Open Access Article
Open Access ArticleCottonseed extract as a coagulant for water treatment†
Mekayla
DePaolis‡
a,
Sophie
De Respino‡
a,
Laxmicharan
Samineni
 b,
Scott
Brighton
a and
Manish
Kumar
b,
Scott
Brighton
a and
Manish
Kumar
 *a
*a
aDepartment of Civil, Architectural and Environmental Engineering, University of Texas at Austin, Austin, Texas 78712, USA. E-mail: manish.kumar@utexas.edu
bDepartment of Chemical Engineering, University of Texas at Austin, Austin, Texas 78712, USA
First published on 29th November 2022
Abstract
Coagulation is an important unit process of water treatment to decrease suspended and dissolved contaminants. However, the use of chemical coagulants, such as alum and ferric chloride, contributes to growing health concerns regarding aluminum exposure and sustainability concerns due to high sludge volumes. Bio-coagulants represent low-cost, sustainable alternatives to chemical coagulants. In this work, cottonseed meal extract, with an average protein content of 77.0 ± 13.5 μg mL−1, was investigated for its coagulation effectiveness and was shown to decrease turbidity by 90%, regardless of initial turbidity (62.5–717 NTU) and the age of extract (2–218 days). Varying doses of cottonseed meal extract protein (0.77–3.83 mg L−1) on turbidity removal were also investigated. The cottonseed meal extract was separated into carbohydrate and protein fractions to determine the active component and it was found that the protein fraction was primarily responsible for coagulation activity. The protein extract was further analyzed to investigate the proteins present in the cottonseed meal extract to identify several proteins including legumin and vicilin. This work details an effective bio-coagulant, cottonseed meal extract, which can achieve high turbidity removal due to its protein components.
Environmental significanceCoagulation is an integral part of water treatment that historically used chemicals to accomplish separation of suspended solids. Due to the high use of chemical and polymer-based materials, coagulation offers an opportunity to increase the sustainability of water treatment by minimizing the production of waste and toxic byproducts. The use of biological materials as potential alternatives to decrease the environmental impact of coagulation has been of wide interest over the years. In this work, we show that cottonseed meal, a large-scale byproduct of the cotton industry, can be used as an effective coagulant or a supplement to upcycle this low-value product and improve the sustainability of water treatment. |
Introduction
Coagulation is an important water treatment process whereby dissolved and suspended colloids are destabilized and aggregated in order to remove them in subsequent treatment processes.1,2 In conventional drinking water treatment, coagulation is commonly accomplished through the use of chemical coagulants, such as aluminum sulfate (alum) and ferric chloride (ferric).3 While chemical coagulants are highly effective at removing colloidal suspensions, there are several concerns regarding their use. The first issue is the link between aluminum exposure and Alzheimer's disease (AD). Aluminum is recognized as a powerful neurotoxicant and while there remains debate over this topic, there are statistically significant relations between aluminum exposure and AD.4,5 While most of the aluminum added during coagulation is removed by filtration and sedimentation, this process can still result in increased aluminum concentration.5 The second concern with the use of chemical coagulants is the large amount of sludge produced. Hydrous oxide, a byproduct of alum and ferric coagulants, is non-biodegradable sludge comprised of 99% water, resulting in a heavy waste that is difficult to dewater, dispose of, and expensive to transport.6 Sludge is often disposed of in landfills due to its non-biodegradability which can lead to landfill concerns like aluminum and iron leachate during acid rain.7,8 The addition of chemical coagulants also results in a decrease in pH of the water and alkalinity consumption, which results in more steps later in the water treatment process to account for these effects.3,6,9 Considering growing health and sustainability concerns with chemical coagulation, investigations of alternative coagulants, such as plant-based bio-coagulants, have increasing importance.Bio-coagulants represent a new class of water treatment materials that provide biodegradable alternatives to conventional chemical coagulants. Bio-coagulants produce a lower volume of sludge than metal salts and do not consume the alkalinity of the water in their coagulative process.10 In areas that lack extensive water treatment infrastructure, bio-coagulants could serve as an effective point-of-use water treatment method, especially if the coagulant is produced in the same area.11 Bio-coagulants could also replace or supplement chemical coagulants to mitigate sustainability concerns, sludge disposal, and cost. However, a disadvantage of bio-coagulants is the addition of organic matter to the water. This can increase microbial activity and require disinfection as an additional treatment process.12 Disinfection of organic-rich water then leads to further issues, such as the creation of toxic disinfection byproducts.13
Some bio-coagulants that have been investigated include common bean seed extract, chitosan, algal alginate, Moringa oleifera seed extract, and various other organic substances.14–18 Bio-coagulants are composed of various constituents including polysaccharides and proteins, both of which can contribute to coagulative mechanisms like adsorption, charge neutralization, and polymer bridging.7,19 Some bio-coagulants, like Moringa oleifera seed extract, have a suspected mechanism of coagulation: cationic protein MO2.1 and chitin-binding protein MoCBP.20,21 However, most coagulation mechanisms of other bio-coagulants remain unknown.22–24
The goal of this work was to investigate the coagulative ability of cottonseed (Gossypium hirsutum) extract and elucidate the potential mechanism for coagulation. Cottonseed, common bean (Phaseolus vulgaris), jack bean (Canavalia ensiformis), and other beans contain vicilin-type storage proteins that exhibit features useful to water treatment.25–27 The vicilin proteins bind to fungal cell walls and plasma membranes, resulting in fungal growth inhibition, a process similar to that of chitin-binding proteins.26 The presence of vicilin in cottonseed is an indicator that it may also show coagulative abilities found in seeds with chitin-binding proteins such as Moringa oleifera.
Cottonseed meal was selected to study as a potential coagulant source due to its genetic similarity to leguminous bio-coagulants and its status as a non-food plant product. Cottonseed meal is a waste byproduct of cotton and cottonseed oil production. For every 45.4 kg of cotton fiber produced, 70.3 kg of cottonseed are produced.28 Cottonseed meal is a byproduct of cottonseed oil production, and the US Department of Agriculture (USDA) National Agricultural Statistics Service estimates that 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679 kg of cottonseed meal will remain in inventory at the end 2022.29 Currently, cottonseed meal is used as a livestock feed protein supplement and a sustainable fertilizer due to its high protein and nutrient content.30,31 The proteins that make this meal useful in agriculture may also lend their properties to coagulative mechanisms that can be utilized in the water treatment process. This work investigated the potential of cottonseed extract for its coagulative abilities and determined the potential mechanism of this plant-based bio coagulant.
679 kg of cottonseed meal will remain in inventory at the end 2022.29 Currently, cottonseed meal is used as a livestock feed protein supplement and a sustainable fertilizer due to its high protein and nutrient content.30,31 The proteins that make this meal useful in agriculture may also lend their properties to coagulative mechanisms that can be utilized in the water treatment process. This work investigated the potential of cottonseed extract for its coagulative abilities and determined the potential mechanism of this plant-based bio coagulant.
A potential drawback to using cottonseed meal as livestock feed is it contains a toxic substance called gossypol that limits its consumption.31–33 While this could also be a concern when considering its use as a water treatment substance, gossypol is insoluble in water and it only makes up 0.9–1.3% of cottonseed meal.34,35 Furthermore, the effects of gossypol can be mitigated by neutralization with soluble iron salts which could be accomplished by using cottonseed serum as a coagulant aid to ferric chloride instead of a complete replacement.34 Cottonseed meal has a high crude protein content of ∼45% and has already been ground and defatted through the processing to produce cottonseed oil.31 A previous study investigated the use of whole, milled cottonseed as a coagulant, after oil extraction by hexane followed by dissolution in distilled water. This work only reported a turbidity decrease of 55.51% for cottonseed serum induced coagulation and did not investigate coagulation mechanisms of the cottonseed meal extract.36 Another study on cottonseed extract in coagulation reported 35–40% turbidity removal of slaughterhouse wastewater.37 No study has investigated the mechanism of coagulation for cottonseed meal or optimized for higher turbidity removals. In this work, we studied the coagulation efficiency and underlying mechanism of cottonseed meal to address this particular gap in literature.
Methods and materials
Cottonseed extract preparation and characterization
Cottonseed extract was prepared using cottonseed meal 6–2–1 from Down to Earth Fertilizer. 1 g of cottonseed meal was mixed with 50 mL of 0.15 M NaCl for 5 minutes using a magnetic stir bar. The mixture was then vacuum filtered using a 20 μm glass fiber filter, retaining the liquid fraction of cottonseed meal extract, termed cottonseed serum for the remainder of this work.Carbohydrates present in cottonseed serum were determined using the total carbohydrate assay kit from Sigma-Aldrich. The standard curve and samples were prepared per the manufacturer's instructions and absorbance was measured at 490 nm using a BioTek plate reader. The total protein concentration of the cottonseed serums was evaluated using the Bradford Dye Reagent from Thermo Scientific. The standard curve and samples were prepared per the manufacturer's instructions using 0.15 M NaCl as background and absorbance was measured at 595 nm using a BioTek plate reader after 2 minutes of incubation.
To study the components of the cottonseed serum separately, acetone precipitation of the proteins within the serum was performed. Acetone at −20 °C was mixed with 10 mL of cottonseed serum at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and vortexed for 15 seconds. This mixture was incubated at −20 °C for at least 60 minutes. Following incubation, the acetone serum mixture was centrifuged at 3803g for 10 minutes at 4 °C. The supernatant, presumably containing the polysaccharide fraction of the serum, was purified using a rotary evaporator for an hour to evaporate the acetone. The water bath temperature, condenser temperature, and vacuum were set to 40 °C, −10 °C, and 390 mbar respectively during evaporation. The protein pellet obtained after evaporation was subjected to acetone precipitation to remove any remaining polysaccharide fraction before suspending in 10 mL of 0.15 NaCl.
1 ratio and vortexed for 15 seconds. This mixture was incubated at −20 °C for at least 60 minutes. Following incubation, the acetone serum mixture was centrifuged at 3803g for 10 minutes at 4 °C. The supernatant, presumably containing the polysaccharide fraction of the serum, was purified using a rotary evaporator for an hour to evaporate the acetone. The water bath temperature, condenser temperature, and vacuum were set to 40 °C, −10 °C, and 390 mbar respectively during evaporation. The protein pellet obtained after evaporation was subjected to acetone precipitation to remove any remaining polysaccharide fraction before suspending in 10 mL of 0.15 NaCl.
Gel electrophoresis and mass spectrometry
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was used to evaluate the protein fractions from cottonseed serum. A protease-inhibited cottonseed serum was utilized as an SDS–PAGE gel sample. Briefly, 0.3 g of cottonseed meal was mixed with 5 mL of a 2 M phenylmethylsulfonyl fluoride (PMSF) solution in 0.15 M NaCl. After mixing for 5 minutes, the solution was vacuum filtered with a 20 μm glass filter to remove solid organics.12 μL of the protease-inhibited cottonseed serum was loaded onto a Novex™ 16% Tricine pre-cast SDS–PAGE gel from Thermo Fisher Scientific. To visualize protein bands, Laemmli buffer was added to the serum before loading and heated to 90 °C for 10 minutes. Coomassie staining was used after the separation of the proteins for 40 minutes at 150 V in a gel electrophoresis setup.
For performing mass spectrometry on the protein fraction, the same amount of serum was loaded and then run at 150 V to form a <5 mm wide band in a 12% hand-cast SDS–PAGE gel. This band was incised and analyzed at the University of Texas Austin Center for Biomedical Research Support Biological Mass Spectrometry Facility (RRID:SCR_021728) where they completed digestion and liquid chromatography-mass spectroscopy (LC-MS) to identify proteins within the serum. Following LC-MS identification, the online ExPASy ProtParam tool was used to find the molecular weight, theoretical pI, amino acid composition, and overall charge for selected proteins.38
Jar tests
To determine the coagulation efficiency of cottonseed serum, jar tests were carried out using a Phipps & Bird Stirrer Model 7790-400. Water used in the jar tests was tap water that was retained in a jug for at least 24 hours to reduce chlorine residuals. The untreated tap water characteristics are included in ESI (Table S1†). Synthetic turbid water stock solution was prepared by suspending kaolin in tap water at a concentration of 5 mg mL−1. The stock solution was mixed for at least 24 hours to ensure suspension and hydration of the kaolin.For each set of jar test experiments, 200 mL of retained tap water was added to each reactor. Kaolin stock solution was added to obtain the desired initial turbidity, using a Hach portable turbidimeter Model 2100P. The turbidimeter was calibrated using formazin standards (0, 20, 100, 800 NTU). The target initial turbidity was 100 NTU unless otherwise noted. The volume of cottonseed serum added to each experiment was converted to protein concentration using the Bradford Dye results. After the desired amount of coagulant was added, the samples were mixed at 200 rpm for 1 minute and then 60 rpm for 30 minutes to simulate conventional coagulation and flocculation. The reactors were allowed to settle for 30 minutes before turbidity values were taken again. DI water was added in place of cottonseed serum as a control. The volume of DI water added was equivalent to the volume of cottonseed serum added for each experiment. In cases where cottonseed serum volume varied, the maximum volume was used for the control. The pH of the initial and final water was recorded using a Mettler Toledo FiveEasy pH probe. Initial and final dissolved organic carbon (DOC) was measured using a TOC-L Shimadzu Total Organic Carbon (TOC) Analyzer. Samples were filtered by 0.45 μm filters and acidified using phosphoric acid to a pH < 2. Experiments were performed in triplicate and standard deviation is included in the Results and discussion section.
Jar tests were also conducted using a groundwater sample. The composition is detailed in ESI (Table S2†). The groundwater was used without pH adjustment (pH 8.50) and at pH 6, 7, and 8. When pH was adjusted, hydrochloric acid was used. For DOC and groundwater matrix experiments, FeCl3 (Sigma-Aldrich, 97%) was used for a coagulant comparison. 1 mL of 1 g L−1 FeCl3 stock solution was used to reach a final concentration of 5 mg L−1 for all FeCl3 experiments. An overview of jar testing parameters and treated water quality is included in ESI (Tables S3 and S4†).
Results and discussion
Cottonseed serum coagulation efficacy
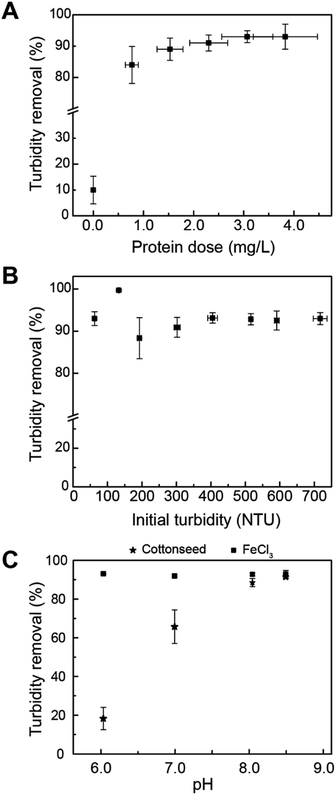
Cottonseed serum was effective in removing up to 90% turbidity with varying serum doses at an initial turbidity of 94 ± 3.9 NTU (Fig. 1A). The protein doses shown in Fig. 1A correlate to additions of 2–10 mL of cottonseed serum into 200 mL of simulated turbid water. The average protein content for cottonseed serum was 77.0 ± 13.5 μg mL−1, resulting in the optimum protein dosing to range from 2–4 mg L−1. Moringa oleifera seed extract has been shown to reduce turbidity by 86% using a protein dose of 7.9 mg L−1.39 Comparatively, cottonseed serum can be used as an effective clarifying agent at lower protein concentrations. A more comprehensive review of various bio-coagulants is included in work done by Kurniawan et al.7 A table summarizing Kurniawan's findings with additional information on coagulation mechanism is included in ESI (Table S5†).During storm events, turbidity levels can increase to over 100 NTU.40,41 To understand how cottonseed serum would perform if utilized for treating source waters with high turbidities, an initial turbidity range of 62.5–717 NTU was explored as this covers the range of most wastewaters and remains below the maximum detection limit of the turbidimeter without requiring dilution. A total protein dose of 1.16 mg L−1 was used for studying the effect of initial turbidity. For waters with initial turbidities greater than 62.5 NTU, the turbidity removal remained near 90% (Fig. 1B), showing cottonseed serum's potential as an initial water treatment step for a variety of feedwater types.
Fig. 1C shows the effect of adjusting the pH on the groundwater matrix. Ferric (5 mg L−1 dose) remains an effective coagulant at all pH values tested, while cottonseed serum effectiveness decreases with decreasing pH. This result may be due to the mixed protein content of cottonseed serum. At pH values above a protein's isoelectric point (pI), proteins have a negative charge and at pH values below the pI, proteins are positively charged.42 The mixed protein content of cottonseed serum likely includes proteins with varying pIs which can lead to charge neutralization and less destabilization of kaolin particles at different pH values.
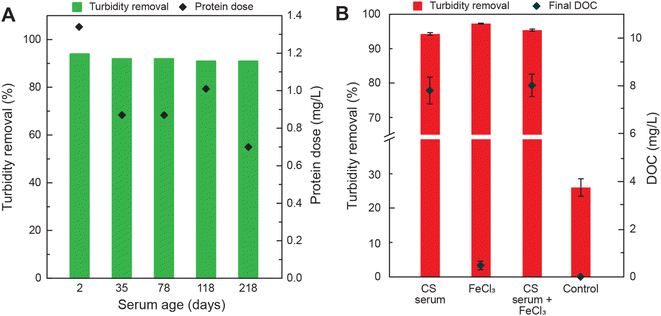
The ability of cottonseed serum to coagulate synthetically turbid waters after being stored for longer time periods at −20 °C was evaluated (Fig. 2A). The protein dose for each experiment is shown in Fig. 1C. After being preserved for over 200 days, cottonseed serum still removed over 90% of turbidity. The protein content of the 218 day-old serum decreased by about 47.7% when compared to the 2 day-old serum but was still effective at treating turbid waters with an average turbidity removal of 91 ± 0.45%. The capability of this material to drastically decrease turbidity, even after over 6 months, shows its ability to withstand transport and storage over long periods of time.
The increase in organic carbon due to the use of biological coagulants is one of the concerns reported in the literature.43–45 A large increase in organic carbon could pose issues in large-scale water treatment as high DOC can decrease the effectiveness of disinfection methods and provide a food source for microorganisms, promoting their growth.46 The dissolved organic carbon (DOC) of the water before and after it was treated with varying amounts of cottonseed serum and DI water (control) was measured to understand the impact of using cottonseed meal as a coagulant. Initial turbidity for DOC experiments was 112.3 ± 23.3 NTU. Utilizing cottonseed serum in combination with a conventional coagulant did not decrease DOC values. A dose of 5 mg L−1 was selected for FeCl3 to minimize sludge production while maintaining high turbidity removal. Depending on environmental factors, the dose of chemical coagulants like alum and ferric can range from 2 to 30 mg L−1.47 A comparison of sludge generation between FeCl3 and cottonseed serum is included in ESI (Fig. S1†). The conventional coagulant and cottonseed serum perform identically in coagulation and sludge generation. However, use of the cottonseed serum imparts an addition of DOC to the water. Due to the high DOC imparted to the treated water by cottonseed serum, it may be best for cottonseed serum to be used as a point of use water treatment method and to avoid storing treated water for long periods of time. pH was measured before and after treatment and information can be found in the ESI (Table S6†). pH increased slightly with increasing cottonseed dose (pH range 8.03–8.6).
Proposed cottonseed serum coagulation mechanism
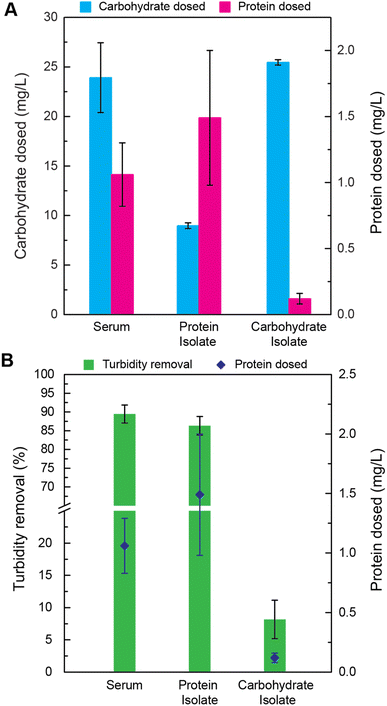
To determine the mechanism of cottonseed serum coagulation, the serum was separated into protein and carbohydrate isolates. Acetone protein separation by precipitation was carried out to separate the fractions. The measured carbohydrate and protein content of each fraction is shown in Fig. 3A. The average carbohydrate content of cottonseed serum had 21 times higher concentration than protein content. It is possible to have many non-covalent interactions between these two macromolecules, including electrostatic interactions.48 A phenomenon termed “associative separation,” which refers to the attraction between two biopolymers, may contribute to the coagulative ability of this serum,48,49 so it was necessary to separate the two macromolecules and evaluate the coagulation ability of each fraction.While the protein fractions still contain a significant amount of carbohydrate, experiments with these isolates can still give insight into which ingredients of the cottonseed serum are actively causing coagulation. The relative amount of carbohydrate to protein in the carbohydrate fraction is large enough to assume that the carbohydrate is the dominant constituent. The protein dose in the carbohydrate fraction was 0.12 mg L−1. Similarly, although the protein fraction has carbohydrates (8.9 mg L−1), the ratio of protein to carbohydrate is larger than in both the carbohydrate isolate and the original cottonseed serum.
The coagulative ability of the cottonseed serum and both its protein and carbohydrate fractions can be seen in Fig. 3B. When the concentration of carbohydrates was relatively high with respect to protein, the turbidity removal of the serum decreased drastically. This indicates that the 0.12 mg L−1 protein dose of the carbohydrate isolate is not sufficient to maintain high turbidity removal. When both carbohydrate and protein are present in the serum it functions as an effective coagulant. This indicates that the proteins within the serum are a necessary component of the coagulation effectiveness. While it cannot be determined whether the total carbohydrate is imperative to the function of the serum, in high concentrations with little to no protein (0.12 mg L−1 protein) it renders the cottonseed serum ineffective.
Cottonseed serum protein identification
To further analyze the proteins present in cottonseed serum responsible for the coagulation activity, gel electrophoresis and mass spectrometry analyses were performed. Using mass spectrometry analysis, 280 total proteins were detected with 1331 total unique peptides. A table of the 65 most abundant proteins can be found in the ESI (Table S7†). Seed storage proteins such as vicilin and legumin proteins were among the most abundant identified peptides. These results substantiate previous studies which report that 60–70% of the protein in cottonseed meal belongs to these families.50–52 These proteins include vicilin GC72-A, vicilin C72, legumin A, and legumin B. The top eight identified proteins selected based on molecular weights of the bands from gel electrophoresis and the corresponding SDS–PAGE gel are shown in Fig. 4A and B.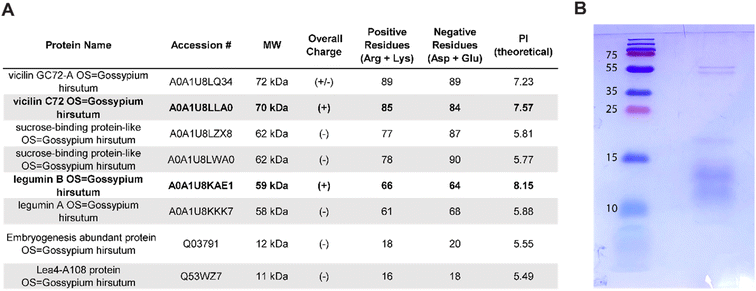 | ||
| Fig. 4 (A) The table shows the top eight proteins with high peptide sequence similarity to proteins present in cottonseed serum sorted by decreasing molecular weight. The protein charge was calculated using ProtParam and positively charged proteins are in bold. A full list of proteins identified is available in the ESI.† (B) The SDS–PAGE gel of a protease inhibited sample of cottonseed serum shows bands near 55 kDa and 10–20 kDa. | ||
Previous studies on legume seed vicilin proteins illustrate their cell wall binding abilities, traditionally responsible for antimicrobial properties, that could be responsible for the coagulation mechanisms in cottonseed serum.26,27 The presence of these vicilin proteins in other seeds that can coagulate waters, across various studies, may indicate that the mechanisms of coagulation in cottonseed are at least partially dependent on vicilin seed storage proteins.27,53–56 2S albumin proteins with antimicrobial properties share high homology to cotton vicilin proteins.55 Chitin binding 2S albumin proteins within M. oleifera contain an amino acid sequence that is highly homologous to the cationic proteins (MO2.1 and MO2.2) within M. oleifera that is believed to be responsible for their coagulative abilities.22,24,57 A sequence alignment and structural alignment between a cottonseed meal serum protein (2S albumin seed storage protein-like) and chitin-binding 2S albumin precursor from M. oleifera shown in Fig. S2† demonstrate the homology between the two proteins.
The top eight most abundant proteins in the cottonseed serum reveal two positively charged proteins, vicilin C72 and legumin B (Fig. 4A). Kaolin particles are negatively charged at pH values greater than 2,58 indicating the importance of electrostatic interactions between kaolin and coagulant. Cationic vicilin C72 and legumin B proteins may provide a charge neutralization mechanism to coagulate kaolin particles and reduce turbidity.
The charge of each investigated protein was calculated using ProtParam on the ExPASy server.38 The results of this analysis can be found in Fig. 4A. Vicilin C72 and legumin B were found to have an overall positive charge. These cationic proteins may cause destabilization of water contaminants in solution through particle bridging or patch flocculation as they are positively charged polymers that can adsorb onto negatively charged contaminants.
Conclusions
Conventional coagulants pose sustainability and health concerns, prompting work in alternative coagulants such as bio-coagulants. This work found that a bio-coagulant, cottonseed serum, can effectively coagulate synthetically turbid waters under a variety of conditions. The primary agent responsible for coagulation was determined to be the protein content of cottonseed serum. Mass spectrometry analysis of the protein fraction found vicilin, vicilin-like proteins, and legumins to be the most abundant. The positively charged proteins found in cottonseed serum at the highest peptide sequence matches (legumin B, vicilin C72) potentially interact with negatively charged contaminants, linking particles together through bridging. Furthermore, previous work has identified a 16.3 kDa protein within cottonseed that exhibited antifungal properties, which may be useful in identifying other applications of the cottonseed serum.56Cottonseed serum was as effective in turbidity removal as a conventional coagulant, ferric chloride (5 mg L−1 dose). Because protein was found to be the primary agent responsible for coagulation, the protein dose can be compared to the ferric dose. A lower protein dose (by mass) achieves a similar reduction in turbidity to the conventional coagulant. Furthermore, the sludge produced by cottonseed meal is a biodegradable organic waste, unlike the conventional coagulants' inorganic sludge.
However, the DOC increase when using cottonseed serum in coagulation poses post-treatment challenges that need to be addressed. Isolating the protein fraction before use in coagulation may reduce DOC levels while maintaining effective coagulation. Future work should address organic load challenges and aim to minimize DOC increase due to cottonseed serum.
This work identified cottonseed meal serum as an effective coagulant and elucidated mechanisms for the coagulative capability. Proteins within cottonseed serum are necessary for it to function as a coagulant, potentially due to vicilin and legumin proteins. Overall, cottonseed serum is as effective as a conventional coagulant and more sustainable due to biodegradable sludge production.
Conflicts of interest
There are no conflicrts to declare.References
- J.-Q. Jiang, The role of coagulation in water treatment, Curr. Opin. Chem. Eng., 2015, 8, 36–44 CrossRef.
- H. Cui, X. Huang, Z. Yu, P. Chen and X. Cao, Application progress of enhanced coagulation in water treatment, RSC Adv., 2020, 10(34), 20231–20244 RSC.
- D. F. Lawler and M. M. Benjamin, Water Quality Engineering, Wiley, 2013 Search PubMed.
- D. McLachlan, Aluminium and the risk for Alzheimer's disease, Environmetrics, 1995, 6(3), 233–275 CrossRef.
- T. P. Flaten, Aluminium as a risk factor in Alzheimer's disease, with emphasis on drinking water, Brain Res. Bull., 2001, 55(2), 187–196 CrossRef CAS PubMed.
- G. Newcombe and D. Dixon, Interface science in drinking water treatment: theory and applications, Academic Press, 2006 Search PubMed.
- S. B. Kurniawan, S. R. S. Abdullah, M. F. Imron, N. S. M. Said, N. I. Ismail and H. A. Hasan, et al., Challenges and opportunities of biocoagulant/bioflocculant application for drinking water and wastewater treatment and its potential for sludge recovery, Int. J. Environ. Res. Public Health, 2020, 17(24), 9312 CrossRef CAS PubMed.
- M. D. Vaverková, Landfill impacts on the environment, Geosciences, 2019, 9(10), 431 CrossRef.
- J. J. Peirce, P. A. Vesilind and R. Weiner, Environmental pollution and control, Butterworth-Heinemann, 1998 Search PubMed.
- N. A. Oladoja, E. I. Unuabonah, O. S. Amuda and O. M. Kolawole, Mechanistic insight into the coagulation efficiency of polysaccharide-based coagulants, Polysaccharides as a green and sustainable resources for water and wastewater treatment, Springer, 2017, pp. 13–35 Search PubMed.
- S. M. Miller, E. J. Fugate, V. O. Craver, J. A. Smith and J. B. Zimmerman, Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment, Environ. Sci. Technol., 2008, 42(12), 4274–4279 CrossRef CAS PubMed.
- S. B. Kurniawan, M. F. Imron, C. E. N. C. E. Chik, A. A. Owodunni, A. Ahmad and M. M. Alnawajha, et al., What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes?, Sci. Total Environ., 2022, 806, 150902 CrossRef CAS PubMed.
- H. Yang, S. Ye, J. Wang, H. Wang, Z. Wang and Q. Chen, et al., The approaches and prospects for natural organic matter-derived disinfection byproducts control by carbon-based materials in water disinfection progresses, J. Cleaner Prod., 2021, 311, 127799 CrossRef CAS.
- H. A. Devrimci, A. M. Yuksel and F. D. Sanin, Algal alginate: A potential coagulant for drinking water treatment, Desalination, 2012, 299, 16–21 CrossRef CAS.
- M. G. Antov, M. B. Šćiban and J. M. Prodanović, Evaluation of the efficiency of natural coagulant obtained by ultrafiltration of common bean seed extract in water turbidity removal, Ecol. Eng., 2012, 49, 48–52 CrossRef.
- B. Ramavandi, Treatment of water turbidity and bacteria by using a coagulant extracted from Plantago ovata, Water Resour. Ind., 2014, 6, 36–50 CrossRef.
- S. Bratskaya, S. Schwarz and D. Chervonetsky, Comparative study of humic acids flocculation with chitosan hydrochloride and chitosan glutamate, Water Res., 2004, 38(12), 2955–2961 CrossRef CAS PubMed.
- T. Okuda, A. U. Baes, W. Nishijima and M. Okada, Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution, Water Res., 2001, 35(2), 405–410 CrossRef CAS PubMed.
- C.-Y. Yin, Emerging usage of plant-based coagulants for water and wastewater treatment, Process Biochem., 2010, 45(9), 1437–1444 CrossRef CAS.
- J. M. Gifoni, J. T. A. Oliveira, H. D. Oliveira, A. B. Batista, M. L. Pereira and A. S. Gomes, et al., A novel chitin-binding protein from Moringa oleifera seed with potential for plant disease control, Pept. Sci., 2012, 98(4), 406–415 CrossRef CAS PubMed.
- U. Gassenschmidt, K. D. Jany, T. Bernhard and H. Niebergall, Isolation and characterization of a flocculating protein from Moringa oleifera Lam, Biochim. Biophys. Acta, Gen. Subj., 1995, 1243(3), 477–481 CrossRef PubMed.
- J. E. Freire, I. M. Vasconcelos, F. B. Moreno, A. B. Batista, M. D. Lobo and M. L. Pereira, et al., Mo-CBP3, an antifungal chitin-binding protein from Moringa oleifera seeds, is a member of the 2S albumin family, PLoS One, 2015, 10(3), e0119871 CrossRef PubMed.
- A. S. Taiwo, K. Adenike and O. Aderonke, Efficacy of a natural coagulant protein from Moringa oleifera (Lam) seeds in treatment of Opa reservoir water, Ile-Ife, Nigeria, Heliyon, 2020, 6(1), e03335 CrossRef PubMed.
- K. A. Ghebremichael, K. Gunaratna, H. Henriksson, H. Brumer and G. Dalhammar, A simple purification and activity assay of the coagulant protein from Moringa oleifera seed, Water Res., 2005, 39(11), 2338–2344 CrossRef CAS PubMed.
- R. M. Santos, A. A. Firmino, C. M. De Sa and C. R. Felix, Keratinolytic activity of Aspergillus fumigatus Fresenius, Curr. Microbiol., 1996, 33(6), 364–370 CrossRef CAS PubMed.
- V. M. Gomes, M.-I. Mosqueda, A. Blanco-Labra, M. P. Sales, K. V. S. Fernandes and R. A. Cordeiro, et al., Vicilin storage proteins from Vigna unguiculata (legume) seeds inhibit fungal growth, J. Agric. Food Chem., 1997, 45(10), 4110–4115 CrossRef CAS.
- G. C. Vieira Bard, V. V. Nascimento, A. E. A. Oliveira, R. Rodrigues, M. Da Cunha and G. B. Dias, et al., Vicilin-like peptides from Capsicum baccatum L. seeds are α-amylase inhibitors and exhibit antifungal activity against important yeasts in medical mycology, Pept. Sci., 2014, 102(4), 335–343 CrossRef CAS PubMed.
- J. Jacob, Cottonseed meal in poultry diets, USDA, available from: https://poultry.extension.org/articles/feeds-and-feeding-of-poultry/feed-ingredients-for-poultry/oilseed-meals-in-poultry-diets/cottonseed-meal-in-poultry-diets/ Search PubMed.
- M. Ash and E. Dohlman, Oil crops situation and outlook yearbook, Electronic outlook report from the Economic Research Service, United States Department of Agriculture, 2006 Search PubMed.
- F. Lewu, T. Volova, S. Thomas and K. Rakhimol, Controlled release fertilizers for sustainable agriculture, Academic Press, 2020 Search PubMed.
- G. M. Rogers, M. H. Poore and J. C. Paschal, Feeding cotton products to cattle, Vet. Clin. Food Anim. Pract., 2002, 18(2), 267–294 CrossRef PubMed.
- R. Kellems, R. Jones, D. Andrus and M. Wallentine, Effect of moisture in total mixed rations on feed consumption and milk production and composition in Holstein cows, J. Dairy Sci., 1991, 74(3), 929–932 CrossRef CAS PubMed.
- Z. He, H. Zhang and D. C. Olk, Chemical composition of defatted cottonseed and soy meal products, PLoS One, 2015, 10(6), e0129933 CrossRef PubMed.
- P. W. Waldroup and J. Kersey, Nutrient composition of cottonseed meal surveyed, Feedstuffs, 2002, 4, 11–22 Search PubMed.
- J. Duke. Dr. Duke's Phytochemical and Ethnobotanical Databases, United States Department of Agriculture, Agricultural Research Service, 2004, vol. 27 Search PubMed.
- Effects of De-Oiled Jute Seed and Cottonseed Extracts as Natural Polymeric Coagulants for Surface Water Treatment, IOP Conference Series: Earth and Environmental Science, ed. Lawal N., Ogedengbe K., Olasoju S. and Abdulrasaq A., IOP Publishing, 2020 Search PubMed.
- T. Werberich, A. G. do Amaral, R. M. Schneider and M. C. Bongiovani, Coagulation/flocculation of slaughterhouse wastewater using cottonseed as coagulant, Nativa, 2016, 4(3), 134–138 CrossRef.
- E. Gasteiger, C. Hoogland, A. Gattiker, M. R. Wilkins, R. D. Appel and A. Bairoch, Protein identification and analysis tools on the ExPASy server, The proteomics protocols handbook, 2005, pp. 571–607 Search PubMed.
- T. Okuda, A. U. Baes, W. Nishijima and M. Okada, Improvement of extraction method of coagulation active components from Moringa oleifera seed, Water Res., 1999, 33(15), 3373–3378 CrossRef CAS.
- R. Mukundan, D. Pierson, E. Schneiderman, D. O'donnell, S. Pradhanang and M. Zion, et al., Factors affecting storm event turbidity in a New York City water supply stream, Catena, 2013, 107, 80–88 CrossRef.
- D. M. Lawler, G. E. Petts, I. D. L. Foster and S. Harper, Turbidity dynamics during spring storm events in an urban headwater river system: The Upper Tame, West Midlands, UK, Sci. Total Environ., 2006, 360(1), 109–126 CrossRef CAS PubMed.
- X. Xia, Protein Isoelectric pPint. Bioinformatics and the Cell: Modern Computational Approaches in Genomics, Proteomics and Transcriptomics, 2007, pp. 207–219 Search PubMed.
- A. Ndabigengesere and K. Subba Narasiah, Quality of water treated by coagulation using Moringa oleifera seeds, Water Res., 1998, 32(3), 781–791 CrossRef CAS.
- A. Benalia, K. Derbal, A. Khalfaoui, R. Bouchareb, A. Panico and C. Gisonni, et al., Use of Aloe vera as an Organic Coagulant for Improving Drinking Water Quality, Water, 2021, 13(15), 2024 CrossRef CAS.
- M. Choudhary, M. B. Ray and S. Neogi, Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water, Sep. Purif. Technol., 2019, 209, 714–724 CrossRef CAS.
- F. W. Pontius, Water quality and treatment: a handbook of community water supplies, 1990, p. 1194 Search PubMed.
- Y. Song, B. Dong, N. Gao and Y. Deng, Comparative evaluation of aluminum sulfate and ferric sulfate-induced coagulations as pretreatment of microfiltration for treatment of surface water, Int. J. Environ. Res. Public Health, 2015, 12(6), 6700–6709 CrossRef CAS PubMed.
- D. J. McClements, Non-covalent interactions between proteins and polysaccharides, Biotechnol. Adv., 2006, 24(6), 621–625 CrossRef CAS PubMed.
- A. K. Ghosh and P. Bandyopadhyay, Polysaccharide-protein interactions and their relevance in food colloids, Complex World Polysaccharides, 2012, 14, 395–406 Search PubMed.
- G. Hu, N. L. Houston, D. Pathak, L. Schmidt, J. J. Thelen and J. F. Wendel, Genomically biased accumulation of seed storage proteins in allopolyploid cotton, Genetics, 2011, 189(3), 1103–1115 CrossRef CAS PubMed.
- Z. He, C. P. Mattison, D. Zhang and C. C. Grimm, Vicilin and legumin storage proteins are abundant in water and alkali soluble protein fractions of glandless cottonseed, Sci. Rep., 2021, 11(1), 1–12 CrossRef PubMed.
- Z. He, K. Klasson, D. Wang, N. Li, H. Zhang and D. Zhang, et al., Pilot-scale production of washed cottonseed meal and co-products, Mod. Appl. Sci., 2016, 10(2), 25–33 CrossRef CAS.
- K. Lewtak, M. J. Fiołka, P. Czaplewska, K. Macur, Z. Kaczyński and T. Buchwald, et al., Sida hermaphrodita seeds as the source of anti-Candida albicans activity, Sci. Rep., 2019, 9(1), 1–17 CrossRef CAS PubMed.
- X. Wang, G. J. Bunkers, M. R. Walters and R. S. Thoma, Purification and characterization of three antifungal proteins from cheeseweed (Malva parviflora), Biochem. Biophys. Res. Commun., 2001, 282(5), 1224–1228 CrossRef CAS PubMed.
- X. Wang and G. J. Bunkers, Potent heterologous antifungal proteins from cheeseweed (Malva parviflora), Biochem. Biophys. Res. Commun., 2000, 279(2), 669–673 CrossRef CAS PubMed.
- R. P.-T. Chung, G. M. Neumann and G. M. Polya, Purification and characterization of basic proteins with in vitro antifungal activity from seeds of cotton, Gossypium hirsutum, Plant Sci., 1997, 127(1), 1–16 CrossRef CAS.
- J. X. Neto, M. L. Pereira, J. T. Oliveira, L. C. Rocha-Bezerra, T. D. Lopes and H. P. Costa, et al., A chitin-binding protein purified from Moringa oleifera seeds presents anticandidal activity by increasing cell membrane permeability and reactive oxygen species production, Front. Microbiol., 2017, 8, 980 CrossRef PubMed.
- S. Li, L. Gao, Y. Cao, X. Gui and Z. Li, Effect of pH on the flocculation behaviors of kaolin using a pH-sensitive copolymer, Water Sci. Technol., 2016, 74(3), 729–737 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2va00205a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |