Self-passivation hole-transporting materials with pyridine-containing cores for antimony chalcogenide solar cells studied under dopant-free conditions†
Shuangting
Xu
,
Jing
Wu
,
Fuling
Guo
 *,
Miaomiao
Wu
,
Sijian
Chen
,
Wangchao
Chen
and
Chengwu
Shi
*,
Miaomiao
Wu
,
Sijian
Chen
,
Wangchao
Chen
and
Chengwu
Shi

School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei, 230009, Anhui, P. R. China. E-mail: guofuling198702@163.com; fuling.guo@hfut.edu.cn
First published on 22nd November 2022
Abstract
Pyridine-containing quinoline derivatives are developed as hole transporting materials for antimony chalcogenide solar cells. This work indicates for the first time that the pyridine heterocycle can passivate the defects of Sb2(S,Se)3 films and suppress charge recombination. The photovoltaic performance and stability are improved with the pyridine-containing hole transporting materials.
The antimony chalcogenide Sb2(S,Se)3 has become a promising and attractive alternative light harvesting material for solar cells. The lower-cost, non-toxic and stable Sb2(S,Se)3 has a strong absorption coefficient (>105 cm−1) and suitable and tunable energy levels.1,2 The deposition of an Sb2(S,Se)3 absorber layer by chemical and physical methods has been well studied.3 In Sb2(S,Se)3 solar cells, the quality of the absorber layer and energy band regulation play crucial roles in efficient charge separation and transport.4 The power conversion efficiency (PCE) of Sb2(S,Se)3 solar cells has reached over 10% by optimizing the Sb2(S,Se)3 films.5 The influence of the interfacial states and modification are also not negligible.6 Defects can easily form at the interface between layers in Sb2(S,Se)3 solar cells. Research on interfacial modification plays an important role in achieving efficient carrier transfer in Sb2(S,Se)3 solar cells.7,8
In this regard, the introduction of an interface layer such as SbCl36 or P3HT9 to reduce the interface defects for Sb2(S,Se)3 solar cells has been investigated. Besides, molecular structure modification of hole transporting materials (HTMs) has also proved to be an effective method for the interfacial modification of Sb-based absorber layers, like PCPDTBT,10 DTPThMe-ThTPA11etc. These thiophene-containing HTMs used the chelation mechanism of Sb–S (thiophene) to passivate the defects of the Sb-based absorber layer. The decreased number of defect states and increased hole extraction efficiency confirmed that the introduction of a thiophene-containing (S-containing) core was an advantageous method for interfacial engineering that enhanced charge transport and suppressed charge recombination. Although the thiophene-containing (S-containing) core was beneficial for interfacial modification, the electron-rich thiophene-containing (S-containing) core can cause an undesirable up-shift in energy level, which could lead to worse stability.12 Based on molecular orbital hybridization theory, the introduction of an electron-rich core increases the possibility of oxidation, therefore the thiophene-containing (S-containing) core is not favorable for the long term stability of Sb2(S,Se)3 solar cells.
Moreover, to overcome the low hole mobility of the commonly used HTM Spiro-OMeTAD in Sb2(S,Se)3 solar cells,13 hygroscopic dopants such as LiTFSI (lithium bis(trifluoro-methanesulfonyl)imide) and t-BP (4-tert-butylpyridine) must be used to improve the hole transporting performance. However, the hygroscopicity and ionic diffusion/migration caused by these dopants can lead to undesirable degradation problems, leading to a shorter device lifetime. Therefore, combined with the previously-mentioned interfacial modification, the development of new HTMs with the dual-functionality of dopant-free and self-passivation is urgently needed.14
Herein, we developed a series of HTMs with an electron deficient unit (pyridine-containing quinoline and isoquinoline, shown in Fig. 1) as a coordination group to passivate the defects in Sb2(S,Se)3 solar cells under dopant-free conditions. Based on molecular orbital theory, incorporation of the electron deficient units could decrease the possibility of oxidation, which is beneficial for an improved stability of organic HTMs. This work demonstrated that the pyridine heterocycle in the quinoline/isoquinoline unit could chelate with Sb3+ in Sb2(S,Se)3 thin films, passivating the uncoordinated Sb3+ defects and improving the interface properties, leading to inhibited charge recombination and improved hole-extraction efficiency. These solar cells showed enhanced power conversion efficiencies (PCEs). Meanwhile, the devices based on quinoline and isoquinoline units showed superior long-term stability compared to the comparison HTMs. This article reveals the ability of pyridine-containing units as a hole transporting material to passivate interface defects in Sb2(S,Se)3 solar cells. Compared with thiophene, the electron deficient pyridine ring has the potential to enable long term stability. These results provide a new idea for the design and preparation of hole transporting materials for Sb2(S,Se)3 solar cells.
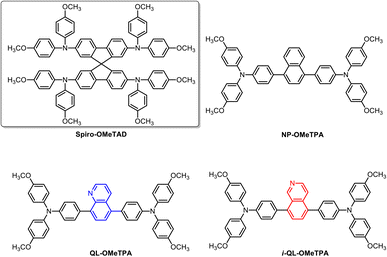 | ||
| Fig. 1 Chemical structure of Spiro-OMeTAD and pyridine-containing quinoline and iso-quinoline based HTMs (QL-OMeTPA, i-QL-OMeTPA); NP-OMeTPA represents naphthalene based HTMs for comparison. | ||
To verify the potential for hole-extraction of the novel materials as HTMs from Sb2(S,Se)3 thin films, the HOMO energy levels of NP-OMeTPA, QL-OMeTPA and i-QL-OMeTPA were measured through photophysical and electrochemical characterization. The UV-vis and photoluminescence emission spectra of these HTMs in CH2Cl2 solution were recorded and are shown in Fig. 2a. Compared to the naphthalene unit, the quinoline and iso-quinoline based HTMs with a pyridine heterocycle showed stronger red-shift in the absorption band and emission spectra, which indicated that the introduction of a pyridine heterocycle was beneficial for stronger π-electron delocalization, leading to efficient ICT (intramolecular charge transfer) for the HTMs.15 This might result in a high charge mobility for the quinoline and iso-quinoline based HTMs. Meanwhile, it could be found that the quinoline-based material showed a slight bathochromic shift in comparison to the iso-quinoline-based material, which might be caused by the effect of substituent position on the aromatic ring and demonstrated that the quinoline-based material might exhibit more advantageous charge mobility.12,16,17 Furthermore, the Eg (optical bandgap) of these materials was calculated from the intersection of the corresponding normalized absorption and emission spectra as 3.08 eV for NP-OMeTPA, 2.83 eV for QL-OMeTPA and 2.87 eV for i-QL-OMeTPA.
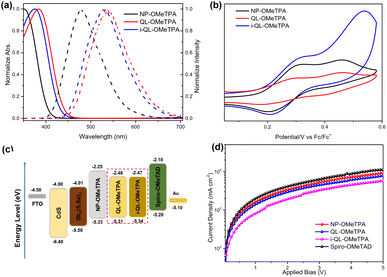 | ||
| Fig. 2 (a) Normalized UV-vis absorption (solid line) and PL emission (dashed line) spectra of these HTMs; (b) CV spectra of these HTMs; (c) energy-level diagram of the Sb2(S,Se)3 layer and these HTMs; (d) the SCLC hole-mobility curves based on the HTM-only devices; the corresponding data are listed in Table S2.† | ||
Cyclic voltammetry (CV) curves were measured to investigate the electrochemical properties of these HTMs. The HOMO (highest occupied molecular orbital) energy levels of these materials could be calculated from the half-wave potential of the CV curves (shown in Fig. 2b). The HOMO energy levels were determined to −5.33 eV for NP-OMeTPA, −5.31 eV for QL-OMeTPA and −5.34 eV for i-QL-OMeTPA.18 Relative to the HOMO energy level of spiro-OMeTAD (−5.20 eV), the downward shifted energy levels of NP-OMeTPA, QL-OMeTPA and i-QL-OMeTPA might provide better optical and thermal stability. Combining the optical bandgaps, the LUMO (Lowest unoccupied molecular orbital) energy levels were calculated as −2.25 eV for NP-OMeTPA, −2.48 eV for QL-OMeTPA and −2.47 eV for i-QL-OMeTPA.
Obviously, as Fig. 2c shows, these novel HTMs exhibited suitable energy alignment for hole extraction and electron blocking from Sb2(S,Se)3 thin films. The hole transporting properties of NP-OMeTPA, QL-OMeTPA and i-QL-OMeTPA were measured using the space-charge-limited current (SCLC) method, shown in Fig. 2d, and the data are summarized in Table S2.† Clearly, i-QL-OMeTPA exhibited a much lower hole mobility of 3.59 × 10−5 cm2 V−1 s−1 than those of NP-OMeTPA (6.36 × 10−5 cm2 V−1 s−1) and QL-OMeTPA (5.41 × 10−5 cm2 V−1 s−1). The obviously weak hole mobility of i-QL-OMeTPA might result in an increased internal series resistance, which could cause disadvantages in photovoltaic performance. Additionally, the hole mobilities of these HTMs were measured at the same concentrations and composition as in the photovoltaic devices. Meanwhile, Spiro-OMeTAD showed a hole mobility of 7.33 × 10−5 cm2 V−1 s−1 under the same conditions.
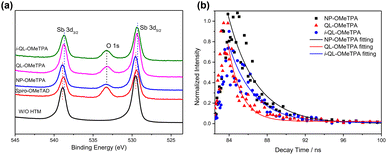
Furthermore, the X-ray photoelectron spectroscopy (XPS) characterization of the pristine Sb2(S,Se)3 and Sb2(S,Se)3 with these HTMs was carried out to investigate the interfacial chemical interaction (the complete XPS spectra are shown in Fig. S1†). The detailed binding energy region of Sb 3d is listed in Fig. 3a.
The Sb 3d peaks of the HTMs without the pyridine heterocycle showed a similar binding energy at ∼538.93 eV of Sb 3d3/2 and ∼529.58 eV of Sb 3d5/2 for pristine Sb2(S,Se)3, and Sb2(S,Se)3 with Spiro-OMeTAD and NP-OMeTPA. With the introduction of the pyridine heterocycle, the Sb 3d peaks of QL-OMeTPA and i-QL-OMeTPA shifted to a lower binding energy at ∼538.68 eV of Sb 3d3/2 and ∼529.38 eV of Sb 3d5/2. The decreased binding energy demonstrated an increased electron density around the Sb atoms. The obvious decrease of binding energy of Sb atoms for the pyridine-containing QL-OMeTPA and i-QL-OMeTPA indicated interactions between pyridine and Sb atoms (as shown in Fig. S2†).19,20 These results suggested the possibility to passivate the defects of unbound Sb atoms. The time-resolved photoluminescence (TRPL) was characterized to investigate the carrier dynamics. The decay curves for the devices of FTO/CdS/Sb2(S,Se)3/HTM structure with NP-OMeTPA, QL-OMeTPA and i-QL-OMeTPA are shown in Fig. 3b and were fitted with a monoexponential equation. The rapid decay times of 1.39 ns for Sb2(S,Se)3 with QL-OMeTPA and 2.63 ns for Sb2(S,Se)3 with i-QL-OMeTPA were obtained compared to 2.97 ns for Sb2(S,Se)3 with NP-OMeTPA. These results of fast dynamics times might suggest efficient charge separation and extraction for the pyridine-containing HTMs,21 which is in good agreement with observations on the coordination passivation of pyridine heterocycles. Among these candidates, QL-OMeTPA might be more effective than i-QL-OMeTPA. These conclusions indicated the superiority of pyridine-containing materials in interfacial passivation and charge carrier transport.
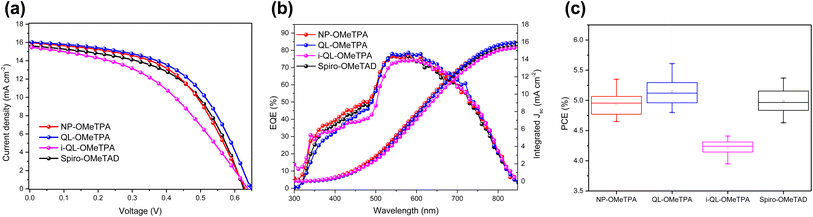
To evaluate the advantages of the pyridine-containing materials, the photovoltaic performance of Sb2(S,Se)3 solar cells with NP-OMeTPA, QL-OMeTPA and i-QL-OMeTPA was investigated under dopant-free conditions. Sb2(S,Se)3 solar cells with Spiro-OMeTAD were fabricated as reference devices under dopant-free conditions. The current density–voltage (J–V) curves of the champion solar cells with NP-OMeTPA, QL-OMeTPA, i-QL-OMeTPA and Spiro-OMeTAD were recorded under standard simulated AM1.5G and are shown in Fig. 4a. The parameters of these devices are summarized in Table 1. The device performance in terms of PCE of these HTMs is shown by the box plots in Fig. 4c. The corresponding average parameters of these solar cells are summarized in Table S1.† The statistical data of Voc, Jsc and FF of these devices are shown by the box plots in Fig. S4.† As the data show, the champion (shown in Fig. 4a, b and Table 1), statistical distribution (shown in Fig. 4c) and average (listed in Table S1†) parameters of these solar cells demonstrate that the pyridine-containing QL-OMeTPA based devices exhibited a higher PCE, due to the superior open-circuit voltage and relatively higher fill factor (shown in Fig S4†).11,22 From Table 1, it can be seen that the pyridine-containing HTM based devices showed a higher open-circuit voltage (Voc) of 646 mV for QL-OMeTPA and 637 mV for i-QL-OMeTPA compared to the Voc of 630 mV for the comparison material NP-OMeTPA and the Voc of 626 mV for the reference material Spiro-OMeTAD. The obviously increased Voc could be attributed to the passivation of the pyridine heterocycle, which was consistent with the conclusion obtained from the XPS characterization. The feasibility of the pyridine heterocycle as a passivating group for Sb2(S,Se)3 solar cells was further verified. The solar cells based on QL-OMeTPA exhibited the highest PCE of 5.61% with a Voc of 646 mV, Jsc (short-circuit current density) of 16.04 mA cm−2 and FF (fill factor) of 50.47%. In comparison, the solar cell based on NP-OMeTPA with a similar molecular structure but without the pyridine heterocycle only showed a PCE of 5.35% with a Voc of 630 mV, Jsc of 15.95 mA cm−2 and FF of 53.17%, which was affected majorly by the decreased Voc. However, for i-QL-OMeTPA, a lower PCE of 4.35% with a Voc of 637 mV, Jsc of 15.47 mA cm−2 and FF of 44.12% could be found. The undesirable PCE might be attributed to the higher internal resistance caused by the lower charge carrier mobility and the inferior driving force caused by the lower HOMO energy level of i-QL-OMeTPA.23 These results demonstrated that the substituent position of the pyridine heterocycle must be particularly considered. As a contrast, the device without a HTM exhibited a PCE of only 3.28% due to a low Voc and FF (shown in Fig. S3†), which indicated the importance of introducing hole transport materials to extract holes. Fig. 4b shows the external quantum efficiency (EQE) spectrum of the Sb2(S,Se)3 solar cells based on these HTMs. Generally, a weak EQE in the short-wavelength range is caused by the absorption of the CdS buffer layer.24 For all these devices, the integrated current based on the EQE and AM 1.5G were very close to the Jsc from the J–V curves. Although Spiro-OMeTAD and NP-OMeTPA retained a relatively high hole mobility, the pyridine-free HTMs did not exhibit a strong interfacial interaction with Sb2(S,Se)3, resulting in inferior hole extraction. The QL-OMeTPA based solar cells underwent better interfacial modification, producing a higher PCE. Meanwhile, the reference Spiro-OMeTAD based device showed a PCE of 5.26% with a Voc of 626 mV, Jsc of 15.63 mA cm−2 and FF of 53.72%.
| HTMs | V oc (mV) | J sc (mA cm−2) | FF (%) | η (%) |
|---|---|---|---|---|
| NP-OMeTPA | 630 | 15.95 | 53.17 | 5.35 |
| QL-OMeTPA | 646 | 16.04 | 50.47 | 5.61 |
| i-QL-OMeTPA | 637 | 15.47 | 44.12 | 4.35 |
| Spiro-OMeTAD | 626 | 15.63 | 53.72 | 5.26 |
To study the interfacial charge transfer, electrical impedance spectroscopy (EIS) was performed. Fig. 5 shows the Nyquist plots of the Sb2(S,Se)3 solar cells based on these HTMs. The plots were fitted with an equivalent circuit. The corresponding data are listed in Table S2.†Rs in the high-frequency components represents the charge transfer resistance caused by the assembled materials of the solar cells, e.g. FTO, Au and Sb2(S,Se)3, and the values are very close together, between 8.00 and 9.87 Ω, except for the value of the i-QL-OMeTPA-based device of 16.64 Ω. The larger Rs resulted from the lower charge carrier mobility, indicating a higher internal resistance, which led to the inferior FF and PCE. Rrec in the low-frequency region indicates the charge-recombination resistance at the Sb2(S,Se)3/HTM interface. Obviously, the devices with pyridine-containing HTMs exhibited a larger Rrec of 3505 Ω for QL-OMeTPA and 4052 Ω for i-QL-OMeTPA compared to that of 1731 Ω for the pyridine-free NP-OMeTPA. The enhanced resistances of the pyridine-containing HTMs related to the decreased charge recombination, showing the superiority of the pyridine heterocycle in the interfacial modification. It was further proven that the coordination of pyridine played a positive role in interface passivation for the Sb2(S,Se)3 solar cells, resulting in the improved Voc. It should also be noted that the devices based on Spiro-OMeTAD exhibited a Rrec of 1608 Ω, showing its inferiority in interface passivation. Combined with the XPS and TRPL analysis, the coordination passivation of the pyridine heterocycle could be an important tool in the improvement of Sb2(S,Se)3 solar cell performance.
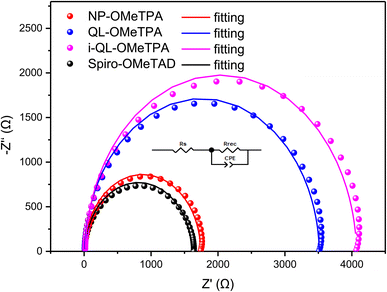 | ||
| Fig. 5 Nyquist plots for the devices based on these HTMs; the inset image is the corresponding equivalent circuit. | ||
To assess the influence of the pyridine unit on the stability of materials and devices, thermogravimetric analysis (TGA) of these synthetic HTMs and study of the long-term stability of their solar cells were carried out. Theoretically, the introduction of electron deficient pyridine should increase the thermal stability.25,26 As Fig. S5a† shows, the temperatures of the 5%(T5) and 10%(T10) weight loss with pyridine were observed for QL-OMeTPA (T5: 295 °C; T10: 335 °C) and i-QL-OMeTPA (T5: 385 °C; T10: 418 °C). However, the comparison NP-OMeTPA without pyridine showed a T5 of 285 °C and T10 of 315 °C, which were lower than those of the pyridine-containing HTMs, as expected by theory. In addition, the T5 and T10 of i-QL-OMeTPA were higher than those of QL-OMeTPA, which might caused by the effect of substituent position in the aromatic ring. However, the obviously weak hole mobility made i-QL-OMeTPA unsuitable for solar cells. Finally, the long-term stability of solar cells based on these synthetic HTMs was investigated. The devices were stored in ambient air. It could be found that the pyridine-containing devices exhibited better stability than the devices without pyridine (shown in Fig. S5b†). After about 10 days of storage, QL-OMeTPA and i-QL-OMeTPA retained 87% and 88% of their initial PCE, while the pyridine-free NP-OMeTPA based devices only retained 84%. The thermal and long-term stability results demonstrated the superiority of pyridine in HTMs. Meanwhile, the reference Spiro-OMeTAD based solar cells maintained 86% of their initial PCE, which was slightly lower than that of pyridine-containing QL-OMeTPA.
In conclusion, we demonstrated a novel strategy of interfacial modification of Sb2(S,Se)3 solar cells. It was demonstrated for the first time that the pyridine heterocycle could act as a passivation group to coordinate with interface defects, which improved charge mobility and inhibited charge recombination. As a result, the Sb2(S,Se)3 solar cell with the pyridine-containing QL-OMeTPA as HTM produced the highest PCE of 5.61% under dopant-free conditions. The advantageous Voc caused by the interfacial passivation of the pyridine heterocycle supported the superior PCE compared to the pyridine-free materials. Combined with the advantages of the pyridine-containing HTMs in stability, this research provides an efficient and simple interfacial modification strategy for high-performance Sb2(S,Se)3 solar cells. This work suggested a promising candidate of molecular structure engineering on hole-transporting materials for high-efficiency Sb2(S,Se)3 solar cells.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52102231, 52002105), the Natural Science Foundation of Anhui Province (2008085QB89), the Fundamental Research Funds for the Central Universities (JZ2022HGTB0254, JZ2021HGTB0105) and the Undergraduate Innovation Entrepreneurship Training Program (S202210359382).Notes and references
- R. Kondrotas, C. Chen and J. Tang, Joule, 2018, 2, 857–878 CrossRef CAS.
- Y. Zhou, L. Wang, S. Chen, S. Qin, X. Liu, J. Chen, D.-J. Xue, M. Luo, Y. Cao, Y. Cheng, E. H. Sargent and J. Tang, Nat. Photonics, 2015, 9, 409–415 CrossRef CAS.
- Y. Wang, R. Liang, C. Qin, L. Ren, Z. Ye and L. Zhu, Sustainable Energy Fuels, 2021, 5, 855–861 RSC.
- X. Wang, X. Shi, F. Zhang, F. Zhou, P. Zeng, J. Song and J. Qu, Appl. Surf. Sci., 2022, 579, 152193 CrossRef CAS.
- Y. Zhao, S. Wang, C. Jiang, C. Li, P. Xiao, R. Tang, J. Gong, G. Chen, T. Chen, J. Li and X. Xiao, Adv. Energy Mater., 2022, 12, 2103015 CrossRef CAS.
- J. Han, S. Wang, J. Yang, S. Guo, Q. Cao, H. Tang, X. Pu, B. Gao and X. Li, ACS Appl. Mater. Interfaces, 2020, 12, 4970–4979 CrossRef CAS.
- O. A. Jaramillo-Quintero, Y. A. Alarcón-Altamirano, R. A. Miranda-Gamboa and M. E. Rincón, Appl. Surf. Sci., 2020, 526, 146705 CrossRef CAS.
- Q. Ye, Y. Xu, W. Chen, S. Yang, J. Zhu and J. Weng, Appl. Surf. Sci., 2018, 440, 294–299 CrossRef CAS.
- D.-H. Kim, S.-J. Lee, M. S. Park, J.-K. Kang, J. H. Heo, S. H. Im and S.-J. Sung, Nanoscale, 2014, 6, 14549–14554 RSC.
- Y. C. Choi and S. I. Seok, Adv. Funct. Mater., 2015, 25, 2892–2898 CrossRef CAS.
- C. Jiang, J. Zhou, R. Tang, W. Lian, X. Wang, X. Lei, H. Zeng, C. Zhu, W. Tang and T. Chen, Energy Environ. Sci., 2021, 14, 359–364 RSC.
- H. Zhang, Y. Wu, W. Zhang, E. Li, C. Shen, H. Jiang, H. Tian and W.-H. Zhu, Chem. Sci., 2018, 9, 5919–5928 RSC.
- P. Yan, D. Yang, H. Wang, S. Yang and Z. Ge, Energy Environ. Sci., 2022, 15, 3630–3669 RSC.
- N. Juneja, S. Mandati, A. Katerski, N. Spalatu, S. Daskeviciute-Geguziene, A. Vembris, S. Karazhanov, V. Getautis, M. Krunks and I. Oja Acik, Sustainable Energy Fuels, 2022, 6, 3220–3229 RSC.
- Y. Hua, B. Xu, P. Liu, H. Chen, H. Tian, M. Cheng, L. Kloo and L. Sun, Chem. Sci., 2016, 7, 2633–2638 RSC.
- A. J. Zucchero, P. L. McGrier and U. H. F. Bunz, Acc. Chem. Res., 2010, 43, 397–408 CrossRef CAS.
- Y. C. HU Bo, Q.-W. Wang, H. Zhang and Y. U. Jian-Kang, Acta Phys. Sin., 2012, 28, 1651–1657 Search PubMed.
- R. Grisorio, B. Roose, S. Colella, A. Listorti, G. P. Suranna and A. Abate, ACS Energy Lett., 2017, 2, 1029–1034 CrossRef CAS.
- D. B. Sulas, A. E. London, L. Huang, L. Xu, Z. Wu, T. N. Ng, B. M. Wong, C. W. Schlenker, J. D. Azoulay and M. Y. Sfeir, Adv. Opt. Mater., 2018, 6, 1701138 CrossRef.
- Y. Xiang, H. Guo, Z. Cai, C. Jiang, C. Zhu, Y. Wu, W.-H. Zhu and T. Chen, Chem. Commun., 2022, 58, 4787–4790 RSC.
- Y. Xue, Y. Wu and Y. Li, J. Power Sources, 2017, 344, 160–169 CrossRef CAS.
- S. Chen, M. Li, Y. Zhu, X. Cai, F. Xiao, T. Ma, J. Yang, G. Shen, A. Ke, Y. Lu, W. Liang, H.-Y. Hsu, C. Chen, J. Tang and H. Song, Adv. Energy Mater., 2022, 2202897 CrossRef.
- J. J. Yoo, G. Seo, M. R. Chua, T. G. Park, Y. Lu, F. Rotermund, Y.-K. Kim, C. S. Moon, N. J. Jeon, J.-P. Correa-Baena, V. Bulović, S. S. Shin, M. G. Bawendi and J. Seo, Nature, 2021, 590, 587–593 CrossRef CAS PubMed.
- J. Li, Y. Ma, G. Chen, J. Gong, X. Wang, Y. Kong, X. Ma, K. Wang, W. Li, C. Yang and X. Xiao, Sol. RRL, 2019, 3, 1800254 CrossRef.
- W. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z.-s. Wang and H. Tian, Adv. Funct. Mater., 2011, 21, 756–763 CrossRef CAS.
- Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2se01448c |
| This journal is © The Royal Society of Chemistry 2023 |