Exploration of atomic interfaces with inherent oxygen vacancies in zirconia for toluene oxidation†
Xueqin
Yang‡
a,
Lin
Chen‡
a,
Xiaolin
Yu
 *b,
Jing
Yu
b,
Dawei
Han
b,
Menglan
Xiao
b,
Maofa
Ge
*b,
Jing
Yu
b,
Dawei
Han
b,
Menglan
Xiao
b,
Maofa
Ge
 b and
Xitian
Yang
*a
b and
Xitian
Yang
*a
aCollege of Forestry, Henan Agricultural University, Zhengzhou, 450002, PR China. E-mail: icecoolyu@iccas.ac.cn; yangxt@henau.edu.cn
bState Key Laboratory for Structural Chemistry of Unstable and Stable Species, Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, PR China
First published on 24th November 2022
Abstract
Constructing interfacial sites in catalysts is an effective strategy to improve catalytic performance. Here, we insert cerium atoms into zirconia via a co-precipitation method. Cerium atoms exist in the form of a single atom in zirconia, and extensive atomic interfaces along with oxygen vacancies were generated, thus greatly improving the low-temperature reducibility and surface oxygen mobility of the inserted catalysts. DFT calculations verified that molecular oxygen was easily adsorbed on the defective tetragonal ZrO2 (011) facets with an energy of −0.53 eV and the distance of the O–O bond was elongated to 1.46 Å, forming the highly active oxygen species with a superoxide-type character. By comparing with pure zirconia and the corresponding impregnated sample, the inserted catalyst not only exhibited excellent catalytic activity, high specific reaction rate and low activation energy, but also possessed satisfactory stability in long-term, water and sulfur resistance tests. In situ DRIFTS spectra further revealed that the defective atomic interface could elevate ring-opening ability and accelerate the toluene reaction process, thus significantly promoting the catalytic performance.
1. Introduction
Volatile organic compounds (VOCs) are a major component of atmospheric pollutants with a very complex composition, which play a vital role in the formation of ozone and secondary organic aerosols.1,2 As a typical VOC, toluene, mainly from industrial emissions, solvent use, motor vehicle exhaust, etc., is harmful to human health and the environment due to its toxicity and reactivity. Catalytic oxidation has been an effective and economical technique for eliminating toluene, which could be completely decomposed into CO2 and water.3,4 In this approach, developing an efficient, stable and low-cost catalyst system is of crucial importance.It is well recognized that constructing metal/oxide interfaces in catalysts is an effective strategy to improve catalytic performance, because the atoms in the interfacial or peripheral regions are more reactive and the ability of molecular oxygen activation is stronger.5,6 For example, Liu et al. constructed an interface architecture by growing Co(OH)2 nanosheets on the KNbO3 perovskite and achieved high catalytic activity for peroxymonosulfate activation.7 Zhang et al. discovered that a unique Co3O4/TiO2 p–n heterojunction with interface defects could induce the improvement of oxygen mobility and the generation of surficial reactive oxygen, thus accelerating the photothermal catalytic degradation of toluene.8 Liu et al. reported a high-efficiency monolithic catalyst with interfaces by in situ growth of MOFs, and it was found that the interfacial interaction could accelerate the fracture of the Co–O bond, which is beneficial to molecular oxygen activation and acetone oxidation.9 However, in traditional loaded metal catalysts, the utilization of metal atoms is low and the surface exposure of interfacial atoms is less, thus leading to a higher cost and poorer catalytic activity.
In recent years, catalysts with atomic interfaces have gradually become a research hotspot in heterogeneous reactions due to their strong synergistic effect, high catalytic activity and excellent selectivity.10–12 Liu et al. modified the electronic state of PdO nanoparticles using a Pd–O–W1 interface at the atomic scale and achieved excellent water resistance for methane combustion.13 Wang et al. stabilized single atomic Au through a Ti–Au–Ti interfacial structure on defective TiO2 nanosheets, and this special structure could reduce the energy barrier and alleviate the competitive adsorption of reactant molecules.14 Li et al. constructed a Cu–N4–C8S2 atomic interface between Cu atoms and a carbon support and revealed the crucial role of the strong synergistic interaction at the atomic interface for enhanced oxygen reduction reactions.11 Therefore, constructing atomic interfaces not only maximized the utilization of atoms, but also generated abundant defects, which is conducive to the activation and mobility of surface oxygen species.
Herein, we report a simple approach to construct atomic interfaces by inserting Ce atoms into a zirconia lattice. The effects of atomic interfaces on the physical–chemical properties and catalytic performance for toluene oxidation were thoroughly explored by comparison with pure zirconia and the corresponding impregnated samples. It was proved that inserting Ce atoms led to the deformation of the lattice structure and the generation of atomic interfaces along with abundant oxygen vacancies, thus resulting in a large improvement in toluene catalytic activity. The influence of atomic interfaces on the reaction mechanism was also studied based on toluene kinetic tests, DFT calculations and in situ DRIFTS spectra.
2. Experimental
2.1 Catalyst preparation
All the reagents were used without further purification. Ammonium solution was purchased from Beijing Chemical Works and Ce(NO3)2·6H2O together with ZrO(NO3)2·2H2O were obtained from Sinopharm Chemical Regent Co., Ltd. A series of Ce doped ZrO2 catalysts with different compositions were synthesized by a co-precipitation method. Typically, the calculated amounts of ZrO(NO3)2·2H2O and Ce(NO3)2·6H2O (Ce/Zr × 100 = 1, 3, 5, and 10 in molar ratio) were dissolved in a beaker filled with 150 mL ultrapure water and stirred vigorously to fully dissolve the sample. The pH of the solution was adjusted to 9 with 25% ammonium solution and then aged for 1 h under stirring conditions. The precipitate was washed with ultrapure water until neutral, dried at 150 °C for 5 h and calcined at 500 °C for 3 h. The obtained catalysts were labeled as CeZr-x (x = 1, 3, 5, and 10), where x is the atomic percentage of cerium relative to the zirconium content. The undoped sample was also synthesized by the same procedure except for the absence of the Ce precursor, denoted as ZrO2. For comparison with the CeZr-10 sample, corresponding Ce impregnated on a ZrO2 catalyst was also prepared, in which the atomic percentage of Ce relative to Zr was 10. In detail, ZrO2 (1.2 g) was dissolved in 30 mL ultrapure water, and then Ce(NO3)2·6H2O (0.43 g) was added. After stirring for 1 h, the impregnated catalyst was obtained followed by centrifugation, washing, drying and calcination, and labeled as CeZr-IM.2.2 Catalyst characterization
Catalysts were characterized using an X-ray diffraction powder diffractometer (XRD), N2 adsorption–desorption isotherms, Raman spectra, high-resolution TEM (HRTEM), X-ray photoelectron spectroscopy (XPS), X-ray absorption spectra (XAS), H2 temperature programmed reduction (H2-TPR), O2 temperature programmed desorption (O2-TPD) and in situ DRIFTS spectra. DFT calculations were also conducted. The detailed characterization and computational method are described in the ESI.†2.3 Catalytic activity test
Catalytic activity was evaluated using a continuous flow fixed-bed quartz reactor. The samples were pressed, crushed and sieved with a 40–60 mesh and then 50 mg catalysts was placed in a quartz tube reactor (4 mm I.D.). The total reactant gas flow rate of 50 mL min−1 consisted of 1000 ppm toluene, 20% O2 and 80% N2, and the gas weight hourly space velocity (WHSV) was 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL gcat−1 h−1. A toluene flow was produced by a bubbler in a thermostatic bath at 0 °C. An online Agilent 6820 gas chromatograph equipped with a flame ionization detector (FID) was used to analyze the concentration of toluene. The toluene conversion (Xtoluene) was obtained using the following eqn (1):
000 mL gcat−1 h−1. A toluene flow was produced by a bubbler in a thermostatic bath at 0 °C. An online Agilent 6820 gas chromatograph equipped with a flame ionization detector (FID) was used to analyze the concentration of toluene. The toluene conversion (Xtoluene) was obtained using the following eqn (1):| Xtoluene = (cinlet − coutlet)/cinlet × 100% | (1) |
For the kinetic test, 10 mg catalysts mixed with 40 mg inert silica sand were used and the total reactant gas flow rate was 50 mL min−1, corresponding to a WHSV of 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL gcat−1 h−1. The specific reaction rate (Rs) was calculated according to eqn (2):
000 mL gcat−1 h−1. The specific reaction rate (Rs) was calculated according to eqn (2):
| Rs = (XtolueneQCf)/(WSBET) | (2) |
3. Results and discussion
3.1 Catalyst characterization
Fig. 1a depicts the XRD patterns of ZrO2, CeZr-x and CeZr-IM catalysts. For pure ZrO2, the characteristic peaks of the monoclinic (PDF # 37–1484) and tetragonal (PDF # 50–1089) phases were obviously observed. With the introduction of cerium atoms, the dominant crystal phase for CeZr-x samples gradually changed from monoclinic to tetragonal zirconia, and the characteristic reflection peaks at around 30° were shifted to a lower 2θ value, which has been taken as an indicative of the successful insertion of cerium atoms into the zirconia lattice.15 However, the impregnated CeZr-IM sample still exhibited a mixed phase structure and the impregnation of cerium did not modify the crystal lattice. For all samples, no characteristic peaks of ceria were detected. The unit cell volumes of CeZr-x catalysts were also calculated and are displayed in Table 1. As shown in Table 1, with increasing the atomic percentage of cerium from 0 to 5%, the unit cell volume increased from 66.71 to 69.04 Å3. This phenomenon is probably attributed to that the substitution of Zr4+ (0.84 Å) by a larger ionic radius Ce4+ (0.97 Å) led to the lattice expansion, and thus the unit cell volume became large.16 However, in comparison with the CeZr-5 sample, a slight reduction in the unit cell volume was also noticed for the CeZr-10 sample. In fact, the lattice contraction or expansion is determined by the competition between the cation radius change and the formation of oxygen vacancies.17,18 In general, a substitution cation with a larger radius could lead to lattice expansion and a larger unit cell volume, but vacancy generation would induce lattice contraction and parameter reduction.5,19 As a consequence, with the increase in the Ce concentration in zirconia, the oxygen content in the lattice diminished, vacancy defects rose, and the modification of steric effects on lattice parameters gradually became negligible. Therefore, it can be concluded that Ce atoms were successfully inserted into the zirconia lattice in the form of a single atom, thereby generating abundant atomic interfaces between isolated Ce atoms and zirconia in CeZr-x samples, along with the plentiful oxygen vacancies.10,11 | ||
| Fig. 1 (a) XRD patterns, (b) Raman spectra, and HRTEM images of (c) CeZr-10 and (d) ZrO2 catalysts, (e) nitrogen adsorption–desorption isotherms and (f) corresponding pore-size distribution curves. | ||
| Samples | Surface area (m2 g−1) | Total pore volume (cm3 g−1) | Pore diameter (nm) | Unit cell volume a (Å3) | Olatt/Oads | Ce3+ (%) |
|---|---|---|---|---|---|---|
| a Estimated unit cell volume calculated from the diffractograms of tetragonal ZrO2 in CeZr-x catalysts. b Unit cell volume of ZrO2 represented by pure tetragonal ZrO2. | ||||||
| ZrO2 | 93.0 | 0.16 | 4.9 | 66.71b | 3.45 | — |
| CeZr-1 | 139.1 | 0.20 | 3.8 | 67.20 | 3.57 | 20.3 |
| CeZr-3 | 114.3 | 0.18 | 4.3 | 67.56 | 4.35 | 23.4 |
| CeZr-5 | 124.4 | 0.16 | 3.8 | 69.04 | 5.00 | 24.6 |
| CeZr-10 | 115.4 | 0.11 | 3.4 | 68.64 | 6.25 | 26.8 |
| CeZr-IM | 90.0 | 0.17 | 5.6 | — | 5.56 | 23.5 |
Raman spectra of ZrO2, CeZr-x and CeZr-IM catalysts are presented in Fig. 1b. For ZrO2 and CeZr-IM samples, a series of bands at around 178, 190, 332, 347, 381, 476, 615 and 635 cm−1 were observed, and the intensity of bands located at 476 cm−1 was stronger than that at 635 cm−1, which is characteristic of monoclinic ZrO2.6,20 As shown in the partially enlarged profile (Fig. S1†), there was a small band at around 459 cm−1 in the CeZr-IM sample, indicating the presence of the CeO2 phase in the impregnated catalyst.21,22 New bands at 145, 260, 318, 458, 595 and 636 cm−1, which are assigned to the Raman-active modes (A1g + 3Eg + 2B1g) of tetragonal ZrO2, appeared in CeZr-x samples.21,23 With an increase in the cerium content, the band intensities of CeZr-x catalysts attributed to tetragonal ZrO2 became stronger, whereas the characteristic bands of the monoclinic phase even disappeared. In addition, the six Raman-active bands of tetragonal ZrO2 broadened and shifted to a lower wavenumber, probably due to the insertion of heteroatoms into the zirconia lattice leading to lattice mismatches and vacancy defects.22,24
In order to visualize the influence of inserted Ce on the zirconia lattice, HRTEM images of CeZr-10 and ZrO2 catalysts were obtained. Fig. 1c shows that the measured lattice spacings over the CeZr-10 catalyst were ca. 2.95 and 2.61 Å, corresponding to the (011) and (002) facets of tetragonal zirconia (t-ZrO2), respectively. The deformation of the lattice structure was observed over the CeZr-10 sample in the enlarged region in Fig. 1c, further verifying that inserting Ce atoms could induce the generation of abundant lattice defects. As shown in Fig. 1d, the interplanar spacings of the ZrO2 sample were also measured and ascribed to the (−111) and (111) facets of monoclinic zirconia (m-ZrO2), which is in agreement with the XRD and Raman results. Therefore, it was further confirmed that the Ce insertion could induce the crystal transformation and the lattice deformation, accompanied by the creation of abundant vacancy defects.
Nitrogen adsorption–desorption isotherms together with pore size distribution curves of ZrO2, CeZr-x and CeZr-IM catalysts are depicted in Fig. 1e and f. According to the IUPAC recommendations, all samples exhibited a type IV isotherm and H4 hysteresis loop, which are affected slightly by the introduction of cerium atoms.25 As shown in Table 1, the specific surface area, pore volume and pore diameter of the ZrO2 sample were 93.0 m2 g−1, 0.16 cm3 g−1 and 4.9 nm, and the impregnated CeZr-IM sample exhibited a similar value. However, inserting Ce atoms could significantly increase the specific surface area and slightly decrease the pore diameter. The specific surface area of CeZr-x samples changed from 102.4 to 139.1 m2 g−1 and the pore diameter is in the range of 3.4–4.3 nm.
In order to investigate the surface chemical environment, X-ray photoelectron spectroscopy (XPS) was conducted. As shown in Fig. 2a, there were two main peaks at around 182.2 and 184.6 eV for all samples, corresponding to the Zr 3d5/2 and Zr 3d3/2 spin-orbital peaks of the tetravalent zirconium cation.26Fig. 2b shows that O 1s spectra could be deconvoluted into three distinct peaks, and the corresponding binding energies were located at 530.0–530.3, 531.5–531.7 and 532.6–533.0 eV, which were assigned to surface lattice oxygen (Olatt), chemisorbed oxygen (Oads) and surface carbonate or hydroxyls, respectively.27,28 The surface Olatt/Oads molar ratios calculated from fitted peaks are listed in Table 1. It can be seen from Table 1 that with elevating the inserted Ce proportion in zirconia, the Olatt/Oads ratio over the CeZr-x surface increased from 3.45 to 6.25, meaning more surficial lattice oxygen species.
 | ||
| Fig. 2 (a) Zr 3d, (b) O 1s and (c) Ce 3d XPS spectra of CeZr-IM, CeZr-10, CeZr-5, CeZr-3, CeZr-1 and ZrO2 catalysts from top to bottom. | ||
As shown in Fig. 2c, Ce 3d spectra of CeZr-x and CeZr-IM samples exhibited eight well-resolved peaks. The peaks marked as v, v′′, and v′′′ and u, u′′, and u′′′ corresponded to the spin–orbit coupling of Ce4+ 3d5/2 and Ce4+ 3d3/2, respectively.29 The v′ and u′ peaks represented Ce3+ 3d5/2 and Ce3+ 3d3/2.30 It is obvious that Ce3+ and Ce4+ oxidation states coexisted in samples. The relative contents of Ce3+ were calculated from the area proportion of Ce3+/(Ce3++Ce4+) and the result is displayed in Table 1. The relative content of Ce3+ on the surface is strongly dependent on the inserted Ce amount and follows the sequence of CeZr-10 (26.8%) > CeZr-5 (24.6%) > CeZr-3 (23.4%) > CeZr-1 (20.3%). Obviously, raising the Ce concentration in the zirconia lattice led to a continuous increase of Ce3+ species in CeZr-x samples, and the CeZr-10 catalyst possesses the most abundant surficial lattice oxygen and Ce3+ species. It is universally acknowledged that trivalent cerium is closely related to oxygen vacancies and the oxygen storage/release process is also easy to occur between Ce3+ and Ce4+.31,32 That is, the more trivalent cerium there is, the more vacancy defects there are. Therefore, it can be concluded that inserting heteroatoms into the zirconia lattice could boost the generation of Ce3+ species along with oxygen vacancies, which is beneficial to the adsorption, migration and activation of surficial oxygen species. For the CeZr-IM sample, the Olatt/Oads ratio and Ce3+ percentage were 5.56 and 23.5%, which are obviously lower than those of the CeZr-10 sample. This phenomenon could be interpreted as the deficiency of the interfaces of isolated Ce atoms with zirconia in the CeZr-IM sample caused by inserting heteroatoms.
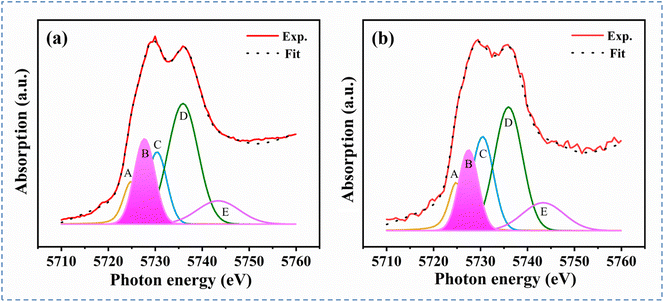
The valence states of cerium over CeZr-10 and CeZr-IM catalysts were further explored using XANES spectra. As shown in Fig. 3, the Ce L3-edge XANES spectra are deconvoluted into five peaks by Gaussian fitting (denoted as peaks A–E). Peak A originates from the dipole–forbidden transition from Ce 2p to 4f.33 Peaks B and C correspond to the transition of Ce3+ species from 2p4f15d0 to 2p4f15d* and the charge transfer of the Ce4+ sate with the 2p41L5d* configuration, where 5d* represents the excited electron of 5d states and L stands for a hole of the ligand orbital.34 Peaks D and E arise from the splitting of Ce4+ with the 2p4f05d* configuration due to the influence of the crystal field.35 The relative concentration of Ce3+ is estimated using the integrated area proportion of peak B to all peaks. The relative proportion of Ce3+ in CeZr-10 and CeZr-IM catalysts is 20.4 and 17.2%. Obviously, these two samples exhibited mixed valence states of cerium, but the CeZr-10 sample contains a higher Ce3+ concentration, further confirming the appearance of atomic interfaces between single Ce atoms and ZrO2 with inherent oxygen vacancies in the CeZr-10 sample.
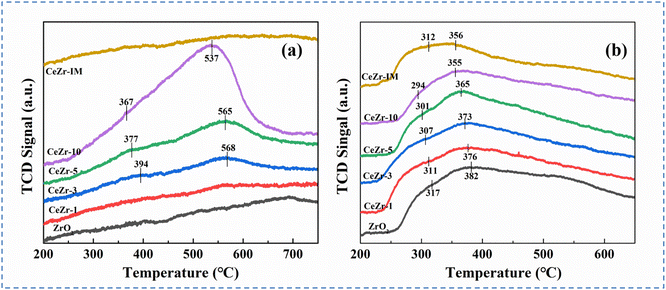
The reducibility and mobility of surficial oxygen species were investigated by H2-TPR and O2-TPD. It can be seen from H2-TPR profiles (Fig. 4a) that no recognizable hydrogen reduction signals were detected for ZrO2, CeZr-1 and CeZr-IM catalysts. However, CeZr-3, CeZr-5 and CeZr-10 samples exhibited two distinct peaks at around 370 and 560 °C, which are attributed to the surface and subsurface reduction, respectively.21,36 Evidently, with an increasing Ce molar percentage from 3 to 10%, these reduction peaks shifted to a lower temperature and whole H2 consumption significantly increased, indicating that a higher amount of heteroatom insertion induced a more reducible and reactive surface, probably due to the extensive atomic interfaces with defects in CeZr-x catalysts.37,38
O2-TPD profiles of all samples are displayed in Fig. 4b. Two broad peaks of oxygen desorption at around 300 and 370 °C were observed, which were assigned to the release of chemisorbed oxygen (O2−, O22− or O−) and superficial lattice oxygen species (O2−) respectively.39–41 Bulk lattice oxygen was not detected in the investigated temperature range. In general, VOC oxidation over transition metal catalysts follows the Mars–van Krevelen (M–K) mechanism, in which the concentration and reactivity of surface lattice oxygen play a decisive role.42,43 It can be seen from Fig. 4b that increasing the Ce concentration in zirconia could lower the temperature of maximal oxygen desorption and increase the intensity of high temperature peaks, implying an enhancement in the amount and mobility of surface lattice oxygen. For the impregnated CeZr-IM sample, the intensity of O2 desorption peaks ascribed to superficial lattice oxygen species was weaker and corresponding desorption temperature was higher than those of the CeZr-10 sample. In consequence, there are abundant active lattice oxygen species over the CeZr-10 sample with defective atomic interfaces, thus facilitating the improvement of catalytic activity.
3.2 Catalyst activity
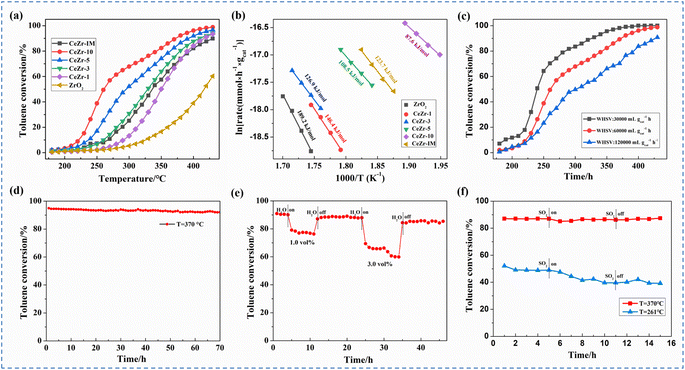
Catalytic activity of samples was evaluated by toluene oxidation and the corresponding conversion curves are displayed in Fig. 5a. Obviously, the inserted CeZr-x with defective atomic interfaces is more active than pure ZrO2 for toluene oxidation. As shown in Table 2, with the increase of inserted Ce, T50 and T90 significantly decreased from 357 and 425 °C of the CeZr-1 catalyst to 263 and 380 °C of the CeZr-10 catalyst. The more the cerium doping amount, the higher the defective interface and catalytic activity. For comparison, the catalytic toluene activity of the impregnated CeZr-IM sample was also evaluated. Remarkably, CeZr-IM displayed lower catalytic activity than CeZr-10 and just showed comparable activity to CeZr-3, implying that the defective Ce–O–Zr atomic interface in the inserted samples plays an essential role in the improvement of catalytic activity.| Samples | T 50 (°C) | T 90 (°C) | R s at 270 °C (mmol h−1 m−2) | E a (kJ mol−1) |
|---|---|---|---|---|
| a Specific toluene reaction rate estimated at 270 °C in a kinetically controlled regime. b Activation energy calculated from Arrhenius-type plots of the toluene oxidation rate. | ||||
| ZrO2 | 425 | — | 0 | 189.2 |
| CeZr-1 | 357 | 425 | 0.35 × 10−4 | 146.4 |
| CeZr-3 | 334 | 418 | 0.76 × 10−4 | 126.9 |
| CeZr-5 | 300 | 400 | 6.89 × 10−4 | 108.5 |
| CeZr-10 | 263 | 380 | 34.2 × 10−4 | 87.6 |
| CeZr-IM | 342 | 440 | 13.9 × 10−4 | 123.7 |
In order to explore the origin of high catalytic performance, kinetic tests over the as-prepared catalysts were also conducted. The specific reaction rate (Rs) and apparent activation energy (Ea) were calculated and are displayed in Table 2. It can be seen from Table 2 that the specific toluene reaction rate estimated at 270 °C was dramatically improved from 0 to 34.2 × 10−4 mmol h−1 m−2 with increasing Ce amounts, and followed the sequence of CeZr-10 > CeZr-5 > CeZr-3 > CeZr-1 > ZrO2. The apparent activation energy was obtained on the basis of the Arrhenius plots of the reaction rate in Fig. 5b and the calculated result is represented in Table 2. With increasing the Ce molar percentage from 0 to 10%, Ea decreased rapidly from 189.2 to 87.60 kJ mol−1, indicating that a higher heteroatom would induce more atomic interfaces with vacancy defects, leading to a great improvement in the intrinsic catalytic performance. In comparison with CeZr-10, the impregnated CeZr-IM displayed a higher activation energy of 123.7 kJ mol−1 and a lower specific reaction of 13.9 × 10−4 mmol h−1 m−2, further verifying the crucial role of defective atomic interfaces in improving the catalytic activity.
Catalytic toluene activity of the CeZr-10 catalyst under different WSHV, high temperature, moisture and SO2 conditions was investigated to explore its potential in practical applications. As shown in Fig. 5c, with the decreasing WHSV from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 30
000 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL gcat−1 h−1, T50 and T90 of the CeZr-10 catalyst dropped to 239 and 328 °C, respectively, which were obviously shifted toward a lower temperature. However, an evident increase in toluene conversion temperature was observed, when WHSV was raised to 120
000 mL gcat−1 h−1, T50 and T90 of the CeZr-10 catalyst dropped to 239 and 328 °C, respectively, which were obviously shifted toward a lower temperature. However, an evident increase in toluene conversion temperature was observed, when WHSV was raised to 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL gcat−1 h−1. To evaluate the catalytic stability, a long-term test for toluene oxidation at high temperature under the same conditions was carried out. Fig. 5d shows that the toluene conversion of the CeZr-10 catalyst at 370 °C only exhibited a slight fluctuation between 94 and 92% during the 70 h long-term test, indicating the excellent high-temperature stability. The moisture and sulfur tolerances of the CeZr-10 sample were also investigated and are displayed in Fig. 5e and f. As shown in Fig. 5e, when 1 vol% water vapor was introduced into the reaction stream, toluene conversion was reduced from 90 to 77%, which was recovered quickly after being replaced by dry air. Further raising the water vapor to 3 vol% led to a continuous declination, and the toluene conversion was maintained at 65%. After removing the water vapor, it recovered to 86%. Fig. 5f depicts the sulfur resistance of the CeZr-10 catalyst during an extended time at different temperatures. With the introduction of 2 ppm SO2 into the toluene oxidation experiment at 261 and 370 °C, the toluene conversion had a slight decrease by 1 and 7%, respectively. Once SO2 was removed, the catalytic conversion at relatively high temperature was restored, while a slight continuous decrease still occurred at relatively low temperature, indicating the excellent sulfur resistance and regeneration capacity at high reaction temperature.
000 mL gcat−1 h−1. To evaluate the catalytic stability, a long-term test for toluene oxidation at high temperature under the same conditions was carried out. Fig. 5d shows that the toluene conversion of the CeZr-10 catalyst at 370 °C only exhibited a slight fluctuation between 94 and 92% during the 70 h long-term test, indicating the excellent high-temperature stability. The moisture and sulfur tolerances of the CeZr-10 sample were also investigated and are displayed in Fig. 5e and f. As shown in Fig. 5e, when 1 vol% water vapor was introduced into the reaction stream, toluene conversion was reduced from 90 to 77%, which was recovered quickly after being replaced by dry air. Further raising the water vapor to 3 vol% led to a continuous declination, and the toluene conversion was maintained at 65%. After removing the water vapor, it recovered to 86%. Fig. 5f depicts the sulfur resistance of the CeZr-10 catalyst during an extended time at different temperatures. With the introduction of 2 ppm SO2 into the toluene oxidation experiment at 261 and 370 °C, the toluene conversion had a slight decrease by 1 and 7%, respectively. Once SO2 was removed, the catalytic conversion at relatively high temperature was restored, while a slight continuous decrease still occurred at relatively low temperature, indicating the excellent sulfur resistance and regeneration capacity at high reaction temperature.
3.3 Theoretical computation
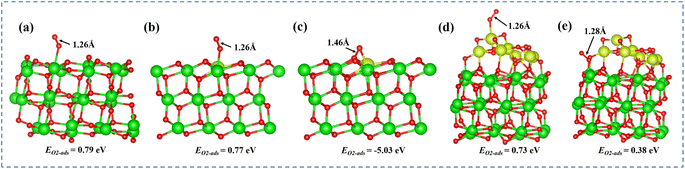
In the toluene oxidation reaction, the activation of molecular oxygen and rapid replenishment of active oxygen are of vital importance. DFT calculations were conducted to further explore the influence of defective atomic interfaces on O2 adsorption and activation capacity. As mentioned above, the predominant crystal phase of pure ZrO2 and impregnated CeZr-IM samples was monoclinic, but the insertion of Ce in the zirconia lattice caused a modification of the crystal structure into a tetragonal phase. Therefore, it was modeled using the most stable m-ZrO2 (−111) and t-ZrO2 (011) surface structures for calculations.44 As shown in Fig. 6a and b, ZrO2 and Ce-doped ZrO2 surfaces without oxygen vacancies exhibit a high adsorption energy of molecular oxygen and the O–O bond length is 1.26 Å, indicating weak adsorption of O2 over both surfaces. With the introduction of oxygen vacancies in Ce-doped ZrO2, molecular oxygen could adsorb strongly on the defective t-ZrO2 (011) facets with an energy of −0.53 eV and the distance of the O–O bond was elongated to 1.46 Å (Fig. 6c), pointing to a superoxide-type character of adsorbed oxygen species over the defective surface.45,46 The impregnated CeZr-IM sample was also simulated by loading a CeO2 cluster on the m-ZrO2 (−111) surface in order to exclude the effect of the interface at the atomic scale. As shown in Fig. 6d and e, the stable adsorption configurations mainly included O2 atop the ceria cluster and interfacial Zr sites. The adsorption energies were still high and corresponded to 0.73 and 0.38 eV, respectively. Therefore, it can be concluded that O2 atoms were strongly adsorbed over defective t-ZrO2 (011) facets and form highly reactive oxygen species, but the other three models without defective Ce–O–Zr atomic interfaces exhibited a weak physical adsorption with a high adsorption energy. That is, the atomic interface with oxygen vacancies could accelerate the O2 adsorption and activation and facilitate the replenishment of active oxygen, thus benefiting the deep oxidation of toluene.473.4 Catalyst mechanism
In order to explore the reaction mechanism and intermediate products in toluene oxidation, in situ DRIFTS spectra of CeZr-10, ZrO2 and CeZr-IM samples were recorded. As shown in Fig. 7a, benzyl alcohol species were formed over the CeZr-10 sample as demonstrated by the weak bands at 1025–1180 cm−1, as a consequence of toluene reacting with surficial oxygen species.48,49 The characteristic bands of benzaldehyde (1436, 1646 and 1702 cm−1) and benzoate (1370, 1479 and 1586 cm−1) were detected and dominant.50,51 Meanwhile, maleic anhydride (1260, 1308, 1823 and 1915 cm−1), as the main ring-opening product of toluene oxidation, was also clearly visible.52,53 Accordingly, it is speculated that the toluene reaction pathway was a successive oxidation. Fig. 7b shows that the characteristic band intensities of all intermediate products over ZrO2 were prominently weaker than that of the CeZr-10 sample. However, as shown in Fig. 7c, the impregnated CeZr-IM sample exhibited very similar spectra to the CeZr-10 sample, but the content of benzoate and maleic anhydride is obviously lower at 250 °C, implying that deeper oxidation was hardly formed over the CeZr-IM sample, probably due to the deficiency of defective atomic interfaces.Based on the infrared contour map of the toluene transient reaction (Fig. 8), the intensities of the main reaction intermediates at two different temperatures were also compared. Obviously, benzoate and maleic anhydride over the CeZr-10 catalyst at 300 °C were stronger than over the other two catalysts. On increasing the reaction temperature to 400 °C, most benzoates on the CeZr-10 surface evolved into maleic anhydride. It should be mentioned that the generation of maleic anhydride is a rate-determining step in the toluene oxidation reaction, which could be transformed into CO2 and H2O readily.52,53 However, for ZrO2 and CeZr-IM catalysts, elevating the temperature resulted in the accumulation of benzoate, but maleic anhydride increased obviously only for the latter. As proved above, there were abundant atomic interfaces between isolated Ce atoms and zirconia along with oxygen vacancies in the CeZr-10 sample, and highly reactive oxygen species were easily generated around defective Ce–O–Zr interfaces, thereby greatly improving the ring-opening ability of toluene. Therefore, complete catalytic oxidation of toluene was easy to occur over the defective CeZr-10 sample, but the deficiency of atomic interfaces in the impregnated sample limited the generation of crucial intermediates, leading to a worse catalytic performance.
 | ||
| Fig. 8 The contour projection of the toluene transient reaction over CeZr-10, ZrO2 and CeZr-IM samples at 300 and 400 °C. | ||
4. Conclusions
In summary, a series of CeZr-x catalysts with defective Ce–O–Zr interfaces were prepared via a simple method. It was proved that inserting Ce atoms could induce the deformation of the lattice structure and the generation of extensive atomic interfaces along with oxygen vacancies. H2-TPR, O2-TPD and DFT calculations verified that molecular oxygen was easily adsorbed and activated over the defective CeZr-x sample, thus boosting the mobility and reaction of surface oxygen species. The activity test showed that inserting Ce atoms could greatly improve the catalytic toluene performance of CeZr-x samples. Impressively, the CeZr-10 catalyst with the best activity possessed satisfactory stability in long-term, water and sulfur resistance tests, which has great potential in practical application. For comparison, pure zirconia and the corresponding impregnated CeZr-IM sample were also prepared, but exhibited worse catalytic activity than the CeZr-10 sample. The impregnated sample just displayed comparable activity to CeZr-3 due to the deficiency of atomic interfaces with oxygen vacancies. In situ DRIFTS spectra further confirmed that the defective Ce–O–Zr interface structure could elevate the formation of the ring-opening product and alter the process of the toluene reaction, leading to a high specific reaction rate and low activation energy. Our findings shed light on the crucial role of atomic interfaces in eliminating toluene and can contribute to the facile design of high-efficiency catalysts.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22006032 and 22076192), the Key Scientific and Technological Project of Henan Province (222102320022), the Special Fund for Topnotch Talents in Henan Agricultural University (30500843) and the Beijing Synchrotron Radiation Laboratory. The authors would like to acknowledge Prof. Chuanhui Zhang from Qingdao University for his help with theoretical computation.Notes and references
- C. He, J. Cheng, X. Zhang, M. Douthwaite, S. Pattisson and Z. Hao, Chem. Rev., 2019, 119, 4471–4568 CrossRef CAS PubMed.
- Y. Zheng, K. Fu, Z. Yu, Y. Su, R. Han and Q. Liu, J. Mater. Chem. A, 2022, 10, 14171–14186 RSC.
- H. Zhang, S. Sui, X. Zheng, R. Cao and P. Zhang, Appl. Catal., B, 2019, 257, 117878 CrossRef CAS.
- J. Tian, C. Wang, J. Wu, D. Sun and Q. Li, Chem. Eng. J., 2023, 451, 138351 CrossRef CAS.
- A. Ruiz Puigdollers, P. Schlexer, S. Tosoni and G. Pacchioni, ACS Catal., 2017, 7, 6493–6513 CrossRef CAS.
- X. Yang, X. Yu, M. Lin, M. Ge, Y. Zhao and F. Wang, J. Mater. Chem. A, 2017, 5, 13799–13806 RSC.
- J. Qi, X. Yang, P.-Y. Pan, T. Huang, X. Yang, C.-C. Wang and W. Liu, Environ. Sci. Technol., 2022, 56, 5200–5212 CrossRef CAS PubMed.
- Y. Yang, S. Zhao, F. Bi, J. Chen, Y. Wang, L. Cui, J. Xu and X. Zhang, Appl. Catal., B, 2022, 315, 121550 CrossRef CAS.
- Y. Zheng, Y. Su, C. Pang, L. Yang, C. Song, N. Ji, D. Ma, X. Lu, R. Han and Q. Liu, Environ. Sci. Technol., 2022, 56, 1905–1916 CrossRef PubMed.
- Y. Pan, C. Zhang, Z. Liu, C. Chen and Y. Li, Matter, 2020, 2, 78–110 CrossRef.
- Z. Jiang, W. Sun, H. Shang, W. Chen, T. Sun, H. Li, J. Dong, J. Zhou, Z. Li, Y. Wang, R. Cao, R. Sarangi, Z. Yang, D. Wang, J. Zhang and Y. Li, Energy Environ. Sci., 2019, 12, 3508–3514 RSC.
- H. Shang, Z. Jiang, D. Zhou, J. Pei, Y. Wang, J. Dong, X. Zheng, J. Zhang and W. Chen, Chem. Sci., 2020, 11, 5994–5999 RSC.
- Z. Hou, L. Dai, J. Deng, G. Zhao, L. Jing, Y. Wang, X. Yu, R. Gao, X. Tian, H. Dai, D. Wang and Y. Liu, Angew. Chem., Int. Ed., 2022, 61, e202201655 CAS.
- J. Wan, W. Chen, C. Jia, L. Zheng, J. Dong, X. Zheng, Y. Wang, W. Yan, C. Chen, Q. Peng, D. Wang and Y. Li, Adv. Mater., 2018, 30, 1705369 CrossRef PubMed.
- S. Letichevsky, C. A. Tellez, R. R. d. Avillez, M. I. P. d. Silva, M. A. Fraga and L. G. Appel, Appl. Catal., B, 2005, 58, 203–210 CrossRef CAS.
- M. Lammert, C. Glissmann and N. Stock, Dalton Trans., 2017, 46, 2425–2429 RSC.
- D. Marrocchelli, S. R. Bishop, H. L. Tuller and B. Yildiz, Adv. Funct. Mater., 2012, 22, 1958–1965 CrossRef CAS.
- A. Nandy, C. S. Tiwary, A. Dutta, K. Chattopadhyay and S. K. Pradhan, Electrochim. Acta, 2015, 170, 360–368 CrossRef CAS.
- X. Yang, X. Yu, M. Jing, W. Song, J. Liu and M. Ge, ACS Appl. Mater. Interfaces, 2019, 11, 730–739 CrossRef CAS PubMed.
- C. Li and M. Li, J. Raman Spectrosc., 2002, 33, 301–308 CrossRef CAS.
- H. Bao, K. Qian, X. Chen, J. Fang and W. Huang, Appl. Surf. Sci., 2019, 467–468, 361–369 CrossRef CAS.
- H. Wang, G.-Q. Yang, Y.-H. Song, Z.-T. Liu and Z.-W. Liu, Catal. Today, 2019, 324, 39–48 CrossRef CAS.
- M. Li, Z. Feng, G. Xiong, P. Ying, Q. Xin and C. Li, J. Phys. Chem. B, 2001, 105, 8107–8111 CrossRef CAS.
- M. Yashima, H. Arashi, M. Kakihana and M. Yoshimura, J. Am. Ceram. Soc., 1994, 77, 1067–1071 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- X. Yang, X. Ma, X. Yu and M. Ge, Appl. Catal., B, 2020, 263, 118355 CrossRef CAS.
- X. Chen, J. Li, S. Cai, J. Chen and H. Jia, J. Mater. Chem. A, 2020, 8, 6619–6630 RSC.
- M. Xiao, X. Yu, Y. Guo and M. Ge, Environ. Sci. Technol., 2022, 56, 1376–1385 CrossRef CAS PubMed.
- J. Xiong, X. Mei, J. Liu, Y. Wei, Z. Zhao, Z. Xie and J. Li, Appl. Catal., B, 2019, 251, 247–260 CrossRef CAS.
- M. Xiao, D. Han, X. Yang, N. Tsona Tchinda, L. Du, Y. Guo, Y. Wei, X. Yu and M. Ge, Appl. Catal., B, 2023, 323, 122173 CrossRef CAS.
- H. Wang, J. X. Liu, L. F. Allard, S. Lee, J. Liu, H. Li, J. Wang, J. Wang, S. H. Oh, W. Li, M. Flytzani-Stephanopoulos, M. Shen, B. R. Goldsmith and M. Yang, Nat. Commun., 2019, 10, 3808 CrossRef PubMed.
- K. X. Cao, X. X. Dai, Z. B. Wu and X. L. Weng, Chin. Chem. Lett., 2021, 32, 1206–1209 CrossRef CAS.
- D. Z. Peng, S. Y. Chen, C. L. Chen, A. Gloter, F. T. Huang, C. L. Dong, T. S. Chan, J. M. Chen, J. F. Lee, H. J. Lin, C. T. Chen and Y. Y. Chen, Langmuir, 2014, 30, 10430–10439 CrossRef CAS PubMed.
- L. Tao, Y. Shi, Y.-C. Huang, R. Chen, Y. Zhang, J. Huo, Y. Zou, G. Yu, J. Luo, C.-L. Dong and S. Wang, Nano Energy, 2018, 53, 604–612 CrossRef CAS.
- Z. Su, W. Si, H. Liu, S. Xiong, X. Chu, W. Yang, Y. Peng, J. Chen, X. Cao and J. Li, Environ. Sci. Technol., 2021, 55, 12630–12639 CrossRef CAS PubMed.
- P. Fornasiero, G. Balducci, R. Di Monte, J. Kăspar, V. Sergo, G. Gubitosa, A. Ferrero and M. Graziani, J. Catal., 1996, 164, 173–183 CrossRef CAS.
- J. Zhong, Y. Zeng, Z. Yin, M. Zhang, D. Yan, Z. Su, P. Chen, M. Fu and D. Ye, J. Mater. Chem. A, 2021, 9, 6890–6897 RSC.
- J. Xiong, Z. G. Li, P. Zhang, Q. Yu, K. X. Li, Y. L. Zhang, Z. Zhao, J. Liu, J. M. Li and Y. C. Wei, Chin. Chem. Lett., 2021, 32, 1447–1450 CrossRef CAS.
- Y. Feng, C. C. Wang, C. Wang, H. B. Huang, H. C. Hsi, E. H. Duan, Y. X. Liu, G. S. Guo, H. X. Dai and J. G. Deng, J. Hazard. Mater., 2022, 424, 127337 CrossRef CAS PubMed.
- X. Yang, X. Yu, M. Lin, X. Ma and M. Ge, Catal. Today, 2019, 327, 254–261 CrossRef CAS.
- D. Han, X. Ma, X. Yang, M. Xiao, H. Sun, L. Ma, X. Yu and M. Ge, J. Colloid Interface Sci., 2021, 603, 695–705 CrossRef CAS PubMed.
- M. Xiao, X. Yang, Y. Peng, Y. Guo, Y. Wei, M. Ge and X. Yu, Nano Res., 2022, 15, 7042–7051 CrossRef CAS.
- Y. Chen, Z. Huang, M. Zhou, Z. Ma, J. Chen and X. Tang, Environ. Sci. Technol., 2017, 51, 2304–2311 CrossRef CAS PubMed.
- Q. Cai, J. A. Lopez-Ruiz, A. R. Cooper, J.-g. Wang, K. O. Albrecht and D. Mei, ACS Catal., 2018, 8, 488–502 CrossRef CAS.
- H. Wu, S. Ma, W. Song and E. J. M. Hensen, J. Phys. Chem. C, 2016, 120, 13071–13077 CrossRef CAS.
- M. Jing, W. Song, L. Chen, S. Ma, J. Deng, H. Zheng, Y. Li, J. Liu and Z. Zhao, J. Phys. Chem. C, 2018, 122, 438–448 CrossRef CAS.
- S. Rong, P. Zhang, F. Liu and Y. Yang, ACS Catal., 2018, 8, 3435–3446 CrossRef CAS.
- Z. Su, W. Yang, C. Wang, S. Xiong, X. Cao, Y. Peng, W. Si, Y. Weng, M. Xue and J. Li, Environ. Sci. Technol., 2020, 54, 12684–12692 CrossRef CAS PubMed.
- H. Sun, Z. Liu, S. Chen and X. Quan, Chem. Eng. J., 2015, 270, 58–65 CrossRef CAS.
- F. Rainone, D. A. Bulushev, L. Kiwi-Minsker and A. Renken, Phys. Chem. Chem. Phys., 2003, 5, 4445–4449 RSC.
- G. Xiao, Z. Guo, B. Lin, M. Fu, D. Ye and Y. Hu, Environ. Sci. Technol., 2022, 56, 10095–10104 CrossRef PubMed.
- H. Sun, X. Yu, Y. Guo, J. Deng and M. Ge, Appl. Surf. Sci., 2022, 591, 153225 CrossRef CAS.
- J. Li, H. Na, X. Zeng, T. Zhu and Z. Liu, Appl. Surf. Sci., 2014, 311, 690–696 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta06736f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |