 The mean Cd levels in both water samples exceeded the criterion maximum concentration (CMC) and criterion continuous concentration (CCC) limits recognized by the USEPA,65 implying that toxic metals, especially Cd, in river water may pose a substantial risk to the surrounding ecosystems (Table 3). In the current investigation, Cd content in both surface and deep waters was significantly greater than some guidelines such as DWSB, EuC, WHO, MCLG, and MCL, despite the seasons, when compared to various standards for drinking water quality.59,60,63,64 When comparing the Cd concentrations in this study with those of the other studies, it was discovered that Cd was substantially higher than those found in the waters of some of the other rivers such as Shitalakhya,2 Old Brahmaputra,71 Turag,66 Karnaphuli,72 Balu,73 Bangshi,74 Damodar,75 Catalan,70 Hawkesbury-Nepean,76 and Trinity River,77 but apparently similar to those found in the Buriganga River.78
Among the sampling sites, B13 showed the highest level of Pb (μg L−1) in the surface water (136 and 91.3 during the dry and wet seasons, respectively), whereas the highest levels of Pb (μg L−1) were detected at B13 (138 in the dry season) and B14 (123 in the wet season) in deep water samples (Table 2). The increased Pb concentration in the current study river could be attributable to garbage discharged by neighboring battery and textile companies, dyeing industry lead-based dyes, or PVC-containing plastic toys.8,9 The first and most troubling component in Bangladesh is Pb, which was found in water samples to be expressively higher than the acceptable limits for drinking water, such as DWSB, EuC, WHO, MCLG, and MCL,59,60,63,64 and aquatic life, such as CMC and CCC,65 as well as in other studies in Bangladesh2,66,71–74 and other countries70,75–77 (Table 3). However, exceptionally high Pb concentration was found in the Buriganga River,78 which is expected to be comparable to the current study, and could be owing to various sources at the study sites, such as gasoline, metropolitan runoffs, and deposition of lead-containing materials from the atmosphere.18
During the dry and wet seasons, the maximum Hg concentrations were detected in surface water at the B13 site (45.8 μg L−1) and the B12 site (35.6 μg L−1). The highest level of Hg was reported at site B12 (45.0 μg L−1 in the dry season) and site B13 (34.6 μg L−1 in the wet season) in the deep-water samples (Table 2). Hg concentrations in water samples were found to be significantly higher than the freshwater quality criteria (CMC and CCC recommendation values) as well as other drinking water standards59,60,63–65 (Table 3). Mercury levels in groundwater and surface water are typically less than 0.5 μg L−1, while higher levels in groundwater may result from local mineral deposits.60 Mercury can also be found in non-ionic organic molecules, as well as in other organic and inorganic compounds. Mercury enters the riverine aquatic environment through a variety of routes. Depositions from the atmosphere can directly introduce inorganic forms into reservoirs.79 Hg(II) and CH3Hg, on the other hand, are carried into water reservoirs by surface runoff and leaching from higher levels of a soil profile to groundwater, which are then recycled into surface waters.80 Mercury adsorption and desorption processes in the aquatic environment play an important role in mercury distribution in different aquatic components. These systems are also necessary for the distribution, transformation, and uptake of Hg by living organisms in bodies of water.81 The mean concentration of Hg was found to be considerably higher than that of the numerous earlier studies conducted in Bangladesh, such as in the Shitalakhya River2 and Old Brahmaputra River.71
The highest value of Cr was observed at B12 and B14 sites (192 μg L−1 in the dry season), while B10, B11, and B14 sites were recorded as the top Cr-rich sites in the wet season (141 μg L−1) (Table 2). In contrast, a greater level of Cr in deep water was found at sites B13 (189 μg L−1) and B14 (156 μg L−1). Concentrations of Cr in water samples from the study area were found to be substantially higher than the CMC and CCC standards for aquatic life protection established by the USEPA.65 The current study's Cr level was compared to numerous drinking water quality criteria, and it was confirmed that Cr levels were higher than the recommended values of the standards such as DWSB, EuC, WHO, MCLG, and MCL.59,60,63,64 The presence of higher levels of Cr in water samples could be due to the effects of untreated wastewater from tanneries on the Buriganga River's west bank (Hazaribagh area of Dhaka City).8 In addition, pigment production, electroplating a thin layer of chromium onto a metal object, and different preservatives for wood processing may have a direct influence on raising Cr levels in the studied river.2 Hence, the waste and effluents emitted by these enterprises are most likely to blame for the higher Cr levels found in the exposed water samples. Furthermore, earlier literature demonstrating worldwide increasing Cr concentrations was accessible from a number of scientists, including Kabir et al.,2 Islam et al.,66 Bhuiyan et al.,71 Islam et al.,72 and Carafa et al.70 (Table 3).
In surface water, the maximum concentration (μg L−1) of Ni was observed at the B13 site (189 and 154 during the dry and wet seasons, respectively). In deep water, the highest concentration of Ni (μg L−1) was found at the B14 site (169 and 138, dry and wet seasons, respectively) (Table 2). However, Ni concentrations were prominent and deliberately higher than the various drinking water reference levels, for instance, DWSB, EuC, and WHO.59,60,63 Moreover, water samples of the Buriganga River showed lower and higher amounts of Ni contents when compared to USEPA-CMC and CCC values (Table 3), respectively, documented by USEPA.65 Although motor oils contain tiny amounts of Ni, the combustion of diesel fuel is the primary source of Ni in urban street dust, and as a result, the urban runoff would be a significant Ni source in nearby surface and deep waters. Accidental spills of Ni-containing items, municipal and industrial waste, and lithogenic causes are all possible sources of Ni in the urban aquatic environment.37 Nickel concentrations in water samples were significantly lower in previous studies66,70,75–77 than in this study, but significantly higher concentration was reported by Bhuiyan et al.71 than the current measured concentrations (Table 3).
For the dry and wet seasons, the greatest concentrations (μg L−1) of Cu in surface water were reported at the B14 site (214 and 178). The highest concentration of Cu in deep water was observed at sites B12 and B14 (211 and 188 μg L−1 during the dry and wet seasons, respectively) (Table 2). All of the Cu contents in all of the water samples collected across the study area were much higher than the DWSB, EuC, WHO, and MCLG standards59,60,63,64 (Table 3). In the current study, an elevated level of Cu could be due to the sorption and desorption, leaching of contaminants, and leakage of sewage from the urban runoff.2,8 The Cu concentration in water of the current study was higher than that of earlier Bangladeshi research2,66,71–74 and other countries70,75–77 (Table 3).
The maximum concentration of Fe in the Buriganga River surface water was determined to be 697 μg L−1 at the B13 site during the dry season, but it dropped to 544 μg L−1 at the B10 site during the wet season. The highest concentration (698 μg L−1) of Fe was found in deep water at the B8 site during the dry season, followed by 563 μg L−1 at the B13 site during the wet season (Table 2). The Fe concentration in the Buriganga River deep water is higher than that in the surface water in this study, and it is always the highest for both seasons and each site. The average Fe content in this investigation was a hundred times greater than DWSB and EuC norms59,63 (Table 3). When the Fe concentrations in this study were compared to those in previous studies, it was determined that they were much greater than those of the aquatic environments like Tigris River,68 Hawkesbury-Nepean,76 and the Trinity River77 (Table 3). The largest Fe concentrations in both water samples are most likely due to the accumulation of rock minerals by the effects of storms, corrosion, wind, and waves, electroplating and smelting activities at the banks of the river, and wastewater application to the riverside agricultural fields.8
In both the dry and wet seasons, the highest level of Mn in surface water was found at sites B14 (190 μg L−1) and B9 (158 μg L−1), respectively, followed by B14 (176 μg L−1) and B11 (168 μg L−1) locations in deep water, respectively (Table 2). It was revealed that the current study's Mn concentration level was extensively greater than DWSB, EuC, and WHO norms59,60,63 (Table 3). Mn concentrations in the current study's waters were compared to those in other studies conducted in Bangladesh and other countries, and it was discovered that the current study's Mn concentration was higher than that in the other studies,72,75–77 it was also interestingly lower than that of Bhuiyan et al.,71 and it was expectedly parallel to that of Bhuiyan et al.78 (Table 3). Manganese may be present in water due to natural sources (rock and soil weathering), extraction of other geological minerals from the earth, and industrial discharges of untreated waste.23
In the Buriganga River, the second highest concentration in surface water was Zn found at the B9 site, with maximum concentrations of 320 and 293 μg L−1 during the dry and wet seasons, respectively (Table 2). However, the maximum Zn level was found in deep water at the B12 site (299 and 259 μg L−1 in the dry and wet seasons, respectively) (Table 2). The mean value of Zn in samples exceeded the USEPA's65 CMC and CCC standards, indicating that Zn in river water posed a significant threat to the riverine ecosystems (Table 3). When compared to various drinking water quality criteria, the Zn level in water was significantly higher than that recommended by DWSB and WHO, regardless of season.59,60 Other researchers observed higher levels of Zn in water samples, such as Kabir et al.2 and Bhuiyan et al.;78 in contrast, lower levels of Zn were found in other investigations70–76 than the present study (Table 3). Zn concentrations varied from location to location and season to season, which could be attributed to changes in water flow, industrial settlement, drainage networks, and waste from agricultural runoff at the sampling sites.2 Moreover, increased anthropogenic activities like brick manufacturing, dredging, power production, transporting industrial emissions, galvanizing, refining, sludge disposal, and energy production also raise the concentration of Zn in the Buriganga River water.
3.2. Source analysis of metals
To determine the interrelationship and potential sources of metals, Pearson's correlation analysis and principal component analysis (PCA) using varimax rotation with Kaiser normalization were used. The results are presented in Tables S5–S8 and Fig. S1–S4.†In the wet season for surface and deep-water data of metals, PCA extracted four and two components (eigenvalues > 1.0) specifying 83.2 and 80.6% of the total variance, respectively. For surface water, the PC1 explained 42.1% of the total variance and was loaded with Pb, Hg, Ni, and Zn. According to Table S4,† Pb was strongly correlated with Hg (r = 0.85) and Zn (r = 0.773). Ni correlated moderately with Hg (r = 0.85) and Pb (r = 0.85). A similar association among these metals means that they may have originated from similar sources such as vehicular pollution. Tire abrasion, combustion of lubricants, fuel additives, and the fumes of vehicles are responsible for releasing these elements in urban areas.82,83 The PC2 accounted for 17.9% of the total variance and was loaded with Cu, Fe, and Zn. Cu showed a moderate correlation with Fe (r = 0.587) and Zn (r = 0.627). Municipal wastewater and landfill leachate together with natural sources (such as weathering of parent rock) may be responsible for releasing these elements into the surface water.84–86 The PC3 explained 12.6% of the total variance and was loaded by As and Cd and moderate correlation was observed among these two elements (r = 0.575) in correlation analysis, demonstrating the agricultural effluents as a source for As and Cd. Arsenic can be found in insecticides, herbicides, and pesticides.87 Cadmium can also be released into the river by overusing Cd-based fertilizers.88,89 The PC4 was found to be loaded with Cr and Mn, and it explained 10.4% of the total variance. There was no correlation between Cr and Mn and other metals, indicating that these two metals may have originated from unlike sources.90
In deep water, the PC1 explained 69.5% of the total variance and was associated with Cd, Pb, As, Hg, Cu, Ni, Fe, and Zn. In correlation analysis, As showed strong correlations with Pb (r = 0.760), Hg (r = 0.879), and Fe (r = 0.797). Cd showed a strong correlation with Pb (r = 0.723), Hg (r = 0.733), and Ni (r = 0.705), whereas Pb showed a strong correlation with Hg (r = 0.921), Ni (r = 0.733), Cu (r = 0.927), Fe (r = 0.799) and Zn (r = 0.737). In addition, a moderate correlation was found for As with Cd (r = 0.591), Cr (r = 0.519), Ni (r = 0.643), Cu (r = 0.690), and Zn (r = 0.591), and for other element pairs such as Cd and Cu (r = 0.595), Cd and Fe (r = 0.580), Cr and Ni (r = 0.564), Cr and Fe (r = 0.511), Ni and Cu (r = 0.576), Ni and Fe (r = 0.539), Ni and Zn (r = 0.504), and Fe and Zn (r = 0.634). The sources of these metals in PC1 are the same as the sources of the metals in surface water in PC1, PC2, and PC3. This is due to the hydraulic characteristics of the river flow, the turbulence of the flow, and secondary flows (especially in river bends). Hence, these elements may be derived from a mixture of vehicular, agricultural, and municipal waste and wastewater. The component PC2 was loaded by As, Cr, Ni, Fe, and Mn and explained 11.0% of the total variance. Arsenic had strong correlations with Fe (r = 0.869) and Mn (r = 0.799). Manganese is also strongly correlated with Fe (r = 0.714). There was a moderate correlation between As and Cr (r = 0.519), As and Ni (r = 0.643), Cr and Fe (r = 0.511), Ni and Fe (r = 0.539), and Ni and Mn (r = 0.687). These elements have been linked to geochemical activities or the sink function of river sediment in terms of metal release into the water column.91 Chromium is moderately correlated with Mn (r = 0.620), indicating the discharge of industrial wastewater.
In the dry season, two components were extracted for both surface and deep waters, explaining 77.9 and 82.7% of the total variance, respectively. For surface water, the first component (PC1) explained 62.5% of the total variance and was loaded with Cd, Pb, As, Hg, Mn, Fe, and Zn. A strong correlation was found between As and Mn (r = 0.754), Cd and Pb (r = 0.919), Cd and Hg (r = 0.797), Cd and Fe (r = 0.816), Cd and Mn (r = 0.836), Pb and Hg (r = 0.769), Pb and Fe (r = 0.753), Pb and Mn (r = 0.849), Hg and Fe (r = 0.886), and Hg and Mn (r = 0.799). In addition, a moderate correlation was observed for As with Cd (r = 0.655), Pb (r = 0.754), Hg (r = 0.627) and Fe (r = 0.571), Zn with Cd (r = 0.694), Fe (r = 0.550), Pb (r = 0.697), and Mn with Zn (r = 0.524). These metals may be associated with a mixture of traffic-related pollution and agricultural effluents, demonstrating a combination of PC1 and PC3 in the wet season. The second component (PC2) was specified by Hg, Cr, Cu, Ni, and Mn and composed 15.4% of the total variance. Manganese showed a strong correlation with Hg (r = 0.799) and Cr (r = 0.799). There was a moderate correlation between Hg and Cr (r = 0.628), Hg and Ni (r = 0.620), Hg and Cu (r = 0.600), Cr and Ni (r = 0.676), As and Pb (r = 0.754), Ni and Cu (r = 0.629), and Ni and Mn (r = 0.594). These metals might be related to a mixture of industrial and municipal wastewater, landfill leachate, and natural sources. PC2 in the dry season can be representative of the combination of PC2 and PC4 in the wet season.
For deep water, the first component (PC1) explained 70.9% of the total variance and was specified by As, Cd, Pb, Hg, Cr, Ni, Cu, and Mn. According to the correlation analysis provided in ESI Table S8,† both strong and moderate correlations were observed between these metals. The sources of these elements in PC1 are the same as the sources of the surface water in PC1, revealing vehicular pollution, municipal waste and wastewater, and agricultural effluents. The second component (PC2) was explained by As, Pb, Hg, Cu, Fe, and Zn and determined by 11.7% of the total variance. Arsenic exhibited a strong correlation with other elements in this PC, indicating natural sources for these elements, which could be derived from river bed sediment.92–94 In addition, the strong correlation between Hg and Cr (r = 0.727) can be justified by the discharge of industrial wastewater. PCA results indicate that the source of metal attribution was mainly anthropogenic actions such as chemical fertilizing, industrial waste, raw materials from households and agro-fields, and so on.
3.3. Pollution level and ecological risks
The cumulative pollution level of the water in Buriganga River was estimated using the heavy metal evaluation index (HEI) and degree of contamination (CD) for the studied metals in this study, which is depicted in Fig. 3. The HEI and CD both indicated a high risk and excessive degree of contamination of the river water in both depths regardless of the seasons. Apparently, there were higher risks and contamination in the surface water during the dry season, where, during the dry season, the HEI was 55.9 ± 8.15 in surface water and 51.4 ± 9.24 in deep water. The highest HEI was observed at site B13 (HEI = 69) in the surface water during the dry season, followed by 67 at B14 in the deep water during the dry season. A similar pattern was also found for CD values, indicating the most polluted location at B13 and B14 in the Buriganga River arising from estimated metals in water (ESI Table S9†). Apparently, the upstream of the Buriganga River is less polluted than the downstream, which might be associated with higher urban, industrial, and other anthropogenic activities in the downstream areas.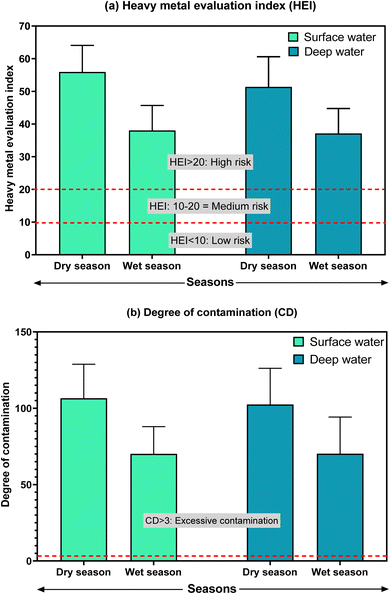 | ||
| Fig. 3 Heavy metal evaluation index (HEI) and degree of contamination (CD) in the surface and deep waters of the Buriganga River during dry and wet seasons. | ||
Ecological risks of individual metals and their cumulative ecological risks are provided in Fig. 4. Most of the studied metals have no ecological risks as the values were less than 40, except for Cd. During the dry season, Cd in surface and deep waters indicated a very high ecological risk (>320), with average values of 474.5 and 420.9, respectively. The highest ecological risk for Cd was found at B13 (ecological risk value of 573.6), followed by B11 (552.6) in the surface water (ESI Table S10†). However, the potential ecological risk (PER) indicated considerable ecological risks both in the surface and deep waters of the Buriganga River during both seasons. Similar to the pollution level, higher ecological risk was observed during the dry season in the surface water. A very high ecological risk (>600) was observed at B11 (ecological risk value of 602.0), B13 (ecological risk value of 626.3), and B14 (ecological risk value of 600.0) in the surface water, while at these points, in the deep water, a considerable ecological risk was observed during the dry season (ESI Table S11†). In line with the pollution and contamination levels, higher ecological risks were observed for the downstream sites of the Buriganga River. As the PER indicated a moderate to very high ecological risk in different locations in the Buriganga River during different seasons at different water depths, there is a big matter of concern, particularly in the very high ecological risk points that may face stress and possibly a matter of extinction of various aquatic species in Buriganga River. Besides, if the pollution from metals continues it will affect the ecological balance in the long run.
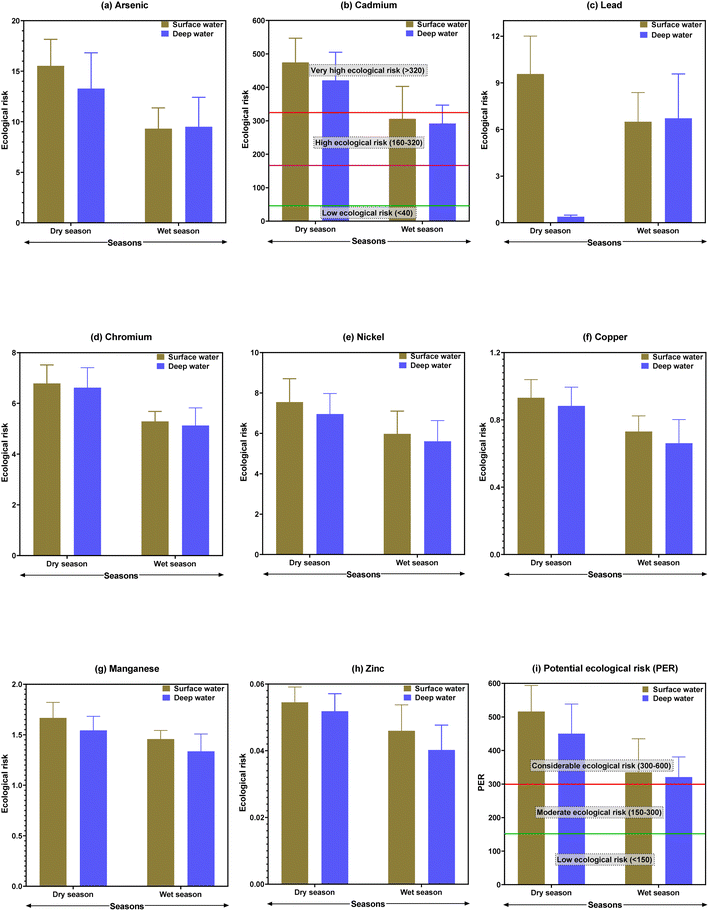 | ||
| Fig. 4 Ecological risks of metals in surface and deep waters from Buriganga River during dry and wet seasons. | ||
3.4. Health risk appraisal
Non-carcinogenic and carcinogenic risks such as HQ, HI, and CR from oral intake and skin contact with water from the Buriganga River are presented in Tables 4 and 5. As shown by the highest HQ values for the examined inhabitants through the ingestion of water (Tables 4 and 5), arsenic contributed 40 and 39% of the total health risk in surface and deep waters, respectively, for both the adult and child receptors (Tables 4 and 5). Previous studies, however, have found a high non-carcinogenic risk of As for residents via oral intake of water.92,95 In the case of surface water samples, Hg also had high HQ values of 2.26 × 100 and 1.56 × 10−1 for residential and recreational receptors, respectively, which contributed 23% of the total risk (HI) (Table 3). Like surface water, Hg also showed elevated values of HQ of 2.17 × 100 and 1.50 × 10−1 for residential and recreational receptors, respectively, which contributed 24% of the total risk in the deep-water case (Table 5). The HQ values of As and Hg from ingestion and dermal exposure pathways were higher than the risk threshold (10−4), indicating that these two elements can cause serious adverse effects on health in residential and recreational adult and child receptors. Considering the health risks for adults and children through oral intake of water, the following descending order of As > Hg > Cd > Pb > Cr > Ni > Mn > Cu > Fe > Zn was found.| Elements | Non-carcinogenic risks for adults | Non-carcinogenic risks for child | Carcinogenic risks for adults | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HQ ingestion | HQ dermal | THI | HQ ingestion | HQ dermal | THI | CR ingestion | CR dermal | TCR | |
| a Bold figures indicate that the risk level of TEs is higher than 1.0 (HQ/HI/THI > 1.0). | |||||||||
| Residential | |||||||||
| As | 3.87 × 100 | 3.04 × 10−2 | 3.90 × 100 | 5.57 × 100 | 4.55 × 10−2 | 5.62 × 100 | 1.31 × 10−3 | 6.99 × 10−6 | 1.32 × 10−3 |
| Cd | 1.22 × 100 | 9.57 × 10−4 | 1.22 × 100 | 1.75 × 100 | 1.43 × 10−3 | 1.75 × 100 | |||
| Pb | 1.07 × 100 | 8.43 × 10−4 | 1.07 × 100 | 1.54 × 100 | 1.26 × 10−3 | 1.55 × 100 | 9.60 × 10−6 | 5.12 × 10−8 | 9.65 × 10−6 |
| Hg | 2.26 × 100 | 1.78 × 10−2 | 2.28 × 100 | 3.25 × 100 | 2.66 × 10−2 | 3.28 × 100 | |||
| Cr | 9.41 × 10−1 | 5.92 × 10−1 | 1.53 × 100 | 1.35 × 100 | 8.84 × 10−1 | 2.24 × 100 | 1.06 × 10−3 | 4.53 × 10−4 | 1.51 × 10−3 |
| Ni | 1.26 × 10−1 | 4.97 × 10−3 | 1.31 × 10−1 | 1.82 × 10−1 | 7.43 × 10−3 | 1.90 × 10−1 | |||
| Cu | 7.77 × 10−2 | 6.11 × 10−4 | 7.83 × 10−2 | 1.12 × 10−1 | 9.13 × 10−4 | 1.13 × 10−1 | |||
| Fe | 1.47 × 10−2 | 1.16 × 10−4 | 1.49 × 10−2 | 2.12 × 10−2 | 1.73 × 10−4 | 2.14 × 10−2 | |||
| Mn | 1.22 × 10−1 | 2.39 × 10−2 | 1.46 × 10−1 | 1.75 × 10−1 | 3.57 × 10−2 | 2.11 × 10−1 | |||
| Zn | 1.57 × 10−2 | 7.39 × 10−5 | 1.57 × 10−2 | 2.25 × 10−2 | 1.10 × 10−4 | 2.27 × 10−2 | |||
| HI | 9.72 × 100 | 6.71 × 10−1 | 1.04 × 10 1 | 1.40 × 10 1 | 1.00 × 100 | 1.50 × 10 1 | 2.38 × 10−3 | 4.60 × 10−4 | 2.84 × 10−3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Recreational | |||||||||
| As | 2.67 × 10−1 | 4.78 × 10−2 | 3.15 × 10−1 | 1.36 × 100 | 7.22 × 10−2 | 1.43 × 100 | 8.69 × 10−5 | 8.93 × 10−6 | 9.58 × 10−5 |
| Cd | 8.40 × 10−2 | 1.50 × 10−3 | 8.55 × 10−2 | 4.28 × 10−1 | 2.27 × 10−3 | 4.30 × 10−1 | |||
| Pb | 7.41 × 10−2 | 1.32 × 10−3 | 7.54 × 10−2 | 3.77 × 10−1 | 2.00 × 10−3 | 3.79 × 10−1 | 6.37 × 10−7 | 6.54 × 10−8 | 7.02 × 10−7 |
| Hg | 1.56 × 10−1 | 2.79 × 10−2 | 1.84 × 10−1 | 7.95 × 10−1 | 4.22 × 10−2 | 8.37 × 10−1 | |||
| Cr | 6.50 × 10−2 | 9.29 × 10−1 | 9.94 × 10−1 | 3.31 × 10−1 | 1.40 × 100 | 1.73 × 100 | 1.13 × 10−5 | 2.89 × 10−4 | 3.01 × 10−4 |
| Ni | 8.74 × 10−3 | 7.80 × 10−3 | 1.65 × 10−2 | 4.45 × 10−2 | 1.18 × 10−2 | 5.63 × 10−2 | |||
| Cu | 5.37 × 10−3 | 9.59 × 10−4 | 6.32 × 10−3 | 2.73 × 10−2 | 1.45 × 10−3 | 2.88 × 10−2 | |||
| Fe | 1.02 × 10−3 | 1.82 × 10−4 | 1.20 × 10−3 | 5.18 × 10−3 | 2.75 × 10−4 | 5.46 × 10−3 | |||
| Mn | 8.40 × 10−3 | 3.75 × 10−2 | 4.59 × 10−2 | 4.28 × 10−2 | 5.67 × 10−2 | 9.95 × 10−2 | |||
| Zn | 1.08 × 10−3 | 1.16 × 10−4 | 1.20 × 10−3 | 5.51 × 10−3 | 1.75 × 10−4 | 5.68 × 10−3 | |||
| HI | 6.71 × 10−1 | 1.05 × 100 | 1.73 × 100 | 3.42 × 100 | 1.59 × 100 | 5.01 × 100 | 9.88 × 10−5 | 2.98 × 10−4 | 3.97 × 10−4 |
| Elements | Non-carcinogenic risks for adult | Non-carcinogenic risks for child | Carcinogenic risks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HQ ingestion | HQ dermal | THI | HQ ingestion | HQ dermal | THI | CR ingestion | CR dermal | TCR | |
| a Bold figures indicate that the risk level of TEs is higher than 1.0 (HQ/HI/THI > 1.0). | |||||||||
| Residential receptor | |||||||||
| As | 3.55 × 100 | 2.79 × 10−2 | 3.58 × 100 | 5.11 × 100 | 4.17 × 10−2 | 5.16 × 100 | 1.20 × 10−3 | 6.41 × 10−6 | 1.21 × 10−3 |
| Cd | 1.11 × 100 | 8.74 × 10−4 | 1.11 × 100 | 1.60 × 100 | 1.31 × 10−3 | 1.60 × 100 | |||
| Pb | 1.10 × 100 | 8.64 × 10−4 | 1.10 × 100 | 1.58 × 100 | 1.29 × 10−3 | 1.58 × 100 | 9.83 × 10−6 | 5.24 × 10−8 | 9.88 × 10−6 |
| Hg | 2.17 × 100 | 1.71 × 10−2 | 2.19 × 100 | 3.12 × 100 | 2.55 × 10−2 | 3.15 × 100 | |||
| Cr | 9.16 × 10−1 | 5.76 × 10−1 | 1.49 × 100 | 1.32 × 100 | 8.60 × 10−1 | 2.18 × 100 | 1.03 × 10−3 | 4.40 × 10−4 | 1.47 × 10−3 |
| Ni | 1.17 × 10−1 | 4.62 × 10−3 | 1.22 × 10−1 | 1.69 × 10−1 | 6.90 × 10−3 | 1.76 × 10−1 | |||
| Cu | 7.22 × 10−2 | 5.68 × 10−4 | 7.28 × 10−2 | 1.04 × 10−1 | 8.49 × 10−4 | 1.05 × 10−1 | |||
| Fe | 1.44 × 10−2 | 1.13 × 10−4 | 1.45 × 10−2 | 2.08 × 10−2 | 1.69 × 10−4 | 2.09 × 10−2 | |||
| Mn | 1.12 × 10−1 | 2.21 × 10−2 | 1.34 × 10−1 | 1.62 × 10−1 | 3.30 × 10−2 | 1.94 × 10−1 | |||
| Zn | 1.43 × 10−2 | 6.76 × 10−5 | 1.44 × 10−2 | 2.06 × 10−2 | 1.01 × 10−4 | 2.07 × 10−2 | |||
| HI | 9.18 × 100 | 6.50 × 10−1 | 9.83 × 100 | 1.32 × 10 1 | 9.71 × 10−1 | 1.42 × 10 1 | 2.24 × 10−3 | 4.47 × 10−4 | 2.69 × 10−3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Recreational receptor | |||||||||
| As | 2.45 × 10−1 | 4.38 × 10−2 | 2.89 × 10−1 | 1.25 × 100 | 6.62 × 10−2 | 1.31 × 100 | 7.97 × 10−5 | 1.62 × 10−5 | 9.59 × 10−5 |
| Cd | 7.67 × 10−2 | 1.37 × 10−3 | 7.81 × 10−2 | 3.91 × 10−1 | 2.07 × 10−3 | 3.93 × 10−1 | |||
| Pb | 7.59 × 10−2 | 1.36 × 10−3 | 7.72 × 10−2 | 3.86 × 10−1 | 2.05 × 10−3 | 3.88 × 10−1 | 6.52 × 10−7 | 6.70 × 10−8 | 7.19 × 10−7 |
| Hg | 1.50 × 10−1 | 2.68 × 10−2 | 1.77 × 10−1 | 7.63 × 10−1 | 4.05 × 10−2 | 8.03 × 10−1 | |||
| Cr | 6.32 × 10−2 | 9.04 × 10−1 | 9.67 × 10−1 | 3.22 × 10−1 | 1.37 × 100 | 1.69 × 100 | 1.08 × 10−5 | 2.81 × 10−4 | 2.92 × 10−4 |
| Ni | 8.11 × 10−3 | 7.24 × 10−3 | 1.54 × 10−2 | 4.13 × 10−2 | 1.09 × 10−2 | 5.22 × 10−2 | |||
| Cu | 4.99 × 10−3 | 8.91 × 10−4 | 5.88 × 10−3 | 2.54 × 10−2 | 1.35 × 10−3 | 2.67 × 10−2 | |||
| Fe | 9.96 × 10−4 | 1.78 × 10−4 | 1.17 × 10−3 | 5.07 × 10−3 | 2.69 × 10−4 | 5.34 × 10−3 | |||
| Mn | 7.75 × 10−3 | 3.46 × 10−2 | 4.24 × 10−2 | 3.94 × 10−2 | 5.23 × 10−2 | 9.18 × 10−2 | |||
| Zn | 9.90 × 10−4 | 1.06 × 10−4 | 1.10 × 10−3 | 5.04 × 10−3 | 1.60 × 10−4 | 5.20 × 10−3 | |||
| HI | 6.34 × 10−1 | 1.02 × 100 | 1.65 × 100 | 3.23 × 100 | 1.54 × 100 | 4.77 × 100 | 9.12 × 10−5 | 2.98 × 10−4 | 3.89 × 10−4 |
Through the dermal pathway, the highest HQ value was obtained for Cr, which contributed 88 and 89%, respectively (Tables 4 and 5). The calculated values of HQ for Cr were higher than those of the studies of Li and Zhang96 and Giri and Singh.97 From dermal contact, HQ values for the studied elements were lower than the threshold limit (HQ < 1), indicating a lower risk through this pathway. Through ingestion from the surface and deep waters, HI values were higher than the threshold limit (HI > 1) for adults only, whereas for recreational HI values, they were higher than the threshold limit (HI > 1) for both adults and children, indicating that oral intake is more severe than dermal contact with water. The assessment of health risks in the current study from the surface and deep waters by ingestion and dermal contact for adults and children is in agreement with the previous studies.92,98–100
The total carcinogenic risk of As and Cr exceeded the USEPA standard limit (1 × 10−4) for the adult and child residents only, indicating that residential receptors were more susceptible to lifetime cancer risk by water intake or contact with their skin. Three elements As, Pb, and Cr were used for CR calculation with Cr having the main contributor to the TCR (CRingestion + CRdermal) via ingestion and dermal pathways (Tables 4 and 5). The TCR value of As in water for residents was (1.32 × 10−3), which was higher than the acceptable limit (1 × 10−4), and the current study is in agreement with the previous studies.96,98,101,102
3.5. Policy implications for sustainable management of river water
This study is an in-depth monitoring of trace elements in an urban river's surface and deep waters, which will assist Bangladeshi policymakers in identifying and deciding on projects in urban regions. They will benefit from the current study by knowing the information and applying it to their decision-making plan, which they will appraise. It will be carried out as part of a development project that will aid in assessing surface and subsurface water quality in Bangladesh. This study is focused on a small amount of the urban river in Bangladesh, which acts as a small step to investigate the impacts of urban activities on the pollution of trace elements in surface and deep waters and the associated risk to human health. A more advanced study could be carried out based on Bangladeshi conditions on the source and factors affecting water quality linked to public health, social acceptability, and sustainability. Further study is, therefore, suggested to examine the impact of poor water quality on individuals and the agricultural sector.4. Conclusions
In summary, the water quality of the studied Buriganga River, located around the industrialized urban area of Dhaka, Bangladesh, is highly deteriorated due to a lack of proper management. Herein, the elemental concentrations of As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn were measured in both surface and deep waters of the river and the obtained concentrations exceeded the safe limits in the water, indicating the contamination of river water. According to the PCA analysis, mostly municipal wastewater and landfill leachate, together with natural sources, were responsible factors for the deterioration of the water quality of the Buriganga River. The potential ecological risk (PER) showed moderate to very high ecological risk in both seasons at different water depths. Health risk assessment revealed that the total hazard index values of As, Cd, Pb, Hg, and Cr in surface and deep waters for both groups of people (adults and children) exceeded the risk threshold, which indicates that there is a possible threat to their health. The carcinogenic risk values of As and Cr in surface water and deep water were a little bit higher than the standard limit (1 × 10−4). This shows that there may be carcinogenic and non-carcinogenic risks in the study area of the river. Furthermore, the analyses showed that children are vulnerable to toxic elements. The study found that, in addition to natural sources, anthropogenic activities such as industrial processes, farm waste disposal, and coal burning were the most prevalent causes of the elevated elemental concentrations in the study area. Depositing harmful elements into adjacent water sources or river water without taking the required corrective action should be prohibited. Toxic element levels in the research area need to be treated and keep an eye on all time monitoring.Conflicts of interest
The authors declare that they do not have any competing interests that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are thankful to the authority of Patuakhali Science and Technology University (PSTU), Dumki, Patuakhali-8602, Bangladesh for sample processing and the Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh for sample analysis.References
- M. Nasiruddin, A. R. M. T. Islam, M. A. B. Siddique, M. Hasanuzzaman, M. M. Hassan, M. A. Akbor, M. Hasan, M. S. Islam, R. Khan, M. A. Amin, S. C. Pal, A. M. Idris and S. Kumar, Environ. Sci. Pollut. Res., 2023, 30, 20934–20958 CrossRef CAS PubMed.
- M. H. Kabir, M. S. Islam, T. R. Tusher, M. E. Hoq, M. Muliadi and S. A. Mamun, Indones. J. Sci. Technol., 2020, 5(3), 395–409 CrossRef.
- Q. Quan, W. Liang, D. Yan and J. Lei, Urban Clim., 2022, 41, 101043 CrossRef.
- J. Dai, H. Feng, K. Shi, X. Ma, Y. Yan, L. Ye and Y. Xia, Chemosphere, 2022, 307, 135833 CrossRef CAS PubMed.
- J. Liu, X. Qu, C. Zhang, W. Dong, C. Fu, J. Wang and Q. Zhang, J. Cleaner Prod., 2022, 377, 134228 CrossRef CAS.
- B. Bai, F. Bai, X. Li, Q. Nie, X. Jia and H. Wu, Environ. Technol. Innovation, 2022, 28, 102944 CrossRef CAS.
- B. Bai, Y. Wang, D. Rao and F. Bai, Front. Earth Sci., 2022, 10, 943853 CrossRef.
- M. N. Hossain, A. Rahaman, M. J. Hasan, M. M. Uddin, N. Khatun and S. M. Shamsuddin, SN Appl. Sci., 2021, 3, 509 CrossRef CAS.
- M. S. Islam, M. K. Ahmed, M. Raknuzzaman, M. Habibullah-Al-Mamun and M. K. Islam, Ecol. Indic., 2015, 48, 282–291 CrossRef CAS.
- M. S. Islam, R. S. Shammi, R. Jannat, M. H. Kabir and M. S. Islam, Chem. Ecol., 2023, 39(2), 173–201 CrossRef CAS.
- M. S. Islam, K. Phoungthong and A. M. Idris, Int. J. Environ. Anal. Chem., 2022 DOI:10.1080/03067319.2022.2071613.
- M. S. Islam, Environ. Sci. Pollut. Res., 2021, 28, 29287–29303 CrossRef PubMed.
- Z. Dai, Z. Ma, X. Zhang, J. Chen, R. Ershadnia, X. Luan and M. R. Soltanian, J. Hydrol., 2022, 614, 128541 CrossRef.
- M. A. Ahsan, F. Satter, M. A. B. Siddique, M. A. Akbor, S. Ahmed, M. Shajahan and R. Khan, Environ. Monit. Assess., 2019, 191, 575 CrossRef CAS PubMed.
- M. H. Kabir, M. S. Islam, M. E. Hoq, T. R. Tusher and M. S. Islam, Arabian J. Geosci., 2020, 13(21), 1135 CrossRef CAS.
- X. Fang, Q. Wang, J. Wang, Y. Xiang, Y. Wu and Y. Zhang, J. Hydrol., 2021, 603, 127146 CrossRef CAS.
- J. Xu, W. Lan, C. Ren, X. Zhou, S. Wang and J. Yuan, Cold Reg. Sci. Technol., 2021, 189, 103335 CrossRef.
- M. S. Islam, T. Kormoker, M. Mazumder, S. E. Anika, M. T. Islam, D. H. Hemy, U. S. Mimi, R. Proshad, M. H. Kabir and A. M. Idris, Toxin Rev., 2022, 41(3), 752–767 CrossRef CAS.
- D. Pan and H. Chen, China Econ. Rev., 2021, 69, 101681 CrossRef.
- M. Longo, R. G. Knox, D. M. Medvigy, N. M. Levine, M. C. Dietze, Y. Kim and P. R. Moorcroft, Geosci. Model Dev., 2019, 12(10), 4309–4346 CrossRef CAS.
- D. Akter, M. S. Islam, M. M. M. Hoque, M. H. Kabir and M. Rehnuma, Bangladesh J. Environ. Sci., 2019, 37, 32–39 Search PubMed.
- Q. Guan, G. Zeng, J. Song, C. Liu, Z. Wang and S. Wu, J. Environ. Manage., 2021, 293, 112961 CrossRef CAS PubMed.
- J. U. Haque, M. A. B. Siddique, M. S. Islam, M. M. Ali, C. Tokatli, A. Islam, S. C. Pal, A. M. Idris, G. Malafaia and A. R. M. T. Islam, Sci. Total Environ., 2023, 857(1), 159383 CrossRef PubMed.
- M. H. Kabir, T. R. Tusher, M. S. Hossain, M. S. Islam, R. S. Shammi, T. Kormoker, R. Proshad and M. Islam, Hum. Ecol. Risk Assess.: Int. J., 2020, 27(5), 1388–1415 CrossRef.
- Y. Ge, Y. Lou, M. Xu, C. Wu, J. Meng, L. Shi, F. Xia and Y. Xu, Environ. Pollut., 2021, 272, 115984 CrossRef CAS PubMed.
- T. Kormoker, A. M. Idris, M. Khan, T. R. Tusher, R. Proshad, M. S. Islam, S. Khadka, S. Rahman, M. H. Kabir and S. Kundu, Toxin Rev., 2022, 41(1), 247–260 CrossRef CAS.
- M. M. Rahman, M. S. Islam, M. H. Kabir, M. M. M. Huq, M. E. Sarker and S. A. Mamun, Ind. Chim. Acta., 2020, 13(2), 100–111 Search PubMed.
- H. Tian, Y. Qin, Z. Niu, L. Wang and S. Ge, J. Indian Soc. Remote Sens., 2021, 49(11), 2863–2874 CrossRef.
- H. Tian, Y. Wang, T. Chen, L. Zhang and Y. Qin, Remote Sens., 2021, 13(19), 3822 CrossRef.
- Y. Tian, Z. Yang, X. Yu, Z. Jia, M. Rosso, S. Dedman and J. Wang, Water Res., 2022, 219, 118551 CrossRef CAS PubMed.
- M. K. Ahmed, M. A. Baki, G. K. Kundu, M. S. Islam, M. M. Islam and M. M. Hossain, SpringerPlus, 2016, 5, 1697 CrossRef PubMed.
- J. Liu, Y. Chen and X. Wang, J. Cleaner Prod., 2022, 336, 130397 CrossRef.
- Z. Jin, S. Ding, Q. Sun, S. Gao, Z. Fu, M. Gong, J. Lin, D. Wang and Y. Wang, J. Hazard. Mater., 2019, 364, 182–191 CrossRef CAS PubMed.
- B. Xiong, R. Li, D. Johnson, Y. Luo, Y. Xi, D. Ren and Y. Huang, Environ. Geochem. Health, 2021, 43(2), 915–930 CrossRef CAS PubMed.
- USEPA, Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A). Interim Final. Office of Emergency and Remedial Response. EPA/540/1-89/002, 1989 Search PubMed.
- USEPA, Exposure Assessment Tools by Media – Water and Sediment, https://www.epa.gov/expobox/exposure-assessment-toolsmedia, 2020 Search PubMed.
- M. H. Kabir, T. Kormoker, M. S. Islam, R. Khan, R. S. Shammi, T. R. Tusher, R. Proshad, M. S. Islam and A. M. Idris, Environ. Sci. Pollut. Res., 2021, 28, 57126–57148 CrossRef CAS PubMed.
- F. Chen, J. Ma, Y. Zhu, X. Li, H. Yu and Y. Sun, J. Hazard. Mater., 2022, 426, 128064 CrossRef CAS PubMed.
- A. Nargis, A. Habib, H. B. Harun, M. S. I. Sarker, R. Jin, G. Liu, W. Liu, A. N. M. Al-Razee, K. Chen and M. Cai, Emerging Contam., 2021, 7, 99–115 CrossRef CAS.
- R. Khan, M. S. Islam, A. R. M. Tareq, K. Naher, A. R. M. T. Islam, M. A. Habib, M. A. B. Siddique, M. A. Islam, S. Das, M. B. Rashid, A. K. M. T. Ullah, M. M. H. Miah, S. U. Masrura, M. B. Doza, M. R. Sarker and A. B. M. Badruzzaman, Environ. Nanotechnol., Monit. Manage., 2020, 14, 100318 Search PubMed.
- Y. N. Jolly, M. J. R. Rakib, R. Kumar, S. Sultana, S. M. M. Rahman, J. Kabir, S. Akter, K. M. Mamun, K. J. Fatema, M. Mehnaz and P. Pal, Reg. Stud. Mar. Sci., 2023, 63, 102988 Search PubMed.
- N. Majed, M. I. H. Real, A. Redwan and H. M. Azam, Int. J. Environ. Sci. Technol., 2022, 19(5), 4181–4200 CrossRef CAS.
- M. K. Bashar, K. Noro, Q. Wang, M. Tokumura, I. Mori, M. Raknuzzaman, A. Hossain and T. Amagai, J. Water Health, 2023, 21(6), 815–825 CrossRef PubMed.
- M. A. Akbor, M. M. Rahman, M. B. Doza, M. M. Haque, M. A. B. Siddique, M. A. Ahsan, S. E. C. Bondad and M. K. Uddin, Desalin. Water Treat., 2020, 193, 284–301 CrossRef CAS.
- M. S. Islam, R. Proshad and S. Ahmed, Hum. Ecol. Risk Assess.: Int. J., 2018, 24(3), 699–720 CrossRef CAS.
- M. A. B. Siddique, M. K. Alam, S. Islam, M. T. M. Diganta, M. A. Akbor, U. H. Bithi, A. I. Chowdhury and A. K. M. A. Ullah, Environ. Nanotechnol., Monit. Manage., 2020, 14, 100366 Search PubMed.
- M. A. B. Siddique, A. R. M. T. Islam, M. S. Hossain, R. Khan, M. A. Akbor, M. Hasanuzzaman, M. W. M. Sajid, M. Y. Mia, J. Mallick, M. S. Rahman, M. M. Rahman and M. D. Doza, Environ. Sci. Pollut. Res., 2021, 29, 8577–8596 CrossRef PubMed.
- M. A. B. Siddique, R. Khan, A. R. M. T. Islam, M. K. Alam, M. S. Islam, M. S. Hossain, M. A. Habib, M. A. Akbor, U. H. Bithi, M. B. Rashid, F. Hossain, I. M. M. Rahman, I. B. Elius and M. S. Islam, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100524 CAS.
- M. A. Habib, A. R. M. T. Islam, M. B. Doza, F. A. Mukta, R. Khan, M. A. B. Siddique, K. Phoungthong and K. Techato, Chemosphere, 2020, 242, 125183 CrossRef CAS PubMed.
- USEPA, Regional Screening Levels (RSLs) – Equations, 2020, https://www.epa.gov/risk/regional-screening-levels-rsls-equations Search PubMed.
- USEPA, RSL Calculator, 2020, https://epa-prgs.ornl.gov/cgi-bin/chemicals/csl_search Search PubMed.
- USEPA, Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Final. OSWER 9285.7-02EP, July 2004 Search PubMed.
- Y. Liu, K. Zhang, Z. Li, Z. Liu, J. Wang and P. Huang, J. Hydrol., 2020, 590, 125440 CrossRef.
- B. M. Saalidong, S. A. Aram, S. Out and P. O. Lartey, PLoS One, 2022, 17(1), e0262117 CrossRef CAS PubMed.
- H. Ngabirano, D. Byamugisha and E. Ntambi, J. Water Resour. Prot., 2016, 8, 1297–1309 CrossRef CAS.
- R. Proshad, T. Kormoker and S. Islam, Toxin Rev., 2021, 40(1), 77–101 CrossRef CAS.
- M. M. Ali, R. Proshad, M. S. Islam, T. Kormoker, M. Rahman, T. R. Tusher and M. A. Al, Hum. Ecol. Risk Assess.: Int. J., 2020, 26(10), 2646–2662 CrossRef CAS.
- L. K. Pandey, J. Park, D. H. Son, W. Kim, M. S. Islam, S. Choi, H. Lee and T. Han, Sci. Total Environ., 2019, 651, 323–333 CrossRef CAS PubMed.
- ECR (The Environment Conservation Rules), Department of Environment, Government of the People's Republic of Bangladesh. Poribesh Bhaban E-16, Agargaon, Shere Bangla Nagar Dhaka 1207, Bangladesh, 1997, pp. 179–226 Search PubMed.
- WHO, Guidelines for Drinking Water Quality, World Health Organization, Geneva, 4th edn, 2011 Search PubMed.
- J. Fu, C. Zhao, Y. Luo, C. Liu, G. Z. Kyzas, Y. Luo, D. Zhao, S. An and H. Zhu, J. Hazard. Mater., 2014, 270, 102–109 CrossRef CAS PubMed.
- U. S. Pravin, P. Trivedi and M. M. Ravindra, Am. J. Chem., 2012, 2, 171–180 CrossRef.
- EC (European Community), The quality of water intended to human consumption, Directive 1998/83/EC, Official Journal L330/05.12.1998, 1998, pp. 32–54 Search PubMed.
- USEPA, National Primary Drinking Water Regulations, 2020, https://www.epa.gov/groundwater-and-drinking-water/national-primary-drinking-water-regulations#Inorganic, 2020f, 2021b, accessed date: 08 January 2021 Search PubMed.
- USEPA, National Recommended Water Quality Criteria - Aquatic Life Criteria Table, 2020, https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-lifecriteria-table#table, 2020g, 2021a, accessed date: 08 January 2021 Search PubMed.
- M. S. Islam, S. Han, M. K. Ahmed and S. Masunaga, J. Water Environ. Nanotechnol., 2014, 12(2), 109–121 CrossRef.
- C. Tokatlı and M. Varol, Environ. Res., 2021, 197, 11105 CrossRef PubMed.
- M. Varol, B. Gökot and A. Bekleyen, Environ. Sci. Pollut. Res., 2013, 20, 6096–6108 CrossRef CAS PubMed.
- A. Ansari, Innov. Infrastruct. Solut., 2023, 8, 98 CrossRef.
- R. Carafa, L. Faggiano, M. Real, A. Munné, A. Ginebreda, H. Guasch, M. Flo, L. Tirapu and P. Carsten von der Ohe, Sci. Total Environ., 2011, 409, 4269–4279 CrossRef CAS PubMed.
- M. A. H. Bhuiyan, S. B. Dampare, M. A. Islam and S. Suzuki, Environ. Monit. Assess., 2015, 187, 4075 CrossRef PubMed.
- F. Islam, M. Rahman, S. S. A. Khan, B. Ahmed, A. Bakar and M. Halder, Pollut. Res., 2013, 32, 715–721 CAS.
- M. A. A. Mokaddes, B. S. Nahar and M. A. Baten, J. Environ. Sci. Nat. Resour., 2013, 5(2), 11–14 Search PubMed.
- M. Rehnuma, M. S. Islam, N. T. Meghla and M. H. Kabir, Bangladesh J. Environ. Sci., 2016, 30, 07–12 Search PubMed.
- D. Pal and S. K. Maiti, Environ. Geochem. Health, 2018, 40, 2303–2324 CrossRef CAS PubMed.
- S. J. Markich and P. L. Brown, Sci. Total Environ., 1998, 217, 201–230 CrossRef CAS PubMed.
- K. W. Warnken and P. H. Santschi, Estuaries Coasts, 2009, 32, 158–172 CrossRef CAS.
- M. A. H. Bhuiyan, S. B. Dampare, M. A. Islam and S. Suzuki, Environ. Monit. Assess., 2015, 187, 4075 CrossRef PubMed.
- B. Gworek, O. Bemowska-Kałabun, M. Kijeńskaa and J. Wrzosek-Jakubowska, Water, Air, Soil Pollut., 2016, 227, 371 CrossRef PubMed.
- C. J. Lina, P. Pongprueksaa, S. E. Lindberg, S. O. Pehkoned, D. Byune and C. Jang, Atmos. Environ., 2006, 40, 2911–2928 CrossRef.
- W. F. Fitzgerald, C. H. Lamborg and C. R. Hammerschmidt, Chem. Rev., 2007, 107, 641–662 CrossRef CAS PubMed.
- G. O. Duodu, A. Goonetilleke and G. A. Ayoko, Environ. Pollut., 2016, 219, 1077–1091 CrossRef CAS PubMed.
- M. Li, Q. Zhang, X. Sun, K. Karki, C. Zeng, A. Pandey and F. Zhang, Chemosphere, 2020, 244, 125410 CrossRef CAS PubMed.
- M. Y. A. Khan, K. M. Gani and G. J. Chakrapani, Environ. Earth Sci., 2017, 76(5), 231 CrossRef.
- C. Sun, Z. Zhang, H. Cao, M. Xua and L. Xu, Chemosphere, 2019, 219, 538–545 CrossRef CAS PubMed.
- B. T. Nguyen, D. D. Do, T. X. Nguyen, V. N. Nguyen, D. T. P. Nguyen, M. H. Nguyen and Q. V. Bach, Environ. Pollut., 2020, 256, 113412 CrossRef CAS PubMed.
- F. Ustaoğlu and M. S. Islam, Ecol. Indicat., 2020, 113, 106237 CrossRef.
- X. Ke, S. Gui, H. Huang, H. Zhang, C. Wang and W. Guo, Chemosphere, 2017, 175, 473–481 CrossRef CAS PubMed.
- R. S. Shammi, M. S. Hossain, M. H. Kabir, M. S. Islam, M. T. I. Taj, M. S. Islam, M. E. Sarker, M. S. Hossain and A. M. Idris, Environ. Sci. Pollut. Res., 2023, 30, 3467–3489 CrossRef CAS PubMed.
- V. L. Mohanta, A. Naz and B. K. Mishra, Hum. Ecol. Risk Assess.: Int. J., 2020, 26(2), 406–429 CrossRef.
- Y. Jia, L. Wang, Z. Qu and Z. Yang, Environ. Sci. Pollut. Control Ser., 2018, 25(7), 7012–7020 CrossRef CAS PubMed.
- J. Xiao, L. Wang, L. Deng and Z. Jin, Sci. Total Environ., 2019, 650, 2004–2012 CrossRef CAS PubMed.
- L. Dai, Z. Wang, T. Guo, L. Hu, Y. Chen, C. Chen and J. Chen, Chemosphere, 2022, 293, 133576 CrossRef CAS PubMed.
- H. O. Nwankwoala, M. T. Harry and T. Warmate, Water Conserv. Manag., 2020, 4(2), 58–62 Search PubMed.
- D. Luan, A. Liu, X. Wang, Y. Xie, Z. Wu and W. Zhang, Discrete Dyn. Nat. Soc., 2022, 184170 Search PubMed.
- S. Li and Q. Zhang, J. Hazard. Mater., 2010, 181, 1051–1059 CrossRef CAS PubMed.
- S. Giri and A. K. Singh, Exposure Health, 2014, 5(4), 173–182 CrossRef CAS.
- N. Saha, M. S. Rahman, M. B. Ahmed, J. L. Zhou, H. H. Ngo and W. Guo, J. Environ. Manage., 2017, 185, 70–78 CrossRef CAS PubMed.
- M. Saleem, J. Iqbal and M. H. Shah, Chemosphere, 2019, 216, 715–724 CrossRef CAS PubMed.
- J. Wang, G. Liu, H. Liu and P. K. Lam, Sci. Total Environ., 2017, 583, 421–431 CrossRef CAS PubMed.
- X. Wang, L. Zhang, Z. Zhao and Y. Cai, Sci. Total Environ., 2018, 634, 158–169 CrossRef CAS PubMed.
- L. Qu, H. Huang, F. Xia, Y. Liu, R. A. Dahlgren and M. Zhang, Environ. Pollut., 2018, 237, 639–649 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3va00094j |
| This journal is © The Royal Society of Chemistry 2023 |