Energy-saving hydrogen production by water splitting coupling urea decomposition and oxidation reactions†
Zehao
Xiao‡
a,
Yinyin
Qian‡
cde,
Tianhui
Tan
a,
Hongxiu
Lu
a,
Canhui
Liu
a,
Bowen
Wang
a,
Qiang
Zhang
 d,
Muhammad Tariq
Sarwar
d,
Muhammad Tariq
Sarwar
 cd,
Ruijie
Gao
cd,
Ruijie
Gao
 *cde,
Aidong
Tang
*acde and
Huaming
Yang
*bcde
*cde,
Aidong
Tang
*acde and
Huaming
Yang
*bcde
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: adtang@csu.edu.cn
bHunan Key Lab of Mineral Materials and Application, School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China. E-mail: hmyang@csu.edu.cn
cEngineering Research Center of Nano-Geomaterials of Ministry of Education, China University of Geosciences, Wuhan 430074, China. E-mail: gaoruijie@cug.edu.cn
dFaculty of Materials Science and Chemistry, China University of Geosciences, Wuhan 430074, China
eKey Laboratory of Functional Geomaterials in China Nonmetallic Minerals Industry, China University of Geosciences, Wuhan 430074, China
First published on 22nd November 2022
Abstract
The efficiency of hydrogen generation via alkaline water splitting is seriously restricted by the high energy barrier of anodic water oxidation with sluggish kinetics. Urea electrocatalysis holds great potential for accomplishing energy-saving hydrogen production and simultaneously relieving urea-rich sewage stress, but its real mechanism is still controversial and the lack of efficient catalysts with sustainable stability limits its further application. In this work, vertical self-supported Ni3S2 nanosheets with boosted activity and better durability were synthesized on the nickel foam. Both experimental and theoretical results reveal a novel mechanism different from most other nickel-based catalysts that Ni3S2 spontaneously reconstructs into surface hydroxyl ligands and directly participates in the decomposition reaction of urea to ammonia, in which hydroxyl-modified Ni sites possess strong binding ability with urea derivatives to further improve self-stability; and an oxidation reaction of urea to N2 provides electrons for maintaining ultra-low potential to avoid the formation of unfavorable NiOOH. Impressively, hydroxyl-modified Ni3S2 requires only 1.339 V to afford 100 mA cm−2, opening up brand-new avenues for the development of urea electrocatalysis with higher nitrogen recycle efficiency and stability.
1 Introduction
Exploring green and renewable energy to alleviate the exhaustion of traditional fossil fuels has widely attracted increasing attention.1,2 Hydrogen (H2) is generally recognized as the most promising energy candidate owing to its environmental friendliness nature and high gravimetric energy density.3,4 Unlike traditional routes for hydrogen production by steam reforming and coal gasification with massive carbon emissions, water splitting as a sustainable technology efficiently converts wind or solar energy resources for large-scale hydrogen generation.5–7 However, anodic oxygen evolution reaction (OER) as a four-electron transfer processes leads to severe sluggish kinetics and demands a huge potential to overcome the energy barrier.8,9 Moreover, the commercial value of oxygen compared to hydrogen is very limited. Therefore, it is promising to rationally append the oxidation of sacrificial easily oxidized organic molecules in the electrolyte for replacing OER.10–13 Among them, urea is widely dispersed in industrial or domestic wastewater and the mixture of its reaction products with H2 has no safety risk. Therefore, constructing the urea-rich water splitting system to simultaneously dispose urea-rich wastewater and reduce the energy consumption of hydrogen production is generally considered as an economic approach.14,15Compared with OER, although urea oxidation reaction (UOR) possesses a lower standard thermodynamic voltage (0.37 V versus reversible hydrogen electrode (vs. RHE)) than that of OER (1.23 V vs. RHE), UOR as a complicated six-electron transfer process also confronts unsatisfactory sluggish kinetics.16,17 Even though nickel-based catalysts are recognized as appropriate UOR competitors, their performances are still hampered due to the self-oxidation of Ni3+ states (such reaction occurs after 1.37 V vs. RHE), which is not favourable because of the too strong binding energy of Ni3+ sites with the CO2 intermediate (∼1242.2 kJ mol−1).18–20 For example, Ni0.9Fe0.1Ox as the best UOR catalyst requires 1.365 V (V vs. RHE) to afford 10 mA cm−2 in 1.0 M KOH with 0.33 M urea.21 However, when such catalyst achieves a higher current density, the required potential exceeds over the nickel oxidation potential region and then such catalyst will transform into unfavorable NiOOH, which does not represent an optimal design principle to reduce the energy barrier. One can image that the inherent reaction mechanism of high-valent Ni3+ results in questionable stability of most nickel-based UOR catalysts. To the best of our knowledge, only few UOR catalysts can maintain the current density at 90% level of the initial state over 50 hours.22 On the other hand, the direct conversion of urea into N2 along UOR leads to poor nitrogen recycle efficiency. Thus, exploring high-performance alternative mechanisms beyond traditional UOR with low energy barrier and high nitrogen recycle efficiency still remains a great challenge.
There have been some alternative mechanisms under alkaline urea conditions, in particular the urea decomposition reaction (UDR) that involves urea adsorption, first NH3 desorption, CO2 desorption and second NH3 desorption (*OH → *OH + *CO(NH2)2 → *COO(NH2) + NH3 → *NH2 + CO2 → *OH + NH3) occurs at hydroxyl-modified nickel sites. More importantly, such unique cyclic mechanism is free of oxidation of nickel sites and accelerates the generation of ammonia at room temperature. Nevertheless, the competition reaction at the anode with UDR is the oxidation of hydroxyl-modified nickel sites. For example, Qiao's group has proved that Ni2Fe(CN)6 can facilitate chemical ammonia production at Ni sites and subsequent ammonia oxidation to N2 at Fe sites.23 However, such catalyst ultimately gets converted into Ni3+ species over six hours, which still cannot avoid the formation of unfavorable NiOOH and the insufficient stability limits its further applications. Therefore, comprehensive understanding of designing nickel-based catalysts with long-time stability based on the above-mentioned discussions for urea electrolysis can be classified into two principles: (1) since UDR occurs without any electron transfer, the simultaneous UOR process should provide electrons to maintain low potential and (2) no matter nickel sites are involved in UDR or UOR, the formation of NiOOH under such low potential region should be avoided. When the catalyst possesses strong binding ability with hydroxyls and urea derivatives, the possible UDR can be continuously activated below the nickel oxidation potential region.
Herein, transition metal sulfides (TMSs) become potential targets for catalysing UDR due to unique spontaneous hydroxyl affinity,24,25 especially Ni3S2 stands out with various valence states and metallic behaviours.26 In fact, the generation of hydroxyl ligands formed from the reconstruction of Ni3S2 under alkaline urea conditions is expected to activate UDR, while UOR can keep low potential to avoid the formation of unfavorable NiOOH; however, such novel mechanism combining UOR and UDR with more favourable kinetics to sustainably improve the activity and stability of urea electrolysis has been rarely reported. On the other hand, the self-supported structure is considered beneficial for maximizing exposed active sites, promoting electronic conductivity and facilitating mass diffusion.27 Therefore, the self-supported Ni3S2 catalyst was successfully synthesized on the nickel foam (NF) in this work. Experimental results reveal a unique anodic reaction pathway of Ni3S2 that involves the chemical decomposition of urea to ammonia on hydroxyl ligands formed via the surface reconstruction of Ni3S2 and a simultaneous oxidation of urea to N2, which provides electrons to keep low anodic potential below the nickel oxidation potential region. Density functional theory (DFT) calculations demonstrate that hydroxyl-modified Ni3S2 possesses metal-like efficient conductivity, thermodynamically favourable CO2 desorption energy for UOR and strong adsorption ability of urea derivatives for UDR to further improve self-stability. Owing to the faster reaction kinetics, the reconstructed Ni3S2 catalyst exhibits an extremely low anodic voltage, only requiring 1.339 V (vs. RHE) to afford 100 mA cm−2 with long-time durability. This work uncovers a novel mechanism of urea electrocatalysis with better stability, boosted activity and higher nitrogen recycle efficiency for simultaneously accomplishing purification of urea-rich sewage and energy-saving hydrogen production.
2 Experimental section
2.1 Synthesis of Ni(OH)2 nanosheets
A piece of NF (1.0 cm × 5.0 cm) was treated by ultra-sonication for 10 min each in 3 M hydrochloric acid, DI water and ethanol to remove the surface oxide layer. Subsequently, above cleaning NF was immerged into 30 mL DI water solution containing 2 mmol Ni(NO3)2·6H2O, 10 mmol urea, and 4 mmol NH4F. After stirring for 10 min, the mixed solution was put into a Teflon-lined stainless steel autoclave, which was then maintained at 120 °C for 6 h. After the temperature was naturally cooled down, the green Ni(OH)2 precursor was rinsed with DI water and dried at 60 °C.2.2 Synthesis of Ni3S2 nanosheets
A piece of the as-prepared Ni(OH)2 precursor was put into 30 mL DI water solution containing 10 mmol Na2S·9H2O. After stirring for 20 min, the mixed solution was put into a Teflon-lined stainless steel autoclave, which was then maintained at 120 °C for 2 h. After the temperature was naturally cooled down, the black Ni3S2 catalyst was rinsed with DI water and kept in DI water to avoid oxidation. The loading mass of Ni3S2 is around 5.8 mg cm−2.2.3 Characterization
The morphologies of the as-prepared catalysts were characterized by scanning electron microscopy (SEM) using a TESCAN MIRA3 and a field emission transmission electron microscope (TEM), high-resolution TEM (HRTEM) and energy-dispersive spectroscopy (EDS) analysis using a Talos F200X. X-ray diffraction (XRD) was performed using a Bruker D8 with Cu-Kα radiation at a scan rate of 5°·min−1. X-ray photoelectron spectroscopy (XPS) was carried out using a Thermo Scientific K-Alpha equipped with a monochromatic Al-Kα X-ray source, and all measured spectra were calibrated by the C 1s peak at 284.8 eV. Ultraviolet-visible spectroscopy (UV-vis) was conducted using a PE lambda 750 at 420 cm. The wetting contact angle was conducted using a JY-82B Kruss DSA. In situ Fourier transform infrared (FTIR) spectroscopy and Raman spectra (λ = 532 nm) were respectively conducted using a Bruker Vertex 80 and a Via-Reflex spectrometer (Renishaw), and the measured potential range was operated by linear sweep voltammetry (LSV) from 1.2 to 1.8 V (vs. RHE) at a scan rate of 0.2 mV s−1 controlled by the electrochemical workstation (CHI760E) in the electrolyte of 1.0 M KOH with 0.5 M urea. Ion chromatography (IC) was conducted using a Thermo Scientific ICS-1100.2.4 Electrochemical measurement
Electrochemical measurements were conducted using a CHI760E electrochemical workstation with a three-electrode system. Hg/HgO (1.0 M KOH) electrode was used as the reference electrode and graphite rod was used as the counter electrode. The area of the working electrode fasted by an electrode clamp immersed in the electrolyte was controlled as 1.0 cm × 1.0 cm. In addition, all data in this work unless specifically noted were corrected by 80% iR compensation and converted to versus RHE potential using the equation (ERHE = 0.098 + 0.059 × pH + EHg/HgO). Before measurements, the as-prepared samples were initially treated by the chronoamperometry (CP) test under 100 mA cm−2 until stable state. Additionally, LSV curves were measured at the scan rate of 2 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were conducted from 0.01 Hz to 100 kHz with 5 mV voltage amplitude. ECSA was calculated from cyclic voltammetric (CV) curves at different scan rates in the non-faradaic region.3 Results and discussion
3.1 Synthesis and structural characterizations
The synthesis process is illustrated in Fig. 1a. Vertical self-supported Ni3S2 nanosheets were synthesized via a two-step hydrothermal strategy, and Ni(OH)2 nanosheets were initially in situ synthesized on the NF substrate (Fig. S1†) based on a previous report (Fig. 1b).28 Then, Ni(OH)2 was sulfurized into Ni3S2 by the second rapid hydrothermal method. In addition, the preparation condition of the Ni3S2 catalyst was optimized (Fig. S2†), and the optimal sulfurization time is 2 h. Notably, such synthesis process can be distinguished from the color change of silvery NF to green Ni(OH)2 to black Ni3S2 (Fig. S3†). The morphology of different samples was characterized by scanning electron microscopy (SEM). As shown in Fig. 1b and S4,† smooth Ni(OH)2 nanosheets with a thickness of tens of nanometers were uniformly loaded on the entire NF substrate. After the sulfurization process, the structure of Ni3S2 nanosheets have no obvious change compared to the Ni(OH)2 precursor (Fig. 1c and S5†). More importantly, layered catalysts vertically anchored on NF are favorable for the exposure and orientation of active edge sites, which is very significant for taking advantage of the self-supported structure in facilitating mass diffusion with electrolytes.29 In contrast, nanosheets obtained by direct sulfurization from the bare NF are very small, indicating that the Ni(OH)2 precursor is essential for the formation of self-supported structure (Fig. S6†). Such unique structure was further verified with transmission electron microscopic (TEM) images (Fig. 1d). Diffraction rings of Ni3S2 in the selected area electron diffraction (SAED) pattern further verified the successful synthesis of Ni3S2. High-resolution TEM image displays lattice fringe spacings of 0.286 nm, 0.237 nm and 0.166 nm very close to the (−110), (111) and (−121) planes of Ni3S2 (Fig. 1e). Moreover, elemental mapping images displayed in Fig. 1f show that the two existing elements are Ni and S, which are uniformly distributed throughout inside the nanosheet. The atomic ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S is measured to be 6.5
S is measured to be 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5, which is approximately consistent with the stoichiometric ratio of Ni
3.5, which is approximately consistent with the stoichiometric ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S in Ni3S2.
S in Ni3S2.
X-ray diffraction (XRD) characterization was employed to identify the phase of Ni(OH)2 and Ni3S2. As shown in Fig. 2a, three strong main peaks were ascribed to the NF substrate (PDF #87-0712). Notably, the peaks of the Ni(OH)2 phase (PDF #40-0215) have completely disappeared and transferred into the Ni3S2 phase (PDF #73-0698) after the sulfurization process. XPS was employed to further probe the surface chemical states of Ni(OH)2 and Ni3S2. As shown in Fig. 2b, the X-ray photoelectron spectroscopy (XPS) survey spectrum shows the co-existence of Ni, S and O, where the O element belongs to the spontaneous surface oxidation under atmosphere. As for the Ni 2p spectrum of Ni3S2 (Fig. 2c), two fitted peaks located at binding energies of 855.6 and 873.3 eV accompanied by two satellite peaks are respectively assigned to Ni 2p3/2 and Ni 2p1/2 related to the surface oxidation of nickel sulfides. On the other hand, the characteristic peak located at the binding energy of 852.6 eV belongs to Ni–S bonds.24,30 As shown in Fig. 2d, the S 2p XPS spectrum exhibits peaks locked at binding energies of 168.1 and 162.1 eV, which are respectively assigned to the S–O and S–S bonds, and the peak locked at the binding energy of 163.2 eV fits well with the metal–sulfur bonds of Ni–S species.31,32 Specifically, the sulfur anion possesses low electronegativity and the ability to get easily polarized, which are beneficial for sharing more dispersed electrons to tune strong positive fields of adjacent metal ions.33,34 Thus, the negative binding energy shift of nickel is caused by the electronegativity and polarization of sulfur anions, indicating that nickel sites receive more electrons and shift to a lower valence state. In summary, these aforementioned results indicate the successful synthesis of the self-supported Ni3S2 catalyst via the proposed facile two-step hydrothermal approach.
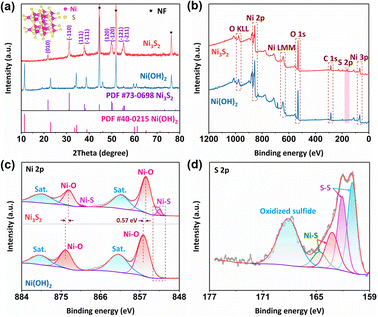 | ||
| Fig. 2 (a) XRD patterns (inset: side-view of the Ni3S2 model), (b) XPS survey and (c) high-resolution Ni 2p spectrum of Ni(OH)2 and Ni3S2. (d) High-resolution S 2p spectrum of Ni3S2. | ||
3.2 Electrocatalytic performance
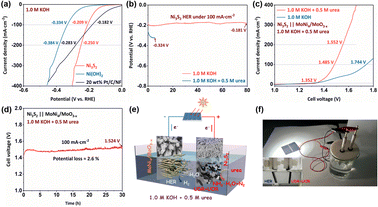
Linear sweep voltammetric (LSV) curves of different samples were respectively investigated in different electrolytes by the standard three-electrode system. As shown in Fig. 3a and S7,† the Ni3S2 catalyst exhibits highly efficient catalytic activity, which only demands 1.339 and 1.364 V (vs. RHE) to afford current densities of 100 and 200 mA cm−2, such performance is much superior to Ni(OH)2 and even precedes most of recently reported efficient catalysts under alkaline urea conditions (Fig. S8 and Table S1†). Tafel plots derived from LSV curves are closely related to dynamic response and reaction kinetics.35,36 Tafel slopes of Ni3S2 and Ni(OH)2 were calculated to be 39.9 and 70.8 mV· dec−1, indicating that the Ni3S2 catalyst owns higher reaction efficiency (Fig. S9†). As for OER, all LSV curves were recorded from negative direction to avoid the influence of anodic peak (Fig. 3b).37 Without urea, the catalytic activity of the Ni3S2 catalyst becomes much more deactivated (Fig. 3c). More importantly, the Ni3S2 catalyst exhibits nearly similar OER activities as the Ni(OH)2 precursor, indicating that nickel sites have mostly converted into the Ni3+ state in NiOOH species during OER. As shown in Fig. 3d, the Ni3S2 catalyst requires a much lower potential to afford high current density under urea conditions before the nickel oxidation potential region, which is further indicated by CV curves (Fig. S10†). Thus, such extremely high performance of the Ni3S2 catalyst may be attributed to the stable Ni2+ state under a low potential region without the generation of NiOOH. This is quite different from the previously reported UOR mechanism of nickel-based catalysts, in which high-valence Ni3+ states are key active sites. Furthermore, the electrochemical active surface area (ECSA) is closely related to inherent catalytic performance, which is calculated from the double layer capacitance (Cdl) under a series of different cyclic voltammetric (CV) curves in the non-faradaic region (Fig. S11†).38,39 As shown in Fig. 3e, the Cdl value of the Ni3S2 catalyst is 9.07 mF cm−2, which is 1.35 times larger than that of Ni(OH)2 (6.74 mF cm−2), indicating that the Ni3S2 catalyst possesses enhanced intrinsic activity and reaction kinetics. Furthermore, LSV curves were normalized by ECSA to exclude the effect of various surface areas.40,41 After ECSA normalization, the catalytic activity of the Ni3S2 catalyst still surpasses Ni(OH)2 (Fig. S12†), indicating that the Ni3S2 catalyst owns a higher intrinsic catalytic activity at each active site. Electrochemical impedance spectroscopy (EIS) measurement was carried out to evaluate the conductivity. Nyquist plots for the Ni(OH)2 precursor and Ni3S2 catalyst in Fig. 3f illustrate that the Ni3S2 catalyst possesses a smaller charge-transfer resistance (Rct) value of 0.35 Ω than that of Ni(OH)2 (4.37 Ω), implying a rapid charge transfer at the solid–liquid interface.42 Long-time stability is a vital index to evaluate whether the catalyst can meet the practical application demand. Therefore, the durability test of the Ni3S2 catalyst was conducted by the chronoamperometry (CP) method under 100 mA cm−2 (Fig. 3g). Notably, the response potential remains highly stable for over 120 h with around 3.4% potential loss (electrolyte is changed every 40 h to avoid excessive urea consumption). In contrast, the primary stage in the OER CP curve exhibits a transient platform potential, which is assigned to the earlier oxidation of sulfur anions and accompanied surface reconstruction of hydroxyl ligands before the generation of NiOOH (Fig. S13†). Nevertheless, the catalytic performance of the Ni3S2 catalyst after 120 h CP test has declined to the nickel oxidation potential region and it will ultimately gets converted into nickel hydroxide derivatives or NiOOH if the test time is extended longer. As expected, the catalytic activity of the Ni3S2 catalyst after the 10 h OER test is significantly inhibited, which further confirms that NiOOH is unfavorable for urea electrolysis (Fig. S14†). Hydrophilicity is a critical factor to evaluate the contact ability between the electrolyte with the surface of the catalyst. The contact angle test displays that a drop of electrolyte fleetly sticks to the surface of Ni3S2, indicating the Ni3S2 catalyst possesses excellent hydrophilicity to ensure adequate contact with the electrolyte (Movie S1†). Finally, SEM, XRD, XPS and TEM characterizations were employed to gain further insights into the structure of Ni3S2 after 120 h CP test. As shown in Fig. S15 and S16,† the characteristic XRD peaks of Ni3S2 and signals of Ni–S bonds and S 2p spectrum still remain but reduce after 120 h CP test. Notably, the FTIR spectra after 120 h CP test shows a newly formed strong peak located at 3638 cm−1, which is assigned to the non-hydrogen-bonded O–H stretching vibration of hydroxyl species (Fig. S17†).43 SEM images show that the Ni3S2 catalyst after the 120 h CP test retains the nanosheet structure (Fig. S18†). In addition, large amounts of amorphous regions with obvious brightness differences in TEM results and an increasing atomic ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S after 120 h CP test are consistent with the above-mentioned results of the possible surface reconstruction of Ni3S2 (Fig. S19†).
S after 120 h CP test are consistent with the above-mentioned results of the possible surface reconstruction of Ni3S2 (Fig. S19†).
The anodic reaction mechanism of the Ni3S2 catalyst was further studied by reaction orders of OH− and urea. As shown in Fig. 4a, the LSV curves at different pH values show a strong dependence on the concentration of OH−. Obviously, the current density linearly increases with the increasing OH− concentration, indicating that the high OH− concentration will promote the hydroxyl adsorption ability and enhance the reaction efficiency. However, the slope of inset double logarithmic plots suggests the reaction order of 1.34 with OH−, which is quite different from 2.00 of the previously reported nickel-based UOR catalysts.44 Moreover, the Tafel slope with varying OH− concentrations based on the onset potential was measured to be 56.6 mV pH−1, which agrees with 59.0 mV pH−1 of the previously reported Ni2Fe(CN)6 catalyst, indicating that the rate-determining step is dependent on the Nernstian-type one electron–proton coupling step (Fig. S20†).23 However, the rate-determining step of the most reported nickel-based UOR catalysts is the desorption of CO2, which demands a two-electron transfer.45–47 Additionally, the LSV curves at different concentrations of urea reveal that sufficient urea condition is necessary to maintain the excellent anodic catalytic activity (Fig. 4b). According to the slope of the inset double logarithmic plots, the reaction order was measured to be 0.31 with respect to urea, which is perfectly consistent with 0.30 of previously reported nickel based catalysts.44 More interestingly, the pungent smell of ammonia can be detected in the electrolyte after the long-time CP test, which is quite different from the traditional UOR pathway that directly converts urea into N2. Therefore, UV-vis spectra were adopted to analyze if ammonia existed in the electrolyte at different time points of the CP test. First, 90 μL Nessler reagent (HgIK) was added to 2 mL thousand-fold diluted electrolyte to detect ammonia (the standard curve was obtained by adding different concentrations of NH3 H2O in 1.0 M KOH as the standard solution). Iodide and mercury of the Nessler reagent can react with ammonia to form reddish brown complex compounds under alkaline conditions and such color possesses strong absorbance at the wavelength of 420 nm, which is directly proportional to ammonia concentrations (Fig. S21†). As the anodic reaction proceeds, the gradual rise of UV-vis absorbance intensity at different reaction time points demonstrates the whole anodic reaction process along the generation of ammonia in the electrolyte (Fig. 4c). Notably, the concentration of ammonia reached 0.082 mol L−1 (≈2920 ppm) after 40 h, indicating the anodic reaction was accompanied by the rapidly accumulated ammonia rate of 2.05 mmol h−1. Considering such reaction is an accumulated ammonia process, we also measured LSV curves in 1.0 M KOH with different amounts of ammonia to investigate whether the Ni3S2 catalyst can oxidize ammonia to N2. However, LSV curves exhibit no response at different concentrations of ammonia, indicating that the Ni3S2 catalyst has no ability to oxidize ammonia to N2, which is due to the lack of critical ammonia oxidation sites of Fe as previously reported (Fig. S22†).23 Moreover, the gas generated during the anodic reaction was passed into saturated lime water to detect CO2, and the turbidity of the solution indicated the formation of CaCO3 (Fig. S23†). According to the previously obtained OER CP curve, the surface reconstruction of Ni3S2 (happens at ∼1.33 V vs. RHE) causes oxidation of sulfur anions and formation of hydroxyl ligands. Subsequently, we employed 0.01 M BaCl2 solution to detect SO42− in the hydrochloric-acidified electrolyte. As shown in Fig. S24,† a large number of bubbles were generated in the electrolyte during the hydrochloric acid acidification, which further proved the existence of CO32−. As shown in Fig. S25,† the turbid BaSO4 precipitation confirmed the presence of SO42− ions. Additionally, the ion chromatography (IC) test in Fig. S26† shows that the concentration of SO42− ions in the electrolyte after the CP test was 0.047 mmol L−1, and such sulfur dissolution (∼0.15 mgS) is bearable compared to the loading mass of Ni3S2 (5.8 mg cm−2). Furthermore, in order to explore the existence of urea oxidation, the CP test was conducted without changing the electrolyte until urea was completely consumed. As shown in Fig. S27,† the inflection time point at 78 h means that urea has been consumed and Ni3S2 will involve in OER. The concentration of ammonia in the electrolyte at this time was measured to be about 0.106 mol L−1, indicating that 0.0053 mol urea participate in UDR and 0.0447 mol urea participate in UOR. Thus, the anodic reaction pathway of Ni3S2 undergoes surface reconstruction of hydroxyl ligands accompanied by dissolution of sulfur anions to leaching SO42−, and UDR from urea to ammonia (CO(NH2)2 + H2O → CO2 + 2NH3) with UOR from urea to N2 (CO(NH2)2 + 6OH− → 5H2O + CO2 + N2 + 6e−). Additionally, LSV curves before and after exchanging the electrolyte illustrate that the fresh solution can maintain the catalytic activity of Ni3S2, indicating that the sufficient urea condition is essential for keeping excellent anodic activity (Fig. S28†).
In situ spectra characterizations were carried out to gain more insights into critical reaction intermediates. As shown in Fig. 4d, once urea is added in the electrolyte for the test, the peak at 1004 cm−1 can be observed, which is assigned to the H2N–C–NH2 bond of adsorbed urea.48 On the other hand, three peaks at 247, 291 and 353 cm−1 belong to A1 and E vibration modes of Ni3S2.49 At initial various potentials, Raman spectra is similar to that under open circuit potential (OCP) condition. However, as the applied potential increases to 1.45 V (vs. RHE), all peaks of Ni3S2 vanish in a very short time, while a strong new peak appears at 475 cm−1, which is attributed to the Ni–O vibration.50 As the applied potential increases over 1.5 V (vs. RHE), characteristic NiOOH doublet peaks appear at 475 and 558 cm−1 (Fig. S29†), indicating that Ni3S2 has reconstructed into NiOOH and the selected potential region for the test (lower than 1.4 V vs. RHE) can avoid the generation of NiOOH. In situ Fourier transform infrared (FTIR) spectroscopy was also conducted to study the reaction intermediates at different potentials (Fig. 4e). Compared with the FTIR spectra at OCP, growing peaks with the increasing potential located at 1222 and 1635 cm−1 were respectively attributed to the C–O stretching vibration of *COO(NH2) and N–H stretching vibration of *NH2 intermediates,23 indicating that observable *COO(NH2) and *NH2 intermediates strongly support the assumptive UDR mechanism. Besides, the other growing peaks located at 1457 cm−1 were attributed to CO32− ions.51 More importantly, the peaks of CO2 vibration are located at 2360 and 2376 cm−1 and no signal of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediates along the traditional UOR path can be found at ∼1720 cm−1.52,53 Furthermore, the strong O–H stretching vibration appears as a broad band at 3340 cm−1, which illustrates that the hydroxyl adsorption ability of Ni3S2 gradually enhances as the potential increases and supports the above-mentioned view of the formation of hydroxyl ligands.54
O intermediates along the traditional UOR path can be found at ∼1720 cm−1.52,53 Furthermore, the strong O–H stretching vibration appears as a broad band at 3340 cm−1, which illustrates that the hydroxyl adsorption ability of Ni3S2 gradually enhances as the potential increases and supports the above-mentioned view of the formation of hydroxyl ligands.54
3.3 Theoretical computation
Based on the above-mentioned analysis, the DFT calculations were employed to gain further insights into the electronic structure and energy barriers with critical intermediates. Initially, the model of hydroxyl adsorbed on the (002) plane of Ni3S2 (Ni3S2–OH*) is built for calculation in Fig. S30.† The relevant UDR reaction pathway over hydroxyl-modified nickel sites and adsorption models of each step are illustrated in Fig. 5a and S31.† The electron density difference of Ni3S2–OH* shown in Fig. 5b indicates the intense electron transfer between Ni sites with neighbor S sites, which fits well with the negative binding energy shift of XPS results. Moreover, extracted 2D data show the alternately connected electron accumulation and depletion areas, which also illustrates the efficient electron transport (Fig. S32†). Such redistribution of electron is beneficial for tuning the valence states of adjacent metal ions and optimizing binding energy.55 As shown in Fig. 5c, the DOS of Ni3S2–OH* spans over the Fermi level and exhibits metal-like excellent conductivity, which is conductive to facilitate electron transfer. On the other hand, the efficiency of the reaction mainly depends on how reactants and intermediates bond to the active sites.56 The desorption of CO2 has been recognized as the rate-determining step of UOR for nickel-based catalysts. Thus, desorption energies of CO2 were evaluated on Ni3S2–OH* and compared with NiOOH. As shown in Fig. 5d, the more favourable desorption energy of CO2 over Ni3S2–OH* (0.42 eV) for UOR is much lower than that of NiOOH (2.02 eV). Furthermore, binding energies of intermediates (*OH, *CO(NH2)2, *OCONH2 and *NH2) and desorption energies of intermediates (NH3 in different steps and CO2) for each step of UDR were calculated. Notably, Ni sites of Ni3S2 have hydroxyl-affinity under alkaline conditions (−2.59 eV) and show strong adsorption ability to urea derivatives (Fig. 5e), which means that hydroxyl-modified Ni sites tend to form ligands with urea derivatives for further enhancing self-stability. As shown in Fig. 5f and desorption models of Fig. S33,† the NH3 desorption in the second step of UDR is the most difficult (0.99 eV). Therefore, the second NH3 desorption step ([HO·M·CO(NH2)2]ads → [M·OCONH2]ads + NH3) is the rate-determining step of UDR, which is quite different from CO2 desorption as the rate-determining step along the UOR mechanism owing to the avoidable formation of NiOOH. However, the fourth NH3 desorption step shows efficient NH3 desorption ability due to the near-zero desorption energy, indicating that such step possesses the highest activity for propelling UDR.3.4 Urea-assisted water splitting performance
Hydrogen evolution reaction (HER) performances of the as-prepared catalysts were also measured in different electrolytes. As shown in Fig. 6a and S34,† the HER LSV curves of Ni3S2 in 1.0 M KOH exhibit an ultra-low overpotential of 209 mV to afford current density of 100 mA cm−2, which is much lower than that of the Ni(OH)2 precursor (334 mV) and such performance of the Ni3S2 catalyst in the high current density region (>150 mA cm−2) even surpasses state-of-the-art 20 wt% Pt/C catalyst. Moreover, Ni3S2 exhibits the lowest Tafel slope of 78.9 mV dec−1 than 110.8 mV dec−1 of Ni(OH)2 and 136.9 mV dec−1 of 20 wt% Pt/C (Fig. S35†). Such Tafel slope of Ni3S2 indicates a Volmer–Heyrovsky mechanism, where the Heyrovsky step (H2O + e− + H* → OH− + 1/2H2) is the rate-determining step.57 However, the HER activity of the Ni3S2 catalyst is significantly inhibited under urea conditions (Fig. S36†), illustrating that the urea adsorption ability of the Ni3S2 catalyst is too strong, which results in insufficient adsorption of H* and fits well with the DFT result that Ni3S2 owns strong urea adsorption ability. Furthermore, the Ni3S2 catalyst exhibits outstanding stability in the long-time HER test (Fig. 6b), and the overpotential even slightly reduces after 80 h, which may be due to the formation of metallic Ni sites that serve as the actual active phase during the electro-reduction process.58 In contrast, the CP test under urea conditions exhibits markedly decreasing HER activity, which also indicates the too strong urea adsorption ability of Ni3S2. To go a target toward practical application, it is necessary to investigate urea-assisted water splitting performance that involves HER at the cathode and the UDR with UOR at the anode in a two-electrode cell system. Considering that the HER activity of the Ni3S2 catalyst is significantly inhibited under urea conditions, we selected an alternative robust MoNi4/MoO3−x catalyst as the cathode for the HER (Fig. S37†). As shown in Fig. S38,† the yellow NiMoO4 precursor turns black after annealing under an Ar/H2 atmosphere. After calcination, the surface of NiMoO4 nanorod was uniformly covered by a large number of nanodots (Fig. S39†). More importantly, the MoNi4/MoO3−x catalyst in different electrolytes requires similar and extremely low overpotentials of 162 and 239 mV to afford current densities of 100 and 200 mA cm−2 (Fig. S40†). As shown in Fig. 6c, the Ni3S2 (+)‖MoNi4/MoO3−x (−) cell system only requires cell voltage of 1.485 V to afford 100 mA cm−2 in 1.0 M KOH with 0.5 M urea, which is much lower than that of 1.744 V in 1.0 M KOH, indicating that such system has excellent energy-saving advantage. Fig. 6d exhibits that the potential loss of the Ni3S2 (+)‖MoNi4/MoO3−x (−) cell system is only 2.6% after 30 h, demonstrating that such cell system shows outstanding stability in practical large-scale H2 production. Impressed by the superior water splitting performance, we assembled a commercial Si solar cell (0.42 W) as the energy source to drive the Ni3S2 (+)‖MoNi4/MoO3−x (−) cell system (Fig. 6e and f). Notably, large amounts of gas bubbles rapidly overflow from both electrodes, which confirms that the Ni3S2 (+)‖MoNi4/MoO3−x (−) cell system possesses extremely high efficiency (Movie S2†). More importantly, the faradaic efficiency was estimated by collecting hydrogen generated at the cathode at the current density of 50 mA cm−2. As shown in Fig. S41,† such cell system possesses a high faradaic efficiency of nearly 97%. These results reflect the promising prospects of Ni3S2 as the appropriate anode in promoting purification of urea-rich sewage and energy-saving hydrogen production.4 Conclusions
In summary, we successfully synthesized self-supported Ni3S2 nanosheets via controllable sulfurization treatment on the NF. Subsequent experimental results demonstrated a unique and more favorable pathway of urea electrocatalysis on hydroxyl ligands formed from the surface reconstruction of Ni3S2 that involved a chemical decomposition reaction of urea to ammonia, and simultaneous oxidation of urea to N2, which provided electrons to keep low anodic potential below the nickel oxidation potential region. Theoretical calculations revealed that hydroxyl-modified Ni3S2 possessed metal-like efficient conductivity, thermodynamically favourable CO2 desorption energy for UOR and strong adsorption ability with urea derivatives for UDR to further promote self-stability. Benefiting from the efficient thermal/kinetic energetics of such unique reaction pathway, reconstructed Ni3S2 exhibits lower potential and better stability than most other nickel-based catalysts, requiring only 1.339 V (vs. RHE) to afford 100 mA cm−2. This work provides inspiration for the novel mechanism of urea electrocatalysis with a higher nitrogen recycle efficiency and opens up a brand new avenue for the design of facile catalysts in accomplishing urea-rich sewage purification with energy-saving hydrogen generation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are greatly grateful for the financial support by the National Natural Science Foundation of China (No. 51674293), the National Postdoctoral Program for Innovative Talents (No. BX2021276), the China Postdoctoral Science Foundation (No. 2020M682519) and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan).References
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS.
- I. Staffell, D. Scamman, A. V. Abad, P. Balcombe, P. E. Dodds, P. Ekins, N. Shah and K. R. Ward, Energy Environ. Sci., 2019, 12, 463–491 RSC.
- J. O. M. Bockris, Int. J. Hydrog. Energy, 2002, 27, 731–740 CrossRef CAS.
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS.
- F. Cai, L. Liao, Y. Zhao, D. Li, J. Zeng, F. Yu and H. Zhou, J. Mater. Chem. A, 2021, 9, 10199–10207 RSC.
- K. L. Zhou, Z. Wang, C. B. Han, X. Ke, C. Wang, Y. Jin, Q. Zhang, J. Liu, H. Wang and H. Yan, Nat. Commun., 2021, 12, 3783 CrossRef CAS PubMed.
- C. G. Morales-Guio, L.-A. Stern and X. Hu, Chem. Soc. Rev., 2014, 43, 6555–6569 RSC.
- B. Tian, H. Shin, S. Liu, M. Fei, Z. Mu, C. Liu, Y. Pan, Y. Sun, W. A. Goddard III and M. Ding, Angew. Chem., Int. Ed., 2021, 60, 16448–16456 CrossRef CAS.
- M. Gopalakrishnan, A. A. Mohamad, M. T. Nguyen, T. Yonezawa, J. Qin, P. Thamyongkit, A. Somwangthanaroj and S. Kheawhom, Mater. Today Chem., 2022, 23, 100632 CrossRef CAS.
- Z. Liu, S. Xue, S. Zhou, J. Li, K. Qu and W. Cai, J. Catal., 2022, 405, 606–613 CrossRef CAS.
- X. Xu, T. Wang, L. Dong, W. Lu and X. Miao, ACS Sustain. Chem. Eng., 2020, 8, 7973–7980 CrossRef CAS.
- W. Chen, Z. Lei, T. Zeng, L. Wang, N. Cheng, Y. Tan and S. Mu, Nanoscale, 2019, 11, 19895–19902 RSC.
- M. N. Meireles, J. A. Alonso, M. T. F. Díaz, L. E. Cadús and F. N. Aguero, Mater. Today Chem., 2020, 15, 100213 CrossRef.
- X. Zhuo, W. Jiang, G. Qian, J. Chen, T. Yu, L. Luo, L. Lu, Y. Chen and S. Yin, ACS Appl. Mater. Interfaces, 2021, 13, 35709–35718 CrossRef CAS PubMed.
- L. Wang, Z. Liu, S. Zhu, M. Shao, B. Yang and J. G. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 41338–41343 CrossRef CAS PubMed.
- L. Zhang, L. Wang, H. Lin, Y. Liu, J. Ye, Y. Wen, A. Chen, L. Wang, F. Ni, Z. Zhou, S. Sun, Y. Li, B. Zhang and H. Peng, Angew. Chem., Int. Ed., 2019, 58, 16820–16825 CrossRef CAS.
- H. Zhang, X. Meng, J. Zhang and Y. Huang, ACS Sustain. Chem. Eng., 2021, 9, 12584–12590 CrossRef CAS.
- D. A. Daramola, D. Singh and G. G. Botte, J. Phys. Chem. A, 2010, 114, 11513–11521 CrossRef CAS.
- S. Hu, C. Feng, S. Wang, J. Liu, H. Wu, L. Zhang and J. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 13168–13175 CrossRef CAS.
- R. P. Forslund, C. T. Alexander, A. M. Abakumov, K. P. Johnston and K. J. Stevenson, ACS Catal., 2019, 9, 2664–2673 CrossRef CAS.
- F. Wu, G. Ou, J. Yang, H. Li, Y. Gao, F. Chen, Y. Wang and Y. Shi, Chem. Commun., 2019, 55, 6555–6558 RSC.
- B. Zhu, Z. Liang and R. Zou, Small, 2020, 16, 1906133 CrossRef CAS.
- S.-K. Geng, Y. Zheng, S.-Q. Li, H. Su, X. Zhao, J. Hu, H.-B. Shu, M. Jaroniec, P. Chen, Q.-H. Liu and S.-Z. Qiao, Nat. Energy, 2021, 6, 904–912 CrossRef CAS.
- Y. Wu, Y. Li, M. Yuan, H. Hao, X. San, Z. Lv, L. Xu and B. Wei, Chem. Eng. J., 2022, 427, 131944 CrossRef CAS.
- G. Ren, Q. Hao, J. Mao, L. Liang, H. Liu, C. Liu and J. Zhang, Nanoscale, 2018, 10, 17347–17353 RSC.
- L.-L. Feng, G. Yu, Y. Wu, G.-D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS.
- A. Kumar, V. Q. Bui, J. Lee, A. R. Jadhav, Y. Hwang, M. G. Kim, Y. Kawazoe and H. Lee, ACS Energy Lett., 2021, 6, 354–363 CrossRef CAS.
- Z. Xiao, M. Yang, J. Wang, Z. Xu, S. Zhang, A. Tang, R. Gao and H. Yang, Appl. Catal., B, 2022, 303, 120913 CrossRef CAS.
- H. Zhou, F. Yu, J. Sun, H. Zhu, I. K. Mishra, S. Chen and Z. Ren, Nano Lett., 2016, 16, 7604–7609 CrossRef CAS PubMed.
- H. Q. Fu, L. Zhang, C. W. Wang, L. R. Zheng, P. F. Liu and H. G. Yang, ACS Energy Lett., 2018, 3, 2021–2029 CrossRef CAS.
- J. Zhou, L. Yu, Q. Zhu, C. Huang and Y. Yu, J. Mater. Chem. A, 2019, 7, 18118–18125 RSC.
- G. Zhang, Y.-S. Feng, W.-T. Lu, D. He, C.-Y. Wang, Y.-K. Li, X.-Y. Wang and F.-F. Cao, ACS Catal., 2018, 8, 5431–5441 CrossRef CAS.
- R. Boppella, J. Tan, J. Yun, S. V. Manorama and J. Moon, Coord. Chem. Rev., 2021, 427, 213552 CrossRef CAS.
- C.-X. Zhao, B.-Q. Li, M. Zhao, J.-N. Liu, L.-D. Zhao, X. Chen and Q. Zhang, Energy Environ. Sci., 2020, 13, 1711–1716 RSC.
- D. Liu, T. Liu, L. Zhang, F. Qu, G. Du, A. M. Asiri and X. Sun, J. Mater. Chem. A, 2017, 5, 3208–3213 RSC.
- Y. Yang, H. Fei, G. Ruan, C. Xiang and J. M. Tour, Adv. Mater., 2014, 26, 8163–8168 CrossRef CAS PubMed.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong, S. Liu, X. Zhuang and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 6702–6707 CrossRef CAS PubMed.
- Y. Li, K. Yin, L. Wang, X. Lu, Y. Zhang, Y. Liu, D. Yan, Y. Song and S. Luo, Appl. Catal., B, 2018, 239, 537–544 CrossRef CAS.
- Z. Xiao, M. Yang, C. Liu, B. Wang, S. Zhang, J. Liu, Z. Xu, R. Gao, J.-J. Zou, A. Tang and H. Yang, Nano Energy, 2022, 98, 107233 CrossRef CAS.
- L. Liao, J. Sun, D. Li, F. Yu, Y. Zhu, Y. Yang, J. Wang, W. Zhou, D. Tang, S. Chen and H. Zhou, Small, 2020, 16, 1906629 CrossRef CAS.
- J. Sun, F. Tian, F. Yu, Z. Yang, B. Yu, S. Chen, Z. Ren and H. Zhou, ACS Catal., 2020, 10, 1511–1519 CrossRef CAS.
- F. Yu, H. Zhou, Y. Huang, J. Sun, F. Qin, J. Bao, W. A. Goddard, S. Chen and Z. Ren, Nat. Commun., 2018, 9, 2551 CrossRef.
- Z. Jia, R. Ding, W. Yu, Y. Li, A. Wang, M. Liu, F. Yang, X. Sun and E. Liu, Adv. Funct. Mater., 2021, 32, 2107674 CrossRef.
- V. Vedharathinam and G. G. Botte, Electrochim. Acta, 2012, 81, 292–300 CrossRef CAS.
- Z. Ji, Y. Song, S. Zhao, Y. Li, J. Liu and W. Hu, ACS Catal., 2022, 12, 569–579 CrossRef CAS.
- V. Vedharathinam and G. G. Botte, Electrochim. Acta, 2013, 108, 660–665 CrossRef CAS.
- L. Zhang, L. Wang, H. Lin, Y. Liu, J. Ye, Y. Wen, A. Chen, L. Wang, F. Ni, Z. Zhou, S. Sun, Y. Li, B. Zhang and H. Peng, Angew. Chem., Int. Ed., 2019, 58, 16820–16825 CrossRef CAS PubMed.
- K. Chen, X. Zhang and D. R. MacFarlane, Chem. Commun., 2017, 53, 7949–7952 RSC.
- J. S. Chen, C. Guan, Y. Gui and D. J. Blackwood, ACS Appl. Mater. Interfaces, 2017, 9, 496–504 CrossRef CAS PubMed.
- H. Q. Fu, M. Zhou, P. F. Liu, P. Liu, H. Yin, K. Z. Sun, H. G. Yang, M. Al-Mamun, P. Hu, H.-F. Wang and H. Zhao, J. Am. Chem. Soc., 2022, 144, 6028–6039 CrossRef CAS.
- M. Manfredi, E. Barberis, A. Rava, E. Robotti, F. Gosetti and E. Marengo, Anal. Methods, 2015, 7, 2313–2322 RSC.
- W.-K. Han, J.-X. Wei, K. Xiao, T. Ouyang, X. Peng, S. Zhao and Z.-Q. Liu, Angew. Chem., Int. Ed., 2022, 61, e202206050 CAS.
- F. Lu and G. G. Botte, Electrochim. Acta, 2017, 246, 564–571 CrossRef CAS.
- M. I. Tejedor-Tejedor, L. Paredes and M. A. Anderson, Chem. Mater., 1998, 10, 3410–3421 CrossRef CAS.
- L. Yang, L. Huang, Y. Yao and L. Jiao, Appl. Catal., B, 2021, 282, 119584 CrossRef CAS.
- C.-T. Dinh, A. Jain, F. P. G. de Arquer, P. De Luna, J. Li, N. Wang, X. Zheng, J. Cai, B. Z. Gregory, O. Voznyy, B. Zhang, M. Liu, D. Sinton, E. J. Crumlin and E. H. Sargent, Nat. Energy, 2019, 4, 107–114 CrossRef CAS.
- T. T. Yang and W. A. Saidi, J. Phys. Chem. Lett., 2020, 11, 2759–2764 CrossRef CAS PubMed.
- Q. Ma, C. Hu, K. Liu, S.-F. Hung, D. Ou, H. M. Chen, G. Fu and N. Zheng, Nano Energy, 2017, 41, 148–153 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Supporting data. See DOI: https://doi.org/10.1039/d2ta07152e |
| ‡ These authors contributed equally: Zehao Xiao, Yinyin Qian. |
| This journal is © The Royal Society of Chemistry 2023 |