Investigation of commercial aluminum alloys as anode materials for alkaline aluminum–air batteries†
Tao
Wang
a,
Yuan
Zhu
a,
Yifan
Li
a,
Kai
Yang
b,
Wenyi
Lu
c,
Ke
Peng
*a and
Zhongliang
Tian
 *a
*a
aSchool of Metallurgy and Environment, Central South University, Changsha 410083, China
bSchool of Metallurgical Engineering, Xi'an University of Architecture and Technology, Xi'an, Shaanxi 710055, China
cBinzhou Institute of Technology, Weiqiao-UCAS Science and Technology Park, Binzhou 256606, Shandong Province, China
First published on 25th November 2022
Abstract
Metal Al is an attractive energy carrier in Al-based batteries with promising recyclability and sustainability in alkaline solutions. However, finding applicable Al anode materials for alkaline Al–air batteries is difficult. In this study, commercial Al alloys are employed as anode materials for Al–air batteries. The self-corrosion behavior, electrochemical properties, composition, and microstructure of the alloys and their relationships are systematically investigated using a hydrogen collection method, electrochemical measurements, and multiple characterization studies. The results show that the 6061 Al alloy (A6061) presents a low hydrogen evolution rate and superior electrochemical performance. The hydrogen evolution reaction is found to be inhibited by increasing the current density. The A6061 anode presents a high anodic efficiency of 89.28%, a specific capacity of 2660.69 mA h g−1, and an energy density of 2119.09 W h kg−1 at 80 mA cm−2. The exceptional performance is due to its crystallographic orientation which is beneficial for Al dissolution and promotes electrochemical activity. This study could provide a better understanding of commercial Al alloy anodes and is conducive to optimizing the Al anode structure.
1 Introduction
With the proposal of the “carbon peaking and carbon neutrality” concept, the industrial era dominated by fossil fuels is destined to be changed.1–3 Reforming the energy system and developing low-carbon industries are of great significance. It is essential to find sustainable, green, as well as efficient energy conversion and storage technologies.4,5 Under this background, the exploitation of various battery technologies is in full swing worldwide.6–12 Aqueous metal–air batteries are considered promising new energy devices, in which the oxygen in the air and metals such as Mg, Zn, Al, and Fe served as fuels.13–20 Among them, metal Al stands out due to its merits of high abundance in the Earth's crust, low cost, and ultra-high theoretical specific capacity (2.9 Ah kg−1) and energy density (8100 W h kg−1).21–24 Hence, aqueous Al–air batteries have been regarded as excellent candidates for underwater power sources, portable energy storage devices, and emergency power supplies.25–28Currently, finding suitable Al anodes with low cost and high performance is urgent.23,29 High-grade Al (≥99.999%) or modified Al alloys are still the key anode materials for Al–air batteries because of the suppressed self-corrosion and parasitic hydrogen evolution reaction (HER) in aqueous alkaline solutions.30–33 However, the cost increases due to the expensive high-grade Al (20–100 times more expensive than primary Al). At the same time, the preparation (alloying, remelting, and processing) of modified Al alloys is complicated, costly, and energy-guzzling.29,30,34–38 Therefore, it is difficult for Al–air batteries to obtain large-scale commercialization at present.
Commercial Al alloys have large outputs and low prices and have been widely used in the construction, transportation, and machinery industries.39–41 They contain various elements and have been carefully heat-treated and processed. More importantly, Al chemistry in aqueous alkaline Al–air systems endows them with recyclability and sustainability, as illustrated in Scheme 1. The product of the anodic reaction is Al(OH)4−, which could be directly recycled to produce Al2O3. After further electrolysis and processing using green and renewable solar or wind energy, primary Al and commercial Al alloys could be obtained. They could be applied in various industries. And when retired, these commercial Al alloys from cars, aircraft, and constructions could be used as anode materials for Al–air batteries to realize energy conversion. Then the cycle repeats. Compared with traditional remelting and hot extrusion methods, this green conversion process may solve the problem that retired Al alloys are difficult to reuse with high quality.42,43 Therefore, it is attractive and meaningful to employ commercial Al alloys as anode materials. Understanding the self-corrosion behavior and battery performance of these alloy anodes is basic and vital. Although some previous studies have reported the application of certain commercial Al alloy anodes, the detailed battery performance and the relationship between performance and the microstructure were not carefully discussed.44–46
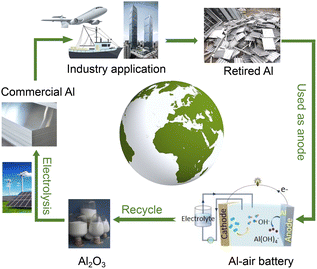 | ||
| Scheme 1 Schematic illustration of the power and resource conversion processes of aluminum bridged by alkaline Al–air batteries. | ||
Commercial Al alloys are commonly classified into different series based on the alloying elements, such as 2xxx (Al–Cu), 3xxx (Al–Mn), 4xxx (Al–Si), 5xxx (Al–Mg), 6xxx (Al–Mg–Si), and 7xxx (Al–Zn). Among the alloying elements, Mg has been reported to be beneficial for the electrochemical properties of Al anodes.47–49 As representative Mg-rich Al alloys, commercial 5052 and 6061 Al alloys (A5052, A6061) have wide applications in industries and display excellent corrosion resistance. They are expected to display high Al utilization. Herein, in this work, we investigated the corrosion behavior, discharge performance, and microstructure of the A5052 and A6061 anodes in detail. A commercially pure Al (A1060) alloy was used for comparison. The results show that the A6061 anode presents a low hydrogen evolution rate and superior electrochemical performance due to its distinct crystallographic orientation. This study would help understand commercial Al alloy anodes and provide guidance for structure optimization of Al anodes for Al–air batteries.
2 Experimental
2.1 Materials
The commercial Al alloys and the air cathodes (MnxOy as the catalysts) were purchased from Wuxi Al-Top New Metal Material Co., Ltd (China) and Changzhou Young-tech New Energy Science & Technology Co., Ltd (China), respectively. Sodium hydroxide (NaOH, analytical grade) was purchased from Sinopharm Chemical Reagent Co., Ltd. The NaOH solution (4 mol L−1) served as the electrolyte.2.2 Self-corrosion tests
The rates of hydrogen evolution and weight loss were tested to illustrate the self-corrosion of different Al anodes. The drainage device is shown in Fig. S1.† The Al samples were cut into 20 mm × 20 mm × 2 mm specimens and sealed with epoxy resin to expose an area of 4 cm2. 80 ml of 4 mol per L NaOH solution in the reaction container was used and the volume of hydrogen was recorded every 5 min for 60 min. The weight of Al samples before and after immersion in the solution was recorded to calculate the weight loss.2.3 Characterization studies
The elemental compositions of the Al alloys were obtained by inductively coupled plasma optical emission spectrometry (ICP-OES, PerkinElmer 8300). The optical micrographs of the Al alloys were captured on an optical microscope (OM, OLYMPUS) and the grain size numbers were calculated by the cut-off method. The Al samples were cut into sheets with a size of 10 mm × 10 mm × 2 mm and sealed with epoxy resin. Before testing, the samples were mechanically polished with silicon carbide sandpaper (180, 1000 to 2000 grit), and then ultrasonically cleaned with ethanol and deionized water for 10 min. Then the samples were polished with diamond polishing paste to brighten the surface. Subsequently, the samples were corroded in Keller solution for 2 min and washed with ultra-pure water and alcohol. The morphologies of the Al surfaces after discharge were characterized using a scanning electron microscope (SEM, JEOL JSM-6701F).2.4 Electrochemical measurements
The open circuit potential curves, EIS spectrum, and Tafel polarization curves were measured using a standard three-electrode system on a CHI660 electrochemical workstation. The self-prepared Al electrode (10 mm × 10 mm) served as the working electrode, Hg/HgO was selected as the reference electrode, and platinum mesh (25 mm × 25 mm) was used as the counter electrode. The open-circuit potential was collected for 600 s. The EIS was carried out from 100 kHz to 0.1 Hz at a scan rate of 5 mV s−1 and the Tafel polarization process was conducted at 1 mV s−1 from −1.0 V to −1.6 V. An Al–air battery was constructed with an Al anode, air cathode and electrolyte chamber (Fig. S2†), in which the electrolyte (100 ml) was circulated at a flow rate of 10 ml min−1 with a pump. The reaction area of the electrodes was about 3 cm2. The galvanostatic discharge tests were conducted at different current densities (5–80 mA cm−2) using a battery discharge system (LAND, CT2001A) for 5 h. All the tests were conducted at room temperature.3 Results and discussion
3.1 Self-corrosion and electrochemical properties
In alkaline solutions, Al reacts with water to generate hydrogen gas. Generally, hydrogen is from two paths. One is the parasitic reaction on the Al anode because of the high activity of metal Al, as eqn (1) shows. Besides, hydrogen may also come from galvanic corrosion. In a galvanic corrosion system, water decomposes to generate hydrogen on the electrolyte/cathodic region interface, as shown in eqn (2). Meanwhile, the anodic Al is corroded, as shown in eqn (3).| Al + H2O + OH− → Al(OH)4− + H2↑ | (1) |
| 2H2O + 2e− → 2OH− + H2↑ | (2) |
| Al + 4OH− → Al(OH)4− + 3e− | (3) |
Both paths would lead to low Al utilization and low energy density.50–52 It is important to understand the HER and self-corrosion characteristics of commercial Al alloy anodes. We first collected the hydrogen gas generated from the alloys in 4 M NaOH solution and calculated the generation rates, as shown inFig. 1(a) and (b). The results show that the hydrogen gas volume is relatively large. The total hydrogen gas volume collected in 60 min for A1060 is 175.0 ml and the rate of HER is calculated to be 0.729 ml cm−2 min−1. The A1060 anode generates hydrogen quickly, indicating that it is corroded severely. For the A5052 and A6061 anodes, the self-corrosion is alleviated. The volumes are 141.3 and 92.3 ml, respectively, corresponding to HER rates of 0.589 and 0.384 ml cm−2 min−1. The self-corrosion of the Al alloy anodes was also tested by a weight-loss method, as Fig. S3† shows. Similarly, the results demonstrate that the self-corrosion of A1060 is more severe, with a weight loss rate of 0.777 mg cm−2 min−1.
 | ||
| Fig. 1 (a) Hydrogen evolution curves, (b) rate of HER, (c) open-circuit potential (OCP) curves, and (d) Tafel plots of the alloy anodes in 4 M NaOH solution. | ||
Fig. 1(c) presents the OCP curves and Tafel plots of the Al alloy anodes. Each OCP curve shows a similar trend. The OCP positively shifts in the beginning and maintains stability gradually. This is because of the breakage of the oxide layer on the Al anode and the accumulation of aluminum hydroxide products.29,53 The OCP values of the alloy anodes are shown in Table S1.† After testing for 600 s, the A6061 anode displays the most negative OCP of −1.417 V, while those of A5052 and A1060 are more positive (−1.292 and −1.261 V, respectively). The Tafel plots and the fitting corrosion potential (Ecorr) and corrosion current density (Icorr) of the alloy anodes are shown in Fig. 1(d) and Table S1.†Ecorr could reflect the electrochemical activity of anodic dissolution. The more negative Ecorr (−1.414 V) of the A6061 anode demonstrates its better electrochemical activity, which is favorable for promoting the battery performance.54Icorr is related to the content of cathodic impurity phases in the Al matrix. The Icorr of the A1060, A5052, and A6061 anodes are 21.1, 22.5, and 22.0 mA cm−2, respectively. The values are very close, which does not match the results of the HER tests and weight loss tests. Considering that A1060 is purer, it can be inferred that the impurity phases are not the only factor that influences the self-corrosion behavior of Al alloys. This would be further discussed later.
Electrochemical impedance spectroscopy (EIS) was also used to characterize the alloy anodes. Fig. 2 shows the obtained Nyquist plots and the fitted equivalent circuit, which include solution resistance (Rs), uncompensated resistance (R), inductance (L), charge transfer resistance (Rct), and constant phase element (CPE). The capacitive reactance arc in the high-frequency region is related to the redox reaction of Al → Al+.55 This stage plays a crucial role in the whole anodic reaction process. Therefore, a higher charge-transfer resistance (Rct,1) means better corrosion resistance. The fitting results in Table S2† show that the A6061 anode displays higher charge-transfer resistance (0.53 Ω), indicating its better corrosion resistance. This is consistent with the Tafel plot results.56 Meanwhile, the curves of impedance modulus vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f (Fig. 2b) and the phase angle vs. log
f (Fig. 2b) and the phase angle vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f plots (Fig. 2c) show that the A6061 anode has the highest impedance value, peak values, and full width. This also indicates that the A6061 alloy anode has better corrosion resistance.57 In the medium-frequency region, the inductive arc (L1) is caused by the desorption of intermediate products, such as Al(OH)ads and OH−. At lower frequencies, the second capacitive reactance arc is ascribed to the fast complementary redox reaction of Al+ → Al3+.58 The second inductive arc (L2) for A5052 and A6061 at low frequency may be related to the formation of an impurity product film, as illustrated in Fig. S4.† The impurities could accumulate on the Al surface and influence the surface environment.
f plots (Fig. 2c) show that the A6061 anode has the highest impedance value, peak values, and full width. This also indicates that the A6061 alloy anode has better corrosion resistance.57 In the medium-frequency region, the inductive arc (L1) is caused by the desorption of intermediate products, such as Al(OH)ads and OH−. At lower frequencies, the second capacitive reactance arc is ascribed to the fast complementary redox reaction of Al+ → Al3+.58 The second inductive arc (L2) for A5052 and A6061 at low frequency may be related to the formation of an impurity product film, as illustrated in Fig. S4.† The impurities could accumulate on the Al surface and influence the surface environment.
3.2 Battery performance
To explore the battery performance, flow-based Al–air batteries were constructed. The galvanostatic discharge curves of the batteries at different current densities (from 10 to 80 mA cm−2) are shown in Fig. 3a–f. The results show that the batteries with A6061 alloy anodes display the highest voltages, and the working voltages with A1060 alloy anodes are the lowest. The high electrochemical activity of the A6061 alloy plays a key role in achieving high discharge voltage. Besides, the higher the current density is, the lower the battery voltage becomes. At low current densities (lower than 40 mA cm−2), first the discharge voltages of the Al alloy anodes decrease and then increase gradually, as the partially magnified discharge curves at 10 mA cm−2 show (Fig. S5†). Because of the high internal resistance caused by the long distance (15 mm) between the Al anode and the air cathode, the battery voltage drops immediately when the Al anodes start to discharge. As for A5052 and A6061, the voltages gradually increase due to the activation of the air cathode. Then the voltages get stable. But for A1060, the voltage keeps dropping for a while. This indicates that the surface passive film on A1060 is more stable and thicker, and thus more difficult to dissolve. Furthermore, the discharge curves fluctuate severely at high current densities of 60 and 80 mA cm−2. This instability could be ascribed to the imbalance between the rapid formation and separation of surface products.59Fig. 3g displays the battery voltage and power density of the alloy anodes at different discharge current densities (detailed data summarized in Tables S3–S5†). With the increase in discharge current density, the voltage decreases, while the power density increases. Generally, A6061 shows higher discharge voltage and power density due to its excellent electrochemical activity and good corrosion resistance. At 80 mA cm−2, though the power density of A6061 reaches 63.69 mW cm−2, the voltage decreases to 0.796 V. As Fig. 3h shows, the specific capacity of the A6061 anode increases from 213.06 mA h g−1 at 5 mA cm−2 to 2660.69 mA h g−1 (80 mA cm−2), which is an improvement in anode efficiency from 10.76% to 89.28%. Similarly, the specific capacities of the A1060 and A5052 anodes also increase at higher current densities. As discussed earlier, Al would be consumed during the discharge process and the self-corrosion reaction. Considering that the specific capacity is calculated from the weight loss of Al, the batteries would present higher specific capacities when the Al anode is mainly consumed by the discharge process. Hence, it could be concluded that the self-corrosion reaction is suppressed when the current density increases. The energy densities of the alloy anodes at different discharge current densities were also calculated and are shown in Fig. 3i. The results show that A6061 displays the highest energy density of 2119.09 W h kg−1 at a high current density of 80 mA cm−2.
3.3 Compositions and microstructure analysis
In order to understand the self-corrosion behavior and electrochemical properties in detail, the elemental compositions and microstructures of the Al alloys were analyzed. The elemental compositions obtained by ICP-OES are shown in Table S6.† A1060 is a commercially pure Al alloy, which represents that the content of Al is not lower than 99.60%, agreeing with the result. Fe occupies the majority of the impurity elements. And a small amount of Si is detected. The contents of impurities gradually increase in Mg-rich alloys. The main impurity elements in A5052 are Mg, Fe, Mn, and Si, in which the Mg content exceeds 2.18%. Compared with A5052, A6061 contains more Si and less Mg. It is noted that the self-corrosion behavior is closely related to the impurity elements.32,60 The backscattered electron images and corresponding elemental distribution maps of the alloys were obtained and the results are shown in Fig. 4. The SEM images show that there are a lot of second phases in the Al matrix. The EDS mappings illustrate that the main compositions of the second phases in the alloys are Mg-rich and (Si, Fe)-rich impurities with uneven microstructures. Some studies have reported that these second phases in 5xxx and 6xxx alloys are mainly (Mg2Si) and (AlFeSi), which would act as cathodes to accelerate hydrogen generation because of their higher Volta potential difference (VPD).61 This explains the high hydrogen evolution rates of the alloy anodes. However, it does not explain the higher electrochemical activity of the A6061 anode. | ||
| Fig. 4 Backscattered electron images of the alloys and corresponding elemental distribution maps. (a) A1060, (b) A5052, and (c) A6061. | ||
Furthermore, the surface morphologies (with discharge products removed) of the alloys after testing at 20 mA cm−2 for 5 h were characterized, as shown in Fig. 5. Because there are fewer impurity elements in A1060, the surface is relatively smooth after discharging as shown in Fig. 5a. But some large craters could be found. It is due to the severe local corrosion originating from the large Fe-rich impurity particles which act as cathodic phases. By observing at larger magnifications as shown in Fig. 5b and c, the surface presents a lot of shallow craters and deep corrosion holes. These shallow craters are from the normal Al dissolution during the discharge process, while the deep corrosion holes come from the impurity dots. As for A5052 and A6061, the whole surface is rougher after discharging because of their high contents of impurity phases. It could be directly observed in Fig. 5f that these deep corrosion holes are caused by local corrosion. As illustrated in Fig. S6,† the impurity phase which presents a higher Volta potential acts as the cathode, and the Al matrix is the anode. After reacting, Al is consumed near the anode/cathode interface, then the impurity phase peels off and a deep corrosion hole is formed. Notably, A6061 presents a different corrosion morphology in which lots of grain boundaries are distributed on the surface (Fig. 5h and i). It demonstrates that A6061 tends to dissolute along a certain grain boundary.
 | ||
| Fig. 5 SEM images of the Al surfaces after discharging at 20 mA cm−2 for 5 h. (a–c) A1060, (d–f) A5052, and (g–i) A6061. | ||
Then the XRD patterns of the alloys were collected to detect the crystallographic orientation. The result in Fig. 6a shows that A5052 and A6061 present more sharp peaks at around 45°, which corresponds to the (220) plane. For A6061, the (220) plane is greatly exposed. Fan and co-workers reported that the electrochemical properties of Al anodes are closely related to their crystallographic orientation.62 Besides, the optical micrographs of the alloys were characterized and are shown in Fig. 6b–d, with the grain size numbers shown in Fig. 6e. The grain of A1060 is slender, which may indicate that it experienced an extrusion process. As for A5052 and A6061, the grains are distributed in equiaxed shapes with different sizes. The grain size numbers calculated by the ASTM E112 cut-off method are 6.5 and 4.5, corresponding to mean grain sizes of 37.8 μm and 75.5 μm (according to GB/T 2394-2017), respectively. Researchers have reported that grain size could affect battery performance.63–66 A smaller grain size is favorable for enhancing the working voltage and specific capacity of the Al anode. However, the former tests show that A6061 which has a larger grain size presents better electrochemical properties. Accordingly, the better performance of the A6061 anode could be mainly ascribed to the crystallographic orientation.
 | ||
| Fig. 6 (a) XRD patterns of A1060, A5052, and A6061. Optical micrographs of (b) A1060, (c) A5052, and (d) A6061 and (e) statistic of grain size number. | ||
4 Conclusions
In summary, typical Mg-based commercial Al alloys are employed as anode materials for Al–air batteries. The self-corrosion behavior, electrochemical performance, elemental composition, and microstructure of the alloy anodes are investigated. Overall, the A6061 anode shows a lower hydrogen evolution rate, higher OCP, and better battery performance. Besides, with an increase in discharge current density, the HER could be inhibited and the A6061-based Al–air battery presents a high anodic efficiency of 89.28%, a specific capacity of 2660.69 mA h g−1, and an energy density of 2119.09 W h kg−1 at 80 mA cm−2. Impurity phases and their contents are found to be factors affecting self-corrosion behavior but the anodic performance is not always positively correlated to impurities. Further structure characterization studies illustrate that the superior performance of the A6061 anode could be attributed to its unique crystallographic orientation, which is beneficial for Al dissolution and promotes electrochemical activity.Conflicts of interest
There are no conflicts of interest.Acknowledgements
This research was supported by the Joint Funds of the National Natural Science Foundation of China (U20A20280) and the University-Industry Collaborative Education Program (202102437022).References
- X. Zhao, X. Ma, B. Chen, Y. Shang and M. Song, Challenges toward carbon neutrality in China: strategies and countermeasures, Resour., Conserv. Recycl., 2022, 176, 105959 CrossRef CAS.
- C. Zou, B. Xiong, H. Xue, D. Zheng, Z. Ge, Y. Wang, L. Jiang, S. Pan and S. Wu, The role of new energy in carbon neutral, Pet. Explor. Dev., 2021, 48, 480–491 CrossRef.
- T. Huo, Y. Ma, W. Cai, B. Liu and L. Mu, Will the urbanization process influence the peak of carbon emissions in the building sector? A dynamic scenario simulation, Energy Build., 2021, 232, 110590 CrossRef.
- Z. Pan, Y. Liu, A. Tahir, O. Christopher Esan, J. Zhu, R. Chen and L. An, A discrete regenerative fuel cell mediated by ammonia for renewable energy conversion and storage, Appl. Energy, 2022, 322, 119463 CrossRef CAS.
- Y. Zhao, B. P. Setzler, J. Wang, J. Nash, T. Wang, B. Xu and Y. Yan, An Efficient Direct Ammonia Fuel Cell for Affordable Carbon-Neutral Transportation, Joule, 2019, 3, 2472–2484 CrossRef CAS.
- J. Wu, C. Yuan, T. Li, Z. Yuan, H. Zhang and X. Li, Dendrite-Free Zinc-Based Battery with High Areal Capacity via the Region-Induced Deposition Effect of Turing Membrane, J. Am. Chem. Soc., 2021, 143, 13135–13144 CrossRef CAS PubMed.
- M. Deng, L. Wang, B. Vaghefinazari, W. Xu, C. Feiler, S. V. Lamaka, D. Höche, M. L. Zheludkevich and D. Snihirova, High-energy and durable aqueous magnesium batteries: recent advances and perspectives, Energy Storage Mater., 2021, 43, 238–247 CrossRef.
- X. Han, X. Li, J. White, C. Zhong, Y. Deng, W. Hu and T. Ma, Metal–Air Batteries: From Static to Flow System, Adv. Energy Mater., 2018, 8, 1801396 CrossRef.
- J. Li, K. S. Hui, S. Ji, C. Zha, C. Yuan, S. Wu, F. Bin, X. Fan, F. Chen, Z. Shao and K. N. Hui, Electrodeposition of a dendrite-free 3D Al anode for improving cycling of an aluminum–graphite battery, Carbon Energy, 2021, 4, 155–169 CrossRef.
- L. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang and K. Xu, “Water-in-Salt” Electrolyte Enables High Voltage Aqueous Li-ion Chemistries, Science, 2015, 350, 938–943 CrossRef CAS PubMed.
- J. Noack, N. Roznyatovskaya, T. Herr and P. Fischer, The Chemistry of Redox-Flow Batteries, Angew. Chem., Int. Ed., 2015, 54, 9776–9809 CrossRef CAS PubMed.
- E. Faegh, B. Ng, D. Hayman and W. E. Mustain, Practical assessment of the performance of aluminium battery technologies, Nat. Energy, 2020, 6, 21–29 CrossRef.
- Q. Liu, Z. Pan, E. Wang, L. An and G. Sun, Aqueous metal–air batteries: Fundamentals and applications, Energy Storage Mater., 2020, 27, 478–505 CrossRef.
- H.-F. Wang and Q. Xu, Materials Design for Rechargeable Metal–Air Batteries, Matter, 2019, 1, 565–595 CrossRef CAS.
- N. Chawla, Recent advances in air-battery chemistries, Mater. Today Chem., 2019, 12, 324–331 CrossRef CAS.
- B. Vaghefinazari, D. Höche, S. V. Lamaka, D. Snihirova and M. L. Zheludkevich, Tailoring the Mg–air primary battery performance using strong complexing agents as electrolyte additives, J. Power Sources, 2020, 453, 227880 CrossRef CAS.
- L. Hu, P. Xiao, L. Xue, H. Li and T. Zhai, The rising zinc anodes for high-energy aqueous batteries, EnergyChem, 2021, 3, 100052 CrossRef CAS.
- S. Yuan, H. Lu, Z. Sun, L. Fan, X. Zhu and W. Zhang, Electrochemical Performance of Mg-3Al Modified with Ga, In and Sn as Anodes for Mg–Air Battery, J. Electrochem. Soc., 2016, 163, A1181–A1187 CrossRef CAS.
- S. Yan, Y. Xue, S. Li, G. Shao and Z. Liu, Enhanced Bifunctional Catalytic Activity of Manganese Oxide/Perovskite Hierarchical Core–Shell Materials by Adjusting the Interface for Metal–Air Batteries, ACS Appl. Mater. Interfaces, 2019, 11, 25870–25881 CrossRef CAS PubMed.
- Y. Xue, S. Sun, Q. Wang, Z. Dong and Z. Liu, Transition metal oxide-based oxygen reduction reaction electrocatalysts for energy conversion systems with aqueous electrolytes, J. Mater. Chem. A, 2018, 6, 10595–10626 RSC.
- X. Liu, H. Jiao, M. Wang, W.-l. Song, J. Xue and S. Jiao, Current progresses and future prospects on aluminium–air batteries, Int. Mater. Rev., 2022, 67, 734–764 CrossRef CAS.
- T. Wang, Z. Tian, Z. You, Z. Li, H. Cheng, W. Li, Y. Yang, Y. Zhou, Q. Zhong and Y. Lai, Hydrogen-bond network manipulation of aqueous electrolytes with high-donor solvent additives for Al–air batteries, Energy Storage Mater., 2022, 45, 24–32 CrossRef.
- M. Jiang, C. Fu, P. Meng, J. Ren, J. Wang, J. Bu, A. Dong, J. Zhang, W. Xiao and B. Sun, Challenges and Strategies of Low-Cost Aluminum Anodes for High-Performance Al-Based Batteries, Adv. Mater., 2021, 34, 2102026 CrossRef PubMed.
- J. Liu, C. Zhang, S. Yuan, W. Yang, Y. Cao, J. Deng, B. Xu and H. Lu, CoP-decorated N,P-doped necklace-like carbon for highly efficient oxygen reduction and Al–air batteries, Chem. Eng. J., 2022, 428, 134283 CrossRef.
- R. Mori, Recent Developments for Aluminum–Air Batteries, Electrochem. Energy Rev., 2020, 3, 344–369 CrossRef CAS.
- J. Ryu, H. Jang, J. Park, Y. Yoo, M. Park and J. Cho, Seed-mediated atomic-scale reconstruction of silver manganate nanoplates for oxygen reduction towards high-energy aluminum–air flow batteries, Nat. Commun., 2018, 9, 3715 CrossRef PubMed.
- H. Cheng, T. Wang, Z. Li, C. Guo, J. Lai and Z. Tian, Anode Interfacial Layer Construction via Hybrid Inhibitors for High-Performance Al–Air Batteries, ACS Appl. Mater. Interfaces, 2021, 13, 51726–51735 CrossRef CAS PubMed.
- A. V. Ilyukhina, B. V. Kleymenov and A. Z. Zhuk, Development and study of aluminum–air electrochemical generator and its main components, J. Power Sources, 2017, 342, 741–749 CrossRef CAS.
- S. Wu, Q. Zhang, J. Ma, D. Sun, Y. Tang and H. Wang, Interfacial design of Al electrode for efficient aluminum–air batteries: issues and advances, Mater. Today Energy, 2020, 18, 100499 CrossRef CAS.
- P. Wu, S. Wu, D. Sun, Y. Tang and H. Wang, A Review of Al Alloy Anodes for Al–Air Batteries in Neutral and Alkaline Aqueous Electrolytes, Acta Metall. Sin., 2020, 34, 309–320 CrossRef.
- P. Goel, D. Dobhal and R. C. Sharma, Aluminum–air batteries: a viability review, J. Energy Storage, 2020, 28, 101287 CrossRef.
- Y.-J. Cho, I.-J. Park, H.-J. Lee and J.-G. Kim, Aluminum anode for aluminum–air battery – Part I: influence of aluminum purity, J. Power Sources, 2015, 277, 370–378 CrossRef CAS.
- Q. Wang, H. Miao, Y. Xue, S. Sun, S. Li and Z. Liu, Performances of an Al–0.15 Bi–0.15 Pb–0.035 Ga alloy as an anode for Al–air batteries in neutral and alkaline electrolytes, RSC Adv., 2017, 7, 25838–25847 RSC.
- Y. Xie, X. Meng, Z. Qin, Y. Chang, D. Mao, L. Wan and Y. Huang, Reversible passivation in primary aluminum–air batteries via composite anodes, Energy Storage Mater., 2022, 49, 537–545 CrossRef.
- M. Chen, X. Zheng, Z. Liu, Q. Zheng and B. Zheng, The role of hot extrusion in improving electrochemical properties of low-cost commercial Al alloy as anode for Al–air battery, J. Electroanal. Chem., 2022, 909, 116127 CrossRef CAS.
- C. Zhang, Z. Cai, R. Wang, P. Yu, H. Liu and Z. Wang, Enhancing the Electrochemical Performance of Al–Mg–Sn–Ga Alloy Anode for Al–Air Battery by Solution Treatment, J. Electrochem. Soc., 2021, 168, 030519 CrossRef CAS.
- M. Wei, K. Wang, Y. Zuo, J. Liu, P. Zhang, P. Pei, S. Zhao, Y. Li and J. Chen, A high-performance Al–air fuel cell using a mesh-encapsulated anode via Al–Zn energy transfer, iScience, 2021, 24, 103259 CrossRef CAS PubMed.
- Z. Sun, H. Lu, L. Fan, Q. Hong, J. Leng and C. Chen, Performance of Al–Air Batteries Based on Al–Ga, Al–In and Al–Sn Alloy Electrodes, J. Electrochem. Soc., 2015, 162, A2116–A2122 CrossRef CAS.
- N. Li, C. Dong, C. Man, X. Li, D. Kong, Y. Ji, M. Ao, J. Cao, L. Yue, X. Liu and M. Du, Insight into the localized strain effect on micro-galvanic corrosion behavior in AA7075-T6 aluminum alloy, Corros. Sci., 2021, 180, 109174 CrossRef CAS.
- G. Li, H. Lu, X. Hu, F. Lin, X. Li and Q. Zhu, Current Progress in Rheoforming of Wrought Aluminum Alloys: A Review, Metals, 2020, 10, 238 CrossRef CAS.
- S. K. Padamata, A. Yasinskiy and P. Polyakov, A Review of Secondary Aluminum Production and Its Byproducts, JOM, 2021, 73, 2603–2614 CrossRef CAS.
- X. Lu, Z. Zhang, T. Hiraki, O. Takeda, H. Zhu, K. Matsubae and T. Nagasaka, A solid-state electrolysis process for upcycling aluminium scrap, Nature, 2022, 606, 511–515 CrossRef CAS PubMed.
- Y. K. Zhong, Y. L. Liu, K. Liu, L. Wang, L. Mei, J. K. Gibson, J. Z. Chen, S. L. Jiang, Y. C. Liu, L. Y. Yuan, Z. F. Chai and W. Q. Shi, In situ anodic precipitation process for highly efficient separation of aluminum alloys, Nat. Commun., 2021, 12, 5777 CrossRef CAS PubMed.
- L. Fan, H. Lu, J. Leng, Z. Sun and C. Chen, The Study of Industrial Aluminum Alloy as Anodes for Aluminum–Air Batteries in Alkaline Electrolytes, J. Electrochem. Soc., 2015, 163, A8–A12 CrossRef.
- R. N. Mutlu, S. Ateş and B. Yazıcı, Al-6013-T6 and Al-7075-T7351 alloy anodes for aluminium–air battery, Int. J. Hydrogen Energy, 2017, 42, 23315–23325 CrossRef CAS.
- P. Katsoufis, V. Mylona, C. Politis, G. Avgouropoulos and P. Lianos, Study of some basic operation conditions of an Al–air battery using technical grade commercial aluminum, J. Power Sources, 2020, 450, 227624 CrossRef CAS.
- J. Ren, J. Ma, J. Zhang, C. Fu and B. Sun, Electrochemical performance of pure Al, Al–Sn, Al–Mg and Al–Mg–Sn anodes for Al–air batteries, J. Alloys Compd., 2019, 808, 151708 CrossRef CAS.
- C. He, B. Luo, Y. Zheng, Y. Yin, Z. Bai and Z. Ren, Effect of Sn on microstructure and corrosion behaviors of Al–Mg–Si alloys, Mater. Charact., 2019, 156, 109836 CrossRef CAS.
- J. Gao, Y. Li, Z. Yan, Q. Liu, Y. Gao, C. Chen, B. Ma, Y. Song and E. Wang, Effects of solid-solute magnesium and stannate ion on the electrochemical characteristics of a high-performance aluminum anode/electrolyte system, J. Power Sources, 2019, 412, 63–70 CrossRef CAS.
- C. Zhou, K. Bhonge and K. T. Cho, Analysis of the effect of hydrogen-evolving side reaction in the aqueous aluminum–air battery, Electrochim. Acta, 2020, 330, 135290 CrossRef CAS.
- H. Wang, D. Y. C. Leung, M. K. H. Leung and M. Ni, Modeling of Parasitic Hydrogen Evolution Effects in an Aluminum–Air Cell, Energy Fuels, 2010, 24, 3748–3753 CrossRef CAS.
- L. D. Chen, J. K. Norskov and A. C. Luntz, Al–Air Batteries: Fundamental Thermodynamic Limitations from First-Principles Theory, J. Phys. Chem. Lett., 2015, 6, 175–179 CrossRef CAS PubMed.
- Y. Zuo, Y. Yu, H. Liu, Z. Gu, Q. Cao and C. Zuo, Electrospun Al2O3 Film as Inhibiting Corrosion Interlayer of Anode for Solid Aluminum–Air Batteries, Batteries, 2020, 6, 1–12 CrossRef.
- X. Liu, P. Zhang, J. Xue, C. Zhu, X. Li and Z. Wang, High energy efficiency of Al-based anodes for Al–air battery by simultaneous addition of Mn and Sb, Chem. Eng. J., 2021, 417, 128006 CrossRef CAS.
- Y. Li, Y. Wang, S. Zhang, L. Miao, M. Wei and K. Wang, Corrosion inhibition of aromatic acids on Al-7075 anode for Al–air batteries with alkaline electrolyte, J. Power Sources, 2022, 523, 231042 CrossRef CAS.
- C. Lv, Q. Zhang, Y. Zhang, Z. Yang, P. Wu, D. Huang, H. Li, H. Wang and Y. Tang, Synergistic regulating the aluminum corrosion by ellagic acid and sodium stannate hybrid additives for advanced aluminum–air battery, Electrochim. Acta, 2022, 417, 140311 CrossRef CAS.
- Y. Zhu, X. Li, D. Zhang and L. Gao, Improvement of electrochemical performance with amphoteric surfactants for Al anode of Al–air battery in alkaline system, J. Power Sources, 2021, 515, 230646 CrossRef CAS.
- L. Yang, Y. Wu, S. Chen, Y. Xiao, S. Chen, P. Zheng, J. Wang and J.-E. Qu, A promising hybrid additive for enhancing the performance of alkaline aluminum–air batteries, Mater. Chem. Phys., 2021, 257, 123787 CrossRef CAS.
- Z. Wu, H. Zhang, S. Tang, J. Zou, D. Yang, Y. Wang, K. Qin, C. Ban, J. Cui and H. Nagaumi, Effect of calcium on the electrochemical behaviors and discharge performance of Al–Sn alloy as anodes for Al–air batteries, Electrochim. Acta, 2021, 370, 137833 CrossRef CAS.
- J. Ren, C. Fu, Q. Dong, M. Jiang, A. Dong, G. Zhu, J. Zhang and B. Sun, Evaluation of Impurities in Aluminum Anodes for Al–Air Batteries, ACS Sustainable Chem. Eng., 2021, 9, 2300–2308 CrossRef CAS.
- Y.-y. Ji, Y.-z. Xu, B.-b. Zhang, Y. Behnamian, D.-h. Xia and W.-b. Hu, Review of micro-scale and atomic-scale corrosion mechanisms of second phases in aluminum alloys, Trans. Nonferrous Met. Soc. China, 2021, 31, 3205–3227 CrossRef CAS.
- L. Fan, H. Lu, J. Leng, Z. Sun and C. Chen, The effect of crystal orientation on the aluminum anodes of the aluminum–air batteries in alkaline electrolytes, J. Power Sources, 2015, 299, 66–69 CrossRef CAS.
- L. Fan, H. Lu and J. Leng, Performance of fine structured aluminum anodes in neutral and alkaline electrolytes for Al–air batteries, Electrochim. Acta, 2015, 165, 22–28 CrossRef CAS.
- S. Linjee, S. Moonngam, P. Klomjit, N. S. Pålsson and C. Banjongprasert, Corrosion behaviour improvement from the ultrafine-grained Al–Zn–In alloys in Al–air battery, Energy Rep., 2022, 8, 5117–5128 CrossRef.
- Y. Han, J. Ren, C. Fu, M. Jiang, S. Lu, J. Zhang and B. Sun, Electrochemical Performance of Aluminum Anodes with Different Grain Sizes for Al–Air Batteries, J. Electrochem. Soc., 2020, 167, 040514 CrossRef CAS.
- L. Fan and H. Lu, The effect of grain size on aluminum anodes for Al–air batteries in alkaline electrolytes, J. Power Sources, 2015, 284, 409–415 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2se01341j |
| This journal is © The Royal Society of Chemistry 2023 |


