Open Access Article
Open Access ArticleSupramolecular alternating copolymers with highly efficient fluorescence resonance energy transfer†
Anwesha
Chakraborty
a,
Pradipta Kumar
Das
b,
Biman
Jana
 *b and
Suhrit
Ghosh
*b and
Suhrit
Ghosh
 *a
*a
aSchool of Applied and Interdisciplinary Sciences, Indian Association for the Cultivation of Science, 2A and 2B Raja S. C. Mullick Road, Kolkata, 700032, India. E-mail: psusg2@iacs.res.in
bSchool of Chemical Sciences, Indian Association for the Cultivation of Science, 2A and 2B Raja S. C. Mullick Road, 700032, Kolkata, India. E-mail: pcbj@iacs.res.in
First published on 19th September 2023
Abstract
This article reports alternating supramolecular copolymerization of two naphthalene-diimide (NDI)-derived building blocks (NDI-1 and NDI-2) under thermodynamic control. Both monomers contain a central NDI chromophore, attached to a hydrocarbon-chain and a carboxylic-acid group. The NDI core in NDI-2 is symmetrically substituted with two butane-thiol groups, which makes it distinct from NDI-1. In decane, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of NDI-1 and NDI-2 shows spontaneous gelation and a typical fibrillar network, unlike the behavior of either of the components individually. The solvent-dependent UV/vis spectrum of the mixed sample in decane shows bathochromically shifted sharp absorption bands and a sharp emission band (holds a mirror-image relationship) with a significantly small Stokes shift compared to those in CHCl3, indicating J-aggregation. In contrast, the aggregated spectra of the individual monomers show broad structureless features, suggesting ill-defined aggregates. Cooling curves derived from the temperature-dependent UV/vis spectroscopy studies revealed early nucleation and a signature of well-defined cooperative polymerization for the mixed sample, unlike either of the individual components. Molecular dynamics simulations predicted the greatest dimer formation tendency for the NDI-1 + NDI-2 (1
1 mixture of NDI-1 and NDI-2 shows spontaneous gelation and a typical fibrillar network, unlike the behavior of either of the components individually. The solvent-dependent UV/vis spectrum of the mixed sample in decane shows bathochromically shifted sharp absorption bands and a sharp emission band (holds a mirror-image relationship) with a significantly small Stokes shift compared to those in CHCl3, indicating J-aggregation. In contrast, the aggregated spectra of the individual monomers show broad structureless features, suggesting ill-defined aggregates. Cooling curves derived from the temperature-dependent UV/vis spectroscopy studies revealed early nucleation and a signature of well-defined cooperative polymerization for the mixed sample, unlike either of the individual components. Molecular dynamics simulations predicted the greatest dimer formation tendency for the NDI-1 + NDI-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by pure NDI-1 and NDI-2. Theoretical studies further revealed a partial positive charge in the NDI ring of NDI-1 when compared to NDI-2, promoting the alternating stacking propensity, which is also favored by the steric factor as NDI-2 is core-substituted with alkyl thiols. Such theoretical predictions fully corroborate with the experimental results showing 1
1), followed by pure NDI-1 and NDI-2. Theoretical studies further revealed a partial positive charge in the NDI ring of NDI-1 when compared to NDI-2, promoting the alternating stacking propensity, which is also favored by the steric factor as NDI-2 is core-substituted with alkyl thiols. Such theoretical predictions fully corroborate with the experimental results showing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (from Job's plot) of the two monomers, indicating alternate stacking sequences in the H-bonded (syn–syn catemer type) supramolecular copolymer. Such alternating supramolecular copolymers showed highly efficient (>93%) fluorescence resonance energy transfer (FRET).
1 stoichiometry (from Job's plot) of the two monomers, indicating alternate stacking sequences in the H-bonded (syn–syn catemer type) supramolecular copolymer. Such alternating supramolecular copolymers showed highly efficient (>93%) fluorescence resonance energy transfer (FRET).
Introduction
Supramolecular polymerization has emerged as a powerful tool for the synthesis of diverse molecular assemblies with precise internal order and functional utility.1 In the recent past the focus has been shifted to supramolecular copolymers,2–7 which represent adaptive multicomponent systems with tuneable photophysical properties and density of surface-displayed functional groups, attractive for functions including supramolecular biomaterials,4 catalysis,5 light harvesting,6 long range exciton transport7 and others. Recent development in this field2,3 helps in directly correlating supramolecular copolymerization with well-established covalent copolymerization in terms of the relationship between the reactivity ratio of the monomers and the nature of the copolymer, microstructure, and other structural aspects. By virtue of this, it has been possible to demonstrate synthesis of well-defined supramolecular blocks, alternating or statistical copolymers in the past few years, mostly from different organic π-systems.2,3 In most of these recent examples, hydrogen-bonding functional groups (amides and others) are attached to a central π-system, and when two or more such monomers are allowed to copolymerize, it becomes essential (not sufficient) that H-bonding functional groups are properly placed in the adjacent dissimilar monomers, so that the aromatic interaction and H-bonding can operate in a synchronized manner, failing which segregated homo-polymerization may dominate.7 Manifestation of such possibilities depends on the relative dimensions of the two (or more) π-systems, involved in the copolymerization, but such stringent structural requirements may not be satisfactory moving from one system to another. We envisaged that, instead of this common strategy in which both the H-bonding group and the π-system are part of the backbone, if the chromophores remain as pendants, then the H-bonded chain can propagate independently, allowing the pendant chromophores to organize freely in their favourable mode. To test such possibilities, we have explored supramolecular copolymerization of two carboxylic-acid appended naphthalene-diimide (NDI)-derived monomers, namely NDI-1 and NDI-2 (Fig. 1a). We had earlier reported NDI-1 and structurally similar derivatives,8 in which extended H-bonding among the carboxylic acid groups in the syn–syn catemer motif produced 1D-supramolecular polymers, but not in pure form as other motifs of H-bonding between the carboxylic acid groups operate simultaneously. We have now introduced a second monomer NDI-2, which is structurally similar to NDI-1 except for the core-substitution,9 by virtue of which it exhibits distinct optical properties which is useful for probing copolymerization by spectroscopy techniques. Furthermore, we have recently reported J-aggregation of amide-functionalized sulphur-substituted NDI derivatives,10 with remarkably high fluorescence quantum yield, which prompted us to use sulphur-substituted NDI for supramolecular copolymerization. In this article we report cooperative supramolecular copolymerization between NDI-1 and NDI-2 under thermodynamic control and reflect on the copolymer structure and sequence and thermodynamic aspects using the experimental and molecular dynamics (MD) simulation results. Furthermore, we show fluorescence resonance energy transfer (FRET)11 between NDI-1 and NDI-2 in the supramolecular copolymers with remarkably high efficiency that has been rarely reported in the literature.6Results and discussion
Supramolecular polymerization and copolymerization
Synthesis of NDI-1 was reported by us elsewhere.8b NDI-2 was prepared by multi-step synthesis (Scheme S1†) and isolated as a red solid with ∼6% overall yield. Gelation12 ability of the two NDI building blocks and their equimolar mixture was tested in decane. Neither NDI-1 nor NDI-2 produced a gel (Fig. 1b) in decane up to a concentration of 5.0 mM. In sharp contrast their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture spontaneously produced a transparent red gel (Fig. 1b) when the hot solution in decane was cooled to room temperature. The critical gelation concentration (CGC) and gel-to-sol transition temperature (Tg) of this mixed gel was estimated to be ∼0.6 mM and ∼80 °C (c = 10 mM), respectively. Rheological properties of the gel were checked by a stress-amplitude sweep measurement in which the variations of the storage modulus (G′) and loss modulus (G′′) were monitored as a function of applied stress (Fig. 1g). Initially G′ (5000 Pa) was significantly larger than G′′ (1200 Pa), as typically observed for a gel phase.13 With increasing stress they remained almost invariant up to a critical point and then crossed each other, revealing the yield stress to be 4.5 Pa. Morphology was examined by atomic force microscopy (AFM) (Fig. 1c–e) with diluted gel/sol samples (c = 0.2 mM). The NDI-1 + NDI-2 (1
1 mixture spontaneously produced a transparent red gel (Fig. 1b) when the hot solution in decane was cooled to room temperature. The critical gelation concentration (CGC) and gel-to-sol transition temperature (Tg) of this mixed gel was estimated to be ∼0.6 mM and ∼80 °C (c = 10 mM), respectively. Rheological properties of the gel were checked by a stress-amplitude sweep measurement in which the variations of the storage modulus (G′) and loss modulus (G′′) were monitored as a function of applied stress (Fig. 1g). Initially G′ (5000 Pa) was significantly larger than G′′ (1200 Pa), as typically observed for a gel phase.13 With increasing stress they remained almost invariant up to a critical point and then crossed each other, revealing the yield stress to be 4.5 Pa. Morphology was examined by atomic force microscopy (AFM) (Fig. 1c–e) with diluted gel/sol samples (c = 0.2 mM). The NDI-1 + NDI-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed gel showed entangled fibrillar morphology with the height of the thinnest fiber ∼40 nm, while NDI-2 showed near spherical irregular particles (height ∼20–30 nm). NDI-1, on the other hand, showed an irregular fibrillar structure as reported by us before due to the possible simultaneous formation of multiple aggregates by different H-bonding motifs of the carboxylic acid group.8 These results strongly suggest formation of supramolecular copolymers of NDI-1 and NDI-2 with the emergence of new physical properties, when compared to those of the individual monomers. This was clearly evident from the UV/vis spectroscopy studies (Fig. 2).
1) mixed gel showed entangled fibrillar morphology with the height of the thinnest fiber ∼40 nm, while NDI-2 showed near spherical irregular particles (height ∼20–30 nm). NDI-1, on the other hand, showed an irregular fibrillar structure as reported by us before due to the possible simultaneous formation of multiple aggregates by different H-bonding motifs of the carboxylic acid group.8 These results strongly suggest formation of supramolecular copolymers of NDI-1 and NDI-2 with the emergence of new physical properties, when compared to those of the individual monomers. This was clearly evident from the UV/vis spectroscopy studies (Fig. 2).
Monomeric spectra of both NDI-1 and NDI-2 in CHCl3 show sharp absorption bands in the range of 300–400 nm and NDI-2 also exhibits an additional intra-molecular charge-transfer band in the window of 450–550 nm. In decane, both chromophores exhibit broad spectra with a concomitant reduction in the peak intensity, indicating aggregation. Interestingly, the UV/vis spectrum of NDI-1 + NDI-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in decane shows distinct features when compared to the aggregated spectra of the individual monomers. Especially noteworthy is the appearance of a sharp, bathochromically shifted (∼30 nm) new charge-transfer band (λmax = 562 nm), indicating J-aggregation10,14 in the supramolecular copolymer. Overall, from the UV/vis data (Fig. 2) it is evident that the aggregated spectrum of NDI-1 + NDI-2 (1
1) in decane shows distinct features when compared to the aggregated spectra of the individual monomers. Especially noteworthy is the appearance of a sharp, bathochromically shifted (∼30 nm) new charge-transfer band (λmax = 562 nm), indicating J-aggregation10,14 in the supramolecular copolymer. Overall, from the UV/vis data (Fig. 2) it is evident that the aggregated spectrum of NDI-1 + NDI-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in decane is totally different than the mathematical sum spectra of the aggregates of the two individual building blocks, which eliminates the possibility of formation of segregated homopolymers or supramolecular block copolymers; rather it strongly evokes the formation of supramolecular copolymers with either statistical or alternating sequences.
1) in decane is totally different than the mathematical sum spectra of the aggregates of the two individual building blocks, which eliminates the possibility of formation of segregated homopolymers or supramolecular block copolymers; rather it strongly evokes the formation of supramolecular copolymers with either statistical or alternating sequences.
To get further insight into the supramolecular copolymerization, a hot solution of NDI-1 + NDI-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in decane (c = 0.2 mM) was cooled from 90 °C to 25 °C and the change in the absorbance spectrum was monitored as a function of temperature (Fig. 3a). At the highest temperature the spectrum appeared similar to that in CHCl3 indicating a monomeric state. With decreasing temperature, the monomeric band (λmax = 523 nm) started disappearing and in lieu the red-shifted J-aggregate band (λmax = 561 nm) appeared gradually. The mole fraction of aggregate (αagg) at each temperature was calculated from the absorption intensity of the J-band (λmax = 561 nm) and plotted as a function of temperature (Fig. 3b). So the constructed cooling curve (Fig. 3b) showed a non-sigmoidal shape that is typically seen for cooperative supramolecular polymerization,15,16 with an elongation temperature (Te) of ∼62 °C. Temperature-dependent UV/vis experiments were carried out for NDI-1 (Fig. S1†) and NDI-2 (Fig. S2†) under identical experimental conditions and cooling curves (Fig. 3) were constructed accordingly. In sharp contrast to the supramolecular copolymer, NDI-2 alone exhibited a peculiar cooling curve (Fig. 3), that neither indicates a cooperative nor isodesmic mechanism. Hence it can be concluded that NDI-2 lacks any well-defined supramolecular polymerization pathway; rather immiscibility driven irregular aggregation leads to the observed change in the UV/vis spectra. NDI-1, on the other hand, showed (Fig. 3) sluggish elongation at much lower temperature, which was studied in detail in our previous report8 and can be attributed to its pathway complexity. By comparing these cooling curves, it is evident that the hetero-nucleation leads to a well-defined distinct supramolecular polymerization pathway with significantly greater propensity than the homo-nucleation for either of the two monomers in their homo-polymerization. To check the stability of the supramolecular copolymer, variable temperature UV/vis spectra of NDI-1 + NDI-2 (1
1) in decane (c = 0.2 mM) was cooled from 90 °C to 25 °C and the change in the absorbance spectrum was monitored as a function of temperature (Fig. 3a). At the highest temperature the spectrum appeared similar to that in CHCl3 indicating a monomeric state. With decreasing temperature, the monomeric band (λmax = 523 nm) started disappearing and in lieu the red-shifted J-aggregate band (λmax = 561 nm) appeared gradually. The mole fraction of aggregate (αagg) at each temperature was calculated from the absorption intensity of the J-band (λmax = 561 nm) and plotted as a function of temperature (Fig. 3b). So the constructed cooling curve (Fig. 3b) showed a non-sigmoidal shape that is typically seen for cooperative supramolecular polymerization,15,16 with an elongation temperature (Te) of ∼62 °C. Temperature-dependent UV/vis experiments were carried out for NDI-1 (Fig. S1†) and NDI-2 (Fig. S2†) under identical experimental conditions and cooling curves (Fig. 3) were constructed accordingly. In sharp contrast to the supramolecular copolymer, NDI-2 alone exhibited a peculiar cooling curve (Fig. 3), that neither indicates a cooperative nor isodesmic mechanism. Hence it can be concluded that NDI-2 lacks any well-defined supramolecular polymerization pathway; rather immiscibility driven irregular aggregation leads to the observed change in the UV/vis spectra. NDI-1, on the other hand, showed (Fig. 3) sluggish elongation at much lower temperature, which was studied in detail in our previous report8 and can be attributed to its pathway complexity. By comparing these cooling curves, it is evident that the hetero-nucleation leads to a well-defined distinct supramolecular polymerization pathway with significantly greater propensity than the homo-nucleation for either of the two monomers in their homo-polymerization. To check the stability of the supramolecular copolymer, variable temperature UV/vis spectra of NDI-1 + NDI-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were monitored as a function of temperature (Fig. 4a). With gradual heating, the J-band at 561 nm started reducing in intensity and in lieu the blue shifted monomeric band re-appeared (Fig. 4a) with other concomitant spectral changes suggesting reversible transformation into the monomeric state at elevated temperature. The melting curve (Fig. 4b) indicated α50 (T) to be ∼60 °C. A consistent result was obtained in μ-DSC experiments (Fig. 4b), revealing a clear phase transition for the 1
1) were monitored as a function of temperature (Fig. 4a). With gradual heating, the J-band at 561 nm started reducing in intensity and in lieu the blue shifted monomeric band re-appeared (Fig. 4a) with other concomitant spectral changes suggesting reversible transformation into the monomeric state at elevated temperature. The melting curve (Fig. 4b) indicated α50 (T) to be ∼60 °C. A consistent result was obtained in μ-DSC experiments (Fig. 4b), revealing a clear phase transition for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gel in decane at ∼55 °C. We further examined NDI-1/NDI-2 composition dependent thermal disassembly of the copolymers by variable temperature UV/vis spectroscopy. To our surprise we noticed almost identical melting curves (Fig. 4c) of the three representative mixed samples (NDI-1
1 gel in decane at ∼55 °C. We further examined NDI-1/NDI-2 composition dependent thermal disassembly of the copolymers by variable temperature UV/vis spectroscopy. To our surprise we noticed almost identical melting curves (Fig. 4c) of the three representative mixed samples (NDI-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI-2 = 1
NDI-2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), constructed from the temperature dependent UV/vis spectra (Fig. S3†). Melting curves of the 1
1), constructed from the temperature dependent UV/vis spectra (Fig. S3†). Melting curves of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample at 1 °C min−1 and 2 °C min−1 were compared while similar results were found (Fig. 4c). Such identical melting curves for different compositions cannot be rationalized if they form a statistical copolymer, as in that case the composition of the copolymer would greatly vary depending on the ratio of the two monomers. Instead, these identical melting curves, irrespective of the monomer feed ratio, suggest the formation of the same alternating copolymer in all three cases. Melting curves for the three different mixed samples (NDI-2/NDI-1 = 1
1 sample at 1 °C min−1 and 2 °C min−1 were compared while similar results were found (Fig. 4c). Such identical melting curves for different compositions cannot be rationalized if they form a statistical copolymer, as in that case the composition of the copolymer would greatly vary depending on the ratio of the two monomers. Instead, these identical melting curves, irrespective of the monomer feed ratio, suggest the formation of the same alternating copolymer in all three cases. Melting curves for the three different mixed samples (NDI-2/NDI-1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with a fixed NDI-2 concentration were compared (Fig. S4†). A similar melting pattern was observed but the transition temperatures as well as α50 (T) were slightly increased with an increasing amount of NDI-1 as the total solute concentration increased in samples with a stoichiometric imbalance. In μ-DSC experiments, two different phase transition temperatures were noticed for 1
3) with a fixed NDI-2 concentration were compared (Fig. S4†). A similar melting pattern was observed but the transition temperatures as well as α50 (T) were slightly increased with an increasing amount of NDI-1 as the total solute concentration increased in samples with a stoichiometric imbalance. In μ-DSC experiments, two different phase transition temperatures were noticed for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (∼39 °C and ∼50 °C) and 2
2 (∼39 °C and ∼50 °C) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (∼47 °C and ∼52 °C) gels (Fig S5†), indicating formation of alternating copolymers, along with a homopolymer of the excess component.
1 (∼47 °C and ∼52 °C) gels (Fig S5†), indicating formation of alternating copolymers, along with a homopolymer of the excess component.
 | ||
Fig. 4 (a) Temperature dependent (heating from 25 °C to 90 °C at a rate of 2 °C min−1) UV/vis spectra of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of NDI-1 + NDI-2 in decane; (b) variation of αagg (estimated from change in intensity at 561 nm for the 1 1 mixture of NDI-1 + NDI-2 in decane; (b) variation of αagg (estimated from change in intensity at 561 nm for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixed sample along with a micro-DSC thermogram (heating) of the 1 1 mixed sample along with a micro-DSC thermogram (heating) of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gel in decane (c = 5 mM) showing disassembly in the similar temperature range. This melting curve, when compared with the cooling curve (Fig. 3b) of the same sample, reveals hysteresis, indicating that there could be pathway complexity in supramolecular polymerization; (c) variation of αagg (calculated from change in intensity at 561 nm) as a function of temperature (heating from 25 °C to 90 °C, c = 0.2 mM) for the three mixed samples heated at a rate of 2 °C min−1 (filled circles) or at a rate of 1 °C min−1 (open circle) only for the 1 1 gel in decane (c = 5 mM) showing disassembly in the similar temperature range. This melting curve, when compared with the cooling curve (Fig. 3b) of the same sample, reveals hysteresis, indicating that there could be pathway complexity in supramolecular polymerization; (c) variation of αagg (calculated from change in intensity at 561 nm) as a function of temperature (heating from 25 °C to 90 °C, c = 0.2 mM) for the three mixed samples heated at a rate of 2 °C min−1 (filled circles) or at a rate of 1 °C min−1 (open circle) only for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample; (d) Job's plot for NDI-1 + NDI-2, constructed from the J-band (561 nm) intensity with different NDI-1/NDI-2 ratios (total concentration fixed at 0.2 mM). 1 sample; (d) Job's plot for NDI-1 + NDI-2, constructed from the J-band (561 nm) intensity with different NDI-1/NDI-2 ratios (total concentration fixed at 0.2 mM). | ||
To examine this further, we probed the behaviour of NDI-1 + NDI-2 with varying compositions such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while the total monomer concentration was kept fixed. All five mixed samples showed spontaneous gelation in decane (c = 2 mM) (Fig. S6†). UV/vis spectra of the diluted gels showed (Fig. S7†) a characteristic sharp absorption band (λmax ∼561 nm) for all five samples, that has been specifically assigned to the copolymer. From these data, Job's plot was constructed, which showed (Fig. 4d) maximum intensity of the J-band for the 1
1, while the total monomer concentration was kept fixed. All five mixed samples showed spontaneous gelation in decane (c = 2 mM) (Fig. S6†). UV/vis spectra of the diluted gels showed (Fig. S7†) a characteristic sharp absorption band (λmax ∼561 nm) for all five samples, that has been specifically assigned to the copolymer. From these data, Job's plot was constructed, which showed (Fig. 4d) maximum intensity of the J-band for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition, strongly indicating an alternating stacking sequence of the two monomers in their supramolecular copolymers.17 In this case, in the mixed samples with a stoichiometric imbalance of the two monomers (say 1
1 composition, strongly indicating an alternating stacking sequence of the two monomers in their supramolecular copolymers.17 In this case, in the mixed samples with a stoichiometric imbalance of the two monomers (say 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of NDI-1/NDI-2), the excess monomer should form additional homo-aggregates as they cannot be incorporated in the supramolecular copolymer with an alternating sequence. Indeed, the AFM images of the NDI-1 + NDI-2 (1
1 of NDI-1/NDI-2), the excess monomer should form additional homo-aggregates as they cannot be incorporated in the supramolecular copolymer with an alternating sequence. Indeed, the AFM images of the NDI-1 + NDI-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) sample showed a significant presence of spherical particles (Fig. S8†), similar to the homo-aggregate of NDI-2 (Fig. 2), in addition to the entangled fibrils of the alternating supramolecular copolymer. Thinking in a similar line one would expect to observe the presence of the homopolymer of NDI-1 along with the supramolecular copolymer in the mixed sample with excess NDI-1 (NDI-1/NDI-2 = 3
2) sample showed a significant presence of spherical particles (Fig. S8†), similar to the homo-aggregate of NDI-2 (Fig. 2), in addition to the entangled fibrils of the alternating supramolecular copolymer. Thinking in a similar line one would expect to observe the presence of the homopolymer of NDI-1 along with the supramolecular copolymer in the mixed sample with excess NDI-1 (NDI-1/NDI-2 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). But this was difficult to identify in the AFM image (Fig. S9†) because the NDI-1 homopolymer too exhibits fibrillar morphology.18
1). But this was difficult to identify in the AFM image (Fig. S9†) because the NDI-1 homopolymer too exhibits fibrillar morphology.18
Fluorescence resonance energy transfer (FRET)
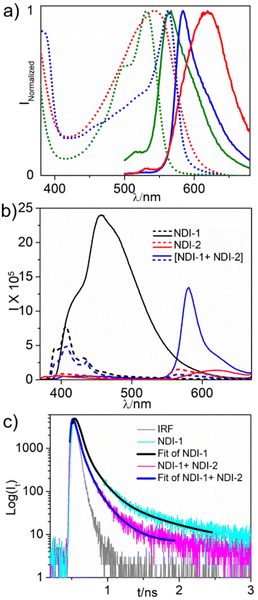
Next, we examined fluorescence properties of the alternating supramolecular copolymer and compared them with those of NDI-2 (Fig. 5a). For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supramolecular copolymer in decane, a sharp emission band was noticed (Fig. 5a) which holds a mirror-image relationship with the absorption band. Emission intensity was significantly higher compared to that of monomeric NDI-2 in CHCl3 (Fig. 5b), indicating enhanced emission because of J-aggregation, which is consistent with our previous report on the J-aggregation of a structurally different sulphur-substituted NDI gelator.10 Furthermore, a significantly smaller Stokes shift (22 nm) in the aggregated state compared to that in the monomeric state (35 nm) was noticed (Fig. 5a) which is considered a typical signature of J-aggregation.14 Interestingly, the homo-aggregate of NDI-2 showed (Fig. 5a) a broad, less intense emission with a large Stokes shift (75 nm), further confirming distinct photophysical properties of the same dye in the supramolecular copolymer. Now the excitation wavelength was changed to 360 nm where the aggregated band appeared for NDI-1. A broad emission band in the window of ∼400–600 nm was noticed (Fig. 5b) that is quite different than the monomeric emission in CHCl3. This issue was deliberated in detail in our previous work8a and assigned to simultaneous formation of multiple aggregates due to pathway complexity, which has been examined in more detail by us in a recent report.8b
1 supramolecular copolymer in decane, a sharp emission band was noticed (Fig. 5a) which holds a mirror-image relationship with the absorption band. Emission intensity was significantly higher compared to that of monomeric NDI-2 in CHCl3 (Fig. 5b), indicating enhanced emission because of J-aggregation, which is consistent with our previous report on the J-aggregation of a structurally different sulphur-substituted NDI gelator.10 Furthermore, a significantly smaller Stokes shift (22 nm) in the aggregated state compared to that in the monomeric state (35 nm) was noticed (Fig. 5a) which is considered a typical signature of J-aggregation.14 Interestingly, the homo-aggregate of NDI-2 showed (Fig. 5a) a broad, less intense emission with a large Stokes shift (75 nm), further confirming distinct photophysical properties of the same dye in the supramolecular copolymer. Now the excitation wavelength was changed to 360 nm where the aggregated band appeared for NDI-1. A broad emission band in the window of ∼400–600 nm was noticed (Fig. 5b) that is quite different than the monomeric emission in CHCl3. This issue was deliberated in detail in our previous work8a and assigned to simultaneous formation of multiple aggregates due to pathway complexity, which has been examined in more detail by us in a recent report.8b
Now considering a significant overlap between the emission spectra of NDI-1 and absorption spectra of NDI-2 (when both are in the aggregated state in decane) (Fig. S10a†), FRET was anticipated in the supramolecular copolymer, which is ubiquitous in natural light harvesting systems. Despite many reports showing such energy transfer in synthetic supramolecular systems,6,19 it is imperative to have a highly ordered organization of the donor and acceptor chromophores to realize highly efficient energy transfer. Now for the alternating supramolecular copolymer, with the same 360 nm excitation, the emission band for the NDI-1 aggregate was almost quenched and in lieu a sharp emission band appeared corresponding to the J-aggregate of NDI-2 which is identical to that obtained by 480 nm excitation (Fig. 5b). However, no such emission band was noticed for the NDI-homopolymer for the 360 nm excitation that eliminates any significant contribution from the direct excitation of NDI-2 (or any other factor) and strongly suggests FRET from the NDI-1 donor to the NDI-2 acceptor when they are co-located in the alternating supramolecular copolymer. This was further evident from the TCSPC studies that showed (Fig. 5c) that fluorescence transients of NDI-1 (donor) decay at a significantly faster rate in the presence of NDI-2 (acceptor), indicating nonradiative resonance type energy transfer between NDI-1 and NDI-2. The average lifetime of NDI-1 was found to be 0.03 ns in the presence of NDI-2 which is significantly less than that of NDI-1 (0.46 ns) in the absence of NDI-2. Energy transfer efficiency was estimated to be ∼98% from the steady state fluorescence spectra, while ∼93% from the TCSPC studies. Such high values, rarely reported in the literature, indicate that the present alternating supramolecular copolymer can be considered a highly promising scaffold for light harvesting. The rate of energy transfer was estimated to be 28 ns−1 (see the ESI† for details). Furthermore, for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample, a sharp and distinct emission band was noticed (Fig. S11†), which further supports alternating copolymerization and the absence of any homopolymer of either NDI-1 or NDI-2.
1 sample, a sharp and distinct emission band was noticed (Fig. S11†), which further supports alternating copolymerization and the absence of any homopolymer of either NDI-1 or NDI-2.
Molecular dynamics (MD) simulations
The internal order, sequence and thermodynamics of supramolecular copolymers and origin of the alternating stacking mode were further examined by theoretical studies. To gain molecular insight regarding this copolymerization behaviour, all-atom molecular dynamics simulations of NDI-1, NDI-2, and their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture in decane were performed. Automated Topology Builder (ATB version 3.0) was used to obtain a suitable structure and forcefield parameters of these molecules.20,21
1 mixture in decane were performed. Automated Topology Builder (ATB version 3.0) was used to obtain a suitable structure and forcefield parameters of these molecules.20,21
GROMACS 2020.6 was used along with a modified gromos54a7 forcefield to perform and analyse MD simulations.22 We have also used VMD23 and PYMOL to visualize and analyse the structures and trajectories obtained from the performed MD simulations.
All the details of the system preparation and MD simulation along with the analysis are included in the ESI.† At first, we took a total of 40 molecules to prepare 3 systems (40 NDI-1, 40 NDI-2, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
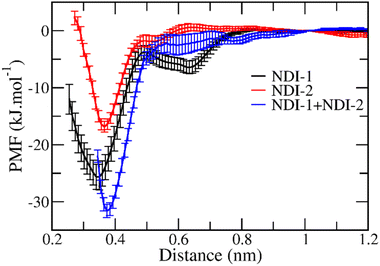
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 NDI-1 and NDI-2). NDI-1 and NDI-2 both have an end-to-end distance of ∼2.1 nm in their fully extended states, calculated from the carboxyl group to the end of the branched chains. All the systems were solvated in decane and properly equilibrated in both NVT and NPT ensembles. During the production run, in all the systems, we observed the formation of different sized oligomers. We took the most stable dimer from each system and constructed a rough binding free energy surface using the umbrella sampling method with the distance between the dimers as the pulling coordinate to obtain the dimer from their individual minima (Fig. 6). These dimers are then used to estimate their stability by evaluating the binding free energy in terms of potential of mean force (PMF) using the umbrella sampling method with the distance between the central rings of the dimers as a pulling coordinate. The free energy was calculated by keeping one of the NDI rings restrained and the distance was varied for the other ring. The free energy was set to be 0 at 1 nm distance to get the idea of relative stability among all 3 dimers. It was evident from the minima of the free energy profiles (Fig. 7) that 1
20 NDI-1 and NDI-2). NDI-1 and NDI-2 both have an end-to-end distance of ∼2.1 nm in their fully extended states, calculated from the carboxyl group to the end of the branched chains. All the systems were solvated in decane and properly equilibrated in both NVT and NPT ensembles. During the production run, in all the systems, we observed the formation of different sized oligomers. We took the most stable dimer from each system and constructed a rough binding free energy surface using the umbrella sampling method with the distance between the dimers as the pulling coordinate to obtain the dimer from their individual minima (Fig. 6). These dimers are then used to estimate their stability by evaluating the binding free energy in terms of potential of mean force (PMF) using the umbrella sampling method with the distance between the central rings of the dimers as a pulling coordinate. The free energy was calculated by keeping one of the NDI rings restrained and the distance was varied for the other ring. The free energy was set to be 0 at 1 nm distance to get the idea of relative stability among all 3 dimers. It was evident from the minima of the free energy profiles (Fig. 7) that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NDI-1 + NDI-2 has a greater dimer formation tendency than both pure NDI-1 and NDI-2 systems. NDI-2 is least biased towards dimer formation and NDI-1 stands in between these two scenarios. The minimum in free energy of the NDI-1-NDI-2 dimer is around −32 kJ mol−1, whereas NDI-1 and NDI-2 have their minimum at −25 kJ mol−1 and −17 kJ mol−1, respectively. The lack of a deeper minimum in the case of NDI-2 compared to the rest suggests its reluctance towards forming any stable dimer. Also, the gradual shift of the minima corresponding to each PMF towards larger distances in the order of NDI-1, NDI-1 + NDI-2, and NDI-2 implies that the bulkier side chain of NDI-2 is directly associated with the compactness of the dimer (Fig. 7).
1 NDI-1 + NDI-2 has a greater dimer formation tendency than both pure NDI-1 and NDI-2 systems. NDI-2 is least biased towards dimer formation and NDI-1 stands in between these two scenarios. The minimum in free energy of the NDI-1-NDI-2 dimer is around −32 kJ mol−1, whereas NDI-1 and NDI-2 have their minimum at −25 kJ mol−1 and −17 kJ mol−1, respectively. The lack of a deeper minimum in the case of NDI-2 compared to the rest suggests its reluctance towards forming any stable dimer. Also, the gradual shift of the minima corresponding to each PMF towards larger distances in the order of NDI-1, NDI-1 + NDI-2, and NDI-2 implies that the bulkier side chain of NDI-2 is directly associated with the compactness of the dimer (Fig. 7).
 | ||
| Fig. 6 Pair formation for each system. (a) Pair between 2 NDI-1; (b) pair between 2 NDI-2; (c) pair between NDI-1 and NDI-2. | ||
We also inspected the stability of trimers (Fig. S12†) formed during the production run of the mixed system, and we observed that the NDI-1 and NDI-2 interface is more stable than the NDI-1 and NDI-1 interface (Fig. S13†). A close inspection of the dimer configurations suggests that the bulky hydrophobic groups stay on one side of the dimer while the hydrogen bonding groups are on the other side. We calculated electrostatic potential on the surfaces of NDI-1 and NDI-2 using the Gaussian package (Gaussian09). Our observations indicate the presence of charge differences in NDI-1 and NDI-2 relative to each other. NDI-1 is partially positively charged on the main NDI ring relative to NDI-2 which is relatively less positive compared to NDI-1, which is conceivable considering the core substitution in NDI-2. This charge difference may play a crucial role in stacking efficiency of the mixed system (Fig. S14†).
We have also been able to identify the larger motif at work behind this efficient copolymerization using all-atom molecular dynamics simulations. For the NDI-1 system, we observed several well-organized NDI-1 oligomers stacking, accommodating around 5 to 8 NDI-1 in each stack (Fig. 8a). Here also, we find that the hydrophobic side chains stay on one side of the stacking while the carboxylic groups stay on the other side. Then, two such stacked clusters come together to form inter-cluster h-bonds in a zig-zag manner, which was proposed in our previous report.8 This situation is mostly steered by the bulky sidechain orientations on one side which allow the hydrogen bonding partners from different stacked clusters to face each other. A more organized stacking through hydrogen bonds was modelled based on this motif for both homogeneous (NDI-1 oligomers) (Fig. 8b) as well as alternate (NDI-1 + NDI-2 oligomers) stacking clusters (Fig. 8c). It is noteworthy that such open chain H-bonding in the syn–syn catemer motif was supported by FT-IR studies (Fig. S16†).24 Furthermore the structure of the supramolecular copolymer (Fig. 8c) was also supported by powder X-ray diffraction studies (Fig. S17†) which showed a sharp peak (2θ = 2.62°) corresponding to a d = 34 Å, which matches with the estimated width of the column (38 Å from MD simulations) consisting of two NDI monomers. In fact, a similar sharp peak with a slightly different d value (30 Å) was also obtained for NDI-1, but not for NDI-2, which corroborates with the MD simulation results suggesting a weak supramolecular polymerization propensity of NDI-2. Therefore, our MD simulations suggest that alternate stacking-based oligomerization followed by hydrogen bonding based association of the stacked oligomers results in fibrilization, which fully corroborate with the experimental results.
Conclusions
Overall, in this manuscript we have shown alternating supramolecular copolymerization of two carboxylic-acid functionalized NDI derivatives (NDI-1 and NDI-2), which differ by the thiol substitution in the NDI core of NDI-2. Due to the possibility of multiple H-bonding motifs (open chain with parallel/anti-parallel orientation, dimers and others) of the carboxylic-acid group as well as other electronic and/or steric factors, neither NDI-1 nor NDI-2 individually is capable of adopting a specific supramolecular polymerization pathway. In sharp contrast, their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture shows typical cooperative supramolecular polymerization, leading to well-defined J-aggregation and gelation in decane, unlike either NDI-1 or NDI-2. Based on experimental and MD-simulation studies, it is evident that these two monomers copolymerize by open-chain H-bonding (zig-zag fashion) with alternating stacking sequences that is favoured by both electronic and steric factors. Basically, thiol substitution makes the NDI-2 ring relatively more electron rich than NDI-1 and imparts steric constraints, both of which favour hetero-nucleation and alternate stacking, reminiscent of the reactivity ratio-based principle for alternate covalent copolymerization. Such a well-defined alternating supramolecular copolymer exhibits FRET with near quantitative efficiency that has been rarely reported in the literature. Despite recent progress in the field, only a handful of examples are known for supramolecular copolymerization under thermodynamic control and most of those designs are system-specific. In this sense the present molecular design is not only simple, but importantly the H-bonding chain formation is decoupled from the π-stacking, which in principle should allow expanding the scope for supramolecular copolymerization with diverse π-systems (with 2 or more monomers) under thermodynamic control for realizing new functional properties such as micro-power energy harvesting, ferroelectricity, photothermal effect, cascade energy transfer and others by fine tuning the structure of the monomers, their internal order in the supramolecular copolymer or chirality. Such efforts are underway in our laboratory.
1 mixture shows typical cooperative supramolecular polymerization, leading to well-defined J-aggregation and gelation in decane, unlike either NDI-1 or NDI-2. Based on experimental and MD-simulation studies, it is evident that these two monomers copolymerize by open-chain H-bonding (zig-zag fashion) with alternating stacking sequences that is favoured by both electronic and steric factors. Basically, thiol substitution makes the NDI-2 ring relatively more electron rich than NDI-1 and imparts steric constraints, both of which favour hetero-nucleation and alternate stacking, reminiscent of the reactivity ratio-based principle for alternate covalent copolymerization. Such a well-defined alternating supramolecular copolymer exhibits FRET with near quantitative efficiency that has been rarely reported in the literature. Despite recent progress in the field, only a handful of examples are known for supramolecular copolymerization under thermodynamic control and most of those designs are system-specific. In this sense the present molecular design is not only simple, but importantly the H-bonding chain formation is decoupled from the π-stacking, which in principle should allow expanding the scope for supramolecular copolymerization with diverse π-systems (with 2 or more monomers) under thermodynamic control for realizing new functional properties such as micro-power energy harvesting, ferroelectricity, photothermal effect, cascade energy transfer and others by fine tuning the structure of the monomers, their internal order in the supramolecular copolymer or chirality. Such efforts are underway in our laboratory.
Data availability
Data generated during this study are available from the authors on reasonable request.Author contributions
AC synthesized the molecules, carried out self-assembly studies and analyzed related data under the supervision of SG. PKD carried out MD simulation stuides and analyzed the data under the supervision of BJ. SG conceptulized the research problem. All authors contributed in manuscript writing and data analysis. Funding was generated by SG and BJ.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AC thanks the DST Inspire program and IACS for a research fellowship. SG thanks the SERB for funding (Grant No: CRG/2020/002395). BJ thanks the SERB for funding (Grant No: CRG/2020/000756).Notes and references
- (a) F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491 CrossRef CAS PubMed; (b) M. Wehner and F. Würthner, Nat. Rev. Chem, 2020, 4, 38 CrossRef CAS; (c) R. D. Mukhopadhyay and A. Ajayaghosh, Science, 2015, 349, 241 CrossRef CAS PubMed; (d) J. Matern, Y. Dorca, L. Sánchez and G. Fernández, Angew. Chem., Int. Ed., 2019, 58, 16730 CrossRef CAS PubMed; (e) S. Datta, S. Takahashi and S. Yagai, Acc. Mater. Res., 2022, 3, 259 CrossRef CAS; (f) L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196 CrossRef CAS PubMed; (g) G. Ghosh, P. Dey and S. Ghosh, Chem. Commun., 2020, 56, 6757 RSC; (h) M. Hartlieb, E. D. H. Mansfield and S. Perrier, Polym. Chem., 2020, 11, 1083 RSC.
- B. Adelizzi, N. J. Van Zee, L. N. J. de Windt, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2019, 141, 6110 CrossRef CAS PubMed.
- (a) W. Wagner, M. Wehner, V. Stepanenko and F. Würthner, J. Am. Chem. Soc., 2019, 141, 12044 CrossRef CAS PubMed; (b) A. Sarkar, R. Sasmal, C. Empereur-mot, D. Bochicchio, S. V. K. Kompella, K. Sharma, S. Dhiman, B. Sundaram, S. S. Agasti, G. M. Pavan and S. J. George, J. Am. Chem. Soc., 2020, 142, 7606 CrossRef CAS PubMed; (c) P. Khanra, A. K. Singh, L. Roy and A. Das, J. Am. Chem. Soc., 2023, 145, 5270 CrossRef CAS PubMed; (d) D. Görl, X. Zhang, V. Stepanenko and F. Würthner, Nat. Commun., 2015, 6, 7009 CrossRef PubMed; (e) B. Adelizzi, A. Aloi, A. J. Markvoort, H. M. M. Ten Eikelder, I. K. Voets, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2018, 140, 7168 CrossRef CAS PubMed; (f) P. Ahlers, K. Fischer, D. Spitzer and P. Besenius, Macromolecules, 2017, 50, 7712 CrossRef CAS; (g) H. Frisch, J. P. Unsleber, D. Lüdeker, M. Peterlechner, G. Brunklaus, M. Waller and P. Besenius, Angew. Chem., Int. Ed., 2013, 52, 10097 CrossRef CAS PubMed; (h) S. H. Jung, D. Bochicchio, G. M. Pavan, M. Takeuchi and K. Sugiyasu, J. Am. Chem. Soc., 2018, 140, 10570 CrossRef CAS PubMed; (i) W. Zhang, W. Jin, T. Fukushima, A. Saeki, S. Seki and T. Aida, Science, 2011, 334, 340 CrossRef CAS PubMed; (j) A. Das, G. Vantomme, A. J. Markvoort, H. M. M. ten Eikelder, M. Garcia-Iglesias, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2017, 139, 7036 CrossRef CAS PubMed; (k) L. N. J. de Windt, C. Kulkarni, H. M. M. ten Eikelder, A. J. Markvoort, E. W. Meijer and A. R. A. Palmans, Macromolecules, 2019, 52, 7430 CrossRef CAS PubMed; (l) S. Chakraborty, H. Kar, A. Sikder and S. Ghosh, Chem. Sci., 2017, 8, 1040 RSC; (m) S. Takahashi and S. Yagai, J. Am. Chem. Soc., 2022, 144, 13374 CrossRef CAS PubMed; (n) L. Aratsu, R. Takeya, B. R. Pauw, M. J. Hollamby, Y. Kitamoto, N. Shimizu, H. Takagi, R. Haruki, S. Adachi and S. Yagai, Nat. Commun., 2020, 11, 1623 CrossRef PubMed; (o) J. Matern, Z. Fernandez and G. Fernandez, Chem. Commun., 2022, 58, 12309 RSC; (p) Y. Dorca, C. Naranjo, G. Ghosh, B. Soberats, J. Calbo, E. Ortí, G. Fernández and L. Sánchez, Chem. Sci., 2022, 13, 81 RSC.
- (a) M. J. Webber, E. A. Appel, E. W. Meijer and R. Langer, Nat. Mater., 2016, 15, 13 CrossRef CAS PubMed; (b) T. Liu, L. van den Berk, J. A. J. Wondergem, C. Tong, M. C. Kwakernaak, B. Ter Braak, D. Heinrich, B. van de Water and R. E. Kieltyka, Adv. Healthcare Mater., 2021, 10, 2001903 CrossRef CAS PubMed; (c) S. Chakraborty, R. Khamrui and S. Ghosh, Chem. Sci., 2021, 12, 1101 RSC; (d) L. Albertazzia, F. J. Martinez-Veracoecheab, C. M. A. Leendersa, I. K. Voetsa, D. Frenkelb and E. W. Meijer, Proc. Natl. Acad. Sci. U.S.A., 2013, 110, 12203 CrossRef PubMed.
- L. N. Neumann, M. B. Baker, C. M. A. Leenders, I. K. Voets, R. P. M. Lafleur, A. R. A. Palmans and E. W. Meijer, Org. Biomol. Chem., 2015, 13, 7711 RSC.
- (a) A. Sarkar, T. Behera, R. Sasmal, R. Capelli, C. Empereur-mot, J. Mahato, S. S. Agasti, G. M. Pavan, A. Chowdhury and S. J. George, J. Am. Chem. Soc., 2020, 142, 11528 CrossRef CAS PubMed; (b) Y. Han, X. Zhang, Z. Ge, Z. Gao, R. Liao and F. Wang, Nat. Commun., 2022, 13, 3546 CrossRef CAS PubMed; (c) R. Liao, F. Wang, Y. Guo, Y. Han and F. Wang, J. Am. Chem. Soc., 2022, 144, 9775 CrossRef CAS PubMed; (d) Q. Song, S. Goia, J. Yang, S. C. L. Hall, M. Staniforth, V. G. Stavros and S. Perrier, J. Am. Chem. Soc., 2021, 143, 382 CrossRef CAS PubMed.
- X. Jin, M. B. Price, J. R. Finnegan, C. E. Boott, J. M. Richter, A. Rao, S. M. Menke, R. H. Friend, G. R. Whittell and I. Manners, Science, 2018, 360, 897 CrossRef CAS PubMed.
- (a) M. R. Molla, D. Gehrig, L. Roy, V. Kamm, A. Paul, F. Laquai and S. Ghosh, Chem.–Eur. J., 2014, 20, 760 CrossRef CAS PubMed; (b) A. Chakraborty, R. Manna, A. Paul and S. Ghosh, Chem.–Eur. J., 2021, 27, 11458 CrossRef CAS PubMed.
- N. Sakai, J. Mareda, E. Vauthey and S. Matile, Chem. Commun., 2010, 46, 4225 RSC.
- H. Kar and S. Ghosh, Chem. Commun., 2016, 52, 8818 RSC.
- P. Rajdev and S. Ghosh, J. Phys. Chem. B, 2019, 123, 327 CrossRef CAS PubMed.
- (a) S. Banerjee, R. K. Das and U. Maitra, J. Mater. Chem., 2009, 19, 6649 RSC; (b) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 4, 1973 CrossRef PubMed.
- (a) D. L. Blair, in Molecular Gels: Structure and Dynamics, ed. R. G. Weiss, RSC, 2018, ch. 2 Search PubMed; (b) F. M. Menger and A. V. Peresypkin, J. Am. Chem. Soc., 2003, 125, 5340–5345 CrossRef CAS PubMed.
- F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376 CrossRef PubMed.
- C. Rest, R. Kandanelli and G. Fernández, Chem. Soc. Rev., 2015, 44, 2543 RSC.
- M. M. J. Smulders, M. M. L. Nieuwenhuizen, T. F. A. de Greef, P. van der Schoot, A. P. H. J. Schenning and E. W. Meijer, Chem.–Eur. J., 2010, 16, 362 CrossRef CAS PubMed.
- S. Ghosh and S. Ramakrishnan, Angew. Chem., Int. Ed., 2005, 34, 5441 CrossRef PubMed.
- In fact, in this case one cannot eliminate the possibility of the NDI-1 polymer growing from the supramolecular copolymer as NDI-1 too showed a well-defined polymerization pathway. This would lead to an interesting block structure consisting of an alternating copolymer of NDI-1 and NDI-2 as the first block and a homopolymer of NDI-1 as the second block. This will be studied in detail in the future.
- V. K. Praveen, C. Ranjith, E. Bandini, A. Ajayaghosh and N. Armaroli, Chem. Soc. Rev., 2014, 43, 4222 RSC.
- A. K. Malde, L. Zuo, M. Breeze, M. Stroet, D. Poger, P. C. Nair, C. Oostenbrink and A. E. Mark, J. Chem. Theory Comput., 2011, 7, 4026 CrossRef CAS PubMed.
- M. Stroet, B. Caron, K. M. Visscher, D. P. Geerke, A. K. Malde and A. E. Mark, J. Chem. Theory Comput., 2018, 14, 5834 CrossRef CAS PubMed.
- N. Schmid, A. P. Eichenberger, A. Choutko, S. Riniker, M. Winger, A. E. Mark and W. F. van Gunsteren, Eur. Biophys. J., 2011, 40, 843 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33 CrossRef CAS PubMed.
- H-bonding between the carboxylic acid groups in the homopolymers and copolymers was probed by FT-IR spectroscopy (Fig. S16†). In CHCl3, a single peak at 3054 cm−1 was noticed for all three samples (NDI-1, NDI-2 or NDI-1 + NDI-2), which can be assigned to the O-H stretching of the free acid. In decane, this band shifted to lower frequency due to H-bonding. For homopolymers of NDI-1 and NDI-2, one major peak was observed at 2985 cm−1 and 3048 cm−1, respectively, while that for the copolymer (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NDI-1 + NDI-2) appeared at 3019 cm−1, suggesting distinct H-bonding in the copolymer compared to the homopolymers, which is conceivable considering the overall structural difference of the copolymer compared to either of the homopolymers.
1 NDI-1 + NDI-2) appeared at 3019 cm−1, suggesting distinct H-bonding in the copolymer compared to the homopolymers, which is conceivable considering the overall structural difference of the copolymer compared to either of the homopolymers.
Footnote |
| † Electronic supplementary information (ESI) available: Sample preparation, materials, methods, instrumental details, experimental details for supramolecular copolymerization, MD simulation details and additional figures. See DOI: https://doi.org/10.1039/d3sc03056c |
| This journal is © The Royal Society of Chemistry 2023 |