Auto-ignition and reaction kinetic characteristics of hydrogen-enriched n-dodecane mixtures under engine-like thermodynamic conditions
Zhihao
Yang
ab,
Changhui
Zhai
 *ab,
Zhen
Gong
c and
Yejian
Qian
c
*ab,
Zhen
Gong
c and
Yejian
Qian
c
aState Key Laboratory of Engine and Powertrain System, Weifang 261061, China. E-mail: yangzhih@weichai.com; zhaich@weichai.com
bWeichai Power Co., Ltd, Weifang 261061, China
cSchool of Automotive and Transportation Engineering, Hefei University of Technology, Hefei 230009, China. E-mail: 18856072762@163.com; qianyjian@163.com
First published on 13th November 2023
Abstract
The hydrogen/diesel dual-fuel engine has attracted extensive attention in recent years. To acquire ignition control methods for a dual-fuel engine, the mutual effects and separate influencing scales of multiple factors on the auto-ignition and reaction kinetic characteristics of a hydrogen/n-dodecane mixture under engine-like thermodynamic conditions were revealed by detailed chemical kinetic mechanism and experimental data based on the colormap and Taguchi methods. The results showed that blending a small amount of n-dodecane induced the initial production of OH and the early oxidation of fuels, which dramatically reduced the IDT of the hydrogen mixture. With a continuous increase in n-dodecane content, the improved reaction rates of the fuels outweighed the reduced reaction rates of active radicals, which resulted in a further reduction in the IDT and an enhancement in NTC behavior. Elevating ϕ and initial pressure apparently reduced the IDT as well. The rates of contribution of temperature, ϕ, pressure, and n-dodecane content on IDT are 62.9%, 27.0%, 5.1%, 5.0%, respectively. The temperature exerted a great influence under all conditions. The ϕ also had a obvious impact in most regions other than a pure hydrogen mixture or under low-temperature/high-ϕ conditions. The effect of pressure was ignorable under low-temperature or pure hydrogen mixture conditions due to the dramatically weakened mixture reactivity. While the influence of n-dodecane fraction was also negligible when it was more than 10% at mid-temperature or 20% at low temperature. Further, the sensitivity analysis showed the sensitivity coefficients of decomposition reactions and H/HO2 related reactions reduced and increased, respectively, with increasing temperature. The sensitivity coefficients of OH/H2O2 related reactions reached their peak values in the NTC region, which represents the region where H2O2/OH radicals were the significant intermediate products in the hydrogen/n-dodecane reaction system. This investigation not only revealed the coupled influence law of multiple factors and the internal interaction mechanism between fuels and active radicals in the hydrogen/n-dodecane reaction system, but also provided fundamental insights into precise ignition control methods for a diesel/hydrogen dual-fuel engine.
1. Introduction
According to the Fourth International Maritime Organization (IMO) Greenhouse Gas (GHG) Study 2020, total shipping generated 1056 million tonnes of CO2 in 2018, or roughly 2.89% of all anthropogenic CO2 emissions worldwide. To reduce the atmospheric and marine pollution caused by ships, the IMO set its GHG reduction strategy to reduce these emissions by a minimum of 40% by 2030 and by a minimum of 70% by 2050 compared to 2018. Thus the world's shipbuilding manufacturers are facing severe challenges of lowering GHG emissions. To achieve these goals, the global shipbuilding industry is striving to propel the adoption of zero-carbon fuels like hydrogen and ammonia.Compared with ammonia, hydrogen has a high flame speed and heat value, which ensure high-efficiency engine combustion performance.1 The wide ignition limit and low ignition energy of hydrogen increase the ignitable scope of the in-cylinder mixture, which is beneficial to stable combustion over wide operating conditions.2 Further, hydrogen has extremely low emission levels because the only combustion pollutant of hydrogen is NOx.3 Therefore, the excellent combustion and emission characteristics significantly broaden the application prospects of hydrogen in internal combustion engines.
Hydrogen-fueled internal combustion engines (HICEs) had already been proposed early in 1933.4 Various investigations suggested that HICEs possess high power performance and reliability with nearly zero carbon emissions.5–9 The development of HICEs in the automotive industry started at the beginning of the 21st century.10–18 However, the characteristics of hydrogen, including extremely low ignition energy (0.02 mJ, 0.24 mJ for diesel), high burning rate (200 cm s−1, 45 cm s−1 for diesel) and shorter quenching distance (0.64 mm, 2 mm for gasoline), may induce abnormal combustion and a high combustion temperature in marine HICEs, which threaten engine operational safety and raise NOx emissions.19,20 To mitigate these issues, HICEs normally apply a lean combustion mode. Spark ignition is practical for automotive engines due to their high rotation speed and small cylinder bores. However, for marine hydrogen engines, which have much larger cylinder bores and leaner fuel/air mixtures, common spark ignition cannot provide sufficient energy to ignite an ultra-lean mixture and cannot ensure stable flame propagation. It requires cylinders with well-penetrated ignition sources and substantially higher ignition energy. As a result, marine HICEs must inject pilot diesel.
In the pilot-diesel/hydrogen dual-fuel engine, the combustion of pilot diesel functions as a huge spark plug with high energy, which ensures continuous reliable ignition and reduces cycle-to-cycle variation.21 This combustion mode of the Otto cycle dramatically reduces engine emissions. Therefore, many researchers have investigated the operating performance of diesel/hydrogen dual-fuel engines. Szwaja et al.22 found that a moderate blending ratio of hydrogen could promote the combustion homogeneity of the in-cylinder mixture, which improved the overall combustion performance. Tsujimura et al.23 showed that the influence of the blended hydrogen on the thermal efficiency of the diesel engine depended on the located engine load. Blending the hydrogen elevated the thermal efficiency at high load; whereas, the variation law showed an opposite trend at low load. Further, with the addition of hydrogen, the CO and soot emissions reduced while the NOx emissions increased. Naber et al.24 investigated the in-cylinder auto-ignition properties of hydrogen in a diesel engine. The results show that hydrogen's auto-ignition characteristics had strong relevance to in-cylinder temperature and diesel injection timing.
It can be concluded from the above research that the operating performance of the dual-fuel engine depends mainly on the blending ratio of diesel and hydrogen and the located in-cylinder thermodynamic conditions. The actual diesel fuel consists of multiple complex compositions, including cycloalkanes, straight-chain alkanes, and aromatic hydrocarbons.25 For the convenience of the theoretical research in the current study, n-dodecane is treated as a surrogate fuel for diesel because the molecular size and boiling characteristics of n-dodecane are close to those of ordinary diesel.26 Therefore, understanding the auto-ignition mechanism of n-dodecane/hydrogen mixtures with wide blending ratios and thermodynamic conditions are critical to revealing the potential control strategies of combustion progress in a diesel/hydrogen dual-fuel engine.
The auto-ignition characteristics of a mixture of hydrogen and low-carbon fuels have been investigated in many studies. Mohamed27 investigated the influence of the addition of hydrogen on the ignition of C1–C7 natural gas blends. The results demonstrate that hydrogen addition has both an inhibiting and a promoting effect in low- and high-temperature regimes, respectively. He28 revealed the auto-ignition kinetics of hydrogen-enriched ammonia mixtures at intermediate temperatures and high pressures via a rapid compression machine. The experiments demonstrate that a higher hydrogen mole fraction increases the reactivity of hydrogen/ammonia mixtures, while the equivalence ratio has a different influence. Jiang et al.29 constructed a skeletal mechanism for the prediction of ignition delay times and laminar premixed flame velocities of hydrogen–methane mixtures under gas turbine conditions. However, only a few studies have concentrated on the ignition progress of a mixture of hydrogen and high-carbon fuels. Li et al.30 studied the ignition properties of premixed iso-octane/air with hydrogen addition through detailed chemistry numerical simulations. They found the addition of a small amount of hydrogen could reduce the minimum ignition energy of iso-octane/air. Whereas the minimum ignition energy was insensitive to hydrogen addition when the blending ratio of hydrogen is above 60%. Aggarwal et al.31 investigated the ignition characteristics of an n-heptane/hydrogen mixture at elevated pressure based on various chemical kinetic mechanisms. The results showed that the addition of n-heptane decreased and increased the IDT of hydrogen mixture at low and high temperature, respectively. Frolov et al.32 found that hydrogen acted as an ignition promoter and an ignition inhibiter, respectively, in the high-temperature (T > 1050 K) and low-temperature (T < 1050 K) reaction system of an n-decane mixture. Furthermore, laminar burning velocity experiments of n-decane/hydrogen mixtures conducted by Xu et al.33 showed that the laminar burning velocity of an n-decane mixture increased linearly with an increasing blending ratio of hydrogen.
Generally speaking, existing research on the auto-ignition properties of hydrogen-enriched mixtures concentrate on a mixture of hydrogen, low-carbon fuels and some high-carbon fuels (n-decane, iso-octane, n-heptane). However, studies of the ignition characteristics of a hydrogen/n-dodecane mixture under wide thermodynamic conditions and various blending ratios were scarce. In addition, the influence of different ambient gas atmospheres on the chemical reaction kinetic property of the mixture with hydrogen and high-carbon fuels also needed to be explored and compared.
Therefore, in the current study, the auto-ignition properties of hydrogen/n-dodecane mixtures (ϕ: 0.5, 1.0, 2.0) were conducted under high pressures (40–80 bar) and low-to-mid temperatures (750–1050 K) to provide basic research data on the IDT for hydrogen/n-dodecane mixtures, and obtain ignition control methods for a hydrogen/diesel dual-fuel engine. The chemical reaction kinetics properties of hydrogen/n-dodecane mixtures were also investigated by detailed kinetic mechanisms to reveal the differences in auto-ignition properties and reaction kinetics characteristics of n-dodecane/hydrogen mixtures under wide thermodynamic conditions and various blending ratios. Finally, the acquired coupled influence mechanisms of specific thermodynamic conditions and mixture compositions on the auto-ignition progress of hydrogen/n-dodecane mixtures were helpful to reveal the optimization directions for further improving the combustion performance of hydrogen/diesel dual-fuel engines.
2. Numerical model and calculation settings
2.1 Mechanism selection and validation
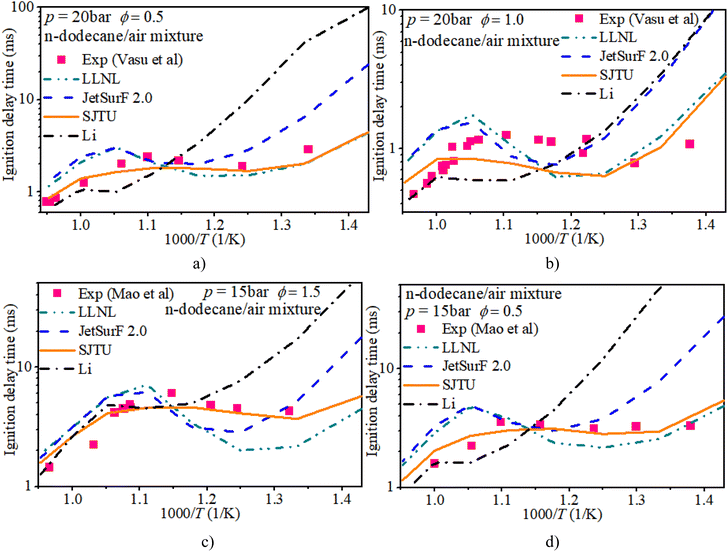
The experimental data were obtained from a shock tube and rapid compression machine.34–36 The zero-dimensional, constant volume and adiabatic reactor in Chemkin-Pro was adopted to reveal the auto-ignition process of n-dodecane/hydrogen mixtures under engine-like conditions. This reactor is a 0-D homogeneous and adiabatic system with equations of mass, energy and species, assuming that fuel molecules are ignited by their own chemical kinetic effects in a homogeneous system, which can be used to calculate the mole fraction and chemical reaction rate of species. The auto-ignition progresses under shock tube conditions are calculated based on the thermodynamic conditions behind reflected shock waves. While the auto-ignition simulations under rapid compression machine conditions rely on the actual variable volume profiles deduced from non-reactive experiments. Although the kinetic mechanisms for the ignition of n-dodecane/hydrogen mixtures are rarely involved, the reaction mechanisms for the ignition of a pure hydrogen mixture or a pure n-dodecane mixture have been studied. LLNL,37 JetsurF 2.0,38 SJTU35 and Li39–42 mechanisms are the representative n-dodecane mechanisms. Further, the n-dodecane mechanism includes the oxidation mechanism of hydrogen. Therefore, the n-dodecane oxidation mechanism can be used to predict the auto-ignition properties of n-dodecane/hydrogen mixtures.In order to confirm the accuracy of the n-dodecane oxidation mechanism in predicting the IDT of hydrogen/n-dodecane mixtures, the IDTs of both hydrogen and n-dodecane calculated from LLNL, JetsurF 2.0, SJTU and Li mechanisms are compared to the experimental measurements from Vasu et al.,34 Mao et al.35 and Gersen et al.36Fig. 1 shows the IDT of a pure n-dodecane mixture with ϕ of 0.5, 1.0, and 1.5 and initial pressures of 15 bar and 20 bar. The LLNL mechanism overestimates the IDT in the mid-temperature region and underestimates the IDT in most low-temperature regions. The SJTU mechanism well captures the IDT in most regions and slightly underestimates the IDT in the NTC region. The JetsurF 2.0 and Li mechanisms exhibit greater discrepancies in estimating the IDT compared to the other mechanisms.
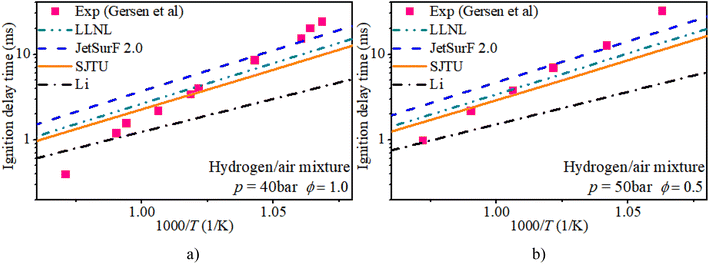
Fig. 2 shows the IDT of a pure hydrogen mixture with ϕ of 0.5 and 1.0 and initial pressures of 40 bar and 50 bar. Almost all mechanisms overestimate the IDT at mid-temperatures and underestimate the IDT at low temperatures. Relatively speaking, the predictions of the IDT of a hydrogen mixture from LLNL and SJTU mechanisms agree well with the measurements from Gersen et al. The JetsurF 2.0 and Li mechanisms exhibit greater discrepancies in estimating the IDT of a hydrogen mixture compared to the LLNL and SJTU mechanisms.
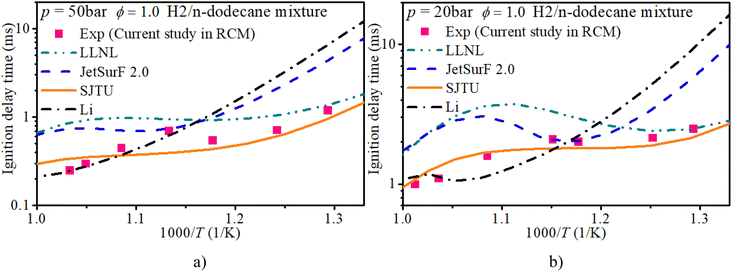
Fig. 3 shows the IDT of an n-dodecane/hydrogen mixture with ϕ of 1.0 and initial pressures of 20 bar and 50 bar. The LLNL mechanism overestimates the IDT in mid-temperature and low-temperature regions. Compared with the measurements from the current rapid compression machine setup, the SJTU mechanism well captures the IDT in most regions and slightly underestimates the IDT in the NTC region. The JetsurF 2.0 and Li mechanisms exhibit greater discrepancies in estimating the IDT compared to the other mechanisms.
In order to select the accurate reaction mechanism to study the chemical kinetics of a hydrogen/n-dodecane mixture, error analysis of experiments and mechanisms was conducted. The calculation methods of errors are derived from He et al.43
The relative error of the ith data point, which reflects the local performance of the mechanisms, is calculated as follows:
 | (1) |
The mean absolute errors are also calculated to evaluate the overall performance of the mechanisms. The expression is as follows:
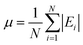 | (2) |
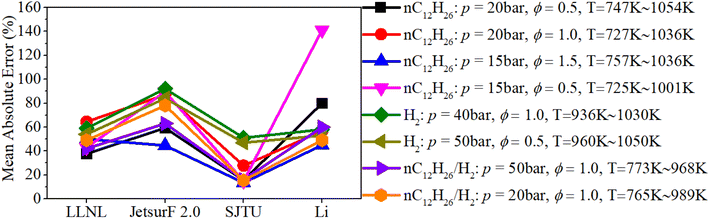
Fig. 4 shows a comparison of the mean absolute errors among LLNL, JetsurF 2.0, SJTU and Li mechanisms. It can be seen that the mean absolute errors of the SJTU mechanism in predicting the IDT of an n-dodecane mixture and n-dodecane/hydrogen mixture are below about 15%, which are obviously lower than that of other mechanisms. Although the mean absolute errors of the SJTU mechanism in predicting the IDT of the hydrogen mixture is close to 50%, this discrepancy cannot obviously influence the prediction accuracy of the reaction progress of hydrogen/n-dodecane due to the dominant role of n-dodecane in mid- and low-temperature reaction systems of a hydrogen/n-dodecane mixture. Therefore, the SJTU mechanism is selected to explore the chemical reaction kinetics of n-dodecane/hydrogen mixtures under engine-like conditions.
2.2 Calculation setup
In order to reveal the auto-ignition characteristics of hydrogen/diesel mixtures under wide in-cylinder thermodynamic conditions, the calculation conditions are obtained based on the actual operating conditions of a diesel/hydrogen engine. This study concentrates on low-temperature to mid-temperature (750–1050 K) and high-pressure (40–80 bar) in-cylinder operating conditions. The mixture compositions are shown in Table 1.| Variable | Range |
|---|---|
| Mole fraction of hydrogen | 100–50% |
| Mole fraction of n-dodecane | 0–50% |
| ϕ | 0.5–2.0 |
| Temperature (K) | 750–1050 |
| Pressure (bar) | 40–80 |
The molar ratio of nitrogen and oxygen is 3.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to simulate the actual air composition in an engine.44,45 In order to comprehensively reveal the auto-ignition properties of hydrogen/n-dodecane under wide blending ratios, mixtures of 100–0, 99–1, 98–2, 95–5, 90–10, 75–25 and 50–50 simulate various molar blending ratios of hydrogen and n-dodecane. For example, the mixture of 90–10 means that the mole fractions of hydrogen and n-dodecane are 90% and 10%, respectively. The ϕ of 0.5, 1.0 and 2.0 represent in-cylinder mixtures with different concentrations. The calculation equation for ϕ is as follows:
1 to simulate the actual air composition in an engine.44,45 In order to comprehensively reveal the auto-ignition properties of hydrogen/n-dodecane under wide blending ratios, mixtures of 100–0, 99–1, 98–2, 95–5, 90–10, 75–25 and 50–50 simulate various molar blending ratios of hydrogen and n-dodecane. For example, the mixture of 90–10 means that the mole fractions of hydrogen and n-dodecane are 90% and 10%, respectively. The ϕ of 0.5, 1.0 and 2.0 represent in-cylinder mixtures with different concentrations. The calculation equation for ϕ is as follows:
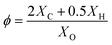 | (3) |
Therefore, the coupled influence mechanisms of pressure, temperature, blending ratio and ϕ on the auto-ignition progress of hydrogen/n-dodecane mixtures under engine-like conditions are analyzed systematically in later sections.
2.3 Taguchi method
The Taguchi method is used to select the combination of parameters that makes the experimental results stable and less volatile, which evaluates a larger number of factors by fewer combinations of parameters. The design method has three steps. The first step is the selection of variables. The temperature, ϕ, pressure, and n-dodecane content are chosen as the key variables. The second step is the selection of 4 levels for each variable, as shown in Table 2. The third step is the experimental design based on the experimental variables and levels, as illustrated in Table 3. The Taguchi method first identified the factors and levels affecting the performance parameters and designed an orthogonal array. After conducting experiments on the specified orthogonal arrays, the data were analyzed by analysis of variance (ANOVA) and the contribution rates of four factors on the IDT of n-dodecane/hydrogen mixtures were determined. The calculation equations for contribution rates are as follows:| Ci = (Si/S) × 100% | (4) |
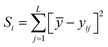 | (5) |
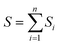 | (6) |
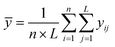 | (7) |
| Factor | Designation | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|---|
| Temperature (K) | A | 750 | 850 | 950 | 1050 |
| ϕ | B | 0.5 | 1.0 | 1.5 | 2.0 |
| Pressure (bar) | C | 40 | 50 | 60 | 70 |
| n-Dodecane content (%) | D | 5 | 20 | 35 | 50 |
| No. | Factor | |||
|---|---|---|---|---|
| A (K) | B | C (bar) | D (%) | |
| 1 | 1 (750) | 1 (0.5) | 1 (40) | 1 (5) |
| 2 | 1 (750) | 2 (1.0) | 2 (50) | 2 (20) |
| 3 | 1 (750) | 3 (1.5) | 3 (60) | 3 (35) |
| 4 | 1 (750) | 4 (2.0) | 4 (70) | 4 (50) |
| 5 | 2 (850) | 1 (0.5) | 2 (50) | 3 (35) |
| 6 | 2 (850) | 2 (1.0) | 1 (40) | 4 (50) |
| 7 | 2 (850) | 3 (1.5) | 4 (70) | 1 (5) |
| 8 | 2 (850) | 4 (2.0) | 3 (60) | 2 (20) |
| 9 | 3 (950) | 1 (0.5) | 3 (60) | 4 (50) |
| 10 | 3 (950) | 2 (1.0) | 4 (70) | 3 (35) |
| 11 | 3 (950) | 3 (1.5) | 1 (40) | 2 (20) |
| 12 | 3 (950) | 4 (2.0) | 2 (50) | 1 (5) |
| 13 | 4 (1050) | 1 (0.5) | 4 (70) | 2 (20) |
| 14 | 4 (1050) | 2 (1.0) | 3 (60) | 1 (5) |
| 15 | 4 (1050) | 3 (1.5) | 2 (50) | 4 (50) |
| 16 | 4 (1050) | 4 (2.0) | 1 (40) | 3 (35) |
3. Results and discussion
3.1 Auto-ignition characteristics of hydrogen/n-dodecane mixtures
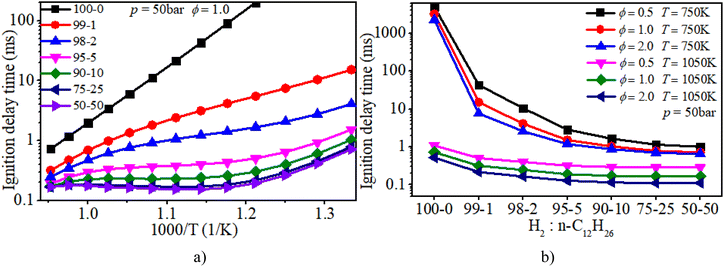
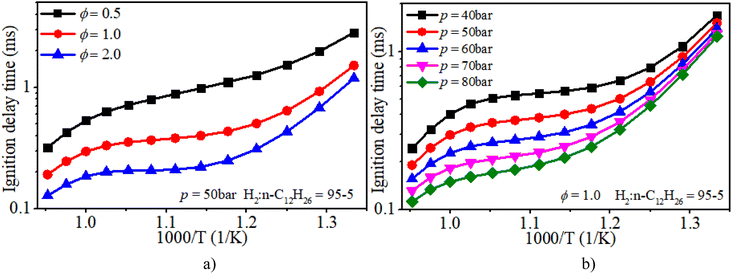
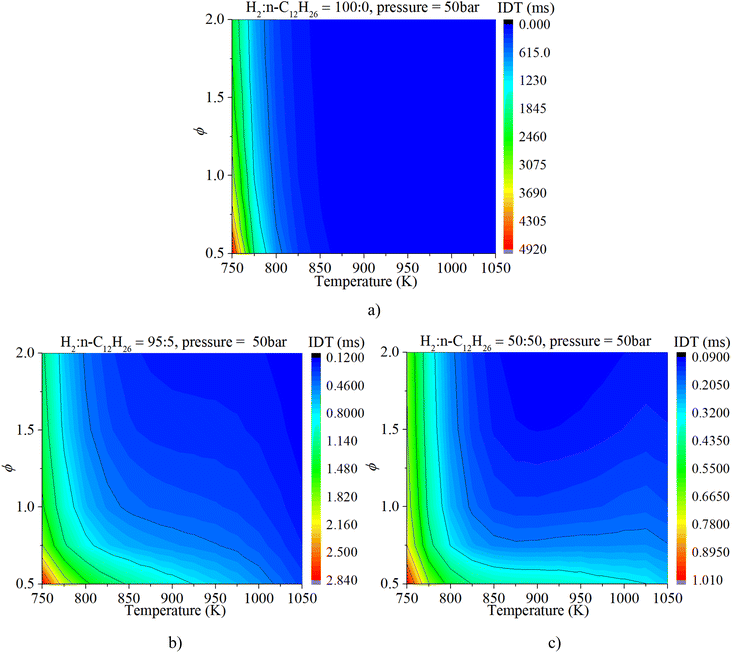
Fig. 6a clearly illuminates the variation law of the IDT with ϕ under low-to-mid-temperature conditions. The ϕ plays a key role in the IDT under all operating conditions. Fig. 6b shows that increasing pressure can apparently accelerate the progress of the auto-ignition reaction due to the increase in reactant concentration per volume and effective collision frequency among molecules. The turning point of the NTC region moves to higher temperature conditions with increasing pressure.
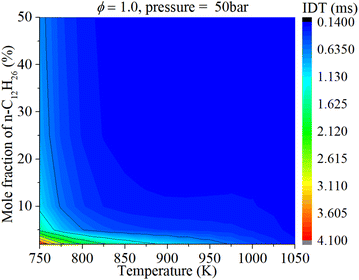
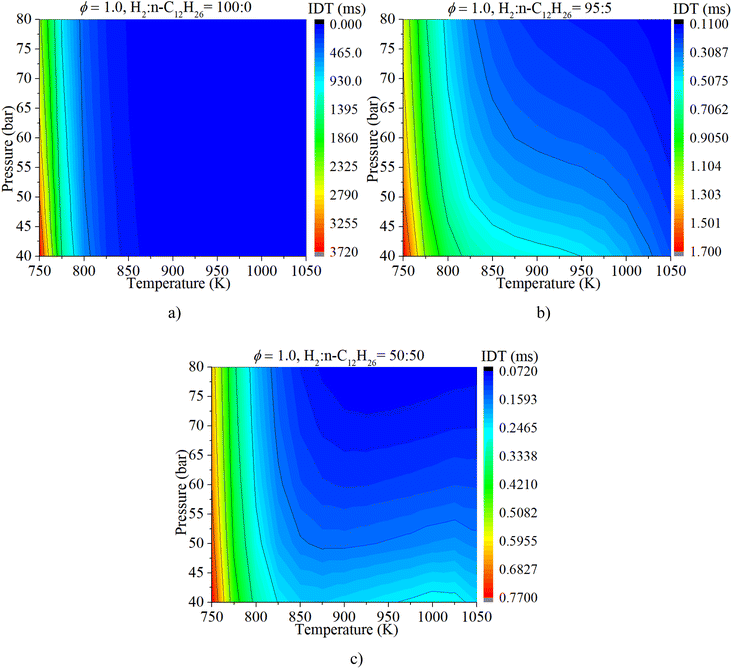
As shown in Fig. 8, NTC behavior is clearly illuminated after blending n-dodecane. With 50% n-dodecane fraction, the IDT is influenced by both pressure and temperature in the temperature range of 850–1050 K. Whereas, it is mainly influenced by temperature when the temperature is below 850 K because the IDT changes apparently with temperature but slightly with pressure. The result with 5% n-dodecane is similar to that with 50% n-dodecane. As for a pure hydrogen mixture, the NTC behavior disappears. The temperature plays a decisive role in the IDT.
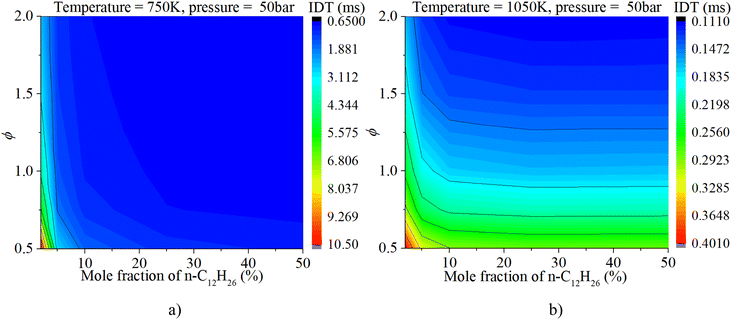
Fig. 9 shows that the IDT is influenced by both ϕ and temperature under low-temperature (750–850 K) and low-ϕ (0.5–1.0) conditions when the n-dodecane fraction is 5% or 50%. Whereas temperature plays a decisive role in the IDT under low-temperature (750–850 K) and high-ϕ (1.0–2.0) conditions. The ϕ is not the significant factor. With an increase in temperature, the IDT is influenced by both temperature and ϕ under higher-temperature (850–1050 K) conditions. As for a pure hydrogen mixture, the temperature remains a more critical factor than ϕ.
The coupled influences of ϕ and n-dodecane content are exhibited in Fig. 10. Under mid-temperature conditions (1050 K), the IDT is influenced by both ϕ and n-dodecane content at low n-dodecane content (0–10%). With an increase in n-dodecane content, the IDT is almost solely affected by ϕ. Under low-temperature conditions (750 K), the IDT is influenced by both ϕ and n-dodecane content at low n-dodecane content (0–20%). With an increase in n-dodecane content, the IDT is less influenced by ϕ and n-dodecane content because of the significantly weakened mixture reactivity.
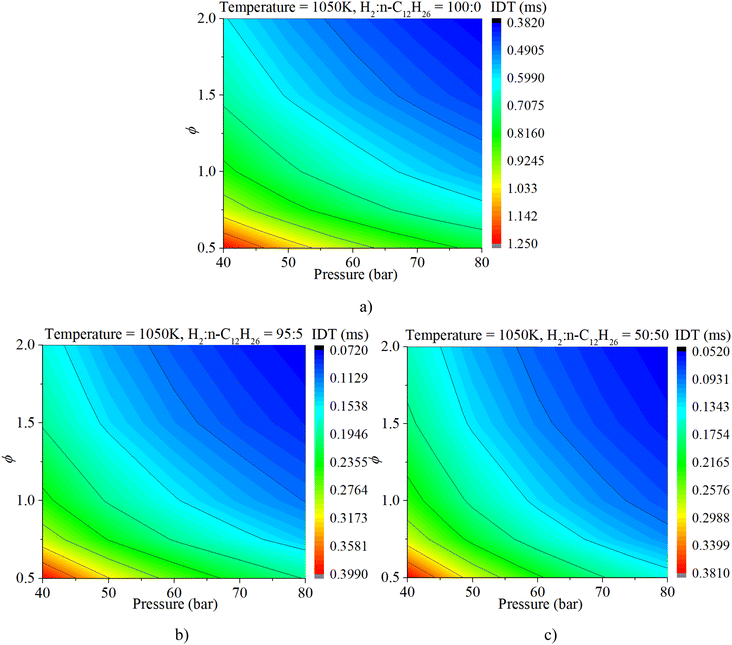
The coupled influences of ϕ and pressure are illustrated in Fig. 11. The IDT is apparently influenced by both ϕ and pressure under various mixture composition conditions. The influence of ϕ on IDT outweighs that of n-dodecane content. With a gradual increase in n-dodecane content, the influence of pressure increases.
| Factor | Contribution rate (%) | |||
|---|---|---|---|---|
| Temperature | ϕ | Pressure | n-Dodecane content | |
| IDT | 62.9 | 27.0 | 5.1 | 5.0 |
3.2 Chemical kinetic properties at mid-temperature and low temperature
| Hydrogen | n-Dodecane |
|---|---|
| R2: H2 + O ⇔ H + OH | R2727: NC12H26(+M) ⇔ C5H11-1 + C7H15-1(+M) |
| R2728: NC12H26(+M) ⇔ 2C6H13-1(+M) | |
| R3: H2 + OH ⇔ H + H2O | R2741: NC12H26 + OH ⇔ C12H25-1 + H2O |
| R2742: NC12H26 + OH ⇔ C12H25-2 + H2O | |
| R28: HO2 + H ⇔ H2 + O2 | R2743: NC12H26 + OH ⇔ C12H25-3 + H2O |
| R2746: NC12H26 + OH ⇔ C12H25-6 + H2O |
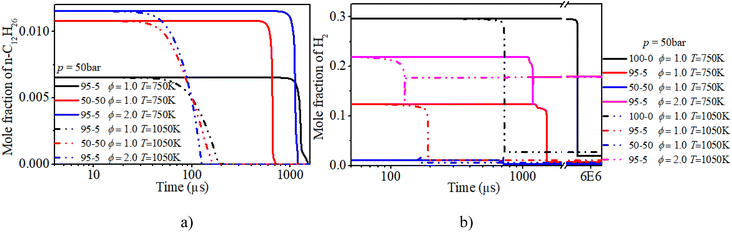
The increases in n-dodecane content and ϕ advance the rapid consumption of n-dodecane. At low temperatures (T = 750 K), the increase in n-dodecane content has a more apparent ignition promotion effect than that of ϕ. Whereas the mid-temperature condition exhibits the opposite trend. Further, the variation trend of the mole fraction of hydrogen is similar to that of n-dodecane. It is worth noting that when the n-dodecane is blended, both hydrogen and n-dodecane experience a slow oxidation process before the ignition time point.
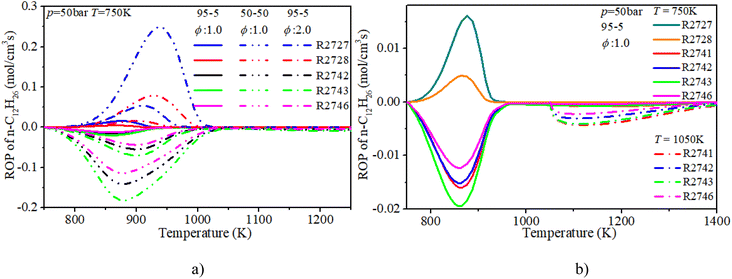
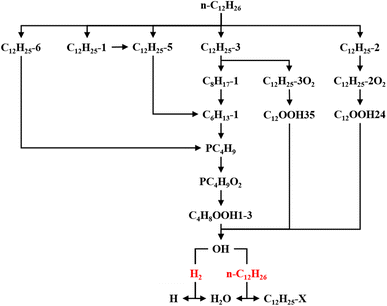
The ROP analysis of n-dodecane shows that the consumption of n-dodecane is dominated by the OH induced H-abstraction reactions (R2741, R2742, R2743, R2746). The formation of n-dodecane is controlled by recombination reactions (R2727, R2728). At low temperature, an increase in n-dodecane content (50–50, ϕ: 1.0) can greatly raise the relevant reaction rates, which is consistent with previous analysis. With an increase in initial temperature, the reaction temperature region of n-dodecane also apparently increases. Further, the reaction duration under mid-temperature conditions is obviously longer than that under low-temperature conditions. Thus the absolute reaction rate under mid-temperature condition is obviously lower than that under low-temperature conditions.
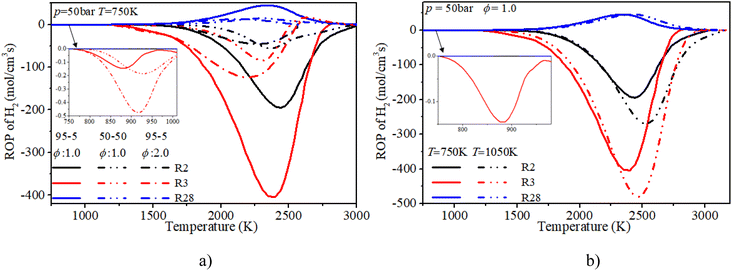
The ROP analysis of hydrogen shows that the consumption of hydrogen is dominated by the O and OH induced H-abstraction reactions (R2, R3). The formation of hydrogen is controlled by the recombination reaction of active radicals (R28). At low temperature, the increase in n-dodecane content (50–50, ϕ: 1.0) can greatly reduce the relevant reaction rates of hydrogen due to the decrease in hydrogen content. With an increase in initial temperature, the reaction region temperature and the reaction rate of hydrogen slightly increase. Further, after blending the n-dodecane, the OH related H-abstraction reaction (R3) is responsible for the initial consumption of hydrogen, which is helpful for shortening the IDT.
Sensitivity analysis on the IDT of an n-heptane/methane mixture was conducted to study the significant reactions in the ignition process. The normalized sensitivity index is defined as follows:44
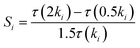 | (8) |
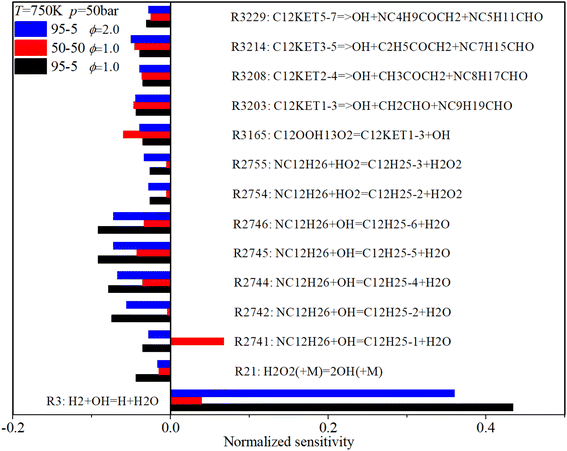
Fig. 17 shows that under low-temperature conditions, key ignition-promoting reactions include the H-abstraction reactions of n-dodecane induced by OH and HO2 radicals (R2741–R2746), the decomposition reaction of H2O2 (R21) and C12KET (R3203, R3208, R3214, R3229) which forms the active OH radical. The main ignition inhibition reaction is R3, which converts the OH radical to an H radical. Increases in n-dodecane content and ϕ reduce the sensitivity coefficients of almost all critical reactions.
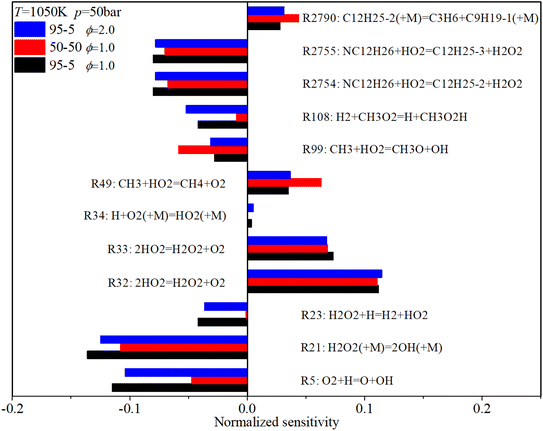
Fig. 18 shows that under mid-temperature conditions, the key ignition-promoting reaction is R21, which converts H2O2 to OH. R5 and R23, which convert H to OH or HO2, also have a considerable ignition-promotion effect. The H-abstraction reactions of n-dodecane, R2754 and R2755, which consume HO2 and form H2O2, can also induce a reduction in the IDT due to the subsequent formation of OH. R49 converts the active H2O and CH3 to stable CH4, which has a positive sensitivity coefficient. The adverse reactions of R32 and R33 convert H2O2 to HO2, also prolonging the IDT. The increases in n-dodecane content and ϕ also reduce the sensitivity coefficients of almost all critical reactions, similar to the low-temperature conditions.
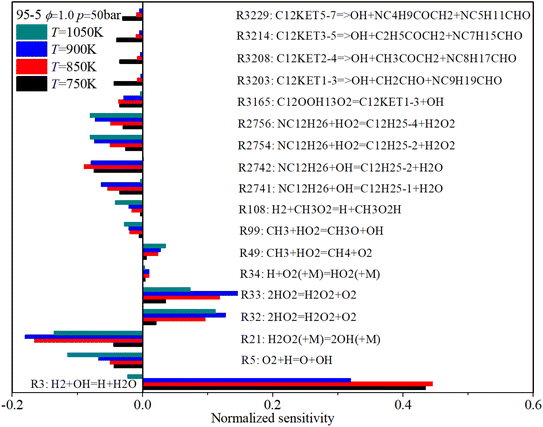
The effect of initial temperature (low temperature: 750 K, NTC region: 850 K and 900 K, mid-temperature: 1050 K) on the sensitivity coefficients is shown in Fig. 19. With an increase in initial temperature, the sensitivity coefficient of the decomposition reactions reduces, which shows the decomposition reactions are weakened. The sensitivity coefficient of those reactions relevant to H and HO2 increases with rising temperature. Whereas the sensitivity coefficient of those reactions relevant to OH and H2O2 reaches its peak value during the NTC region, which means the H2O2 and OH radicals are the significant intermediate products in the NTC region.
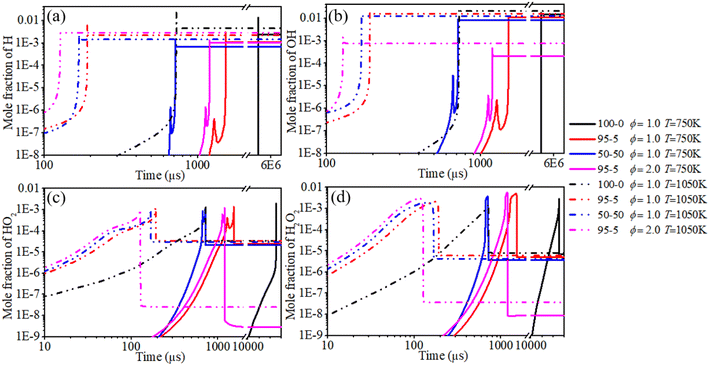
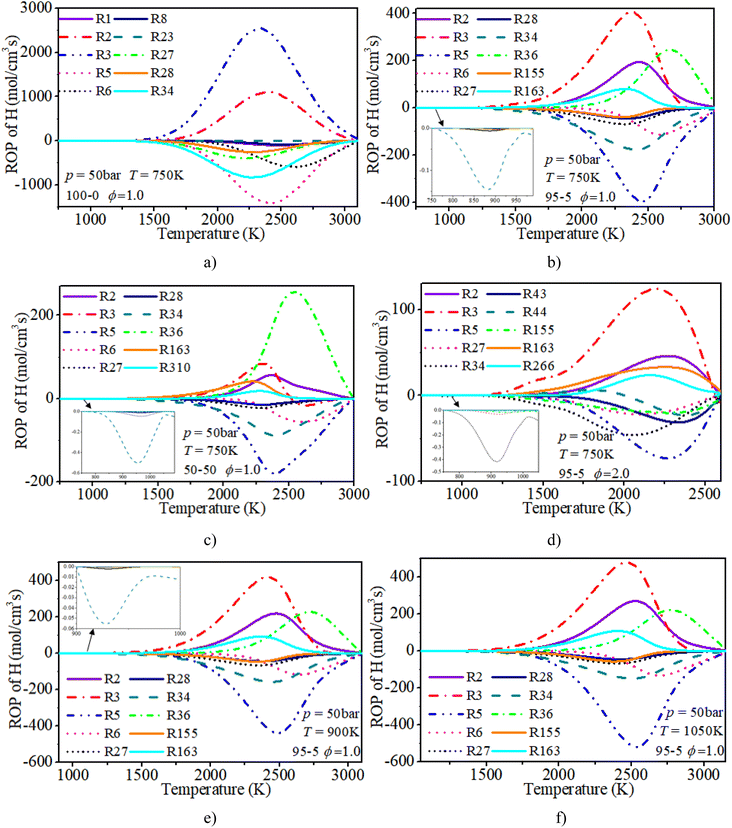
In order to systematically reveal the influence of the addition of n-dodecane on the reaction pathway of active radicals, ROP analyses of H and OH radicals are shown in Fig. 21 and 22, respectively. The key reactions of active radicals are shown in Table 6. The results show that the initial consumption of H radicals is induced by R34 (H + O2(+M) ⇔ HO2(+M)) after blending the n-dodecane. An increase in n-dodecane content further enhances this reaction process. Near the ignition time point, the oxidation reactions (R5: O2 + H ⇔ O + OH and R34: H + O2(+M) ⇔ HO2(+M)) are the dominant consumption reactions of H. For a pure hydrogen mixture, R2: H2 + O ⇔ H + OH and R3: H2 + OH ⇔ H + H2O are the main reactions forming H radicals. After blending the n-dodecane, R36: CO + OH ⇔ CO2 + H and R163: HCO + M ⇔ H + CO + M also become key reactions forming H radicals. However, with an increase in n-dodecane content, the reaction rates of the H radical reduce apparently due to the decrease in hydrogen content. Further, an increase in initial temperature slightly elevates the reaction temperature of the H radical.
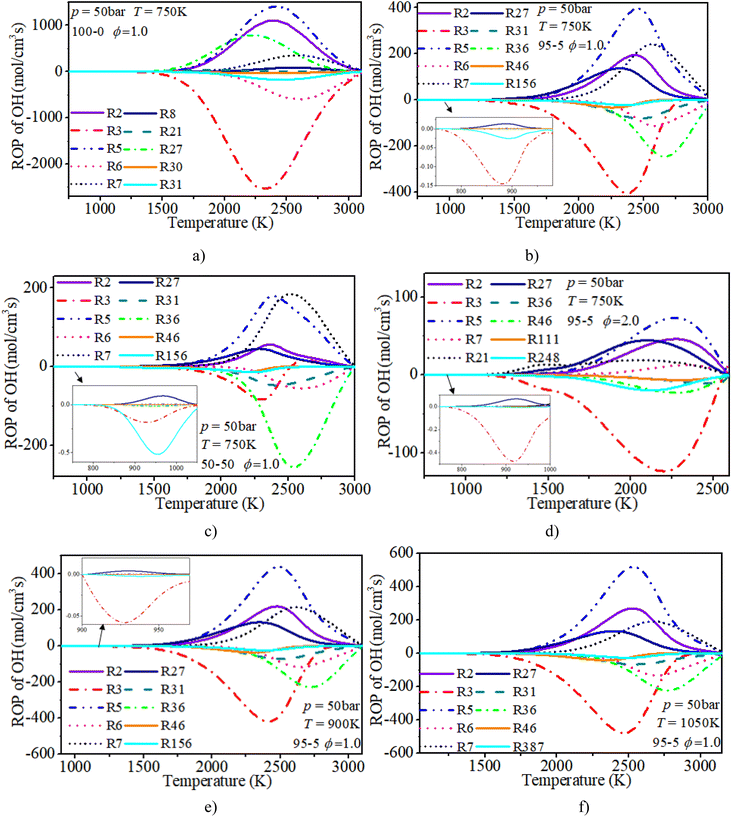
| H & OH | |
|---|---|
| R1: H2 + M ⇔ 2H + M | R2: H2 + O ⇔ H + OH |
| R3: H2 + OH ⇔ H + H2O | R5: O2 + H ⇔ O + OH |
| R6: H + OH + M ⇔ H2O + M | R7: O + H2O ⇔ 2OH |
| R8: O + H + M ⇔ OH + M | R21: H2O2(+M) ⇔ 2OH(+M) |
| R23: H2O2 + H = H2 + HO2 | R27: HO2 + H ⇔ 2OH |
| R28: HO2 + H ⇔ H2 + O2 | R30: OH + HO2 ⇔ H2O + O2 |
| R31: OH + HO2 ⇔ H2O + O2 | R34: H + O2(+M) ⇔ HO2(+M) |
| R36: CO + OH ⇔ CO2 + H | R43: CH3 + H(+M) ⇔ CH4(+M) |
| R44: CH4 + H ⇔ CH3 + H2 | R46: CH4 + OH ⇔ CH3 + H2O |
| R111: CH3OH(+M) ⇔ CH3 + OH(+M) | R155: CH2O + H ⇔ HCO + H2 |
| R156: CH2O + OH ⇔ HCO + H2O | R163: HCO + M ⇔ H + CO + M |
| R248: C2H4 + OH ⇔ C2H3 + H2O | R266: C2H2 + H(+M) ⇔ C2H3(+M) |
| R310: C2H2 + O ⇔ HCCO + H | R387: HCCO + OH ⇔ H2 + 2CO |
The ROP analysis of the OH radical shows that the initial consumption of OH radicals is induced by R3: H2 + OH ⇔ H + H2O after blending n-dodecane. An increase in n-dodecane content further enhances this reaction process. In other words, the addition of n-dodecane enhances the whole-system reactivity, which is beneficial to the progress of OH-relevant reactions. For a pure hydrogen mixture, near the ignition time point, the oxidation reactions (R3: H2 + OH ⇔ H + H2O and R6: H + OH + M ⇔ H2O + M) are the dominant consumption reactions of the OH radical. R2: H2 + O ⇔ H + OH, R5: O2 + H ⇔ O + OH and R27: HO2 + H ⇔ 2OH are the main reactions forming the OH radical. After blending n-dodecane, R31: OH + HO2 ⇔ H2O + O2 and R36: CO + OH ⇔ CO2 + H also become key consumption reactions of the OH radical. R7: O + H2O ⇔ 2OH becomes the key reaction forming an OH radical. However, with an increase in n-dodecane content, the reaction rates of the OH radical reduce apparently due to the decrease in hydrogen content. The increase in initial temperature slightly elevates the reaction temperature and the reaction rate of the OH radical.
4. Conclusions
In the current study, the coupled influence mechanisms of specific thermodynamic conditions and mixture compositions on the auto-ignition characteristics of n-dodecane/hydrogen mixtures were revealed by detailed chemical reaction kinetic mechanisms. Chemical reaction kinetic properties, including mole fractions of fuels and active radicals, ROP, sensitivity and reaction pathway analyses, were carried out to acquire the internal interaction mechanisms among hydrogen and n-dodecane and multiple active radicals during the auto-ignition progress of hydrogen/n-dodecane mixtures. The conclusions can be summarized as follows:(1) Under engine-like conditions, blending a small amount of n-dodecane dramatically reduced the IDT of a hydrogen mixture. However, the decrease in amplitude of the IDT gradually reduced with a continuous increase in n-dodecane content. Increasing ϕ and initial pressure also obviously reduced the IDT. Further, raising the content of n-dodecane apparently highlighted the NTC behavior. The progress of the auto-ignition of hydrogen/n-dodecane mixtures relied on the coupled effects of fuel composition and located thermodynamic conditions. The initial temperature exerted a great influence on the IDT under all conditions. The ϕ also had a obvious impact on the IDT in most regions other than a pure hydrogen mixture or under low-temperature/high-ϕ conditions. The effect of pressure and n-dodecane content on the IDT depended on the located conditions. The effect of pressure was ignorable under low-temperature or pure hydrogen mixture conditions due to the dramatically weakened mixture reactivity. The influence of n-dodecane fraction was negligible when the n-dodecane fraction was greater than 10% at mid-temperature or 20% at low temperature. The contribution rates of temperature, ϕ, pressure, and n-dodecane content on the IDT are 62.9%, 27.0%, 5.1%, 5.0%, respectively.
(2) The addition of n-dodecane induced the initial production of OH radical, which was responsible for the advanced slow oxidation of n-dodecane and hydrogen. With an increase in n-dodecane content, the relevant reaction rates of the fuels was further improved, which outweighed the reduced reaction rates of active radicals. Thus the IDT was reduced. The variation laws of mole fractions of active radicals (H, OH, HO2, H2O2) were similar to those of n-dodecane and hydrogen. Further, with an increase in initial temperature, the temperatures of the reaction region of n-dodecane obviously increased. Whereas the temperatures of the reaction regions of hydrogen and H and OH radicals were slightly elevated.
(3) The main ignition-promoting reactions were the reactions related to OH and HO2 and H2O2 (H-abstraction reactions of n-dodecane induced by OH and HO2 radicals (R2741–R2746), decomposition reaction of H2O2 (R21) and C12KET (R3203, R3208, R3214, R3229) which formed the active OH radical) under low-temperature conditions. Under mid-temperature conditions, the key ignition-promoting reactions were still those reactions related to OH and HO2 and H2O2 (R5 and R23 which covert H to OH or HO2, H-abstraction reactions of n-dodecane (R2754 and R2755) which consume HO2 and form H2O2, and R21). With an increase in initial temperature, the sensitivity coefficients of decomposition reactions and those reactions relevant to H and HO2 reduced and increased, respectively. Further, the sensitivity coefficients of those reactions relevant to OH and H2O2 reached their peak values in the NTC region, which meant the H2O2 and OH radicals were the significant intermediate products.
Abbreviations
| IDT | Ignition delay time |
| NOx | Nitrogen oxide |
| NTC | Negative temperature coefficient |
| ROP | Rate of production |
| ϕ | Overall equivalence ratio of mixture |
| p | Initial pressure |
| T | Initial temperature |
| ANOVA | Analysis of variance |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work in this study received financial support from the Science Fund of State Key Laboratory of Engine and Powertrain System (No. SKLEPS-202104), the Fundamental Research Funds for the Central Universities (Grant No. JZ2023HGQA0139).References
- A. A. Konnov, A. Mohammad and V. R. Kishore, et al., A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+air mixtures, Prog. Energy Combust. Sci., 2018, 68, 197–267 CrossRef.
- A. Ghosh, N. M. Munoz-Munoz and D. A. Lacoste, Minimum ignition energy of hydrogen-air and methane-air mixtures at temperatures as low as 200 K, Int. J. Hydrogen Energy, 2022, 47(71), 30653–30659 CrossRef CAS.
- S. T. P. Purayil, M. O. Hamdan and S. A. B. Al-Omari, et al., Review of hydrogen–gasoline SI dual fuel engines: Engine performance and emission, Energy Rep., 2023, 9, 4547–4573 CrossRef.
- R. A. Erren and W. H. Campbell, Hydrogen: a commercial fuel for internal combustion engines and other purposes, J. Inst. Fuel, 1933, 6, 277 Search PubMed.
- P. Huyskens, et al., The technical implementation of a retrofit hydrogen PFI system on a passenger car, SAE Technical Paper, 2011 Search PubMed.
- M. He, Z. Jia and F. Liu, Development prospect of hydrogen engine vehicles in China, in 2008 4th International Conference on Wireless Communications, Networking and Mobile Computing, IEEE, 2008 Search PubMed.
- G. Kiesgen, et al., The new 12-cylinder hydrogen engine in the 7 series: the H2 ICE age has begun, SAE Technical Paper, 2006 Search PubMed.
- D. Sainz, et al., Conversion of a commercial gasoline vehicle to run bi-fuel (hydrogen-gasoline), Int. J. Hydrogen Energy, 2012, 37(2), 1781–1789 CrossRef CAS.
- S. J. Szwabowski, et al., Ford hydrogen engine powered P2000 vehicle, SAE Technical Paper, 2002 Search PubMed.
- S. Danner and S. Fuerst, BMW hydrogen 7 series–a safe way to a clean future, in Fisita 2006 World Congress, Yokohama, JP, 2006 Search PubMed.
- D. Nguyen, et al., Effect of supercharger system on power enhancement of hydrogen fueled spark-ignition engine under low-load condition, Int. J. Hydrogen Energy, 2021, 46(9), 6928–6936 CrossRef CAS.
- N. Saravanan and G. Nagarajan, Hydrogen-diesel dual fuel combustion in a direct injection diesel engine, Int. J. Renew. Energy Technol., 2011, 2(3), 259–278 CrossRef.
- Y. Ye, et al., Numerical study of the effect of injection timing on the knock combustion in a direct-injection hydrogen engine, Int. J. Hydrogen Energy, 2020, 45(51), 27904–27919 CrossRef CAS.
- H. Rottengruber, et al., Direct-injection hydrogen SI-engine-operation strategy and power density potentials, SAE Trans., 2004, 1749–1761 Search PubMed.
- T. Wallner, et al., Fuel economy and emissions evaluation of BMW Hydrogen 7 Mono-Fuel demonstration vehicles, Int. J. Hydrogen Energy, 2008, 33(24), 7607–7618 CrossRef CAS.
- A. Wimmer, et al., H2-direct injection–a highly promising combustion concept, SAE Technical Paper, 2005 Search PubMed.
- N. S. Matthias, T. Wallner and R. Scarcelli, A hydrogen direct injection engine concept that exceeds US DOE light-duty efficiency targets, SAE Int. J. Engines, 2012, 5(3), 838–849 CrossRef.
- T. Wallner, S. Ciatti and B. Bihari, Investigation of injection parameters in a hydrogen DI engine using an endoscopic access to the combustion chamber, SAE Technical Paper, 2007 Search PubMed.
- K. K. Rana, S. Natarajan and S. Jilakara, Potential of hydrogen fuelled IC engine to achieve the future performance and emission norms, 2015 Search PubMed.
- R. Sivabalakrishnan, et al., Study of knocking effect in compression ignition engine with hydrogen as a secondary fuel, Chin. J. Eng., 2014, 2014(102390), 1–82014 Search PubMed.
- M. Xu, W. Cheng and Z. Li, et al., Pre-injection strategy for pilot diesel compression ignition natural gas engine, Appl. Energy, 2016, 179, 1185–1193 CrossRef CAS.
- S. Szwaja and K. Grab-Rogalinski, Hydrogen combustion in a compression ignition diesel engine, Int. J. Hydrogen Energy, 2009,(34), 4413–4421 CrossRef CAS.
- T. Tsujimura and Y. Suzuki, The utilization of hydrogen in hydrogen/diesel dual fuel engine, Int. J. Hydrogen Energy, 2017, 42(19), 14019–14029 CrossRef CAS.
- J. D. Naber and S. Szwaja, Statistical approach to characterize combustion knock in the hydrogen fueled SI engine, J. KONES, 2007, 14, 443–450 Search PubMed.
- P. Kundu, C. Xu and S. Som, et al., Implementation of multi-component diesel fuel surrogates and chemical kinetic mechanisms for engine combustion simulations, Transp. Eng., 2021, 3, 100042 CrossRef.
- W. Zhou, S. Zhou and H. Xi, et al., Chemical Kinetic Study on Dual-Fuel Combustion: The Ignition Properties of n-Dodecane/Methane Mixture, Int. J. Chem. Eng., 2021, 7100812 CAS.
- A. A. E. Mohamed, A. Bikram Sahu, S. Panigrahy, G. Bourque and H. Curran, The Ignition of C1–C7 Natural Gas Blends and the Effect of Hydrogen Addition in the Low and High Temperature Regimes, J. Eng. Gas Turbines Power, 2022, 144(12), 121009 CrossRef CAS.
- X. He, B. Shu and D. Nascimento, et al., Auto-ignition kinetics of ammonia and ammonia/hydrogen mixtures at intermediate temperatures and high pressures, Combust. Flame, 2019, 206, 189–200 CrossRef CAS.
- Y. Jiang, G. del Alamo and A. Gruber, et al., A skeletal mechanism for prediction of ignition delay times and laminar premixed flame velocities of hydrogen-methane mixtures under gas turbine conditions, Int. J. Hydrogen Energy, 2019, 44, 18573–18585 CrossRef CAS.
- Z. Li, W. Han and D. Liu, et al., Laminar flame propagation and ignition properties of premixed iso-octane/air with hydrogen addition, Fuel, 2015,(158), 443–450 CrossRef CAS.
- S. Aggarwal, O. Awomolo and K. Akber, et al., Ignition characteristics of heptane-hydrogen and heptane-methane fuel blends at elevated pressures, Int. J. Hydrogen Energy, 2011, 36, 15392–15402 CrossRef CAS.
- S. M. Frolov, S. N. Medvedev and V. Ya. Basevich, et al., Self-ignition of hydrocarbon-hydrogen-air mixtures, Int. J. Hydrogen Energy, 2013, 38, 4177–4184 CrossRef CAS.
- C. Xu, Q. Wang and X. Li, et al., Effect of hydrogen addition on the laminar burning velocity of n-decane/air mixtures: Experimental and numerical study, Int. J. Hydrogen Energy, 2022, 47, 19263–19274 CrossRef CAS.
- S. S. Vasu, D. F. Davidson and Z. Hong, et al., n-Dodecane oxidation at high-pressures: Measurements of ignition delay times and OH concentration time-histories, Proc. Combust. Inst., 2009, 32(1), 173–180 CrossRef CAS.
- Y. Mao, M. Raza and Z. Wu, et al., An experimental study of n-dodecane and the development of an improved kinetic model, Combust. Flame, 2020, 212, 338–402 CrossRef.
- S. Gersen, H. Darmeveil and H. Levinsky, et al., The effects of CO addition on the autoignition of H2, CH4 and CH4/H2 fuels at high pressure in an RCM, Combust. Flame, 2012, 159, 3472–3475 CrossRef CAS.
- S. Sarathy, C. Westbrook and M. Mehl, et al., Comprehensive chemical kinetic modeling of the oxidation of 2-methylalkanes from C7 to C20, Combust. Flame, 2011, 158, 2338–2357 CrossRef CAS.
- H. Wang, E. Dames, B. Sirjean, D. A. Sheen, R. Tango, A. Violi, J. Y. W. Lai, F. N. Egolfopoulos, D. F. Davidson, R. K. Hanson, C. T. Bowman, C. K. Law, W. Tsang, N. P. Cernansky, D. L. Miller and R. P. Lindstedt, A high-temperature chemical kinetic model of n-alkane (up to n-dodecane), cyclohexane, and methyl-, ethyl-, n-propyl and n-butyl-cyclohexane oxidation at high temperatures, JetSurF version 2.0, 2010, https://web.stanford.edu/group/haiwanglab/JetSurF/JetSurF2.0/index.html Search PubMed.
- L. Xiangyuan, Y. Xiaoxia and S. Jiangtao, et al. , Chem. J. Chin. Univ., 2020, 41, 512–520 Search PubMed.
- L. Xiangyuan, S. Jiangtao and L. Yiwei, et al. , Chem. J. Chin. Univ., 2020, 41, 772–779 Search PubMed.
- L. Yiwei, S. Jiangtao and W. Jingbo, et al. , Chem. J. Chin. Univ., 2021, 42, 1871–1880 Search PubMed.
- H. Ren, J. Wang and X. Li, Combustion Dynamics, Sichuan, CDS1.0, Center for Combustion Dynamics, Sichuan University, 2021, https://cds.scu.edu.cn/ Search PubMed.
- Y. He, Y. Wang and C. Grégoire, et al., Ignition characteristics of dual-fuel methane-n-hexane-oxygen-diluent mixtures in a rapid compression machine and a shock tube, Fuel, 2019, 249, 379–391 CrossRef CAS.
- Z. Gong, L. Y. Feng and L. C. Li, et al., Shock tube and kinetic study on ignition characteristics of methane/n-hexadecane mixtures, Energy, 2020, 201, 117609 CrossRef CAS.
- Z. Gong, L. Y. Feng and W. J. Qu, et al., Auto-ignition characteristics of methane/n-heptane mixtures under carbon dioxide and water dilution conditions, Appl. Energy, 2020, 278, 115639 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |