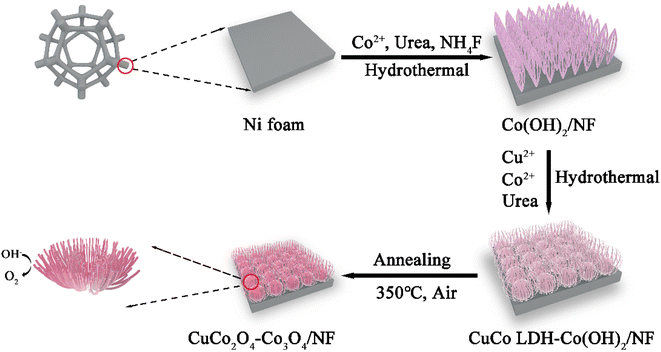
Chrysanthemum-like spinel nanomaterials assembled with bundled nanowires as an efficient catalyst for the oxygen evolution reaction†
Hongliang
Yu
,
Jianzhi
Wang
,
Tong
Xu
,
Ningbo
Yu
,
Li
Wu
,
Siming
Chen
,
Ning
Cai
,
Yanan
Xue
,
Hui
Li
* and
Faquan
Yu
 *
*
Key Laboratory for Green Chemical Process of Ministry of Education, Hubei Key Laboratory for Novel Reactor and Green Chemistry Technology, Hubei Engineering Research Center for Advanced Fine Chemicals, School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, LiuFang Campus, No.206, Guanggu 1st road, Wuhan 430205, Hubei, P. R. China. E-mail: sodium2008@163.com; fyu@wit.edu.cn; Fax: +86 27 87194465; Tel: +86 27 87194980
First published on 5th December 2022
Abstract
The fabrication of low-cost and high-performance electrocatalysts is essential for water splitting to achieve a sustainable oxygen evolution reaction. In this work, spinel oxides (CuCo2O4 and Co3O4) are mounted on nickel foam (NF) to form chrysanthemum-like hybrid crystal structures assembled from nanowires. The as-synthesized CuCo2O4–Co3O4/NF shows a high surface area, a large number of mesoporous structures and excellent charge transfer channels, which is conducive to the generation and overflow of O2. And the synergistic effect, as well as the transfer of electron density between CuCo2O4 and Co3O4, contributes to the enhancement of OER activity. As a result, CuCo2O4–Co3O4/NF exhibits excellent OER performance with an overpotential of only 267 mV at 10 mA cm−2, a low Tafel slope (114.6 mV dec−1) and excellent stability with a scarce loss of the initial catalyst activity over 24 h under alkaline conditions. This work provides an effective method to show higher catalytic activity by combining two spinel oxides.
Introduction
With the growth of the fossil fuel crisis and environmental problems, the development of renewable energy sources has become a global common view.1 Hydrogen has been considered as an ideal substitute for traditional fossil fuels, and electrocatalytic water splitting is believed to be a convenient and efficient method to produce hydrogen. Electrochemical water splitting, which includes the hydrogen evolution reaction (HER) on the cathode and the oxygen evolution reaction (OER) on the anode, is an efficient and sustainable way to produce hydrogen (H2) and oxygen (O2).2 However, four proton-coupled electron transfer and slow kinetics of the OER process result in high overpotential and a bottleneck in the entire process.3 Normally, noble metal oxides like ruthenium dioxide (RuO2) and iridium dioxide (IrO2) benchmark electrocatalysts are considered to have the best electrochemical activity for the OER.4 However, the high cost, scarcity and poor stability of precious metals have seriously hindered the large-scale application of renewable energy systems.5 Therefore, it is urgent to explore efficient OER catalysts with low cost, earth abundance and good stability for widespread applications.In recent decades, it has been found that transition metals and their derivatives (such as metal sulfides, metal phosphides, and metal oxides or hydroxides) have high activity and low price, which are very suitable as substitutes for noble metal catalysts.1,3,6 Among transition metal oxides, especially spinel oxides AB2O4 (A, B = 3d transition metals, such as MnCo2O4, CoFe2O4, CoAl2O4 and NiCo2O4) have attracted considerable attention as OER catalysts.1,7 In spinel oxides, oxygen is closely packed in a cubic form, divalent metal cations occupy one eighth of tetrahedral sites, and trivalent metal cations are filled in one half of octahedral sites. And the remaining unoccupied gaps endow the spinel with an open structure to accommodate the migration of cations.8 At the same time, the mixed valence of metal cations also provides donor–acceptor sites to adsorb oxygen and promote the occurrence of electrolytic water reactions.9 In addition, the introduction of other metal atoms into the crystal lattice can greatly affect the electronic structure of the original catalyst and enhance the intrinsic activity as well as the conductivity of the catalyst.
Among them, a Cu-based electrocatalyst has attracted a lot of attention due to its biomimetic chemistry similar to that of O2.10,11 The introduction of Co into the Cu-based electrocatalyst to form CuCo2O4 spinel oxides can not only change the 3d orbitals of Cu and the surrounding environment of the 3d electrons,12 but also increase the adsorption energy of water and change the Gibbs free energy of the adsorption intermediate, thereby improving the catalytic activity of the catalyst.11,13 However, powder catalyst materials easily agglomerate and fall off during the test process, resulting in the reduction of catalyst activity and stability. A self-supporting electrode can directly grow the catalyst on a common substrate surface (such as NF or carbon cloth), which can perfectly solve the problem caused by powder catalysts. Moreover, it has a larger specific surface area and faster carrier release, which can enhance the interaction and synergy between various species.14 Nevertheless, the spinel oxides on the substrate are mostly two-dimensional layered structures, with a large layer thickness, few active centers and low catalytic activity, which is difficult to meet the industrial requirements of high activity.15
Another special metal oxide Co3O4 is considered to be one of the best OER catalysts due to its large cobalt reserves, low price and high oxygen evolution efficiency in alkaline solution.16 Zhu et al. prepared a hollow Co3O4 microtube array with a hierarchical structure and only required a battery voltage of 1.63 V to reach a current density of 10 mA cm−2.17 Kuang et al. synthesized a copper–cobalt mixed oxide, which can reach a current density of 10 mA cm−2 at only 1.61 V for overall water splitting in alkaline medium.18 It is generally believed that the 3d empty or semi-empty orbital of Co can interact with the π electrons of oxygen and reduce the adsorption energy of O*, OH* and OO* in the center of the 3d band.3,7,19 Using one-dimensional Co3O4 microtube array materials as templates, 3D catalysts were grown on a conductive substrate to form a three-dimensional (3D) structure, which can also increase the surface area and active sites of the catalyst to a greater extent, promoting the diffusion of reactants and generated gases.20
Inspired by this, we prepared a chrysanthemum-like CuCo2O4–Co3O4 electrocatalyst on Co3O4 microtube arrays by a two-step hydrothermal reaction and annealing in air. A 3D chrysanthemum-like morphology forms outward active sites and a large specific surface area, which accelerates electron transfer and facilitates the desorption of generated oxygen. And the synergistic effect of redox pairs of active catalytic centers makes it show higher catalytic activity. The CuCo2O4–Co3O4 electrocatalyst exhibits outstanding OER activity with a low overpotential of 267 mV at 10 mA cm−2, a small Tafel slope of 114.6 mV dec−1, and excellent durability in alkaline medium. It is a promising way to optimize the electronic configuration and greatly enhance the OER activity.
Experimental section
Materials
Cobalt sulfate heptahydrate (CoSO4·7H2O, 98%), copper sulfate nonahydrate (CuSO4·9H2O, 98%), urea (CH4N2O, 99%), ammonium fluoride (NH4F, 96%), potassium hydroxide (KOH, 85%), Ni foam (NF, purity ≥99%, porosity ≥90%), concentrated hydrochloric acid (HCl, 37%) and ethanol (CH3CH2OH, 99.7%) were purchased from Sinopharm Chemical Reagent Co., Ltd. Commercial RuO2 was purchased from Aladdin Reagent Co. Ltd. Nafion (5 wt%) was purchased from Dupont China Holding Co., Ltd. All the chemical reagents were of analytical grade and used as received without further purification. Water deionized with a Millipore system was used throughout all experiments.Synthesis of Co(OH)2/NF
The NF was treated before the experiment. Firstly, the NF was ultrasonically washed in absolute ethanol and HCl solution (3.0 M) sequentially for 20 minutes to remove greasy dirt and the oxide layer, and then cleaned with deionized water. After that, 2.81 g (10 mmol) of CoSO4·7H2O, 3.00 g (50 mmol) of CH4N2O and 0.74 g (20 mmol) of NH4F were dissolved into 50 mL of deionized water by an ultrasonic treatment to form a homogeneous solution. Then, both the solution and the cleaned NF were placed into a 50 mL Teflon autoclave and then sealed and kept at 120 °C for 5 h. After cooling down to room temperature, the obtained Co(OH)2/NF was washed several times with deionized water and dried using a freeze dryer.Synthesis of CuCo LDH-Co(OH)2/NF
In a typical synthesis, 337.32 mg (1.20 mmol) of CoSO4·7H2O, 149.814 mg (0.60 mmol) of CuSO4·9H2O, and 360.36 mg (6 mmol) of CH4N2O were dissolved into 30 mL of deionized water by an ultrasonic treatment to form a homogeneous solution. Then, both the solution and the prepared Co(OH)2/NF were placed into a 50 mL Teflon autoclave and then sealed and kept at 120 °C for 6 h. After cooling down to room temperature, the samples were removed from the autoclave, washed with water several times and freeze-dried to obtain CuCo LDH-Co(OH)2/NF.Synthesis of CuCo2O4–Co3O4/NF
The prepared CuCo LDH-Co(OH)2/NF was calcined at 350 °C for 2 h with a heating ramp of 5 °C min−1 in air to obtain CuCo2O4–Co3O4/NF.Synthesis of other control samples
For comparison, CuCo2O4/NF and Co3O4/NF were prepared under identical conditions of CuCo2O4–Co3O4/NF, except replacing CuCo LDH-Co(OH)2/NF with CuCo LDH/NF and Co(OH)2/NF, respectively. CuO–Co3O4/NF and Co3O4–Co3O4/NF were also prepared under the same conditions as CuCo2O4–Co3O4/NF but without CoSO4·7H2O and CuSO4·9H2O, respectively.Material characterization
The morphology of the synthesized materials was characterized by field emission scanning electron microscopy (FESEM, GeminiSEM 300) with an energy dispersive spectroscopy (EDX) detector and transmission electron microscope (TEM, JEOL JEM 2100). High-resolution TEM (HRTEM, JEOL JEM 2100) was used to obtain the information on the lattice fringe. X-ray powder diffraction (XRD, X'Pert Pro MRD) was carried out with Cu Kα radiation at a scan rate of 5° min−1. X-ray photoelectron spectroscopy (XPS, ESCALAB XI+) was used to analyze the valence state with Al Kα monochromatized radiation.Electrochemical measurements
All the electrochemical measurements were conducted in a classical three-electrode glass cell using an Electrochemical Workstation (CHI 760E) in 1 M KOH (pH = 14) aqueous solution at room temperature. The three electrode system is composed of a graphite rod as the counter electrode, a saturated calomel electrode (SCE) electrode as the reference electrode, and the sample (CuCo2O4–Co3O4/NF, CuCo2O4/NF, Co3O4/NF, CuO–Co3O4/NF, Co3O4–Co3O4/NF, CuCo LDH-Co(OH)2/NF, RuO2/NF or blank Ni Foam) as the working electrode. In all tests, the potentials were carefully calibrated to a reversible hydrogen electrode (RHE) potential in 1.0 M KOH electrolyte solution. The SCE electrode was calibrated with respect to the RHE: E(RHE) = E(SCE) + 0.059 × pH + E0(SCE). Linear sweep voltammetry (LSV) was conducted from 0 to 0.7 V (vs. SCE) at a scan rate of 2 mV s−1 with 85% iR-compensation. Electrochemical impedance spectroscopy (EIS) analyses were conducted at a fixed potential of 0.50 V and performed in the frequency range of 0.01 Hz to 10 kHz with an amplitude of 5 mV. The electrochemical double layer capacitance (Cdl) of the synthesized samples was measured from 1.043 to 1.143 V (in the non-faradaic region) versus the RHE with different scan rates (10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 mV s−1) to estimate the effective electrode surface area. The Cdl value is half of the linear slope. A long-term durability and stability test was performed using chronopotentiometry with a polarization current density of 10 mA cm−1. The electrochemical stability was actualized using CV sweeps at 50 mV s−1 between −0.1 V and 0.1 V versus the RHE for 5000 cycles at 25 °C.For comparison, RuO2 was deposited onto NF as the working electrode with a loading of about 2.7 mg cm−2. Typically, 4 mg RuO2 (99.9%) and 40 μL Nafion solution (5 wt%) were ultrasonically scattered in 960 μL solvent (720 μL ultrapure water and 240 μL ethanol), and then the ink solution was loaded onto a cleaned NF, and dried under an infrared drying lamp.
Results and discussion
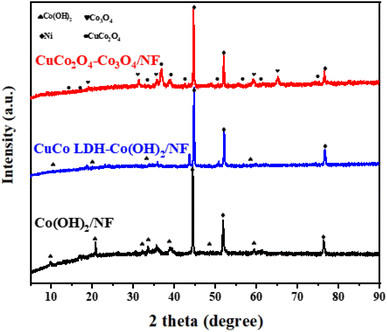
The preparation of chrysanthemum-like CuCo2O4–Co3O4 on nickel foam (NF) is mainly divided into three steps, as displayed in Scheme 1. Initially, uniform and vertical Co(OH)2 nanowire arrays (Co(OH)2/NF) were grown on the surface of Ni foam by the first solvothermal method. Subsequently, CuCo LDH-Co(OH)2 was deposited onto the surface of NF by the second hydrothermal reaction in an alkaline environment. Finally, the obtained CuCo LDH-Co(OH)2/NF was annealed in air to get chrysanthemum-like CuCo2O4–Co3O4/NF.The crystalline phases of Co(OH)2/NF, CuCo LDH-Co(OH)2/NF and CuCo2O4–Co3O4/NF were characterized by X-ray powder diffraction (XRD). As a comparison, the XRD patterns of CuCo2O4/NF, Co3O4/NF, CuO–Co3O4/NF and Co3O4–Co3O4/NF were also recorded. As shown in Fig. 1 and S1,† the three high-intensity peaks of all the products at 44.5°, 51.8° and 76.4° can be indexed to the (111), (200) and (220) planes of Ni (JCPDS No.04-0850)21 derived from NF. In Fig. 1, the diffraction peaks of Co(OH)2/NF nanowire arrays at 9.50°, 19.16°, 38.03° and 58.19° can be assigned to the (001), (002), (102) and (110) planes of Co(OH)2 (JCPDS No.51-1731).22 For CuCo LDH-Co(OH)2/NF, the peaks of Co(OH)2 still remain, while the new reflection peaks at 35.8° may be attribute to CuCo LDH.23 The diffraction peaks of CuCo2O4–Co3O4/NF at 19°, 31.27°, 36.85°, 59.36° and 66.23° are well ascribed to the (111), (220), (311), (551) and (440) crystal planes of Co3O4 (JCPDS No.42-1467),24 respectively, while the diffraction peaks at 19.07°, 31.36°, 36.96° and 38.96° are assigned to the (111), (220), (311) and (222) planes of spinel CuCo2O4 (JCPDS No. 01-1155),25 which verifies the formation of CuCo2O4–Co3O4/NF. In Fig. S1,† the XRD patterns of CuCo2O4/NF, Co3O4/NF, CuO–Co3O4/NF and Co3O4–Co3O4/NF show the peaks of CuCo2O4, Co3O4 and CuO respectively, which proved that the comparison samples were also successfully prepared.
The scanning electron microscope (SEM) images of the as-prepared samples are displayed in Fig. 2, S2 and S3.† For Co(OH)2/NF, it can be seen from Fig. S2† that the Co(OH)2 grown on the surface of NF has an obvious sea urchin like structure. Each “sea urchin ball” is surrounded by nanowires, which are connected to each other. After the growth of CuCo LDH, the “sea urchin ball” disappeared, evolved into a flower like structure, and the rudiment of chrysanthemum has appeared. It is speculated that the addition of Cu2+ caused the rearrangement of Co(OH)2. In Fig. 2a, flower like CuCo2O4–Co3O4 balls are grown on the NF. As shown in Fig. 2b, the flower structure is like a “chrysanthemum”, and each “chrysanthemum” is connected with each other. The “petals” of the “chrysanthemum” are actually composed of nanowires, and the ends of each nanowire are bound together, further increasing the mass transfer channel.1,2 As shown in Fig. 2d, the EDX mapping of CuCo2O4–Co3O4/NF verifies the presence of Cu, Co and O, and the ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O atoms is close to 1
O atoms is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (Fig. S4†).
8 (Fig. S4†).
The structure and morphology of CuCo2O4–Co3O4 were further investigated by TEM and HRTEM. It can be seen in Fig. 3a–c that these CuCo2O4–Co3O4 nanowires are composed of numerous primary nanoparticles, and mesoporous structures can be observed among the tiny particles. These structures are beneficial to the diffusion or immersion of electrolyte ions during the redox reaction, provide channels for efficient diffusion of evolved O2, and even offer more edges to facilitate the OER.9 The HRTEM images of CuCo2O4–Co3O4 (Fig. 3c and d) confirm that CuCo2O4 and Co3O4 nanoflakes were well crystallized, and the lattice fringes with an interplanar spacing of 0.286 and 0.244 nm are assigned to the spacing of CuCo2O4 (220) and Co3O4 (311) planes, respectively. Meanwhile the lattice fringes with an interplanar spacing of 0.465 and 0.467 nm can be indexed to CuCo2O4 (111) and Co3O4 (111), respectively. The hybrid crystal structure can produce a rich interface structure, which can optimize the valence electron state of the active center and improve the electronic conductivity of the catalyst.26,27 These results illustrated that CuCo2O4 and Co3O4 were successfully loaded on NF, which are consistent with the results of XRD patterns.
The chemical valence states in CuCo2O4/NF, Co3O4/NF and CuCo2O4–Co3O4/NF were analyzed by X-ray photoelectron spectroscopy (XPS), as shown in Fig. 4. The Cu 2p spectrum shows two peaks at binding energies of 933.7 and 953.6 eV, which could be assigned to Cu 2p3/2 and Cu 2p1/2, respectively. Two satellite peaks could also be detected at 941.6 and 961.6 eV.16 In the Co 2p spectra (Fig. 4c), the fitting peaks at 778.97 eV and 793.96 eV are assigned to Co3+, while the other two fitting peaks at 780.26 eV and 795.43 eV are attributed to Co2+.28 The spectrum of O 1s could be resolved into two peaks at binding energies of 529.4 eV and 530.8 eV, which correspond to metal–oxygen bonding and defect sites. In addition, by comparing the valence distribution of each element of the target sample, CuCo2O4/NF (Fig. S5†), and Co3O4/NF (Fig. S6†), it can be found that this electron density distribution is conducive to enhancing the electron transfer between interfaces, thus improving the catalytic activity of CuCo2O4–Co3O4/NF.16,29
 | ||
| Fig. 4 XPS full-spectrum survey (a) and core-level spectra of Cu 2p (b), Co 2p (c) and O 1s (d) for CuCo2O4–Co3O4/NF. | ||
The high surface area and mesoporous structure of CuCo2O4–Co3O4 were further analyzed by Brunauer–Emmett–Teller (BET). The N2 adsorption–desorption isotherm of CuCo2O4–Co3O4 is a Type IV isotherm with a H1 hysteresis loop in the P/P0 range of 0.5–1.0 (Fig. S7†), identifying the mesoporous structure of as-synthesized CuCo2O4–Co3O4. The BET surface area is 31.5 m2 g−1 for CuCo2O4–Co3O4. The high specific surface area of CuCo2O4–Co3O4 is beneficial to expose many more electrochemically active sites and promote the mass transport of the electrolyte. According to the pore size distribution curve of CuCo2O4–Co3O4 calculated by using the BJH model, most of the pore sizes are 4.9 nm and 10 nm, confirming the mesoporous structure of the sample. Hence, it can be concluded from the isotherm and pore size distribution that the as-synthesized CuCo2O4–Co3O4 possesses a high surface area and mesoporous structure.
The electrocatalytic performance of the obtained catalysts for the OER was tested in a conventional three-electrode configuration in a 1.0 M KOH aqueous electrolyte at room temperature. As shown in the linear scan voltammogram (LSV, Fig. 5a), the CuCo2O4–Co3O4/NF electrode requires only 267 mV versus the RHE to achieve a current density of 10 mA cm−2, which is superior to that of CuCo2O4/NF (318 mV), Co3O4/NF (299 mV), CuO–Co3O4/NF (323 mV), Co3O4–Co3O4/NF (288 mV), CuCo LDH-Co(OH)2/NF (284 mV), RuO2/NF (339 mV) and NF (345 mV). In addition, CuCo2O4–Co3O4/NF only needs an overpotential of 407 mV to reach a high current density of 100 mA cm−2 (Fig. 5b). The excellent OER performance of CuCo2O4–Co3O4/NF can be attributed to the unique chrysanthemum-like structure with a three-dimensional mesoporous structure and the synergistic effect between active substances, which is beneficial to the formation and diffusion of oxygen. Some recently studied materials similar to CuCo2O4–Co3O4/NF are listed in Table S1.† By comparing the performance of the catalysts, such as A2.7B-MOF-FeCo1.6 (η10 = 288 mV),30 CoMn LDH@Cu(OH)2/CF (η10 = 280 mV),31 CoBDC–Fc0.17 (η10 = 291 mV),32 P-doped Co@NC-3/1 (η10 = 340 mV),33 O–CoSe2-HNT (η10 = 252 mV),34etc., it was found that CuCo2O4–Co3O4/NF showed excellent OER activity in an alkaline electrolyte.
To evaluate the intrinsic catalytic activity, Fig. 5b presents the Tafel slopes of the different samples. The data from the LSV can be fitted according to the Tafel equation: η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(|j|),35 where η is the overpotential, j is the current density, a is the constant and b represents the Tafel slope. CuCo2O4–Co3O4/NF exhibits the smallest Tafel slope of 114.59 mV dec−1, which is close to that of RuO2/NF (109.7 mV dec−1) and outperforms that of CuCo2O4/NF (155.02 mV), Co3O4/NF (135.69 mV dec−1), CuO–Co3O4/NF (132.26 mV dec−1), Co3O4–Co3O4/NF (142.53 mV dec−1), CuCo LDH-Co(OH)2/NF (140.87 mV dec−1) and NF (140.91 mV dec−1), respectively. The results indicate favorable OER kinetics on the CuCo2O4–Co3O4/NF surface.
log(|j|),35 where η is the overpotential, j is the current density, a is the constant and b represents the Tafel slope. CuCo2O4–Co3O4/NF exhibits the smallest Tafel slope of 114.59 mV dec−1, which is close to that of RuO2/NF (109.7 mV dec−1) and outperforms that of CuCo2O4/NF (155.02 mV), Co3O4/NF (135.69 mV dec−1), CuO–Co3O4/NF (132.26 mV dec−1), Co3O4–Co3O4/NF (142.53 mV dec−1), CuCo LDH-Co(OH)2/NF (140.87 mV dec−1) and NF (140.91 mV dec−1), respectively. The results indicate favorable OER kinetics on the CuCo2O4–Co3O4/NF surface.
The electrochemical impedance spectroscopy (EIS) spectrum of CuCo2O4–Co3O4/NF was recorded to illustrate the interfacial properties between the electrode and the electrolyte. As shown in Fig. 5c, all the samples show almost the same solution resistance (Rs).36 The Rs values can be neglected in contrast with the large charge transfer resistance (Rct). As is known to all, a smaller semicircle in the low frequency region reflects a faster charge transfer process in electrodes, namely a smaller Rct. The Rct of CuCo2O4–Co3O4/NF was estimated to be 1.20 Ω at an overpotential of 300 mV versus the RHE, which is much smaller than those of CuCo2O4/NF (5.85 Ω), Co3O4/NF (1.75 Ω), CuO–Co3O4/NF (31.05 Ω), Co3O4–Co3O4/NF (1.48 Ω) and CuCo LDH-Co(OH)2/NF (1.67 Ω), respectively. In CuCo2O4–Co3O4/NF, the connection between the nanowires forms more electron transport pathways, which is conducive to the rapid transfer of charge. In addition, the interface interaction produced in the formation process makes it have excellent electrical conductivity.37 Generally, electrochemical activity is highly dependent on the nature and number of uncoordinated metal sites. Thus, the electrochemical surface areas (ECSAs) were measured, as shown in Fig. 5d. The ECSA values were estimated from the double electric layer capacitances (Cdl),38 which were derived from cyclic voltammograms (CV) at different scan rates (Fig. S8†) and proportional to the active sites of the catalyst. The Cdl value of CuCo2O4–Co3O4/NF (26.68 mF cm−2) is significantly larger than those of CuCo2O4/NF (14.65 mF cm−2), Co3O4/NF (16.67 mF cm−2), CuO–Co3O4/NF (17.32 mF cm−2), Co3O4–Co3O4/NF (16.63 mF cm−2) and CuCo LDH-Co(OH)2/NF (12.62 mF cm−2), respectively. It is verified that the 3D chrysanthemum bundle grown on NF provides a higher surface area for redox reactions.
In order to investigate the stability and durability of the CuCo2O4–Co3O4/NF electrocatalyst, continuous CV scans were conducted between −0.1 and 0.1 V (versus the RHE) at an accelerated sweep rate of 50 mV s−1. Long-term electrolysis was carried out at a controlled current density of 10 mA cm−2 for 20 h in 1 M KOH solution. The performance of the CuCo2O4–Co3O4/NF electrocatalyst just decreased by 5.2% during the 20 h test (Fig. 6a). In Fig. 6b, the overpotential at 10 mA cm−2 almost retained its initial value after 5000 cycles. The high stability of the electrode was further confirmed by XRD (Fig. S9†), SEM (Fig. S10†) and XPS (Fig. S11†). After electrolysis, most of the characteristic diffraction peaks, the micromorphology and the main valence state of the original structure were retained. These results demonstrate that the CuCo2O4–Co3O4/NF electrocatalyst is stable for the OER in strong alkaline media for a long time.
Conclusions
In summary, a chrysanthemum-like hybrid electrocatalyst was successfully prepared via a facile hydrothermal reaction and annealing in air. Spinel oxide nanowires (CuCo2O4–Co3O4) were assembled into a chrysanthemum like hybrid crystal structure and grown on NF. CuCo2O4–Co3O4/NF exhibited excellent OER performance with an overpotential of only 267 mV to obtain a current of 10 mA cm−2 in an alkaline electrolyte with a low Tafel slope (114.59 mV dec−1) and excellent stability with a scarce loss of the initial catalyst activity over 20 h under alkaline conditions. The special structure of CuCo2O4–Co3O4/NF shows a high surface area and excellent charge transfer channels, endowing it with a high surface area and mesoporous structure for the easy evolution of O2. And the synergistic effect, as well as the transfer of electron density between CuCo2O4 and Co3O4, contributes to its high OER activity. This study provides a pathway for developing high-performance OER electrocatalysts, resulting in new advances in the field of the oxygen evolution reaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22078251 and 51808414) and the Research Project of Hubei Provincial Department of Education of China (D20191504) as well as by the Graduate Innovation Fund of Wuhan Institute of Technology (CX2021012).References
- B. M. Hunter, H. B. Gray and A. M. Müller, Earth-Abundant Heterogeneous Water Oxidation Catalysts, Chem. Rev., 2016, 116(22), 14120–14136, DOI:10.1021/acs.chemrev.6b00398.
- G. Zhao, K. Rui, S. X. Dou and W. Sun, Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review, Adv. Funct. Mater., 2018, 28(43), 1803291, DOI:10.1002/adfm.201803291.
- L. Zhang, Q. Fan, K. Li, S. Zhang and X. Ma, First-Row Transition Metal Oxide Oxygen Evolution Electrocatalysts: Regulation Strategies and Mechanistic Understandings, Sustainable Energy Fuels, 2020, 4(11), 5417–5432, 10.1039/D0SE01087A.
- J. Hu, C. Zhang, X. Meng, H. Lin, C. Hu, X. Long and S. Yang, Hydrogen Evolution Electrocatalysis with Binary-Nonmetal Transition Metal Compounds, J. Mater. Chem. A, 2017, 5(13), 5995–6012, 10.1039/C7TA00743D.
- Q. Shi, C. Zhu, D. Du and Y. Lin, Robust Noble Metal-Based Electrocatalysts for Oxygen Evolution Reaction, Chem. Soc. Rev., 2019, 48(12), 3181–3192, 10.1039/C8CS00671G.
- S. Chandrasekaran, L. Yao, L. Deng, C. Bowen, Y. Zhang, S. Chen, Z. Lin, F. Peng and P. Zhang, Recent Advances in Metal Sulfides: From Controlled Fabrication to Electrocatalytic, Photocatalytic and Photoelectrochemical Water Splitting and Beyond, Chem. Soc. Rev., 2019, 48(15), 4178–4280, 10.1039/C8CS00664D.
- A. Fan, C. Qin, X. Zhang, X. Dai, Z. Dong, C. Luan, L. Yu, J. Ge and F. Gao, Phosphorus-Doped FeNi Alloys/NiFe2O4 Imbedded in Carbon Network Hollow Bipyramid as Efficient Electrocatalysts for Oxygen Evolution Reaction, ACS Sustainable Chem. Eng., 2019, 7(2), 2285–2295, DOI:10.1021/acssuschemeng.8b04997.
- T. W. Kim, M. A. Woo, M. Regis and K.-S. Choi, Electrochemical Synthesis of Spinel Type ZnCo2O4 Electrodes for Use as Oxygen Evolution Reaction Catalysts, J. Phys. Chem. Lett., 2014, 5(13), 2370–2374, DOI:10.1021/jz501077u.
- L. Wang, J. Wang, M. Wang, P. Li, J. Tong and F. Yu, AgO-Decorated Multi-Dimensional Chrysanthemum-like NiCo2O4 Mounted on Nickel Foam as a Highly Efficient and Stable Electrocatalyst for the Oxygen Evolution Reaction, Nanoscale, 2020, 12(13), 7180–7187, 10.1039/C9NR10141A.
- M. Jahan, Z. Liu and K. P. Loh, A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR, Adv. Funct. Mater., 2013, 23(43), 5363–5372, DOI:10.1002/adfm.201300510.
- S.-K. Lee, S. D. George, W. E. Antholine, B. Hedman, K. O. Hodgson and E. I. Solomon, Nature of the Intermediate Formed in the Reduction of O2 to H2O at the Trinuclear Copper Cluster Active Site in Native Laccase, J. Am. Chem. Soc., 2002, 124(21), 6180–6193, DOI:10.1021/ja0114052.
- B. Chi, H. Lin and J. Li, Cations Distribution of CuxCo3−xO4 and Its Electrocatalytic Activities for Oxygen Evolution Reaction, Int. J. Hydrogen Energy, 2008, 33(18), 4763–4768, DOI:10.1016/j.ijhydene.2008.05.032.
- P. Li, J. Wang, N. Cai, L. Wang, J. Tong and F. Yu, Self-Supported Electrocatalysts for Efficient Oxygen Evolution Reaction: Hierarchical CuOx@CoO Nanorods Grown on Cu Foam, ChemCatChem, 2020, 12(6), 1639–1646, DOI:10.1002/cctc.201902170.
- Z. Yin, S. Zhang, W. Chen, M. Xinzhi, Y. Zhou, Z. Zhang, X. Wang and J. Li, Hybrid-Atom-Doped NiMoO4 Nanotubes for Oxygen Evolution Reaction, New J. Chem., 2020, 44(40), 17477–17482, 10.1039/D0NJ02305A.
- T. Zhang, Z. Li, L. Wang, P. Sun, Z. Zhang and S. Wang, Spinel MnCo2O4 Nanoparticles Supported on Three-Dimensional Graphene with Enhanced Mass Transfer as an Efficient Electrocatalyst for the Oxygen Reduction Reaction, ChemSusChem, 2018, 11(16), 2730–2736, DOI:10.1002/cssc.201801070.
- A. Maiti, Cobalt-Based Heterogeneous Catalysts in an Electrolyzer System for Sustainable Energy Storage, Dalton Trans., 2020, 49(33), 11430–11450, 10.1039/D0DT01469A.
- Y. P. Zhu, T. Y. Ma, M. Jaroniec and S. Z. Qiao, Self-Templating Synthesis of Hollow Co3O4 Microtube Arrays for Highly Efficient Water Electrolysis, Angew. Chem., Int. Ed., 2017, 56(5), 1324–1328, DOI:10.1002/anie.201610413.
- M. Kuang, P. Han, Q. Wang, J. Li and G. Zheng, CuCo Hybrid Oxides as Bifunctional Electrocatalyst for Efficient Water Splitting, Adv. Funct. Mater., 2016, 26(46), 8555–8561, DOI:10.1002/adfm.201604804.
- S. Anantharaj, S. R. Ede, K. Karthick, S. S. Sankar, K. Sangeetha, P. E. Karthik and S. Kundu, Precision and Correctness in the Evaluation of Electrocatalytic Water Splitting: Revisiting Activity Parameters with a Critical Assessment, Energy Environ. Sci., 2018, 11(4), 744–771, 10.1039/C7EE03457A.
- X. Lu and C. Zhao, Electrodeposition of Hierarchically Structured Three-Dimensional Nickel–Iron Electrodes for Efficient Oxygen Evolution at High Current Densities, Nat. Commun., 2015, 6(1), 6616, DOI:10.1038/ncomms7616.
- Y. Yang, K. Zhang, H. Lin, X. Li, H. C. Chan, L. Yang and Q. Gao, MoS2–Ni3S2 Heteronanorods as Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting, ACS Catal., 2017, 7(4), 2357–2366, DOI:10.1021/acscatal.6b03192.
- S. Tang, X. Li, M. Courté, J. Peng and D. Fichou, Hierarchical Cu(OH)2@Co(OH)2 Nanotrees for Water Oxidation Electrolysis, ChemCatChem, 2020, 12(16), 4038–4043, DOI:10.1002/cctc.202000558.
- R. Guo, L. Nengzi, Y. Chen, Y. Li, X. Zhang and X. Cheng, Efficient Degradation of Sulfamethoxazole by CuCo LDH and LDH@fibers Composite Membrane Activating Peroxymonosulfate, Chem. Eng. J., 2020, 398, 125676, DOI:10.1016/j.cej.2020.125676.
- D. Cai, D. Wang, B. Liu, L. Wang, Y. Liu, H. Li, Y. Wang, Q. Li and T. Wang, Three-Dimensional Co3O4@NiMoO4 Core/Shell Nanowire Arrays on Ni Foam for Electrochemical Energy Storage, ACS Appl. Mater. Interfaces, 2014, 6(7), 5050–5055, DOI:10.1021/am500060m.
- W. Kang, Y. Tang, W. Li, Z. Li, X. Yang, J. Xu and C.-S. Lee, Porous CuCo2O4 Nanocubes Wrapped by Reduced Graphene Oxide as High-Performance Lithium-Ion Battery Anodes, Nanoscale, 2014, 6(12), 6551–6556, 10.1039/C4NR00446A.
- L. Jiang, S. Zhang, S. A. Kulinich, X. Song, J. Zhu, X. Wang and H. Zeng, Optimizing Hybridization of 1T and 2H Phases in MoS2 Monolayers to Improve Capacitances of Supercapacitors, Mater. Res. Lett., 2015, 3(4), 177–183, DOI:10.1080/21663831.2015.1057654.
- R. Kappera, D. Voiry, S. E. Yalcin, B. Branch, G. Gupta, A. D. Mohite and M. Chhowalla, Phase-Engineered Low-Resistance Contacts for Ultrathin MoS2 Transistors, Nat. Mater., 2014, 13(12), 1128–1134, DOI:10.1038/nmat4080.
- S. Zhu, J. Lei, Y. Qin, L. Zhang and L. Lu, Spinel Oxide CoFe2O4 Grown on Ni Foam as an Efficient Electrocatalyst for Oxygen Evolution Reaction, RSC Adv., 2019, 9(23), 13269–13274, 10.1039/C9RA01802F.
- X. Xiong, C. You, Z. Liu, A. M. Asiri and X. Sun, Co-Doped CuO Nanoarray: An Efficient Oxygen Evolution Reaction Electrocatalyst with Enhanced Activity, ACS Sustainable Chem. Eng., 2018, 6(3), 2883–2887, DOI:10.1021/acssuschemeng.7b03752.
- Z. Xue, Y. Li, Y. Zhang, W. Geng, B. Jia, J. Tang, S. Bao, H.-P. Wang, Y. Fan, Z. Wei, Z. Zhang, Z. Ke, G. Li and C.-Y. Su, Modulating Electronic Structure of Metal-Organic Framework for Efficient Electrocatalytic Oxygen Evolution, Adv. Energy Mater., 2018, 8(29), 1801564, DOI:10.1002/aenm.201801564.
- J. Wang, H. Yu, X. Wang, C. Chen, S. Li, N. Cai, W. Chen, Y. Xue, H. Li and F. Yu, Tri-Metal-Based Hollow Nanorods-on-Microrod Arrays as Efficient Water Splitting Electrocatalysts, J. Ind. Eng. Chem., 2022, 105, 427–434, DOI:10.1016/j.jiec.2021.10.007.
- Z. Xue, K. Liu, Q. Liu, Y. Li, M. Li, C.-Y. Su, N. Ogiwara, H. Kobayashi, H. Kitagawa, M. Liu and G. Li, Missing-Linker Metal-Organic Frameworks for Oxygen Evolution Reaction, Nat. Commun., 2019, 10(1), 5048, DOI:10.1038/s41467-019-13051-2.
- Y. Li, B. Jia, Y. Fan, K. Zhu, G. Li and C.-Y. Su, Bimetallic Zeolitic Imidazolite Framework Derived Carbon Nanotubes Embedded with Co Nanoparticles for Efficient Bifunctional Oxygen Electrocatalyst, Adv. Energy Mater., 2018, 8(9), 1702048, DOI:10.1002/aenm.201702048.
- B. Jia, Z. Xue, Q. Liu, Q. Liu, K. Liu, M. Liu, T.-S. Chan, Y. Li, Z. Li, C.-Y. Su and G. Li, Hierarchical Nanotubes Constructed from CoSe2 Nanorods with an Oxygen-Rich Surface for an Efficient Oxygen Evolution Reaction, J. Mater. Chem. A, 2019, 7(25), 15073–15078, 10.1039/C9TA03606G.
- Z. Chen, X. Duan, W. Wei, S. Wang and B.-J. Ni, Recent Advances in Transition Metal-Based Electrocatalysts for Alkaline Hydrogen Evolution, J. Mater. Chem. A, 2019, 7(25), 14971–15005, 10.1039/C9TA03220G.
- H. Wan, X. Liu, H. Wang, R. Ma and T. Sasaki, Recent Advances in Developing High-Performance Nanostructured Electrocatalysts Based on 3d Transition Metal Elements, Nanoscale Horiz., 2019, 4(4), 789–808, 10.1039/C8NH00461G.
- C. Hu, L. Zhang and J. Gong, Recent Progress Made in the Mechanism Comprehension and Design of Electrocatalysts for Alkaline Water Splitting, Energy Environ. Sci., 2019, 12(9), 2620–2645, 10.1039/C9EE01202H.
- H. Sun, Z. Yan, F. Liu, W. Xu, F. Cheng and J. Chen, Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution, Adv. Mater., 2020, 32(3), 1806326, DOI:10.1002/adma.201806326.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2se01297a |
| This journal is © The Royal Society of Chemistry 2023 |