Towards the thermal stability of dye-sensitized solar cells for wavelength-selective greenhouses using the polymorphism of light-scattering layers†
Daniel
Ursu
a,
Melinda
Vajda
ab,
Elisei
Ilieş
b,
Radu
Ricman
b,
Magdalena
Marinca
b,
Szilard
Bularka
b,
Marinela
Miclau
 *a and
Aurel
Gontean
*b
*a and
Aurel
Gontean
*b
aNational Institute for Research and Development in Electrochemistry and Condensed Matter, Timisoara 300569, Romania. E-mail: marinela.miclau@gmail.com
bPolitehnica University Timisoara, Timisoara 300006, Romania. E-mail: aurel.gontean@upt.ro
First published on 6th December 2023
Abstract
Using laboratory-size yellow dye-sensitized solar cells based on iodide/triiodide redox electrolytes, the effect of light-scattering layers on the thermal stability of these cells was studied up to 60 °C. In this work, the effect of the light-scattering layer and its polymorphism on the thermal stability is explored for the first time, opening a new perspective to achieve the long-term thermal stability of dye-sensitized solar cells under real conditions. Moreover, the commercial yellow dye was studied from the point of view of its thermal stability at the TiO2/dye/electrolyte interface. Due to the dual function of the yellow dye in our DSSCs, namely, as a photosensitizer and as a UV filter, an electrolyte with a high I3− concentration was used, which simultaneously favored a good photovoltaic efficiency and thermal stability. Herein, the dual function of the light-scattering layer was highlighted; the improvement of photon harvesting and the stability of yellow dye anchored on a rutile polymorph allow us to achieve excellent temperature coefficients for the short circuit current (+0.38%/°C) and the maximum power factor (−0.22%/°C), superior or comparable to other commercial generations of solar cells.
1. Introduction
More recently, there have been energy and food crises requiring solutions in both short and long terms. The use of the same land for both solar cells and plant culture, proposed by Agriculture 4.0, has boosted the research on agrivoltaic systems, one of them being photovoltaic greenhouses.1 Owing to the intense use and the best photovoltaic efficiency, silicon-based solar cells have been proposed as the most promising candidates for photovoltaic greenhouses. The experimentally proven concept is a greenhouse roof built with wavelength-selective photovoltaic (WSPV) panels made with WS luminescent absorbers positioned between Si-PV panels. The coverage rate, defined as the ratio of the horizontal surface of the greenhouse covered by solar panels located on the roof, was between 10% and 60% depending on the requested light crops.2 Although the geometry of this roof tried to solve the most important requirement of plant crops, namely, maximizing the accessibility of photosynthetic active radiation (PAR) into the greenhouse, these photovoltaic (PV) cells do not transmit sunlight, generating a permanent shadow region with detrimental effects on production, crop growth, or the amount of biomass. Moreover, the power efficiency of a WSPV module is conditioned by the power efficiency of PV cells and the luminescent material, and is reduced by the low absorption of solar radiation and the self-absorption of the emitted light from the luminescent absorber, a best conversion efficiency of 5% being reported.3In this context, the search for new solutions inspired research into the possibility of using other generations of solar cells, even if they have a lower photovoltaic efficiency. Because of their construction and operation principle, dye-sensitized solar cells (DSSCs) can be easily adapted to the conditions imposed by the optimal operation of greenhouses.4 Simplicity, low cost, and modeling of light absorption through dye selectivity, because the photosensitizers can be finely modified for wavelength-selective absorption, are the attractive advantages of DSSCs.5–13 The choice of DSSC dyes in greenhouses can extend beyond energy generation and play a significant role in optimizing the plant growth conditions. This approach allows for a more controlled and sustainable cultivation environment by manipulating the light spectrum, reducing harmful radiation, and potentially lowering plant disease risks.14 However, the most studied DSSCs based on visible light conversion, although they are most efficient from the perspective of photovoltaic efficiency, drastically reduce PAR inside the greenhouse with negative effects on plants. In this context, our previous work has experimentally demonstrated that the requirements imposed by a greenhouse can be provided by a DSSC using a yellow dye and a complex architecture of TiO2 photoanode with a brookite light-scattering layer as follows: (i) a conversion efficiency of 4.91% for 1 sun illumination, two times higher than the best efficiency reported for the same dye until then;15 (ii) partially transparency throughout the PAR domain; (iii) a coverage rate of 100% and (iv) a photovoltaic efficiency of nearly 5% at an irradiance between 50 mW cm−2 and 100 mW cm−2, corresponding to the maximum light intensity in all seasons.16
Based on these excellent premises, the next step in the large-scale implementation of this concept is the optimization of yellow-based DSSCs for outdoor conditions. Thus far, the significant previous research efforts on the thermal stability of DSSCs have only focused on studying more efficient visible-responsive DSSCs, namely, the mechanisms of deterioration of visible absorbing dye molecules and electrolytes under outdoor conditions.17,18 Moreover, until now, the development of materials with light-scattering capacity for DSSCs aimed to increase the photon harvesting capacity of dye molecules for the improvement of the solar cell efficiency, without considering and exploring a possible effect on the anchoring capacity of the dye or thermal stability, and more if its polymorphism could determine an additional functionality.
In this work, our aim is to explore for the first time the thermal stability of yellow dye-sensitized solar cells from the perspective of integration in wavelength-selective greenhouses determining the temperature coefficients. The effect of the light-scattering layer and its polymorphism on the thermal stability is explored, opening a new perspective for achieving the long-term thermal stability of dye-sensitized solar cells under real conditions. Moreover, the commercial yellow dye has been studied from the point of view of its thermal stability at the TiO2/dye/electrolyte interface. Using laboratory-size yellow dye-sensitized solar cells based on iodide/triiodide redox electrolytes, the effect of the light-scattering layer on the thermal stability has been studied up to 60 °C, the maximum possible temperature achieved by a DSSC under 1 solar irradiation (100 mW cm−2). Beneficial positive evolution of the short circuit current as a function of the temperature for all yellow DSSCs is due to its high absorption of UV radiation (355–400 nm), close to 98%, protecting against UV exposure that causes cell degradation. A dual function of the light-scattering layer was also highlighted; the improvement of photon harvesting and the stability of yellow dye anchored on a rutile polymorph allow us to achieve an excellent temperature coefficient for the maximum power and a promising new feature for the large-scale practical exploitation of yellow DSSCs in a wavelength-selective greenhouse. In this light, the maximum power point tracking (MPPT) of the energy conversion equipment must be compatible with different types of solar cells, and hence, accurate models are useful in the process of designing them. To model our DSSC cells, we developed a method that generates a SPICE temperature-dependent model with relative errors below 5%.
2. Experimental section
2.1. Fabrication of DSSCs with TiO2 polymorphic particles for light scattering
The crystalline phase and morphological structure of TiO2 polymorphs have been explained in detail in our previous work.16,19 In Fig. 1, the anatase, brookite, and rutile crystalline phases used as light-scattering layers are highlighted, and Table 1 summarizes the structural parameters.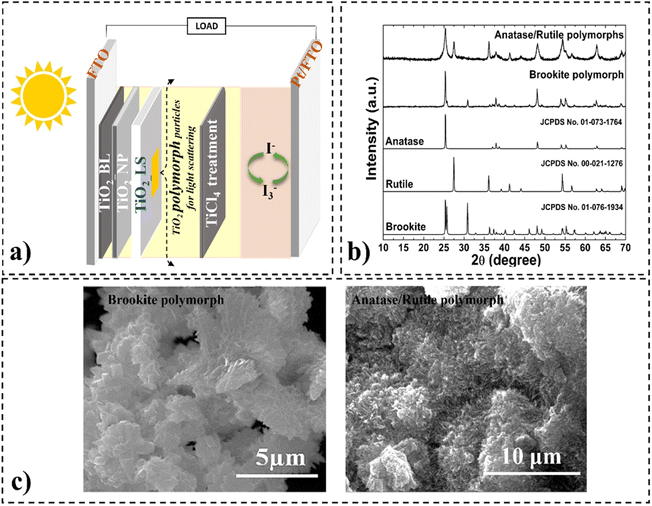 | ||
| Fig. 1 Scheme of DSSCs constructed with TiO2 polymorphs used as light-scattering layers (a), XRD patterns (b) and SEM images (c) of TiO2 polymorphs. | ||
| Light scattering layer (LS) | Space group | Particle size range | Particle shape | Band gap |
|---|---|---|---|---|
| Brookite (BLS) | Pbca | 330–950 nm | Quasi-microcube | 3.32 eV |
| Anatase/rutile (RLS) 54%/46% | I41/amd/P42/mnm | ∼23 nm | Dendritic structure consists of agglomerated nanorods | 3.36 eV |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 titanium diisopropoxide bis(acetylacetonate) (75 wt% in isopropanol) in tert-butanol (99.8%, Sigma-Aldrich) followed by calcination at 450 °C for 60 minutes at a rate of 1 °C min−1; (ii) the second layer consists of TiO2 nanoparticles crystallized in an anatase form, obtained by a hydrothermal method at 100 °C for 12 h and calcination at 500 °C for 60 minutes at a rate of 1 °C min−1; and (iii) the final treatment with 40 mM TiCl4 is applied on the photoanode surface and sintered at 450 °C for 1 h.
5 titanium diisopropoxide bis(acetylacetonate) (75 wt% in isopropanol) in tert-butanol (99.8%, Sigma-Aldrich) followed by calcination at 450 °C for 60 minutes at a rate of 1 °C min−1; (ii) the second layer consists of TiO2 nanoparticles crystallized in an anatase form, obtained by a hydrothermal method at 100 °C for 12 h and calcination at 500 °C for 60 minutes at a rate of 1 °C min−1; and (iii) the final treatment with 40 mM TiCl4 is applied on the photoanode surface and sintered at 450 °C for 1 h.
The DSSC1_BLS photoanode with 3.75 μm thickness is composed of 4 layers: 3 layers previously presented, and another layer placed before the TiCl4 treatment step that contains brookite phase-based TiO2 as a scattering layer.
The obtained photoanodes are introduced into a 0.3 mM solution of DN-F01 (Dyenamo Yellow) in absolute ethanol for a 5 h immersion time. The photoanode and platinized counter electrode obtained by deposition of H2PtCl6 followed by calcination at 400 °C for 30 min were encapsulated using a Meltonix 1170-60 thick spacer. According to our previous work,16 two different electrolytes were used to fill the space between the electrodes, namely, the electrolyte E1, which consists of a solution of 0.6 M 1-butyl-3-methyl-immidazolium iodide, 0.03 M I2, 0.10 M guanidinium thiocyanate and 0.5 M 4 tert-butylpyridine in acetonitrile/valeronitrile (85/15) and the electrolyte E2 that differs only in the I3−/I− ratio (2 M 1-butyl-3-methyl-immidazolium iodide and 0.05 M I2).
The synthesized materials were characterized by X-ray diffraction (XRD) for crystal structure and phase purity determination using a PANalytical PW 3040/60 X'Pert PRO diffractometer. The measurements were made with Cu-Kα radiation at 1.5418 Å wavelength in the 2θ range between 10 and 80°. The SEM analysis was performed for the surface morphology inspection using a FEI Inspect S scanning electron microscope. The optical bandgap of the powders was obtained from the diffuse reflectance spectra measured using a Lambda 950 UV-Vis-NIR spectrophotometer equipped with a 150 mm integrating sphere at room temperature. The 4000–400 cm−1 domain was used for recording FTIR spectra using the KBr pellet procedure and a JASCO-430 Fourier transform spectrometer.
2.2. Description of thermal stability testing stand
To analyze the thermal stability and to acquire solar cell parameters at different temperatures, using a LOT LSH302 solar simulator and a VotschLabEvent T/20/40/3 climatic chamber, we designed an automated system around a Keithley 2450 source meter. The setup can measure up to five solar cells using only one source meter and allows remote control over the Internet, and therefore, the data acquisition process can be monitored from anywhere.20J–V curves were obtained using the system at different temperatures and different levels of irradiation on the cell surface. For the preliminary evaluation of solar cells, with the J–V curves, basic parameters such as ISC, VOC, P curve, MPP (Vmpp, Impp) maximum power point, and Impp (current at maximum power point), and Vmpp (voltage at maximum power point) were calculated using the system. The method required to generate the SPICE model of our solar cells is presented in the ESI.†
3. Results and discussions
Based on the J–V curves, the beneficial effect of the light-scattering layer on the photovoltaic performance, mostly JSC, was confirmed for both architectures of DSSCs, namely, DSSC1 and DSSC2 that are different, irrespective of the electrolyte (Fig. 2).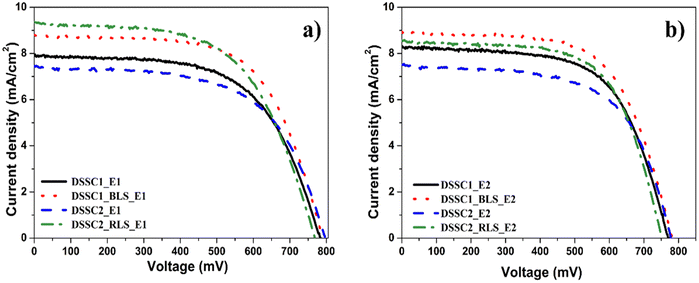 | ||
| Fig. 2 J–V curves of DSSC1 and DSSC2 without and with the light-scattering layer using (a) E1 electrolyte and (b) E2 electrolyte at room temperature. | ||
The TiO2 micro-sized aggregate films have a higher light-scattering capacity, due to their larger diameters. Thus, the distance covered by the incident light within the photoanode is significantly extended by a better scattering effect, which leads to an increase in photon harvesting by the dye molecules and provides a higher photocurrent.
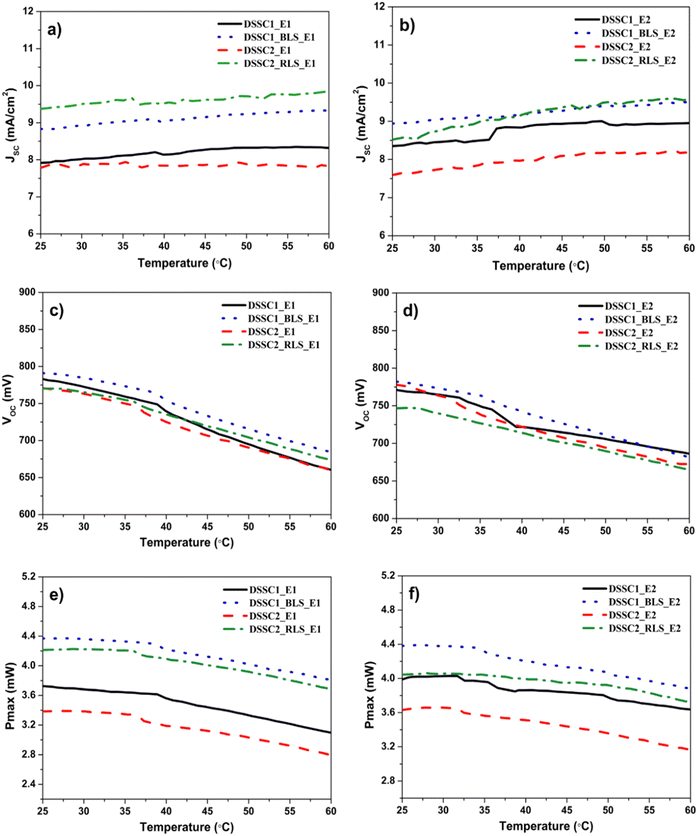
The effects of the light-scattering layer and its polymorphism on the thermal stability of the DSSCs loaded with the yellow dye are highlighted in all photovoltaic parameters, as shown in Fig. 3 (a)–(f) and Table 2. To quantify the impact of temperature variation, three temperature coefficients usually considered in photovoltaic cell performance studies, namely, the temperature coefficient for the short circuit current (α), the open circuit voltage (β), and the maximum power (γ) will be used in the following analysis of the variation of JSC, VOC, η and Pmax as a function of temperature (Fig. 3(a)–(f)). To our knowledge, the temperature coefficients of DSSCs are reported here for the first time. The beneficial positive evolution of the temperature coefficient for the short circuit current (α) for all yellow DSSCs is due to its high absorption of UV radiation (355–400 nm), close to 98% (ref. 16 and 19) highlighted in the previous work. Together with TiO2, the yellow dye also acts as a filter extending UV absorption domain with direct effect on decreasing the depletion of I3− from the electrolyte, and thus, no reduction in JSC was observed by diffusion limitation.21 Low UV exposure of the electrolyte in the yellow DSSC has made it possible to use high I3− concentration in both electrolytes E1 and E2 that simultaneously favor good photovoltaic efficiency and thermal stability.
| Cell type | Datasheet values | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I SC 25 °C [mA] | I MP 25 °C [mA] | V OC 25 °C [V] | V MP 25 °C [V] | P MAX 25 °C [mW] | (α) [%/°C] | (β) [%/°C] | (γ) [%/°C] | ||
| a The standard deviation data for each DSSC are obtained based on three cells. | |||||||||
| DSSC1_E1 | 7.93 ± 0.02 | 6.66 ± 0.01 | 0.783 ± 0.001 | 0.559 ± 0.002 | 3.73 ± 0.01 | 0.16 | –0.48 | –0.49 | In this study |
| DSSC1_BLS_E1 | 8.81 ± 0.02 | 7.62 ± 0.00 | 0.791 ± 0.000 | 0.573 ± 0.000 | 4.37 ± 0.00 | 0.16 | –0.42 | –0.40 | |
| DSSC2_E1 | 7.81 ± 0.03 | 5.98 ± 0.01 | 0.766 ± 0.002 | 0.568 ± 0.001 | 3.39 ± 0.01 | 0.01 | –0.45 | –0.54 | |
| DSSC2_RLS_E1 | 9.35 ± 0.02 | 7.59 ± 0.05 | 0.769 ± 0.001 | 0.549 ± 0.005 | 4.21 ± 0.00 | 0.10 | –0.39 | –0.39 | |
| DSSC1_E2 | 8.34 ± 0.01 | 6.98 ± 0.00 | 0.771 ± 0.001 | 0.573 ± 0.001 | 4.00 ± 0.01 | 0.24 | –0.34 | –0.28 | |
| DSSC1_BLS_E2 | 8.94 ± 0.00 | 7.84 ± 0.03 | 0.782 ± 0.000 | 0.557 ± 0.004 | 4.38 ± 0.00 | 0.18 | –0.39 | –0.35 | |
| DSSC2_E2 | 7.56 ± 0.03 | 6.09 ± 0.00 | 0.777 ± 0.001 | 0.594 ± 0.002 | 3.61 ± 0.01 | 0.24 | –0.40 | –0.40 | |
| DSSC2_RLS_E2 | 8.54 ± 0.02 | 7.68 ± 0.02 | 0.748 ± 0.002 | 0.529 ± 0.005 | 4.07 ± 0.03 | 0.38 | –0.33 | –0.22 | |
| Mono c-Si [IBC mono] | — | — | — | — | — | 0.06 | –0.28 | –0.38 | 25 |
| HIT [panasonic] | — | — | — | — | — | 0.03 | –0.25 | –0.29 | |
| Poly c-Si [IBC poly] | — | — | — | — | — | 0.041 | –0.31 | –0.411 | |
| CIGS [solibro] | — | — | — | — | — | 0 | –0.29 | –0.38 | |
In the case of E1 electrolytes characterized by 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of I3−/I−, α is positively influenced only by the rutile polymorph, and no changes are observed for the brookite polymorph. In accordance with the previous literature results, the drop in open circuit voltage for all DSSCs is caused by increasing the charge recombination rate with the increase in temperature.22 However, β and γ are clearly improved by the light-scattering layer, and a more significant increase was observed for the rutile polymorph.
1 of I3−/I−, α is positively influenced only by the rutile polymorph, and no changes are observed for the brookite polymorph. In accordance with the previous literature results, the drop in open circuit voltage for all DSSCs is caused by increasing the charge recombination rate with the increase in temperature.22 However, β and γ are clearly improved by the light-scattering layer, and a more significant increase was observed for the rutile polymorph.
In the case of the E2 electrolyte with a concentration of the reducing agent below that of the E1 electrolyte, the effect of polymorphism on thermal stability is clearly highlighted. If the brookite polymorph induced a negative effect, the rutile polymorph provided an excellent maximum power factor, superior or comparable to many commercial generations of solar cells (Table 2). An inflection point was also observed in the approximate range from 35 °C to 40 °C in both electrolytes, but weaker in intensity in the case of electrolyte 2 and diminished by the polymorphic light-scattering layers in DSSC1_BLS and DSSC2_RLS.
These preliminary results appear to be correlated with a lower iodide concentration23 and a rutile polymorph being beneficial for thermal stability probably due to the inhibition of the dye replacement at the surface by I−.
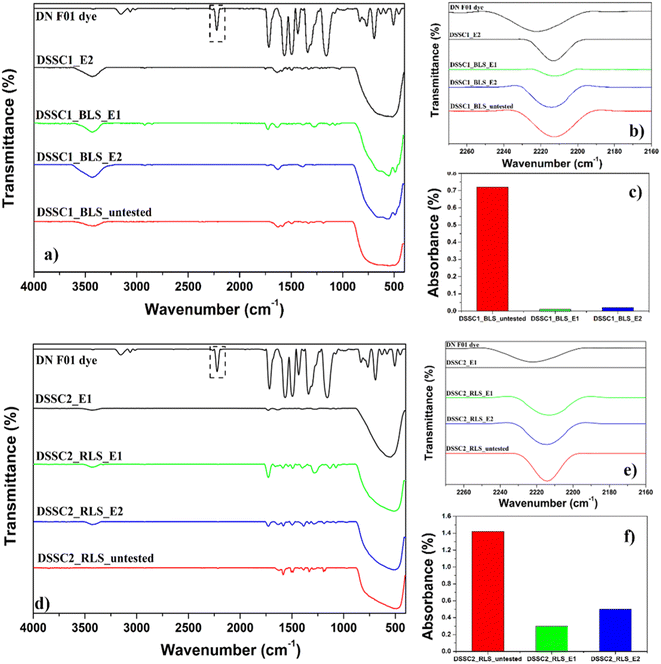
For a deeper understanding, the effect of the light-scattering layer and its polymorphism on dye anchoring stability will be mainly explored. Based on the DSSC mechanism, the possible modifications induced by temperature could be the formation of new species at the TiO2 interface or the break of bonds between TiO2 and the dye molecules followed by the dissolution of the dye in the ionic solution of the electrolyte. To explore these assumptions, FTIR analysis of the dye-loaded photoanodes, before and after testing of the DSSCs in the temperature range between 25 °C and 60 °C under 1 sun illumination was proposed. All FTIR spectra highlight the molecular fingerprint of TiO2 polymorphs, dye, and water (Fig. 4(a)–(f)). The first peak observed at 3438 cm−1 corresponds to the stretching vibrations of the hydroxyl group O–H of the interlayer water molecules.24
The broad and intense band between 890 cm−1 and 410 cm−1 is assigned to Ti–O and Ti–O–Ti stretching and bending vibrations with the characteristic values of the TiO2 polymorphs, such as 481 cm−1 for the pure anatase polymorph,26 620 cm−1 for the rutile polymorph,27 and 491 cm−1 and 555 cm−1 for the brookite polymorph, respectively.28 Furthermore, the high NaOH molarity used in the preparation of RLS favored the formation of surface hydroperoxo species, TiOOH, which exhibits an absorption band at 970 cm−1 due to vibration modes.29 Based on the similar vibrational ‘fingerprint’ of the photoanodes, before and after testing of DSSCs in the temperature range from 25 °C to 60 °C under 1 sun illumination, the formation of new species at the TiO2 interface due to the electrolyte and enabled by the temperature is excluded (Fig. 4(a)–(f)). Consequently, the break bonds between TiO2 and the dye molecules followed by the dissolution of the dye in the ionic solution of the electrolyte were investigated. It is much more significant that, from the perspective of dye anchoring, the evolution of the absorption band at ∼2200 cm−1, corresponding to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration of the UV dye,30 has provided information about the effect of polymorphism on thermal stability. As evidenced by Fig. 4(b) and (e), the rutile polymorph promotes an approximately 2-fold increase in the absorbance value compared to the brookite polymorph.
N stretching vibration of the UV dye,30 has provided information about the effect of polymorphism on thermal stability. As evidenced by Fig. 4(b) and (e), the rutile polymorph promotes an approximately 2-fold increase in the absorbance value compared to the brookite polymorph.
Commonly, one of the most frequently used anchors in DSSCs is carboxylic acid, and previous studies have shown that this anchoring group is sensitive to surface defects of the TiO2 surface. The high anchoring of yellow dye molecules for the rutile polymorph can be explained in relation to intrinsic defects, more reactive and more stable on the surface than in the bulk, the COO group being adsorbed on Ti cations located near an oxygen vacancy.31–33 However, the presence of the hydroxyl groups given by the TiOOH surface species of the rutile polymorph will also contribute to the adsorption of the dye onto titania surfaces via hydrogen bonding. However, most of all, these anchoring paths increase the thermal stability of yellow dye on the rutile surface. Due to the rutile polymorph, the undesirable break bonds between TiO2 and the dye molecules, one of the main limitations of outdoor performance and stability, are significantly reduced.
The beneficial evolution is illustrated in Fig. 4(c) and (f). For example, in the case of E2, 36.6% of the anchored dye is still identified on the TiO2 surface after testing up to 60 °C under 1 sun illumination compared to 4.2% of the anchored dye for the brookite polymorph. The improvement in the thermal stability of the anchored dye is directly reflected in the high α (+0.38%/°C) and an excellent maximum power factor (−0.22%/°C), superior or comparable to other commercial generations of solar cells (Table 2).
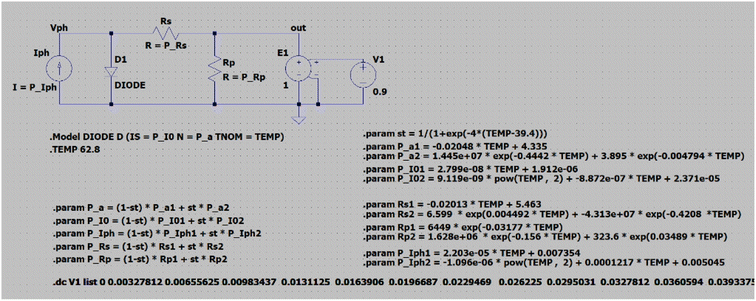
The DSSC is an electrochemical device, electrical characteristics of which are described in detail taking into account series and parallel or shunt resistance depending on the proposed model, the most popular ones being described in the study of Sarkar et al.,34 and all models having a current source as the main component, where the generated current is proportional with the irradiation. For our method, we chose the model with one diode and a series/parallel resistor, which offers good accuracy and keeps the complexity relatively low (Fig. 5).
Based on the equation for this model, eqn (1), and to model a cell based on this, the following five parameters34,35 were calculated, namely, the current generated by light energy (Iph), the saturation current of diode (I0), the ideality factor of diode (a), the series resistor (Rs) and the parallel resistor (Rp):
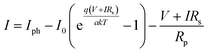 | (1) |
In relation to the working principle of DSSCs, the series resistance RS involves three resistances, namely, R1 related to the diffusion of iodide and triiodide within the electrolyte between the photoanode and the Pt counter electrode, R2, the charge-transfer resistance that occurs at the Pt counter electrode, and R3, the sheet resistance of the TCO glass substrate.
The parallel resistance, Rp, provides information about the back electron transfer rate between TiO2 and electrolyte at the TiO2/dye/electrolyte interface.
In our DSSCs, R2 and R3 are unaffected by the polymorphism of the light-scattering layer and the temperature is related to the Pt and FTO properties; the main resistance that influences Rs being R1, despite a slight increase in the values of RS, is determined by the light-scattering layer. As expected, the temperature has an impact on the diffusion resistance of I3− ions in the electrolyte, and a sharp decrease in the RS value is observed at ∼40 °C for E1 and below 35 °C for E2, explained by its high I3−/I− ratio, which improves the rate of I3− reduction and therefore decreases Rs (Fig. 6(a) and (b)).
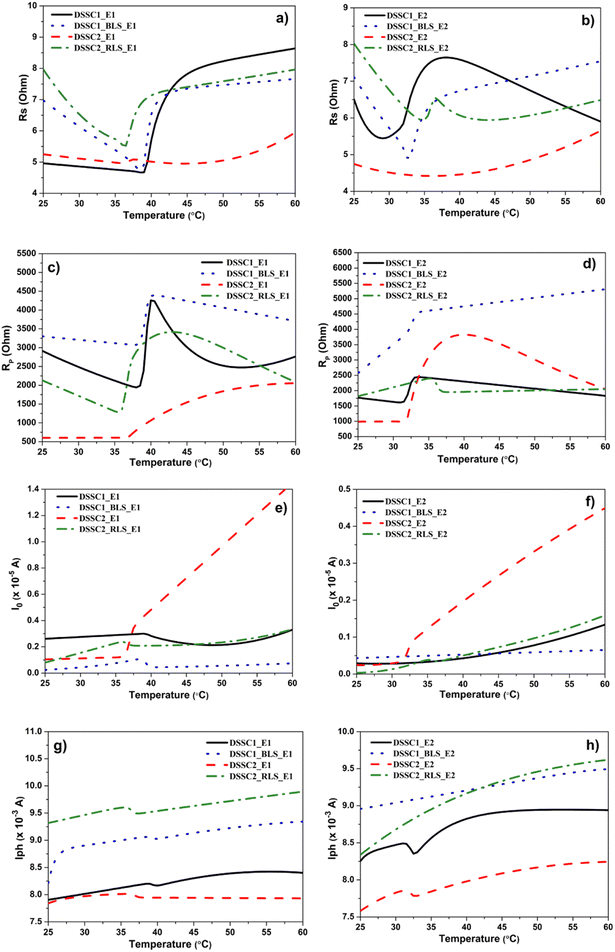 | ||
| Fig. 6 Variation in (a and b) Rs, (c and d) Rp, (e and f) I0 and (g and h) Iph as a function of temperature. | ||
More importantly, from the viewpoint of the recombination processes at the TiO2/dye/electrolyte interface, Rp has a similar evolution depending on the temperature in DSSC1_BLS_E1 and DSSC2_RLS_E1 caused by the E1 electrolyte (Fig. 6(c) and (d)).
In Fig. 6(c), RP decreases up to ∼37 °C, and then is followed by a steep increase up to ∼42 °C, and a continuous decline for the rest of the temperature range. According to FTIR results, the lowest RP values for the rutile polymorph and the implicit increase in the recombination rate could be explained by the higher dye loading capacity (Fig. 4(f)).
In the case of the E2 electrolyte, the recombination process between TiO2 and electrolyte remains more intense in the DSSC2_RLS_E2 cell, with the difference that the temperature continuously reduces this process for DSSC1_BLS_E2, leading to an improvement in cell performance.
The values of the reverse saturation current, I0, are of the order of microamperes, confirming the high quality of all DSSCs (Fig. 6(e) and (f)), and the evolution of the photogenerated current (Iph) as a function of temperature also highlighted the beneficial effect of the light-scattering layer, but more importantly the increase in the range from ∼40 °C to 60 °C for the rutile polymorph (Fig. 6(g) and (h)).
From a practical point of view, the maximum accuracy must be reached near the maximum power point (MPP) where the system works.
Extended cell characterization (Fig. 7 and 8) illustrates this area for different cells.
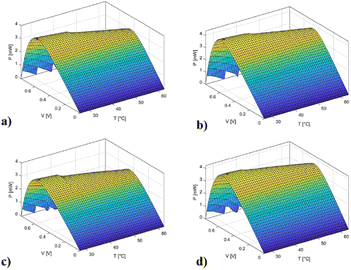 | ||
| Fig. 7 PV curves with respect to the temperature of (a) DSSC1_E1; (b) DSSC1_BLS_E1; (c) DSSC2_E1, and (d) DSSC2_RLS_E1. | ||
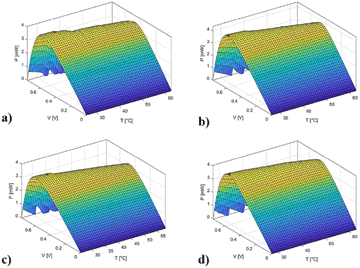 | ||
| Fig. 8 PV curves with respect to the temperature of (a) DSSC1_E2; (b) DSSC1_BLS_E2; (c) DSSC2_E2, and (d) DSSC2_RLS_E2. | ||
4. Conclusions
To the best of our knowledge, no report has yet explored the thermal stability of yellow dye-sensitized solar cells from the perspective of integration in wavelength-selective greenhouses determining the temperature coefficients. The effect of the polymorphism of light-scattering layers on the thermal stability of DSSCs up to 60 °C using laboratory-size yellow dye-sensitized solar cells based on iodide/triiodide redox electrolytes was demonstrated. Due to the dual function of the yellow dye in our DSSCs, namely, as a photosensitizer and as a UV filter together with TiO2, an electrolyte with a high I3− concentration was used, which simultaneously favored good photovoltaic efficiency and thermal stability. The study revealed that the anchoring paths provided by the rutile polymorph for yellow dye are responsible for increasing the thermal stability of DSSCs. Therefore, the undesirable break bonds between TiO2 and the dye molecules, one of the main limitations of outdoor performance and stability, are significantly reduced. Here, a dual function of the light-scattering layer was highlighted; the improvement of photon harvesting and the stability of yellow dye anchoring on a rutile polymorph allow us to achieve excellent temperature coefficients for the short circuit current (+0.38%/°C) and a maximum power factor (−0.22%/°C), superior or comparable to other commercial generations of solar cells. Our algorithm performs better for the J–V curve than Villava's method,35 but is more time consuming. The errors are below 5%, but the precision can be further improved if additional experimental data are acquired on solar cells (for example, acquiring the dark J–V curve).In future work, we will continue to develop the stability of these yellow dye-sensitized solar cells implemented in a modular photovoltaic tile with consideration of the evolution of plants under conditions imposed by the greenhouse roof. This direction is necessary as thermal stability together with the optimization of the production cost constitutes critical factors for the large-scale practical exploitation of DSSCs for wavelength-selective greenhouses, as an agrivoltaic system.
Author contributions
Daniel Ursu: investigation, writing – original draft. Melinda Vajda: investigation, writing – original draft, writing – review & editing. Elisei Ilies: data formal analysis, software. Radu Ricman: data formal analysis, software. Magdalena Marinca: data formal analysis, software. Szilard Bularka: data formal analysis, software. Marinela Miclau: conceptualization, writing – original draft, writing – review & editing, validation, supervision. Aurel Gontean: validation, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, UEFISCDI Project No. PN-III-P2-2.1-PED-2019-2091, PNCDI III.References
- J. Schallenberg-Rodriguez, J.-J. Rodrigo-Bello and B. D. Río-Gamero, Energy Rep., 2023, 9, 5420–5431 CrossRef.
- J.-R. Aira, S. Gallardo-Saavedra, M. Eugenio-Gozalbo, V. Alonso-Gómez, M.-Á. Muñoz-García and L. Hernández-Callejo, Agronomy, 2021, 11, 1097 CrossRef CAS.
- C. Corrado, S. W. Leow, M. Osborn, E. Chan, B. Balaban and S. A. Carter, Sol. Energy Mater. Sol. Cells, 2013, 111, 74–81 CrossRef CAS.
- S. A. Badawy, E. Abdel-Latif, M. A. Assiri, T. E. Ali and M. R. Elmorsy, Dyes Pigm., 2023, 217, 111447 CrossRef CAS.
- D. Ursu, M. Miclau, R. Banica and N. Vaszilcsin, Mater. Lett., 2015, 143, 91–93 CrossRef CAS.
- D. Ursu, N. Vaszilcsin, R. Bănica and M. Miclau, J. Mater. Eng. Perform., 2016, 25, 59–63 CrossRef CAS.
- D. Ursu, M. Vajda and M. Miclau, J. Alloys Compd., 2019, 802, 86–92 CrossRef CAS.
- M. Vajda, D. Ursu, N. Duteanu and M. Miclau, Mater. Lett., 2020, 275, 128151 CrossRef CAS.
- M. Vajda, D. Ursu, C. Mosoarca, N. Duteanu and M. Miclau, Int. J. Energy Res., 2021, 45, 5309–5317 CrossRef CAS.
- M. Khan, M. A. Iqbal, M. Malik, S. U. M. Hashmi, S. Bakhsh, M. Sohail, M. T. Qamar, M. Al-Bahrani, R. Y. Capangpangan, A. C. Alguno and J. R. Choi, Sci. Rep., 2023, 13, 3123 CrossRef CAS PubMed.
- G. Spinelli, M. Freitag and I. Benesperi, Sustainable Energy Fuels, 2023, 7, 916–927 RSC.
- T. F. Yadeta and T. Imae, Appl. Surf. Sci., 2023, 637, 157880 CrossRef CAS.
- J.-h. Bae, H.-J. Jeon, S.-H. Cho, Y.-b. Cho, S.-E. Lee and T.-O. Kim, Appl. Surf. Sci., 2023, 621, 156823 CrossRef CAS.
- M. Raviv and Y. Antignus, Photochem. Photobiol., 2004, 79, 219–226 CAS.
- N. Roslan, M. E. Ya'acob, M. A. M. Radzi, Y. Hashimoto, D. Jamaludin and G. Chen, Renewable Sustainable Energy Rev., 2018, 92, 171–186 CrossRef.
- D. Ursu, M. Vajda and M. Miclau, Int. J. Energy Res., 2022, 46, 18550–18561 CrossRef CAS.
- A. G. Kontos, T. Stergiopoulos, V. Likodimos, D. Milliken, H. Desilvesto, G. Tulloch and P. Falaras, J. Phys. Chem. C, 2013, 117, 8636–8646 CrossRef CAS.
- S. Ruiz Raga and F. Fabregat-Santiago, Phys. Chem. Chem. Phys., 2013, 15, 2328–2336 RSC.
- D. Albulescu, D. Ursu, L.-M. Rusnac, S. Nitu, M. Miclau and M. Vajda, Crystals, 2022, 12, 98 CrossRef CAS.
- E. Ilies, M. Marinca, S. Bularka, R. Ricman, M. Vajda and D. Albulescu, IEEE 28th International Symposium for Design and Technology in Electronic Packaging, 2022, pp. 74–77 Search PubMed.
- A. Ebenezer Anitha and M. Dotter, Energies, 2023, 16, 5129 CrossRef CAS.
- A. Usami, S. Seki, Y. Mita, H. Kobayashi, H. Miyashiro and N. Terada, Sol. Energy Mater. Sol. Cells, 2009, 93, 840–842 CrossRef CAS.
- Z. Zhang, S. Ito, J.-E. Moser, S. M. Zakeeruddin and M. Grätzel, ChemPhysChem, 2009, 10, 1834–1838 CrossRef CAS PubMed.
- P. Praveen, G. Viruthagiri, S. Mugundan and N. Shanmugam, Spectrochim. Acta, Part A, 2014, 117, 622–629 CrossRef CAS PubMed.
- B. R. Paudyal and A. G. Imenes, Sol. Energy, 2021, 224, 425–439 CrossRef CAS.
- S. El-Sherbiny, F. Morsy, M. Samir and O. A. Fouad, Appl. Nanosci., 2014, 4, 305–313 CrossRef CAS.
- A. N. Kadam, R. S. Dhabbe, M. R. Kokate, Y. B. Gaikwad and K. M. Garadkar, Spectrochim. Acta, Part A, 2014, 133, 669–676 CrossRef CAS PubMed.
- M. N. Iliev, V. G. Hadjiev and A. P. Litvinchuk, Vib. Spectrosc., 2013, 64, 148–152 CrossRef CAS.
- S. Yurdakal, S. Çetinkaya, V. Augugliaro, G. Palmisano, J. Soria, J. Sanz, M. J. Torralvo, S. Livraghi, E. Giamello and C. Garlisi, Catal. Sci. Technol., 2020, 10, 5000–5012 RSC.
- L. G. Wade, Organic Chemistry, Prentice Hall, 2003 Search PubMed.
- F. Parrino and L. Palmisano, Titanium Dioxide (TiO2) and Its Applications, Elsevier, 2021 Search PubMed.
- Q.-Y. Liu, H.-D. Wang, R. Tang, Q. Cheng and Y.-J. Yuan, ACS Appl. Nano Mater., 2021, 4, 8674–8679 CrossRef CAS.
- L. Ojamäe, C. Aulin, H. Pedersen and P.-O. Käll, J. Colloid Interface Sci., 2006, 296, 71–78 CrossRef PubMed.
- M. N. I. Sarkar, Sustainable Energy Res., 2016, 3, 13 Search PubMed.
- M. G. Villalva, J. R. Gazoli and E. R. Filho, IEEE Trans. Power Electron., 2009, 24, 1198–1208 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se01084h |
| This journal is © The Royal Society of Chemistry 2024 |