New insights on oxidative desulfurization for low sulfur residual oil production†
Shouvik
Mitra
a,
Surya Murali
Racha
a,
Biswajit
Shown
*b,
Sukumar
Mandal
b and
Asit Kumar
Das
b
aRefining R&D, Reliance Research and Development Centre, Reliance Industries Ltd., Ghansoli 400701, Navi Mumbai, India
bRefining R&D Centre, Reliance Jamnagar Refinery and Petrochemical Complex, Reliance Industries Ltd., Jamnagar, Gujarat 361142, India. E-mail: biswajit.shown@ril.com
First published on 21st November 2022
Abstract
Combustion of heavy fuel oils releases sulfur oxides (SOx) which are known to cause various types of diseases which are harmful to human health, and also contribute to acid rain. The International Maritime Organization (IMO) regulations to reduce emission of SOx from ships came into force in 2005. With the new regulation in force, from 1st January 2020 (IMO-2020) the upper limit of sulfur in fuel oil used in ships operating outside designated emission control areas is now restricted to 0.5 wt%. Currently, installation of scrubbers, catastrophic blending and hydrodesulfurization are some of the techniques available to the shipping industries to reduce the sulfur content and meet the specifications of IMO-2020 grade residual oil. However, the high capital cost for scrubber installation, demand for use of higher temperature/pressure and hydrogen consumption in hydrodesulfurization are some of the intrinsic drawbacks. In this regard, oxidative desulfurization (ODS) could be a solution to produce IMO-2020 grade residual oil. Herein, we present for the first time, a systematic evaluation and new insights to produce IMO-2020 compliant residual oil using a heterogeneous catalyst system together with a techno-economic evaluation. The V2O5/Al2O3 heterogeneous catalyst was used in combination with cumene hydroperoxide (CHP) to desulfurize residual oil at atmospheric pressure. The ability to recycle CHP makes the ODS process economically viable, and a potential competitor to the currently existing processes being used, to produce IMO-2020 compliant residual oil. This systematic study, gives new insights and the techno-economic evaluation shows the potential of ODS to produce IMO-2020 compliant residual oil for the shipping industries and this study could be utilized for future developmental purposes.
Introduction
In the last few decades, uses of transportation fuels have increased enormously. With the limited options of fuel reserves, use of low-quality fossil fuel feedstocks has become inevitable. Low-quality fossil fuel feedstocks are often associated with high amounts of sulfur contamination.1–3 Although, these low-quality feedstocks are available at a cheaper price, they are associated with the emissions of toxic gases such as SOx and NOx.1 A comparison of these two toxic gases, shows that the SOx emissions are associated with environmental hazards and are detrimental to health. The SOx primarily cause acid rain after reactions with atmospheric oxygen and water.1,4,5 Considering the hazardous effects which SOx may pose, stringent specifications of sulfur are being maintained upstream of the feedstocks. It is worth noting that the transportation sectors (road transport, aviation, shipping) are the major contributors for unwanted SOx emissions. As a result, new policies have emerged to mitigate the problems of sulfur emission. The sulfur content of the transportation fuels (gasoline and diesel) is now restricted to 10 ppm across the world.6 Light/middle distillates are mainly used as a feedstock for transportation fuels, whereas heavier fractions/fuel oils are the primary feedstocks for transportation by ship. Sulfur, nitrogen and heavy metals are present as major contaminants. To restrict the SOx emission, the International Maritime Organization (IMO) has capped the upper limit of sulfur specifications in marine fuels and heavy fuel oil (The International Convention for the Prevention of Pollution from Ships: MARPOL Annex VI). The upper limit of sulfur content is now restricted to 0.1 wt% in Sulfur Emission Control Areas (SECAs) and 0.5 wt% outside designated emission control areas beyond 2020, which is well below the previously maintained sulfur specifications (3.5 wt%).2 Although this limiting of the sulfur specification is beneficial for the environment it brings severe technical and economic challenges to the refiners.In this context, researchers have extensively used a hydrodesulfurization technique to produce low sulfur fuel oils. However, the high temperature, pressure and very high hydrogen consumption required for hydrodesulfurization are some of the major disadvantages of the process.4,7,8 In addition, refractory sulfur compounds such as dibenzothiophene (DBT), and 4,6-dimethyldibenzothiophene (DMDBT) are not easy to remove via hydrogenation.1,4,9 Therefore, oxidative desulfurization (ODS) techniques have been emerged as an alternative to hydrodesulfurization. The ODS involves oxidation of a sulfur containing hydrocarbon to sulfoxides or sulfones in the presence of a catalyst, followed by extraction of relatively higher polar compounds such as sulfones or sulfoxides in the presence of polar solvents.1,4,9 It is quite evident that the major advantages of the ODS process are its low reaction temperature, and low operating pressure throughout the process. The ODS was first reported as early as 1967,10 however, it was not given much attention before the current stringent regulations required the production of low sulfur fuel oils. In the last decade a variety of oxidative systems have been investigated for use as a homogeneous catalyst, for example, hydrogen peroxide (H2O2) with organic acids, H2O2 with a heteropoly acid, and other non- H2O2 systems.11–15 Although homogeneous catalyst systems are known to demonstrate a high desulfurization capacity, they have intrinsic drawbacks too. Separation of the homogeneous catalyst from the reaction mixture appears to be a difficult task, and as a result the pH of the solution is drastically decreased. Moreover, the oxygen contents are higher in the desulfurized fuel oil compared to its untreated feedstocks. Attempts have been made to use extraction coupled oxidative desulfurization for the separation of organic acids after reactions,16 but the membrane-based separation process is ineffective for heavier residual oils. In contrast heterogeneous catalyst systems are preferred from the industrial point of view because of their easy separation and reusability.11 In this regard, transition metal-based catalysts such as molybdenum (Mo),17–21 nickel (Ni) promoted,22,23 vanadium (V),24,25 and tungsten (W)26,27 have been tested on alumina (Al2O3), silica (SiO2), zeolites, and active carbon supports.28–31 Although these studies have shown encouraging results, a systematic study from industrial perspectives is required. Despite a handful of papers on oxidative desulfurization techniques of fuel oil to produce IMO-2020 grade residual oil being available in the literature, information on the practical integration of systematic approach, mechanism and economic evaluation required from the industrial point of view is scarcely available. Herein, we report for the first time a systematic study to produce IMO-2020 grade residual fuel oil together with a mechanism and a techno-economic evaluation.
In our detailed study both homogeneous and heterogeneous catalysts were tested. The homogeneous catalyst system was able to remove sulfur efficiently, but the desulfurized product was out of specification as per the IMO-2020 residual oil standard. To meet the IMO-2020 specification, we evaluated a heterogeneous catalyst system for desulfurization, and V2O5/Al2O3 in combination with cumene hydroperoxide (CHP) was found to be a suitable catalyst-oxidant combination to produce IMO-2020 grade residual oil. The oxidation mechanism and extraction of oxidized sulfur compounds was studied systematically. To produce a competitive technology, the catalyst, oxidant and extraction solvent were recycled in the course of the ODS process. With the recycling of catalyst, oxidant and extraction solvent, our results highlighted the promising potential of ODS to produce IMO-2020 grade residual oil with a good profit margin.
Experimental
Materials and methods
Commercially available extruded γ-Al2O3 (surface area >250 m2 g−1) was used as the support material. Analytical grade 30% H2O2, tertiary butyl hydrogen peroxide (TBHP) solution, CHP solution, ammonium heptamolybdate, ammonium metatungstate, ammonium metavanadate, oxalic acid, and methanol (MeOH) were purchased and used as received. The supported catalyst V2O5/Al2O3 was prepared using an incipient wetness impregnation method in the laboratory. Residual oil (temperature range: 380–590 °C, average aromatic content: 70%) with a sulfur content of >1.50 wt%, was collected from a refinery.Preparation of the catalyst
The V2O5/Al2O3 was prepared using a wetness impregnation method.32 15 wt% aqueous solution of ammonium metavanadate was prepared at 80 °C by the addition of oxalic acid.32 The resultant solution was added to 100 g of extruded γ-Al2O3 followed by soaking at room temperature for 4 h. Once the ammonium metavanadate solution was soaked, it was heated at 80 °C for 6 h. The resultant greenish material was then calcined at 800 °C for 8 h to obtain a yellowish orange colored V2O5/Al2O332. The as prepared catalyst was systematically characterized. Phase purity and crystallinity were determined using powder X-ray diffraction (PXRD). Surface functionality and thermal stability was determined using Fourier transform infrared spectra (FTIR) and thermogravimetric analysis (TGA), respectively. The surface area and morphology of the catalyst were evaluated using BET surface area analysis and scanning electron microscopy (SEM), respectively. The V2O5 loading on the catalyst was determined using ICP-MS analysis (for further details, see the ESI†).Homogeneous catalysts
The oxidizing agent, H2O2 was added dropwise into a glass reactor containing 50 mL of residual oil. About 4–30% (v/v) of organic acid, either formic or acetic acid, was added to the mixture. The entire solution was mixed well and then heated at 60 °C for about 2 h. The mixture was allowed to cool down to room temperature, and the oxidized sulfur compounds were extracted with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) solvent (either acetonitrile (ACN) or MeOH) at room temperature for 1 h. After phase separation, the treated residual oil was collected from the separating funnel and measurement of the sulfur content was carried out.
1 (v/v) solvent (either acetonitrile (ACN) or MeOH) at room temperature for 1 h. After phase separation, the treated residual oil was collected from the separating funnel and measurement of the sulfur content was carried out.
Heterogeneous catalysts
A solid catalyst (V2O5/Al2O3) of various wt% was added to 50 mL of residual oil in a glass reactor, and mixed to ensure uniform distribution. Subsequently, oxidant (TBHP/H2O2/CHP) (various quantities % (v/v) were tested) was added to the reaction mixture. The entire mixture was heated at 80 °C (for H2O2 at 60 °C) for about 3 h under continuous stirring. Afterwards, the mixture was allowed to cool down to room temperature. The extracting solvent (MeOH) was added to the solution and mixed at room temperature for 1 h. The solution was allowed to separate into two phases, where the solvent and extracted components remained on the top layer, while the heavier desulfurized residual oil settled at the bottom. The treated desulfurized residual oil was collected from the bottom of separating funnel and analyzed to determine the total sulfur using the ASTM D5453 method. Comparison of fuel properties (sulfur content, total acid number (TAN), pour point, conradson carbon residue (CCR)) between the feed and treated residual oil is detailed in Section 1 (ESI†).Results and discussion
The ODS is often preferred over hydrodesulfurization because it converts and removes substituted DBTs at low temperature and low pressure. In the presence of a catalyst and oxidant, refractory sulfur compounds are oxidized to sulfones or sulfoxides, followed by extraction in a polar solvent using liquid–liquid extraction (LLE).1,4,9 At the same time, nitrogen containing compounds are oxidized into nitrogen oxides in this process. The refractory sulfur compounds and general scheme of ODS are summarized in Fig. 1.To produce IMO-2020 grade residual fuel oil, homogeneous catalysts were tested first. Hydrogen peroxide was selected as the oxidant, and during the process H2O2 was converted to O2 and H2O. Formic acid or acetic acid in combination with H2O2 were used as the homogeneous oxidative catalyst which would produce performic acid or peracetic acid, respectively, in situ.2 After completion of the reaction, the oxidized components were extracted with methanol or ACN.2,33 The extracting solvents were selected based on the miscibility of the residual oil with the solvents, which are summarized in Table T1 (ESI†). Based on phase separation, MeOH and ACN were selected for the extraction step because of their low boiling points compared to N-methyl-2-pyrrolidone (NMP), poly(ethylene glycol) (PEG) and tetraethylene glycol (TEG). The data presented in Table T1 (ESI†) also suggest that at least a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
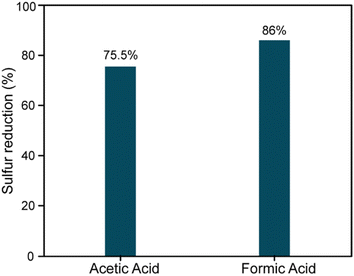
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of feed to solvent is required to achieve the maximum extraction of the oxidized sulfones from the feed. About 75% desulfurization of residual fuel oil was achieved when acetic acid was used as the organic acid, and about 86% desulfurization was achieved when formic acid was used as the organic acid (Fig. 2). Although Otaibi et al. showed that there was more desulfurization with acetic acid,34 but in this research, we observed more desulfurization using formic acid. The reason behind this phenomenon could be the greater miscibility of the performic acid formed in situ compared to that formed with peracetic acid with the residual oil feed in course of the reaction. A simple solvent extraction process was also tried for desulfurization of residual oil. Considering the low solubility of thiophenes, benzothiophenes (BTs), DBTs and substituted DBTs in ACN.
1 ratio of feed to solvent is required to achieve the maximum extraction of the oxidized sulfones from the feed. About 75% desulfurization of residual fuel oil was achieved when acetic acid was used as the organic acid, and about 86% desulfurization was achieved when formic acid was used as the organic acid (Fig. 2). Although Otaibi et al. showed that there was more desulfurization with acetic acid,34 but in this research, we observed more desulfurization using formic acid. The reason behind this phenomenon could be the greater miscibility of the performic acid formed in situ compared to that formed with peracetic acid with the residual oil feed in course of the reaction. A simple solvent extraction process was also tried for desulfurization of residual oil. Considering the low solubility of thiophenes, benzothiophenes (BTs), DBTs and substituted DBTs in ACN.
MeOH and ethanol, respectively, the process was not effective (only 6% desulfurization was achieved).1,35 The detailed reaction conditions and the results are summarized in Table T2 (ESI†). The amount of oxidative catalyst was also varied in course of the ODS process, and with the increase in oxidant strength, the desulfurization (%) was enhanced. It was interesting to note that 30% (v/v) of H2O2 and 30% (v/v) of both the organic acids were found to be suitable for desulfurization.
The majority of the literature on oxidative desulfurization of heavy fuel oils (with a high sulfur content) is based on homogeneous catalysis because of the simple reactions and high degree of desulfurization (Table T3, ESI†). Our results were promising and consistent with those found in the literature, but the homogeneous catalysis had its own drawbacks.11 The pH of the reaction mixture was reduced to 2.3–2.5 after the addition of the acidic catalyst, which would certainly induce corrosion in the reactor and pipelines at a larger scale and thus would require compatible metallurgy. In addition, the catalyst remained in the liquid phase, which was difficult to separate and reuse for subsequent reactions.11 Therefore, fresh catalyst was required for every new reaction. To combat these challenges, a heterogeneous catalyst was preferred over a homogeneous catalyst for the ODS reaction. Subsequently, considerable attention has been given by the researchers to the heterogeneous catalyst assisted ODS processes. Use of a metal supported catalyst for desulfurization of diesel with O2 gas has already been reported,36 and the oxidized components were often captured in an adsorbent to obtain the desulfurized product. Meanwhile transition metal oxides on an active support/without an active support are some of the widely explored heterogeneous catalysts for the ODS process. Although MoO3/MCM-41-TBHP, MoO3/Al2O3-TBHP, oxovanadium catalysts–TBHP, bis(glycerol)oxotitanium (IV)-organohydro peroxides were some of the catalyst combinations used for the desulfurization of heavy oil,37–40 but the degree of desulfurization was often low (Table T3, ESI†).
Keeping these observations in mind, a V2O5/Al2O3 catalyst was prepared using an incipient wetness impregnation method. We anticipated that V2O5 would be beneficial for oxidation reactions and the degree of desulfurization would be greater, because V2O5 is already known for its good oxidation ability. During the preparation of V2O5/Al2O3, a drastic change in the color from dark green (ammonium metavanadate) to yellowish orange upon calcination, which signified the formation of V2O5 on the Al2O3 support.32 The PXRD pattern of V2O5/Al2O3 confirmed that the crystalline phase of V2O5 was on the Al2O3 support (2θ = 15.37°, 20.35°, 21.75°, 26.32°, 30.89°, 32.51°, 34.50°, 45.45°, 47.44°, 51.23°, 61.18° and 62.18°) (Fig. S1, ESI†).32 The crystalline peaks mentioned previously could be attributed to the crystalline planes of V2O5 (ICDD ID: 01-70-8747). Characteristic V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and V–O–V stretching at 1019 cm−1 and 505 cm−1 also indicated the formation of V2O5 on the Al2O3 (Fig. S2, ESI†). Prior to the evaluation of the surface area, the thermal stability of V2O5/Al2O3 was measured using TGA. The TGA analysis showed that the V2O5/Al2O3 was stable up to 800 °C and only ∼13% weight loss observed. From all the results obtained, ∼9% weight loss up to 150 °C signified the removal of moisture and desorbed water molecules from the surface of the catalyst (Fig. S4, ESI†). The Al2O3 support was found to be porous in nature with a BET surface area of 279.5 m2 g−1, whereas the V2O5/Al2O3 showed a BET surface area of 228.9 m2 g−1 (Fig. 3a). A significant reduction in surface area was attributed to the formation of V2O5 on to the support (Fig. 3a). At the same time the BJH pore diameter of V2O5/Al2O3 showed an average pore diameter of 4.54 nm (Fig. S3, ESI†). Formation of V2O5 on Al2O3 was further supported by the analysis of the SEM image. The V2O5/Al2O3 SEM image revealed the rod shaped particles of V2O5 (Fig. 3b and Fig. S6, ESI†), which was distinctly different when compared to the surface of pristine Al2O3 (Fig. S5, ESI†). Meanwhile the EDX analysis confirmed that Al, O, and V were the major chemical constituents of V2O5/Al2O3. Loading of V2O5 was further confirmed by digestion of the sample. Based on the calibration curve, the concentration of the stock solution was found to be 31.9 ppm which correlated to the 10 wt% loading of V2O5 on to Al2O3 and was consistent with the results of our experiment (Fig. S7, ESI†). This systematic characterization of V2O5/Al2O3 was used in heterogeneous catalysis in combination with TBHP and CHP as the oxidant. Although other heterogeneous catalyst combinations such as Ti-silicate, MoO3/Al2O3, WO3/Al2O3, WO3/Ti-silicate, MoO3–WO3/Al2O3, V2O5/TiO2 were investigated, V2O5/Al2O3 showed the most promising results in desulfurization (Table T5, ESI†). We anticipate that a high surface area, porosity in combination with the Lewis acidity of the catalyst could be a plausible reason for such a phenomenon.41,42 Therefore, V2O5/Al2O3 was chosen for the optimization of the reaction conditions. With 5 wt% of the V2O5/Al2O3 catalyst and 30% (v/v) of TBHP/CHP as oxidant, the desired desulfurization was achieved (69.1% with TBHP and 73.2% with CHP). With the increase in the amount of oxidant, the degree of desulfurization was increased as expected.34 This could be attributed to the optimum oxygen requirement for the conversion of sulfurs to sulfur oxides and sulfones. When the oxidant amount was reduced to 25% (v/v) and 20% (v/v), the degrees of desulfurization were also reduced to 55.5%, 52.4%, and 69.5%, 66.1%, respectively for TBHP and CHP. However, to match the specification of the IMO-2020 grade residual oil, at least 30% (v/v) of oxidant was required, because of the 1.55 wt% of sulfur in the feed. Since our desired sulfur specification (<0.5 wt%) was already achieved at 30% (v/v) CHP concentration, hence we judiciously maintained the oxidant concentration at 30% (v/v). Although TBHP and CHP could both be used as oxidants, the degree of desulfurization was higher in the presence of CHP. Therefore, CHP was selected as the oxidant for rest of the optimization experiments. On a similar note, considering the low-cost option of H2O2, the ODS reactions were conducted with H2O2 and mixed oxidant (H2O2 + CHP) under identical reaction conditions. However, we were unable to separate the desulfurized residual fuel oil after the solvent extraction step. The desulfurization results with TBHP and CHP are summarized in Table 1 and Fig. 4a, respectively.
O and V–O–V stretching at 1019 cm−1 and 505 cm−1 also indicated the formation of V2O5 on the Al2O3 (Fig. S2, ESI†). Prior to the evaluation of the surface area, the thermal stability of V2O5/Al2O3 was measured using TGA. The TGA analysis showed that the V2O5/Al2O3 was stable up to 800 °C and only ∼13% weight loss observed. From all the results obtained, ∼9% weight loss up to 150 °C signified the removal of moisture and desorbed water molecules from the surface of the catalyst (Fig. S4, ESI†). The Al2O3 support was found to be porous in nature with a BET surface area of 279.5 m2 g−1, whereas the V2O5/Al2O3 showed a BET surface area of 228.9 m2 g−1 (Fig. 3a). A significant reduction in surface area was attributed to the formation of V2O5 on to the support (Fig. 3a). At the same time the BJH pore diameter of V2O5/Al2O3 showed an average pore diameter of 4.54 nm (Fig. S3, ESI†). Formation of V2O5 on Al2O3 was further supported by the analysis of the SEM image. The V2O5/Al2O3 SEM image revealed the rod shaped particles of V2O5 (Fig. 3b and Fig. S6, ESI†), which was distinctly different when compared to the surface of pristine Al2O3 (Fig. S5, ESI†). Meanwhile the EDX analysis confirmed that Al, O, and V were the major chemical constituents of V2O5/Al2O3. Loading of V2O5 was further confirmed by digestion of the sample. Based on the calibration curve, the concentration of the stock solution was found to be 31.9 ppm which correlated to the 10 wt% loading of V2O5 on to Al2O3 and was consistent with the results of our experiment (Fig. S7, ESI†). This systematic characterization of V2O5/Al2O3 was used in heterogeneous catalysis in combination with TBHP and CHP as the oxidant. Although other heterogeneous catalyst combinations such as Ti-silicate, MoO3/Al2O3, WO3/Al2O3, WO3/Ti-silicate, MoO3–WO3/Al2O3, V2O5/TiO2 were investigated, V2O5/Al2O3 showed the most promising results in desulfurization (Table T5, ESI†). We anticipate that a high surface area, porosity in combination with the Lewis acidity of the catalyst could be a plausible reason for such a phenomenon.41,42 Therefore, V2O5/Al2O3 was chosen for the optimization of the reaction conditions. With 5 wt% of the V2O5/Al2O3 catalyst and 30% (v/v) of TBHP/CHP as oxidant, the desired desulfurization was achieved (69.1% with TBHP and 73.2% with CHP). With the increase in the amount of oxidant, the degree of desulfurization was increased as expected.34 This could be attributed to the optimum oxygen requirement for the conversion of sulfurs to sulfur oxides and sulfones. When the oxidant amount was reduced to 25% (v/v) and 20% (v/v), the degrees of desulfurization were also reduced to 55.5%, 52.4%, and 69.5%, 66.1%, respectively for TBHP and CHP. However, to match the specification of the IMO-2020 grade residual oil, at least 30% (v/v) of oxidant was required, because of the 1.55 wt% of sulfur in the feed. Since our desired sulfur specification (<0.5 wt%) was already achieved at 30% (v/v) CHP concentration, hence we judiciously maintained the oxidant concentration at 30% (v/v). Although TBHP and CHP could both be used as oxidants, the degree of desulfurization was higher in the presence of CHP. Therefore, CHP was selected as the oxidant for rest of the optimization experiments. On a similar note, considering the low-cost option of H2O2, the ODS reactions were conducted with H2O2 and mixed oxidant (H2O2 + CHP) under identical reaction conditions. However, we were unable to separate the desulfurized residual fuel oil after the solvent extraction step. The desulfurization results with TBHP and CHP are summarized in Table 1 and Fig. 4a, respectively.
 | ||
| Fig. 3 (a) Comparison of the BET surface area between Al2O3 and V2O5/Al2O3. (b) The SEM image of V2O5/Al2O3. | ||
| Sr. No | Oxidant | Amount of oxidant %, (v/v) | Desulfurization % |
|---|---|---|---|
| 1 | TBHP | 20 | 52.4 |
| 2 | TBHP | 25 | 55.5 |
| 3 | TBHP | 30 | 69.1 |
| 4 | CHP | 20 | 66.1 |
| 5 | CHP | 25 | 69.5 |
| 6 | CHP | 30 | 73.2 |
A brief kinetic study was also carried out with respect to time, where the catalyst (V2O5/Al2O3) and CHP amounts was kept fixed at 5 wt% and 30% (v/v), respectively. Meanwhile, the ODS reaction was carried out at two different temperatures (60 °C and 80 °C).34 The reaction mixture was removed after 0.5 h, 1 h, 1.5 h, 2 h and 2.5 h and the oxidized sulfoxides or sulfones were extracted using MeOH. The final product was subjected to sulfur content measurement following the ASTM D5453 standard (for further details see Section 1, ESI†) method to check the degree of desulfurization. Our results showed that, the desulfurization rate was increased with respect to time as expected, and 2 h was the optimum time for this ODS reaction. In addition, 80 °C was found to be the most suitable temperature between the two used, because the oxidation kinetics were faster at a higher temperature (the required oxygen was generated easily for ODS), and greater desulfurization was achieved.34 A room temperature or low temperature reaction (below 60 °C) was therefore excluded, as faster oxidation kinetics were found at slightly elevated temperatures. Meanwhile, temperatures above 80 °C were avoided because of the thermal decomposition of CHP near 100 °C. The effect of time and temperature is shown in Fig. 4b. The final step was comprised of extraction of sulfoxides or sulfones in the presence of polar solvents such as MeOH by phase separation. At this point it is worth mentioning that the catalyst could be reused, and the solvent could be recycled as well. In this regard, the catalyst was washed thoroughly with toluene, dried and reused. During the reaction, the catalyst showed good activity and retained its performance even after three catalytic cycles (Fig. 4c). The amount of catalyst and pressure were the two other important parameters which were varied in the course of our process. Although the amount of catalyst has an impact on desulfurization, the effect of pressure was insignificant (Table T4 and Fig. S8, ESI†). Use of 5 wt% of the catalyst demonstrated greater desulfurization compared to use of 2.5 wt% of catalyst which was expected. To obtain further insights, a systematic kinetic study was carried out using DBT as a model compound at two different temperatures (see Section 1, ESI†).43,44 It was interesting to note that the DBT concentration was reduced gradually with respect to time (Fig. S9 and S10, ESI†) and the kinetics were faster at 80 °C than at 60 °C (Fig. S11, ESI†). In the presence of V2O5/Al2O3 (catalyst) and CHP (oxidant), DBT was converted to sulfone or sulfoxide. The sulfoxide or sulfone formed was extracted in ACN which resulted in the reduction of the DBT concentration in heptane phase (revealed from the UV-Vis spectra). It is worth noting that the results of the kinetic study with the model compound agreed with those of the kinetic study with the residual oil feed, where a higher temperature yielded greater desulfurization.
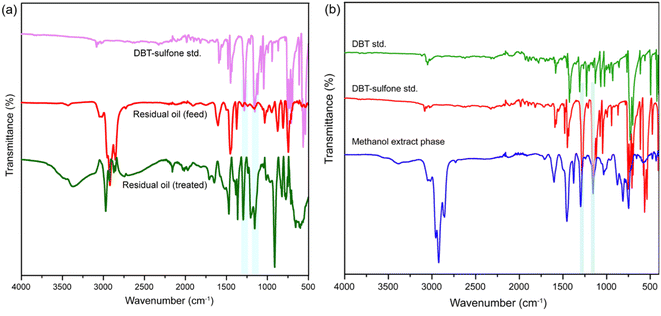
It was evident from papers found in the literature, that the refractory sulfur compounds were oxidized to sulfoxides and sulfones in the presence of catalysts and oxidants. Sulfoxides and sulfones being the more polar counterpart are easily extracted in polar solvents. To verify the mechanism in the presence of V2O5/Al2O3 as the catalyst, and CHP as the oxidant, the feed residual oil and the treated residual oil were isolated and subjected to direct FTIR analysis without solvent extraction. As a comparison the same experiment was also carried out using a DBT-sulfone standard. A characteristic sulfone S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency was noted in both the oxidized feed and the DBT-sulfone standard at 1290 cm−1 and 1150 cm−1, respectively (Fig. 5a).45 In contrast, such a type of characteristic sulfone stretching frequency was completely absent in the feed residual oil (Fig. 5a). This confirmed the formation of sulfone in the treated feed using V2O5/Al2O3 as a catalyst, and CHP as an oxidant in the ODS reaction. Similarly, the oxidized refinery grade residual oil was subjected to a solvent extraction step in the presence of MeOH and the MeOH phase was analyzed by FTIR. The FTIR spectra of the extracted compounds in the MeOH phase clearly demonstrated the presence of characteristic sulfone stretching frequency at 1290 cm−1 and 1150 cm−1 and this was consistent with DBT-sulfone standard.45 However, such characteristic sulfone stretching was absent with the DBT standard (Fig. 5b). Therefore, the results of both the FTIR analyses suggested the formation of sulfone had occurred in the ODS reaction and subsequent extraction of the sulfone in polar solvent MeOH produced the desulfurized residual oil. The FTIR analysis was further supported by the results of the gas chromatography with sulfur chemiluminescent detection (GC-SCD) analysis (Fig. S12, ESI†). The GC-SCD analysis of residual oil feed showed no signature peak of sulfones, whereas the oxidized residual oil (without an extraction step) showed a signature sulfone peak and this was consistent with the DBT-sulfone GC-SCD peak as well (Fig. S12, ESI†). Collectively, these results illustrated that, in the presence of V2O5/Al2O3 and CHP, the refractory sulfur compounds were oxidized to sulfones and were subsequently extracted by polar solvents.
O stretching frequency was noted in both the oxidized feed and the DBT-sulfone standard at 1290 cm−1 and 1150 cm−1, respectively (Fig. 5a).45 In contrast, such a type of characteristic sulfone stretching frequency was completely absent in the feed residual oil (Fig. 5a). This confirmed the formation of sulfone in the treated feed using V2O5/Al2O3 as a catalyst, and CHP as an oxidant in the ODS reaction. Similarly, the oxidized refinery grade residual oil was subjected to a solvent extraction step in the presence of MeOH and the MeOH phase was analyzed by FTIR. The FTIR spectra of the extracted compounds in the MeOH phase clearly demonstrated the presence of characteristic sulfone stretching frequency at 1290 cm−1 and 1150 cm−1 and this was consistent with DBT-sulfone standard.45 However, such characteristic sulfone stretching was absent with the DBT standard (Fig. 5b). Therefore, the results of both the FTIR analyses suggested the formation of sulfone had occurred in the ODS reaction and subsequent extraction of the sulfone in polar solvent MeOH produced the desulfurized residual oil. The FTIR analysis was further supported by the results of the gas chromatography with sulfur chemiluminescent detection (GC-SCD) analysis (Fig. S12, ESI†). The GC-SCD analysis of residual oil feed showed no signature peak of sulfones, whereas the oxidized residual oil (without an extraction step) showed a signature sulfone peak and this was consistent with the DBT-sulfone GC-SCD peak as well (Fig. S12, ESI†). Collectively, these results illustrated that, in the presence of V2O5/Al2O3 and CHP, the refractory sulfur compounds were oxidized to sulfones and were subsequently extracted by polar solvents.
It was extremely important to compare the properties of the desulfurized residual oil with the IMO-2020 grade residual oil. Although the major specification was, the upper limit of sulfur in the desulfurized fuel, but this was not typically restricted to the sulfur specification only. There were other parameters such as pour point, TAN and CCR (%) which need to be monitored to satisfy the required specification.46 Pour point was used to determine the flowability of a fuel, below that temperature, the fuel lost its flow property. The lower the pour point value is, the greater the flow property of the fuel oil would be. As shown in Table 2, that the standard IMO-2020 grade residual oil has a pour point of 30 °C, and the desulfurized refinery grade residual oil has pour points of 3 °C and 9 °C after treatment with TBHP and CHP, respectively. Therefore, the flow property of desulfurized residual fuel oil could be restored well below the pour point mentioned in the IMO-2020 grade of residual oil. This showed the promising potential for using desulfurized residual oil in relatively low temperature regions as well. The TAN dictates the acidity of the residual fuel oil and is another important quality measurement parameter. The TAN indicated that naphthenic acid was present in the sample and it was primarily related to high temperature naphthenic acid corrosion.47 Although the permissible TAN limit of the IMO-2020 grade residual oil was restricted to 2.5, the TAN value of desulfurized refinery grade residual was limited to 0.87 and 1.89 only when TBHP and CHP, respectively, were used as oxidant in the heterogeneous catalysis. Our results justified that unwanted naphthenic acid corrosion could be reduced in ODS in the presence of a heterogeneous catalyst. This was in sharp contrast with the results obtained in homogeneous catalysis where the resultant TAN value of the desulfurized refinery grade fuel oil was well above the standard value of 2.5, which could induce naphthenic acid corrosion. Lastly the CCR (%) showed the tendency of a fuel oil to form coke after being subjected to evaporation and pyrolysis. The CCR value of standard IMO-2020 grade residual oil is 18%, and the CCR of the desulfurized product also lies within the standard range (Table 2). Hence, unwanted coking and choking problems could be avoided with the desulfurized product after the ODS reaction. All these parameters are summarized in Table 2. It was also evident from the literature that the presence of oxygen in excess could impart unwanted corrosion or deposition by choking (enhanced CCR%). Therefore, an ODS reaction was carried out using a DBT standard with our heterogeneous catalyst (V2O5/Al2O3 as catalyst and CHP as oxidant). Under the identical reaction conditions, DBT was oxidized to DBT-sulfone. Neither DBT-sulfone, nor the reaction mixture contributed to a significant CCR value (Table T6, ESI†). Collectively these results indicated the promising potential of the desulfurized refinery grade residual oil, obtained via the ODS process, to be used as IMO-2020 grade residual oil.
| Parameter | Residual oil standard (IMO-2020) | Feed used for study | Desulfurized feed | |
|---|---|---|---|---|
| TBHP | CHP | |||
| Density (kg m−3) | 0.991–1.01 | 1.07 | 1.0 | 1.0 |
| Pour point (°C) | 30 | NA | 3 | 9 |
| TAN | 2.5 | 0 | 0.87 | 1.89 |
| CCR (%) | 18–20 | 7.1 | 15.33 | 17.04 |
| S (%) | 0.5 | 1.55 | 0.52 | 0.41 |
From industrial perspective, recycling is an extremely important phenomenon in terms of the process as well as the economic point of view. Recycling of catalyst and solvent was established earlier in the experimental work, where the catalyst restored its catalytic activity even after three cycles. Although the catalyst was recyclable, the major cost contribution would come from the oxidant, CHP. In this context, we anticipated that the recycling of CHP would promote the economic viability of the process. The primary role of CHP was to supply oxygen for the oxidation of refractory sulfur compounds to sulfoxides and sulfones. After ODS, the refractory sulfur compounds were oxidized to sulfones and sulfoxides, whereas the CHP was converted to cumyl alcohol, α-methyl styrene (AMS) and cumene.48 In addition, there could be minor amount of unreacted CHP. Altogether cumyl alcohol, AMS, and cumene could be recycled to CHP via a two-step process49 which included: (a) catalytic hydrogenation using a RANEY®/Ni catalyst at 150 °C, 20 bar, (b) followed by oxidation in the presence of O2 at 150 °C. The CHP recycling process is shown schematically in Fig. 6a.
In order to quantify the recycling of CHP, the reaction mixture was subjected to an ASTM D1160 distillation study up to a 215 °C cut, because all the recyclable components have boiling points less than 215 °C. Once the distillation was completed, the samples were subjected to GC-MS analysis. Based on the GC-MS results, it was confirmed that 80% of the CHP was recyclable from each batch process (Fig. 6b and Table 3). Considering the CHP regeneration as 80%, 36% of fresh (top-up) CHP was required for each batch per ton of desulfurized residual oil production. Based on these experimental results a brief techno-economic evaluation was carried out (Section 7, ESI†). Refinery grade residual oil is typically available at USD 350 per ton (average) and methanol being the low boiling solvent, could be recovered up to 95% with a minor handling loss. Our leading results concluded that about 36% of fresh CHP was required in each step. Considering the 95% solvent and 80% oxidant recycling, cash cost per ton of IMO-2020 grade residual oil was found to be about USD 697 per ton. Meanwhile the very low sulfur fuel oil (VLSFO) was available at an average price of USD 900 per ton.50 Considering other major expenses, a significant product margin of USD 203 per ton could be attained with EBITDA 51 MMUSD/year and a payback period of three years (Table T7 in Section 7, ESI†). This profit margin is lucrative for the oxidative desulfurization of residual oil to produce IMO grade residual oil, however further fine tuning could be done when considering the miscellaneous factors. Currently the desulfurization techniques that have been explored, and that are available to the shipping industries are installation of scrubbers, catastrophic blending and hydrodesulfurization to reduce the sulfur content to meet the specification of the IMO-2020 grade residual oil. However, high capital cost, higher payback period associated with the installation of scrubbers and high-temperature/pressure/hydrogen consumption in hydrodesulfurization is the major set-back for their practical implications for small shipping industries.4,7,8,51 In contrast, the ODS process is a low-temperature/pressure-based process and also economically feasible to produce the IMO-2020 grade residual fuel oil. Judicious recycling and a good profit margin could make ODS a potential competitor to the currently existing technologies to produce IMO-2020 grade residual oil for the shipping industries.
| Component | Residual oil phase | |
|---|---|---|
| Area (%) | Recyclable | |
| 1-Methylethyl benzene (cumene) | 5.63 | Yes |
| Phenol | 1.35 | No |
| α-Methyl styrene | 63.02 | Yes |
| Acetone | 1.42 | No |
| α,α-Dimethyl benzene methanol (cumyl alcohol) | 11.39 | Yes |
| Acetophenone | 6.51 | No |
| Others | 10.68 | No |
| Recyclable amount of CHP | 80% | |
Conclusions
In summary, we present an oxidative desulfurization process of residual oil to produce IMO-2020 grade residual oil using V2O5/Al2O3 as a catalyst and CHP as an oxidant. Although homogeneous catalysis was quite effective in desulfurization, the separation of the catalyst and the low pH of the reaction mixture in the post-reaction, were some of its intrinsic drawbacks. Meanwhile use of a heterogeneous catalyst was able to overcome these challenges. The unique combination resulted in a good degree of desulfurization and the resultant desulfurized residual oil was compliant with the IMO-2020 residual oil grade standard. In addition, the catalyst, the oxidizing agent and the solvent were all recyclable. Moreover ODS process was mostly restricted to model compounds, therefore valuable insights required for the process development are scarcely available. Moreover, the ODS processing of heavier fuel oil is mostly restricted to homogeneous catalysis because of its simple process, which rarely addressed its drawbacks. Therefore, we anticipated that using real residual oil as feed, and a heterogeneous catalyst system could be a potential solution to address these issues. Our systematic analysis revealed that, the heterogeneous catalyst and oxidant combination was not only effective in desulfurization of the residual oil feed, but it could also be recycled. Although economic viability was equally important from an industrial point of view, which none of the literature has addressed so far. Thanks to CHP, with a fair potential for recycling, a significant profit margin made the overall process economically viable. In an ideal case, the oxidant should be recycled completely, but practically 80% of CHP could be recycled, leaving scope for further improvement. Although installation of scrubbers, catastrophic blending and hydrodesulfurization are some of the currently used techniques for IMO-2020 grade residual oil production, but the process still has challenges to overcome. At this stage, it is worth mentioning that a significant profit margin in the ODS process makes it a potential competitor for all the existing processes. We believe that systematic evaluation and new insights into the oxidative desulfurization process could be useful for future developments to produce IMO-2020 compliant residual oil.Author contributions
Shouvik Mitra: conceptualization, experiments, investigation, methodology development, writing – original draft and editing. Surya Murali Racha: experiments and rechecking. Biswajit Shown: valuable discussions, and review of work and manuscript. Sukumar Mandal: valuable suggestions and review. Asit Kumar Das: valuable suggestions and final review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Reliance Industries Limited for funding and the experimental facility. The authors thank Dr Bikash Garai (NYU, Abu Dhabi) for the SEM and ICP-MS analysis.Notes and references
- A. Rajendran, T. Y. Cui, H. X. Fan, Z. F. Yang, J. Feng and W. Y. Li, J. Mater. Chem. A, 2020, 8, 2246 RSC.
- S. Houda, C. Lancelot, P. Blanchard, L. Poinel and C. Lamonier, Catalysts, 2018, 8, 344 CrossRef.
- A. Marafi, H. Albazzaz and M. S. Rana, Catal. Today, 2019, 329, 125 CrossRef CAS.
- V. C. Srivastava, RSC Adv., 2012, 2, 759 RSC.
- W. L. Fang, Inventory of U. S. Greenhouse Gas Emissions and Sinks, 1990–2003, Clean Air Markets Division, 2004 Search PubMed.
- L. Hao, M. J. Hurlock, X. Li, G. Ding, K. W. Kriegsman, X. Guo and Q. Zhang, Catal. Today, 2020, 350, 64 CrossRef CAS.
- D. D. Whitehurst, I. Isoda and I. Mochida, Adv. Catal., 1998, 42, 345 CAS.
- K. Chan, J. Jung, J. Lee, B. Sang, C. Kyungil and H. M. Sang, Appl. Catal., A, 2000, 200, 233 CrossRef.
- R. Javadli and A. D. Klerk, Appl. Petrochem. Res., 2012, 1, 3 CrossRef CAS.
- J. F. Ford, T. A. Rayne, D. G. Adiington, US Pat., US3341448, 1967, pp. 1–3 Search PubMed.
- M. N. Hossain, H. C. Park and H. S. Choi, Catalysts, 2019, 9, 229 CrossRef.
- (a) S. Otsuki, T. Nonaka, N. Takashima, W. Qian, A. Ishihara, T. Imai and T. Kabe, Energy Fuels, 2000, 14, 1232 CrossRef CAS; (b) L. Chen, J. T. Ren and Z. Y. Yuan, Chem. Eng. J., 2022, 450, 138330 CrossRef CAS; (c) L. Chen and Z. Y. Yuan, J. Ind. Eng. Chem., 2022, 108, 1 CrossRef CAS; (d) L. Chen, Z. P. Hu, J. T. Ren, Z. Wang and Z. Y. Yuan, Micro. Meso. Mater., 2021, 315, 110921 CrossRef CAS; (e) L. Chen, J. T. Ren and Z. Y. Yuan, Chem. Eng. J., 2020, 387, 124164 CrossRef CAS.
- S. W. Li, W. Wang and J. S. Zhao, Sustainable Energy Fuels, 2020, 4, 2422 RSC.
- E. Rafiee and E. N. Nobakht, J. Mol. Catal. A Chem., 2015, 398, 17 CrossRef CAS.
- H. Lu, Y. Zhang, Z. Jiang and C. Li, Green Chem., 2010, 12, 1954 RSC.
- B. Bertleff, R. Goebel, J. Claubnitzer, W. Korth, M. Skiborowski, P. Wasserscheid, A. Jess and J. Albert, ChemCatChem, 2018, 10, 4602 CrossRef CAS.
- P. Sikarwar, U. K. A. Kumar, V. Gosu and V. Subbaramaiah, J. Environ. Chem. Eng., 2018, 6, 1736 CrossRef CAS.
- W. A. W. A. Bakar, R. Ali, A. A. A. Kadir and W. N. A. W. Mokhtar, Fuel Process. Technol., 2012, 101, 78 CrossRef CAS.
- A. Akbari, M. Omidkhah and J. Towfighi Darian, Ultrason. Sonochem., 2015, 23, 231 CrossRef CAS.
- J. L. García-Gutiérrez, G. A. Fuentes, M. E. Hernández-Terán, P. García, F. Murrieta-Guevara and F. Jiménez-Cruz, Appl. Catal., A, 2008, 334, 366 CrossRef.
- C. Yang, K. Zhao, Y. Cheng, G. Zeng, M. Zhang, J. Shao and L. Lu, Sep. Purif. Technol., 2016, 163, 153 CrossRef CAS.
- S. Subhan, A. Rahman, M. Yaseen, H. U. Rashid, M. Ishaq, M. Sahibzada and Z. Tong, Fuel, 2019, 237, 793 CrossRef CAS.
- H. Naseri, G. Mazloom, A. Akbari and F. Banisharif, Microporous Mesoporous Mater., 2021, 325, 111341 CrossRef CAS.
- I. S. Tomskii, M. V. Vishnetskaya, P. A. Vakhrushin and L. A. Tomskaya, Pet. Chem., 2017, 57, 908 CrossRef CAS.
- L. Cedeño, H. Gomez-Bernal, A. Fraustro-Cuevas, H. D. Guerra-Gomez and R. Cuevas-García, Catal. Today, 2008, 133, 244 CrossRef.
- M. C. Capel-Sanchez, P. Perez-Presas, J. M. Campos-Martin and J. L. G. Fierro, Catal. Today, 2010, 157, 390 CrossRef CAS.
- Z. Long, C. Yang, G. Zeng, L. Peng, C. Dai and H. He, Fuel, 2014, 130, 19 CrossRef CAS.
- V. Dumont, L. Oliviero, F. Maugé and M. Houalla, Catal. Today, 2008, 130, 195 CrossRef CAS.
- P. Zhang, L. Kang, M. Zhu and B. Dai, Sustainable Energy Fuels, 2020, 4, 4293 RSC.
- Z. Jiang, H. Lü, Y. Zhang and C. Li, Chin, J. Catal., 2011, 32, 707 CrossRef CAS.
- J. Xiao, L. Wu, Y. Wu, B. Liu, L. Dai, Z. Li, Q. Xia and H. Xi, Appl. Energy, 2014, 113, 78 CrossRef CAS.
- Y. Jeon, S. W. Row, A. Dorjgotov, S. D. Lee, K. Oh and Y. G. Shul, Korean J. Chem. Eng., 2013, 30, 1566 CrossRef CAS.
- A. Farshi and P. Shiralizadeh, Pet. Coal, 2015, 57, 1337 Search PubMed.
- R. L. Al Otaibi, D. Liu, X. Hou, L. Song, Q. Li, M. Li, H. O. Almigrin and Z. Yan, Appl. Petrochem. Res., 2015, 5, 355 CrossRef CAS PubMed.
- Y. Tian, Y. Yao, Y. Zhi, L. Yan and S. Lu, Energy Fuels, 2015, 29, 618 CrossRef CAS.
- K. M. Dooley, D. Liu, A. M. Madrid and F. C. Knopf, Appl. Catal., A, 2013, 468, 143 CrossRef CAS.
- Q. Fang, J. Wang, W. H. Wang and M. Bao, RSC Adv., 2019, 9, 21473 RSC.
- A. Ishihara, D. Wang, F. Dumeignil, H. Amano, E. W. Qian and T. Kabe, Appl. Catal., A, 2005, 279, 279 CrossRef CAS.
- A. S. Ogunlaja, R. S. Walmsley, C. D. Sautoy, N. Torto and Z. R. Tshentul, Energy Fuels, 2013, 27, 7714 CrossRef CAS.
- K. E. Litz, J. L. Vreeland, US Pat., US20120022272A1, 2012, pp. 1–9 Search PubMed.
- W. A. W. A. Bakar, R. Ali, A. A. A. Kadir and W. N. A. W. Mokhtar, Fuel Process. Technol., 2012, 101, 78 CrossRef CAS.
- W. N. W. Abdullah, R. Ali and W. A. W. A. Bakar, J. Taiwan Inst. Chem. Eng., 2016, 58, 344 CrossRef CAS.
- U. Arellano, J. A. Wang, M. T. Timko, L. F. Chen, S. P. Paredes Carrera, M. Asomoza, O. A. González Vargas and M. E. Llanos, Fuel, 2014, 126, 16 CrossRef CAS.
- G. Abdi, M. Ashok Kumar and A. Alizadeh, Fuel, 2017, 210, 639 CrossRef CAS.
- V. Toteva, A. Goergiev and L. Topalova, Fuel Process. Technol., 2009, 90, 965 CrossRef CAS.
- B. Klussmann, Hydrocarbon Processing, 2021 Search PubMed.
- J. Yu, L. Jiang and F. Gan, Anti-Corros. Methods Mater., 2008, 55, 257 CrossRef CAS.
- H. Y. Hou, C. M. Shu and T. L. Tsai, J. Hazard. Mater., 2008, 152, 1214 CrossRef CAS PubMed.
- Process Principles, Allied/UOP Phenol Process with AMS Hydrogenation Technology Manual.
- https://shipandbunker.com/prices/av/global/av-glb-global-average-bunker-priceaccessed on 16th September 2022.
- R. Bergqvist, M. Turesson and A. Weddmark, Eur. Transp. Res. Rev., 2015, 7, 7032 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2se01363k |
| This journal is © The Royal Society of Chemistry 2023 |