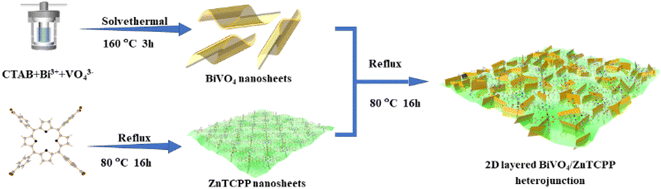
Construction of a 2D layered BiVO4/zinc porphyrin (ZnTCPP) S-scheme heterostructure boosting photocatalytic N2 oxidation performance†
Desen
Zhou
a,
Shuai
Shao
a,
Xuan
Zhang
a,
Tingmin
Di
b,
Jun
Zhang
 *a,
Tielin
Wang
*a and
Cunwen
Wang
a
*a,
Tielin
Wang
*a and
Cunwen
Wang
a
aKey Laboratory of Green Chemical Process of Ministry of Education, Key Laboratory of Novel Reactor and Green Chemical Technology of Hubei Province, School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430205, P. R. China. E-mail: junzhang@wit.edu.cn; witwangtl@hotmail.com
bHubei Key Laboratory of Plasma Chemistry and Advanced Materials, School of Materials Science and Engineering, Wuhan Institute of Technology, Wuhan 430205, P. R. China
First published on 29th November 2022
Abstract
Nitric acid is considered to be an important chemical raw material; however, the traditional production of nitric acid is an energy-intensive process. Photocatalysis provides an ideal way to oxidize nitrogen to nitrate through a green process, but still faces the limitations of low visible light utilization and rapid carrier compounding. Herein, a unique S-scheme heterojunction of a 2D/2D BiVO4/metalloporphyrin (ZnTCPP) layered composite with high visible-light photocatalytic N2 oxidation activity is reported. The nitrate yield of the optimal sample (BZ-20) reaches 4.72 mg g−1 h−1 and the apparent quantum efficiency reaches 0.80% at 420 nm, which represents the highest photocatalytic activity for nitrate production so far. Experimental and density functional theory calculation results indicate that a unique S-scheme charge transfer path can be formed at the BiVO4/ZnTCPP interfaces to ensure superior separation and redox ability of photogenerated electrons and holes, resulting in high photocatalytic performance. This work provides a new insight into metalloporphyrin-based photocatalysts for efficient N2 photofixation driven by the S-scheme charge transfer route.
Introduction
The consumption of fossil fuels has caused a range of serious environmental problems, from water and air pollution to global climate change.1 Thus, there is an urgent need to develop green and effective approaches for energy conversion and environmental protection. Among various renewable energy methods, solar light induced photocatalytic reactions offer a promising way for environmental remediation and renewable energy production.2 Until now, numerous catalytic reactions have been carried out in the presence of an appropriate photocatalyst and light irradiation3 such as organic pollutant degradation,4 water splitting to produce H2 and O2,5 CO2 reduction to hydrocarbon fuels,6 virus inactivation7 and selective organic synthesis reactions.8 Recently, photocatalytic nitrogen fixation has received lots of attention due to its mild reaction conditions, environmental friendliness and utilization of solar energy.9,10 The common products of photocatalytic N2 fixation are NH3 or NH4+ obtained through the N2 reduction reaction (NRR)11–13 and NO or NO3− obtained through the N2 oxidation reaction (NOR).14–16 Ammonia and nitrates are important raw materials in modern industry, and compared with the traditional Haber-Bosch and Ostwald processes, the photocatalytic N2 fixation shows great promise for sustainable environmental nitrogen fixation.17To date, numerous semiconductor photocatalysts such as g-C3N4, TiO2, CdS, BiVO4, Fe2O3, etc.,18–22 have been developed to boost the efficiency of photocatalytic N2 fixation. However, these traditional semiconductor photocatalysts often have a limited photoresponse range and poor energy band regulation, which restricts the effective utilization of solar light. Consequently, photocatalytic heterostructures that can respond to UV, visible light and full solar spectrum have become an important and challenging strategy for high solar-light induced photocatalytic performance.23,24 Among the strategies, modification with various light-harvesting units, especially conjugated organic macrocycles (porphyrins and phthalocyanines), has attracted considerable attention.25–27 In natural light harvesting systems, porphyrin derivatives have strong light absorption ability, the Soret absorption band is at 400–500 nm and the Q absorption band is at 500–700 nm.28 They are also the major components of the energy conversion pathway.29 In addition, by adjusting the surrounding ligands and mental coordination centers, the band gap structures and optoelectronic properties of the corresponding porphyrin derivatives can be regulated. Thus, porphyrins have been often coupled with other semiconductors to improve the light absorption ability, selectivity or activity in many photocatalytic reactions including water splitting, CO2 photoreduction, organic pollutant degradation, dye sensitized solar cells and so on.30–33 For example, Fe tetra(4-carboxyphenyl) porphyrin (TCPP) was coupled with g-C3N4, and the obtained g-C3N4/FeTCPP heterogeneous catalysts exhibit high activity and selectivity for CO2 reduction under visible-light irradiation.34 Ma et al. loaded porphyrin-based polymers on a hollow TiO2 surface by hyper-crosslinking and subsequently coordinated with Pd(II), and the prepared composites exhibited higher CO2 adsorption capability than O2 and high photocatalytic activity for CO2 reduction in an aerobic environment.35 Mourzina et al. fabricated a series of Sn porphyrin derivatives coupled with TiO2, and the Sn(IV) meso-tetra(4-pyridyl) porphyrin dichloride (SnTPyP)/TiO2/Pt composites exhibited enhanced photocatalytic hydrogen evolution activity under visible-light irradiation.36
Although porphyrin-based photocatalysts have already been used for solar energy conversion in many reactions, the utilization of porphyrin-based heterostructures for photocatalytic N2 fixation has been rarely studied. Herein, we take advantage of a 2D–2D layered composite and the wide light-absorption capability of porphyrins by combining ultrathin BiVO4 nanosheets with metalloporphyrin (Zn tetra(4-carboxyphenyl) porphyrin ZnTCPP) nanosheets. Due to the suitable work functions and band structures, a unique S-scheme charge transfer path at BiVO4/ZnTCPP interfaces can be formed under light irradiation. Consequently, photogenerated charge carriers can be efficiently separated driven by the internal electric field (IEF), and the band edges bend at the interface and the S-scheme heterostructure, resulting in high photocatalytic N2 oxidation activity.
Results and discussion
The synthesis process of the 2D/2D BiVO4/ZnTCPP composite is schematically illustrated in Fig. 1 (see more details in the ESI†). The nominal weight contents of ZnTCPP in the composite materials are 0%, 10%, 20%, and 50%, respectively, and the corresponding samples are marked as BVO, BZ-10, BZ-20 and BZ-50. The crystal structures of the prepared samples were investigated by XRD, and the characterization results are shown in Fig. S1.† It can be seen that the diffraction peaks of pure BiVO4 correspond well to the orthorhombic phase BiVO4 (JCPDS No. 73-0474). As shown in Fig. S1,† pure ZnTCPP exhibits several peaks at about 5.5°, 7.6°, 10.7° and 21.5°, which can be assigned to (100), (110) (002) and (004) planes, respectively.25,37 The BiVO4/ZnTCPP composite samples show the apparent diffraction peaks of orthorhombic BiVO4 and some small peaks of ZnTCPP can be also observed, indicating that ZnTCPP has been successfully grafted with BiVO4 nanosheets. The FT-IR spectra of pure BiVO4, ZnTCPP and BZ-20 samples are shown in Fig. S2.† For pure BiVO4, the vibration peaks near 658 cm−1 can be attributed to Bi–O bending and the peaks at 740 and 875 cm−1 can be assigned to the V–O deformation bending vibration and symmetrical stretching of BiVO4.38,39 For pure ZnTCPP, a strong CO bond vibration around 1710 cm−1, a COO− band around 1415 cm−1 and an O–H bond stretching vibration band at 1240 cm−1 can be observed.40 In addition, the Zn–N bond in the metalloporphyrin can be found around 995 cm−1, and the N–H bond of the pyrrole ring is located around 964 cm−1.41 As expected, the characteristic peaks of BiVO4 and ZnTCPP can be both observed over the sample BZ-20, indicating the formation of hybrid heterostructures. In addition, the characteristic peaks at 1415 cm−1 and 1240 cm−1 were significantly weakened, which may be due to the interaction between the carboxyl and hydroxyl groups on the surface of ZnTCPP and the hydroxyl groups on the surface of BiVO4. Such strong interaction could facilitate the transfer and separation of photogenerated carriers between the two phases.Fig. 2a–f present the SEM and TEM images of the prepared samples. The prepared ZnTCPP exhibits a flake-like 2D morphology with a size of several micrometers, which is larger than that of BiVO4 nanosheets. Through AFM tests, the thicknesses of BiVO4 and ZnTCPP nanosheets are measured to be about 1.52 and 1.18 nm, respectively (Fig. S3†). The BZ-20 composite shows an obvious lamellar stacking morphology, which is caused by mutual stacking and accumulation between BiVO4 and ZnTCPP nanosheets. The TEM images further reveal the formation of a layered stacking structure of the BZ-20 sample. As shown in Fig. 2f, the image reveals a lattice spacing of 0.65 nm, which can be assigned to the (004) plane of the Zn-TCPP crystal, since it is consistent with the value of 0.64 nm based on the X-ray structure model (Fig. S4†). Such a close contact and contact interface between ZnTCPP and BiVO4 could provide a basis for the realization of effective interfacial charge transfer between the two components. The elemental mapping results shown in Fig. 2g illustrate the distinct distribution of Bi, Zn, V, O, C and N elements, which further confirms the binary nature of BiVO4 and ZnTCPP in the BZ-20 sample.
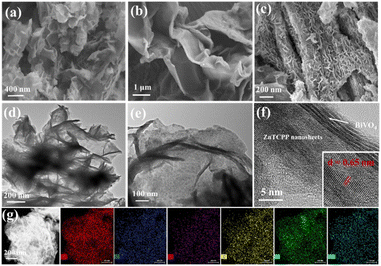 | ||
| Fig. 2 (a–c) SEM images of BiVO4, ZnTCPP and BZ-20, respectively, (d–f) TEM and HRTEM images of BZ-20, and (g) EDX mappings of the BZ-20 sample. | ||
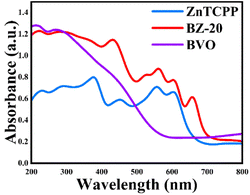
The optical absorption abilities of the as-prepared samples were evaluated by UV-vis diffuse reflectance spectroscopy. As shown in Fig. 3, the BVO sample shows obvious absorption at 200–550 nm, but the absorption gradually decreases to zero at 550–800 nm. Pure ZnTCPP exhibits obvious strong absorption peaks between 200 and 800 nm due to the characteristic absorption of porphyrin compounds in the ultraviolet-visible light region. The strong peak near 400–500 nm is the Soret band, and the several absorption peaks in the range of 500–700 nm can be attributed to the Q bands.42 The band gap values (Eg) of the prepared samples are estimated by using the Kubelka–Munk43 equation shown in Fig. S5,† and the Eg values of BVO and ZnTCPP are calculated to be 2.21 and 1.90 eV, respectively. Compared with BVO and ZnTCPP, the composite sample shows a significant enhancement of absorption in the entire region, and thus can be greatly beneficial to the subsequent photocatalytic reaction.
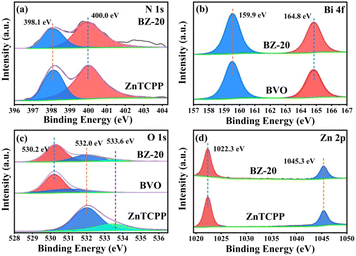
Fig. 4a–d show the XPS spectra of N 1s, Bi 4f, O 1s and Zn 2p of different samples. For N 1s, the strong peaks of ZnTCPP and BZ-20 located near 398.1 and 400.0 eV can be attributed to pyridinic N (CN–C) and tertiary N (N–C3), respectively.44 The small signals at about 402.9 eV in BZ-20 can be assigned to the oxidized N-species with N–O bonds. For Bi 4f, the binding energies near 159.9 and 164.8 eV can be attributed to the Bi 4f7/2 and Bi 4f5/2 of Bi3+ in BiVO4.45 For O 1s, the BZ-20 sample exhibits the peaks for the Bi–O–Bi band (530.2 eV) and the C–O and CO bonds in the peripheral carboxyl groups of ZnTCPP (532.0 and 533.6 eV). In addition, the two strong peaks of Zn 2p are at 1022.3 and 1045.3 eV, respectively, corresponding to the Zn 2p3/2 and Zn 2p1/2 of Zn2+. Fig. S6 and Table S1† show the BET surface areas, pore volumes and pore diameters of the different samples. Due to the high surface area of ZnTCPP, the BZ-20 composite sample exhibits an enhanced specific surface area, pore volume and pore diameter, which could be beneficial to photocatalytic reactions.
Fig. 5a shows the surface photovoltage (SPV) of ZnTCPP, BVO and BZ-20 samples. The pristine BiVO4 sample shows a clear response in the range from 350 to 450 nm corresponding to the bandgap of BiVO4.16 Due to the wide absorption ability, the ZnTCPP and BZ-20 composite samples exhibit two responses in the range of 350–450 nm and 500–600 nm, and such results are consistent with the UV-vis diffuse reflectance spectra. In addition, the surface photovoltage intensity of BZ-20 is much higher, indicating the increased photogenerated charges migrated to the surface.46 Moreover, time-resolved photoluminescence (TR-PL) spectra were recorded to further investigate the lifetime and decay dynamics of the photogenerated electrons. As shown in Fig. 5b and Table S2,† the average PL lifetime (τave) of the BZ-20 composite sample (2.79 ns) is shorter than that of the pure BiVO4 sample (4.91 ns), which indicates the high separation and migration abilities of photogenerated electrons and holes.47
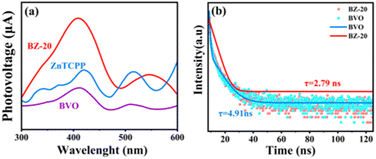 | ||
| Fig. 5 (a) Surface photovoltage spectra and (b) time-resolved fluorescence decay curves of different samples. | ||
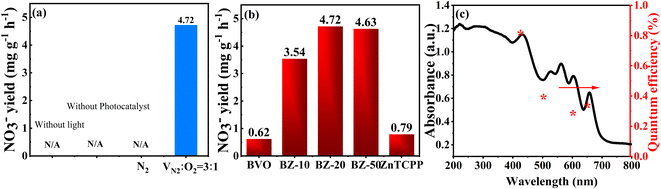
The control experiments shown in Fig. 6a indicated that NO3− ions can only be detected in the presence of a photocatalyst and light irradiation. No nitrate was generated when using Ar as the feed gas, which suggested that the obtained NO3− originated from N2 gas through the photocatalytic reaction under the coaction of the photocatalyst and light irradiation. In addition, an isotope tracer experiment was also carried out by using 15N2 as the purge gas to further confirm the source of the N element. After the photocatalytic reaction under the same reaction conditions, the product was analyzed by using a Stable Isotope Quantifier (Thermo Delta V advantage). For the BZ-20 sample, the 15N abundance in NO3− was about 79.5%. Moreover, the testing concertation of 15N element could be obtained from δ15N as follows:14
| δ15N = Csample15N/Cstandard15N | (1) |
The δ15N value of the BZ-20 sample was 1.124 according to the above formula, indicating that the product contained heavier isotopes than the standard sample, which confirmed that the N element in the product NO3− comes from the 15N2 source. The photocatalytic NOR activities of the different samples under visible-light irradiation are presented in Fig. 6b. It can be seen that pristine BiVO4 and ZnTCPP show very low NO3− production rates of 0.62 and 0.79 mg h−1 g−1, respectively. Interestingly, all the composite samples exhibit an enhanced activity. The highest NO3− production activity was achieved over the BZ-20 sample (4.72 mg h−1 g−1), which was 7.6 and 6.0 times higher than those of pure BiVO4 and ZnTCPP, respectively. As depicted in Fig. 6c, the apparent QE of the BZ-20 sample was calculated to be 0.80% at 420 nm, which represents the highest photocatalytic activity for nitrate production so far (Table 1). Moreover, the apparent QE of BZ-20 could even reach 0.31% at 650 nm, suggesting that the composite sample exhibits effective wide absorption ability. Moreover, the NO3− production rate of the BZ-20 sample can still retain about 85% after four reaction cycles (Fig. S7†), implying good stability.
| Photocatalyst | NO3− yield activity | Apparent quantum efficiency | Light source | Ref. | |
|---|---|---|---|---|---|
| Pd/H–TiO2 | 4.58 μmol g−1 h−1 | 0.99% (350 nm) | Thermal-assisted photocatalysis (200 °C) | 48 | |
| Pothole-rich WO3 | 1.92 mg g−1 h−1 | 0.11% (380 nm) | 300 W xenon lamp | 14 | |
| PF–g–C3N4 | 109.96 μmol L−1 h−1 g−1 | — | 300 W xenon lamp | 49 | |
| WO3/CdS | 14.2 μmol L−1 h−1 g−1 | — | 300 W xenon lamp | 50 | |
| W18O49 nanowires | 0.54 mol g−1 h−1 | — | AM 1.5G, 100 mW cm−2 | 51 | |
| BiVO4/rGO | 1.45 mg g−1 h−1 | 0.64% (420 nm) | 300 W xenon lamp (>420 nm) | 16 | |
| P25(TiO2)-carbon paper | 3.51 mg m−2 h−1 | — | Mercury lamp with UV light | 52 | |
| BiVO4/ZnTCPP | 4.72 mg g−1 h−1 | 0.80% (420 nm) | 300 W xenon lamp (>420 nm) | This work | |
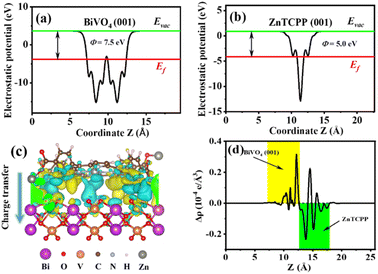
In order to investigate the enhanced mechanism for the NOR process over the BiVO4/ZnTCPP composite, density functional theory calculations were carried out. Firstly, the work function (ϕ) values of BiVO4 (001) and ZnTCPP (001) have been estimated, which can be used to study the electron transfer in the heterostructures.53 As shown in Fig. 7a and b, the work functions of BiVO4(001) and ZnTCPP(001) are 7.5 and 5.0 eV, respectively. Thus, BiVO4 exhibits a lower Fermi level than ZnTCPP, resulting in electron transfer from ZnTCPP to BiVO4 to reach an equal Fermi level. In addition, the charge density difference of the BiVO4/ZnTCPP interface is provided in Fig. 7c and d to visualize this process. It can be observed that the charge redistribution is mainly concentrated near the contact interface of BiVO4 and ZnTCPP, and the electrons mainly transfer from ZnTCPP to BiVO4 through the interface. A charge transfer of 0.02 e can be quantified by Bader charge analysis. As a consequence, the net charge accumulation led to the creation of an internal electrical field (IEF) at the BiVO4/ZnTCPP interface, and the direction of the electric field is from ZnTCPP to BiVO4, which facilitates the separation of photogenerated charge carriers in the photocatalytic reactions (as shown in Fig. S8†).54
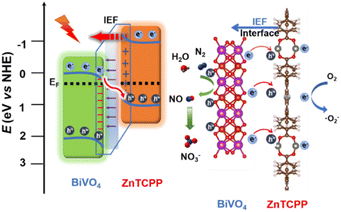
From the above analysis, the proposed photocatalytic mechanism of the BiVO4/ZnTCPP heterojunction is shown in Fig. 8 according to the band structures of BiVO4 and ZnTCPP.55,56 Due to the lower Fermi level of BiVO4, when the close contact has been formed between the two phases, the electrons will transfer from ZnTCPP to BiVO4 thermodynamically. As a result, an IEF pointing from ZnTCPP to BiVO4 is created, and the energy bands of ZnTCPP and BiVO4 bend upwards and downwards, respectively. Under irradiation, the valence band (VB) electrons of both ZnTCPP and BiVO4 could be excited to the conduction bands (CBs). Benefitting from the suitable staggered band structures, the interfacial IEF and bent bands, the photogenerated electrons in the CB of BiVO4 could spontaneously recombine with the holes in the VB of ZnTCPP, which exhibits a clear S-scheme charge transfer process in the ZnTCPP/BiVO4 heterojunctions. Besides, as displayed in Fig. 9a, the BZ-20 heterojunction photocatalyst exhibits the highest photocurrent density, implying the most efficient separation and transfer of the photogenerated electrons and holes. Meanwhile, as shown in Fig. 9b, the arc radius of the EIS spectrum of the BiVO4/ZnTCPP composite is remarkably smaller than that of pure ZnTCPP and BiVO4, suggesting that the formation of an S-scheme heterojunction between BiVO4 and ZnTCPP is beneficial to improve charge separation efficiency and photosensitivity.47
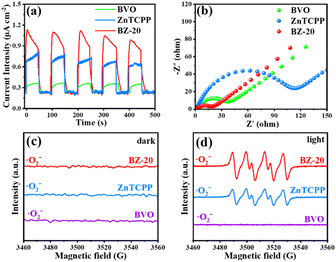 | ||
| Fig. 9 (a) Photocurrent density curves and (b) EIS spectra of BVO, ZnTCPP and BZ-20. (c) and (d) EPR spectra of ·O2− radicals generated over BVO, ZnTCPP and BZ-20 under dark and light conditions. | ||
In order to further understand the electron transfer in the composite, electron paramagnetic resonance (EPR) measurement was carried out using 5,5-dimethyl-pyrrolidine N-oxide (DMPO) as the spin trap. As shown in Fig. 9c and d, under dark conditions, the signals of DMPO-·O2− cannot be detected for all the samples. Under light irradiation, no signal can be observed for the BiVO4 sample; however, the ZnTCPP sample exhibits the characteristic peaks of DMPO-·O2−, indicating that only the CB of ZnTCPP has sufficient reduction capacity to reduce O2 to ·O2−. Obviously, the BZ-20 sample also exhibits the typical DMPO-·O2− signals, and the intensity is found to be stronger than that of pure ZnTCPP. These results fully suggest the formation of an S-scheme heterojunction and better separation ability for photogenerated electrons and holes.57 Such S-scheme charge transfer could efficiently consume the photoexcited charge carriers, and the holes accumulated in the VB of BiVO4 could participate in the N2 oxidation reactions; meanwhile, the electrons in the CB of ZnTCPP will be consumed by O2.58 Indeed, control experiments (Fig. S9†) showed that the electron acceptor (0.02 M NaIO3) could improve the NOR activity of the BZ-20 sample; in contrast, the hole acceptor (20% ethanol) reduces the activity, suggesting that the NOR process over the BZ-20 composite could involve a VB mechanism.59 The involved equations are as follows:
| N2 + 2H2O + 4h+ = 2NO + 4H+ |
| 4NO + 2H2O + 3O2 → 4HNO3 |
Conclusion
In summary, 2D/2D layered BiVO4/ZnTCPP organic–inorganic composite photocatalysts were prepared by the heat reflux method and applied to photocatalytic nitrogen oxidation. FT-IR and XPS results confirmed the strong interaction between BiVO4 and ZnTCPP, which facilitated the transfer of photogenerated electrons and holes between the two phases. Meanwhile, the light absorption of the composite sample was significantly enhanced in the range of 200–800 nm due to the strong absorption ability of porphyrins, which greatly improved the light utilization efficiency. The nitrate yield of the BZ-20 sample reached 4.72 mg g−1 h−1, which was 7.6 and 5.9 times that of pure BVO and ZnTCPP. Theoretical calculations showed that the work function of BiVO4 was greater than that of ZnTCPP, and the IEF could be formed due to the electron transfer from ZnTCPP to BiVO4. The suitable staggered band arrangement with an internal electric field in BiVO4/ZnTCPP induces an S-scheme charge transfer path, resulting in high separation and redox ability of photogenerated electrons and holes. Thus, a remarkably enhanced photocatalytic N2 oxidation activity could be obtained.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22005228) and the Science Foundation of Wuhan Institute of Technology (K2021002).Notes and references
- S. Wan, J. Xu, S. Cao and J. Yu, Interdiscip. Mater., 2022, 1, 294–308 Search PubMed.
- Z. Luo, X. Ye, S. Zhang, S. Xue, C. Yang, Y. Hou, W. Xing, R. Yu, J. Sun, Z. Yu and X. Wang, Nat. Commun., 2022, 13, 2230 Search PubMed.
- X. Wu, H. Fan, W. Wang, L. Lei, X. Chang and L. Ma, J. Mater. Chem. A, 2022, 10, 17817–17826 Search PubMed.
- T. Chen, L. Liu, C. Hu and H. Huang, Chin. J. Catal., 2021, 42, 1413–1438 Search PubMed.
- J. Zhang, S. Shao, D. Zhou, T. Di and T. Wang, ACS Appl. Energy Mater., 2021, 4, 6340–6347 CrossRef CAS.
- G. Tang, H. Zhang, T. Song, S. Yin, G. Mao, B. Long, A. Ali and G.-J. Deng, J. Mater. Chem. A, 2022, 10, 16891–16899 RSC.
- I. De Pasquale, C. Lo Porto, M. Dell'Edera, M. L. Curri and R. Comparelli, Curr. Opin. Chem. Eng., 2021, 34, 100716 Search PubMed.
- T. Xia, W. Gong, Y. Chen, M. Duan, J. Ma, X. Cui, Y. Dai, C. Gao and Y. Xiong, Angew. Chem., Int. Ed., 2022, 61, e202204225 Search PubMed.
- J. Y. Zheng, L. Jiang, Y. H. Lyu, S. P. Jian and S. Y. Wang, Energy Environ. Mater., 2022, 5, 452–457 CrossRef CAS.
- A. J. Medford and M. C. Hatzell, ACS Catal., 2017, 7, 2624–2643 CrossRef CAS.
- J. Zhang, Z.-H. Pan, Y. Yang, P.-F. Wang, C.-Y. Pei, W. Chen and G.-B. Huang, Chin. J. Catal., 2022, 43, 265–275 Search PubMed.
- G. Zhang, X. Yuan, B. Xie, Y. Meng, Z. Ni and S. Xia, Chem. Eng. J., 2022, 433, 133670 Search PubMed.
- H. Li, M. Xia, B. Chong, H. Xiao, B. Zhang, B. Lin, B. Yang and G. Yang, ACS Catal., 2022, 12, 10361–10372 Search PubMed.
- Y. Liu, M. Cheng, Z. He, B. Gu, C. Xiao, T. Zhou, Z. Guo, J. Liu, H. He, B. Ye, B. Pan and Y. Xie, Angew. Chem., Int. Ed., 2019, 58, 731–735 Search PubMed.
- Y. Yu, C. Wang, Y. Yu, Y. Huang, C. Liu, S. Lu and B. Zhang, J. Mater. Chem. A, 2020, 8, 19623–19630 RSC.
- S. Shao, J. Zhang, L. K. Li, Y. H. Qin, Z. Q. Liu and T. L. Wang, Chem. Commun., 2022, 58, 2184–2187 Search PubMed.
- J. Li, X. Guo, L. Gan, Z.-F. Huang, L. Pan, C. Shi, X. Zhang, G. Yang and J.-J. Zou, ACS Appl. Energy Mater., 2022, 5, 9241–9265 CrossRef CAS.
- X. Rong, H. Chen, J. Rong, X. Zhang, J. Wei, S. Liu, X. Zhou, J. Xu, F. Qiu and Z. Wu, Chem. Eng. J., 2019, 371, 286–293 CrossRef CAS.
- J. Li, P. Liu, Y. Tang, H. Huang, H. Cui, D. Mei and C. Zhong, ACS Catal., 2020, 10, 2431–2442 CrossRef CAS.
- C. Dong, S. Lu, S. Yao, R. Ge, Z. Wang, Z. Wang, P. An, Y. Liu, B. Yang and H. Zhang, ACS Catal., 2018, 8, 8649–8658 CrossRef CAS.
- S. Gao, B. Gu, X. Jiao, Y. Sun, X. Zu, F. Yang, W. Zhu, C. Wang, Z. Feng, B. Ye and Y. Xie, J. Am. Chem. Soc., 2017, 139, 3438–3445 Search PubMed.
- B. Chang, Y. Guo, H. Liu, L. Li and B. Yang, J. Mater. Chem. A, 2022, 10, 3134–3145 Search PubMed.
- S. Ning, Y. Sun, S. Ouyang, Y. Qi and J. Ye, Appl. Catal. B, 2022, 310, 121063 CrossRef CAS.
- X. Niu, A. Shi, D. Sun, S. Xiao, T. Zhang, Z. Zhou, X. a. Li and J. Wang, ACS Catal., 2021, 11, 14058–14066 Search PubMed.
- M. Zhao, Y. Wang, Q. Ma, Y. Huang, X. Zhang, J. Ping, Z. Zhang, Q. Lu, Y. Yu, H. Xu, Y. Zhao and H. Zhang, Adv. Mater., 2015, 27, 7372–7378 CrossRef CAS PubMed.
- H. Yang, L. Jia, Z. Zhang, B. Xu, Z. Liu, Q. Zhang, Y. Cao, Z. Nan, M. Zhang and T. Ohno, Chin. J. Catal., 2022, 405, 74–83 CrossRef CAS.
- Y. Benseghir, A. Sole-Daura, D. R. Cairnie, A. L. Robinson, M. Duguet, P. Mialane, P. Gairola, M. Gomez-Mingot, M. Fontecave, D. Iovan, B. Bonnett, A. J. Morris, A. Dolbecq and C. Mellot-Draznieks, J. Mater. Chem. A, 2022 10.1039/d2ta04164b.
- Z. Liang, H.-Y. Wang, H. Zheng, W. Zhang and R. Cao, Chem. Soc. Rev., 2021, 50, 2540–2581 Search PubMed.
- Y. Zhang, K. Ren, L. Wang, L. Wang and Z. Fan, Chin. Chem. Lett., 2022, 33, 33–60 CrossRef CAS.
- J. L. Shi, R. Chen, H. Hao, C. Wang and X. Lang, Angew.Chem., 2020, 132, 9173–9178 CrossRef.
- J.-H. Qin, P. Xu, Y.-D. Huang, L.-Y. Xiao, W. Lu, X.-G. Yang, L.-F. Ma and S.-Q. Zang, Chem. Commun., 2021, 57, 8468–8471 RSC.
- R. Chen, Y. Wang, Y. Ma, A. Mal, X.-Y. Gao, L. Gao, L. Qiao, X.-B. Li, L.-Z. Wu and C. Wang, Nat. Commun., 2021, 12, 1–9 CrossRef PubMed.
- F. Zhang, J. Ma, Y. Tan, G. Yu, H. Qin, L. Zheng, H. Liu and R. Li, ACS Catal., 2022, 12, 5827–5833 CrossRef CAS.
- L. Lin, C. Hou, X. Zhang, Y. Wang, Y. Chen and T. He, Appl. Catal. B, 2018, 221, 312–319 CrossRef CAS.
- Y. Ma, X. Yi, S. Wang, T. Li, B. Tan, C. Chen, T. Majima, E. R. Waclawik, H. Zhu and J. Wang, Nat. Commun., 2022, 13, 1400 CrossRef CAS PubMed.
- E. Koposova, X. Liu, A. Pendin, B. r. Thiele, G. Shumilova, Y. Ermolenko, A. Offenhäusser and Y. Mourzina, J. Phys. Chem. C, 2016, 120, 13873–13890 CrossRef CAS.
- Z. Zhao, J. Bian, L. Zhao, H. Wu, S. Xu, L. Sun, Z. Li, Z. Zhang and L. Jing, Chin. J. Catal, 2022, 43, 1331–1340 Search PubMed.
- K. Huang, T. Hu and Y. Wang, J. Solid State Chem., 2021, 294, 121864 CrossRef CAS.
- G. Li, Y. Bai and W. F. Zhang, Mater. Chem. Phys., 2012, 136, 930–934 CrossRef CAS.
- R. Rahimi, S. Shariatinia, S. Zargari, M. Y. Berijani, A. Ghaffarinejad and Z. S. Shojaie, RSC Adv., 2015, 5, 46624–46631 RSC.
- G. Huang, W. L. Wang, X. X. Ning, Y. Liu, S. K. Zhao, Y.-A. Guo, S. J. Wei and H. Zhou, Ind. Eng. Chem. Res., 2016, 55, 2959–2969 CrossRef CAS.
- C. Zhang, M. Han, L. Yu, L. Qu and Z. Li, J. Electroanal. Chem., 2021, 890, 115232 CrossRef CAS.
- Z. Xia, R. Yu, H. Yang, B. Luo, Y. Huang, D. Li, J. Shi and D. Xu, Int. J. Hydrogen Energy, 2022, 47, 13340–13350 CrossRef CAS.
- J. Jing, J. Yang, Z. Zhang and Y. Zhu, Adv. Energy Mater., 2021, 11, 2101392 Search PubMed.
- Q. Zhang, M. Liu, W. Zhou, Y. Zhang, W. Hao, Y. Kuang, H. Liu, D. Wang, L. Liu and J. Ye, Nano Energy, 2021, 81, 105651 CrossRef CAS.
- D. Zhou, J. Zhang, Z. Jin, T. Di and T. Wang, Chem. Eng. J., 2022, 138108, DOI:10.1016/j.cej.2022.138108.
- C. Cheng, B. He, J. Fan, B. Cheng, S. Cao and J. Yu, Adv. Mater., 2021, 33, 2100317 CrossRef CAS PubMed.
- X. Zhang, R. Shi, Z. Li, J. Zhao, H. Huang, C. Zhou and T. Zhang, Adv. Energy Mater., 2022, 12, 2103740 Search PubMed.
- Y. Li, M. Ti, D. Zhao, Y. Zhang, L. Wu and Y. He, J. Alloys Compd., 2021, 870, 159298 CrossRef CAS.
- P. Xia, X. Pan, S. Jiang, J. Yu, B. He, P. M. Ismail, W. Bai, J. Yang, L. Yang, H. Zhang, M. Cheng, H. Li, Q. Zhang, C. Xiao and Y. Xie, Adv. Mater., 2022, 34, 2200563 CrossRef CAS PubMed.
- W. Ren, Z. Mei, S. Zheng, S. Li, Y. Zhu, J. Zheng, Y. Lin, H. Chen, M. Gu and F. Pan, Research, 2020, 2020, 3750314 CrossRef CAS PubMed.
- S. Shao, J. Zhang, L. Li, Y. Qin, Z.-Q. Liu and T. Wang, Chem. Commun., 2022, 58, 2184–2187 RSC.
- S.-J. Yuan, J.-J. Chen, Z.-Q. Lin, W.-W. Li, G.-P. Sheng and H.-Q. Yu, Nat. Commun., 2013, 4, 2249 Search PubMed.
- F. Xu, K. Meng, B. Cheng, S. Wang, J. Xu and J. Yu, Nat. Commun., 2020, 11, 4613 CrossRef CAS PubMed.
- Q. Han, L. Li, W. Gao, Y. Shen, L. Wang, Y. Zhang, X. Wang, Q. Shen, Y. Xiong, Y. Zhou and Z. Zou, ACS Appl. Mater. Interfaces, 2021, 13, 15092–15100 CrossRef CAS PubMed.
- J. Jing, J. Yang, W. Li, Z. Wu and Y. Zhu, Adv. Mater., 2022, 34, 2106807 Search PubMed.
- Y. Zhang, J. Qiu, B. Zhu, M. V. Fedin, B. Cheng, J. Yu and L. Zhang, Chem. Eng. J., 2022, 444, 136584 CrossRef CAS.
- H. Wang, R. Zhao, J. Qin, H. Hu, X. Fan, X. Cao and D. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 44249–44262 CrossRef CAS PubMed.
- S. Chen, D. Liu and T. Peng, Sol. RRL, 2021, 5, 2000487 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials synthesis procedures, materials characterization, DFT calculation methods and additional figures and tables. See DOI: https://doi.org/10.1039/d2ta07289k |
| This journal is © The Royal Society of Chemistry 2023 |