Open Access Article
Open Access ArticleEfficient manipulation of Förster resonance energy transfer through host–guest interaction enables tunable white-light emission and devices in heterotopic bisnanohoops†
Yanqing
Fan
,
Shimin
Fan
,
Lin
Liu
 ,
Shengzhu
Guo
,
Jing
He
,
Xiaonan
Li
,
Zhe
Lian
,
Weijie
Guo
,
Xuebo
Chen
,
Shengzhu
Guo
,
Jing
He
,
Xiaonan
Li
,
Zhe
Lian
,
Weijie
Guo
,
Xuebo
Chen
 ,
Ying
Wang
,
Ying
Wang
 and
Hua
Jiang
and
Hua
Jiang
 *
*
College of Chemistry, Beijing Normal University, Beijing 100875, P. R. China. E-mail: jiangh@bnu.edu.cn
First published on 25th September 2023
Abstract
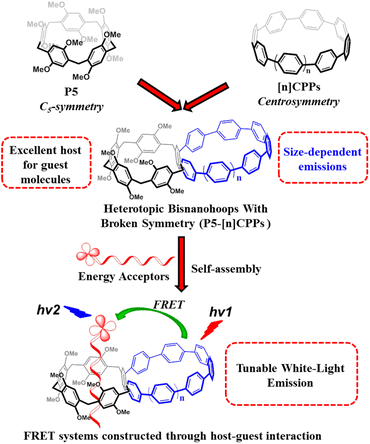
In this study, we synthesized and reported the heterotopic bisnanohoops P5-[8,10]CPPs containing cycloparaphenylenes (CPPs) and a pillar[5]arene unit, which act not only as energy donors but also as a host for binding energy acceptors. We demonstrated that a series of elegant FRET systems could be constructed successfully through self-assembly between donors P5-[8,10]CPPs and acceptors with different emissions via host–guest interaction. These FRET systems further allow us to finely adjust the donors P5-[8,10]CPPs and acceptors (BODIPY-Br and Rh-Br) for achieving multiple color-tunable emissions, particularly white-light emission. More importantly, these host–guest complexes were successfully utilized in the fabrication of white-light fluorescent films and further integrated with a 365 nm LED lamp to create white LED devices. The findings highlight a new application of carbon nanorings in white-light emission materials, beyond the common recognition of π-conjugated molecules.
Introduction
Förster resonance energy transfer (FRET)1 is a distance-dependent photophysical process, in which the energy is transferred from an excited donor to proximal ground-state acceptor fluorophores by means of nonradiative dipole–dipole coupling.1 Efficient FRET requires a substantial spectral overlap between the emission spectrum of the donor and the excitation spectrum of the acceptor, as well as an optimal distance between them.2 Upon excitation of the donor, there is a partial transfer of energy between the donor and acceptor fluorophores, resulting in emission from both interactive partners3 and enabling color-tunable emissions and even white-light emission.4 On the other hand, white-light emission can also be developed through appropriate integrations of fluorophores that emit the three primary colors (red, green and blue) or two complementary colors within a single molecular backbone via covalent linkages.5 However, in addition to the intricate synthesis process, a significant obstacle for achieving white-light emission is the tendency for molecular arrangements to exhibit undesired non-panchromatic emission due to intramolecular FRET or through-bond energy transfer.6 Therefore, an efficient strategy for fabricating FRET systems based on non-covalent bonds has been developed through host–guest interactions, which not only circumvents complex synthesis processes but also minimizes potential emission changes caused by covalent functionalization.7 During the past few decades, various FRET systems based on host–guest interactions8 have been designed and successfully applied in the construction of white-light emitting materials.5d,9Host–guest systems characterized by non-covalent interactions10 have garnered significant attention in recent years due to their facile syntheses, complementary host and guest properties, optical tenability, various supramolecular structures and unique complexation behaviors.11 Such host–guest interaction plays a crucial role in the development of tunable white-light emission because it provides an effective approach to generate white-light emission by self-assembly of multiple donor–acceptor molecules.12 In recent decades, there have been various macrocyclic molecules,13 such as crown ethers,14 cyclodextrins,15 calixarenes,16 cucurbiturils,17 cycloparaphenylenes18 and pillararenes,19 which have been applied to investigate host–guest interaction. Among them, pillar[5]arene is a very attractive host due to its C5-symmetric structure and an overall cylindrical or pillar-like cavity,20 which enable pillar[5]arene and its derivatives to demonstrate excellent host–guest complexing abilities towards electron-poor or neutral guest molecules.21 Therefore, they provide an effective pathway for constructing FRET systems and further fabricating tunable fluorescent materials.10a,22 However, few examples have been investigated regarding the white-light emission achieved through host–guest interactions based on macrocycles.
Strained cycloparaphenylenes (CPPs) consisting of para-linked phenylenes23 not only exhibit a common absorbance maximum while their emissions are red-shifted as their sizes decrease,24 but also can bind fullerenes in their curved cavity.25 We recently reported a [12]CPP nanoring able to bind non-fullerene guests thus forming ternary complexes with a narcissistic chiral self-recognition.26 In the present work, by taking advantage of the exceptional size-dependent photophysical properties of CPPs and the host–guest interaction of pillar[5]arene, we incorporated a pillar[5]arene unit into the CPP framework to generate heterotopic bisnanohoops P5-[8,10]CPPs with broken symmetry, in which the CPP moieties would serve as energy donors while the pillar[5]arene would act as a host for binding various energy acceptors with different emissions for achieving color-tunable emissions including white light by manipulating FRET systems through host–guest interactions between energy donors and acceptors. It is worth noting that the size-dependent photophysical properties of CPPs suggest that their fluorescent colors can be tuned by simply altering their size without changing their excitation wavelengths. In specific, the P5-[8]CPP and P5-[10]CPP not only display green and blue emissions upon excitation at the same wavelength, respectively, but also can host energy acceptors emitting red emission in an organized assembly for achieving FRET systems that emit tunable colors in the visible spectral range (400–700 nm), in particular, white-light emission without cutting off the margin region through a filter. It is very important for developing white-light emitting materials through simultaneously using energy donors with different emissions because a slight deviation in excitation wavelength would destroy the balance of colors comprising white-light emission.
In this article, we synthesized heterotopic bisnanohoops (P5-[8]CPP and P5-[10]CPP) through the incorporation of a pillar[5]arene unit into the CPP framework (Fig. 1) and demonstrated that these elegant FRET systems could be efficiently constructed through self-assembly between P5-[8,10]CPP donors and BODIPY- or rhodamine-based acceptors (BODIPY-R or Rh-R) via host–guest interaction with a high energy transfer efficiency from P5-[8,10]CPPs to BODIPY-R/Rh-R. The combinations of the donors emitting blue or green colors with the acceptors emitting green or red colors through host–guest interactions allow us to finely regulate FRET systems for obtaining multiple color-tunable emissions, particularly white-light emission (Fig. 1). More importantly, these host–guest complexes were not only utilized in the fabrication of white-light fluorescent films but also integrated with a 365 nm LED lamp to create white LED devices. To the best of our knowledge, it is unprecedented that CPPs have been employed for regulating white-light emission. Overall, this work presents a straightforward and efficient strategy for achieving regulation of multiple color emissions, including white light.
Results and discussion
Synthesis and photophysical properties of P5-[8,10]CPPs
In order to construct heterotopic bisnanohoops P5-[8,10]CPPs, we incorporated a pillar[5]arene unit into the CPP framework. Thus, P5-[8]CPP and P5-[10]CPP were designed; their synthesis is outlined in Scheme 1 and the detailed procedures are included in the ESI.† The key precursor P5-[n]CPP-OMe was obtained from the Suzuki cross-coupling reaction between P5-OTf and compound C7 (or C9). A mixture of equimolar amounts of P5-OTf and compound C7 (or C9) was refluxed with Pd(PPh3)4 as a catalyst in THF/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide crude P5-[n]CPP-OMe, which was directly subjected to a reductive aromatization reaction without further purification in the presence of H2SnCl4 to generate the desired products P5-[8]CPP and P5-[10]CPP (ref. 27) with a two-step yield of 26% and 25%, respectively. The structures of P5-[n]CPPs were confirmed by 1H NMR, 13C NMR and MALDI-TOF MS.
1) to provide crude P5-[n]CPP-OMe, which was directly subjected to a reductive aromatization reaction without further purification in the presence of H2SnCl4 to generate the desired products P5-[8]CPP and P5-[10]CPP (ref. 27) with a two-step yield of 26% and 25%, respectively. The structures of P5-[n]CPPs were confirmed by 1H NMR, 13C NMR and MALDI-TOF MS.
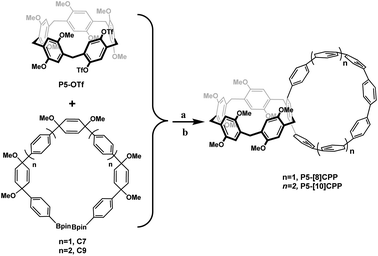 | ||
Scheme 1 Reaction conditions: (a) Pd(PPh3)4, K2CO3, THF/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), N2, 70 °C, 36 h; (b) SnCl2·H2O, HCl, THF, room temperature, 24 h, yield (two steps): P5-[8]CPP, 26%; P5-[10]CPP, 25%. 1), N2, 70 °C, 36 h; (b) SnCl2·H2O, HCl, THF, room temperature, 24 h, yield (two steps): P5-[8]CPP, 26%; P5-[10]CPP, 25%. | ||
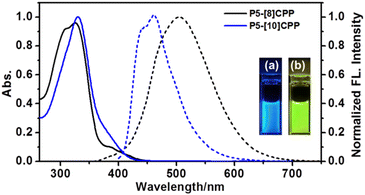
The photophysical properties of P5-[8]CPP and P5-[10]CPP were examined by UV-vis and fluorescence spectroscopies, and the photophysical parameters are summarized in Table 1. Similar to the [n]CPPs, the P5-[n]CPPs have a common absorption maximum at around 330 nm (Fig. 2) from HOMO → LUMO+1 and HOMO → LUMO+2 transitions (Fig. S2 and S3†), which correspond to the calculated absorption maxima (341–366 nm) with large oscillator strength (f) values (Fig. S4 and S5†). A shoulder peak appearing at 380 nm is assigned to the HOMO → LUMO transition for P5-[8]CPP, as well as the HOMO → LUMO forbidden transition for P5-[10]CPP. In addition, in this series, there is a red-shifting fluorescence emission as the size of the hoop decreases (visible as a peak at 460 and 510 nm for P5-[10]CPP and P5-[8]CPP, respectively). Impressively, the fluorescence quantum yields of P5-[8,10]CPPs displayed a significant enhancement reaching 43% for P5-[8]CPP and 74% for P5-[10]CPP, respectively, compared to those of pristine [8]CPP (10%) and [10]CPP (65%). The phenomena were mainly ascribed to symmetry breaking caused by introduction of the pillar[5]arene unit into the CPP backbone. Additionally, fluorescence lifetimes (τ) of P5-[n]CPPs (6.3 and 2.9 ns for P5-[8]CPP and P5-[10]CPP, respectively) decrease in comparison to those of [n]CPPs (17.6 and 6.6 ns for [8]CPP and [10]CPP, respectively) (Tables 1 and S7†). In order to explain enhancement of fluorescence quantum yields, we thus optimized the S1 state of P5-[n]CPPs (Fig. S6†) and calculated the theoretical kr, which was related to two key parameters (oscillator strength and transition energy). The theoretical kr values were calculated to be 1.27 × 108 s−1 for P5-[8]CPP and 3.64 × 108 s−1 for P5-[10]CPP, which were consistent with the experimental ones (Table S5†). It is well known that the oscillator strength originates from the overlap of the frontier molecular orbitals.28 As shown in Fig. S6,† the HOMOs and LUMOs of P5-[n]CPPs at the S1 state all tend to concentrate on cycloparaphenylene portions, so the frontier molecular orbitals exhibit maximum overlap, which results in large oscillator strengths (fmax = 0.5 and 1.23). In addition, the transition energies of P5-[n]CPPs were also calculated to be 5.0 eV, and the synergistic effect between oscillator strength and transition energy led to higher kr values for P5-[n]CPPs. Furthermore, the competition between kr and knr contributed to their high fluorescence quantum yield.
| Compounds | λ ex/nm | λ em/nm | Φ | Lifetime (ns) |
|---|---|---|---|---|
| P5-[8]CPP | 330 | 510 | 43% | 6.3 |
| P5-[10]CPP | 332 | 460 | 74% | 2.9 |
Investigation of the FRET process
It is widely acknowledged that pillar[5]arene exhibits a notable capacity to accommodate numerous alkyl derivatives with moderate to high affinity.29 Accordingly, BODIPY and rhodamine30 were carefully selected and functionalized with different alkyl chains (Fig. 3A). The functionalized fluorescent dyes were utilized as energy acceptors for constructing FRET systems with P5-[n]CPPs through host–guest interactions between the functionalized alkyl chains and pillar[5]arene (Fig. 3B). First, the host–guest interaction between P5-[10]CPP and BODIPY-Br was investigated by 1H NMR titration experiments. As indicated in Fig. S10,† the related 1H NMR signals on BODIPY-Br displayed obvious upfield shifts, while the protons on part of pillar[5]arene in P5-[10]CPP showed an obvious downfield shift upon addition of BODIPY-Br into a solution of P5-[10]CPP in CDCl3 (Fig. S11 and S12†). These observed changes in chemical shifts indicated that the electron-rich cavity of the pillar[5]arene unit within P5-[10]CPP is capable of binding BODIPY-Br to form a host–guest complex with a binding constant 3.20 × 105 M−1 (Ka was calculated from the fluorescence titration experiment), which is higher than those of most previously reported pillar[5]arene derivatives for similar guest molecules.31,32 The enhancements in Ka values may be attributed to the alteration of electron density within it's electron-rich cavity caused by functionalization of pillar[5]arene, which subsequently affects the binding affinity towards guests. These results provided an opportunity for the development of FRET-based P5-[n]CPPsvia host–guest interactions.Generally, one of the most important criteria that should be satisfied for FRET to take place is a significant overlap between the absorption spectrum of the acceptor fluorophore and the emission spectrum of donors. Hence, the investigations on the absorption and emission spectra of P5-[10]CPP and BODIPY-Br revealed that the emission spectrum of P5-[10]CPP (λ = 460 nm) highly overlap with the absorption spectrum of BODIPY-Br (λ = 500 nm) (Fig. S13†). Therefore, it shows great potential to construct a fluorescence energy transfer system through the self-assembly of P5-[10]CPP and BODIPY-Br (Fig. 3B). In order to explore the fluorescence energy transfer from P5-[10]CPP to BODIPY-Br, the fluorescence titration experiment was carried out by gradually adding BODIPY-Br into a solution of P5-[10]CPP in DCM (Fig. 3C(IV)). The results indicate that the gradual addition of BODIPY-Br to a solution of P5-[10]CPP led to a decrease in fluorescence emission of the donor at 460 nm and a simultaneous increase in fluorescence emission of the acceptor at 522 nm, confirming successful energy transfer from the P5-[10]CPP moiety to the BODIPY one, which is consistent with expectations for FRET. The energy transfer efficiency was calculated to be 78% (Table 2). The Ka value was extracted to be 3.20 × 105 M−1 (Fig. 3D(IV)). Additionally, the FRET from P5-[10]CPP to BODIPY-Br was further confirmed by fluorescence decay profiles (Fig. S29†), and the results showed that the lifetime of P5-[10]CPP slightly decreased from 2.90 ns to 2.80 ns upon addition of BODIPY-Br under identical measurement conditions. Furthermore, in order to confirm that FRET resulted from the self-assembly via host–guest interaction between P5-[10]CPP and BODIPY-Br, a control experiment was carried out by gradually adding ester group-decorated BODIPY (BODIPY-EA) into a solution of P5-[10]CPP (Fig. S25†), and the findings showed that the gradual addition of BODIPY-EA to a solution of P5-[10]CPP does not induce a decrease in fluorescence emission at 460 nm, but rather results in an increase in fluorescence emission at 522 nm, which can be attributed to the amplification of BODIPY-EA fluorescence resulting from an increase in its concentration. These observations have revealed that the FRET phenomenon arises from self-assembly through host–guest interactions between the donor (P5-[10]CPP) and acceptor molecules (BODIPY-Br) (Fig. 3B).
| Donors | Acceptors | K a/M−1 (× 105) | Energy transfer efficiency |
|---|---|---|---|
| P5-[8]CPP | Rh-Br | 1.57 ± 0.05 | 53% |
| BODIPY-Br | 1.01 ± 0.16 | 26% | |
| P5-[10]CPP | BODIPY-Br | 3.20 ± 0.09 | 78% |
| BODIPY-Im | 1.04 ± 0.07 | 57% | |
| BODIPY-N3 | 1.06 ± 0.07 | 60% | |
| Rh-Br | 2.62 ± 0.10 | 21% |
A series of similar FRET systems were also successfully constructed by self-assembly of donors (P5-[8]CPP or P5-[10]CPP) with other acceptors (BODIPY-R or Rh-R) (Fig. 3B). The fluorescence titration experiments (Fig. 3C, D and S19–S24†) demonstrated that energies were successfully transferred from the P5-[n]CPPs moiety to the BODIPY (or rhodamine) moiety. The energy transfer efficiency and comparable binding constants were calculated and are listed in Table 2. Furthermore, the alterations in their lifetimes provide additional evidence of energy transfer from donors to acceptors (Fig. S27–S32 and Table S7†). These observations further confirmed the successful fabrication of FRET systems from self-assembly of P5-[n]CPPs and BODIPY-R (or Rh-R).
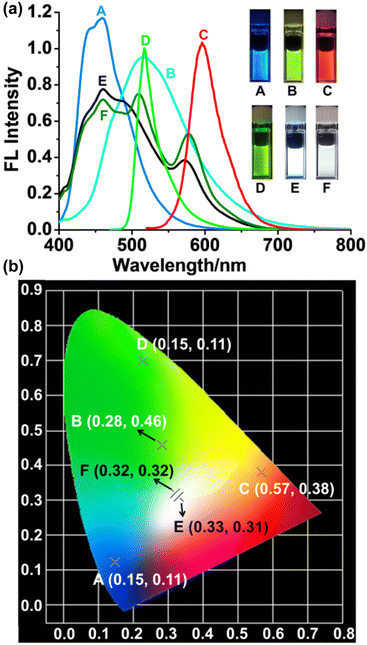
Moreover, it is very apparent from the fluorescent features of these fluorophores (P5-[10]CPP, P5-[8]CPP, BODIPY-Br and Rh-Br) that these compounds emit the three primary colors (red, green and blue, Fig. 4a). The suitable combination of these fluorophores in an organized assembly would cover the visible spectral window (400–700 nm), leading to white-light emission without cutting off the margin region through a filter. By fine adjusting self-assembly between different energy donors and acceptors, various FRET systems with tunable multiple color emissions from purple to blue, green, yellow, orange, then to near-white, and even to pure white were readily prepared without complex synthesis and structural modification (Fig. S33–S44 and Table S8†). In particular, upon mixing P5-[10]CPP, P5-[8]CPP and Rh-Br in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or P5-[10]CPP, P5-[8]CPP, BODIPY-Br and Rh-Br in a molar ratio of 1
1 or P5-[10]CPP, P5-[8]CPP, BODIPY-Br and Rh-Br in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pure white-light emission could be readily obtained (Fig. 4a). In addition, the 1931 Commission Internationale de l'Eclairage (CIE) chromaticity coordinates for the corresponding fluorescence spectra were calculated using “GOCIE” software.33 The observed CIE coordinates for the 1
1, pure white-light emission could be readily obtained (Fig. 4a). In addition, the 1931 Commission Internationale de l'Eclairage (CIE) chromaticity coordinates for the corresponding fluorescence spectra were calculated using “GOCIE” software.33 The observed CIE coordinates for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of P5-[10]CPP, P5-[8]CPP and Rh-Br and for the 1
1 mixture of P5-[10]CPP, P5-[8]CPP and Rh-Br and for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of P5-[10]CPP, P5-[8]CPP, Rh-Br and BODIPY-Br are (0.33, 0.31) and (0.32, 0.33), respectively, which all match well with the CIE coordinates of the natural white light (0.33, 0.33) (Fig. 4b).
1 mixture of P5-[10]CPP, P5-[8]CPP, Rh-Br and BODIPY-Br are (0.33, 0.31) and (0.32, 0.33), respectively, which all match well with the CIE coordinates of the natural white light (0.33, 0.33) (Fig. 4b).
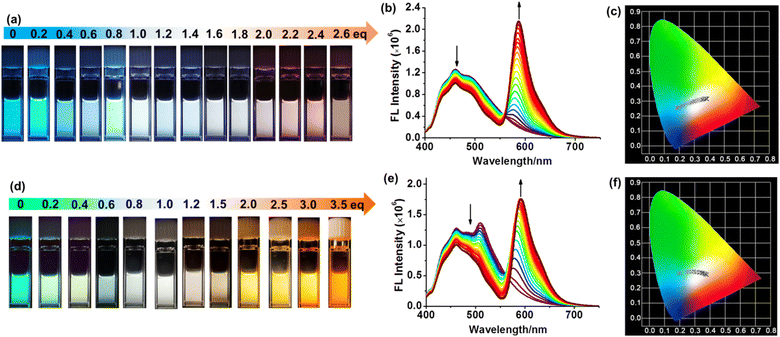
We next conducted a meticulous investigation of the tunable white-light emission process through fluorescence titration experiments, which was carried out by gradually introducing Rh-Br into a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio mixture of P5-[10]CPP and P5-[8]CPP in DCM. The results suggest that the gradual addition of Rh-Br leads to a progressive decrease in the intensity of the fluorescence emission band within the range of 400 nm to 550 nm, accompanied by a simultaneous increase in fluorescence intensity at 580 nm (Fig. 5b). Throughout the process, the addition of varying ratios of the acceptor (Rh-Br) into the donor (a 1
1 molar ratio mixture of P5-[10]CPP and P5-[8]CPP in DCM. The results suggest that the gradual addition of Rh-Br leads to a progressive decrease in the intensity of the fluorescence emission band within the range of 400 nm to 550 nm, accompanied by a simultaneous increase in fluorescence intensity at 580 nm (Fig. 5b). Throughout the process, the addition of varying ratios of the acceptor (Rh-Br) into the donor (a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of P5-[10]CPP and P5-[8]CPP) resulted in a diverse range of emissions spanning from blue to white, and even to red hues (Fig. 5a). In particular, when the ratio of Rh-Br, P5-[10]CPP and P5-[8]CPP is 1
1 mixture of P5-[10]CPP and P5-[8]CPP) resulted in a diverse range of emissions spanning from blue to white, and even to red hues (Fig. 5a). In particular, when the ratio of Rh-Br, P5-[10]CPP and P5-[8]CPP is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the FRET system exhibits white-light emission. These findings in the emission of the FRET system are consistent with the CIE chromaticity diagram, where the emission spectrum shifts from blue to white and even pink hues (Fig. 5c).
1, the FRET system exhibits white-light emission. These findings in the emission of the FRET system are consistent with the CIE chromaticity diagram, where the emission spectrum shifts from blue to white and even pink hues (Fig. 5c).
Moreover, we conducted fluorescence titration experiments to investigate the impact of Rh-Br in mixtures of varying ratios of P5-[10]CPP, P5-[8]CPP and BODIPY-Br on the regulation of white-light emission in the FRET process. First, the fluorescence titration experiments were carried out by dropwise adding Rh-Br into the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio mixture of P5-[8]CPP, P5-[10]CPP and BODIPY-Br in DCM. The results indicate that the gradual addition of Rh-Br led to a gradual decrease in the intensity of the fluorescence emission band ranging from 400 nm to 550 nm, while the fluorescence intensity at 593 nm gradually increased (Fig. 5e). Simultaneously, the mixture of Rh-Br and P5-[10]CPP/P5-[8]CPP/BODIPY (1
1 ratio mixture of P5-[8]CPP, P5-[10]CPP and BODIPY-Br in DCM. The results indicate that the gradual addition of Rh-Br led to a gradual decrease in the intensity of the fluorescence emission band ranging from 400 nm to 550 nm, while the fluorescence intensity at 593 nm gradually increased (Fig. 5e). Simultaneously, the mixture of Rh-Br and P5-[10]CPP/P5-[8]CPP/BODIPY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in different ratios yields multiple color emissions from blue-green to white, even orange (Fig. 5d). When Rh-Br, P5-[10]CPP, P5-[8]CPP, and BODIPY-Br reached a ratio of 1
1) in different ratios yields multiple color emissions from blue-green to white, even orange (Fig. 5d). When Rh-Br, P5-[10]CPP, P5-[8]CPP, and BODIPY-Br reached a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the FRET system displayed white-light emission. In addition, the CIE chromaticity diagram demonstrated a well-matched correlation with these changes in multi-color emission, transitioning from the blue-green region to white and even orange (Fig. 5f). Similarly, upon gradual addition of Rh-Br into the 1
1, the FRET system displayed white-light emission. In addition, the CIE chromaticity diagram demonstrated a well-matched correlation with these changes in multi-color emission, transitioning from the blue-green region to white and even orange (Fig. 5f). Similarly, upon gradual addition of Rh-Br into the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 or 1
0.5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio mixture of P5-[10]CPP, P5-[8]CPP and BODIPY-Br, respectively, a similar change in fluorescence emission could also be observed (Fig. S47 and S49†), which are consistent with the CIE chromaticity diagram, changing from a blue-green region to a white, even an orange one (Fig. S47 and S49†). The evidence obtained from these experiments suggests that different donor ratios demonstrate a consistent FRET process for Rh-Br, resulting in the emission of fluorescence shifting from blue-green to white or even orange, as well as enabling the creation of a white-light system.
2 ratio mixture of P5-[10]CPP, P5-[8]CPP and BODIPY-Br, respectively, a similar change in fluorescence emission could also be observed (Fig. S47 and S49†), which are consistent with the CIE chromaticity diagram, changing from a blue-green region to a white, even an orange one (Fig. S47 and S49†). The evidence obtained from these experiments suggests that different donor ratios demonstrate a consistent FRET process for Rh-Br, resulting in the emission of fluorescence shifting from blue-green to white or even orange, as well as enabling the creation of a white-light system.
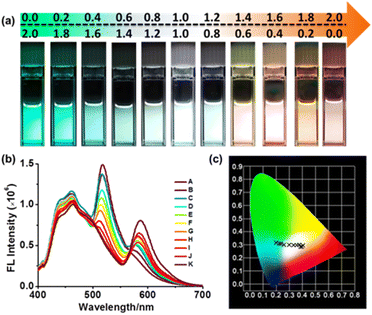
To further investigate the impact of different ratios of acceptors Rh-Br and BODIPY-Br on the regulation of white-light emission in FRET processes, we prepared various ratios of acceptors by increasing the ratio of Rh-Br to BODIPY-Br from 0.0 to 2.0 equivalents. As shown in Fig. 6b, upon the addition of the aforementioned acceptors to a solution containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio mixture of P5-[10]CPP and P5-[8]CPP in DCM, respectively, the fluorescence emission in the range of 400–550 nm exhibited a decrease, while the fluorescence intensity at 593 nm showed an increase. The fluorescence exhibited a color shift from green to white and even pink hues, which was in great agreement with the CIE chromaticity diagram (Fig. 6a, S51 and S52†). More importantly, when Rh-Br, BODIPY-Br, P5-[10]CPP and P5-[8]CPP reached a ratio of 1
1 ratio mixture of P5-[10]CPP and P5-[8]CPP in DCM, respectively, the fluorescence emission in the range of 400–550 nm exhibited a decrease, while the fluorescence intensity at 593 nm showed an increase. The fluorescence exhibited a color shift from green to white and even pink hues, which was in great agreement with the CIE chromaticity diagram (Fig. 6a, S51 and S52†). More importantly, when Rh-Br, BODIPY-Br, P5-[10]CPP and P5-[8]CPP reached a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, that system displayed white-light emission. These findings further suggest that different acceptor-to-donor ratios can facilitate efficient FRET processes with multi-color emission and result in white-light emission.
1, that system displayed white-light emission. These findings further suggest that different acceptor-to-donor ratios can facilitate efficient FRET processes with multi-color emission and result in white-light emission.
Finally, we prepared the PMMA films of P5-[10]CPP, P5-[8]CPP, the mixture of P5-[10]CPP, P5-[8]CPP and Rh-Br (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the mixture of P5-[10]CPP, P5-[8]CPP, BODIPY-Br and Rh-Br (1
1), and the mixture of P5-[10]CPP, P5-[8]CPP, BODIPY-Br and Rh-Br (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. Under a UV lamp excited at λ = 365 nm, the PMMA films of P5-[8]CPP and P5-[10]CPP exhibit green and blue emissions, respectively. Significantly, the PMMA film exhibits white-light emission upon doping with 1 equivalent of Rh-Br or a 1
1), respectively. Under a UV lamp excited at λ = 365 nm, the PMMA films of P5-[8]CPP and P5-[10]CPP exhibit green and blue emissions, respectively. Significantly, the PMMA film exhibits white-light emission upon doping with 1 equivalent of Rh-Br or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Rh-Br and BODIPY-Br in the 1
1 mixture of Rh-Br and BODIPY-Br in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio mixture of P5-[10]CPP and P5-[8]CPP, thus enabling its potential use in solid-state lighting applications (Fig. 7A). Inspired by this, a white-light-emitting diode was fabricated by coating a 365 nm light-emitting diode (LED) bulb with a solution containing PMMA and the aforementioned mixture (1
1 ratio mixture of P5-[10]CPP and P5-[8]CPP, thus enabling its potential use in solid-state lighting applications (Fig. 7A). Inspired by this, a white-light-emitting diode was fabricated by coating a 365 nm light-emitting diode (LED) bulb with a solution containing PMMA and the aforementioned mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio mixture of P5-[10]CPP, P5-[8]CPP and Rh-Br; and 1
1 ratio mixture of P5-[10]CPP, P5-[8]CPP and Rh-Br; and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio mixture of P5-[10]CPP, P5-[8]CPP, BODIPY-Br and Rh-Br) with white-light emission (Fig. 7B). With the application of a 3 V voltage, these LEDs emit bright white light (Fig. 7C), suggesting that P5-[n]CPPs-based drop-cast films with white-light emission may hold potential for future applications in OLED light-emitting devices.
1 ratio mixture of P5-[10]CPP, P5-[8]CPP, BODIPY-Br and Rh-Br) with white-light emission (Fig. 7B). With the application of a 3 V voltage, these LEDs emit bright white light (Fig. 7C), suggesting that P5-[n]CPPs-based drop-cast films with white-light emission may hold potential for future applications in OLED light-emitting devices.
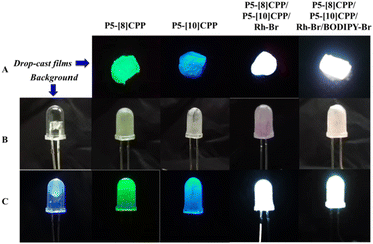 | ||
| Fig. 7 (A) Drop-cast films; (B) photographs of uncoated LED bulb and coated LED bulb before illumination; (C) photographs of uncoated LED bulb and coated LED bulb after illumination. | ||
Conclusions
In conclusion, we synthesized heterotopic bisnanohoops (P5-[8,10]CPPs) by imbedding a pillar[5]arene unit into the CPP skeleton. The P5-[8,10]CPPs showed excellent photophysical properties. Importantly, a series of elegant FRET systems containing donors (P5-[8,10]CPPs) and acceptors (BODIPY-R or Rh-Br) were constructed by host–guest interactions. Within these FRET systems, the fluorescence energy was transferred from the CPP unit to the BODIPY (or rhodamine) part with the energy transfer efficiency reaching 78%. Significantly, by fine regulating the self-assemblies between donors (P5-[8,10]CPP) and acceptors (BODIPY-Br and Rh-Br) in these FRET systems, multiple color-tunable emissions, particularly white-light emission, were readily achieved without complex synthesis and structural modification. More importantly, these host–guest interactions could not only be utilized in the fabrication of white-light fluorescent films but also integrated with a 365 nm LED lamp to create white LED devices. The findings highlight a new application of carbon nanorings in white-light emitting materials, beyond the common recognition of π-conjugated molecules. The present studies suggest that carbon nanorings can offer a highly efficient and versatile platform for developing white-light emitting devices with potential applications in lighting, displays and sensing technologies.Data availability
The data that support the findings of this study are available in the ESI† or on request from the corresponding author.Author contributions
Yanqing Fan designed the experiments, synthesized the compounds, carried out the characterization, and collected and analyzed the data. Hua Jiang and Yanqing Fan wrote the manuscript. Shimin Fan, Jing He, Shengzhu Guo, Zhe Lian, Xiaonan Li and Weijie Guo synthesized partial compounds. Ying Wang analyzed the data; Lin Liu and Xuebo Chen carried out theoretical calculations. Hua Jiang supervised the project. All authors participated in the discussion and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22271019 and 21971020) and Beijing Natural Science Foundation (2212008).Notes and references
- (a) T. Förster, Ann. Phys., 1948, 437, 55–75 CrossRef; (b) T. Förster, Discuss. Faraday Soc., 1959, 27, 7–17 RSC; (c) L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X. P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110–5139 RSC; (d) W. R. Algar, N. Hildebrandt, S. S. Vogel and I. L. Medintz, Nat. Methods, 2019, 16, 815–829 CrossRef CAS PubMed; (e) Z. Lian, J. He, L. Liu, Y. Q. Fan, X. B. Chen and H. Jiang, Nat. Commun., 2023, 14, 2752 CrossRef CAS PubMed.
- (a) H. Q. Peng, L. Y. Niu, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Rev., 2015, 115, 7502–7542 CrossRef CAS PubMed; (b) Z. Y. Zhang, Z. Q. Zhao, L. W. Wu, S. Lu, S. L. Ling, G. P. Li, L. T. Xu, L. Z. Ma, Y. L. Hou, X. C. Wang, X. P. Li, G. He, K. Wang, B. Zou and M. M. Zhang, J. Am. Chem. Soc., 2020, 142, 2592–2600 CrossRef CAS PubMed; (c) Z. M. Hudson, D. J. Lunn, M. A. Winnik and I. Manners, Nat. Commun., 2014, 5, 3372 CrossRef PubMed; (d) A. Ajayaghosh, V. K. Praveen, C. Vijayakumar and S. J. George, Angew. Chem., Int. Ed., 2007, 46, 6260 CrossRef CAS PubMed; (e) C. Vijayakumar, K. Sugiyasu and M. Takeuchi, Chem. Sci., 2011, 2, 291 RSC; (f) C. Giansante, G. Raffy, C. Schäfer, H. Rahma, M. Kao, A. G. L. Olive and A. Del Guerzo, J. Am. Chem. Soc., 2011, 133, 316 CrossRef CAS PubMed; (g) D. K. Maiti, R. Bhattacharjee, A. Datta and A. Banerjee, J. Phys. Chem. C, 2013, 117, 23178 CrossRef CAS; (h) S. Bhattacharyya, B. Jana and A. Patra, ChemPhysChem, 2015, 16, 796 CrossRef CAS PubMed; (i) J. Xu, A. Takai and M. Takeuchi, Chem.–Eur. J., 2016, 22, 13014 CrossRef CAS PubMed.
- (a) S. Kim, S. Yoon and S. Y. Park, J. Am. Chem. Soc., 2012, 134, 12091 CrossRef CAS PubMed; (b) Q. Zhao, Y. Chen, S. Li and Y. Liu, Chem. Commun., 2018, 54, 200 RSC.
- (a) Y. L. Hou, Z. Y. Zhang, S. Lu, J. Yuan, Q. Y. Zhu, W. P. Chen, S. L. Ling, X. P. Li, Y. Z. Zheng, K. L. Zhu and M. M. Zhang, J. Am. Chem. Soc., 2020, 142, 18763–18768 CrossRef CAS PubMed; (b) H. F. Liu, C. X. Guo, Z. Y. Zhang, C. Q. Mu, Q. Feng and M. M. Zhang, Chem.–Eur. J., 2023, 29, e202203926 CrossRef CAS PubMed; (c) P. Pallavi, B. Sk, P. Ahir and A. Patra, Chem.–Eur. J., 2018, 24, 1151–1158 CrossRef CAS PubMed.
- (a) C. Vijayakumar, V. K. Praveen and A. Ajayaghosh, Adv. Mater., 2009, 21, 2059–2063 CrossRef CAS; (b) P. Pallavi, S. Bandyopadhyay, J. Louis, A. Deshmukh and A. Patra, Chem. Commun., 2017, 53, 1257–1260 RSC; (c) X. Zhang, S. Rehm, M. M. Safont-Sempere and F. Werthner, Nat. Chem., 2009, 1, 623–629 CrossRef CAS PubMed; (d) R. Wang, J. Peng, F. Qiu and Y. Yang, Chem. Commun., 2011, 47, 2787–2789 RSC; (e) M. Bälter, S. Li, M. Morimoto, S. Tang, J. Hernando, G. Guirado, M. Irie, F. M. Raymo and J. Andréasson, Chem. Sci., 2016, 7, 5867–5871 RSC; (f) Z. Chen, C. L. Ho, L. Q. Wang and W. Y. Wong, Adv. Mater., 2020, 32, 1903269 CrossRef CAS PubMed; (g) D. F. Li, W. D. Hu, J. Wang, Q. W. Zhang, X. M. Cao, X. Ma and H. Tian, Chem. Sci., 2018, 9, 5709–5715 RSC; (h) Z. K. He, W. J. Zhao, J. W. Y. Lam, Q. Peng, H. L. Ma, G. D. Liang, Z. G. Shuai and B. Z. Tang, Nat. Commun., 2017, 8, 416 CrossRef PubMed; (i) J. G. Wang, X. G. Gu, H. L. Ma, Q. Peng, X. B. Huang, X. Y. Zheng, S. H. P. Sung, G. G. Shan, J. W. Y. Lam, Z. G. Shuai and B. Z. Tang, Nat. Commun., 2018, 9, 2963 CrossRef PubMed.
- (a) A. V. Macatangay, J. F. Endicott and X. Song, J. Phys. Chem. A, 1998, 102, 7537 CrossRef CAS; (b) E. Aharon, M. Kalina and G. L. Frey, J. Am. Chem. Soc., 2006, 128, 15968 CrossRef CAS PubMed; (c) S. Kundu, B. Sk, P. Pallavi, A. Giri and A. Patra, Chem.–Eur. J., 2020, 26, 5557–5582 CrossRef CAS PubMed; (d) S. Park, J. E. Kwon, S. H. Kim, J. Seo, K. Chung, S. Y. Park, D. J. Jang, B. M. Medina, J. Gierschner and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 14043–14049 CrossRef CAS PubMed.
- A. J. P. Teunissen, C. Perez-Medina, A. Meijerink and W. J. M. Mulder, Chem. Soc. Rev., 2018, 47, 7027–7044 RSC.
- (a) H. Q. Peng, Y. Z. Chen, Y. Zhao, Q. Z. Yang, L. Z. Wu, C. H. Tung, L. P. Zhang and Q. X. Tong, Angew. Chem., Int. Ed., 2012, 51, 2088–2092 CrossRef CAS PubMed; (b) Z. Xu, S. Peng, Y. Y. Wang, J. K. Zhang, A. I. Lazar and D. S. Guo, Adv. Mater., 2016, 28, 7666–7671 CrossRef CAS PubMed; (c) P. Z. Chen, Y. X. Weng, L. Y. Niu, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, Angew. Chem., Int. Ed., 2016, 55, 2759–2763 CrossRef CAS PubMed; (d) S. Guo, Y. Song, Y. He, X. Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2018, 57, 3163–3167 CrossRef CAS PubMed.
- (a) V. K. Praveen, C. Ranjith and N. Armaroli, Angew. Chem., Int. Ed., 2014, 53, 365–368 CrossRef CAS PubMed; (b) A. Khan, S. Mallik and A. L. Koner, J. Org. Chem., 2023, 88, 6765–6775 CrossRef CAS PubMed; (c) X. H. Jin, C. Chen, C. X. Ren, L. X. Cai and J. Zhang, Chem. Commun., 2014, 50, 15878–15881 RSC.
- (a) F. C. Ho, Y. J. Huang, C. C. Weng, C. H. Wu, Y. K. Li, J. I. Wu and H. C. Lin, ACS Appl. Mater. Interfaces, 2020, 12, 53257–53273 CrossRef CAS PubMed; (b) W. C. Geng, J. L. Sessler and D. S. Guo, Chem. Soc. Rev., 2020, 49, 2303–2315 RSC; (c) X. F. Ji, M. Ahmed, L. L. Long, N. M. Khashab, F. H. Huang and J. L. Sessler, Chem. Soc. Rev., 2019, 48, 2682–2697 RSC; (d) H. J. Schneider and A. K. Yatsimirsky, Chem. Soc. Rev., 2008, 37, 263–277 RSC.
- (a) M. A. Beatty and F. Hof, Chem. Soc. Rev., 2021, 50, 4812–4832 RSC; (b) O. Chatterjee, A. Pramanik and A. L. Koner, Org. Mater., 2022, 4, 228–239 CrossRef CAS; (c) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed; (d) D. A. Roberts, B. S. Pilgrim and J. R. Nitschke, Chem. Soc. Rev., 2018, 47, 626–644 RSC.
- (a) Y. Liu, M. Nishiura, Y. Wang and Z. Hou, J. Am. Chem. Soc., 2006, 128, 5592 CrossRef CAS PubMed; (b) H. Wu, L. Ying, W. Yang and Y. Cao, Chem. Soc. Rev., 2009, 38, 3391 RSC; (c) S. Tang, J. Pan, H. A. Buchholz and L. Edman, J. Am. Chem. Soc., 2013, 135, 3647 CrossRef CAS PubMed; (d) N. Zhang, C. Sun, X. Jiang, X. Xing, Y. Yan, L. Cai, M. Wang and G. Guo, Chem. Commun., 2017, 53, 9269 RSC; (e) H. Wang, Y. Li, Y. Zhang, J. Mei and J. Su, Chem. Commun., 2019, 55, 1879 RSC; (f) H. Wang, X. F. Ji, Z. T. Li and F. H. Huang, Adv. Mater., 2017, 29, 1606117 CrossRef PubMed; (g) X. F. Ji, B. B. Shi, H. Wang, D. Y. Xia, K. C. Jie, Z. L. Wu and F. H. Huang, Adv. Mater., 2015, 27, 8062–8066 CrossRef CAS PubMed; (h) G. J. He, D. Guo, C. He, X. L. Zhang, X. W. Zhao and C. Y. Duan, Angew. Chem., Int. Ed., 2009, 48, 6132–6135 CrossRef CAS PubMed; (i) B. B. Shi, K. C. Jie, Y. J. Zhou, J. Zhou, D. Y. Xia and F. H. Huang, J. Am. Chem. Soc., 2016, 138, 80–83 CrossRef CAS PubMed; (j) Y. Q. Fan, J. Liu, Y. Y. Chen, X. W. Guan, J. Wang, H. Yao, Y. M. Zhang, T. B. Wei and Q. Lin, J. Mater. Chem. C, 2018, 6, 13331–13335 RSC; (k) Q. W. Zhang, D. F. Li, X. Li, P. B. White, J. Mecinović, X. Ma, H. Ågren, R. J. M. Nolte and H. Tian, J. Am. Chem. Soc., 2016, 138, 13541–13550 CrossRef CAS PubMed; (l) D. Li, J. Wang and X. Ma, Adv. Opt. Mater., 2018, 6, 1800273 CrossRef.
- D. Y. Xia, P. Wang, X. F. Ji, N. M. Khashab, J. L. Sessler and F. H. Huang, Chem. Rev., 2020, 120, 6070–6123 CrossRef CAS PubMed.
- A. Inthasot, S. T. Tung and S. H. Chiu, Acc. Chem. Res., 2018, 51, 1324–1337 CrossRef CAS PubMed.
- (a) X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS PubMed; (b) D. F. Li, F. F. Lu, J. Wang, W. D. Hu, X. M. Cao, X. Ma and H. Tian, J. Am. Chem. Soc., 2018, 140, 1916–1923 CrossRef CAS PubMed.
- H. W. Tian, Y. C. Liu and D. S. Guo, Mater. Chem. Front., 2020, 4, 46–98 RSC.
- Y. Xia, S. Y. Chen and X. L. Ni, ACS Appl. Mater. Interfaces, 2017, 10, 13048–13052 CrossRef PubMed.
- (a) E. S. Hirst and R. Jasti, J. Org. Chem., 2012, 77, 10473–10478 CrossRef CAS PubMed; (b) H. Y. Zhao, Y. C. Ma, L. Cao, S. Q. Huang, J. P. Zhang and X. Y. Yan, J. Org. Chem., 2019, 84, 5230–5235 CrossRef CAS PubMed; (c) S. E. Lewis, Chem. Soc. Rev., 2015, 44, 2221–2304 RSC.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed; (b) T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed; (c) K. Kato, S. X. Fa, S. Ohtani, T. H. Shi, A. M. Brouwer and T. Ogoshi, Chem. Soc. Rev., 2022, 51, 3648–3687 RSC; (d) P. J. Cragg and K. Sharma, Chem. Soc. Rev., 2012, 41, 597–607 RSC.
- (a) K. Du and A. C. H. Sue, Synlett, 2019, 30, 2209–2215 CrossRef CAS; (b) J. Ding, J. Chen, W. Mao, J. Huang and D. Ma, Org. Biomol. Chem., 2017, 15, 7894–7897 RSC; (c) M. Guo, X. Wang, C. Zhan, P. Demay-Drouhard, W. Li, K. Du, M. A. Olson, H. Zuilhof and A. C. H. Sue, J. Am. Chem. Soc., 2018, 140, 74–77 CrossRef CAS PubMed; (d) P. Demay-Drouhard, K. Du, K. Samanta, X. Wan, W. Yang, R. Srinivasan, A. C. H. Sue and H. Zuilhof, Org. Lett., 2019, 21, 3976–3980 CrossRef CAS PubMed; (e) C. H. Liu, J. B. Yao, C. Xiao, T. Zhao, N. Selvapalam, C. S. Zhou, W. H. Wu and C. Yang, Org. Lett., 2021, 23, 3885–3890 CrossRef CAS PubMed.
- (a) J. R. Wu, G. X. Wu, D. X. Li and Y. W. Yang, Angew. Chem., Int. Ed., 2023, 62, e202218142 CrossRef CAS PubMed; (b) Z. Q. Wang, X. Wang and Y. W. Yang, Adv. Mater., 2023, 35, 2210551 CrossRef PubMed; (c) H. T. Z. Zhu, Q. Li, W. J. Zhu and F. H. Huang, Acc. Mater. Res., 2022, 3, 658–668 CrossRef CAS; (d) L. E. Khalil-Cruz, P. Liu, F. H. Huang and N. M. Khashab, ACS Appl. Mater. Interfaces, 2021, 13, 31337–31354 CrossRef CAS PubMed.
- (a) S. Y. Sun, M. Geng, L. Huang, Y. M. Chen, M. P. Cen, D. Lu, A. W. Wang, Y. Wang, Y. J. Shi and Y. Yao, Chem. Commun., 2018, 54, 13006–13009 RSC; (b) C. B. Huang, L. Xu, J. L. Zhu, Y. X. Wang, B. Sun, X. P. Li and H. B. Yang, J. Am. Chem. Soc., 2017, 139, 9459–9462 CrossRef CAS PubMed; (c) P. P. Jia, L. Xu, Y. X. Hu, W. J. Li, X. Q. Wang, Q. H. Ling, X. L. Shi, G. Q. Yin, X. P. Li, H. T. Sun, Y. R. Jiang and H. B. Yang, J. Am. Chem. Soc., 2021, 143, 399–408 CrossRef CAS PubMed; (d) Q. Li, Y. T. Wu, J. J. Cao, Y. Liu, Z. J. Wang, H. T. Z. Zhu, H. H. Zhang and F. H. Huang, Angew. Chem., Int. Ed., 2022, 61, e202202381 CrossRef CAS PubMed.
- (a) Z. Liu, S. M. Nalluri and J. F. Stoddart, Chem. Soc. Rev., 2017, 46, 2459–2478 RSC; (b) R. Jasti, J. Bhattacharjee, J. B. Neaton and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 17646–17647 CrossRef CAS PubMed; (c) Y. Segawa, A. Yagi, K. Matsui and K. Itami, Angew. Chem., Int. Ed., 2016, 55, 5136–5158 CrossRef CAS PubMed; (d) J. He, M. H. Yu, Z. Lian, Y. Q. Fan, S. Z. Guo, X. N. Li, Y. Wang, W. G. Wang, Z. Y. Cheng and H. Jiang, Chem. Sci., 2023, 14, 4426–4433 RSC; (e) J. He, M. H. Yu, M. F. Pang, Y. Q. Fan, Z. Lian, Y. Wang, W. G. Wang, Y. J. Liu and H. Jiang, Chem.–Eur. J., 2022, 28, e202103832 CrossRef CAS PubMed; (f) E. Kayahara, L. Sun, H. Onishi, K. Suzuki, T. Fukushima, A. Sawada, H. Kaji and S. Yamago, J. Am. Chem. Soc., 2017, 139, 18480–18483 CrossRef CAS PubMed; (g) W. Zhang and J. S. Moore, Angew. Chem., Int. Ed., 2006, 45, 4416–4439 CrossRef CAS PubMed; (h) U. H. Bunz, S. Menning and N. Martin, Angew. Chem., Int. Ed., 2012, 51, 7094–7101 CrossRef CAS PubMed; (i) M. A. Majewski and M. Stepien, Angew. Chem., Int. Ed., 2019, 58, 86–116 CrossRef CAS PubMed; (j) X. N. Li, L. Y. Jia, W. G. Wang, Y. Wang, D. Sun and H. Jiang, J. Mater. Chem. C, 2023, 11, 1429–1434 RSC.
- (a) E. R. Darzi and R. Jasti, Chem. Soc. Rev., 2015, 44, 6401–6410 RSC; (b) Y. Segawa, A. Fukazawa, S. Matsuura, H. Omachi, S. Yamaguchi, S. Irle and K. Itami, Org. Biomol. Chem., 2012, 10, 5979–5984 RSC; (c) T. C. Lovell, C. E. Colwell, L. N. Zakharovb and R. Jasti, Chem. Sci., 2019, 10, 3786–3790 RSC.
- (a) T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342–8344 CrossRef CAS PubMed; (b) Z. L. Qiu, C. Tang, X. R. Wang, Y. Y. Ju, K. S. Chu, Z. Y. Deng, H. Hou, Y. M. Liu and Y. Z. Tan, Angew. Chem., Int. Ed., 2020, 59, 20868–20872 CrossRef CAS PubMed; (c) Y. Nakanishi, H. Omachi, S. Matsuura, Y. Miyata, R. Kitaura, Y. Segawa, K. Itami and H. Shinohara, Angew. Chem., Int. Ed., 2014, 53, 3102–3106 CrossRef CAS PubMed; (d) A. Stergiou, J. Rio, J. H. Griwatz, D. Arčon, H. A. Wegner, C. P. Ewels and N. Tagmatarchis, Angew. Chem., Int. Ed., 2019, 58, 17745–17750 CrossRef CAS PubMed; (e) Y. Z. Xu, B. Z. Wang, R. Kaur, M. B. Minameyer, M. Bothe, T. Drewello, D. M. Guldi and M. V. Delius, Angew. Chem., Int. Ed., 2018, 57, 11549–11553 CrossRef CAS PubMed.
- Y. Q. Fan, J. He, L. Liu, G. Q. Liu, S. Z. Guo, Z. Lian, X. N. Li, W. J. Guo, X. B. Chen, Y. Wang and H. Jiang, Angew. Chem., Int. Ed., 2023, e202304623 CAS.
- During our preparation of this manuscript, these bisnanohoops were reported by the Yam and Du groups, respectively. (a) Y. Z. Xu, F. Steudel, M. Y. Leung, B. Xia, M. V. Delius and V. W. W. Yam, Angew. Chem., Int. Ed., 2023, 62, e202302978 CrossRef CAS PubMed; in this article, they demonstrated that bismacrocycles could bind pyridinium salts and C60 derivatives within the two distinct cavities to obtain a 1:2 complex; (b) Y. Zhou, G. L. Zhuang and P. W. Du, Chin. Chem. Lett., 2023 DOI:10.1016/j.cclet.2023.108593 ; in this article, they employed bismacrocycles comprising pillar[5]arene and [10]CPP for the binding of C60 and imidazole derivatives, respectively..
- (a) J. Jiang, D. H. Hu, M. Hanif, X. L. Li, S. J. Su, Z. Q. Xie, L. L. Liu, S. T. Zhang, B. Yang and Y. G. Ma, Adv. Opt. Mater., 2016, 4, 2109–2118 CrossRef CAS; (b) J. K. Li, X. Y. Chen, W. L. Zhao, Y. L. Guo, Y. Zhang, X. C. Wang, A. C. H. Sue, X. Y. Cao, M. Li, C. F. Chen and X. Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202215367 CrossRef CAS PubMed.
- (a) H. C. Zhang, Z. N. Liu and Y. L. Zhao, Chem. Soc. Rev., 2018, 47, 5491–5528 RSC; (b) L. Jiang, X. Huang, D. Chen, H. Yan, X. Y. Li and X. Z. Du, Angew. Chem., Int. Ed., 2017, 56, 2655–2659 CrossRef CAS PubMed; (c) G. C. Yu, C. Y. Han, Z. B. Zhang, J. Z. Chen, X. Z. Yan, B. Zheng, S. Y. Liu and F. H. Huang, J. Am. Chem. Soc., 2012, 134, 8711–8717 CrossRef CAS PubMed; (d) Y. Cao, X. Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y. Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762–10769 CrossRef CAS PubMed; (e) J. C. Ji, X. Q. Wei, W. H. Wu, C. Y. Fan, D. Y. Zhou, K. Kanagaraj, G. Cheng, K. Luo, X. G. Meng and C. Yang, J. Am. Chem. Soc., 2022, 144, 1455–1463 CrossRef CAS PubMed; (f) C. Xiao, W. H. Wu, W. T. Liang, D. Y. Zhou, K. Kanagaraj, G. Cheng, D. Su, Z. H. Zhong, J. J. Chruma and C. Yang, Angew. Chem., Int. Ed., 2020, 59, 8094–8098 CrossRef CAS PubMed; (g) W. J. Li, X. Q. Wang, D. Y. Zhang, Y. X. Hu, W. T. Xu, L. Xu, W. Wang and H. B. Yang, Angew. Chem., Int. Ed., 2021, 60, 18761–18768 CrossRef CAS PubMed; (h) W. J. Li, Z. Hu, L. Xu, X. Q. Wang, W. Wang, G. Q. Yin, D. Y. Zhang, Z. Sun, X. Li, H. Sun and H. B. Yang, J. Am. Chem. Soc., 2020, 142(39), 16748–16756 CrossRef CAS PubMed; (i) J. F. Chen, X. Yin, B. Wang, K. Zhang, G. Meng, S. Zhang, Y. Shi, N. Wang, S. Wang and P. Chen, Angew. Chem., Int. Ed., 2020, 59, 11267–11272 CrossRef CAS PubMed; (j) H. Zhu, Q. Li, B. Shi, H. Xing, Y. Sun, S. Lu, L. Shangguan, X. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2020, 142, 17340–17345 CrossRef CAS PubMed; (k) K. Kato, Y. Kurakake, S. Ohtani, S. Fa, M. Gon, K. Tanaka and T. Ogoshi, Angew. Chem., Int. Ed., 2022, 61, e202209222 CrossRef CAS PubMed.
- (a) A. B. Nepomnyashchii, A. J. Pistner, A. J. Bard and J. Rosenthal, J. Phys. Chem. C, 2013, 117, 5599–5609 CrossRef CAS PubMed; (b) M. Kondo, S. Furukawa, K. Hirai and S. Kitagawa, Angew. Chem., Int. Ed., 2010, 49, 5327–5330 CrossRef CAS PubMed; (c) A. Singh, W. T. Yip and R. L. Halterman, Org. Lett., 2012, 14, 4046–4049 CrossRef CAS PubMed; (d) R. Bandichhor, A. D. Petrescu, A. Vespa, A. B. Kier, F. Schroeder and K. Burgess, J. Am. Chem. Soc., 2006, 128, 10688–10689 CrossRef CAS PubMed; (e) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC; (f) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC; (g) Z. X. Shi, X. Han, W. B. Hu, H. Bai, B. Peng, L. Ji, Q. L. Fan, L. Li and W. Huang, Chem. Soc. Rev., 2020, 49, 7533–7567 RSC.
- (a) Y. L. Wang, G. C. Ping and C. J. Li, Chem. Commun., 2016, 52, 9858–9872 RSC; (b) C. Li, S. Chen, J. Li, K. Han, M. Xu, B. Hu, Y. Yu and X. Jia, Chem. Commun., 2011, 47, 11294 RSC; (c) X. Shu, J. Fan, X. Wang, W. Chen, X. Jia and C. Li, Org. Biomol. Chem., 2012, 10, 3393 RSC; (d) X. Shu, W. Chen, D. Hou, Q. Meng, R. Zheng and C. Li, Chem. Commun., 2014, 50, 4820–4823 RSC.
- (a) T. Ogoshi, T. A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed; (b) G. Yu, B. Hua and C. Han, Org. Lett., 2014, 16, 2486–2489 CrossRef CAS PubMed; (c) B. Hua and G. Yu, Tetrahedron Lett., 2014, 55, 6274–6276 CrossRef CAS.
- (a) CIE, Commission Internationale de l'Eclairage Proceedings, 1931, Cambridge University Press, Cambridge, 1932 Search PubMed; (b) L. A. Jones, J. Opt. Soc. Am., 1943, 33, 534 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR, 13C NMR and MS spectra; titration experiment details. See DOI: https://doi.org/10.1039/d3sc04358d |
| This journal is © The Royal Society of Chemistry 2023 |