Unravelling the redox behaviour of a carbon nitride polymer-based ternary heterostructure for CO2 photoreduction promoted by amine coupling†
Pankaj Kumar
Prajapati
 ,
Neha
Saini
,
Deepak Kumar
Chauhan
and
Kamalakannan
Kailasam
,
Neha
Saini
,
Deepak Kumar
Chauhan
and
Kamalakannan
Kailasam
 *
*
Advanced Functional Nanomaterials, Institute of Nano Science and Technology (INST), Mohali, Punjab-140306, India. E-mail: kamal@inst.ac.in; Tel: +91-172-2297046
First published on 28th November 2022
Abstract
Imine compounds are essential commodity chemicals used in synthetic organic chemistry and pharmaceuticals. To date, the oxidative coupling of amines produces imines in a pure oxygen atmosphere. Here, we have presented a sustainable and more economical route for producing imines by replacing O2 with inexpensive and likely inert CO2. A noble metal-free ZnV2O6@WO3–g–C3N4 heterojunction has dual photocatalysis ability, converting CO2 into CO and CH4 in 1674 and 683 μmol g−1 yields, respectively, within six hours with the simultaneous oxidative coupling of amines into imines with a maximum of 55% yield for N-benzylidene benzylamine. Also, CO and CH4 yields were 5.3 and 2.9 times higher than those of pristine g-C3N4. The reusability demonstrated the reliability of the present heterojunction, which showed four consecutive uses of the recovered photocatalyst with relatively similar yields. Besides these, extensive corroboration of mechanistic understanding was made through thorough characterization of the intermediates formed during this simultaneous photocatalytic transformation. Thus, replacing molecular oxygen with CO2 to oxidize amines has vast opportunities, as CO2 can be used as an oxidant for sustainable organic transformations into fine chemicals and fuels.
1 Introduction
The cost-effective production of fine chemicals and fuels is essential from an energy perspective.1 The production of energy chemicals utilizing waste or unwanted chemicals as precursors is highly desirable. Meanwhile, imines are functional raw materials in natural product synthesis, medicinal chemistry, synthetic organic chemistry, catalysis, analytical chemistry, photochromism, etc.2 Imines are produced from the oxidative coupling of amines under different catalytic conditions using oxidizing agents, such as oxygen and air. Traditionally, imines were produced from oxidative coupling of amines using carbonyl compounds and 2-iodoxybenzoic acid (or N-t-butylphenylsulfinimidoyl chloride).3,4 These materials are expensive and produce harmful by-products under harsh conditions, increasing the complexity and energy consumption.Later, the oxidative coupling of amines was improved by replacing expensive and hazardous oxidizing agents with more convenient and environmentally benign dioxygen and air as oxidizing agents. Furthermore, earth-abundant solar light replaced the harsh conditions, which decreased the cost of the oxidative coupling of amines to imines.5 Many pioneering studies on oxidative coupling of amines have been performed using various photocatalytic materials, including metal and non-metal semiconductors. Yang et al. reported a solvent-free ordered mesoporous Au/TiO2 catalyzed oxidative coupling of amines under visible light.6 Khampuanbut et al. discussed the energy band alignment and mechanistic insight into visible light-assisted good conversion of amines into imines using a WO3/BiOBr heterojunction photocatalyst.7 Xu et al. evidenced the charge separation in a porphyrin-based metal–organic framework for oxidative coupling of amines into imines under visible light irradiation.8 Battula et al. fabricated a truxene-based conjugated microporous polymer for homocoupling amines using O2 under natural sunlight.9 Although the oxidative coupling of amines into imines is a meaningful organic transformation, improvements are still needed to make the process more convenient, economical, and energy-efficient.
Recently, the emission of greenhouse gases, mainly carbon dioxide (CO2), emerged as a severe environmental issue. CO2 is the most abundant component of greenhouse gas emitted by different chemical processes in various industries. The excessive emission of CO2 from wetlands, crude oil exploration, rapid population growth, rapid industrialization, fossil fuel consumption, etc., has impacted the environment and raised the Earth's temperature.10 However, the non-toxicity, cheapness, and easy availability of CO2 make it generous and a suitable C1 feedstock for producing fine chemicals and fuels. The photoreduction of CO2 produces different C1 products, such as CO, CH4, CH3OH, HCOOH, and HCHO, using various hybrid and nanocomposite photocatalysts.11 Water is used as the proton source, while amines are used as the sacrificial donor or hole scavenger for the photoreduction of CO2. Several novel strategies have been developed for CO2 as a C1 feedstock with different organic transformations, such as cycloaddition with epoxides,12 carboxylation of various olefinic and halogenated compounds,13 oxidants for alcohols,14 C–N couplings,15etc., under visible light irradiation.
Replacing O2 with the major greenhouse gas CO2 has attracted great interest because of the cost-effectiveness and ease of availability of CO2 over expensive O2. Very few reports are available in the literature on the oxidative coupling of amines with simultaneous reduction of CO2 under UV-Vis light. Luo et al. achieved 82.1% conversion of benzylamine with CO2 reduction to CH3OH at 0.5 MPa using a solid solution of CuMoxW(1−x)O4 under visible light.16 Markushyna et al. investigated benzylamine oxidation with CO2 using a C3N4 semiconductor, but the maximum yield remained at only 34% for N-benzylidene benzylamine.17 Recently, Wang et al. fabricated CdS quantum dot decorated ordered mesoporous carbonaceous frameworks to efficiently reduce CO2 with the oxidative coupling of amines into imines under visible light irradiation.18 Although the above-mentioned photocatalytic systems provided moderate conversion and yields of the corresponding products for the simultaneous reduction of CO2 with oxidation of amines, significant development is desirable to achieve high efficiency for simultaneous photoreduction of CO2 with the oxidative conversion of amines into imines.
Among the various photocatalytic semiconductors, g-C3N4 has tremendous opportunities for photocatalytic applications, including CO2 photoreduction and oxidation of organic compounds.19 The band positions and significant basic sites at the g-C3N4 surface provide excellent photocatalytic activity for CO2 photoreduction for solar fuel production. Recently, g-C3N4-based metal oxide composites have been developed and exhibited excellent photocatalytic activity for CO, CH4, CH3OH, and HCOOH production from CO2 photoreduction.20 The heterojunction of g-C3N4 with metal oxides improved charge separation at the interface, resulting in the heterojunction photocatalyst's excellent oxidation and reduction ability.21–23 The heterojunction of g-C3N4 with WO3 was reported for a variety of CO2 photoreduction due to the highly positive oxidation potential of the valence band (VB) of WO3, which efficiently oxidized water or amine compounds provided necessary protons and electrons to reduce CO2.24,25 In addition, boosting the efficiency of CO2 photoreduction, the composite of g-C3N4 and metal nanoparticles with efficient CO2 adsorption and a suitable band position for CO2 reduction is necessary.
A metal vanadate with strong visible light harvesting, a narrow band gap, and excellent photocurrent density could be a suitable semiconductor for various photocatalytic applications.26 Among them, ZnVxOy-based semiconductors have vast opportunities for CO2 photoreduction, which have not been explored yet.27 Recently, ZnV2O6 proved to be a suitable component of heterojunction photocatalysts with g-C3N4 and graphene oxide (GO) for the photoreduction of CO2 to solar fuels.28,29 However, there is not much literature available for ZnV2O6 and g-C3N4-based heterojunctions. The favorable band gap and band positions of the individual components provide an impetus to develop a new heterojunction that augurs well for the simultaneous transformation, which is the need of the hour. Keeping this in mind, the construction of a heterojunction from ZnV2O6, g-C3N4, and WO3 would be an excellent approach to improving charge transfer and subsequent efficient oxidation and reduction processes for such transformations.
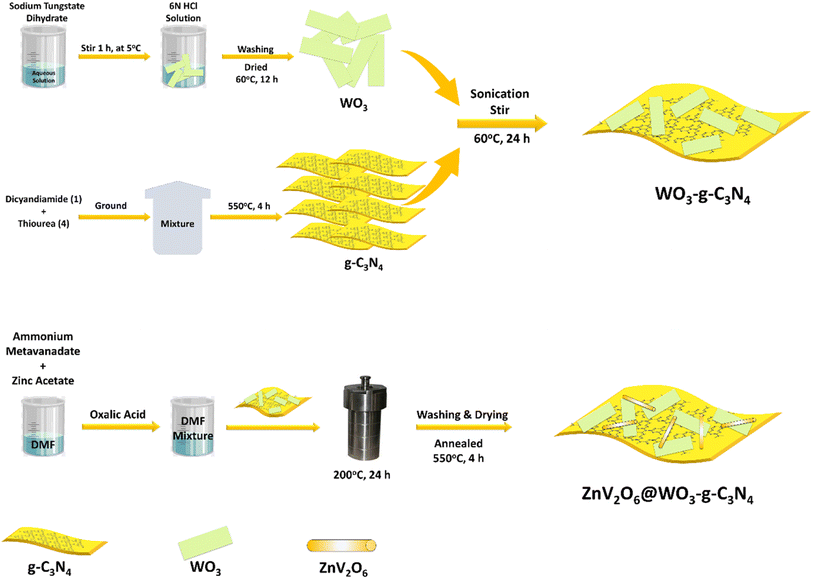
Continuing our approach for the one-pot production of chemicals and fuels from CO2, we constructed an efficient, strong oxidizing and reducing tendency and highly stable ZnV2O6@WO3–g–C3N4 heterojunction via the sol–gel hydrothermal method. Different concentrations of ZnV2O6 on the WO3–g–C3N4 composite were synthesized and studied for the simultaneous oxidative coupling of amines with the photoreduction of CO2 to fine chemicals and solar fuels. We found that 20 wt% loading of ZnV2O6 on the WO3–g–C3N4 nanocomposite, i.e., ZWC-20 nanocomposite, showed the highest photocatalytic activity under optimized conditions (Scheme 1). The heterojunction afforded good yields of the corresponding imines and C1 products (CO and CH4) within six hours of visible light irradiation. The detailed characterization of the heterojunction photocatalyst and the products was performed with various spectroscopic, microscopic, and chromatographic techniques.
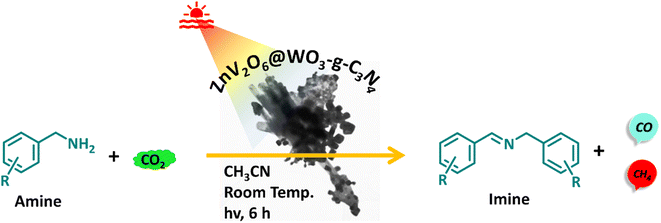 | ||
| Scheme 1 Photoreduction of CO2 with an oxidative coupling of amines under visible light irradiation. | ||
2 Experimental section
2.1 Catalysts' synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of dicyandiamide and thiourea was ground using a mortar and pestle for 30 min. This mixture was placed in a crucible and capped for the condensation process. The mixture in the crucible was annealed at 550 °C for 4 h under static air. After cooling, the yellow solid was ground and used further.
4 ratio of dicyandiamide and thiourea was ground using a mortar and pestle for 30 min. This mixture was placed in a crucible and capped for the condensation process. The mixture in the crucible was annealed at 550 °C for 4 h under static air. After cooling, the yellow solid was ground and used further.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio of WO3 and g-C3N4. In the typical synthesis, 1 g of g-C3N4 was sonicated for 2 h to exfoliate the graphitic nanosheets in ethanol. Then, 200 mg of WO3 nanosheets were added to this mixture and stirred overnight or till the evaporation of the solvent. Furthermore, the mixture was placed in an oven at 60 °C for 24 h to complete dryness and used for further modification. This heterojunction photocatalyst was coded as WCN.
5 ratio of WO3 and g-C3N4. In the typical synthesis, 1 g of g-C3N4 was sonicated for 2 h to exfoliate the graphitic nanosheets in ethanol. Then, 200 mg of WO3 nanosheets were added to this mixture and stirred overnight or till the evaporation of the solvent. Furthermore, the mixture was placed in an oven at 60 °C for 24 h to complete dryness and used for further modification. This heterojunction photocatalyst was coded as WCN.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ammonium metavanadate. At the same time, 1 g of WO3–g–C3N4 dispersed in 20 mL of DMF was added with vigorous stirring for 30 min. This solution was added to the zinc acetate and ammonium metavanadate solution and stirred for one hour. Finally, this mixture was transferred to a 100 mL Teflon-lined autoclave and heated at 200 °C for 24 h. After cooling, the precipitates were separated using centrifugation, followed by washing with absolute ethanol, and then the wet precipitates were placed in an oven at 80 °C for 12 h. The dried content was annealed at 550 °C for 4 h and collected after cooling. The composites of ZnV2O6 on WO3–g–C3N4 were coded as ZWC-20, ZWC-10, ZWC-20, ZWC-30, and ZWC-40 based on the wt% of ZnV2O6.
1 ratio of ammonium metavanadate. At the same time, 1 g of WO3–g–C3N4 dispersed in 20 mL of DMF was added with vigorous stirring for 30 min. This solution was added to the zinc acetate and ammonium metavanadate solution and stirred for one hour. Finally, this mixture was transferred to a 100 mL Teflon-lined autoclave and heated at 200 °C for 24 h. After cooling, the precipitates were separated using centrifugation, followed by washing with absolute ethanol, and then the wet precipitates were placed in an oven at 80 °C for 12 h. The dried content was annealed at 550 °C for 4 h and collected after cooling. The composites of ZnV2O6 on WO3–g–C3N4 were coded as ZWC-20, ZWC-10, ZWC-20, ZWC-30, and ZWC-40 based on the wt% of ZnV2O6.
2.2 Photocatalytic experiment
The photocatalytic experiments for one-pot production of imines from amines and C1 products from CO2 photoreduction were performed in a 10 mL round-bottom flask. The system was equipped with a 350 W xenon lamp solar simulator. Initially, 0.1 mmol of benzylamine as a model substrate, 10 mg of the photocatalyst, and 3 mL of solvent were charged to a 10 mL round-bottom flask. The round-bottom flask was closed with a rubber septum and purged with CO2 gas continuously for 30 min. The reaction vessel was appropriately closed with parafilm wrapping on the mouth of the round-bottom flask to avoid any leakage. Then the light source was irradiated on the reaction mixture, and the reaction progress was monitored by collecting small aliquots from the reaction mixture and injecting them into a GC-MS and GC-FID to detect the corresponding products of the reaction. GC-MS and 1H and 13C NMR spectroscopy confirmed the products of benzylamine oxidation.2.3 Photoelectrode preparation
The synthesized semiconductors and nanocomposite materials were coated on fluorine-doped tin oxide (FTO) plates by a previously reported printing method.31 The materials' paste was prepared by blending 25 mg of photocatalyst with 50 mg of ethyl cellulose, 0.1 mL terpineol, 0.02 mL of acetic acid, and ∼1 mL of ethanol for homogeneity. This mixture was then manually printed over a FTO plate. The as-prepared plates were dried at 150 °C under a vacuum for 2 h to eliminate the binding agents. Furthermore, the modified FTO plates were utilized for photo-electrochemical measurements.3 Results and discussion
3.1 Catalysts characterization
The desired heterostructure was fabricated via three-step synthesis (Scheme 2). Initially, melamine was heated at 550 °C to synthesize g-C3N4. WO3 was synthesized using sodium tungstate as the precursor for precipitation under acidic conditions at low temperatures. Then, the composite of ZnV2O6, WO3, and g-C3N4 was fabricated via the sol–gel-hydrothermal method and subsequent post-annealing process. The synthesized nanomaterials were characterized using various spectroscopic and microscopic techniques.Powder X-ray diffraction was employed to analyze the crystal parameters of the synthesized nanomaterials (Fig. 1). XRD of g-C3N4 showed a (002) plane of conjugated aromatic units with the stacked interlayer structure because of its graphitic nanosheet structure, identified by the JCPDS card no. 87-1526.32 The high crystallinity of WO3 confirmed its intense crystal planes of a cubic crystal lattice. The diffraction peaks at 2θ values 13.9°, 22.8°, 24.4°, 26.7°, 28.1°, 33.7°, 36.4°, 50.1°, and 55.3° were attributed to the (100), (001), (110), (101), (200), (111), (201), (220), and (202) planes for the hexagonal crystal lattice of WO3 matching with the JCPDS card no. 33-1387.33 The nanocomposite construction of WO3 and g-C3N4 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio, i.e., WCN, showed the diffraction plane of both g-C3N4 and WO3. However, due to the low concentration, the diffraction planes of WO3 were observed with less intensity. In addition, the diffraction signals at 2θ values 20.3°, 27.3°, 28.9°, 29.4°, 32.8°, 37.3°, 38.8°, 44.4°, 47.4°, and 51.9° were attributed to the (−201), (110), (−202), (201), (111), (−112), (−311), (003), (311), and (020) planes that correspond to the orthorhombic crystal lattice of ZnV2O6, confirmed by the JCPDS card no. 01-074-1262.29 As the construction of the heterojunction was reflected in its photocatalytic activity, the heterojunctions of ZnV2O6 and WCN, i.e., ZWC derivatives, were also analyzed by PXRD. ZWC derivatives showed the additional diffraction planes of ZnV2O6 along with WCN (Fig. S1†). Although ZnV2O6 was loaded on the WCN surface, the diffraction planes of ZnV2O6 were not observed in ZWC-05 and ZWC-10 nanocomposites due to the low loading content. The higher loading of ZnV2O6, i.e., ZWC-20 sample, exhibited (−202), (201), and (111) diffraction planes, indicating the incorporation of ZnV2O6 with WCN.
5 ratio, i.e., WCN, showed the diffraction plane of both g-C3N4 and WO3. However, due to the low concentration, the diffraction planes of WO3 were observed with less intensity. In addition, the diffraction signals at 2θ values 20.3°, 27.3°, 28.9°, 29.4°, 32.8°, 37.3°, 38.8°, 44.4°, 47.4°, and 51.9° were attributed to the (−201), (110), (−202), (201), (111), (−112), (−311), (003), (311), and (020) planes that correspond to the orthorhombic crystal lattice of ZnV2O6, confirmed by the JCPDS card no. 01-074-1262.29 As the construction of the heterojunction was reflected in its photocatalytic activity, the heterojunctions of ZnV2O6 and WCN, i.e., ZWC derivatives, were also analyzed by PXRD. ZWC derivatives showed the additional diffraction planes of ZnV2O6 along with WCN (Fig. S1†). Although ZnV2O6 was loaded on the WCN surface, the diffraction planes of ZnV2O6 were not observed in ZWC-05 and ZWC-10 nanocomposites due to the low loading content. The higher loading of ZnV2O6, i.e., ZWC-20 sample, exhibited (−202), (201), and (111) diffraction planes, indicating the incorporation of ZnV2O6 with WCN.
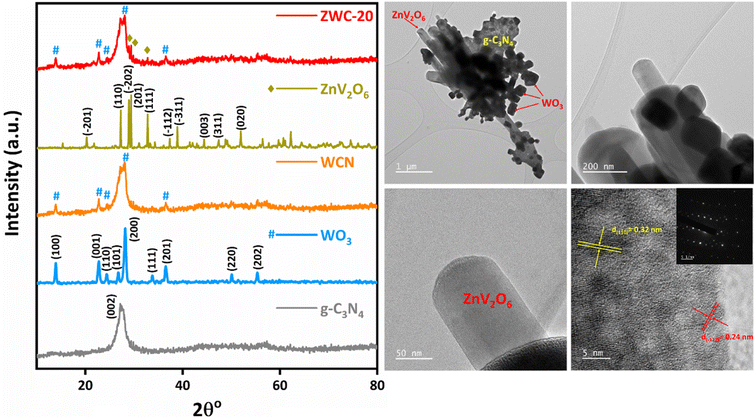 | ||
| Fig. 1 Powder XRD of g-C3N4, WO3, WCN, ZnV2O6, and ZWC-20 nanocomposites. HR-TEM images and SAED pattern of the ZWC-20 nanocomposite. | ||
The morphological aspects of ZnV2O6, WCN, and ZWC-20 nanocomposites were analyzed through field-emission scanning electron microscopy (FE-SEM) (Fig. S2†). FE-SEM of ZnV2O6 showed that nanorods are embedded in ZnV2O6 granules. The WCN composite showed rectangular sheets of WO3 settled on g-C3N4 sheets. Some sheets of g-C3N4 were found cracked during WCN nanocomposite formation. These cracked sheets of g-C3N4 with rectangular WO3 nanosheets doped with ZnV2O6 in the ZWC-20 nanocomposite were observed. The nanorods of ZnV2O6 were observed at WCN sheets, thus confirming the formation of a ZWC-20 heterojunction. High-resolution transmittance electron microscopy (HR-TEM) scanned the rough imperfection and morphology of WCN and ZWC-20 nanocomposites. The nanosheets of WO3 were observed at the g-C3N4 surface, and the composite WCN showed some crystalline features, confirmed in the SAED pattern with bright dots of the corresponding planes (Fig. S3†). Also, the elemental mapping indicates the amount of W, C, N, and O in WCN. In the ZWC-20 nanocomposite, the nanorods of ZnV2O6 were observed on g-C3N4 nanosheets. The TEM images of the ZWC-20 nanocomposite confirmed the nanorod morphology of ZnV2O6. The high-resolution TEM image exhibited the interplanar spacing of 0.32 and 0.24 nm matching with the (110) and (−112) planes of ZnV2O6, respectively. In addition, the SAED pattern of ZWC-20 showed bright dots indicating the highly crystalline nature of the composite. Also, the elemental mapping and EDX pattern showed Zn, V, W, C, N, and O in the ZWC-20 nanocomposite (Fig. S4†).
X-ray photoelectron spectroscopy (XPS) determined the surface properties of the synthesized nanomaterials (Fig. 2). The deconvoluted C-1s XPS spectra for g-C3N4 showed three peaks. The three signals at 288.0, 287.6, and 284.4 eV correspond to N–CN, CN, and CC in the graphitic sheets of g-C3N4, respectively. In the heterojunction of g-C3N4 with WO3 and ZnV2O6, there was a shift in the binding energies of C-1s XPS peaks. Especially when compared to g-C3N4, C-1s XPS signals shifted to relatively higher binding energies in the WCN composite. In contrast, as an interesting observation, C-1s XPS peaks were shifted to lower binding energies in the ZWC-20 nanocomposite than in WCN, indicating that ZnV2O6 and WO3 constructed an interface that shifted the C-1s peaks to lower binding energies. Still, they remained slightly higher (288.2 eV for N–CN and 287.8 eV for CN) than g-C3N4. In addition, an additional peak observed at 285.5 eV in C-1s XPS spectra might be due to the interaction between ZnV2O6 and g-C3N4. The deconvoluted N-1s XPS spectra indicated three characteristic signals of –NH2 groups, graphitic N–(C)3, and C–NC at 400.4, 399.5, and 398.2 eV, respectively. In WCN, –NH2 and C–NC peaks shifted to relatively higher binding energies, i.e., 400.6 and 398.3 eV, respectively, while the graphitic N–(C)3 XPS peak moved to 399.3 eV, which might be due to the construction of an interface between g-C3N4 and WO3.34 The graphitic N–(C)3 signal was more pronounced and shifted to lower binding energy in ZWC-20 than in g-C3N4 and WCN. In addition, the C–NC peak shifted by −0.5 eV in the ZWC-20 nanocomposite, indicating a change in the environment of graphitic C3N4 sheets. The characteristic two XPS signals, W-4f5/2 and W-4f7/2, were found at 37.5 and 35.4 eV in the XPS spectra of WO3. These two signals correspond to W6+ without any other oxidation state in WO3.35 Similar binding energies were obtained for the elements in WCN and ZWC-20 nanocomposites, indicating that the heterojunction remained intact with the chemical properties of WO3. However, a shift to lower binding energies was observed in WCN and ZWC-20 nanocomposites, which corresponds to the facile excitation of the electrons at the surface of the photocatalyst. ZnV2O6 and ZWC-20 nanocomposites showed two characteristic XPS peaks for Zn-2p, 2p1/2, and 2p3/2 signals at 1044.9 and 1021.7 eV corresponding to Zn2+ with no significant change in the binding energies of 2p1/2 and 2p3/2 peaks.36 XPS of V-2p for ZnV2O6 and ZWC-20 nanocomposites showed two characteristic signals for 2p1/2 and 2p3/2. The peaks 2p1/2 and 2p3/2 were found at 524.4 and 517.0 eV, respectively, in the XPS of ZnV2O6. There was no marginal change in the binding energies of V-2p1/2 and 2p3/2, indicating that the environment of V-2p was relatively similar to the ZWC-20 nanocomposite. It confirms that there was no change in the crystal lattice of ZnV2O6. The ZnV2O6 nanorods were decorated at the WCN composite in the ZWC-20 nanocomposite. All the components, ZnV2O6, WO3, WCN, and ZWC-20 nanocomposites, showed two deconvoluted XPS signals for surface oxygen and lattice oxygen. There was no marginal change in the binding energies, and these two XPS peaks were found at 531.8 and 530.2 eV, respectively, for the ZWC-20 nanocomposite. This indicates no change in the crystal lattice of WO3 and ZnV2O6 in WCN and ZWC-20. ZnV2O6, WO3, g-C3N4, WCN, and ZWC-20 nanocomposite survey spectra confirmed all the elements (Fig. S5†).
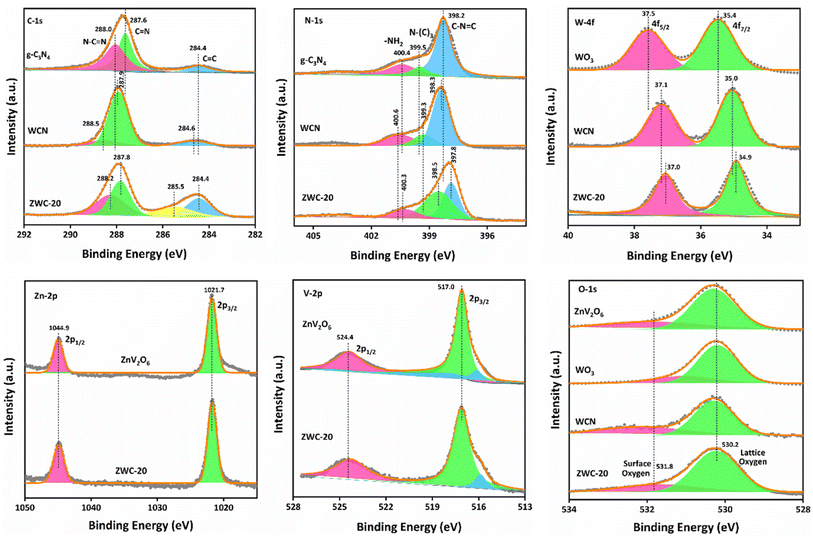 | ||
| Fig. 2 HR-XPS spectra of the C-1s, N-1s, W-4f, Zn-2p, V-2p, and O-1s of ZnV2O6, WO3, g-C3N4, WCN, and ZWC-20 nanocomposites. | ||
Ultra-violet-visible (UV-Vis) spectroscopy determined the optical behaviour of the synthesized nanomaterials (Fig. 3a). g-C3N4 exhibited a steep and strong absorption in the UV region due to π → π* and n → π* transitions in the heptazine ring. A broad absorption above 400 nm arose from the electronic transition from the N-2p orbitals of the valence band (VB) to the C-2p orbitals of the conduction band (CB) in g-C3N4 with a band gap of 2.68 eV responsible for its photocatalytic activity (Fig. S6†).37 WO3 absorbs in the UV region as well as in the visible region, showing a band gap of 2.53 eV. ZnV2O6 had multiple absorption bands; two absorption bands at 260 and 350 nm in the UV-region and another at 490 nm were observed in the visible region showing a band gap of 2.09 eV. The broad absorption tail, i.e., the Urbach tail, was observed due to the excitation of defect states with energies in the forbidden energy region.38,39 Constructing a nanocomposite of g-C3N4 and WO3, i.e., WCN, exhibited relatively similar absorption in the UV and visible regions. Nevertheless, the ternary heterojunction ZWC-20 showed good absorption in visible light with a band gap of 2.3 eV. This indicates that incorporating ZnV2O6 and WO3 with g-C3N4 would improve the visible light absorption and be a suitable candidate for photocatalytic application.
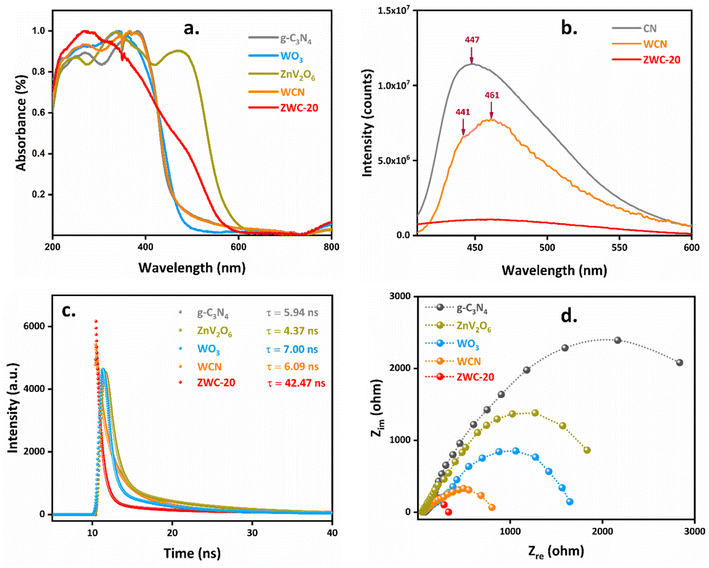 | ||
| Fig. 3 (a) UV-Vis spectra, (b) PL spectra, (c) time-resolved PL spectra, and (d) EIS spectra of the samples used in this study. | ||
As mentioned above, the band gaps of g-C3N4, WO3, and ZnV2O6 were 2.68, 2.53, and 2.09 eV, respectively. Mott–Schottky (M–S) and VB-XPS were employed to calculate the potentials of CB and VB to predict the electron transfer mechanism. M–S plots could determine the semiconductors' flat-band potentials (Efb), with the help of which the band edge potentials of the CB and VB can be calculated. VB-XPS is another crucial technique to determine the band edge potentials of the CB and VB, as the binding energy determined by VB-XPS refers to the energy difference between the Fermi and VB edge levels of the particular semiconductor.40 M–S plots determined that the individual components g-C3N4, WO3, and ZnV2O6 were n-type semiconductors. The Efb of g-C3N4, WO3, and ZnV2O6 was calculated to be −0.71, −0.06, and −0.46 V (vs. Ag/AgCl), respectively, from M–S plots (Fig. S7a–c†) using the equation:
| ERHE = Eθ(Ag/AgCl) + 0.0591 × pH + Efb(Ag/AgCl) |
E fb was −0.10, 0.54, and 0.16 V (vs. NHE) for g-C3N4, WO3, and ZnV2O6, respectively, where Eθ(Ag/AgCl) was 0.1989 and pH was 7.41 The energy differences between the VB edge level and Fermi level determined by VB-XPS for g-C3N4, WO3, and ZnV2O6 were 1.71, 2.56, 1.28 eV, respectively (Fig. S7d–f†). The calculated VB for g-C3N4, WO3, and ZnV2O6 was 1.60, 3.10, and 1.44 V (vs. NHE), respectively.42 Therefore, the CB calculated was −1.08, 0.57, and −0.65 V (vs. NHE), respectively, from the equation Eg = EVB − ECB.
Moreover, photoluminescence (PL) spectroscopy provided information regarding the charge transfer and charge carrier's recombination of the semiconductors and composite photocatalysts correlated with the photocatalytic activity (Fig. 3b). The three bands at 420, 439, and 459 nm in the PL spectra of WO3 correspond to the near band–edge transition and oxygen vacancies (Fig. S8†).43 ZnV2O6 exhibited three emission bands at 411, 434, and 457 nm due to band gap excitation, interstitial Zn atoms, and excitations between Zn and singly charged surface oxygen, respectively.44 An intense PL emission peak at 447 nm for g-C3N4 was found due to the n → π* electronic transition, showing the poor separation of charge carriers and rapid recombination of electron–hole pairs, which may refer to decreased catalytic activity in visible light.45 The constructed WCN composite exhibited two emission peaks at 441 and 461 nm with relatively less intensity, indicating an improvement in the separation of charge carriers.46 As expected, the ternary heterojunction ZWC-20 showed a less intense and broad emission peak corresponding to the lowest recombination of charge carriers. This refers to the highest separation of electron–hole pairs, and the highest activity is expected under visible light conditions. Thus, the heterojunction ZWC-20 showed the best optical properties with band edge potentials of the CB and VB suitable for oxidation and reduction reactions.
Furthermore, the time-resolved photoluminescence (TRPL) decay curves were recorded at an emission wavelength of 460 nm (Fig. 3c). The decay curves of g-C3N4, ZnV2O6, WO3, and WCN comprised two components, short-lived (τ1) and long-lived lifetime (τ2). The fast decay with a time constant of τ1 was 10.45–26.67 ns, followed by a slow decay (τ2 = 26.48–40.00 ns). The average lifetimes (τav) of g-C3N4, ZnV2O6, WO3, and WCN were 5.94, 4.37, 7.00, and 6.09 ns, respectively. This indicates a slightly extended excitation diffusion lifetime. The π*–n transition of sp2 bonded carbon in g-C3N4 resulted in a shorter carrier lifetime (5.94 ns).47 In the case of ZnV2O6, we observed the shortest lifetime of 4.37 ns. In comparison, WO3 showed a slightly extended lifetime of 7.00 ns. The shorter lifetime of pristine g-C3N4, ZnV2O6, and WO3 indicates the less probability of the charge carrier's participation in the photocatalytic reaction. The prolonged charge carrier lifetime of 6.09 ns for WCN compared to g-C3N4 comes from the interface between g-C3N4 and WO3. In ZWC-20, a fast decay with a time constant of τ1 ranged between 10.41–14.27 ns, followed by a slow decay with a time constant (τ2) of 20.01–40.00 ns, was found. The τav for ZWC-20 is unprecedented and was about 42.47 ns, a long extended excitation diffusion lifetime. The decoration of ZnV2O6 on the WCN surface inhibited the charge transfer interaction, resulting in an extended lifetime in ZWC-20 heterojunctions.48 This means that the more extensive decay time of ZWC-20 corresponds to the prolonged lifetime of charge carriers, which refers to its higher photocatalytic activity than g-C3N4 and WCN nanocomposites.
Electrochemical impedance spectroscopy (EIS) could further study the charge transfer kinetics and charge carrier's separation in the nanomaterials (Fig. 3d). The arc radius is the facial charge transfer resistance function across the catalyst's interface. The large arc radius of g-C3N4 indicates extensive facial charge transfer resistance, which corresponds to the minimum efficiency of the charge transfer. Consequently, the photocatalytic activity of g-C3N4 showed the lowest photocatalytic activity. The composite of WO3 and g-C3N4, i.e., WCN, showed a smaller semicircle arc radius with decreased facial charge transfer resistance, which implies improved charge transfer efficiency. Furthermore, the decoration of ZnV2O6 on the WCN composite, i.e., ZWC-20 nanocomposite, showed the smallest semicircle arc radius with minimum facial charge transfer resistance, which corresponded to the high efficiency of the separation of charge carriers and inhibited the recombination of electron–hole pairs. This suggested that the ZWC-20 nanocomposite with minimum facial charge transfer resistance has the highest photocatalytic activity among the synthesized nanomaterials. This result was also supported by the PL spectra of the synthesized nanomaterials, which showed that the ZWC-20 nanocomposite photocatalyst would be superior in activity compared to the other photocatalysts studied in the present system.
The additional optical properties of the semiconductors and the nanocomposites were investigated using cyclic voltammetry (CV) and linear sweep voltammetry (LSV) in the potential range of −1.5 to 1.5 V and −1.5 to 0 V (Fig. S9†). In CV, g-C3N4 showed a strong anodic peak at positive and a cathodic peak at negative potentials (Fig. S9a†). The anodic peak at 1.37 V corresponds to the oxidation of the electrolyte, while the cathodic peak at −1.12 V corresponds to the reduction with a current density of −0.89 mA.49 This indicates that the g-C3N4 surface would be favorable for oxidation and reduction reactions.50 In ZnV2O6, two oxidation peaks in the forward scan were found at 0.31 and 1.09 V with relatively weak oxidation properties. Also, a reduction peak at −0.7 V was found with a current density of −0.16 mA. WO3 showed a current density of 0.22 mA at −0.28 V in the forward scan, ascribed to its strong oxidation properties under reaction conditions.51 In addition, WO3 had a −0.95 mA current density, higher than g-C3N4 and ZnV2O6. However, in WCN and ZWC-20 nanocomposites, an anodic oxidation peak and cathodic reduction peak were found. WCN showed a reduction peak at −1.09 V with a current density of −0.46 mA, while ZWC-20 had a reduction peak at −1.08 V with a current density of −0.52 mA. In addition, ZWC-20 had a higher current density of −1.45 mA than WCN, which had a current density of −1.15 mA.52 WCN and ZWC-20 nanocomposite photocatalysts have the highest current density of −1.15 mA and −1.45 mA, respectively, which correspond to a large amount of the charge carriers' transportation efficiency. This suggested that the charge carriers can transport easily through WCN and ZWC-20 nanocomposites and thus enhanced the mobility values compared to g-C3N4, WO3, and ZnV2O6.53 In addition, the ZWC-20 nanocomposite showed improved absorption with a reduced band gap with increase in the number of charge carriers and revealed better mobility than the other samples. Thus, the ZWC-20 nanocomposite photocatalyst having a large number of charge carriers with high current density exhibited the highest photocatalytic activity for the desired photocatalytic experiments. Similarly, LSV showed the highest current density of −1.51 mA with the highest photocatalytic activity under experimental conditions (Fig. S9b†).
Fourier transform infrared (FTIR) spectroscopy was utilized to determine the surface functionalities of the synthesized nanomaterials (Fig. 4a). g-C3N4 exhibited a vibrational peak at 807 cm−1, characteristic of the breathing mode of tri-s-triazine units, confirming its heptazine basic units.54 The stretching bands between 1250 and 1650 cm−1 were assigned to the C–N and CN stretching vibrational modes with a broad IR peak above 3300 cm−1 for some uncondensed amine groups in the framework of g-C3N4. The intense vibrations between 500–1000 cm−1 were ascribed to the O–W–O stretching modes in WO3. Another weak vibrational peak at 1635 cm−1 corresponds to the hydroxyl bending modes, including hydroxyl stretching modes found above 3300 cm−1. Thus, the composite WCN showed the vibrational peaks of WO3 and g-C3N4, indicating heterostructure formation. ZnV2O6 showed the characteristic V–O–Zn stretching modes at 484 and 607 cm−1.55 Also, the V–O–V asymmetric vibrations of VO43− tetrahedral units were found at 823 and 1018 cm−1.56 In addition, adsorbed H2O stretching and bending modes were obtained at 3436 and 1631 cm−1. Furthermore, the ternary heterojunctions of ZnV2O6 and WCN, i.e., ZWC derivatives, showed two stretching modes of Zn–O–V below 700 cm−1. However, the intensity was low with lower loading of ZnV2O6 (5 and 10 wt%) (Fig. S10†). A significant increase in the vibrational frequencies was found in composites with higher ZnV2O6, i.e., ZWC-20 to ZWC-40 heterojunctions, indicating the deposition of ZnV2O6 on the WCN nanocomposite, thus confirming the formation of a ternary heterojunction.
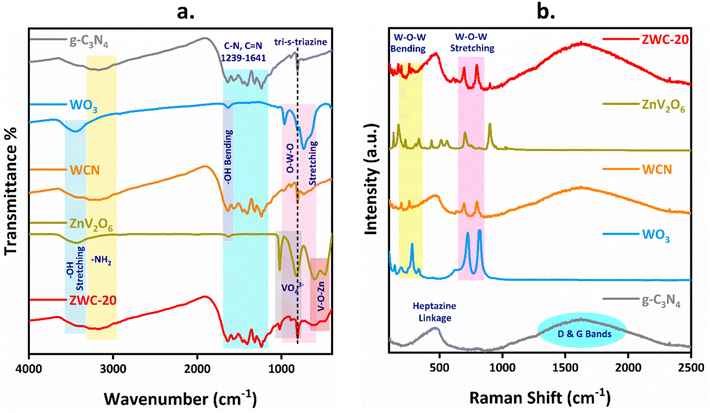 | ||
| Fig. 4 (a) FTIR and (b) Raman spectra of g-C3N4, WO3, WCN, ZnV2O6, and ZWC-20 heterojunction photocatalysts. | ||
The Raman spectra of the synthesized nanomaterials are shown in Fig. 4b. The Raman peak at 469 cm−1 was a group of multiple peaks showing the in-plane bending mode of the heptazine linkage and the symmetric N-breathing mode of heptazine units.57 The broad peak centred at 1621 cm−1 is ascribed to the stretching vibrations of C–N and analogous to the typical D- and G-bands of graphitic carbon nitride-based materials.58 The Raman spectra exhibited various characteristic peaks of WO3. The peaks below 200 cm−1, i.e., 143 and 194 cm−1, are the characteristics of (W2O2)n chains, while the Raman bands at 280 and 334 cm−1 are ascribed the W–O–W bending modes of bridging oxide ions. In addition, the highly intense Raman bands at 723 and 818 cm−1 demonstrated the W–O–W stretching mode of the WO3 network.59 The composite of WO3 and g-C3N4, i.e., WCN, showed similar Raman bands to g-C3N4 along with WO3 Raman bands. The intense Raman bands of WO3 have shifted slightly towards lower frequencies (695 and 797 cm−1) in WCN, indicating the construction of an interface between WO3 and g-C3N4. The Raman spectra of ZnV2O6 showed two characteristic peaks, 702 and 900 cm−1, with multiple peaks ranging between 100 and 1100 cm−1.60 In addition, the ternary nanocomposite ZWC-20 showed the Raman band at a relatively similar frequency of 900 cm−1 for ZnV2O6 along with strong Raman bands at 695 and 794 cm−1 for WO3. This confirmed the formation of the ZWC-20 heterostructure of g-C3N4, WO3, and ZnV2O6 semiconductors.
The surface area of the nanomaterials could be determined using the Brunauer–Emmett–Teller (BET) theory of multilayer adsorption (Fig. S11†). The N2 adsorption–desorption isotherm of g-C3N4 was identified as type II with an H3 hysteresis loop. The BET surface area of g-C3N4 was 66 m2 g−1, with an average pore size of 23 nm and a total pore volume of 0.35 cm3 g−1. This indicates that g-C3N4 had mesopores with a significant surface area. The decoration of WO3 on g-C3N4 in WCN changed the isotherm from type-II to type-III with H3 hysteresis. The incorporation of WO3 on g-C3N4 reduced the surface area to 15 m2 g−1. Also, the total pore volume and average pore diameter were reduced to 0.14 cm3 g−1 and 3.8 nm, respectively. The reduced pore volume and diameter are ascribed to the deposition of WO3 particles on the surface of g-C3N4. In the ZWC-20 nanocomposite, i.e., the decoration of ZnV2O6 on the WCN composite, the surface area was diminished to 4 m2 g−1 with a total pore volume of 0.03 cm3 g−1 and an average pore diameter of 3.4 nm. This indicates that the decoration of WO3 and ZnV2O6 on g-C3N4 nanosheets diminished the surface area, total pore volume, and average pore diameter.
3.2 Visible-light-driven production of imines and solar fuels
The photocatalytic experiments were performed in a 10 mL round-bottom flask equipped with a 350 W xenon lamp. Benzylamine was used as a model sacrificial donor for CO2 reduction to CO. In addition, the in situ generated hydrogen from benzylamine oxidation reduces CO2 to CH4. Blank experiments with just benzylamine and irradiation with a Xe-lamp without a photocatalyst showed no reaction (Table 1, entry 1). While adding a catalytic amount of g-C3N4, we observed the formation of N-benzylidene benzylamine along with CO and CH4. Using 10 mg of g-C3N4, we achieved 27% N-benzylidene benzylamine as the coupled product with 316 and 236 μmol g−1 of CO and CH4, respectively (entry 2) (Fig. 5a). This indicates that the catalyst was necessary for the reaction to take place. Other semiconductors, WO3 and ZnV2O6, were screened, and WO3 showed a low yield (5%) of imine and, as expected, did not generate CO (entry 3). However, a low yield of CH4 (84 μmol g−1) was observed, and it might be due to some basic nature of benzylamine, which absorbed more CO2 in the solution. In contrast, ZnV2O6 has suitable CB and VB positions for CO2 reduction and amine oxidation; thus, it resulted in a relatively higher N-benzylidene benzylamine yield (14%) but afforded CO (151 μmol g−1) and CH4 (120 μmol g−1) (entry 4). Therefore, the semiconductors used in this reaction were suitable for visible light-assisted oxidation and reduction (redox) reactions.| Entry | Photocatalyst | Solvent | Yield | ||
|---|---|---|---|---|---|
| Imineb (%) | COc (μmol g−1) | CH4c (μmol g−1) | |||
| a Reaction conditions: benzylamine: 0.1 mmol; solvent: 3 mL; photocatalyst: 10 mg; simulated solar light; temperature: 25 °C; atmospheric pressure; reaction time: 6 h. b Yield determined by GC-MS. c Yield determined by GC-FID. | |||||
| 1 | — | CH3CN | 0 | 0 | 0 |
| 2 | g-C3N4 | CH3CN | 27 | 316 | 236 |
| 3 | WO3 | CH3CN | 5 | 0 | 84 |
| 4 | ZnV2O6 | CH3CN | 14 | 151 | 120 |
| 5 | WO3–g–C3N4 | CH3CN | 29 | 892 | 219 |
| 6 | ZnV2O6@WO3 | CH3CN | 27 | 492 | 137 |
| 7 | ZnV2O6@g-C3N4 | CH3CN | 9 | 728 | 194 |
| 8 | ZWC-05 | CH3CN | 29 | 218 | 741 |
| 9 | ZWC-10 | CH3CN | 35 | 236 | 455 |
| 10 | ZWC-20 | CH3CN | 55 | 1674 | 683 |
| 11 | ZWC-30 | CH3CN | 52 | 754 | 513 |
| 12 | ZWC-40 | CH3CN | 52 | 580 | 433 |
| 13 | ZWC-20 | H2O | 0 | 633 | 477 |
| 14 | ZWC-20 | THF | 10 | 102 | 93 |
| 15 | ZWC-20 | 1,4-Dioxane | 38 | 500 | 433 |
| 16 | ZWC-20 | DMF | 50 | 705 | 590 |
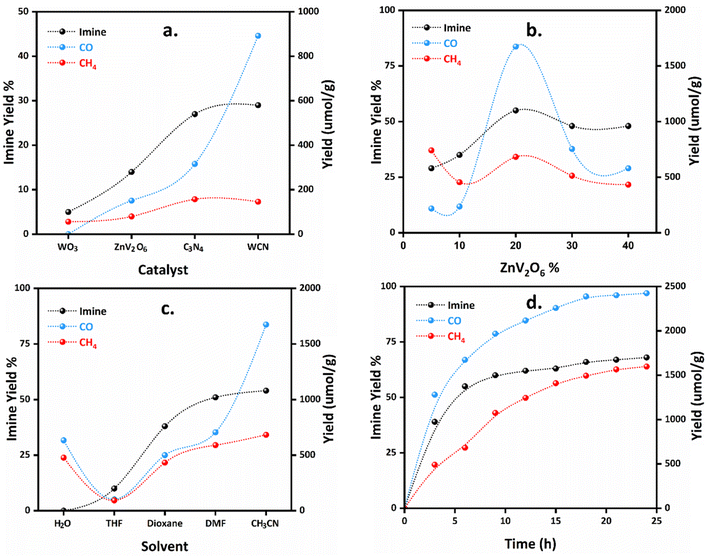 | ||
| Fig. 5 Optimization using (a) individual semiconductors, (b) ZnV2O6 loadings, (c) solvents, and (d) timeline for the redox reaction. | ||
The construction of the heterojunction always helps improve the photocatalytic conversion of the substrate molecules by inhibiting the recombination of charge carriers at the interface between the semiconductors. Thus, the constructed heterojunctions of g-C3N4, WO3, and ZnV2O6 were screened for the current photocatalytic reaction (Fig. 5b). At first, the constructed WCN nanocomposite resulted in a 29% yield of N-benzylidene benzylamine with 892 and 219 μmol g−1 yields of CO and CH4, respectively (entry 5). This indicates that the WCN heterojunction did not improve the imine yield; however, the CO yield improved much compared to CH4. In addition, other nanocomposites ZnV2O6@WO3 and ZnV2O6@g-C3N4 were also investigated for their photocatalytic activity. ZnV2O6@WO3 resulted in 492 μmol g−1, 137 μmol g−1, and 27% yields of CO, CH4, and imine, respectively (entry 6). The ZnV2O6@g-C3N4 composite afforded 728 μmol g−1, 194 μmol g−1, and 9% yields of CO, CH4, and imine, respectively (entry 7). This indicates that the binary composites exhibited moderate photocatalytic activity for the redox reaction. Furthermore, the WCN nanocomposite was modified by loading ZnV2O6 as it has suitable CO2 reduction and oxidation potentials. ZWC nanocomposites with different ZnV2O6 concentrations were screened for this reaction. ZWC-05 with 5 wt% loading of ZnV2O6 on the WCN composite showed a 29% N-benzylidene benzylamine yield. It was also found that the yields of CO and CH4 were 218 and 741 μmol g−1 (entry 8). The ZWC-10 nanocomposite showed an enhanced yield of 35% of N-benzylidene benzylamine. The yields of CO and CH4 were 236 and 455 μmol g−1 (entry 9). Increasing ZnV2O6 on WCN, i.e., ZWC-20 nanocomposite, enhanced the products' yields. The highest yield for N-benzylidene benzylamine was 55% within 6 h of the photocatalytic reaction. Also, the yield of CO and CH4 was 1674 μmol g−1 and 683 μmol g−1, respectively (entry 10). This indicates that increasing the ZnV2O6 content on the WCN composite dramatically enhanced the yields of imine and CO2 reduction products. Higher concentrations of ZnV2O6, ZWC-30, and ZWC-40 nanocomposites did not show improved yields. Both ZWC-30 and ZWC-40 resulted in a 52% yield of N-benzylidene benzylamine (entry 11). However, the CO yield was slightly higher (754 μmol g−1) with ZWC-30 than with ZWC-40 (580 μmol g−1) but less than half that achieved with ZWC-20. Similarly, the CH4 yield was reduced from 513 to 433 μmol g−1 for ZWC-30 and ZWC-40 nanocomposites (entry 12). Thus, the ZWC-20 nanocomposite was selected as an optimum photocatalyst for simultaneous photoreduction of CO2 with benzylamine oxidation under visible light irradiation.
Further optimization of the reaction conditions was also carried out extensively. In this context, the effect of solvent plays a crucial role in the photocatalyst's activity. Thus, we selected five different solvents: water, tetrahydrofuran (THF), 1,4-dioxane, N,N-dimethylformamide (DMF), and acetonitrile, based on the previously reported literature for CO2 photoreduction and amine coupling (Fig. 5c). In water, no imine formation occurred, while CO and CH4 yields were 633 and 477 μmol g−1, respectively (Table 1, entry 13). While using THF as a solvent, the yield of N-benzylidene benzylamine was improved by up to 10%, but CO and CH4 yields were minimized to 102 and 93 μmol g−1, respectively, which is not a significant increase in yield (entry 14). In 1,4-dioxane, imine's yield was 38%, with 500 and 433 μmol g−1 yields of CO and CH4, respectively (entry 15). When using DMF, N-benzylidene benzylamine crossed 50% yield, which means that DMF would be a good solvent for this reaction (51% yield). However, the yields of CO and CH4 were not improved much, i.e., CO and CH4 obtained were 705 and 590 μmol g−1, respectively (entry 16). The highest yields were achieved with acetonitrile as the solvent. The N-benzylidene benzylamine yield was 55% in acetonitrile, while CO and CH4 yields were improved to 1674 and 683 μmol g−1. This indicates that benzylamine oxidation was effectively achieved in dioxane, DMF, and acetonitrile, while the CO yield was much higher in acetonitrile than in the other solvents. Furthermore, the reaction was prolonged to 24 h and achieved a maximum of 68% yield of N-benzylidene benzylamine with 2424 and 1598 μmol g−1 of CO and CH4, respectively (Fig. 5d). This concluded that prolonging the reaction did not provide significant results and a photocatalytic reaction time of 6 h is quite enough to provide significant activity.
In addition, we performed experiments with different substrate concentrations to investigate the optimum and effective concentration under identical conditions (Fig. S12†). We used 0.02 mmol and 0.05 mmol of benzylamine and found 61% and 59% yields of N-benzylidene benzylamine within six hours. In comparison, 0.1 mmol of benzylamine afforded a 55% yield of N-benzylidene benzylamine. However, a higher amine concentration (0.15 mmol) resulted in ∼54% yield. This indicates that there was no significant enhancement in the yield by varying the concentrations of benzylamine in the photocatalytic experiments. Thus, 0.1 mmol of benzylamine was a reasonable and acceptable concentration of the substrate for the desired redox photocatalytic reaction.
3.3 Scavenger and stability experiments
The effect of electron and hole scavengers could also be studied for the photoreduction of CO2 with benzylamine oxidation (Fig. 6a). We used silver nitrate and ammonium oxalate as electron and hole scavengers, respectively. When 0.025 mmol of silver nitrate was used, the yields of CO and CH4 were diminished to 468 and 214 μmol g−1, while the yield of N-benzylidene benzylamine was 54%. This indicates that silver nitrate quenched the electrons from benzylamine donors, which were readily available for the reduction of CO2; thus, the yields of CO and CH4 were diminished. Moreover, when 0.025 mmol of ammonium oxalate was employed, the holes were quenched, resulting in a very low 7% yield of N-benzylidene benzylamine; however, the yields of CO and CH4 were nearly similar under the optimized conditions, i.e., 1616 and 607 μmol g−1, respectively. Thus, the photocatalytic reduction of CO2 and oxidative coupling of amines could be quenched by silver nitrate and ammonium oxalate, consequently diminishing the corresponding oxidation and reduction products' yields.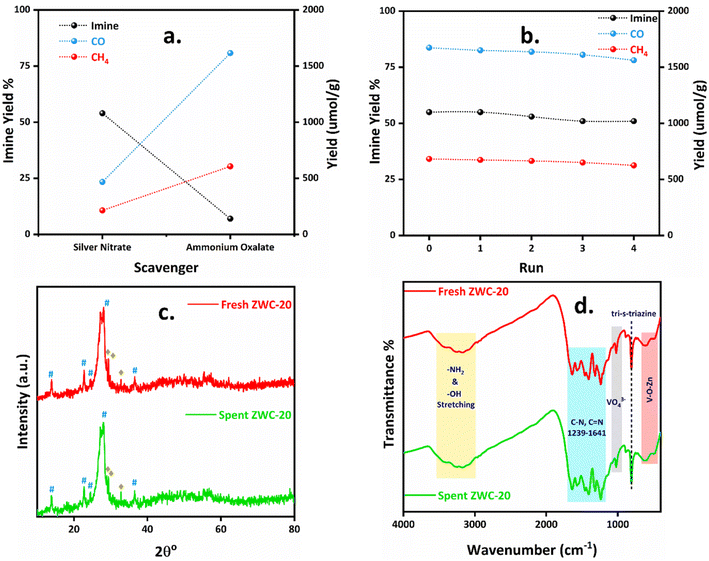 | ||
| Fig. 6 (a) Scavenger study, (b) recycling experiments, (c) XRD (# for WO3 and ♦ for ZnV2O6) and (d) FTIR spectra of fresh and spent ZWC-20 nanocomposites after four cycles. | ||
The applicability of the photocatalyst depends on its reusability and how it retains its photocatalyst activity. So, the reusability of the photocatalyst needs to be determined to check its durability. The photocatalytic reaction of CO2 photoreduction and amine oxidation was performed with the ZWC-20 photocatalyst. The photocatalyst was recovered by washing with ethanol, dried in an oven at 60 °C for 24 h, and reused for successive photocatalytic reactions. The recycling experiments were performed for four cycles and achieved similar CO, CH4, and N-benzylidene benzylamine yields (Fig. 6b). This indicates that the ZWC-20 photocatalyst exhibited good photocatalytic activity and was stable enough for the present reaction conditions.
Furthermore, the recovered photocatalyst was characterized using XRD and FTIR spectroscopy. After four cycles, the diffraction pattern of the spent ZWC-20 nanocomposite was similar to that of the fresh ZWC-20 nanocomposite (Fig. 6c). It showed the diffraction peaks of WO3, ZnV2O6, and g-C3N4 with a minor change in intensity. This indicates that the ZWC-20 nanocomposite remained intact after four cycles of the photocatalytic experiments. Also, FTIR spectra were recorded for the spent ZWC-20 nanocomposite (Fig. 6d). Similarly, there was no change in the pattern of tri-s-triazine, C–N, CN, and –NH2 groups' peaks of the spent ZWC-20 nanocomposite. In addition, the VO43− and V–O–Zn bindings were relatively similar to those of the fresh ZWC-20 nanocomposite. Thus, the analysis of the recovered ZWC-20 photocatalyst confirmed that the photocatalyst was stable enough for the photocatalytic reduction of CO2 with the oxidative coupling of amines.
In addition, the recovered ZWC-20 nanocomposite was analyzed using HR-XPS analysis (Fig. 7a and b, and S13†). The recovered ZWC-20 nanocomposite exhibited binding energy deviation of the elements C-1s, N-1s, O-1s, and Zn-2p (except W-4f and V-2p) compared to the fresh catalyst. This deviation in C-1s, N-1s, and O-1s might be due to the significant interaction between the substrate molecules and the nanocomposite's surface. Also, HR-TEM analysis found a similar morphology to the ZWC-20 nanocomposite (Fig. 7c–f), where ZnV2O6 had a nanorod-like morphology wrapped in g-C3N4 nanosheets. The SAED pattern reveals that the nanocomposite retained the high crystallinity after four cycles of consecutive runs of the recovered nanocomposite photocatalyst, demonstrating high stability under reaction conditions. In addition, the recovered photocatalyst's EDX pattern confirmed that there is no marginal leaching of Zn, V, and W metals compared to the fresh one, indicating high stability up to four cycles of photocatalytic experiments (Fig. S14†).
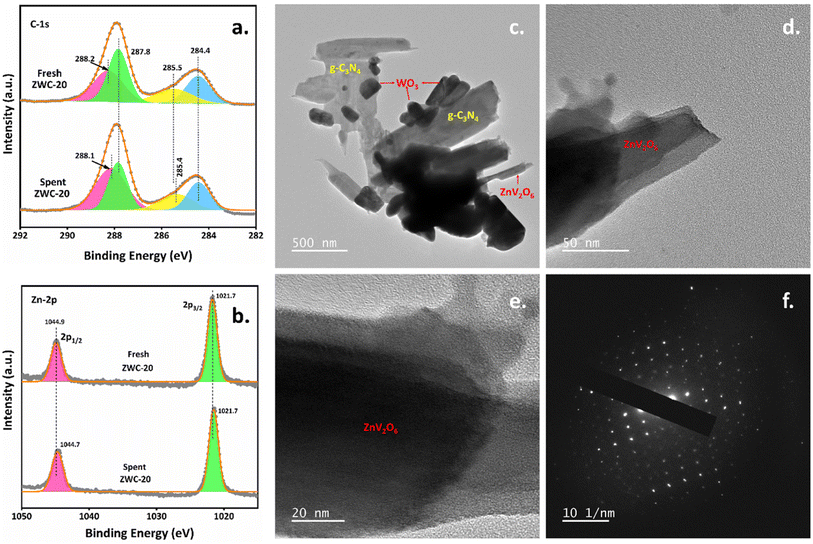 | ||
| Fig. 7 C-1s and Zn-2p XPS spectra, HR-TEM images, and SAED pattern of the spent ZWC-20 nanocomposite. | ||
3.4 Comparison with reported photocatalysts
The photoreduction of CO2 coupled with the oxidation of benzylamine is seldom reported. Compared to the previously reported literature, we found that the reported system, ZWC-20, achieved good conversion and yields of amines (55%) and C1 (1674 μmol g−1 of CO and 683 μmol g−1 of CH4) products, respectively, within a shorter reaction time (6 h). However, a prolonged reaction time afforded 68% yield of imine and 2424 and 1598 μmol g−1 of CO and CH4, respectively. This shows the supremacy of the developed ZWC-20 heterojunction photocatalyst (Table 2).3.5 Product and intermediate identification
The products of benzylamine oxidative coupling with CO2 photoreduction were confirmed using GC-MS and 1H and 13C NMR spectra. The GC-MS spectra of N-benzylidene benzylamine showed a molecular ion peak at an m/z value of 91, corresponding to (Fig. S15†). Also, the 1H NMR spectra of N-benzylidene benzylamine showed a de-shielded imine proton at an 8.34 ppm chemical shift (Fig. S16a†). In addition, the de-shielded imine carbon peak was found at 162 ppm in 13C NMR spectra, confirming the product formation (Fig. S16b†). The CO2 photoreduction products, i.e., CO and CH4, were identified using a calibrated GC-FID equipped with a capillary column. In addition, CO and CH4 were identified using GC-MS (Shimadzu, SH-Rt-Msieve 5A, 30 m, 0.32 mm, 30 μm, and temp. 30–250 °C), which showed fragmentation at the corresponding m/z values. Two m/z values, 16 and 28, were observed for CO, while CH4 exhibited a base peak as a molecular ion peak (m/z = 16) (Fig. S17†).
(Fig. S15†). Also, the 1H NMR spectra of N-benzylidene benzylamine showed a de-shielded imine proton at an 8.34 ppm chemical shift (Fig. S16a†). In addition, the de-shielded imine carbon peak was found at 162 ppm in 13C NMR spectra, confirming the product formation (Fig. S16b†). The CO2 photoreduction products, i.e., CO and CH4, were identified using a calibrated GC-FID equipped with a capillary column. In addition, CO and CH4 were identified using GC-MS (Shimadzu, SH-Rt-Msieve 5A, 30 m, 0.32 mm, 30 μm, and temp. 30–250 °C), which showed fragmentation at the corresponding m/z values. Two m/z values, 16 and 28, were observed for CO, while CH4 exhibited a base peak as a molecular ion peak (m/z = 16) (Fig. S17†).
Furthermore, several experiments were performed to understand the mechanistic route, and the intermediates were detected using GC-MS and NMR studies. Benzylamine has converted into N-benzylidene benzylamine via benzaldehyde intermediate formation, which GC-MS detected. Within 2 h of the reaction, the benzaldehyde intermediate was found at a retention time of 4.3 min in the total ion chromatogram (TIC) (Fig. S18†). The fragmentation pattern confirmed the benzaldehyde intermediate formation in the reaction system. This indicates the reaction pathway where a certain amount of benzylamine got oxidized to benzaldehyde. Then the remaining benzylamine is coupled with the benzaldehyde intermediate in a dehydrogenated manner to produce N-benzylidene benzylamine as the desired product. This was also observed in NMR studies where the aldehydic protons were found at a chemical shift of 9.85 ppm in 1H NMR, while the aldehydic carbon was detected at a 192 ppm chemical shift (Fig. S19a and b†). This confirmed the benzaldehyde intermediate formation during benzylamine coupling with the CO2 photoreduction reaction.
3.6 Isotopic labelling (13CO2)
To better understand the carbon source in the products CO and CH4, photocatalytic oxidative coupling using labelled CO2 (13CO2) was performed. In the isotope labelling experiment, we used labelled CO2 (13CO2) to find the source of C1 products. Under identical conditions in a 13CO2 atmosphere, we detected two peaks of 13CO and 13CH4 in the TIC and fragmentation patterns of 13CO and 13CH4 with m/z values of 29 and 17, respectively (Fig. S20a–c†). This confirms that CO and CH4 were produced from the photoreduction of CO2.3.7 Substrate scope
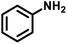
In order to prove the significance of this simultaneous redox reaction, various substituted benzylamines and other unsaturated amines at the β-position to the amine group were investigated (Table 3). Benzylamine as the model substrate resulted in a maximum of 55% yield with the highest CO (1674 μmol g−1) and CH4 (683 μmol g−1) yields (entry 1). Benzylamines having electron-donating groups at the para position resulted in relatively higher yields than electron-withdrawing groups. The yields of 4,4′-dimethyl benzylidene benzylamine and 4,4′-dimethoxy benzylidene benzylamine were 53% and 49%, but CO and CH4 achieved moderate yields (entries 2 & 3). 4,4′-Dichloro and 4,4′-difluoro benzylidene benzylamine achieved 49% and 48% yields. Furfuryl-derived amine was also investigated for this reaction under similar conditions. Furfuryl amine afforded a 51% yield of the coupled product. Also, we employed aniline as the substrate, where there was no imine formation and significantly less CO (107 μmol g−1) and CH4 (75 μmol g−1) yields.| Entry | Substrate | Imine | YieldImineb (%) | YieldCOc (μmol g−1) | YieldCH4c (μmol g−1) |
|---|---|---|---|---|---|
| a Reaction conditions: amine substrate: 0.1 mmol; catalyst: 10 mg; acetonitrile: 2 mL; reaction time: 6 h; light source: 350 W xenon lamp. b Yield determined by GC-MS. c Yield determined by GC-FID. | |||||
| 1 |
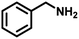
|
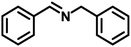
|
55 | 1674 | 683 |
| 2 |
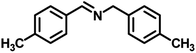
|
|
53 | 767 | 347 |
| 3 |
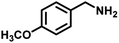
|
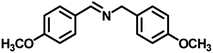
|
49 | 550 | 382 |
| 4 |
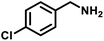
|
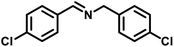
|
49 | 444 | 614 |
| 5 |
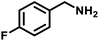
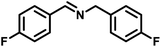
|
|
48 | 604 | 441 |
| 6 |
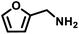
|
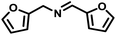
|
51 | 1531 | 594 |
| 7 |
|
— | 107 | 75 | |
3.8 Mechanism of the simultaneous photocatalytic redox reaction
As mentioned earlier, the band gaps of g-C3N4, WO3, and ZnV2O6 were 2.68, 2.53, and 2.09 eV, respectively. Based on the band edge potentials calculated above in Section 3.1, the energy band structure for g-C3N4, WO3, and ZnV2O6 is proposed (Scheme 3). The CB of g-C3N4 (−1.08 eV) and ZnV2O6 (−0.65 eV) calculated above was more negative than that of WO3 (0.57 eV). Also, the Efb of WO3 (0.54 V vs. NHE) is lower than that of g-C3N4 (−0.10 V vs. NHE) and ZnV2O6 (0.16 V vs. NHE), and M–S plots confirmed that these three semiconductors are n-type semiconductors. The Efb of g-C3N4 was higher than that of WO3; this tends to transfer electrons from g-C3N4 to the WO3 surface, making WO3 highly electronegative and g-C3N4 highly electropositive.63 Thus, at equilibrium, the induced electric field is directed from g-C3N4 to WO3. When the photocatalytic system is irradiated with visible light, photogenerated electrons and holes are produced, and the electrons get excited to the CB. In contrast, the photogenerated holes are left in the VB of the semiconductors. The CB (0.54 V vs. NHE) of WO3 is positive in energy, which does not favour the reduction of CO2 to any C1 products (CO2/C1 ≥ −0.24 eV, for CH4). Also, when the photogenerated electrons from the CB of g-C3N4 transferred to the CB of WO3, it could not result in any C1 product. However, the highly positive VB (2.56 eV) of WO3 favours the oxidation of amines under visible light irradiation. In addition, from CV, WO3 showed strong oxidizing properties, which shows that the photogenerated holes in the VB of WO3 are entirely involved in the oxidation process. Thus, the possibility of the photoreduction of CO2 over WO3 is ruled out.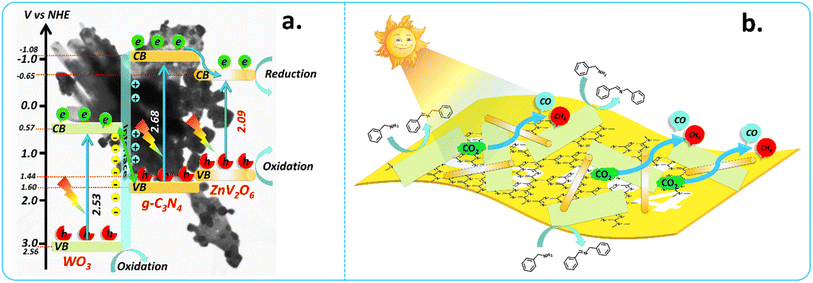 | ||
| Scheme 3 (a) Band energy diagram depicting the CB and VB in the ternary heterojunction and (b) representation of photocatalytic CO2 reduction along with amine oxidation. | ||
In another way, the photogenerated electrons in the CB of WO3 tend to migrate to the VB of g-C3N4, where they recombine with the photogenerated holes and thus result in photogenerated holes in the VB of WO3 with strong oxidation ability, suitable for the oxidative coupling of amines into the corresponding imines. The excited photogenerated electrons in the CB of g-C3N4 are readily available for reduction and might show high activity in visible light. In PL spectra, it was also confirmed that the WCN heterojunction had a high separation of charge carriers and favours better charge separation than the individual semiconductor components. In addition, WO3 and g-C3N4 were n-type semiconductors; thus, this photocatalytic system showed electron transfer in a step manner and favoured the S-scheme mechanism.64 The readily available photogenerated electrons in the CB of g-C3N4 transferred to the lower energy CB of ZnV2O6, where the available excess of electrons reduced the adsorbed CO2 into CO and CH4 using benzylamine as a sacrificial donor and hole scavenger.28 Here, benzylamine (sacrificial donor and proton source) boosted the yields of CO and CH4 from the photoreduction of CO2. Thus, the substrate molecules utilized simultaneous oxidation and reduction (redox) reactions more sustainably, making the entire redox photocatalytic process atom economical. This detailed mechanistic study using various techniques on this heterojunction system provides more understanding of the advantage of individual components and as a hetero-composite for applying this system in various other challenging redox reactions to be carried out, e.g., for the biomass component conversion strategy.
4 Conclusion
The present work demonstrated the use of a cost-effective and readily available carbon waste CO2 to oxidize amines to the corresponding imines with simultaneous reduction of CO2 to value-added C1 products under simulated solar light irradiation. A ternary ZnV2O6@WO3–g–C3N4 nanocomposite heterojunction had an excellent ability for the photoreduction of CO2 and oxidative coupling of amines into CO and CH4 and imines, respectively. The present photocatalytic system provided a good yield of CO (1674 μmol g−1) and CH4 (683 μmol g−1), along with a 55% yield of N-benzylidene benzylamine within six hours of light irradiation. In addition, the recovered photocatalyst exhibited reusability without significant change in the activity up to four consecutive runs of the photocatalytic reaction. The present methodology developed a one-pot system with added benefits of atom economy via simultaneous oxidation and reduction reactions.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are thankful to the Institute of Nano Science and Technology (INST), Mohali, for providing instrumental facilities to carry out the scientific work.References
- C. Higman and S. Tam, Chem. Rev., 2014, 114, 1673–1708 CrossRef CAS PubMed.
- S. I. Murahashi, Angew. Chem., Int. Ed. Engl., 1995, 34, 2443–2465 Search PubMed.
- T. Mukaiyama, A. Kawana, Y. Fukuda and J.-i. Matsuo, Chem. Lett., 2001, 30, 390–391 Search PubMed.
- K. Nicolaou, C. J. Mathison and T. Montagnon, J. Am. Chem. Soc., 2004, 126, 5192–5201 CrossRef CAS PubMed.
- X. Li, Q. Su, Z. Liu, K. Luo, G. Li and Q. Wu, Chem. Res. Chin. Univ., 2020, 36, 1017–1023 CrossRef CAS.
- J. Yang and C.-Y. Mou, Appl. Catal. B, 2018, 231, 283–291 CrossRef CAS.
- A. Khampuanbut, S. Santalelat, A. Pankiew, D. Channei, S. Pornsuwan, K. Faungnawakij, S. Phanichphant and B. Inceesungvorn, J. Colloid Interface Sci., 2020, 560, 213–224 Search PubMed.
- C. Xu, H. Liu, D. Li, J.-H. Su and H.-L. Jiang, Chem. Sci., 2018, 9, 3152–3158 RSC.
- V. R. Battula, H. Singh, S. Kumar, I. Bala, S. K. Pal and K. Kailasam, ACS Catal., 2018, 8, 6751–6759 CrossRef CAS.
- S. I. Seneviratne, M. G. Donat, A. J. Pitman, R. Knutti and R. L. Wilby, Nature, 2016, 529, 477–483 CrossRef CAS PubMed.
- G. Chen, G. I. Waterhouse, R. Shi, J. Zhao, Z. Li, L. Z. Wu, C. H. Tung and T. Zhang, Angew. Chem., Int. Ed., 2019, 58, 17528–17551 CrossRef CAS PubMed.
- P. K. Prajapati, A. Kumar and S. L. Jain, ACS Sustainable Chem. Eng., 2018, 6, 7799–7809 CrossRef CAS.
- S. Saini, H. Singh, P. K. Prajapati, A. K. Sinha and S. L. Jain, ACS Sustainable Chem. Eng., 2019, 7, 11313–11322 CrossRef CAS.
- M.-Y. Qi, Q. Lin, Z.-R. Tang and Y.-J. Xu, Appl. Catal. B, 2022, 307, 121158 Search PubMed.
- P. K. Prajapati, S. Saini, N. Nandal and S. L. Jain, J. CO2 Util., 2021, 45, 101402 CrossRef CAS.
- C. Luo, T. Yang, Q. Huang, X. Liu, H. Ling, Y. Zhu, G. Xia, W. Zou and H. Wang, Nanomaterials, 2020, 10, 1303 Search PubMed.
- Y. Markushyna, P. Lamagni, J. Catalano, N. Lock, G. Zhang, M. Antonietti and A. Savateev, ACS Catal., 2020, 10, 7336–7342 CrossRef CAS.
- F. Wang, T. Hou, X. Zhao, W. Yao, R. Fang, K. Shen and Y. Li, Adv. Mater., 2021, 33, 2102690 Search PubMed.
- G. Wang, T. Zhang, W. Yu, Z. Sun, X. Nie, R. Si, Y. Liu and Z. Zhao, CCS Chem., 2022, 4, 2793–2805 Search PubMed.
- Q. Xu, Z. Xia, J. Zhang, Z. Wei, Q. Guo, H. Jin, H. Tang, S. Li, X. Pan and Z. Su, Carbon Energy, 2022, 1–43 Search PubMed.
- H. Shi, G. Chen, C. Zhang and Z. Zou, ACS Catal., 2014, 4, 3637–3643 CrossRef CAS.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2017, 29, 1605148 Search PubMed.
- K. Chu, Q.-q. Li, Y.-p. Liu, J. Wang and Y.-h. Cheng, Appl. Catal. B, 2020, 267, 118693 CrossRef CAS.
- T. Ohno, N. Murakami, T. Koyanagi and Y. Yang, J. CO2 Util., 2014, 6, 17–25 Search PubMed.
- L.-f. Hong, R.-t. Guo, Y. Yuan, X.-y. Ji, Z.-d. Lin, J.-w. Gu and W.-g. Pan, Energy Fuels, 2021, 35, 11468–11478 CrossRef CAS.
- L. Wang, X. Shi, Y. Jia, H. Cheng, L. Wang and Q. Wang, Chin. Chem. Lett., 2021, 32, 1869–1878 CrossRef CAS.
- M. Du, Y. Chen, W. Wang, X. Xu, Y. Li, Y. Zhang, Z. Li and Z. Zou, Appl. Catal. B, 2022, 317, 121722 CrossRef CAS.
- A. Bafaqeer, M. Tahir and N. A. S. Amin, Appl. Catal. B, 2019, 242, 312–326 CrossRef CAS.
- A. Bafaqeer, M. Tahir, A. Ali Khan and N. A. Saidina Amin, Ind. Eng. Chem. Res., 2019, 58, 8612–8624 Search PubMed.
- A. Kumar, P. K. Prajapati, M. Aathira, A. Bansiwal, R. Boukherroub and S. L. Jain, J. Colloid Interface Sci., 2019, 543, 201–213 CrossRef CAS PubMed.
- P. Wang, S. M. Zakeeruddin, P. Comte, R. Charvet, R. Humphry-Baker and M. Grätzel, J. Phys. Chem. B, 2003, 107, 14336–14341 CrossRef CAS.
- L. Shi, L. Liang, F. Wang, M. Liu, K. Chen, K. Sun, N. Zhang and J. Sun, ACS Sustainable Chem. Eng., 2015, 3, 3412–3419 CrossRef CAS.
- S. Wei, J. Zhao, B. Hu, K. Wu, W. Du and M. Zhou, Ceram. Int., 2017, 43, 2579–2585 CrossRef CAS.
- Y. Bao and K. Chen, Nano-Micro Lett., 2016, 8, 182–192 Search PubMed.
- B. Paul, B. Bhuyan, D. D. Purkayastha and S. S. Dhar, Catal. Commun., 2015, 69, 48–54 CrossRef CAS.
- A. Malik, P. K. Prajapati, B. M. Abraham, S. Bhatt, P. Basyach and S. L. Jain, Catal. Sci. Technol., 2022, 12, 2688–2702 RSC.
- S. Le, T. Jiang, Q. Zhao, X. Liu, Y. Li, B. Fang and M. Gong, RSC Adv., 2016, 6, 38811–38819 Search PubMed.
- F. Kröger and H. Vink, in Solid State Physics, Elsevier, 1956, vol. 3, pp. 307–435 Search PubMed.
- S. Hong, S. J. Burkhow, R. M. Doughty, Y. Cheng, B. J. Ryan, A. Mantravadi, L. T. Roling, M. G. Panthani, F. E. Osterloh and E. A. Smith, Chem. Mater., 2021, 33, 1667–1682 CrossRef CAS.
- Y. Yu, P. Zhang, Y. Kuang, Y. Ding, J. Yao, J. Xu and Y. Cao, J. Phys. Chem. C, 2014, 118, 20982–20988 CrossRef CAS.
- T. Tachikawa, T. Ochi and Y. Kobori, ACS Catal., 2016, 6, 2250–2256 CrossRef CAS.
- L. Wang, Y. Dong, J. Zhang, F. Tao and J. Xu, J. Solid State Chem., 2022, 308, 122878 CrossRef CAS.
- J. Hao, B. Qi, J. Wei, D. Li and F. Zeng, Fuel, 2021, 287, 119439 CrossRef CAS.
- M. Aslam, I. M. Ismail, T. Almeelbi, N. Salah, S. Chandrasekaran and A. Hameed, Chemosphere, 2014, 117, 115–123 CrossRef CAS PubMed.
- M. Alenizi, R. Kumar, M. Aslam, F. Alseroury and M. Barakat, Sci. Rep., 2019, 9, 1–8 CrossRef CAS PubMed.
- X. Han, D. Xu, L. An, C. Hou, Y. Li, Q. Zhang and H. Wang, Int. J. Hydrogen Energy, 2018, 43, 4845–4855 CrossRef CAS.
- L. Lin and S. Zhang, Chem. Commun., 2012, 48, 10177–10179 RSC.
- G. Rajender, B. Choudhury and P. Giri, Nanotechnology, 2017, 28, 395703 CrossRef PubMed.
- C. Rajkumar, P. Veerakumar, S.-M. Chen, B. Thirumalraj and K.-C. Lin, ACS Sustainable Chem. Eng., 2018, 6, 16021–16031 Search PubMed.
- S. Siwal, N. Devi, V. K. Perla, S. K. Ghosh and K. Mallick, Catal. Struct. React., 2019, 5, 1–9 CAS.
- Y. Yin, C. Lan, H. Guo and C. Li, ACS Appl. Mater. Interfaces, 2016, 8, 3861–3867 CrossRef CAS PubMed.
- W. Li, Q. Chen and Q. Zhong, J. Mater. Sci., 2020, 55, 10712–10724 Search PubMed.
- S. C. Rasmussen, R. L. Schwiderski and M. E. Mulholland, Chem. Commun., 2011, 47, 11394–11410 RSC.
- C. Ji, S.-N. Yin, S. Sun and S. Yang, Appl. Surf. Sci., 2018, 434, 1224–1231 CrossRef CAS.
- S. E. Arasi, P. Devendran, R. Ranjithkumar, S. Arunpandiyan and A. Arivarasan, Mater. Sci. Semicond. Process., 2020, 106, 104785 CrossRef.
- M. M. Sajid, N. A. Shad, S. B. Khan, Z. Zhang and N. Amin, J. Alloys Compd., 2019, 775, 281–289 Search PubMed.
- S. Tonda, S. Kumar, S. Kandula and V. Shanker, J. Mater. Chem. A, 2014, 2, 6772–6780 Search PubMed.
- W.-J. Ong, L.-L. Tan, S.-P. Chai and S.-T. Yong, Dalton Trans., 2015, 44, 1249–1257 Search PubMed.
- D. Vernardou, H. Drosos, E. Spanakis, E. Koudoumas, C. Savvakis and N. Katsarakis, J. Mater. Chem., 2011, 21, 513–517 Search PubMed.
- A. Bafaqeer, M. Tahir and N. A. S. Amin, Appl. Surf. Sci., 2018, 435, 953–962 Search PubMed.
- T. Yang, Q. Yu and H. Wang, Catal. Lett., 2018, 148, 2382–2390 CrossRef CAS.
- P. K. Prajapati, D. Tripathi, M. K. Poddar, P. Gupta and S. L. Jain, Sustainable Energy Fuels, 2022, 6, 2996–3007 RSC.
- M. Sun, Y. Zhou, T. Yu and J. Wang, Int. J. Hydrogen Energy, 2022, 47, 10261–10276 Search PubMed.
- Q. Xu, L. Zhang, B. Cheng, J. Fan and J. Yu, Chem, 2020, 6, 1543–1559 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta07642j |
| This journal is © The Royal Society of Chemistry 2023 |