Accelerating filtration by introducing an oscillation paradigm and its atomistic origin†
Na
Li
a,
Zemeng
Feng
a,
Huijuan
Lin
a,
Jixin
Zhu
 *ab and
Kui
Xu
*a
*ab and
Kui
Xu
*a
aSchool of Flexible Electronics (Future Technologies), Institute of Advanced Materials (IAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing 211816, P. R. China. E-mail: iamkxu@njtech.edu.cn
bState Key Laboratory of Fire Science, University of Science and Technology of China, 443 Huangshan Road, Hefei, 230027 P. R. China. E-mail: zhujixin@ustc.edu.cn
First published on 7th December 2022
Abstract
Developing high efficiency and anti-fouling filtration technologies is critical to meet the global water crisis. Improving the filtration efficiency while maintaining selectivity and reducing membrane contamination is a huge challenge. To overcome this challenge, we introduced an oscillation paradigm into filter membranes and discovered a previously unknown mechanism by molecular dynamics simulations. An ultrahigh permeability 355.04 L per cm2 per day per MPa is achieved and the salt rejection rate can be maintained at almost 99%, when using a designed covalent organic framework membrane (Hex-Aza-COF-2) in our simulations. A new concept of “spatial permeability” is proposed, which attributes the ultrahigh permeability to the loss of hydrogen bonds at the liquid/membrane interface caused by the oscillation, resulting in a larger effective pore for water molecule transport and a shorter residence time of water molecules in the membrane. The newly proposed oscillation filtration paradigm breaks the permeability-selectivity trade-off rule, overcomes the drawbacks of low permeability of traditional selectivity membranes, and proposes a new theory for designing high-performance filtration membranes.
1. Introduction
With the development of the economy and the increase in water demand, the importance of clean water resources cannot be ignored. More than 97% of liquid water on earth cannot be used directly due to the existence of salt, and frequent droughts also exacerbate the shortage of freshwater.1–5 The use of seawater is considered an effective way to solve this water crisis. Traditionally, reverse osmosis techniques for desalination have been limited by permeability-selectivity trade-off rules and inefficiencies.6,7 The improvement of filtration efficiency usually occurs at the cost of a decrease in selectivity. Improving the filtration efficiency while maintaining selectivity is a huge challenge. To overcome this challenge, a potential viable method is introducing a new filtration paradigm and designing new membrane materials, simultaneously.Recently, numerous studies have shown that membranes made using graphene,8–11 MoS2,12–15 titanium carbide16 and boron nitride17 exhibit good desalination performance by simulations14 and experiments.18 However, the pristine membranes mentioned above are intrinsically impermeable to water molecules or have inhomogeneous pore sizes. We should make them porous with a controlled homogeneous pore size for water desalination. This process is usually accomplished by bombarding the membrane with ions. However, reasonable control of the pore size is usually hard to achieve with plasma/ion bombardment, and pore size control is at the root of ion selection.19 Second, ion bombardment sometimes causes irreversible mechanical damage to expensive nanomembranes, making them unusable for seawater desalination, which is also a key factor preventing the large-scale application of the above membrane materials.
A straightforward solution to the above problem is to use membranes with intrinsic nanopores. Covalent organic frameworks (COFs), as an emerging class of periodically crystalline porous polymers, hold promise as high-performance selective permeation membranes due to their ordered channels, excellent stability, and adjustable pore size.20–27 The high porosity and well-defined in-plane pores endow COF membranes with fast water permeability and high separation precision compared to conventional amorphous polymers,28–32 with great potential to drive the upper limit of permeability and selectivity. As reported, Xiao et al. experimentally demonstrated that COF membranes are promising building blocks for the production of high-permeability reverse osmosis (RO) membranes.33 Zhang et al. investigated a series of two-dimensional functional COF membranes for seawater desalination.34 All these studies indicate that COF membranes are very promising materials for seawater desalination applications.
Meanwhile, in traditional filtration processes, the membranes are generally fixed,35–38 and suffer from inefficiency and permeability-selectivity trade-off rules. Therefore, seeking new filtration methods to improve efficiency while breaking the permeability-selectivity trade-off rule is a quite important topic. Zhang et al. achieved a high permeability of 176 L per cm2 per day per MPa for the first time using the rotary filtration of CNT systems, and the selectivity can be maintained above 95%.39 Their rotary filtration method opens a new door for improving membrane filtration efficiency. However, the usage of the rotary filtration method is limited to tube systems (e.g., CNTs), and it is difficult to use with a wider range of planar membranes. A more general filtration paradigm, which can work not only with tube membranes but also with planar membranes, is urgently needed.9,35–37,40 Here, we propose a new solution: introducing oscillation into filtration membranes. Since surface oscillation will affect the diffusion of interfacial fluids, wetting properties, and thus fluid transport, these effects are no exception for desalination using nanoporous membranes.41–44 At the same time, oscillation filtration can effectively prevent membrane pollution and achieve the effect of self-cleaning.45,46 Therefore, the oscillation filtration paradigm is expected to have a large impact on the water treatment performance of membranes.
In this work, we apply an oscillation to the Hex-Aza-COF-2 (and for simplicity, we'll define it as COF-O) membrane for desalination, by making the membrane move overall with a specific pattern. In the practical process, the membrane can be oscillated by using a electromechanical oscillator.47 Here, we make the membrane move in a simple harmonic oscillation with the center of mass (COM) position M = (x, y, z) as a function of time as M(t) = M0 + A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(ω × t), where M0 = (x0, y0, z0) represents its initial COM positions, A is the specified amplitude vector with components (Ax, Ay, Az), the oscillation period T = 2π/ω, and t is the time. During the filtration process, the permeability and selectivity of the membranes at different states (different pore sizes, different oscillation amplitudes A and periods T, or no-oscillation) are examined and recorded. Furthermore, the inner atomistic mechanism of the oscillation filtration membrane to improve the permeability is revealed, which opens a new door for the design of high-efficiency reverse osmosis seawater desalination devices.
sin(ω × t), where M0 = (x0, y0, z0) represents its initial COM positions, A is the specified amplitude vector with components (Ax, Ay, Az), the oscillation period T = 2π/ω, and t is the time. During the filtration process, the permeability and selectivity of the membranes at different states (different pore sizes, different oscillation amplitudes A and periods T, or no-oscillation) are examined and recorded. Furthermore, the inner atomistic mechanism of the oscillation filtration membrane to improve the permeability is revealed, which opens a new door for the design of high-efficiency reverse osmosis seawater desalination devices.
2. Simulation details
Molecular dynamics simulation methods have proven to be very effective tools to study permeation processes in porous membranes involving molecular transport in confined spaces at the nanoscale.48–50 The COF oscillation filtration system is shown in Fig. 1a and b. And to verify the accuracy of the experiment, we tested two different COF membrane materials; the atomic structures of the two kinds of COF units (COF-O and COF-H) are shown in Fig. 1c, and the difference is that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group becomes the C–H functional group. The size of the simulation box in the x–y–z dimensions is 5.7 nm × 6.6 nm × 30.0 nm with the COF membrane located in the x–y plane, and the simulation box contains four parts, including a graphene piston applying pressure to the brine, a COF filter membrane, and brine containing sodium and chloride ions at a molar concentration of 0.5 M,51–53 slightly lower than the salinity of seawater (∼35 g L−1).54 The lower salinity was chosen because the concentration of water flowing into the pure water region would effectively increase the salinity of the seawater side during the simulation. The synthesis route and structural properties of the COF membrane are shown in Fig. S1.† The oscillation period T is in the range of 0–4.0 ps, and the amplitude A is in the range of 0–2.0 Å. In the practical process, frequency < 25 Hz and amplitude < 0.015 m were generally used.55 For the COF-H material, we chose the pressure of 50–250 MPa, which is commonly used in most simulation studies,13,15,35 while for the COF-O material, in order to save computing resources, we chose the pressure of 100 MPa to explore the influence of amplitude, period and offset on the water treatment performance of the membrane. Periodic boundary conditions are used for all three dimensions of the simulation frame. The COF membrane is set to rigid throughout the simulation. However in the actual filtration process, the stability of the membrane can be enhanced by embedding the supporting layer to avoid the delamination of the COF layer.56 Representative movies of the COF-O atomic trajectory with a 0% offset oscillating membrane and fixed membrane are provided in movie S1.†
O functional group becomes the C–H functional group. The size of the simulation box in the x–y–z dimensions is 5.7 nm × 6.6 nm × 30.0 nm with the COF membrane located in the x–y plane, and the simulation box contains four parts, including a graphene piston applying pressure to the brine, a COF filter membrane, and brine containing sodium and chloride ions at a molar concentration of 0.5 M,51–53 slightly lower than the salinity of seawater (∼35 g L−1).54 The lower salinity was chosen because the concentration of water flowing into the pure water region would effectively increase the salinity of the seawater side during the simulation. The synthesis route and structural properties of the COF membrane are shown in Fig. S1.† The oscillation period T is in the range of 0–4.0 ps, and the amplitude A is in the range of 0–2.0 Å. In the practical process, frequency < 25 Hz and amplitude < 0.015 m were generally used.55 For the COF-H material, we chose the pressure of 50–250 MPa, which is commonly used in most simulation studies,13,15,35 while for the COF-O material, in order to save computing resources, we chose the pressure of 100 MPa to explore the influence of amplitude, period and offset on the water treatment performance of the membrane. Periodic boundary conditions are used for all three dimensions of the simulation frame. The COF membrane is set to rigid throughout the simulation. However in the actual filtration process, the stability of the membrane can be enhanced by embedding the supporting layer to avoid the delamination of the COF layer.56 Representative movies of the COF-O atomic trajectory with a 0% offset oscillating membrane and fixed membrane are provided in movie S1.†
In molecular dynamics modelling, we take the Lennard-Jones (LJ) potential parameters of COF atoms in this work from the Dreiding force field force field,28,57–59 which has been widely used in the study of COFs or metal–organic skeletal materials. The standard SPC/E water model60,61 is used to simulate water molecules and its correctness and soundness have been systematically and extensively verified by many calculations of the fundamental physical properties of water. Lennard-Jones and Coulomb potentials are used to calculate intermolecular interactions between ions and atoms.9 Long-range Coulomb interactions are treated by the particle–particle–particle–particle–grid (PPPM) method.62 The parameters in the LJ potential are listed in Fig. S2.† Some parameters between different types of atoms are calculated according to the Lorentz–Berthelot mixing rule.63 MD simulations are performed using the LAMMPS package in the NVT system synthesis at a constant temperature of 300 K controlled by a Nosé–Hoover thermostat.64 The time step is 1 fs, and all simulation results are collected within 5 ns. Considering that the equilibrium state before desalination is very important for collecting accurate MD data, the whole system was equilibrated for 1.0 ns before collecting the data, while the system could reach equilibrium at 0.5 ns. All the water permeance and salt rejection data are averaged from three simulations with different initial geometric configurations.
3. Results and discussion
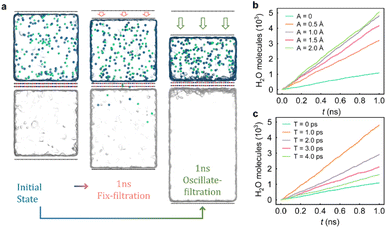
Permeability and selectivity are key indicators to evaluate the performance of RO membranes. Fig. 2a displays snapshots of the oscillating membrane system and fixed membrane system after the 1 ns filtration process. It can be clearly seen that the oscillating membrane allows the transport of more water molecules and fewer ions, meaning its permeability and selectivity are improved simultaneously. Fig. 2b and c show the relationship between the number of penetrated water molecules and the simulation time for a fully stacked bilayer COF-O structure with a pore size of 1.2 nm (larger than the hydrated ion kinetic diameter < 1.0 nm) at different oscillation amplitudes A and periods T. We can see that the permeated water molecules increase linearly with time in all cases, proving that the COF-O membrane reaches steady state filtration.As shown in Fig. 3a, with the oscillation period T fixed at 1.0 ps, while the oscillation amplitude increases from A = 0 (no oscillation) to A = 2.0 Å, the water flux increases from 82.04 L per cm2 per day per MPa to 355.04 L per cm2 per day per MPa without loss of selectivity (Fig. 3a), which is 2 times the water flux obtained from the rotary filtration method of CNTs.39 When fixing the amplitude A = 1 Å and varying the oscillation period T, a similar trend can be observed. The water flux gradually increases from 106.96 L per cm2 per day per MPa to 327.88 L per cm2 per day per MPa as the period T gradually decreases from 4.0 ps to 1.0 ps (Fig. 3c). The water flux at the A = 1 Å, T = 1.0 ps oscillating case reaches as high as ∼4.0 times larger than the fixed case. In particular, the fully stacked oscillating COF-O membrane can maintain an ultrahigh selectivity above 93% in all examined cases (Fig. 3a and c). This is due to the boundary slip of hydrated ions near the oscillation membrane, which makes the salt rejection no longer strictly depend on the small and uniform pore size, and this was also confirmed by the study of shear membranes by Zhang et al.39
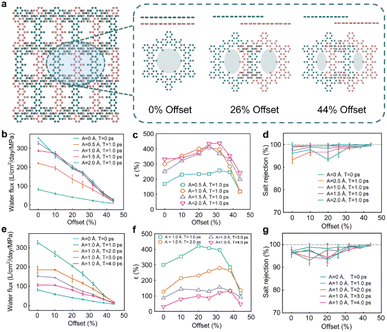
In general, the salt rejection rate is defined as R = (1 − Cp/Cf) × 100%, where Cp and Cf are the salt concentrations of the permeate and feed solutions, respectively. Here, we define the enhancement rate of water flux and salt rejection as ε = (Poscillation − Pfix)/Pfix, δ = Roscillation − Rfix, where Poscillation and Pfix are the water flux of the oscillating and fixed membranes. Roscillation and Rfix are the salt rejection rates of the membrane in the oscillating and fixed states, respectively. To our surprise, Fig. 3b shows that with the increase in the oscillation amplitude A, the water flux enhancement rate ε and the salt rejection enhancement rate δ increase simultaneously, and the highest water flux enhancement rate reaches 332.8% at amplitude A = 2 Å and period T = 1.0 ps. Fig. 3d shows the effect of the oscillation period T on the water flux enhancement rate ε and the salt rejection enhancement rate δ, and the water flux enhancement rate and the salt rejection enhancement rate increase linearly as the period T decreases. Therefore, the above results prove that this novel oscillation-induced filtration paradigm achieves the goal of significantly increasing the water permeability without losing any selectivity by increasing the oscillation amplitude A or decreasing the oscillation period T. Subsequently, we further discussed the effect of pore size on permeability with the proposed oscillation filtration system. As in reality, COF membranes tend to stack with offsets.28 A recent study showed that despite exhibiting the same pattern in a powder X-ray diffraction (XRD) test, COF stacking with a small horizontal offset is more stable than fully overlapping stacking,28,33,65 where the offset is the amount of misalignment between two COF membranes in the vertical direction. The offset exists when there are two or more layers of nanosheets in the filtration system. The different offsets will cause a change in the pore size (Fig. 4a), which is expected to have a large effect on the water flux. Therefore, we constructed a series of bilayer COF-O membranes with 7 different offsets from 0% to 50% for the oscillating filtration systems. (All 7 models are shown in Fig. S15†)
The permeability of the oscillation COF-O membrane at different offsets as a function of oscillation amplitude A and period T are shown in Fig. 4b and e. It can be seen that as the offset increases (that is, the pore size decreases), the water permeability decreases gradually. In the fixed state (A = 0 Å, T = 0 ps), as the offset increases to 44% (that is, the pore size decreases to 0.65 nm), the permeability decreases to 8.02 L per cm2 per day per MPa from 82.04 L per cm2 per day per MPa. For all the cases with different offsets, the water permeability increases significantly when the oscillation is applied compared with the fixed state. A larger oscillation amplitude A and smaller period T lead to a stronger enhancement of permeability. For the 44% offset case, the permeability increases from 8.02 L per cm2 per day per MPa to 27.37 L per cm2 per day per MPa in the fixed state and oscillating state (A = 2 Å, T = 1.0 ps), respectively, exhibiting a 341.2% increase.
Therefore, for oscillation-induced permeability, even with a quite small pore size, we can still obtain a high permeability by regulating the oscillation periods and amplitudes. From Fig. 4d and g, we can see that as the offsets increase, the salt rejection rate slightly increases, reaching nearly 100% in the 44% offset case. Details of the water flux and salt rejection rate at different offsets are listed in Fig. S3–S14.† It is also very interesting to note that the oscillation-induced water flux enhancement rate at offsets of 20–30% (the pore size is 0.8–1.0 nm) is higher than other offsets (Fig. 4c and f). Overall, the permeability acquired by the oscillating membrane filtration is significantly higher than that of the fixed membrane filtration for all the pore sizes tested.
To reveal the atomistic origin of the oscillation-induced ultrahigh permeability, we compared the two-dimensional number density maps of water molecules inside the pores of fixed and oscillating COF-O membranes to obtain the profile characteristics of water passing through the pores. Fig. 5a shows that for the fixed membrane, water molecules tend to gather in the nanopore, and the density profile is relatively larger. However, for the oscillating membrane, no aggregation of water molecules was observed in the pore and the distribution was more uniform (Fig. 5c). Density cloud maps of COF-O membranes with several other offsets are shown in Fig. S16.† Similar features can be observed in the radial distribution map of water molecules in Fig. 5b and S17.† Thus, this oscillation pattern reduces the aggregation of water molecules in the membrane pore and facilitates the rapid passage of water molecules.
The structures of water molecules in the COF-O membrane channel were further investigated by hydrogen bonding (HB). HB was defined by the following two geometric criteria: (i) the distance between the acceptor and donor was less than 3.5 Å, and (ii) the hydrogen donor–acceptor angle was less than 30°.66 The average HB number per water molecule along the Z axis for the 0% offset COF-O membrane filtration system was calculated and is shown in Fig. 5d. The results show that the HB structure of water molecules in the channel undergoes a series of breaks and reconstructions when water molecules pass through the channel, thus reducing their transport efficiency.53 The existence of the ordered hydrogen bonding network is the key factor preventing water molecules from crossing the membrane.28,29
According to the geometric criterion of HB, the calculated average number of HBs in the feed solution and pure water is comparable. The presence of Na+ and Cl− makes the corresponding values on the feed solution side (∼2.72) lower than those on the pure water side (∼3.00) for the oscillating COF-O membrane with a 0% offset. Interestingly, a valley is observed as the water molecules approach the membrane pore, and the number of HBs decreases to 1.01 and 2.01 for the oscillating and fixed cases, respectively. Moreover, according to the hydrogen bond distribution results under different offsets in Fig. S16,† it can be seen that the number of HBs in the oscillating membrane with different offsets is much smaller than that in the fixed membrane, and the peak value under the fixed membrane conditions is relatively high. This result indicates that the interaction between water molecules and the fixed membrane is much stronger than that of the oscillating membrane, making it easier for water molecules to be trapped in the channels of the fixed membrane. This can also be confirmed in the simulated snapshots of the fixed membrane (Fig. 6c) and the oscillating membrane (Fig. 6a).
Therefore, when water molecules approach the membrane pores, the water molecules first need to break their HB from the bulk solution and form new HBs with other water molecules after passing through the pores. This “break-form” process limits the movement of water molecules, and makes the water molecules trap inside the fixed membrane for a longer time, leading to a lower water flux passing through the fixed membrane. It is worth noting that when oscillation is applied, the number of HBs is reduced due to the collision of water molecules with the membrane. It is more difficult for water molecules close to the membrane to form stable HBs with the pore wall, which reduces the transport resistance of water molecules, and shortens the residence time of water molecules in the membrane, so as to reduce the accumulation of water molecules in the membrane and achieve the purpose of rapid separation. This conclusion can be confirmed by the trajectory of water molecules and ions passing through the membrane in Fig. 6. Compared with the fixed membrane, the retention time of water molecules in the oscillating membrane is nearly 5 times shorter, and the water molecules can pass through the membrane hole quickly. This can also be confirmed by the velocity distribution of water molecules approaching the membrane pore in Fig. 7a and S18.† At the same time, we compared the anti-fouling ability of the COF membrane before and after oscillation using the average number of solute ions adsorbed on the membrane and the residence time in membrane pores. As shown in Fig. S21 and S22,† the number of solute ions adsorbed in the fixed membrane is about 8.7 times higher than that in the oscillating membrane. As a result, the oscillation makes it difficult for ions to adsorb on the membrane, which is conducive to anti-fouling. (Fig. S23†) The movement trajectory of solute ions in the membrane pores can be seen that the residence and accumulation time of ions in the oscillating membrane pores is 10 times shorter than that of the fixed membrane, thereby reducing the blockage of the membrane pores and achieving the effect of anti-fouling.
Since the oscillation-induced high permeability essentially depends on the difference in permeation space caused by the variation in the number of HBs at the solid–liquid interface, we propose a new separation mechanism called “spatial permeability”. Obviously, the water flux of spatial permeability no longer depends only on the pore size, and the oscillation period T of the membrane and the amplitude A will also have a significant impact on the water flux. We found that as the oscillation period decreases or the amplitude increases, the COF-O membrane with a small pore size can still achieve extremely high permeability. Therefore, compared with fixed membranes, oscillating membranes can achieve high permeability by adjusting the amplitude and period in addition to the pore size. At present, the permeation principle of all fixed porous reverse osmosis membranes generally relies on the difference in membrane pore size. “Spatial permeability” can effectively bypass the pore size limitation to obtain ultrahigh water flux, and will further reduce the energy consumption requirements of reverse osmosis membranes.
To determine whether the HB loss induced by the oscillation filtration paradigm is influenced by the membrane thickness as well as the functional groups in the pore, we further investigated the COF-O filtration system with 1/2/4/8 layers of membrane thickness and another COF membrane structure called COF-H. The structural model comparison of COF-O and COF-H is shown in Fig. 1c. The difference between COF-O and COF-H is the inner functional group, and the O atoms are replaced by H atoms. As expected, at A = 1 Å and T = 1.0 ps, the permeability of COF-O filtration systems with different thicknesses in the oscillating state is always higher than that in the fixed state, and the hydrogen bond reduction mechanism mentioned above is also reasonable. (Fig. S19†) At the same time, the permeability of the COF-H membranes with an offset of 32% in the oscillating state was 104.8 L per cm2 per day per MPa, which was 13 times higher than that in the fixed state (8.05 L per cm2 per day per MPa) and slightly lower than 32% offset of COF-O (113.26 L per cm2 per day per MPa). At various reverse osmosis pressures from 50–250 MPa, we consistently observed this high permeation enhancement. In addition, consistent with what was discovered before, the selectivity of the oscillating COF-H membrane is also well maintained (Fig. 7b). From the distribution of the number of hydrogen bonds, it can be seen that the number of hydrogen bonds in the pores of the oscillating membrane is much smaller than that of the fixed membrane (Fig. 7c), similar to the COF-O results. Thus, we can conclude that for the membrane pores with different chemical environments, the solid/liquid interface will experience significant hydrogen bond loss due to oscillation. Therefore, compared with fixed membranes, the oscillation filtration paradigm shows better water treatment performance, and the proposed spatial permeability mechanism above could be widely applicable to COF membranes with different thicknesses and different functional groups.
In Fig. 7d, we compare the water flux and salt rejection of the oscillating COF-O membranes with those of other representative membranes including nanoporous graphene membranes,13 MoS2 membranes,13 covalent triazine backbones (CTFs),57 TiC2,16 Ti3C2,16 and rotated CNTs.39 Some optimum data for desalination by oscillating COF-O membranes with different offsets obtained in this work are shown in Fig. 7d. It can be seen that for salt rejection rates above 95%, the permeability of the oscillating COF-O membranes is as high as 355.04 L per cm2 per day per MPa, which is 700 to 2000 times greater than that of the best reported commercial membranes67 and 5 times greater than the reported permeability of 64.2 L per cm2 per day per MPa for CTF.57 In addition, this water permeability can be more than 2 times as large as the currently reported maximum water permeability of rotated CNTs.39 In particular, by adjusting the membrane pore sizes, as well as the oscillation amplitude A and period T, we can easily obtain our desired water permeability and salt rejection rate. This oscillation filtration paradigm is expected to be applied to COF membranes with larger pore sizes, thus achieving higher permeability and selectivity goals. In this study, we report a new oscillation-induced separation paradigm that achieves an ultrahigh permeability of 355.04 L per cm2 per day per MPa by making COF membranes move in a simple harmonic oscillation, and the salt rejection rate can be maintained above 99%. At the same time, we further reveal the intrinsic mechanism of the permeability enhancement of oscillating filtration, and propose a new concept of “spatial permeability”, which attributes the ultrahigh permeability to the oscillation-induced loss of hydrogen bonds at the liquid/membrane interface that increases the effective pore area for water molecule transport, and the residence time of water molecules in the membrane becomes shorter, which greatly improves the permeation efficiency.
This novel oscillation-induced separation paradigm for COF membranes bypasses the traditional limitations of drilling holes, pore size, and membrane pollution, and achieves the goal of extremely high permeability and selectivity by manipulating oscillation amplitude A and period T other than pore size. In conclusion, this study may further revolutionize the design of the next generation of seawater desalination technology, triggering an upsurge in theoretical and experimental research on oscillating membranes, and opening a new door for the design of high-efficiency reverse osmosis seawater desalination devices.
4. Conclusions
In this study, we report a new oscillation-induced separation paradigm that achieves an ultrahigh permeability of 355.04 L per cm2 per day per MPa by making COF membranes move in a simple harmonic oscillation, and the salt rejection rate can be maintained above 99%. At the same time, we further reveal the intrinsic mechanism of the permeability enhancement of oscillating filtration, and propose a new concept of “spatial permeability”, which attributes the ultrahigh permeability to the oscillation-induced loss of hydrogen bonds at the liquid/membrane interface that increases the effective pore area for water molecule transport, and the residence time of water molecules in the membrane becomes shorter, which greatly improves the permeation efficiency. This novel oscillation-induced separation paradigm for COF membranes bypasses the traditional limitations of drilling holes, pore size, and membrane pollution, and achieves the goal of extremely high permeability and selectivity by manipulating oscillation amplitude A and period T other than pore size. In conclusion, this study may further revolutionize the design of the next generation of seawater desalination technology, triggering an upsurge in theoretical and experimental research on oscillating membranes, and opening a new door for the design of high-efficiency reverse osmosis seawater desalination devices.Author contributions
Na Li: investigation, data curation, formal analysis, writing – original draft. Zemeng Feng: data curation, writing – original draft. Huijuan Lin: writing – original draft. Jixin Zhu: formal analysis, funding acquisition. Kui Xu: conceptualization, investigation, formal analysis, funding acquisition, resources, supervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
K. Xu acknowledges the support from the National Natural Science Foundation of China (Grant number 22202102) and Natural Science Foundation of Jiangsu Province (Grant number BK20200702). J. X. Zhu acknowledges the support from the National Natural Science Foundation of China (Grant numbers 51872139, and 52172204).References
- Y. Wang, Z. Cao and A. Barati Farimani, npj 2D Mater. Appl., 2021, 5, 66 CrossRef.
- J. Eliasson, Nature, 2015, 517, 6 CrossRef CAS PubMed.
- M. M. Mekonnen and A. Y. Hoekstra, Sci. Adv., 2016, 2, e1500323 CrossRef PubMed.
- J. T. Mueller and S. Gasteyer, Nat. Commun., 2021, 12, 3544 CrossRef CAS PubMed.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- J. K. Holt, H. G. Park, Y. Wang, M. Stadermann, A. B. Artyukhin, C. P. Grigoropoulos, A. Noy and O. Bakajin, Science, 2006, 312, 1034–1037 CrossRef CAS PubMed.
- M. Di Vincenzo, A. Tiraferri, V. E. Musteata, S. Chisca, R. Sougrat, L. B. Huang, S. P. Nunes and M. Barboiu, Nat. Nanotechnol., 2021, 16, 190–196 CrossRef CAS PubMed.
- J. Muscatello, F. Jaeger, O. K. Matar and E. A. Muller, ACS Appl. Mater. Interfaces, 2016, 8, 12330–12336 CrossRef CAS PubMed.
- Z. Cao, G. Markey and A. Barati Farimani, J. Phys. Chem. B, 2021, 125, 11256–11263 CrossRef CAS PubMed.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- D. Cohen-Tanugi, L. C. Lin and J. C. Grossman, Nano Lett., 2016, 16, 1027–1033 CrossRef CAS PubMed.
- J. P. K. Abal, R. F. Dillenburg, M. H. Köhler and M. C. Barbosa, ACS Appl. Nano Mater., 2021, 4, 10467–10476 CrossRef CAS.
- Z. Cao, V. Liu and A. Barati Farimani, ACS Energy Lett., 2020, 5, 2217–2222 CrossRef CAS.
- P. O. Oviroh, T. C. Jen, J. Ren, L. M. Mohlala, R. Warmbier and S. Karimzadeh, Langmuir, 2021, 37, 7127–7137 CrossRef CAS PubMed.
- M. Heiranian, A. B. Farimani and N. R. Aluru, Nat. Commun., 2015, 6, 8616 CrossRef CAS PubMed.
- K. Meidani, Z. Cao and A. Barati Farimani, ACS Appl. Nano Mater., 2021, 4, 6145–6151 CrossRef CAS.
- H. Gao, Q. Shi, D. Rao, Y. Zhang, J. Su, Y. Liu, Y. Wang, K. Deng and R. Lu, J. Phys. Chem. C, 2017, 121, 22105–22113 CrossRef CAS.
- Y. Wen, R. Dai, X. Li, X. Zhang, X. Cao, Z. Wu, S. Lin, C. Y. Tang and Z. Wang, Sci. Adv., 2022, 8, eabm4149 CrossRef CAS PubMed.
- I. E. Roslon, R. J. Dolleman, H. Licona, M. Lee, M. Siskins, H. Lebius, L. Madauss, M. Schleberger, F. Alijani, H. S. J. van der Zant and P. G. Steeneken, Nat. Commun., 2020, 11, 6025 CrossRef CAS PubMed.
- A. Halder, M. Ghosh, M. A. Khayum, S. Bera, M. Addicoat, H. S. Sasmal, S. Karak, S. Kurungot and R. Banerjee, J. Am. Chem. Soc., 2018, 140, 10941–10945 CrossRef CAS PubMed.
- R. Shi, L. Liu, Y. Lu, C. Wang, Y. Li, L. Li, Z. Yan and J. Chen, Nat. Commun., 2020, 11, 178 CrossRef CAS PubMed.
- W. Wang, V. S. Kale, Z. Cao, S. Kandambeth, W. Zhang, J. Ming, P. T. Parvatkar, E. Abou-Hamad, O. Shekhah, L. Cavallo, M. Eddaoudi and H. N. Alshareef, ACS Energy Lett., 2020, 5, 2256–2264 CrossRef CAS.
- S. S. Han, H. Furukawa, O. M. Yaghi and W. A. Goddard III, J. Am. Chem. Soc., 2008, 130, 11580–11581 CrossRef CAS PubMed.
- H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268–272 CrossRef CAS PubMed.
- Y. Lan, X. Han, M. Tong, H. Huang, Q. Yang, D. Liu, X. Zhao and C. Zhong, Nat. Commun., 2018, 9, 5274 CrossRef PubMed.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- R. Wang, J. Guo, J. Xue and H. Wang, Small Struct., 2021, 2, 2100061 CrossRef CAS.
- W. Zhou, M. Wei, X. Zhang, F. Xu and Y. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 16847–16854 CrossRef CAS PubMed.
- Y. Zhang, T. Fang, Q. Hou, Z. Li and Y. Yan, Phys. Chem. Chem. Phys., 2020, 22, 16978–16984 RSC.
- X. Feng, L. Chen, Y. Dong and D. Jiang, Chem. Commun., 2011, 47, 1979–1981 RSC.
- Z. Xia, Y. Zhao and S. B. Darling, Adv. Mater. Interfaces, 2020, 8, 2001507 CrossRef.
- J. Li, X. Zhou, J. Wang and X. Li, Ind. Eng. Chem. Res., 2019, 58, 15394–15406 CrossRef CAS.
- A. Xiao, X. Shi, Z. Zhang, C. Yin, S. Xiong and Y. Wang, J. Membr. Sci., 2021, 624, 119122 CrossRef CAS.
- K. Zhang, Z. He, K. M. Gupta and J. Jiang, Environ. Sci.: Water Res. Technol., 2017, 3, 735–743 RSC.
- Z. Cao, V. Liu and A. Barati Farimani, Nano Lett., 2019, 19, 8638–8643 CrossRef CAS PubMed.
- V. Mortazavi, A. Moosavi and A. Nouri-Borujerdi, J. Mol. Liq., 2022, 355, 118953 CrossRef CAS.
- Z. Li, Q. Han and Y. Qiu, Desalination, 2022, 528, 115610 CrossRef CAS.
- X. Yu, J. Hou, H. Wu, J. Rong, X. Wang, K. Xu and J. Feng, J. Mater. Chem. A, 2021, 9, 21158–21166 RSC.
- Z. Zhang, S. Li, B. Mi, J. Wang and J. Ding, Sci. Adv., 2020, 6, eaba9471 CrossRef CAS PubMed.
- B. Liu, A. W.-K. Law and K. Zhou, J. Membr. Sci., 2018, 550, 554–562 CrossRef CAS.
- Y. Noh and N. R. Aluru, Nano Lett., 2022, 22, 419–425 CrossRef CAS PubMed.
- M. Ma, F. Grey, L. Shen, M. Urbakh, S. Wu, J. Z. Liu, Y. Liu and Q. Zheng, Nat. Nanotechnol., 2015, 10, 692–695 CrossRef CAS PubMed.
- S. Marbach, D. S. Dean and L. Bocquet, Nat. Phys., 2018, 14, 1108–1113 Search PubMed.
- H. Qiu, R. Shen and W. Guo, Nano Res., 2010, 4, 284–289 CrossRef.
- L. Pu, J. Zhang, C. Wang, Y. Pan, Y. Zhao, Y. Bu, Q. Zhang, B. Pan and G. Gao, J. Membr. Sci., 2021, 638, 119722 CrossRef CAS.
- H. G. Gomaa, S. Rao and M. A. Taweel, J. Membr. Sci., 2011, 381, 64–73 CrossRef CAS.
- A. Ullah, K. Shahzada, S. W. Khan and V. Starov, Desalination, 2020, 491 Search PubMed.
- G. Lei, D. Chen, X. Zhang and H. Liu, J. Mol. Liq., 2021, 331, 115813 CrossRef CAS.
- T. Z. X. Hong, M. Dahanayaka, B. Liu, A. W.-K. Law and K. Zhou, Appl. Surf. Sci., 2021, 546, 149080 CrossRef CAS.
- X. Zhang, M. Wei, F. Xu and Y. Wang, J. Membr. Sci., 2020, 601, 117899 CrossRef CAS.
- E. Chen, L. Jia, C. Chen, F. Huang and L. Zhang, J. Mol. Liq., 2021, 325, 115209 CrossRef CAS.
- J.-W. Shen, J. Li, F. Liu, L. Zhang, L. Liang, H. Wang and J.-Y. Wu, J. Membr. Sci., 2020, 595, 117611 CrossRef CAS.
- C. Chen, L. Jia, J. Li, L. Zhang, L. Liang, E. Chen, Z. Kong, X. Wang, W. Zhang and J.-W. Shen, Desalination, 2020, 491, 114560 CrossRef CAS.
- P. G. Demingos, R. A. Pagnussatti and A. R. Muniz, J. Phys. Chem. B, 2021, 125, 7311–7319 CrossRef CAS PubMed.
- H. G. Gomaa and S. Rao, J. Membr. Sci., 2011, 374, 59–66 CrossRef CAS.
- J. Shen, J. Yuan, B. Shi, X. You, R. Ding, T. Zhang, Y. Zhang, Y. Deng, J. Guan, M. Long, Y. Zheng, R. Zhang, H. Wu and Z. Jiang, J. Mater. Chem. A, 2021, 9, 23178–23187 RSC.
- L. C. Lin, J. Choi and J. C. Grossman, Chem. Commun., 2015, 51, 14921–14924 RSC.
- M. Tong, Q. Yang, Q. Ma, D. Liu and C. Zhong, J. Mater. Chem. A, 2016, 4, 124–131 RSC.
- X. Zhang, M. Wei, Z. Zhang, X. Shi and Y. Wang, Desalination, 2022, 526, 115548 CrossRef CAS.
- P. Mark and L. Nilsson, J. Phys. Chem. A, 2001, 105, 9954–9960 CrossRef CAS.
- X. Ma, X. Zhu, C. Huang and J. Fan, J. Membr. Sci., 2022, 647, 120334 CrossRef CAS.
- Y. I. Yang, Q. Shao, J. Zhang, L. Yang and Y. Q. Gao, J. Chem. Phys., 2019, 151, 070902 CrossRef PubMed.
- J. Delhommelle and P. MilliÉ, Mol. Phys., 2001, 99, 619–625 CrossRef CAS.
- S. Plimpton, J. Comput. Phys., 1995, 117, 1–19 CrossRef CAS.
- Y. Li, Q. Wu, X. Guo, M. Zhang, B. Chen, G. Wei, X. Li, X. Li, S. Li and L. Ma, Nat. Commun., 2020, 11, 599 CrossRef CAS PubMed.
- A. Luzar and D. Chandler, Nature, 1996, 379, 55–57 CrossRef CAS.
- J. R. Werber, C. O. Osuji and M. Elimelech, Nat. Rev. Mater., 2016, 1, 16018 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta06245c |
| This journal is © The Royal Society of Chemistry 2023 |