Stable Zr(IV) coordination polymers with electroactive metal-terpyridine units for enhanced electrochemical sensing dopamine†
Tingting
Yan‡
,
Xiao-Yu
Zhang‡
,
Yue
Zhao
 and
Wei-Yin
Sun
and
Wei-Yin
Sun
 *
*
Coordination Chemistry Institute, State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210023, China. E-mail: sunwy@nju.edu.cn
First published on 14th November 2022
Abstract
Coordination polymers (CPs) have attracted remarkable attention in electrochemical sensing for their high selectivity and sensitivity. However, CPs with desired electroactivity and stability for electrochemical sensing in aqueous solution still remain to be developed. Herein, two stable Zr(IV)-based CPs, [M2Zr2(HCOO)8(cptpy)4]n·solvent [M = Fe, named Fe-CP; M = Ni, named Ni-CP; HCOO− = formate; Hcptpy = 4′-(4-carboxylphenyl)-2,2′:6′,2′′-terpyridine], with incorporated electroactive metal-terpyridine moieties were explored, using electrochemical dopamine (DA) sensing as a model. Compared with electroactive ligands, Fe-CP and Ni-CP have a large diffusion coefficient and fast electron transfer rate towards DA oxidation, resulting in enhanced signals. The CPs show a low detection limit of 40 nM for Fe-CP and 62 nM for Ni-CP as well as a wide detection range with high selectivity, satisfactory reproducibility and stability. Additionally, the capacity of DA detection on these CP modified electrodes in simulated urine further demonstrates their feasible application in real systems.
1 Introduction
Coordination polymers (CPs) or metal–organic frameworks (MOFs) have been widely applied in electrochemical sensing because of their large surface area, tunable chemical and physical properties, high sensitivity, selectivity and practicality.1 To enhance the electrochemical activity of CPs for better application in electrochemical sensing, varied strategies have been developed including use of electroactive ligands and/or electroactive metal nodes and incorporating electroactive species.2 In addition, the stability is also important for the application of electroactive CPs in aqueous solution for electrochemical sensing.3 There have been several strategies developed to improve the chemical stability of CPs, such as using nitrogen- and/or oxygen-donor linkers and metal cations with high valence states as nodes to construct CPs,4–6 post-synthetic modification of CPs, etc.7,8 Nevertheless, CPs possessing desired redox activity and chemical stability still need to be developed for electrochemical sensing applications.Zirconium (Zr)-based CPs have been reported to have high stability,9–12 and can be endowed with activity for electrochemical sensing by using redox active organic ligands and/or post synthetic modification.13,14 For example, porphyrins or metalloporphyrins have been widely utilized as electroactive ligands,15–17 because of their π-conjugated macrocyclic structures with a capacity for electron transfer and redox activity.16,18 To explore the desired electroactive ligands for construction of Zr(IV)-based CPs, herein, we employed metal-terpyridine complexes as electroactive ligands to construct Zr(IV)-based CPs for electrochemical sensing. 4′-(4-Carboxylphenyl)-2,2′:6′,2′′-terpyridine (Hcptpy) was utilized to react with metal salts to give metal-terpyridine complex ligands, namely [M(cptpy)2], which can be further connected by using Zr(IV) to generate CPs. It is noteworthy that cptpy− has a large π-conjugated system, contributing to facilitating electron transport and consolidating the CP structure.19,20 For example, a Cu(II) complex of cptpy− has catalytic activity for electrochemical detection of NaNO2 and H2O2.21 Furthermore, integrating molecular metal-terpyridine complexes into CPs could result in improved properties, because CPs with orderly arranged metal-terpyridine moieties as active sites could allow the transfer of analytes easily to the active sites,22 amplifying the response current to analytes and thereby improving the electrochemical sensing sensitivity.23–25
Based on these, herein, two Zr(IV)-based CPs incorporating Fe(cptpy)2 (Fe-L) and Ni(cptpy)2 (Ni-L) ligands were employed considering the earth-abundant metals Fe/Ni and their redox active properties,19,20 taking electrochemical dopamine (DA) sensing as a model. DA is an important biomolecule that will affect cognition, motivation and endocrine functions.26–28 The abnormal variation of DA would cause serious neurodegenerative diseases,29–31 so the monitoring of the DA level is significant.32 As illustrated in Fig. 1, compared with the performance of individual electroactive ligands Fe-L and Ni-L, Fe-CP and Ni-CP exhibit signal amplification owing to the effective electron transfer via the redox hopping mechanism in the CPs that have high density of accessible and ordered active sites,2,33,34 leading to high response current to the oxidization of DA to dopamine quinone (DAQ). The results show that the electroactive Fe-CP and Ni-CP have high sensitivity, a low detection limit and a wide detection range for electrochemical DA sensing.
2 Experimental section
2.1 Preparation of the electroactive ligands and CP modified electrodes
Before modification, glassy carbon electrodes (GCE, 3 mm in diameter) were polished with 0.05 μm alumina slurry and then washed with deionized water. 1 mg of the electroactive ligand or CP was suspended in 1 mL deionized water containing 10 μL Nafion (5 wt%). The mixture was then sonicated for 1 hour for good dispersion. Then, 5 μL of the resulting suspension was pipetted onto the freshly polished GCE and dried under ambient air conditions.2.2 Electrochemical measurements of the electroactive ligands and CP modified electrodes
Differential pulse voltammetry (DPV) and cyclic voltammetry (CV) measurements were performed on a CHI 730E electrochemical workstation (Shanghai CH Instruments, China). A standard three-electrode cell was used for the electrochemical experiments. The Fe-L, Ni-L, Fe-CP or Ni-CP modified electrode was used as the working electrode. A saturated calomel electrode (SCE) and platinum (Pt) electrode were used as the reference and counter electrodes, respectively. All potentials in this work are reported with respect to the SCE. Each sample solution in 0.1 M phosphate buffered saline (PBS) solution (5 mL) was bubbled with purified N2 for 5 min to remove oxygen before measurement. For DA added measurements, the experiments were performed after 30 seconds of immersion.3 Results and discussion
3.1 Characterization of electroactive CPs
Crystallographic analysis proves that the synthesized Fe-CP has the same one-dimensional (1D) chain structure (Fig. 2A, B, S1 and Table S1†) as the previously reported Ni-CP.35,36 The phase purity of the synthesized CPs was ensured by using powder X-ray diffraction (PXRD) patterns (Fig. 2C). The stability of these CPs in an aqueous medium was investigated by soaking the CPs in 0.1 M PBS with a pH range of 5.6–8.0 for varied times, and the unchanged PXRD patterns certify that the structures of Fe-CP (Fig. 2D and S2B†) and Ni-CP (Fig. S2A and C†) are intact, reflecting their high stability. In addition, zeta potentials for the electroactive ligands and their CPs dispersed in 0.1 M PBS at different pH values were also measured (Table S2†). The zeta potentials between −9.4 and −12.5 mV indicate that the CPs have a negatively charged character and are stable in 0.1 M PBS at varied pH values.37,38 In addition, scanning electron microscopy (SEM) images and the corresponding elemental mapping analyses further ensure the uniform distribution of the metal elements in the CPs after being soaked in 0.1 M PBS at pH 7.4 (Fig. 2E and F), confirming the maintenance of their structures in PBS, which is important for electrochemical sensing applications.The valence state of the Fe sites in Fe-L and Fe-CP was investigated by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 2G and S3,† the Fe 2p1/2 and Fe 2p3/2 peaks at 721.4 and 708.6 eV belong to Fe(II),39–41 which appeared in both Fe-L and Fe-CP, indicating that the formation of the CP did not affect the valence of the Fe sites in the ligand. The redox activity of the electroactive ligands and their CPs was investigated by cyclic voltammetry (CV) with respect to the Fe(I/II) and Fe(II/III) redox couples.42–44 As shown in Fig. S4,† four redox peaks corresponding to Fe(I/II), Fe(II/III), Fe(II/I) and Fe(III/II) were observed for both Fe-CP and Fe-L, confirming that the redox properties of the Fe centers in Fe-L are maintained in Fe-CP after integration.42 It was noticed that these four redox peaks are a little more positively shifted for Fe-CP compared to those for Fe-L, which is probably caused by the variation of electron density of the Fe center after the coordination of Fe-L with Zr(IV) in Fe-CP.45,46 The CV measurements were also applied to Ni-L and Ni-CP (Fig. S5†), and the redox properties of the divalent Ni centers in Ni-L and Ni-CP are similar, and the valence of Ni(II) was further confirmed by the XPS results (Fig. S6†).35
The electrical conductivity of the electroactive ligands and their CPs was investigated (Fig. S7 and S8†). As listed in Table S3,† after the integration of the electroactive ligands into CPs, the electrical conductivity was only reduced a little and the values still have the same order of magnitude. The results indicate that the Zr(IV) core connected CPs could maintain the electrical conductivity of the discrete electroactive ligands.19
3.2 Feasibility of sensing
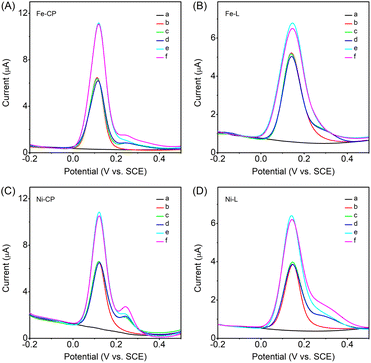
Differential pulse voltammetry (DPV) was adopted owing to its high sensitivity by minimizing the capacitive background current.47 The electroactive Fe-L, Ni-L, Fe-CP and Ni-CP show no intrinsic activity within the electrochemical measurement window of interest (black curves) (Fig. 3), which would not obscure the electrochemical signals from the analytes. The intrinsic redox activity of these CPs and ligands in 0.1 M PBS was also investigated by CV measurements. As shown in Fig. S9,† in the potential range of −0.2 V to 0.5 V, the ligands and CP modified electrodes exhibit no or neglectable peak current, further confirming that the intrinsic activity of the ligands and CPs has no influence on the electrochemical signals produced by the analytes in the potential range used for detection.On the other hand, DA detection is usually interfered with by ascorbic acid (AA) and uric acid (UA) because their oxidation peaks are almost indistinguishable,48 and thus the feasibility of the ligands and CPs was also investigated in the presence of AA and UA. As shown in Fig. 3A, the Fe-CP modified electrode exhibits a sharp oxidation peak at 0.11 V vs. SCE in the presence of 100 μM DA (curve b). After the addition of 100 μM UA, there was a small oxidation peak at 0.25 V vs. SCE (curve c); after continued addition of 100 μM AA, there was no change in the DPV curve (curve d), showing that Fe-CP had no response to AA. On increasing the concentration of DA to 200 μM, a large enhanced oxidation peak current was observed (curve e), and when another 100 μM UA was added into the system, there was only a little increase in the UA oxidation peak current (curve f), implying that the Fe-CP modified electrode has an excellent selectivity and response to DA. As shown in Fig. 3B, the Fe-L modified electrode also has a good response to DA (curve b) and a small response to UA at low concentration (curve c), but no response to AA (curve d). It was found that the oxidation peak potential of DA on the Fe-L modified electrode was more positive, around 0.15 V vs. SCE, and there was only a limited increase in the oxidation peak current after the concentration of DA was doubled (curve e), and no UA oxidation peak current was detected after the concentration of UA was doubled (curve f). Furthermore, the oxidation peak current of DA (200 μM) for Fe-CP is about 1.5 times that for Fe-L. The observed difference in the electrochemical response toward DA, AA and UA could be attributed to the different interactions between the analytes and ligands/CPs,49 in terms of negatively charged ligands and CPs (Table S2†), negatively charged AA,50 neutral UA,51 and positively charged DA52 at physiological pH 7.4, which may facilitate their electrostatic interactions, resulting in different selectivities and responses to the analytes on the ligands and CP modified electrodes. The same trend could also be found on the Ni-CP (Fig. 3C) and Ni-L (Fig. 3D) modified electrodes, and the oxidation peak current of DA (200 μM) for Ni-CP was about 1.6 times that for Ni-L. Additionally, the influence of the glassy carbon electrode (GCE) on the performance of the ligands and CPs could also be excluded since the GCE has a response to DA, AA and UA (Fig. S10†), while the modified ligands and CPs only have a significant response to DA. The results demonstrate that the sensor constructed by using the CPs could be applied for DA detection, and the CPs comprising electroactive metal-terpyridine complexes could exhibit enhanced electrochemical sensing performance compared with the electroactive ligands.
3.3 Performance comparison of the electroactive ligands and their CPs
The DA detection performance of the electrochemical sensor constructed by using the electroactive ligands and their CPs was examined in different pH environments. As shown in Fig. 4, an Fe-CP modified electrode shows a narrower DA oxidation peak than an Fe-L modified one within the applied pH range, implying the better diffusion of DA on the Fe-CP modified electrode.53 The wide DA oxidation peak also affects the ability of Fe-L to distinguish DA and UA, and as shown in Fig. 4A, the Fe-L modified electrode could not distinguish the oxidization peak of DA and UA when the concentration of DA increased, while the Fe-CP modified electrode could still recognize the oxidization peak of DA and UA in the pH range from 5.6 to 8.0 even at high DA concentrations (Fig. 4B). The same conclusion could be obtained for the Ni-L and Ni-CP modified electrodes (Fig. S11†), implying that the electroactive CP modified electrodes possess a better electrochemical sensing performance. Furthermore, the oxidization peak potential and current of DA on the ligands and CP modified electrodes varied with the pH (Fig. S12†). The DA oxidation peak potential of the CP modified electrodes is close to 0 V (vs. SCE) compared with that of the ligand modified electrode at the same pH, indicating that DA is easy to be oxidized on the CP modified electrode. Meanwhile, both the electroactive ligand and CP modified electrodes show a negatively shifted DA oxidation peak potential with increasing pH (Fig. S12A and B†).54 The DA oxidation peak current increased with the increase of pH until the pH reached 7.4, and then the DA oxidation peak current decreased (Fig. S12C and D†). This decrease may be attributed to the release of protons from the positively charged DA upon increasing the pH. Therefore, pH 7.4 was selected as the optimal one, which is consistent with the physiological pH. Furthermore, the DA oxidation peak current on the CP modified electrodes was larger than that on their ligands within the whole pH range, which may be attributed to the large surface area of CPs benefiting the easy access of DA to the abundant active sites, and the efficient charge transfer over the periodic array based on the redox hopping mechanism,31,32 facilitating the signal amplification and resulting in enhanced current response.Next, the diffusion coefficient and electron transfer rate towards the electrochemical DA redox reaction on the ligands and CP modified electrodes were examined. As shown in Fig. S13 and S14,† there are two ranges of lower (5–80 mV s−1) and higher (100–1000 mV s−1) scan rates on the ligand and CP modified electrodes. Based on the Randles–Sevcik equation, linear relationships were obtained between the oxidation peak current of DA and the square root of the scan rate in the two ranges of scan rates, respectively, and the diffusion coefficient could be obtained from the slope (Table 1). The diffusion coefficient in the lower scan rate range is larger than that in the higher scan rate range for the ligand and CP modified electrodes, because the reversibility of the electrochemical redox of DA on the modified electrode weakened when the scan rate increased, which could be found from the increasing peak-to-peak separations (Fig. S15†).55 It was also found that the reversibility of the electrochemical redox of DA on the CP modified electrodes is better than that on the ligand modified ones because the former had smaller peak-to-peak potential separation. Additionally, the linear plots observed in the lower scan rate range suggest that the diffusion controlled process drives the electrochemical oxidation of DA on the ligand and CP modified electrodes.56 The large diffusion coefficient on CP modified electrodes further indicated that the CPs are in favour of the DA transport to active sites, thus contributing to an enhanced electrochemical oxidation current of DA.23
| Scan rate (mV s−1) | Fe-L | Fe-CP | Ni-L | Ni-CP |
|---|---|---|---|---|
| Diffusion coefficient (cm 2 s −1 ) | ||||
| 5–80 | 8.78 × 10−6 | 1.56 × 10−5 | 6.44 × 10−6 | 1.20 × 10−5 |
| 100–1000 | 8.68 × 10−6 | 1.55 × 10−5 | 5.33 × 10−6 | 1.09 × 10−5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Heterogeneous electron transfer rate constants (cm s −1 ) | ||||
| 5–80 | 7.90 × 10−3 | 9.99 × 10−3 | 6.72 × 10−3 | 9.01 × 10−3 |
| 100–1000 | 7.80 × 10−3 | 9.96 × 10−3 | 6.13 × 10−3 | 8.61 × 10−3 |
The heterogeneous electron transfer rate of DA redox reaction on the ligand and CP modified electrodes was calculated based on the Gileadi method (see the ESI, Fig. S16†).57 As shown in Table 1, the heterogeneous electron transfer rates for the electroactive ligand and their CP modified electrodes are within the same order of magnitude, and the heterogeneous electron transfer rate constants obtained in the scan rate range of 5 to 80 mV s−1 are faster than that in the scan rate range of 100 to 1000 mV s−1, which is because the irreversibility of DA redox reaction on the ligand and CP modified electrodes increased at higher scan rates leading to a slow heterogeneous electron transfer rate.55Fe-CP has the fastest heterogeneous electron transfer rate followed by Ni-CP, Fe-L and Ni-L, which led to the same order of their final electrochemical DA oxidation peak current. The higher heterogeneous electron transfer rate constants for CPs than the ligands demonstrate that the integration of electroactive ligands into CPs could result in enhanced electrochemical performance for the DA redox reaction, which could be attributed to the rapid transport of DA in the periodic structure of the CPs and the easy access to the abundant active sites provided by the ordered and repeated electroactive ligands, favourable to the charge transfer based on the redox hopping mechanism,58–60 thus resulting in enhanced electrochemical DA oxidation peak current.1,4,33,34 Besides, the amount of Fe and Ni in the final electrode determined by ICP-OES is 5.180 × 10−5 mmol cm−2 (calcd 6.922 × 10−5 mmol cm−2) for Fe-CP and 4.515 × 10−5 mmol cm−2 (calcd 6.230 × 10−5 mmol cm−2) for Ni-CP, while the surface concentration of electrochemically accessible Fe or Ni sites estimated by CV (Fig. S17 and S18†) is 7.660 × 10−7 mmol cm−2 for Fe-CP and 6.128 × 10−7 mmol cm−2 for Ni-CP. Based on these data, the percentage of deposited catalyst that is electrochemically accessible is 1.48% for Fe-CP and 1.36% for Ni-CP, which is higher than that in the ligands of Fe-L (0.69%) and Ni-L (0.60%) (Table S4†). The results indicate that the CPs with a 1D chain structure may improve the utilization of the electroactive metal sites in the ligands.
3.4 Assay performance
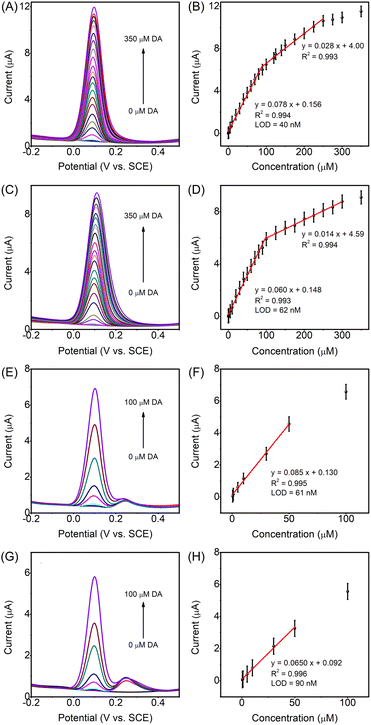
After the comparison between the electroactive ligands and their CPs, the conclusion that CPs have better electrochemical DA sensing performance could be reached. Therefore, the assay performance to DA with different concentrations in 0.1 M pH 7.4 PBS was explored on CP modified electrodes. The DA oxidation peak current on the CP modified electrode increased linearly with the increasing DA concentration from 0.05 to 350 μM in two different linear regions, as shown in Fig. 5A and C. For Fe-CP modified electrodes (Fig. 5A and B), the sensitivity for the first linear range was 1.12 μA μM−1 cm−2 in the DA concentration range from 50 nM to 90 μM and a detection limit (LOD) of 40 nM was estimated (see the ESI†), which is lower than that of other CP material modified electrodes (GCE) (Table S5†). The sensitivity for the second linear range was 0.40 μA μM−1 cm−2 in the range of DA concentration from 90 μM to 250 μM. At low DA concentration, there are abundant active sites available for DA, while as the DA concentration increased, multilayer DA gathered around inaccessible active sites, thus compromising the sensitivity.59–61 The same conclusion could be drawn for the Ni-CP modified electrode with two sensitivity values of 0.86 μA μM−1 cm−2 in the range of DA concentration from 100 nM to 100 μM (LOD = 62 nM), and of 0.20 μA μM−1 cm−2 in the range of DA concentration from 100 μM to 300 μM (Fig. 5C and D). Furthermore, the investigation on the detectable DA concentration of these CP modified electrodes in the presence of UA and AA with high concentration (100 μM) was conducted in 0.1 M pH 7.4 PBS. As shown in Fig. 5E–H, the detectable concentration of DA on the Fe-CP and Ni-CP modified electrodes both was 500 nM, which was very low relative to the added concentration of 100 μM UA and 100 μM AA. For these CP modified electrodes, the linear relationship between the DA oxidation peak current and the DA concentration (500 nM to 50 μM) with a low LOD and high sensitivity, respectively, was still retained, further indicating the high sensitivity and selectivity of the CP modified electrodes for DA detection.The selectivity of Fe-CP and Ni-CP modified electrodes towards DA in the presence of other typical interferents was further explored. It can be seen that the interference species induced negligible interference with a relative standard deviation (RSD) of 5% in DA detection on both Fe-CP and Ni-CP modified electrodes (Fig. S19†), indicating the potential application of the modified electrodes for selective DA detection in a complex system. On the other hand, to estimate the repeatability of Fe-CP and Ni-CP modified electrodes, 10 independent electrodes modified with these ligands and CPs were measured with a RSD of 3.5% for the Fe-CP modified electrode and a RSD of 4.0% for the Ni-CP modified one (Fig. S20†), implying good reproducibility. In addition, it was found that there are memory effects with the sensor. After the measurement at a higher concentration followed by rinsing with running water, the sensor can be used for DA measurement at a lower concentration. Furthermore, the stability of Fe-CP and Ni-CP modified electrodes was evaluated every 7 days (Fig. S21†). The electrode was rinsed by using running water after each measurement and stored under ambient air conditions. The DA oxidation peak current on the Fe-CP modified electrode maintained 96.9% of its initial DA oxidation peak current after 28 days, and for the Ni-CP modified electrode, the DA oxidation peak current maintained 96.7% of its initial value after 28 days, showing good stability. Additionally, the measurement of 90 CV cycles of DA redox was performed on the Fe-CP, Ni-CP, Fe-L and Ni-L modified electrodes, respectively, and the oxidation peak current toward DA exhibited insignificant variation (Fig. S22†), further demonstrating the high stability of the ligands and CPs. Finally, after the recycle test, the structures of the CPs were investigated by PXRD (Fig. S23†), showing that CPs remained intact. The states of Fe or Ni active sites in the CPs were analyzed by XPS (Fig. S24†), further confirming the stability of the CPs. SEM and the corresponding elemental mapping, and EDS analysis further confirmed the uniform distribution of the metal elements in the ligands and CPs (Fig. S25–S27†), demonstrating the high stability of the ligands and CPs for electrochemical DA sensing.
3.5 Detection of DA in simulated urine
Simulated urine was chosen to investigate the analytical reliability and potential application of these CP modified electrodes.62,63 The simulated urine (pH 6.0) was prepared according to the reported studies55,63 (see the ESI†). As shown in Fig. 6, a good linear relationship between the DA oxidation peak currents and DA concentrations (1 μM to 100 μM) was obtained for both Fe-CP and Ni-CP modified electrodes, although the LOD in the simulated urine was higher than the one obtained in 0.1 M PBS for these CP modified electrodes. The results show that the complicated chemical species and varied pH values in simulated urine have not hindered the ability of these CP modified electrodes for DA detection, indicating the acceptable reliability and potential application of these CP materials for DA detection in a complex system.4 Conclusions
In summary, the electrochemical DA sensing performance of the electroactive metal-terpyridine complexes before and after their integration into the stable Zr(IV) coordination polymers was compared. The results showed that integrating molecular metal-terpyridine complexes into CPs could result in improved properties. Compared with electroactive ligands, the CPs have a large diffusion coefficient towards DA oxidation and a fast heterogeneous electron transfer rate to DA oxidation, which may be attributed to the periodic architecture of the CPs with ordered incorporation of electroactive ligands as active centers where DA could transfer easily to the abundant active sites, contributing to the enhanced signal amplification and obtaining increased electrochemical response current, the low detection limit and the wide detection range. In addition, these CP modified electrodes show long-term stability and excellent reproducibility, and good feasibility in a complex system. This work not only benefits the understanding of the relationship between structure and performance, but also provides a strategy to develop CPs for electrochemical sensing applications in aqueous solutions.Author contributions
T. T. Y., conceptualization, data curation, investigation, visualization, writing – original draft; X. Y. Z., data curation, investigation, visualization; Y. Z., data curation, formal analysis; W.-Y. S., conceptualization, investigation, supervision, validation, funding, writing – review and editing. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (grant nos. 22231006 and 22171131) for financial support of this work. This work was also supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- S. Liu, C. Lai, X. Liu, B. Li, C. Zhang, L. Qin, D. Huang, H. Yi, M. Zhang, L. Li, W. Wang, X. Zhou and L. Chen, Coord. Chem. Rev., 2020, 424, 213520 CrossRef CAS.
- S. Zhang, F. L. Rong, C. P. Guo, F. H. Duan, L. H. He, M. H. Wang, Z. H. Zhang, M. M. Kang and M. Du, Coord. Chem. Rev., 2021, 439, 213948 CrossRef CAS.
- J. Calbo, M. J. Golomb and A. Walsh, J. Mater. Chem. A, 2019, 7, 16571–16597 RSC.
- S. Banerjee, R. I. Anayah, C. S. Gerke and V. S. Thoi, ACS Cent. Sci., 2020, 6, 1671–1684 CrossRef CAS PubMed.
- W. J. Schultz, J. Neurophysiol., 1998, 80, 1–27 CrossRef CAS.
- K. C. Berridge and T. E. Robinson, Brain Res. Rev., 1998, 28, 309–369 CrossRef CAS PubMed.
- R. Pivonello, D. Ferone, G. Lombardi, A. Colao, S. W. J. Lamberts and L. J. Hofland, Eur. J. Endocrinol., 2007, 156, 513–521 Search PubMed.
- A. Carlsson, Pharmacol. Rev., 1959, 11, 490–493 CAS.
- M. K. Siedlecka, E. Bączyńska and M. J. Niedziółka, Anal. Chem., 2019, 91, 10908–10913 CrossRef PubMed.
- K. M. Lohr, S. T. Masoud, A. Salahpour and G. W. Miller, Eur. J. Neurosci., 2017, 45, 20–33 CrossRef PubMed.
- J. Huang, Y. Liu, H. Hou and T. You, Biosens. Bioelectron., 2008, 24, 632–637 CrossRef CAS.
- X. J. Liao, H. M. Fu, T. T. Yan and J. P. Lei, Biosens. Bioelectron., 2019, 146, 111743 CrossRef CAS.
- Z. Hu, Y. Wang and D. Zhao, Chem. Soc. Rev., 2021, 50, 4629–4683 RSC.
- J. N. Lu, J. Liu, L. Z. Dong, S. L. Li, Y. H. Kan and Y. Q. Lan, Chem.–Eur. J., 2019, 25, 15830–15836 CrossRef CAS.
- Z. Chen, S. L. Hanna, L. R. Redfern, D. Alezi, T. Islamoglu and O. K. Farha, Coord. Chem. Rev., 2019, 386, 32–49 CrossRef CAS.
- M. L. Ding, X. C. Cai and H. L. Jiang, Chem. Sci., 2019, 10, 10209–10230 RSC.
- T. T. Yan, L. Y. Zhu, H. X. Ju and J. P. Lei, Anal. Chem., 2018, 90, 14493–14499 CrossRef CAS.
- L. Feng, G. S. Day, K. Y. Wang, S. Yuan and H. C. Zhou, Chem, 2020, 6, 2902–2923 CAS.
- M. O. Cichocka, Z. Z. Liang, D. W. Feng, S. Back, S. Siahrostami, X. Wang, L. Samperisi, Y. J. Sun, H. Y. Xu, N. Hedin, H. Q. Zheng, X. D. Zou, H. C. Zhou and Z. H. Huang, J. Am. Chem. Soc., 2020, 142, 15386–15395 CrossRef CAS PubMed.
- L. T. Yang, P. Y. Cai, L. L. Zhang, X. Y. Xu, A. A. Yakovenko, Q. Wang, J. D. Pang, S. Yuan, X. D. Zou, N. Huang, Z. H. Huang and H. C. Zhou, J. Am. Chem. Soc., 2021, 143, 12129–12137 CrossRef CAS PubMed.
- S. Pal, S. S. Yu and C. W. Kung, Chemosensors, 2021, 9, 306 CrossRef CAS.
- Y. C. Chen, W. H. Chiang, D. Kurniawan, P. C. Yeh, K. Otake and C. W. Kung, ACS Appl. Mater. Interfaces, 2019, 11, 35319–35326 CrossRef CAS PubMed.
- Z. Y. Zhou, S. Mukherjee, S. J. Hou, W. J. Li, M. Elsner and R. A. Fischer, Angew. Chem., Int. Ed., 2021, 60, 20551–20557 CrossRef CAS.
- S. Biswas, Y. L. Chen, Y. Xie, X. Sun and Y. Wang, Microchim. Acta, 2020, 187, 661 CrossRef CAS PubMed.
- J. J. Chen, Y. F. Zhu and S. Kaskel, Angew. Chem., Int. Ed., 2021, 60, 5010–5035 CrossRef CAS.
- X. Zhang, M. C. Wasson, M. Shayan, E. K. Berdichevsky, J. R. Noordberg, Z. Singh, E. K. Papazyan, A. J. Castro, P. Marino, Z. Ajoyan, Z. J. Chen, T. Islamoglu, A. J. Howarth, Y. Y. Liu, M. B. Majewski, M. J. Katz, J. E. Mondloch and O. K. Farha, Coord. Chem. Rev., 2021, 429, 213615 CrossRef CAS.
- W. Q. Liu, C. J. Lin, J. A. Weber, C. L. Stern, R. M. Young, M. R. Wasielewski and J. F. Stoddart, J. Am. Chem. Soc., 2020, 142, 8938–8945 CrossRef CAS.
- Z. L. Wu, J. Dong, W. Y. Ni, B. W. Zhang, J. Z. Cui and B. Zhao, Inorg. Chem., 2015, 54, 5266–5272 CrossRef CAS PubMed.
- F. F. Zhu, J. F. Fang, Y. J. Xiong, Q. Cheng, Y. Li and S. T. Yue, Inorg. Chem. Commun., 2016, 64, 71–73 CrossRef CAS.
- X. F. Li, X. Wang, Y. Y. Wu, X. W. Zhao, H. Y. Li and Y. M. Li, J. Solid State Chem., 2019, 269, 118–124 CrossRef CAS.
- M. L. Aubrey, B. M. Wiers, S. C. Andrews, T. Sakurai, S. E. L. Reyes, S. M. Hamed, C. Yu, L. E. Darago, J. A. Mason, J. Baeg, F. Grandjean, G. J. Long, S. Seki, J. B. Neaton, P. D. Yang and J. R. Long, Nat. Chem., 2018, 17, 625–632 CAS.
- T. T. Yan, P. Wang, Z. H. Xu and W. Y. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 13645–13652 CrossRef CAS PubMed.
- C. S. Liu, J. J. Li and H. Pang, Coord. Chem. Rev., 2020, 410, 213222 CrossRef CAS.
- S. Tajik, H. Beitollahi, F. G. Nejad, I. Sheikhshoaie, A. S. Nugraha, H. W. Jang, Y. Yamauchi and M. Shokouhimehr, J. Mater. Chem. A, 2021, 9, 8195–8220 RSC.
- X. Y. Zhang, C. F. Xie, S. Q. Wang, X. M. Cheng, Y. Zhang, Y. Zhao, Y. Lu and W. Y. Sun, Inorg. Chem., 2022, 61, 1590–1596 CrossRef CAS PubMed.
- M. E. Mahmoud, H. Audi, A. Assoud, T. H. Ghaddar and M. Hmadeh, J. Am. Chem. Soc., 2019, 141, 7115–7121 CrossRef.
- A. Mikolajczyk, A. Gajewicz, B. Rasulev, N. Schaeublin, E. M. Gardner, S. Hussain, J. Leszczynski and T. Puzyn, Chem. Mater., 2015, 27, 2400–2407 CrossRef CAS.
- N. Gibson, O. Shenderova, T. J. M. Luo, S. Moseenkov, V. Bondar, A. Puzyr, K. Purtov, Z. Fitzgerald and D. W. Brenner, Diamond Relat. Mater., 2009, 18, 620–626 CrossRef CAS.
- A. Maurin and M. Robert, J. Am. Chem. Soc., 2016, 138, 2492–2495 CrossRef CAS PubMed.
- M. Descostes, F. Mercier, N. Thromat, C. Beaucaire and M. G. Soyer, Appl. Surf. Sci., 2000, 165, 288–302 CrossRef CAS.
- C. Gao, Y. Su, X. Quan, V. K. Sharma, S. Chen, H. Yu, Y. Zhang and J. Niu, Appl. Catal., B, 2020, 276, 119016 CrossRef CAS.
- Y. N. Wang, T. Liu, L. X. Chen and D. B. Chao, Inorg. Chem., 2021, 60, 5590–5597 CrossRef CAS.
- Y. N. Wang, L. X. Chen, T. Liu and D. B. Cha, Dalton Trans., 2021, 50, 6273–6280 RSC.
- S. Gonell, J. L. Fillol and A. J. M. Miller, ACS Catal., 2021, 11, 615–626 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2001 Search PubMed.
- M. W. Hu, J. Y. Shen, Z. Yu, R. Z. Liao, G. G. Gurzadyan, X. C. Yang, A. Hagfeldt, M. Wang and L. C. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 30409–30416 CrossRef CAS.
- H. L. Rui, J. Y. Shen, Z. Yu, L. H. Li, H. X. Han and L. C. Sun, Angew. Chem., Int. Ed., 2021, 60, 16156–16163 CrossRef CAS.
- B. Si and E. Song, Chemosensors, 2018, 6, 1 CrossRef.
- F. Wu, W. Fang, X. Yang, J. Xu, J. Xia and Z. Wang, J. Chin. Chem. Soc., 2019, 66, 522–528 CrossRef CAS.
- J. J. Warren and J. M. Mayer, J. Am. Chem. Soc., 2010, 132, 7784–7793 CrossRef CAS.
- A. A. Elela, J. Adv. Res., 2017, 8, 513–527 CrossRef PubMed.
- J. L. Berfield, L. C. Wang and M. E. A. Reith, J. Biol. Chem., 1999, 274, 4876–4882 CrossRef CAS.
- S. Biswas, Y. L. Chen, Y. Xie, X. Sun and Y. Wang, Anal. Chem., 2020, 92, 4566–4572 CrossRef CAS PubMed.
- S. Pradhan, S. Biswas, D. K. Das, R. Bhar, R. Bandyopadhyay and P. Pramanik, New J. Chem., 2018, 42, 564–573 RSC.
- M. Ko, L. Mendecki, A. M. Eagleton, C. G. Durbin, R. M. Stolz, Z. Meng and K. A. Mirica, J. Am. Chem. Soc., 2020, 142, 11717–11733 CrossRef CAS.
- C. Liu, X. Bo and L. Guo, Sens. Actuators, B, 2019, 297, 126741 CrossRef CAS.
- H. Muhammad, I. A. Tahiri, M. Muhammad, Z. Masood, M. A. Versiani, O. Khaliq, M. Latif and M. Hanif, J. Electroanal. Chem., 2016, 775, 157–162 CrossRef CAS.
- D. Feng, Z. Y. Gu, J. R. Li, H. L. Jiang, Z. Wei and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed.
- Y. Chen, X. Sun, S. Biswas, Y. Xie, Y. Wang and X. Hu, Biosens. Bioelectron., 2019, 141, 111470 CrossRef CAS PubMed.
- Y. Chen, W. Huang, C. Wang, X. Zhai, T. Zhang, Y. Wang and X. Hu, ACS Sustainable Chem. Eng., 2020, 8, 13226–13235 CrossRef CAS.
- S. Biswas, R. Das, M. Basu, R. Bandyopadhyay and P. Pramanik, RSC Adv., 2016, 6, 100723–100731 RSC.
- M. Satyanarayana, K. K. Reddy and K. V. Gobi, Electroanalysis, 2014, 26, 2365–2372 CrossRef CAS.
- A. K. Ellerbee, S. T. Phillips, A. C. Siegel, K. A. Mirica, A. W. Martinez, P. Striehl, N. Jain, M. Prentiss and G. M. Whitesides, Anal. Chem., 2009, 81, 8447–8452 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials, reagents, apparatus, the synthesis methods of the electroactive ligands and their CPs, crystal data and structure refinements for Fe-CP, TGA, PXRD, XPS spectra, electrical conductivity measurements, measurements of zeta potential, CV measurements, interference test, preparation of artificial urine, DPV responses and CV curves, SEM images, elemental mapping, EDS spectra, calculation of the electrical conductivity, LOD diffusion coefficient and heterogenous electron transfer rate , and comparison of electroanalytical DA performances of CPs modified on a GCE. CCDC 2203773. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ta06797h |
| ‡ T. T. Y. and X.-Y. Z. contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |