Open Access Article
Open Access ArticleUtilization of the nickel hydroxide derived from a spent electroless nickel plating bath for energy storage applications
Aasiya
Shaikh
 *a,
Balwant Kr
Singh
b,
Kartikay
Purnendu
*a,
Balwant Kr
Singh
b,
Kartikay
Purnendu
 c,
Prapunj
Kumari
a,
P. Ram
Sankar
a,
Girdhar
Mundra
a and
Sivasambu
Bohm
d
c,
Prapunj
Kumari
a,
P. Ram
Sankar
a,
Girdhar
Mundra
a and
Sivasambu
Bohm
d
aDesign and Manufacturing Technology Division, Raja Ramanna Centre for Advanced Technology (RRCAT), Indore-452013, Madhya Pradesh, India. E-mail: aasiya@rrcat.gov.in; Tel: +91-731-248-8091
bCentre for Advanced Materials Research, University of Texas El Paso, TX 79968, USA
cDepartment of Metallurgical Engineering and Materials Science, Indian Institute of Technology-Bombay, Mumbai-400076, Maharashtra, India
dDepartment of Chemistry, Molecular Sciences Research Hub, Imperial College London, White City Campus, Wood Lane, London W12 0BZ, UK
First published on 16th December 2022
Abstract
In this study, a simple one-step approach has been proposed to treat a spent electroless nickel plating bath by precipitating nickel metal as nickel hydroxide. Beta phase nickel hydroxide β-Ni(OH)2 generated after the treatment of an electroless nickel plating bath was characterized by material characterization techniques including X-ray diffraction (XRD), scanning electron microscopy (SEM), FT-IR and X-ray photoelectron spectroscopy (XPS). The electrochemical performance of β-Ni(OH)2 was evaluated using cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) techniques. A specific capacitance of 332 F g−1@5 mV s−1 is obtained from the CV data with an energy density of 11.5 W h kg−1 and power density of 207.5 W kg−1. The GCD results show a specific capacitance of 330 F g−1, which is in close agreement with the value obtained from the CV data. This work represents a scalable approach for the synthesis of β-Ni(OH)2 from plating waste and converting it into a value-added product. The β-Ni(OH)2 powder obtained from plating waste has comparable electrochemical properties to that of pristine β-Ni(OH)2 and other transition metal hydroxides produced by other chemical methods.
Sustainability spotlightThe current electroless nickel plating industry practice is to remove the plating bath from the production line after multiple usage. The treatment of this spent bath is a great concern for the plating industry. The normal procedure for the treatment of waste electroless nickel plating baths is hydroxide precipitation, which generates a large amount of solid nickel hydroxide waste. This solid waste is used in landfill, adding extra costs for handling and storage, making the treatment procedure exorbitant. This study proposes circular material economy for generated nickel hydroxide waste which has potential application in supercapacitors and battery applications. This work represents a sustainable approach for the synthesis of β-Ni(OH)2 from plating waste and converting it into a value-added product. |
Introduction
Protective metallic coatings are coatings consisting of metallic elements or metal alloys. These protective coatings are normally applied to substrates by using spray paint, electrochemical or chemical methods. They are commonly used in several industrial applications for different purposes, including technological aspects like corrosion protection to decorative applications.1 Nickel plating is one such plating technology, which adds durability, hardness, conductivity and heat resistance in addition to corrosion resistance and an enhanced aesthetic look to the underlying metallic substrates.1,2 It is one of the most commonly used protective coating procedures and has been practiced in the metal finishing industry for the past few decades. Nickel plating has applications in all major industrial sectors, such as aerospace and aviation, automobiles, petrochemicals and telecommunications.3 There are different types of nickel plating which give different desired properties to the substrate, including bright nickel plating, sulphamate or dull nickel plating4 and electroless nickel plating.3 Both bright nickel and dull nickel plating are electroplating technologies involving different metallic precursors of nickel–metal (nickel sulphate and nickel sulphamate), which result in different appearances and properties of the final electroplating products.5 Bright nickel plating results in a smooth and ductile coating that seals off the surface of the component and protects it from a corrosive environment by enhancing the corrosion resistance properties. It is also used as a base coating for other metallic coatings like chrome and gold.6,7 Nickel sulphamate or dull nickel plating offers a much denser coating compared to bright nickel coating with high tensile strength, excellent corrosion resistance, high-temperature tolerance and ease of machinability.8Electroless nickel plating (ENP) or autocatalytic nickel plating deposits a nickel–phosphorus (NiP) or nickel boron (NiB) alloy instead of pure metallic nickel on the substrate. The properties of electroless nickel plating vary with the percentage of alloying elements like phosphorous and boron. Unlike the conventional electroplating process, the electroless nickel plating process involves the deposition of a nickel alloy from an aqueous plating bath without passing an electric current through the system.9 It utilizes chemical reducing agents like sodium hypophosphite, sodium borohydride and dimethylamine borane to reduce nickel metal ions on the substrate.10 This plating technique offers uniform thickness, corrosion and wear resistance, lubricity, solderability and magnetic properties.9 However, the conundrum associated with this feasible plating technology is the limited lifespan of the electroless nickel plating bath. Additional replenishment of the nickel source and the reducing agent is required to maintain the optimum concentration levels for the autoreduction process. Multiple usages of electroless nickel plating baths over a long period of time cause accumulation of byproducts, contaminant build-up, an increase in phosphorous content resulting in poor deposition rates, alteration in the characteristics of the deposit like internal stress, pitting, blistering, roughness and porosity. By normal industrial practices, once the electroless nickel bath is no longer productive, it is discarded and removed from the production line.11 The treatment of this spent bath solution is a great concern for the electroplating industry.12 The normal procedures adopted by the industries for the regeneration and treatment of waste electroless nickel plating baths include regeneration by ion exchange resin, photoelectrocatalytic treatment,13 chemical precipitation or hydroxide precipitation,14 and electrochemical methods like electrowinning,15 electrocoagulation,16 electrodialysis,17 and electrodeionization.18
The ion exchange procedure is based on a reversible interchange of ions between solid and liquid phases with resins removing ions from the spent plating solution and releasing other ions of the same chemical charge into the spent bath. The ion exchange resin becomes exhausted and needs regeneration using the concentrated electrolyte.18 Electrodialysis is a continuous electrically driven separation process that utilizes ion-selective membranes to carry out the separation of ions. During this process, ions are transported from a low concentration chamber to a high concentration chamber. This method requires regeneration of the dialysis membrane and frequent membrane replacement making it economically inviable.19 An electrodeionization process is a combination of electrodialysis and an ion exchange resin method. In the electrocoagulation treatment method, the spent plating bath is electrocoagulated using aluminum electrodes to produce sludge containing nickel hydroxide and aluminum hydroxide. This sludge is digested in acid to produce a nickel-rich concentrated solution which is utilized for electrowinning to recover pure nickel metal.16 In the electrowinning process, nickel metal ions are extracted as nickel metal by electrodeposition at the cathode using suitable reaction conditions. This process involves three steps: precipitation, leaching and electrodeposition.15 Chemical precipitation or a hydroxide precipitation method is based on the low solubility of metal hydroxides at higher pH values. It is followed by physical separation like sedimentation, flotation and filtration to separate the nickel hydroxide precipitate.16 As the name suggests, the hydroxide precipitation treatment method involves the precipitation of nickel metal ions from the electroless nickel bath as nickel hydroxide Ni(OH)2 at an elevated pH value using sodium/potassium hydroxide. The process is simple and effective but generates a large amount of solid waste mainly consisting of nickel hydroxide Ni(OH)2, which is then used in landfills or dumped into the ground, adding extra costs for handling and storage and making the treatment procedure exorbitant.
Hydroxides and oxides of nickel–metal have been used by researchers since the first half of the twentieth century for battery applications, however with recent advances in nanotechnology their usage is no longer limited to batteries and they have found diverse applications in various advanced technologies such as electrocatalysis,20,21 photocatalysis,22,23 electrochromic devices,24 electrochemical sensors,25 batteries26 and supercapacitors.27
A supercapacitor or electrochemical capacitor is an energy storage device that stores electrical charges between electrode–electrolyte interfaces.28 Supercapacitors have gained much attention recently due to their unique characteristics including a high power density, long cycle life, fast charge/discharge process and low cost. Due to these unique features, supercapacitors have potential applications in transport, renewable energy systems like solar and wind, pulsed laser technology and mobile phones.29 Depending upon the charge storage mechanism, supercapacitors are classified as two types: electrochemical double-layer capacitors (EDLCs) and pseudocapacitors.30 EDLCs involve charge storage at an electrode–electrolyte interface by reversible ion adsorption, while pseudocapacitors store chemical charge liberated in the redox reaction at the electrode surface.31 Carbonaceous materials (carbon blacks, activated carbon, graphene, and carbon nanotubes)32–36 are normally used as EDLC electrodes. Transition metal hydroxides/oxide including RuO2, IrO2, MnO2, Co3O4, NiO, Fe2O3, Fe3O4, Co(OH)2 and Ni(OH)2etc. give rise to the phenomenon of pseudocapacitance.37–39 Among these materials, oxide and hydroxides of nickel have drawn great interest as active electrode materials for supercapacitors due to their high theoretical capacitance (2358 F g−1), excellent electrochemical properties, stability in alkaline medium and lower toxicity compared to other metal hydroxide and oxides.27,40 Different nickel hydroxide synthesis procedures have been reported in the literature however most of them require special reaction conditions, costly equipment and chemicals.41–43
Electroless nickel plating is a routine plating activity at the chemical treatment lab (CTL) of Raja Ramanna Centre for Advanced Technology (RRCAT), which mainly deals with research and development activities in lasers and particle accelerators. In this article, we are reporting the effective chemical treatment of spent electroless nickel baths and utilization of the generated plating waste as an active electrode material for supercapacitors. A similar approach to utilizing waste materials for energy storage applications has been reported in the literature, but it is mostly limited to the production of carbon and its different allotropes.44–46 There are no previous literature reports on the utilization of solid waste nickel hydroxide obtained from spent electroless nickel baths for energy storage applications. This research provides a scaled-up approach for the production of nickel hydroxide from nickel plating waste with comparable electrochemical performances as that of pristine nickel hydroxide and other transition metal hydroxides produced by different synthesis methods. The findings of this research increase awareness towards the conversion of waste to wealth and the utilization of waste material for a sustainable future.
Experimental details
Chemicals and reagents
Sodium hydroxide NaOH (98%) was obtained from high purity laboratory chemicals (HPLC) private limited. An electroless nickel plating bath (medium phosphorus ∼6–8%) was procured from Growel or Grauer and Weil India Limited and was utilized for 8 metal turnovers (MTOs) to obtain a spent electroless nickel bath. Isopropanol and ethanol were purchased from Advent Chem Bio Private Limited and Ultrapure US. 5 wt% Nafion perfluorinated resin solution (Sigma-Aldrich) was used as a binder for electrode preparation. Battery grade conducting carbon black (99%) was used as the conducting filler in electrode preparation. The potassium hydroxide (KOH) used as an electrolyte for the electrochemical study was supplied by Merck. The nickel standard solution for atomic absorption spectroscopy was obtained from Certipur Merck and it contained Ni(NO3)2 in HNO3 0.5 mol L−1 (1000 mg L−1). Ultrapure water was used throughout the experimentation. An ECT electroless nickel plating bath containing a high phosphorus content of ∼10–13% was supplied by Komal Agency.Instrumentation
Fourier-transform infrared spectroscopy (FTIR) analysis of nickel hydroxide was carried out using the Jasco FT/IR-660 plus spectrometer. All electrochemical experiments were performed at room temperature using an Autolab PGSTAT302 (AUT73043) electrochemical working station. Atomic absorption spectroscopy (AAS) of the supernatant liquid left after settling of the nickel hydroxide precipitate was performed using a novAA 300 Analytik Jena spectrometer. The AAS calibration analysis was carried out using the nickel standard supplied by Certipur Merck using a 50 mm burner setup and air acetylene flame. The XRD spectrum of nickel hydroxide powder was determined by using a PANalytical X-ray diffractometer (1.54 Å Cu Kα) in the range of 2θ = 10–90°. The morphology of the nanoparticles was analyzed using a scanning electron microscope (FIB-FEG-SEM) of Carl Zeiss Auriga Compact-4558. The XPS analysis was done using Kratos Analytical (AXIS Supra) with an analysis pressure of <2 × 10−9 Torr and fitted with a monochromatic Al K-alpha (75 W) as the X-ray source. Spectral analysis was performed using the peak fitting software (XPSPEAK version 4.1). X-ray fluorescence analysis (XRF) analysis was carried out using Bruker S1-Titan 600.Synthesis of nickel hydroxide from the spent electroless nickel bath
The chemical synthesis of nickel hydroxide from the spent electroless plating bath was carried out using a simple precipitation reaction. 100 mL solution of the spent electroless nickel bath was moved to a clean and dry glass beaker, to this solution, 10 wt% sodium hydroxide solution was added slowly while continuously stirring the resultant reaction mixture.The starting pH of the spent electroless nickel bath is 5 with a light green color which changes to a greenish-blue color (pH 9–10), the solution remains transparent and clear as shown in stage II of Fig. 1. Further addition of 10 wt% solution of sodium hydroxide changes the reaction mixture color to dark blue with a strong evolution of ammonia gas and takes the reaction to stage III at pH of 12. This dark blue colored slightly turbid solution is converted to a lime green precipitate of nickel hydroxide as the pH of the reaction mixture is increased from 12 to 14 by the addition of 10 wt% sodium hydroxide solution, as depicted in stages IV and V in Fig. 1. The precipitate of nickel hydroxide is allowed to settle (stage VI) in the glass beaker and the resultant supernatant solution is decanted and tested for traces of metallic nickel remaining in the supernatant solution, which will provide the efficiency of the nickel removal as nickel hydroxide using sodium hydroxide. The nickel hydroxide precipitate is then washed with copious amounts of water, followed by drying in a hot air oven at 70 °C for 8–10 h. The final dried nickel hydroxide precipitate is then converted into a fine green powder, which is used for further analysis. A similar procedure is repeated with 5 L of the spent electroless nickel bath to obtain ∼35 g of dried nickel hydroxide powder. The reproducibility and selectivity of the adopted synthesis method were checked by repeating the synthesis procedure three times with spent electroless nickel plating baths procured from different suppliers with different chemical compositions.
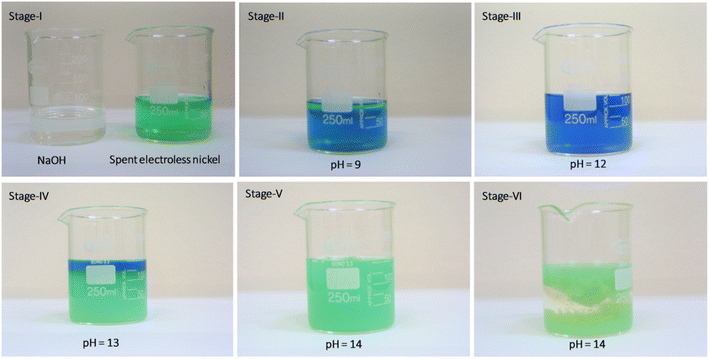 | ||
| Fig. 1 Different stages of treatment of the spent electroless nickel bath using 10 wt% sodium hydroxide solution and generation of plating waste rich in nickel hydroxide content. | ||
Electrode fabrication for supercapacitor application
The electrodes of the supercapacitor were fabricated by following this procedure. The as-synthesized nickel hydroxide is mixed with conducting carbon black in the ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. The mixture is ground in a mortar and pestle for 30 min to obtain the uniformly mixed active electrode material. 20 mg of this mixture is suspended in ultrapure ethanol and sonicated for 30 min to get a uniform suspension of metal hydroxide and conducting carbon particles. 20 μL of 5 wt% Nafion perfluorinated resin binder was added to this suspension and sonicated/mixed for another 10 min. The slurry of active electrode material and Nafion binder was coated on graphite paper using the layer-by-layer brush coating technique. The coated graphite paper is heated in a hot air oven at 80 °C overnight. The process was repeated to make other electrodes. For the asymmetric supercapacitor configuration, a negative electrode was made by using only conducting carbon black and Nafion binder (carbon black: 20 mg and 5 μL Nafion binder). 3 M potassium hydroxide (KOH) was used as an aqueous electrolyte in atmospheric conditions.
15. The mixture is ground in a mortar and pestle for 30 min to obtain the uniformly mixed active electrode material. 20 mg of this mixture is suspended in ultrapure ethanol and sonicated for 30 min to get a uniform suspension of metal hydroxide and conducting carbon particles. 20 μL of 5 wt% Nafion perfluorinated resin binder was added to this suspension and sonicated/mixed for another 10 min. The slurry of active electrode material and Nafion binder was coated on graphite paper using the layer-by-layer brush coating technique. The coated graphite paper is heated in a hot air oven at 80 °C overnight. The process was repeated to make other electrodes. For the asymmetric supercapacitor configuration, a negative electrode was made by using only conducting carbon black and Nafion binder (carbon black: 20 mg and 5 μL Nafion binder). 3 M potassium hydroxide (KOH) was used as an aqueous electrolyte in atmospheric conditions.
Results and discussion
Characterization of the supernatant effluent
Atomic absorption spectroscopy (AAS) is used to quantify the nickel removal efficiency of the sodium hydroxide from the spent electroless nickel plating bath. Calibration for this analysis was carried out using a nickel standard solution obtained from Certipur Merck. The concentration of the nickel metal in the supernatant solution (6 ppm) is calculated using the calibration plot, as shown in Fig. 2.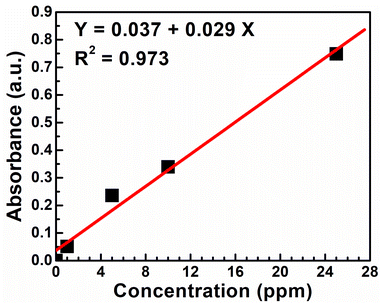 | ||
| Fig. 2 The atomic absorption spectroscopy calibration curve using different nickel standard solutions. | ||
Material characterization of the nickel hydroxide
Nickel hydroxide exists in different polymorphic forms like α-Ni(OH)2 and β-Ni(OH)2 and its crystal structure is made up of edge-sharing Ni(OH)6 octahedron layers. The factors which mainly distinguish α-Ni(OH)2 from β-Ni(OH)2 are the distance between the Ni(OH)6 octahedral layers and the presence of ions/water molecules intercalated between these octahedron layers. The interlayer distance in β-Ni(OH)2 is ∼0.46 nm with no intercalation of water molecules between the octahedral layers, while for α-Ni(OH)2 it is ∼0.75 nm with intercalation of water molecules and oxyanions between these layers.47–49 The first confirmation of the successful removal of nickel metal as hydroxides from an electroless nickel bath is obtained from XRD analysis. The XRD pattern of nickel hydroxide in Fig. 3(a) shows major diffraction peaks at 2θ = 19.70°, 33.22°, 38.36°, 51.49°, 59.09°, 62.49°, 69.43° and 72.76°, corresponding to the (001), (100), (101), (102), (110), (111), (103) and (201) planes of the β-Ni(OH)2 phase (JCPDS #14-0117).25 The average crystallite size of nickel hydroxide was calculated using Scherrer's eqn (1):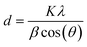 | (1) |
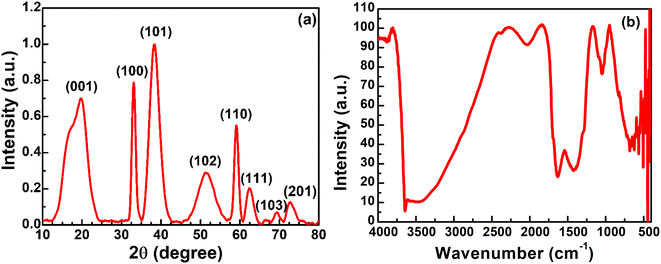 | ||
| Fig. 3 (a) The X-ray diffraction pattern of nickel hydroxide powder and (b) the FT-IR spectrum of nickel hydroxide powder. | ||
The band around 554 cm−1 is due to the lattice vibrations of δ(OH) bending.49 The bands at 442 and 421 cm−1 arise due to the lattice vibration of the Ni–O bond.51 The peak observed around 1631 cm−1 is ascribed to the bending vibration of water molecules.50 The band at 681 cm−1 corresponds to the δ(O–H) wagging vibration.42 The band at 1047 cm−1 shows the signal of the metal–oxygen metal (M–O–M) bond between Ni and O. The bands at 1420 cm−1 and 2034 cm−1 could arise from the –CH2 symmetric bending vibration and C–H stretching vibration of carbon-based plating additives, which are normally incorporated in plating baths and might be adsorbed on the nickel hydroxide surface.
Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analysis were performed to study the microstructure and composition of the nickel hydroxide powder Ni(OH)2 obtained from the spent electroless nickel plating bath. The SEM micrograph of β-Ni(OH)2 in Fig. 4(a) and (b) shows the presence of flaky particles in agglomeration.
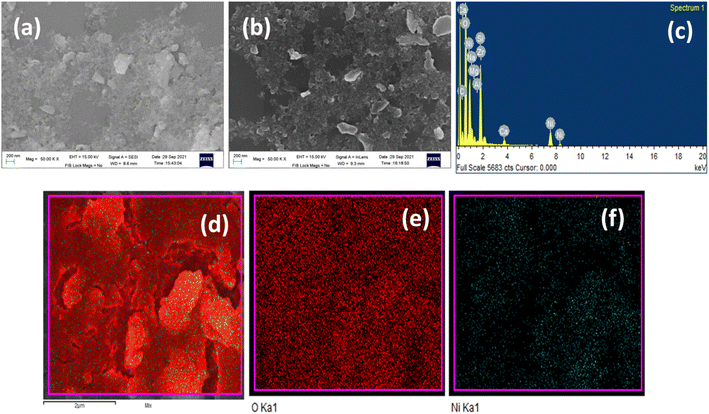 | ||
| Fig. 4 (a) and (b) SEM of Ni(OH)2 powder, (c) EDX spectrum of Ni(OH)2 powder and EDX elemental area mapping of nickel hydroxide powder: (d) Ni(OH)2, (e) oxygen in Ni(OH)2 and (f) nickel in Ni(OH)2. | ||
XPS analysis was performed to determine the surface composition of nickel hydroxide powder. The XPS survey spectrum of nickel hydroxide in Fig. 5(a) shows peaks associated with the presence of nickel and oxygen. The high-resolution Ni 2p XPS spectrum in Fig. 5(b) shows two major peaks at 856.1 and 873.8 eV, which can be identified as Ni 2p3/2 and Ni 2p1/2, indicating the presence of nickel in the +2 oxidation state (Ni2+).52 The spin energy separation observed between these two peaks is about 17.6 eV, which is a typical characteristic of the β-Ni(OH)2 phase.53 In addition to the peaks associated with Ni 2p3/2 and Ni 2p1/2, two satellite peaks were observed at 861.8 and 880.1 eV, which signify the presence of nickel in the hydroxide form.52 The high-resolution XPS spectrum of O 1s as depicted in Fig. 5(c) has a peak at 531.8 eV, indicating oxygen in the Ni–OH bond.54 The composition of β-Ni(OH)2 powder was also determined using XRF analysis. β-Ni(OH)2 powder contains Ni (96.5%) and other impurities like Fe (0.6%), Si (0.6%), P (0.7%) and S (0.9%). The presence of these impurities can also result in peak broadening in XRD results.
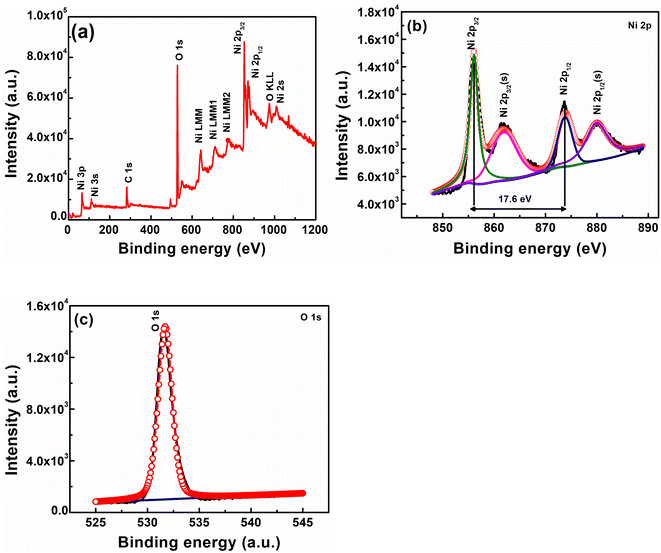 | ||
| Fig. 5 (a) XPS survey spectrum of nickel hydroxide powder, (b) high-resolution Ni 2p spectrum of Ni(OH)2, and (c) high-resolution O 1s spectrum of Ni(OH)2. | ||
Electrochemical characterization of nickel hydroxide
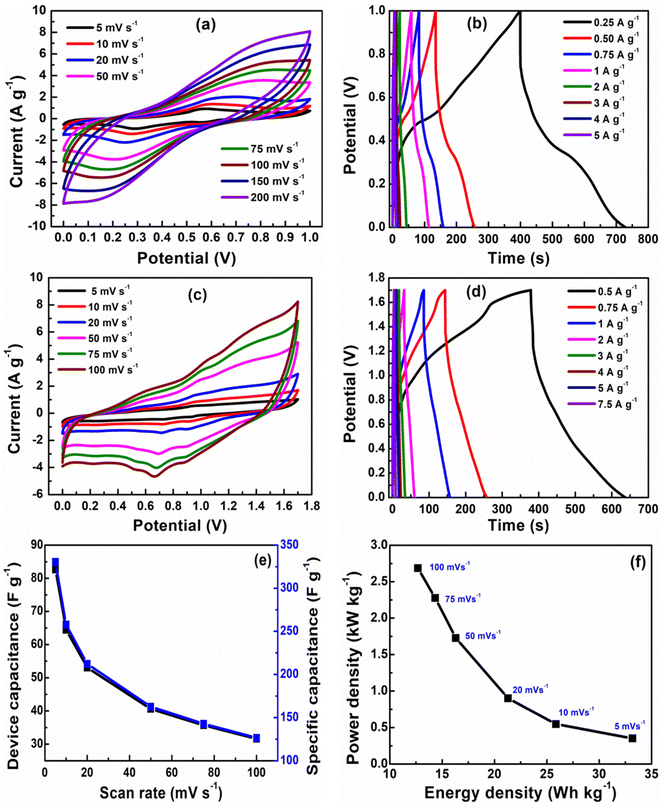
The capacitive performance of nickel hydroxide obtained from the spent electroless nickel plating bath was evaluated by electrochemical methods like cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD). Two different devices (a symmetric supercapacitor and an asymmetric supercapacitor) were fabricated to study the electrochemical properties. A two-cell electrode configuration is used to evaluate electrochemical properties for both the symmetric supercapacitor and asymmetric supercapacitor in 3 M KOH electrolyte.The device capacitance (Cd), energy density (Ed) and power density (Pd) for Ni(OH)2 electrode materials were calculated by using eqn (2)–(4):39,55
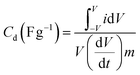 | (2) |
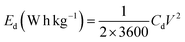 | (3) |
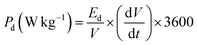 | (4) |
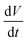 is a scan rate (V s−1) and m is the mass of both electrodes. The specific capacitance is calculated by using the formula Cs = 4 × Cd. The mass loading of nickel hydroxide and carbon black electrode on graphite paper (2 × 2 cm2) was 2.6 mg and 2 mg, respectively. The asymmetric supercapacitor was made by using nickel hydroxides as a positive electrode and carbon black as a negative electrode. The size of each graphite paper was 2 × 2 cm2. To increase the potential window of the supercapacitor, the weight of each positive and negative electrode was obtained by using the charge balance eqn (5). The voltammetric charge (q) of each electrode was calculated by eqn (6) where Cs, ΔV and m were the specific capacitance (F g−1), potential window (V) and mass (mg) of each electrode. To secure the charge balance, the mass ratio of the positive and negative electrodes was calculated by eqn (6) and (7)56–58
is a scan rate (V s−1) and m is the mass of both electrodes. The specific capacitance is calculated by using the formula Cs = 4 × Cd. The mass loading of nickel hydroxide and carbon black electrode on graphite paper (2 × 2 cm2) was 2.6 mg and 2 mg, respectively. The asymmetric supercapacitor was made by using nickel hydroxides as a positive electrode and carbon black as a negative electrode. The size of each graphite paper was 2 × 2 cm2. To increase the potential window of the supercapacitor, the weight of each positive and negative electrode was obtained by using the charge balance eqn (5). The voltammetric charge (q) of each electrode was calculated by eqn (6) where Cs, ΔV and m were the specific capacitance (F g−1), potential window (V) and mass (mg) of each electrode. To secure the charge balance, the mass ratio of the positive and negative electrodes was calculated by eqn (6) and (7)56–58| q+ = q− | (5) |
| q = CsΔVm | (6) |
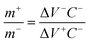 | (7) |
According to the specific capacitance and potential window, the weight of nickel hydroxide of carbon black was 2.4 mg and 3.6 mg, respectively.
Fig. 6(a) shows a cyclic voltammogram (CV) curve for a symmetric supercapacitor at different scan rates ranging from 5–200 mV s−1 in 3 M KOH electrolyte and a potential window of 0–1 V. Unlike electric double-layer capacitors (EDLCs), which normally produce rectangular CV curves, nickel hydroxide CV curves show prominent redox reaction peaks, indicating reversible faradaic reactions occurring at the surface of the electrode. The nearly symmetric redox reaction peaks arise from the pseudocapacitance behavior of Ni(OH)2 electrodes in the 3 M KOH electrolyte. The faradaic process occurring on the electrode surface can be represented by eqn (8). The oxidation of the Ni(OH)2 to NiOOH can be observed in the forward anodic peak occurring at 0.56 V, while the cathodic peak representing the reverse reaction occurs at 0.29 V.59
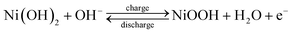 | (8) |
The peak potentials for anodic peaks shift towards the right side, while the cathodic peaks shift towards the left side with increasing scan rates due to the mass transfer limitation of diffusing electrolyte ions at fast scan rates.40,60 The specific capacitance of 332 F g−1 is obtained at a scan rate of 5 mV s−1 from the CV data of a symmetric supercapacitor with the energy density of 11.5 W h kg−1 and power density of 207.5 W kg−1. The cyclic voltammogram (CV) curve for the asymmetric supercapacitor device recorded in the potential range of 0–1.7 V at scan rates of 5–100 mV s−1 is represented in Fig. 6(c). The CV profile of asymmetric supercapacitor is quasi-rectangular due to the contribution of electric double-layer capacitance arising from conducting carbon and pseudocapacitance of Ni(OH)2. The specific capacitance of 330 F g−1 at a scan rate of 5 mV s−1 is obtained from the CV data of an asymmetric supercapacitor device with the energy density of 33 W h kg−1 and power density of 351 W kg−1. The higher energy density and power density of the asymmetric supercapacitor are attributed to the wider operating potential window of the asymmetric supercapacitor due to the presence of conducting carbon black. The galvanostatic charge–discharge (GCD) profiles for the symmetric and asymmetric supercapacitors at various current densities are illustrated in Fig. 6(b) and (d). The specific capacitances of 330 F g−1 and 305 F g−1 are obtained for symmetric and asymmetric supercapacitor devices which are in close agreement with the value obtained from the CV data.
The device capacitance and specific capacitance of the asymmetric supercapacitor at different scan rates are shown in Fig. 6(e). The capacitance decreased with increasing scan rates because at lower scan rates, electrolyte ions have more time to diffuse into electrode materials, while at a higher scan rate the movement of electrolyte ions is limited to the surface of electrode materials. The value of energy density and power density (11 W h kg−1 and 125 W kg−1) for the symmetric capacitor is lower than that of the (33 W h kg−1 and 351 W kg−1) energy density and power density of the asymmetric supercapacitor due to dependence on the potential window, as shown in Fig. 6(f). The capacitive performance of β-Ni(OH)2 reported in this work is comparable to nickel hydroxide obtained by precipitation and hydrothermal methods61,62 and other transition metal hydroxides of Mn and Cu.63,64
Conclusions
This work reports a simple method to treat a spent electroless nickel plating bath using sodium hydroxide to form nickel hydroxide. The nickel hydroxide generated after the treatment of an electroless nickel plating bath was mainly composed of the β-Ni(OH)2 phase and was characterized by different material characterization techniques. The electrochemical performance of β-Ni(OH)2 powder was evaluated using electrochemical techniques like cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) in the symmetric and asymmetric configurations in different potential windows. A specific capacitance of 332 F g−1@5 mV s−1 is obtained for the symmetric configuration which mainly arises from the pseudocapacitance behavior of β-Ni(OH)2. The GCD results show a specific capacitance of 330 F g−1 which is close to the value obtained from the CV data of the symmetric supercapacitor configuration. The β-Ni(OH)2 powder obtained from the plating waste has comparable electrochemical properties to those of previously reported reports.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
Authors are thankful to the staff members of the chemical treatment lab (CTL), RRCAT. Also, the authors acknowledge access to the experimental facilities provided by IIT Bombay.References
- W. Giurlani, G. Zangari, F. Gambinossi, M. Passaponti, E. Salvietti, F. Di Benedetto, S. Caporali and M. Innocenti, Coatings, 2018, 8, 1–25 Search PubMed.
- K. Mazur, A. Stefańska and M. Hebda, Mater. Sci., 2018, 54, 387–394 CrossRef CAS.
- C. A. Loto, Silicon, 2016, 8, 177–186 CrossRef CAS.
- C. S. Lin, P. C. Hsu, L. Chang and C. H. Chen, J. Appl. Electrochem., 2001, 31, 925–933 CrossRef CAS.
- V. A. Pérez Jiménez, V. Hernández-Montoya, L. A. Ramírez-Montoya, F. Castillo-Borja, R. Tovar-Gómez and M. A. Montes-Morán, J. Environ. Manage., 2021, 284, 112024 CrossRef PubMed.
- C. Sherwin, S. Bhat and S. P. Hebbar, AIP Conf. Proc., 2020, 2236, 040007 CrossRef CAS.
- C. M. Whittington and W. Y. Lo, Trans. Inst. Met. Finish., 2019, 97, 64–66 CrossRef CAS.
- O. Sadiku-Agboola, Int. J. Phys. Sci., 2012, 7, 349–360 Search PubMed.
- F. Delaunois, V. Vitry and L. Bonin, Electroless Nickel Plating Fundamentals to Applications, CRC Press Taylor & Francis Group, New York, 2020 Search PubMed.
- R. Sun, G. Yu, Z. Xie, B. Hu, J. Zhang, X. He and X. Zhang, Int. J. Electrochem. Sci., 2015, 10, 7893–7904 CAS.
- R. Taheri, Evaluation of Electroless Nickel-Phosphorus (EN) Coatings, University of Saskatchewan, 2003 Search PubMed.
- X. Yu and J. Jiang, J. Environ. Manage., 2019, 245, 447–453 CrossRef CAS PubMed.
- J. Zhang, R. Djellabi, S. Zhao, M. Qiao, F. Jiang and M. Yan, J. Hazard. Mater., 2020, 394, 122559 CrossRef CAS PubMed.
- V. Coman, B. Robotin and P. Ilea, Resour., Conserv. Recycl., 2013, 73, 229–238 CrossRef.
- P. Laokhen, N. Ma-Ud, T. Yingnakorn, T. Patcharawit and S. Khumkoa, J. Met., Mater. Miner., 2022, 32, 95–100 CrossRef CAS.
- K. I. Dermentzis, D. I. Marmanis, A. K. Christoforidis, N. C. Kokkinos and D. K. Stergiopoulos, Recovery of metallic nickel from waste sludge produced by electrocoagulation of nickel bearing electroplating effluents, 4th International Conference on Sustainable Solid Waste Management, Cyprus, 2016, pp. 0–7 Search PubMed.
- C. L. Li, H. X. Zhao, T. Tsuru, D. Zhou and M. Matsumura, J. Membr. Sci., 1999, 157(2), 241–249 CrossRef CAS.
- H. Lu, Y. Wang and J. Wang, J. Cleaner Prod., 2015, 92, 257–266 CrossRef CAS.
- C. L. Li, H. X. Zhao, T. Tsuru, D. Zhou and M. Matsumura, J. Membr. Sci., 1999, 157, 241–249 CrossRef CAS.
- L. A. Stern and X. Hu, Faraday Discuss., 2014, 176, 363–379 RSC.
- E. Farjami, M. A. Rottmayer and L. Jay Deiner, J. Mater. Chem. A, 2013, 1, 15501–15508 RSC.
- L. Mao, Q. Ba, X. Jia, S. Liu, H. Liu, J. Zhang, X. Li and W. Chen, RSC Adv., 2019, 9, 1260–1269 RSC.
- Y. P. Xie, Y. Zheng, Y. Yang, R. Jiang, G. Wang, Y. Zhang, E. Zhang, L. Zhao and C. Y. Duan, J. Colloid Interface Sci., 2018, 514, 634–641 CrossRef CAS PubMed.
- J. Liu, S. Y. Chiam, J. Pan, L. M. Wong, S. F. Y. Li and Y. Ren, Sol. Energy Mater. Sol. Cells, 2018, 185, 318–324 CrossRef CAS.
- H. Yang, G. Gao, F. Teng, W. Liu, S. Chen and Z. Ge, J. Electrochem. Soc., 2014, 161, B216–B219 CrossRef CAS.
- B. Ash, V. S. Nalajala, A. K. Popuri, T. Subbaiah and M. Minakshi, Nanomaterials, 2020, 10, 1–22 CrossRef PubMed.
- S. W. Kim, I. H. Kim, S. I. Kim and J. H. Jang, Chem.–Asian J., 2017, 12, 1291–1296 CrossRef CAS PubMed.
- Y. Wen, K. Kierzek, X. Chen, J. Gong, J. Liu, R. Niu, E. Mijowska and T. Tang, Waste Manage., 2019, 87, 691–700 CrossRef CAS PubMed.
- B. T. Al-Abawi, N. Parveen and S. A. Ansari, Sci. Rep., 2022, 12, 1–11 CrossRef PubMed.
- Y. L. Zhang and Z. S. Tang, Waste Manage., 2020, 106, 250–260 CrossRef CAS PubMed.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 RSC.
- S. Trano, F. Corsini, G. Pascuzzi, E. Giove, L. Fagiolari, J. Amici, C. Francia, S. Turri, S. Bodoardo, G. Griffini and F. Bella, ChemSusChem, 2022, 15, e202200294 CrossRef CAS PubMed.
- F. Elizalde, J. Amici, S. Trano, G. Vozzolo, R. Aguirresarobe, D. Versaci, S. Bodoardo, D. Mecerreyes, H. Sardon and F. Bella, J. Mater. Chem. A, 2022, 10, 12588–12596 RSC.
- R. Colombo, N. Garino, D. Versaci, J. Amici, M. L. Para, E. Quartarone, C. Francia, F. Bella and S. Bodoardo, J. Mater. Sci., 2022, 57, 15690–15704 CrossRef CAS.
- Y. Long, X. An, H. Zhang, J. Yang, L. Liu, Z. Tian, G. Yang, Z. Cheng, H. Cao, H. Liu and Y. Ni, Chem. Eng. J., 2023, 451, 138877 CrossRef CAS.
- L. Fagiolari, M. Sampò, A. Lamberti, J. Amici, C. Francia, S. Bodoardo and F. Bella, Energy Storage Mater., 2022, 51, 400–434 CrossRef.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- B. K. Singh, A. Shaikh, S. Badrayyana, D. Mohapatra, R. O. Dusane and S. Parida, RSC Adv., 2016, 6, 100467–100475 RSC.
- B. K. Singh, A. Shaikh, R. O. Dusane and S. Parida, J. Energy Storage, 2020, 31, 101631 CrossRef.
- L. Aguilera, Y. Leyet, R. Peña-Garcia, E. Padrón-Hernández, R. R. Passos and L. A. Pocrifka, Chem. Phys. Lett., 2017, 677, 75–79 CrossRef CAS.
- S. S. Waghmare, P. B. Patil, S. K. Baruva, M. S. Rajput, R. J. Deokate and S. H. Mujawar, AIP Conf. Proc., 2018, 1942, 3–7 CrossRef.
- G. J. A. A. De Soler-Illia, Chem. Mater., 1999, 11, 3140–3146 CrossRef CAS.
- P. E. Sharel, D. Liu, R. A. Lazenby, J. Sloan, M. Vidotti, P. R. Unwin and J. V. Macpherson, J. Phys. Chem. C, 2016, 120, 16059–16068 CrossRef.
- H. Hwang, J. H. Lee, M. A. Ahmed and J. W. Choi, J. Environ. Manage., 2021, 298, 113436 CrossRef CAS PubMed.
- S. I. El-Hout, S. Y. Attia, S. G. Mohamed and S. M. Abdelbasir, J. Environ. Manage., 2022, 304, 114222 CrossRef CAS PubMed.
- L. Chen, T. Ji, L. Mu, Y. Shi, H. Wang and J. Zhu, J. Environ. Manage., 2017, 196, 168–177 CrossRef CAS PubMed.
- X. Wang, H. Luo, P. V. Parkhutik, A. C. Millan and E. Matveeva, J. Power Sources, 2003, 115, 153–160 CrossRef CAS.
- M. Aghazadeh, M. Ghaemi, B. Sabour and S. Dalvand, J. Solid State Electrochem., 2014, 18, 1569–1584 CrossRef CAS.
- X. Yi, H. Sun, N. Robertson and C. Kirk, Sustainable Energy Fuels, 2021, 5, 5236–5246 RSC.
- A. Numan, N. Duraisamy, F. Saiha Omar, D. Gopi, K. Ramesh and S. Ramesh, Prog. Nat. Sci.: Mater. Int., 2017, 27, 416–423 CrossRef CAS.
- M. Taibi, S. Ammar, N. Jouini, F. Fiévet, P. Molinié and M. Drillon, J. Mater. Chem., 2002, 12, 3238–3244 RSC.
- J. Li, H. Hao, J. Wang, W. Li and W. Shen, J. Alloys Compd., 2019, 782, 516–524 CrossRef CAS.
- D. Kong, L. Cao, Z. Fang, F. Lai, Z. Lin, P. Zhang and W. Li, Ionics, 2019, 25, 4341–4350 CrossRef CAS.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4, 1–7 CAS.
- B. K. Singh, A. Shaikh, R. O. Dusane and S. Parida, Nano-Struct. Nano-Objects, 2019, 17, 239–247 CrossRef.
- N. R. Chodankar, D. P. Dubal, G. S. Gund and C. D. Lokhande, Energy Technol., 2015, 3, 625–631 CrossRef CAS.
- S. Balasubramaniam, A. Mohanty, S. K. Balasingam, S. J. Kim and A. Ramadoss, Comprehensive Insight into the Mechanism, Material Selection and Performance Evaluation of Supercapatteries, Springer, Singapore, 2020, vol. 12 Search PubMed.
- S. E. Moosavifard, M. F. El-Kady, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, ACS Appl. Mater. Interfaces, 2015, 7, 4851–4860 CrossRef CAS PubMed.
- B. Shruthi, B. J. Madhu, V. B. Raju, S. Vynatheya, B. V. Devi, G. V. Jayashree and C. R. Ravikumar, J. Sci.: Adv. Mater. Devices, 2017, 2, 93–98 Search PubMed.
- H. Cui, J. Xue, W. Ren and M. Wang, J. Nanopart. Res., 2014, 16, 2601 CrossRef.
- L. Zhao, S. Lei, Q. Tu, L. Rao, W. Zen, Y. Xiao and B. Cheng, J. Energy Storage, 2021, 43, 103171 CrossRef.
- Q. Li, H. Ni, Y. Cai, X. Cai, Y. Liu, G. Chen, L. Z. Fan and Y. Wang, Mater. Res. Bull., 2013, 48, 3518–3526 CrossRef CAS.
- A. You, M. A. Y. Be and I. In, AIP Adv., 2013, 3, 082118 CrossRef.
- X. Zhu, Y. Chen, R. Xie, H. Zhong and W. Zhao, 2021, 9, 1–11.
| This journal is © The Royal Society of Chemistry 2023 |