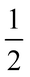Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
R-Nb2O5 has an ‘idealized’ V2O5 structure and Wadsley–Roth-like structural stability during Li-ion battery cycling†
Kausturi
Parui
 ,
Alexander D.
Lee
,
Shornam
Gandhi
and
Megan M.
Butala
,
Alexander D.
Lee
,
Shornam
Gandhi
and
Megan M.
Butala
 *
*
Department of Materials Science and Engineering, University of Florida, Gainesville, FL 32611, USA. E-mail: mbutala@ufl.edu
First published on 10th February 2023
Abstract
The adoption of batteries across diverse applications requires electrode materials with a wider range of performance metrics, such as cost, safety, and material availability. Along the path to discovering new commercially viable materials, a fundamental understanding of chemical and atomic structure features that provide structural stability and effective ion transport is essential. In support of new understanding, we report the cycling behavior of metastable R-Nb2O5. R-Nb2O5 adopts an ‘idealized’ V2O5 structure, in which [NbO6] octahedra alternate in edge- and corner-sharing resulting in ReO3-like slabs, whereas Wadsley–Roth materials have ReO3-like blocks, linked through edge-sharing octahedra at intersecting crystallographic shear planes. We find that this slab structure is stable during cycling, with minor atomic structure changes and cycling curves that are symmetric on discharge and charge, resembling the behavior of Wadsley–Roth materials more than other related materials, such as ReO3, V2O5, or Nb3O7F. Based on our findings, R-Nb2O5 can serve as a ‘structural bridge’ between Wadsley–Roth block structures and V2O5, through which we can relate inter- and intra-polyhedral structures to cycling behavior and structural stability during cycling.
1 Introduction
Early transition metal oxides featuring crystallographic shear planes are a technologically and fundamentally compelling family of Li-ion battery electrode candidates. These materials, sometimes referred to as Wadsley–Roth or Magnéli phases,1–5 have demonstrated excellent performance as high potential anodes. With operating potentials between 1 V and 1.5 V and excellent rate capabilities, they offer a pathway to safe, high-power Li-ion batteries as alternatives to graphite and Li4Ti5O12.6–8 Wadsley–Roth (WR) materials can also have multi-electron redox, in which more than one redox reaction occurs per redox active transition metal, contributing to high energy densities, e.g., reduction of Nb5+ to Nb3+, allowing for storage of >1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol Li per transition metal, M.5,9–12
mol Li per transition metal, M.5,9–12
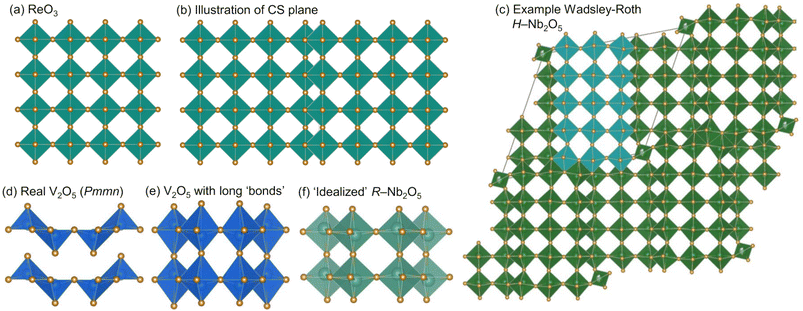
In addition to being technologically relevant, WR materials provide a foundation for identifying atomic and electronic structure features that support effective transport of ions and electrons.9,13,14 WR materials have ReO3-like blocks, in which [MO6] octahedra are corner-sharing (Fig.1a and c) with square channels that provide fast Li transport. These blocks are connected across crystallographic shear planes, at which [MO6] octahedra are edge-sharing (Fig. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b and c). During lithiation of ReO3, [ReO6] octahedra rotate around corner-sharing vertices.4,15 In contrast, in WR materials, the edge-sharing octahedra at crystallographic shear planes stabilize against this rotation, keeping square channels accessible for fast Li+ transport.9,11 In addition to structural stability, edge-sharing octahedra have a favorable overlap of d-electron orbitals, which results in high electronic conductivity upon even minor reduction, contributing to their high-rate capabilities.11,16,17
1b and c). During lithiation of ReO3, [ReO6] octahedra rotate around corner-sharing vertices.4,15 In contrast, in WR materials, the edge-sharing octahedra at crystallographic shear planes stabilize against this rotation, keeping square channels accessible for fast Li+ transport.9,11 In addition to structural stability, edge-sharing octahedra have a favorable overlap of d-electron orbitals, which results in high electronic conductivity upon even minor reduction, contributing to their high-rate capabilities.11,16,17
Among WR and related materials studied as battery electrodes, there are many niobates,16,18 including those with the chemical formula Nb2O5.17,19–24 We report here, for the first time, the cycling behavior and charge storage mechanism of yet another polymorph R-Nb2O5. ‘R’ represents a “neutral designation”,19,25,26 and is not related to the crystal system or preparation conditions. This metastable polymorph adopts an idealized V2O5 structure (Fig. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f and 2)2 and was first identified by Gruehn.25R-Nb2O5 can be described relative to WR block structures as having ReO3-like slabs (rather than blocks) connected by parallel (rather than perpendicular) shear planes (Fig. 2a).2,27
1f and 2)2 and was first identified by Gruehn.25R-Nb2O5 can be described relative to WR block structures as having ReO3-like slabs (rather than blocks) connected by parallel (rather than perpendicular) shear planes (Fig. 2a).2,27
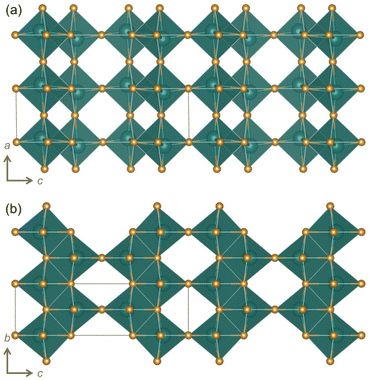 | ||
| Fig. 2 Visualizations of R-Nb2O5 based on the original report (ICSD collection code 25765)25 viewed along the (a) [010] and (b) [100] directions. | ||
WR block structures can be described by the connectivity of [MO6] octahedra, specifically in terms of the number of corner-sharing octahedra in a given direction. For example, a block that is m corner-sharing octahedra wide, n high, and infinite in the third direction would be represented as (m × n)∞.3 For H-Nb2O5, this representation would be (3 × 5)∞ (Fig. 1c). Borrowing the WR structure notation,3 the structure of R-Nb2O5 can be represented as (2 × ∞)∞.
Both α-V2O5 and ReO3 can be considered as ‘parent structures’ for R-Nb2O5.27,28 Like ReO3, V2O5 also undergoes polyhedral rotations during lithiation that result in extensive atomic structure changes.29,30 V2O5 has layers composed of edge- and corner-sharing [VO5] square pyramids (Fig. 1d). Across the layers, there is a long V–O distance (≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 Å); when a ‘bond’ is drawn between these atoms, V2O5 can be visualized as a three-dimensionally-connected structure (Fig. 1e). The larger ionic radius of Nb relative to that of V results in the octahedral, rather than square-pyramidal, coordination of Nb in R-Nb2O5, approaching a relatively idealized structure.2,31 Given the different atomic structure evolutions and stabilizations of ReO3 and WR block structures, we analogously find that R-Nb2O5 has improved structural stability upon lithiation relative to V2O5. In addition to being stable against polyhedral rotation during lithiation, we also find that R-Nb2O5 has minimal atomic structure evolution during cycling, akin to the relatively small changes in WR materials, for example, unit cell volume changes ≤
2.8 Å); when a ‘bond’ is drawn between these atoms, V2O5 can be visualized as a three-dimensionally-connected structure (Fig. 1e). The larger ionic radius of Nb relative to that of V results in the octahedral, rather than square-pyramidal, coordination of Nb in R-Nb2O5, approaching a relatively idealized structure.2,31 Given the different atomic structure evolutions and stabilizations of ReO3 and WR block structures, we analogously find that R-Nb2O5 has improved structural stability upon lithiation relative to V2O5. In addition to being stable against polyhedral rotation during lithiation, we also find that R-Nb2O5 has minimal atomic structure evolution during cycling, akin to the relatively small changes in WR materials, for example, unit cell volume changes ≤![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6%.10,11
6%.10,11
The ‘ideality’ of R-Nb2O5 relative to that of α-V2O5 can be illustrated by comparing their distributions of M–O distances. For R-Nb2O5, they range from 1.7 Å to 2.3 Å (this work); this is narrower than the range for V2O5, which is from 1.58 Å to 2.8 Å,32 and broader than that for ReO3, in which there is a single Re–O bond length of 1.9 Å.33 Nb3O7F is another slab structure, denoted as (3 × ∞)∞, that has also been studied as a Li-ion electrode15 and has a similar distribution as R-Nb2O5, from 1.85 Å to 2.2 Å.34
Overall, we find that the cycling behavior and structural stability of R-Nb2O5 are more similar to WRs than to ReO3,4,35 α-V2O5,29 or Nb3O7F.15R-Nb2O5 has symmetric discharge and charge profiles that do not significantly change between the first and later cycles. In addition, atomic structure changes are minimal, with no evidence of the polyhedral rotation that ReO3 or V2O5 undergo during lithiation. We observed unit cell changes of less than 5% and a reduced off-centering of Nb in cycled products.9–11
2 Results and discussion
2.1 Synthesis and characterization of R-Nb2O5
The R polymorph of Nb2O5 was prepared through soft chemical methods, specifically, through the drying and decomposition of hydrated precursor H3ONb3O8.36 H3ONb3O8 was produced from KNb3O8, a layered niobate, through ion-exchange in nitric acid.36–38 KNb3O8 was prepared using molten salt synthesis in an excess of KCl, which facilitates the mass transport among precursors to form a phase pure product.18,39Bragg peak positions of the prepared R-Nb2O5 are consistent with the structure originally reported by Gruehn,25 which is evident in neutron diffraction data (POWGEN at Oak Ridge National Laboratory, Fig. 3) as well as high-resolution synchrotron X-ray diffraction (XRD) data (11-BM at Argonne National Laboratory, Fig. S1†). In both types of diffraction data, peak broadening is evident. Scanning electron microscope (SEM) images show anisotropic particles with sub-micron features in two dimensions and one longer axis in the order of microns (Fig. 4c and d). This indicates that finite size effects give rise to the reflection-dependent broadening in diffraction data.
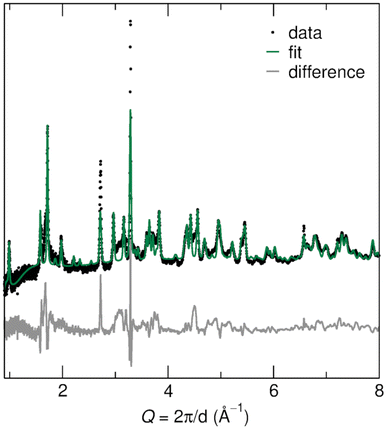 | ||
| Fig. 3 Rietveld refinement against neutron diffraction data for the prepared R-Nb2O5 shows good agreement between the measured and calculated patterns. The largest differences occur in regions with diffuse scattering, as well as for two reflections that are underestimated in the calculated data. Details of the refined structure parameters are provided in the ESI.† | ||
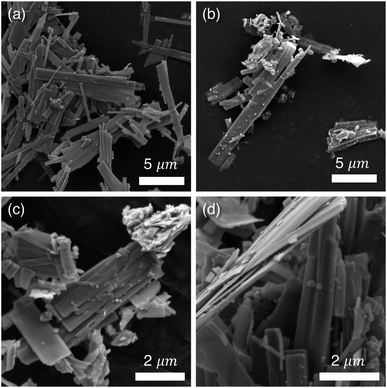 | ||
| Fig. 4 SEM images of (a) precursor KNb3O8, (b) intermediate product of ion-exchange, H3ONb3O8, and (c, d) R-Nb2O5. The elongated, rectangular particles of R-Nb2O5 result from its precursors. The anisotropic particle size has crystallographic origins and results in the reflection-character-dependent broadening in the diffraction data (Fig. 3). | ||
Gruehn's reported R-Nb2O5 structure was used as a starting model for refinements of the atomic structure model against neutron and X-ray diffraction data [International Crystal Structure Database (ICSD) collection code 25765].25 Rietveld refinement against neutron diffraction data resulted in minor changes to lattice parameters and atom positions, resulting in a fit that captures the data well (Fig. 3). Similar parameters were found from the refinement of synchrotron XRD data (Fig. S1†). Details of refinement parameters and the resulting structures are provided in Tables S1 and S2.†
The differences between the measured data and calculated fit have two primary origins: diffuse scattering near Q of 1.5 Å−1 and 3 Å−1, and peak intensity mismatch near Q of 2.9 Å−1 and 3.25 Å−1 (Fig. 3). The diffuse scattering features suggest low crystallinity, disorder, or a combination. Even after accounting for anisotropic particle shape in refinements, there is a remaining intensity mismatch for the (113), (11![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), and (020) reflections, which are especially narrow and accordingly must be associated with the long axis of the particles. Similar reflection-dependent broadening has also been identified for KNb3O8,18 the precursor from which this R-Nb2O5 was prepared (Fig. 4a).
), and (020) reflections, which are especially narrow and accordingly must be associated with the long axis of the particles. Similar reflection-dependent broadening has also been identified for KNb3O8,18 the precursor from which this R-Nb2O5 was prepared (Fig. 4a).
The elongated rectangular morphology of R-Nb2O5 comes from the morphology of the KNb3O8 and H3ONb3O8 precursors (Fig. 4a and b). The layered nature of the KNb3O8 atomic structure drives preferential growth along specific crystallographic directions.18 The underlying particle shape is retained through ion-exchange and low temperature heating of the prepared product. This synthetic route did not allow for the preparation of larger or more isotropic R-Nb2O5 particles, which was beyond the scope of this work, but variations of particle size and shape may be accessible from more isotropic KNb3O8 precursors or through alternative synthetic methods.
A notable feature of the crystal structure of R-Nb2O5 is the off-centering of Nb within the octahedra, as well as the pattern of displacements from the center between neighboring octahedra. In V2O5, the nearest neighbor V–O distances range from 1.58 Å to 2.8 Å, and off-centering of V5+ away from the center of an octahedron occurs as pairs (two displaced lower, two displaced higher, Fig. 1d).32 In R-Nb2O5, Nb is also off-centered in the octahedra, but with a narrower distribution of Nb–O distances, ranging from 1.7 Å to 2.3 Å. In addition, off-centering occurs in a zig-zag pattern, alternating up and down for each octahedron, along the c direction (Fig. 2a).
2.2 Battery cycling behavior
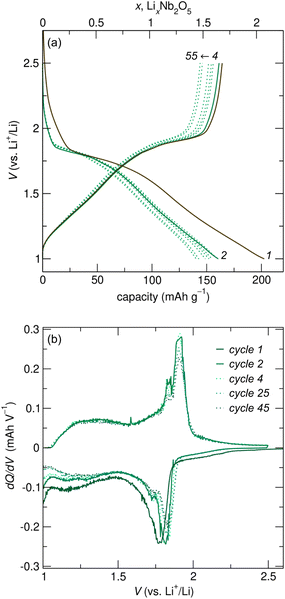
R-Nb2O5 was cycled against Li metal in coin cells at various rates. At a relatively slow cycling rate of C/20 (based on the reaction of 2 mol Li per mol Nb2O5 in 20 h), galvanostatic cycling shows a plateau at ≈ 1.8 V for 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≤
≤ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≤
≤ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a sloping region at low potentials (Fig. 5a), suggestive of two-phase and solid solution lithiation mechanisms at high and low potentials, respectively. The plateau for R-Nb2O5 is slightly higher than that for H-Nb2O5, which is at ≈
1, and a sloping region at low potentials (Fig. 5a), suggestive of two-phase and solid solution lithiation mechanisms at high and low potentials, respectively. The plateau for R-Nb2O5 is slightly higher than that for H-Nb2O5, which is at ≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 V.20 Over the first ≈55 cycles, unoptimized coin cells show gradual capacity fade (Fig. 5a).
1.6 V.20 Over the first ≈55 cycles, unoptimized coin cells show gradual capacity fade (Fig. 5a).
Between the first and second discharge of R-Nb2O5, there are minor differences in the cycling profiles. Specifically, there is additional capacity before the plateau in the first discharge compared to later discharges. This difference is likely related to the irreversible capacity over the first cycle, with ≈0.3 mol Li per mol Nb2O5 remaining at the end of the first charge. This type of slight irreversibility is common in WR and related materials,18 and has been previously attributed to sluggish diffusion of Li+ from specific sites within WR-type structures, as well as an electronic transition from an initially electronic insulator to having delocalized electron states upon slight Nb reduction.10,11,17 Additionally, the differential capacity plot highlights minor differences in the shape of the first discharge curve relative to that of later cycles (Fig. 5). Even so, discharge and charge curves are generally symmetric, indicating similar reaction pathways on discharge and charge. This is evident in the positions of plateaus and sloped regions in galvanostatic cycling (Fig. 5a), and the corresponding peaks and diffuse features in the differential capacity plot (Fig. 5b). The symmetric cycling processes on discharge and charge are in contrast to those of the ‘parent’ compounds of R-Nb2O5, ReO3 and V2O5, in which extensive and irreversible atomic structure changes during the first lithiation result in significant differences in the profile shape of the first discharge and following cycles.29,30,35
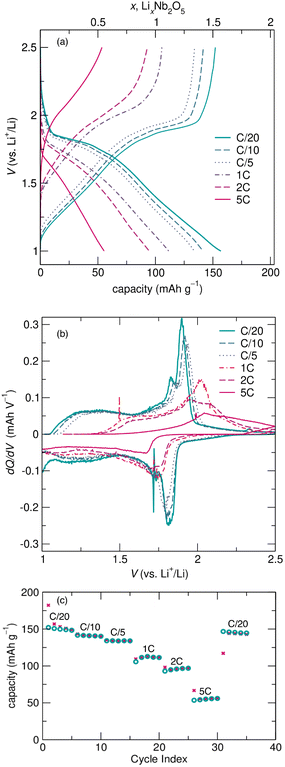
At faster cycling rates, electrochemical curves show good reversibility and similar electrochemical features to those after cycling at slower rates, as well as lower capacities and higher overpotentials. Changes in cycling features with increasing rate are evident in Fig. 6a, which shows the second cycle at each rate from variable rate galvanostatic cycling. The increased overpotential and more sloped nature of the cycling curves with increasing rate are highlighted in the differential capacity plot (Fig. 6b). R-Nb2O5 has excellent reversibility and good capacity retention, visible in cycling at C/10 and 1C in the ESI† (Fig. S3 and S4).
The decrease in capacity with increasing rate is more significant for R-Nb2O5 than for typical block Wadsley–Roth materials. The reversible capacity of ≈150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mA h
mA h ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1 at C/20 reduces to about 50
g−1 at C/20 reduces to about 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mA h
mA h ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1 at 5C (Fig. 6c), whereas typical WR block materials, e.g., TiNb2O7 and PNb9O25, maintain more capacity with proportional increases in cycling rates.11,40 While the capacities and rate capabilities of R-Nb2O5 do not meet those of typical WR block materials, they do exceed the reversibility and capacity retention of other related materials, such as ReO3,4,35 and even ‘slab’ material Nb3O7F.15
g−1 at 5C (Fig. 6c), whereas typical WR block materials, e.g., TiNb2O7 and PNb9O25, maintain more capacity with proportional increases in cycling rates.11,40 While the capacities and rate capabilities of R-Nb2O5 do not meet those of typical WR block materials, they do exceed the reversibility and capacity retention of other related materials, such as ReO3,4,35 and even ‘slab’ material Nb3O7F.15
Considering that R-Nb2O5 has an ‘idealized’ V2O5 structure, there is a significant difference between their electrochemical behaviors during lithiation. The asymmetry observed in the charge and discharge profiles for α-V2O5 during lithiation, due to phase transformations into ε-, δ-, and γ- polymorphs, is significantly different than that in the highly symmetric profiles we find for R-Nb2O5.29,41 The origins of these differences are discussed further in the following section.
2.3 Structural evolution with cycling
To understand the structural stability and cycling mechanism of R-Nb2O5, we analyzed ex situ synchrotron XRD data after the first discharge and the first charge (Fig. 7). For this, loose powder cathodes were cycled in Swagelok cells at C/20 and extracted after the first discharge (to 0.5 V, Li3Nb2O5) and first charge (to 2.5 V, LiNb2O5). Cycling for loose powder Swagelok cells is qualitatively similar to that from coin cells (Fig. S2†). The deeper discharge relative to that of coin cells resulted in a higher first discharge capacity, but a similar reversible capacity, which suggests that the low potential capacity is associated with the formation of a solid-electrolyte interphase, storage by a conductive carbon additive, or a combination thereof.42,43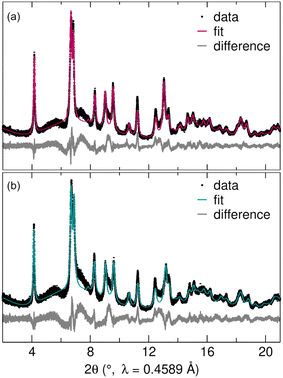 | ||
| Fig. 7 Rietveld refinements of high-resolution synchrotron XRD data following (a) the first discharge and (b) the first charge of R-Nb2O5 indicate agreement between the measured and calculated patterns. Due to the poor sensitivity of X-rays to light elements, Li was excluded from the structural model and only the Nb–O framework was refined (Table 1). | ||
Synchrotron XRD data of cycling products were used in Rietveld refinements of atomic structure models to determine the nature of these minor structural changes. For both states of charge, the R-Nb2O5 structure refined against neutron diffraction data of the prepared powder was used as the starting model. Due to the poor sensitivity of X-rays to light elements, Li was not included in the structural model, with an emphasis instead on the Nb–O framework. As in the pristine R-Nb2O5, reflection-dependant broadening was captured using isotropic crystallite size parameters, with a mix of Lorentzian and Gaussian shapes, and the preferred orientation (modeled with spherical harmonics) to account for the broadening caused by the anisotropic crystallite size. Additional information on Rietveld refinements and refined structures are provided in the ESI† (Tables S1, S2, S3, and S4).
There were no new reflections in the first discharge and charge products compared to pristine R-Nb2O5 (Fig. 3 and 7). Rather, changes in lattice parameters resulted in shifted peak positions, with the Nb–O framework retained through Li+ intercalation and deintercalation. As in the pristine R-Nb2O5, XRD data of cycled products have diffuse scattering and broad peaks. The qualitative similarity of diffraction data for the pristine and cycled products is reflected in the quantitative results of Rietveld refinements (Table 1 and Fig. 8). Lattice parameters undergo minor contraction (a and c) and expansion (b), resulting in 4.66% and 3.56% increases in the unit cell volume (Vuc) of the discharge and charge products, respectively, relative to those of pristine R-Nb2O5.
| Composition | Pristine Nb2O5 | Discharged Li3Nb2O5 | Charged LiNb2O5 |
|---|---|---|---|
| a Percent change relative to that of the pristine one. b Baur's distortion index (based on bond length variation)44 calculated with VESTA.45 | |||
| a | 3.9752(6) Å | 3.94788(19) Å | 3.9313(3) Å |
| b | 3.8233(3) Å | 4.04267(15) Å | 4.0058(2) Å |
| c | 12.7064(19) Å | 12.6633(7) Å | 12.6987(10) Å |
| β | 90.603(13)° | 90.0034(10)° | 89.966(11)° |
| V uc | 193.11(4) Å3 | 202.107(17) Å3 | 199.98(2) Å3 |
| % Change Vuca | — | 4.66% | 3.56% |
| Octahedral volume | 10.437 Å3 | 10.81 Å3 | 10.6955 Å3 |
| Octahedral distortion indexb | 0.073 | 0.024 | 0.029 |
While lattice parameters are similar for pristine and cycled R-Nb2O5, there are notable differences in the [NbO6] octahedra for the pristine and cycled products (Fig. 8). As mentioned, Nb in R-Nb2O5 is less off-centered than V in V2O5 (Fig. 1d and f), but still has a range of Nb–O bond lengths. Baur's distortion index is a single-value quantity that can be used to describe deviations from a perfect octahedron, with 6 M–O bonds of the same length, which would have a Baur's index of 0.44 Specifically, Baur's index represents octahedral (or tetrahedral) distortions as the deviation of bond lengths from an average bond length.44 In the cycled products, the reduction in Baur's index reflects the decrease in Nb off-centering with the reduction of Nb5+ during lithiation (Fig. 8). In pristine Nb2O5, Nb5+ has an electronic configuration [Kr]5s24d0; at the ex situ states of charge evaluated, there is at least some reduction of Nb away from this d0 electron configuration. The reduced oxidation state of Nb relieves the second-order Jahn-Teller distortion,10,11,17 which is commonly active for fully oxidized early transition metals, such as Nb.46 The reduction of Nb, and thus Nb off-centering, following discharge and charge results in nearly identical crystal structures at these states of charge (Fig. 8, Tables 1, and S1, S2, S3, and S4).
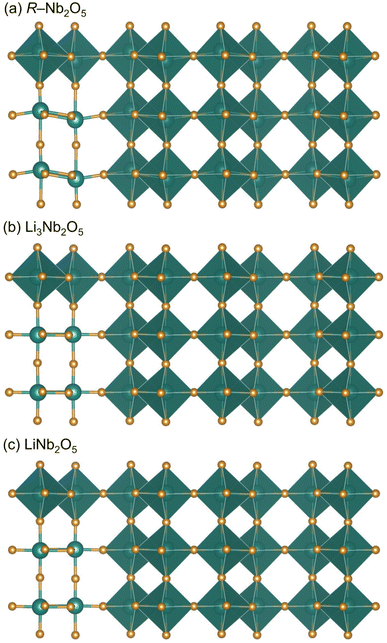 | ||
| Fig. 8 Refined structures of (a) R-Nb2O5, and (b) lithiated product of the first discharge to 0.5 V, and (c) the following first charge to 2.5 V. | ||
In contrast to ReO3 and V2O5, R-Nb2O5 has minimal structure evolution during the first discharge (Fig. 8).29,35 The difference in cycling behavior and structural stability between R-Nb2O5 and Nb3O7F is especially interesting as both have slab structures with ReO3-like regions connected by parallel, non-intersecting crystallographic shear-planes.27 The difference in their behavior is most likely a consequence of the difference in the width of their slabs. The extended-defect-based structures can be described as (2 × ∞)∞ for R-Nb2O5, and (3 × ∞)∞ for Nb3O7F.3
3 Conclusions
Based on our findings, R-Nb2O5 can serve as a ‘structural bridge’ between Wadsley–Roth block structures and V2O5 in terms of polyhedral distortions, polyhedral connectivity, and the consequences thereof for structural stability during cycling. Relative to V2O5, we show that bridging layers to form a three-dimensionally connected structure, as in its ‘idealized’ structure, stabilizes it against polyhedral rotations associated with irreversible structural changes. For WR structures, this work demonstrates for the first time that a (2 × ∞)∞ slab structure, with only parallel (rather than intersecting) shear planes, is stable against polyhedral rotations at corner-sharing vertices during electrochemical cycling. This is in contrast to what has been found for Nb3O7F, in which non-intersecting crystallographic shear planes that link three-octahedron-wide slabs, denoted as (3 × ∞)∞, do not provide structural stability during lithiation. Thus, the combination of three-dimensionally connected polyhedra and non-intersecting shear planes with a smaller slab width results in the stability of R-Nb2O5 against unfavorable distortions, making it a high voltage anode candidate, and moreso, a link between heavily studied material families.In the context of published work on related structures, we illustrate how lithiation in isotypic materials induces varying degrees of structural changes and phase transformations, which have direct impacts on Li+ diffusion pathways. The highly symmetric charge discharge profile for R-Nb2O5 indicates that similar sequences of phase transformations occur during lithiation and delithiation. Furthermore, the minimal structural changes result in the retention of square channels that resemble the fast Li+ transport pathways in WR block structures. Future work to further characterize Li+ and electronic conduction, as well as long-term cycling stability, will provide additional insights into bridge classes of early transition metal oxide electrode materials, including Wadsley–Roth block structures and V2O5.
4 Experimental
4.1 Synthesis
Phase pure R-Nb2O5 was prepared from precursors synthesized using the molten salt reaction by ion-exchange methods adapted from reported methods.18,36,47 Precursor KNb3O8 was prepared by ball-milling K2CO3 (Sigma Aldrich, ≥ 99%), Nb2O5 (Sigma Aldrich, 99.99%), and KCl (Sigma Aldrich, ≥99%) in molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 in ethanol (Thermo Fisher, reagent grade) for 30 min. Ethanol was evaporated under ambient conditions and the resulting mixture was heated in a covered alumina crucible at 800 °C for 5 h in air, with a heating rate of 3° min−1 and allowed to cool passively with a furnace. The reacted powder was washed with warm deionized (DI) water to remove KCl; the mixture was centrifuged and decanted to isolate KNb3O8 powder, which was dried in air.
12 in ethanol (Thermo Fisher, reagent grade) for 30 min. Ethanol was evaporated under ambient conditions and the resulting mixture was heated in a covered alumina crucible at 800 °C for 5 h in air, with a heating rate of 3° min−1 and allowed to cool passively with a furnace. The reacted powder was washed with warm deionized (DI) water to remove KCl; the mixture was centrifuged and decanted to isolate KNb3O8 powder, which was dried in air.
The prepared KNb3O8, ≈1 g, was stirred in 100 mL of 7 M HNO3 for 3 days to prepare H3ONb3O8via ion-exchange.36 As before, the solid product was isolated and washed using DI water, centrifugation, and decanting. After drying at room temperature, H3ONb3O8 powder was heated in air at 600 °C for 2 h, yielding R-Nb2O5.36 Engineering controls (i.e., ventilation hood) and personal protective equipment should always be used when handling hazardous materials, such as strong acids.
4.2 X-ray and neutron diffraction
The phase purity of the intermediates and final products was initially evaluated using Rigaku Miniflex and Panalytical X'Pert Powder diffractometers. High-resolution synchrotron XRD was carried out through mail-in from beamline 11-BM at the Advanced Photon Source at Argonne National Laboratory. Synchrotron X-rays had a calibrated wavelength of 0.4589 Å and powders were measured in transmission mode in 0.8 mm diameter Kapton capillaries sealed with epoxy at both ends.For ex situ synchrotron XRD of cycled R-Nb2O5, powder cathodes (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mass ratio of Nb2O5 and SuperP) were extracted from Swagelok half-cells cycled to a specified state of charge. The powders were washed with dimethyl carbonate (DMC), dried under vacuum, and ground with an agate mortar and pestle in an Ar-filled glove box. The samples were sealed under Ar in secondary containment for shipping and removed just prior to measurements to minimize exposure to moisture.
2 mass ratio of Nb2O5 and SuperP) were extracted from Swagelok half-cells cycled to a specified state of charge. The powders were washed with dimethyl carbonate (DMC), dried under vacuum, and ground with an agate mortar and pestle in an Ar-filled glove box. The samples were sealed under Ar in secondary containment for shipping and removed just prior to measurements to minimize exposure to moisture.
Neutron diffraction data of the as-prepared R-Nb2O5 were collected using a POWGEN diffractometer at the Spallation Neutron Source (SNS) at Oak Ridge National Laboratory (ORNL) through the mail-in program. The powders were loaded into a 6 mm diameter vanadium can. Data were collected in time-of-flight for 2 h with a center wavelength of 1.5 Å.
Structural data were refined using GSAS-II48 and Topas Academic v7.49 VESTA was used to visualize the crystal structures and for quantitative descriptions of [NbO6] octahedra.45
4.3 Electron microscopy
Scanning electron microscopy (SEM) was carried out using a TESCAN MIRA3 microscope with a beam voltage of 8 keV and a working distance of 15 mm. The samples were prepared on carbon tape and sputtered with a thin layer of gold to prevent charging.4.4 Battery assembly and electrochemical studies
R-Nb2O5 was cycled in half-cell batteries with a Li metal anode (MTI, 99.9%). For preliminary cycling and ex situ synchrotron XRD, loose powder cathodes (Nb2O5 and SuperP in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mass ratio, mixed in an agate mortar and pestle) were assembled in Swagelok cells. For coin cells, cast cathode films were prepared by mixing R-Nb2O5 with Ketjen Black (MSE Supplies) and polyvinylidene floride (PVDF) in a 7.5
2 mass ratio, mixed in an agate mortar and pestle) were assembled in Swagelok cells. For coin cells, cast cathode films were prepared by mixing R-Nb2O5 with Ketjen Black (MSE Supplies) and polyvinylidene floride (PVDF) in a 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio, respectively; N-methyl-2-pyrollidone was used as a solvent for PVDF during casting on copper foil (MSE Supplies) using a 15 μm doctor blade.
1 mass ratio, respectively; N-methyl-2-pyrollidone was used as a solvent for PVDF during casting on copper foil (MSE Supplies) using a 15 μm doctor blade.
For both cell configurations, the electrolyte was 1 M LiPF6 in a mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) of ethylene carbonate and DMC. For Swagelok cells, the cathode and anode were separated by two Whatman glass filter dryer (GFD) disks; for coin cells, the separator was polypropylene (Celgard 2500, 25 μm). The cells were assembled in an Ar-filled glovebox. Swagelok cells were cycled galvanostatically at various rates in terms of C, such that C is based on the reaction of 2 mol of Li. Coin cells were cycled galvanostatically at rates of C/20, C/10 and 1C with an upper potential limit of 2.5 V and a lower potential limit of 1 V. Additionally, cells were cycled with varying cycling rates of C/20, C/10, C/5, 1C, 2C, and 5C for 5 consecutive cycles, and a final set at C/20.
1 by volume) of ethylene carbonate and DMC. For Swagelok cells, the cathode and anode were separated by two Whatman glass filter dryer (GFD) disks; for coin cells, the separator was polypropylene (Celgard 2500, 25 μm). The cells were assembled in an Ar-filled glovebox. Swagelok cells were cycled galvanostatically at various rates in terms of C, such that C is based on the reaction of 2 mol of Li. Coin cells were cycled galvanostatically at rates of C/20, C/10 and 1C with an upper potential limit of 2.5 V and a lower potential limit of 1 V. Additionally, cells were cycled with varying cycling rates of C/20, C/10, C/5, 1C, 2C, and 5C for 5 consecutive cycles, and a final set at C/20.
Author contributions
KP: conceptualization; data curation; formal analysis; investigation; visualization; writing – original draft, reviewing and editing. ADL: investigation; especially synthesis and initial characterization (laboratory XRD). SG: investigation; especially characterization (SEM imaging). MMB: conceptualization; formal analysis; project administration; resources; supervision; visualization; writing – original draft, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge MMB's startup support. Use of the Advanced Photon Source at Argonne Nationals Laboratory was supported by the U. S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Synchrotron XRD was carried out at Argonne National Laboratory at beamline 11-BM through the general user proposal mail-in program (GUP-75192). The neutron scattering portion of this research used resources at Oak Ridge National Laboratory's Spallation Neutron Source, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory (mail-in POWGEN data through IPTS-29055). This research made use of the Research Service Center at the University of Florida and the Materials Science and Engineering undergraduate research laboratory. The authors thank POWGEN staff for support with mail-in experiments, especially Dr Alicia M. Manjon Sanz. The authors also thank Dr Allyson Fry-Petit for helpful discussions about Topas and Dr Kit McColl for exciting discussions on early transition metal oxide materials. Furthermore, all authors acknowledge the broad and consistent support of all members of the Butala Research Group, especially John D. Langhout.References
- A. Magnéli, Structures of the ReO3-type with Recurrent Dislocations of Atoms: ‘Homologous Series’ of Molybdenum and Tungsten Oxides, Acta Crystallogr., 1953, 6, 495–500 CrossRef.
- S. Andersson and A. D. Wadsley, Crystallographic Shear and Diffusion Paths in Certain Higher Oxides of Niobium, Tungsten, Molybdenum and Titanium, Nature, 1966, 211, 581–583 CrossRef CAS.
- L. R. Eyring and L. T. Tai, The Structural Chemistry of Extended Defects, Annu. Rev. Phys. Chem., 1973, 24, 189–206 CrossRef CAS.
- R. Cava, A. Santoro, D. W. Murphy, S. M. Zahurak and R. S. Roth, Structural Aspects of Lithium Insertion in Oxides: LixReO3 and Li2FeV3O8, Solid State Ionics, 1981, 5, 323–326 CrossRef CAS.
- A. A. Voskanyan and A. Navrotsky, Shear Pleasure: The Structure, Formation, and Thermodynamics of Crystallographic Shear Phases, Annu. Rev. Mater. Res., 2021, 51, 521–540 CrossRef CAS.
- R. Kanno, Y. Kawamoto, Y. Takeda, S. Ohashi, N. Imanishi and O. Yamamoto, Carbon Fiber as a Negative Electrode in Lithium Secondary Cells, J. Electrochem. Soc., 1992, 139, 3397 CrossRef CAS.
- M. G. Verde, L. Baggetto, N. Balke, G. M. Veith, J. K. Seo, Z. Wang and Y. S. Meng, Elucidating the Phase Transformation of Li4Ti5O12 Lithiation at the Nanoscale, ACS Nano, 2016, 10, 4312–4321 CrossRef CAS PubMed.
- M. M. Thackeray and K. Amine, Li4Ti5O12 Spinel Anodes, Nat. Energy, 2021, 6, 683 CrossRef CAS.
- K. J. Griffith, A. C. Forse, J. M. Griffin and C. P. Grey, High-Rate Intercalation without Nanostructuring in Metastable Nb2O5 Bronze Phases, J. Am. Chem. Soc., 2016, 138, 8888–8899 CrossRef CAS PubMed.
- K. J. Griffith, K. M. Wiaderek, G. Cibin, L. E. Marbella and C. P. Grey, Niobium Tungsten Oxides for High-rate Lithium-ion Energy Storage, Nature, 2018, 559, 556–563 CrossRef CAS PubMed.
- M. B. Preefer, M. Saber, Q. Wei, N. H. Bashian, J. D. Bocarsly, W. Zhang, G. Lee, J. Milam-Guerrero, E. S. Howard, R. C. Vincent, B. C. Melot, A. Van der Ven, R. Seshadri and B. S. Dunn, Multielectron Redox and Insulator-to-Metal Transition upon Lithium Insertion in the Fast-Charging, Wadsley–Roth Phase PNb9O25, Chem. Mater., 2020, 32, 4553–4563 CrossRef CAS.
- R. C. Vincent, Y. Luo, J. L. Andrews, A. Zohar, Y. Zhou, Q. Yan, E. M. Mozur, M. B. Preefer, J. N. Weker, A. K. Cheetham, J. Luo, L. Pilon, B. C. Melot, B. Dunn and R. Seshadri, High-Rate Lithium Cycling and Structure Evolution in Mo4O11, Chem. Mater., 2022, 34, 4122–4133 CrossRef CAS.
- J. Muller, J. Joubert and M. Marezio, Synthese et Structure Cristalline d’un Nouvel Oxyde Mixte “FeV3O8” (FexV1−xO2; x ≃ 0.25), J. Solid State Chem., 1979, 27, 191–199 CrossRef CAS.
- A. R. Balakrishna, Crystallographic Design of Intercalation Materials, J. Electrochem. Energy Convers. Storage, 2022, 19, 040802 CrossRef CAS.
- N. H. Bashian, M. B. Preefer, J. Milam-Guerrero, J. J. Zak, C. Sendi, S. A. Ahsan, R. C. Vincent, R. Haiges, K. A. See, R. Seshadri and B. C. Melot, Understanding the Role of Crystallographic Shear on the Electrochemical Behavior of Niobium Oxyfluorides, J. Mater. Chem. A, 2020, 8, 12623–12632 RSC.
- M. Saber, M. B. Preefer, S. K. Kolli, W. Zhang, G. Laurita, B. Dunn, R. Seshadri and A. Van der Ven, Role of Electronic Structure in Li Ordering and Chemical Strain in the Fast Charging Wadsley–Roth Phase PNb9O25, Chem. Mater., 2021, 33, 7755–7766 CrossRef CAS.
- C. P. Koçer, K. J. Griffith, C. P. Grey and A. J. Morris, Lithium Diffusion in Niobium Tungsten Oxide Shear Structures, Chem. Mater., 2020, 32, 3980–3989 CrossRef PubMed.
- K. McColl, K. J. Griffith, R. L. Dally, R. Li, J. E. Douglas, K. R. Poeppelmeier, F. Corà, I. Levin and M. M. Butala, Energy Storage Mechanisms in Vacancy-ordered Wadsley–Roth Layered Niobates, J. Mater. Chem. A, 2021, 9, 20006–20023 RSC.
- H. Schäfer, R. Gruehn and F. Schulte, The Modifications of Niobium Pentoxide, Angew. Chem., Int. Ed., 1966, 5, 40–52 CrossRef.
- T. Li, G. Nam, K. Liu, J.-H. Wang, B. Zhao, Y. Ding, L. Soule, M. Avdeev, Z. Luo and W. Zhang, et al., A Niobium Oxide with a Shear Structure and Planar Defects for High-power Lithium Ion Batteries, Energy Environ. Sci., 2022, 15, 254–264 RSC.
- H. Nakayama, M. Nose, S. Nakanishi and H. Iba, Electrochemical Reactions of Layered Niobate Material as Novel Anode for Sodium Ion Batteries, J. Power Sources, 2015, 287, 158–163 CrossRef CAS.
- J. W. Kim, V. Augustyn and B. Dunn, The Effect of Crystallinity on the Rapid Pseudocapacitive Response of Nb2O5, Adv. Energy Mater., 2012, 2, 141–148 CrossRef CAS.
- V. Augustyn, P. Simon and B. Dunn, Pseudocapacitive Oxide Materials for High-rate Electrochemical Energy Storage, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- A. A. Lubimtsev, P. R. Kent, B. G. Sumpter and P. Ganesh, Understanding the Origin of High-rate Intercalation Pseudocapacitance in Nb2O5 Crystals, J. Mater. Chem. A, 2013, 1, 14951–14956 RSC.
- R. Gruehn, Eine weitere neue Modifikation des niobpentoxids, J. Less-Common Met., 1966, 11, 119–126 CrossRef.
- O. Aalling-Frederiksen, M. Juelsholt, A. S. Anker and K. M. Jensen, Formation and growth mechanism for Niobium Oxide Nanoparticles: Atomistic Insight from in-situ X-ray Total Scattering, Nanoscale, 2021, 13, 8087–8097 RSC.
- S. Andersson, On the Description of Complex Inorganic Crystal Structures, Angew. Chem., Int. Ed. Engl., 1983, 22, 69–81 CrossRef.
- M. Israelsson and L. Kihlborg, The Crystal Structure of Monoclinic Wolfram Vanadium Oxide, W3V5O20, an 0-D Structure Related to R-Nb2O5, J. Solid State Chem., 1970, 1, 469–477 CrossRef.
- L. R. De Jesus, J. L. Andrews, A. Parija and S. Banerjee, Defining Diffusion Pathways in Intercalation Cathode Materials: Some Lessons from V2O5 on Directing Cation Traffic, ACS Energy Lett., 2018, 3, 915–931 CrossRef CAS.
- P. Schofield, Y. Luo, D. Zhang, W. Zaheer, D. Santos, G. Agbeworvi, J. D. Ponis, J. V. Handy, J. L. Andrews and E. J. Braham, et al., Doping-Induced Pre-Transformation to Extend Solid-Solution Regimes in Li-Ion Batteries, ACS Energy Lett., 2022, 7, 3286–3292 CrossRef CAS.
- L. Kihlborg, Crystal Structure of (MO0.3V0.7)2O5 of R-Nb2O5 type and a comparison with Structures of V2O5 and V2MoO8, Acta Chem. Scand., 1967, 21, 2495 CrossRef CAS.
- V. Shklover, T. Haibach, F. Ried, R. Nesper and P. Novak, Crystal Structure of the Product of Mg2+ Insertion into V2O5 Single Crystals, J. Solid State Chem., 1996, 123, 317–323 CrossRef CAS.
- K. Meisel, Rheniumtrioxyd. III. Mitteilung. Ueber die Kristallstruktur des Rheniumtrioxyds, Z. Anorg. Allg. Chem., 1932, 207, 121–128 CrossRef CAS.
- S. Andersson, Crystal Structure of Nb3O7F, Acta Chem. Scand., 1964, 18, 2339–2344 CrossRef CAS.
- N. H. Bashian, S. Zhou, M. Zuba, A. M. Ganose, J. W. Stiles, A. Ee, D. S. Ashby, D. O. Scanlon, L. F. J. Piper, B. Dunn and B. C. Melot, Correlated Polyhedral Rotations in the Absence of Polarons during Electrochemical Insertion of Lithium in ReO3, ACS Energy Lett., 2018, 3, 2513–2519 CrossRef CAS.
- R. Nedjar, M. Borel and B. Raveau, H3ONb3O8 and HNb3O8: New protonic oxides with a layer structure involving ion exchange properties, Mater. Res. Bull., 1985, 20, 1291–1296 CrossRef CAS.
- M. Gasperin, Structure du Triniobate (V) de Potassium KNb3O8, un Niobate Lamallaire, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, B38, 2024–2026 CrossRef CAS.
- R. Nedjar, M. M. Borel, A. Leclaire and B. Raveau, The Sodium Niobate NaNb3O8: A Novel Lamellar Oxide Synthesized by Soft Chemistry, J. Solid State Chem., 1987, 71, 182–188 CrossRef CAS.
- Z. Y. Zhan, C. Y. Xu, L. Zhen, W. S. Wang and W. Z. Shao, Large-Scale Synthesis of Single-Crystalline KNb3O8 Nanobelts via a Simple Molten Salt Method, Ceram. Int., 2010, 36, 679–682 CrossRef CAS.
- K. J. Griffith, I. D. Seymour, M. A. Hope, M. M. Butala, L. K. Lamontagne, M. B. Preefer, C. P. Kocer, G. Henkelman, A. J. Morris and M. J. Cliffe, et al., Ionic and electronic conduction in TiNb2O7, J. Am. Chem. Soc., 2019, 141, 16706–16725 CrossRef CAS PubMed.
- C. Delmas, H. Cognac-Auradou, J. Cocciantelli, M. Ménétrier and J. Doumerc, The LixV2O5 System: An Overview of the Structure Modifications induced by the Lithium Intercalation, Solid State Ionics, 1994, 69, 257–264 CrossRef CAS.
- K. A. See, M. Lumley, G. D. Stucky, C. P. Grey and R. Seshadri, Reversible Capacity of Conductive Carbon Additives at Low Potentials: Caveats for Testing Alternative Anode Materials for Li-Ion Batteries, J. Electrochem. Soc., 2017, 164, A327–A333 CrossRef CAS.
- M. M. Butala, K. R. Danks, M. A. Lumley, S. Zhou, B. C. Melot and R. Seshadri, MnO Conversion in Li-Ion Batteries: In Situ Studies and the Role of Mesostructuring, ACS Appl. Mater. Interfaces, 2016, 8, 6496–6503 CrossRef CAS PubMed.
- W. H. Baur, The Geometry of Polyhedral Distortions. Predictive Relationships for the Phosphate Group, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30, 1195–1215 CrossRef CAS.
- K. Momma and F. Izumi, VESTA3 for Three-dimensional Visualization of Crystal, Volumetric and Morphology Data, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- P. S. Halasyamani, Asymmetric Cation Coordination in Oxide Materials: Influence of Lone-Pair Cations on the Intra-octahedral Distortion in d0 Transition Metals, Chem. Mater., 2004, 16, 3586–3592 CrossRef CAS.
- L. Li, J. Deng, R. Yu, J. Chen, X. Wang and X. Xing, Phase Evolution in Low-dimensional Niobium Oxide Synthesized by a Topochemical Method, Inorg. chem., 2010, 49, 1397–1403 CrossRef CAS PubMed.
- B. H. Toby and R. B. Von Dreele, GSAS-II: the Genesis of a Modern Open-source All Purpose Crystallography Software Package, J. Appl. Crystallogr., 2013, 46, 544–549 CrossRef CAS.
- A. A. Coelho, TOPAS and TOPAS-Academic: An Optimization Program Integrating Computer Algebra and Crystallographic Objects Written in C++, J. Appl. Crystallogr., 2018, 51, 210–218 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional details and results from Rietveld refinements of atomic structure models as well as cycling data in Swagelok cells, representative of cycling from which ex situ discharged and charged powders were prepared, and coin cells cycled at C/10 and 1C. See DOI: https://doi.org/10.1039/d2ta08653k |
| This journal is © The Royal Society of Chemistry 2023 |