Open Access Article
Open Access Article2,3-Diarylmaleate salts as a versatile class of diarylethenes with a full spectrum of photoactivity in water†‡
Iumzhana A.
Bolotova
ab,
Alexander O.
Ustyuzhanin
ab,
Ekaterina S.
Sergeeva
ab,
Anna A.
Faizdrakhmanova
 ab,
Yu
Hai
c,
Andrey V.
Stepanov
ab,
Igor A.
Ushakov
a,
Konstantin A.
Lyssenko
d,
Lei
You
ab,
Yu
Hai
c,
Andrey V.
Stepanov
ab,
Igor A.
Ushakov
a,
Konstantin A.
Lyssenko
d,
Lei
You
 *c and
Andrey G.
Lvov
*c and
Andrey G.
Lvov
 *ab
*ab
aLaboratory of Photoactive Compounds, A. E. Favorsky Irkutsk Institute of Chemistry, Siberian Branch of the Russian Academy of Sciences, 1 Favorsky St., Irkutsk, 664033, Russia. Web: http://www.lvovchem.ruE-mail: lvov-andre@yandex.ru
bIrkutsk National Research Technical University, 83, Lermontov St., Irkutsk, 664074, Russia
cState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: lyou@fjirsm.ac.cn
dDepartment of Chemistry, Lomonosov Moscow State University, Moscow, 119992, Russia
First published on 18th August 2023
Abstract
There is incessant interest in the transfer of common chemical processes from organic solvents to water, which is vital for the development of bioinspired and green chemical technologies. Diarylethenes feature a rich photochemistry, including both irreversible and reversible reactions that are in demand in organic synthesis, materials chemistry, and photopharmacology. Herein, we introduce the first versatile class of diarylethenes, namely, potassium 2,3-diarylmaleates (DAMs), that show excellent solubility in water. DAMs obtained from highly available precursors feature a full spectrum of photoactivity in water and undergo irreversible reactions (oxidative cyclization or rearrangement) or reversible photocyclization (switching), depending on their structure. This finding paves a way towards wider application of diarylethenes in photopharmacology and bioinspired technologies that require aqueous media for photochemical reactions.
Introduction
Light is a unique energy source for chemical reactions providing the opportunity to induce them in a selected volume within the selected period of time.1–4 Varying the properties of a molecule by its reversible or irreversible photochemical transformation is of great interest for the development of new methods and technologies in materials chemistry, biology and medicine.5,6 Solubility of photoactive organic molecules in water is a crucial requirement in many bio-related applications.7,8 For example, bioimaging beyond the diffraction limit with high-resolution fluorescence microscopy became possible in aqueous solutions using appropriate photoswitchable dyes as labels.9–13 Among photoactive molecules, diarylethenes (DAEs) became one of the most powerful molecular tools for photochemical applications. There are three modes of DAE's chemical activity associated with light-induced cyclization (Scheme 1A): reversible switching between initial and closed-ring isomers,14 irreversible oxidative cyclization towards tricyclic aromatics (Mallory reaction);15 irreversible photorearrangement to bicyclic aromatics.16 The classical Mallory reaction is used mainly for building polyaromatic compounds that contain a fragment of phenanthrene or its analogues (Scheme 1A(a)).17–21 The photoswitching of DAEs finds application in the development of stimuli-responsive materials and light-controllable processes (Scheme 1A(b)).22–27 The photorearrangement with formation of bicyclic aromatics is a recently discovered transformation (Scheme 1A(c)) that is promising for various applications, including organic synthesis,28–30 photopharmacology31 and click-chemistry.32 | ||
| Scheme 1 Three types of DAE photoreactions (A). Water-soluble DAEs for various bio-related applications (B). | ||
Water-soluble DAEs bearing solubilizing charged groups are in demand for various photopharmacological and biomedical applications (Scheme 1B). The only example of the Mallory reaction in aqueous solutions was reported for light-induced generation of a DNA/RNA intercalator by wDAE-1 with a protonated amidine substituent.33 Reversible switching was successfully performed for DAEs that comprised of, among others,7 phosphate and phosphonate (wDAE-2 (ref. 34)), pyridinium (wDAE-3 (ref. 35 and 36), wDAE-4 (ref. 37)), ammonium (wDAE-5 (ref. 38)) and carboxylate groups (wDAE-6 (ref. 39)). The corresponding dyes were successfully applied as photocontrollable enzyme inhibitors, DNA ligands, dyes for bioimaging, and agents for the control of physiological functions of living organisms. It should be noted that all examples of water-soluble DAEs contain charged groups in the pendant aromatic rings. This approach is not universal and requires a significant change in the synthesis strategy depending on the desired structure. Introduction of a solubilizing group on the central part of the molecule is an alternative approach that has never been probed for the synthesis of water-soluble DAEs. It can be expected that this method should allow the incorporation of any type of aromatic moiety “by choice” and pave a way towards the modular construction of water-soluble molecules with desired properties and functions. Herein, we introduce a first general class of water-soluble photoactive molecules – 2,3-DiArylMaleate (DAM) salts (Scheme 1B). In the structure of DAMs, two solubilizing carboxylate groups are directly attached to the central double bond and could be combined with various aromatic moieties while retaining the entire spectrum of DAE photoactivity in water.
Results and discussion
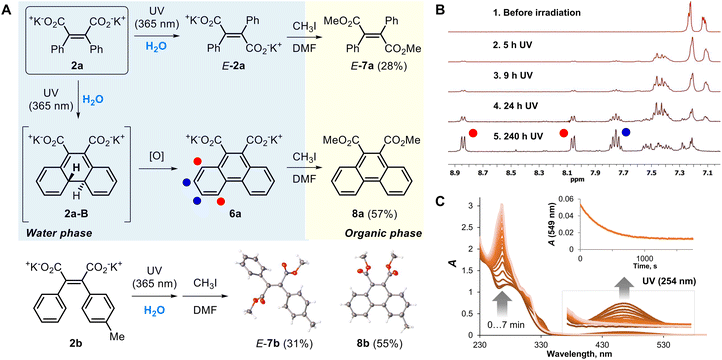
To date, DAM salts have never been considered as an individual class of organic molecules, although these entities were often involved as intermediates in the synthesis/cleavage of 3,4-di(het)arylfuran-2,5-diones 1 or related maleimides (Scheme 2).40,41 Straightforward hydrolysis of 1 in aqueous alkali is not suitable for DAM isolation. We assumed that this problem could be overcome by cleavage of 3,4-di(het)arylfuran-2,5-diones 1 in dry DMF with potassium carbonate (K2CO3). First of all, we attempted trapping DAMs by in situ alkylation in the reaction starting from 1e (Scheme 2). The treatment of 1e by K2CO3 at the heating followed by the addition of methyl iodide or 2-bromo-1-phenylethan-1-one led to the disappearance of the starting material and formation of the corresponding dithienylmaleic acid esters 5a and 5b. These products were isolated with 42–63% yields. The experiments clearly showed the generation of the potassium 2,3-dithienylmaleate by cleavage of the maleic anhydride cycle of 1e.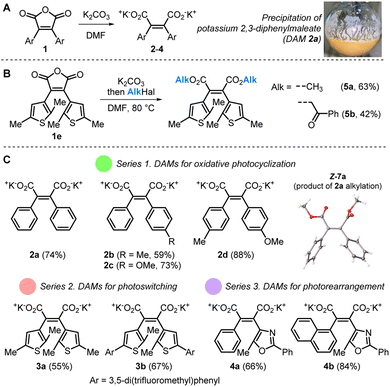 | ||
| Scheme 2 Synthesis of the DAM salts from accessible 3,4-diarylfuran-2,5-diones (A). Trapping of DAM salt by alkylating agents (B). Scope of the DAM salts (C). | ||
To synthesize a range of multipurpose water-soluble DAMs, we used eight 3,4-di(het)arylfuran-2,5-diones 1 bearing two benzene rings (towards substrates for Mallory oxidative cyclization), two thienyl rings (for photoswitchable products) and both phenyl/naphthyl and oxazolyl rings (towards photorearrangement substrates). As expected, treatment of these compounds with K2CO3 in dry DMF with heating results in the disappearance of the starting material. To ensure complete consumption of K2CO3, a slight excess of 1 was exploited (for details see Section III.1 in the ESI‡). In all cases, the reaction was accompanied by abundant precipitation of DAM salts, which were isolated by filtration. The desired salts 2–4 were obtained in 55–88% yields as stable solids and were fully characterized by 1H and 13C NMR spectroscopy in deuterated water (D2O). DAM 3b bearing two lipophilic 3,5-di(trifluoromethyl)phenyl moieties was an exception. This compound was readily soluble in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/acetonitrile mixture.
1 water/acetonitrile mixture.
The obtained DAM salts were isolated exclusively as Z-isomers, that is predetermined by the structure of their substrates 1. The Z-configuration was additionally confirmed by NOESY NMR for 4a (Fig. S13‡) and by X-ray crystallography data for stilbene Z-7a obtained by direct alkylation of DAM 2a (Scheme 2). The thermodynamic stability of Z-stilbenes is determined by the high activation energy barrier of Z- to E-isomerization.42,43
It was expected that the DAMs 2a–d as close analogues of stilbene should undergo oxidative cyclization to phenanthrene derivatives (Scheme 1A(a)).15,44 Irradiation of 2a by UV (λ = 365 nm) in D2O led to new products according to NMR spectroscopy (Fig. 1B). Complete disappearance of the substrate 2a was reached after 240 h of reaction. The major product in the reaction mixture was identified as phenanthrene derivative 6a with characteristic doublets of H1(8) and H4(5) at 8.05 and 8.85 ppm (Fig. 1A). Two minor photoproducts with NMR signals in the 7.20–7.40 ppm range were also detected in the reaction mixture. The preparative photoreaction of 2a in water led to a mixture with the same composition. To isolate and characterize the products, we “transferred” the products from the aqueous phase to the organic one by trapping potassium salts with methyl iodide and separation by column chromatography (Fig. 1A). Two products where obtained: the expected phenanthrene 8a (57% yield) and methyl diphenylmaleate 7a in the E-configuration (31% yield). A similar reaction outcome was obtained for DAM salts 2b–d (yields 55–66% for phenanthrenes 8b–d; Fig. S4–S6‡). The structures of DAM 4b photoproducts E-7b and 8b were supported by X-ray crystallography (Fig. 1A). In addition, the molecular structures were obtained for photoproducts E-7c and 8d (for details, see Section IV in the ESI‡).
The main photoproducts of photolysis of DAM salts 2a–d were found to be expected phenanthrenes 6, formed by 6π-photocyclization followed by oxidation by oxygen species.45 The side process is Z-/E-isomerization, that is quasi-irreversible probably due to a low quantum yield. Photolysis of 2a in the buffer solution (pH = 7.00) allowed us to detect the elusive 4a,4b-dihydrophenanthrene intermediate of the Mallory reaction,45,46 a closed-ring isomer 2a-B (Fig. 1A). These species are usually highly unstable and could be isolated in specific cases only.46,47 This entity has absorbance maxima at 459 nm due to a conjugated system of π-bonds (Fig. 1B). Simulation of the spectrum by DFT calculations gave the value of 490 nm for this species (Table S3‡). The intermediate 2a-B was thermally unstable and underwent fast bleaching in the dark. In the presence of air, the half-life of these species was ca. 200 s, while in nitrogen-saturated water, t1/2(2a-B) = 300 s (Fig. S17‡). According to the literature data,47,48 the product of unsubstituted stilbene photocyclization (4a,4b-dihydrophenanthrene) features a similar stability, suggesting that the effect of carboxylate anions on the stability of 2a-B is minor.
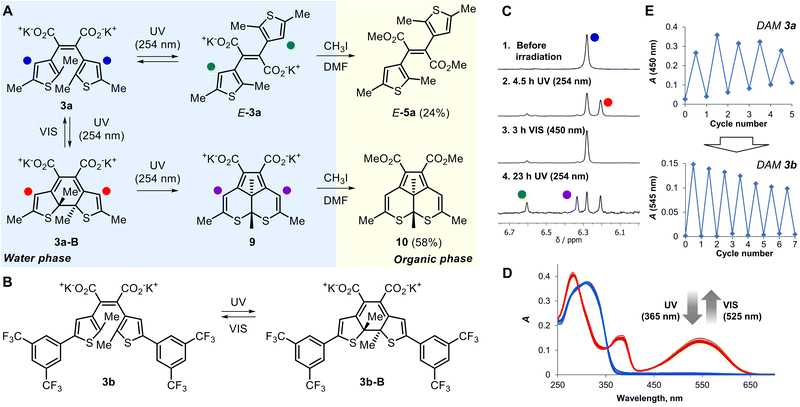
The next probed photochemical reaction was the reversible switching of thiophene-derived DAMs 3a,b between initial and closed-ring isomers (Scheme 1Ab).14 Irradiation of 3a with UV (254 nm) for 4.5 h in D2O resulted in the formation of the closed-ring isomer 3a-B (Fig. 2A) with an absorbance maximum at 450 nm (Fig. S21‡) with conversion ≈ 40%, that was clearly observed by the shift of thiophene protons in the NMR spectra (Fig. 2C). Subsequent irradiation with blue light resulted in the restoration of the initial spectrum. However, prolonged irradiation with UV to reach higher conversion results in two products: a 2a1,5a-dihydro-5,6-dithiaacenaphthylene derivative 9 and the E-isomer of 3a (see Section III.4 in the ESI‡). The first one is the result of formal 1,2-dyotropic rearrangement, limiting the photoswitching of thiophene-based diarylethenes.49,50
The structures of both products were proved again by transferring the preparative reaction mixture to the organic phase followed by isolation of products 10 and E-5a. After 23 h of preparative photolysis of 3a with subsequent methylation, the yields of 10 and E-5a were 58% and 24%, respectively.
Similarly to the diphenyl-substituted DAMs 2a–d, dithienylethene derivative 3a undergoes Z-/E-isomerization as well as irreversible phototransformation. These side reactions are highly undesirable for photoswitch applications. To bypass the 1,2-dyotropic rearrangement, we incorporated additional 3,5-di(trifluoromethyl)phenyl groups50 to the thiophene rings in 3a (DAM 3b, Fig. 1B). As expected, 3b displayed an enhanced light-induced switching between the initial state and the closed-ring isomer 3b-B, which was tracked by UV-Vis and NMR spectroscopy. The open-ring form 3b is colorless with an intense band at 315 nm (ε = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1) (Fig. 2D). Upon UV irradiation, a prominent band of the closed-ring isomer 3b-B at 545 nm (ε = 10
500 M−1 cm−1) (Fig. 2D). Upon UV irradiation, a prominent band of the closed-ring isomer 3b-B at 545 nm (ε = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1) emerges, which completely disappears upon green light irradiation. According to NMR spectroscopy, the conversion of 3b to 3b-B in the photostationary state was 84% (Fig. S10‡). The cyclization (ΦOC) and cycloreversion (ΦCO) quantum yields were 0.083 and 0.004, respectively. These values suggest that incorporation of carboxylate groups into the chromophore system reduces the efficiency of photoreactions in comparison with typical cyclopentene and perfluorocyclopentene diarylethenes.50
900 M−1 cm−1) emerges, which completely disappears upon green light irradiation. According to NMR spectroscopy, the conversion of 3b to 3b-B in the photostationary state was 84% (Fig. S10‡). The cyclization (ΦOC) and cycloreversion (ΦCO) quantum yields were 0.083 and 0.004, respectively. These values suggest that incorporation of carboxylate groups into the chromophore system reduces the efficiency of photoreactions in comparison with typical cyclopentene and perfluorocyclopentene diarylethenes.50
In contrast to 3a, 3b revealed switching between two isomers accompanied by a minor impact of side processes (Fig. 2E). In particular, the Z-/E-isomerization of 3b was suppressed significantly. After two cycles, no signs of the E-product were found in NMR spectra (Fig. S10‡). In absorbance studies, only a minor contribution of the E-isomer (the shoulder at 370–380 nm) was detected after 7 cycles (Fig. S24‡) in contrast to 3a (Fig. S22‡). Although diarylethenes with a completely suppressed cyclization pathway were described previously,51,52 the effect of incorporating aryl groups on the E-/Z-isomerization is unknown to the best of our knowledge. We suggest that the difference in 3a/3b performance is a result of the twofold increase in the extinction coefficients in the UVA region, a 10-fold decrease in ΦCO, and an enhancement of ΦOC in the case of 3b (Table S1‡).
The drawback of the 3b photoswitch is the decreased hydrophilicity due to the presence of four CF3 groups. It is poorly soluble in water, so the experiments were performed in water/acetonitrile solutions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). According to the previous results by Hecht et al.,50 the number of lipophilic electron-withdrawing groups in the diarylethene molecule could be reduced without loss of fatigue resistance. This should be taken into account in the future design of DAM photoswitches.
1). According to the previous results by Hecht et al.,50 the number of lipophilic electron-withdrawing groups in the diarylethene molecule could be reduced without loss of fatigue resistance. This should be taken into account in the future design of DAM photoswitches.
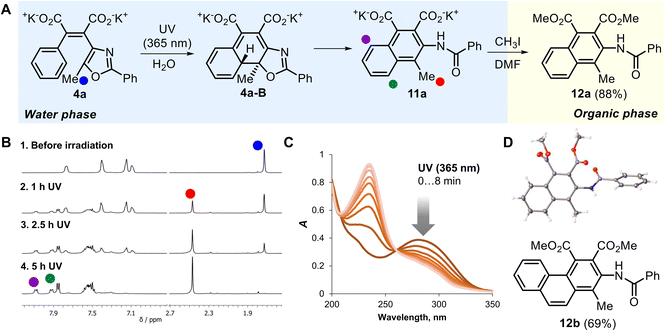
Finally, we studied the DAMs 4a,b as analogues of DAEs that undergo irreversible photorearrangement with formal cycloreversion of the oxazole ring (Scheme 1A(c)).16,281H NMR monitoring of 4a photolysis disclosed an almost quantitative conversion to a new product with characteristic doublets at 7.95 and 8.05 ppm, showing the formation of the naphthalene system of 11a (Fig. 3A and B). The side Z-/E-isomerization was negligible, unlike DAMs 2a–d and 3a and similarly to 3b. UV-Vis spectroscopy also disclosed the nearly quantitative conversion to a sole product accompanied by the isosbestic point at 260 nm (Fig. 3C).
Similarly to the related diarylethenes,28,53 a key intermediate of the photorearrangement 4a-B was not observed during photolysis due to a fast aromatization-driven migration of hydrogen, which triggers the irreversible process. Unexpectedly, the quantum yield of the irreversible rearrangement was relatively high, Φ = 0.33. This value is comparable to quantum yields of the related DAEs, measured in acetonitrile (Φ = 0.34 for the furanone derivative54 and 0.15 for the cyclopentenone derivative53). The significant increase of the cyclization efficiency in comparison with photochromic DAMs 3a,b (ΦOC = 0.051–0.083) could be explained by the stabilization of the photoactive conformation of diarylethene55–57 (a possible approach is a fixation of the oxazole ring by non-covalent interactions of water with a nitrogen atom and carboxylate group58).
Preparative photolysis of 4a with subsequent alkylation by methyl iodide afforded naphthalene derivative 12a with 88% yield (the structure was proved by X-ray crystallography, Fig. 3D). The related result was obtained for DAM 4b (Fig. S12‡), which formed the corresponding phenanthrene in the irreversible photorearrangement with good yield (alkylated product 12b was isolated with 69% yield). It should be noted that intermediate 4b-B is relatively stable59 and was detected by its prominent absorbance band at 427 nm (Fig. S26‡).
Conclusions
In conclusion, we introduce the first versatile class of water-soluble diarylethenes, namely, potassium 2,3-diarylmaleates (DAMs). A technologically simple method for their synthesis from accessible 3,4-di(het)arylfuran-2,5-diones was suggested. Three types of efficient photochemical reactions of DAM salts in water were demonstrated. The first one is the Mallory reaction of diphenyl-substituted DAMs, leading to the phenanthrene derivatives with good yields. The second photoreaction is the reversible cyclization/cycloreversion, which was performed for thiophene-based DAMs. Finally, photolysis of DAMs bearing phenyl/naphthyl and oxazolyl groups resulted in rearrangement products. Such a combination of various modes of photoactivity in water is unique for diarylethenes. Due to availability and versatility, DAMs could be considered as promising photoactive agents for the development of new bioinspired and photopharmacological applications that require solubility in water.†Data availability
The data related to this study are available from the corresponding author upon reasonable request.Author contributions
I. A. B., A. O. U., E. S. S., A. A. F., Y. H., and A. V. S. synthesized 3,4-di(het)arylfuran-2,5-diones and DAM salts. I. A. B. and A. O. U. studied the photochemical properties of DAM salts. I. A. U. performed NMR spectroscopy. K. A. L. performed X-ray crystallography. A. G. L. performed DFT calculations. L. Y. and A. G. L. supervised the project and supported writing of the manuscript. All authors read and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Russian Science Foundation (grant no. 21-13-00391). L. Y. is grateful to the National Natural Science Foundation of China for support (grant no. 22071247). A. G. L. thanks Dr Arailym M. Nalibayeva for her assistance.Notes and references
- R. S. Stoll and S. Hecht, Angew. Chem., Int. Ed., 2010, 49, 5054–5075 CrossRef CAS PubMed.
- A. J. Teator, D. N. Lastovickova and C. W. Bielawski, Chem. Rev., 2016, 116, 1969–1992 CrossRef CAS PubMed.
- W. Szymański, J. M. Beierle, H. A. V. Kistemaker, W. A. Velema and B. L. Feringa, Chem. Rev., 2013, 113, 6114–6178 CrossRef PubMed.
- V. M. Lechner, M. Nappi, P. J. Deneny, S. Folliet, J. C. K. Chu and M. J. Gaunt, Chem. Rev., 2022, 122, 1752–1829 CrossRef CAS PubMed.
- A. Goulet-Hanssens, F. Eisenreich and S. Hecht, Adv. Mater., 2020, 32, 1905966 CrossRef CAS.
- V. García-López, D. Liu and J. M. Tour, Chem. Rev., 2020, 120, 79–124 CrossRef PubMed.
- J. Volarić, W. Szymanski, N. A. Simeth and B. L. Feringa, Chem. Soc. Rev., 2021, 50, 12377–12449 RSC.
- R. Weinstain, T. Slanina, D. Kand and P. Klán, Chem. Rev., 2020, 120, 13135–13272 CrossRef CAS PubMed.
- B. Roubinet, M. L. Bossi, P. Alt, M. Leutenegger, H. Shojaei, S. Schnorrenberg, S. Nizamov, M. Irie, V. N. Belov and S. W. Hell, Angew. Chem., Int. Ed., 2016, 55, 15429–15433 CrossRef CAS PubMed.
- X. Chai, H.-H. Han, A. C. Sedgwick, N. Li, Y. Zang, T. D. James, J. Zhang, X.-L. Hu, Y. Yu, Y. Li, Y. Wang, J. Li, X.-P. He and H. Tian, J. Am. Chem. Soc., 2020, 142, 18005–18013 CrossRef CAS.
- K. Uno, A. Aktalay, M. L. Bossi, M. Irie, V. N. Belov and S. W. Hell, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2100165118 CrossRef CAS PubMed.
- B. Roubinet, M. Weber, H. Shojaei, M. Bates, M. L. Bossi, V. N. Belov, M. Irie and S. W. Hell, J. Am. Chem. Soc., 2017, 139, 6611–6620 CrossRef CAS PubMed.
- K. Uno, M. L. Bossi, M. Irie, V. N. Belov and S. W. Hell, J. Am. Chem. Soc., 2019, 141, 16471–16478 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- K. B. Jørgensen, Molecules, 2010, 15, 4334–4358 CrossRef PubMed.
- A. G. Lvov, J. Org. Chem., 2020, 85, 8749–8759 CrossRef CAS PubMed.
- F. Saal, F. Zhang, M. Holzapfel, M. Stolte, E. Michail, M. Moos, A. Schmiedel, A.-M. Krause, C. Lambert, F. Würthner and P. Ravat, J. Am. Chem. Soc., 2020, 142, 21298–21303 CrossRef CAS PubMed.
- J. Tomada, T. Dienel, F. Hampel, R. Fasel and K. Amsharov, Nat. Commun., 2019, 10, 3278 CrossRef PubMed.
- R. K. Mohamed, S. Mondal, J. V. Guerrera, T. M. Eaton, T. E. Albrecht-Schmitt, M. Shatruk and I. V. Alabugin, Angew. Chem., Int. Ed., 2016, 55, 12054–12058 CrossRef CAS PubMed.
- A. T. Turley, P. K. Saha, A. Danos, A. N. Bismillah, A. P. Monkman, D. S. Yufit, B. F. E. Curchod, M. K. Etherington and P. R. McGonigal, Angew. Chem., Int. Ed., 2022, 61, e202202193 CrossRef CAS PubMed.
- M. Krzeszewski, H. Ito and K. Itami, J. Am. Chem. Soc., 2022, 144, 862–871 CrossRef CAS PubMed.
- Y. Hai, H. Ye, Z. Li, H. Zou, H. Lu and L. You, J. Am. Chem. Soc., 2021, 143, 20368–20376 CrossRef CAS PubMed.
- F. Eisenreich, M. Kathan, A. Dallmann, S. P. Ihrig, T. Schwaar, B. M. Schmidt and S. Hecht, Nat. Catal., 2018, 1, 516–522 CrossRef CAS.
- S. M. Büllmann, T. Kolmar, N. F. Zorn, J. Zaumseil and A. Jäschke, Angew. Chem., Int. Ed., 2022, 61, e202117735 CrossRef PubMed.
- A. G. Lvov, A. V. Yadykov, K. A. Lyssenko, F. W. Heinemann, V. Z. Shirinian and M. M. Khusniyarov, Org. Lett., 2020, 22, 604–609 CrossRef CAS PubMed.
- C. Jurissek, F. Berger, F. Eisenreich, M. Kathan and S. Hecht, Angew. Chem., Int. Ed., 2019, 58, 1945–1949 CrossRef CAS PubMed.
- O. Babii, S. Afonin, C. Diel, M. Huhn, J. Dommermuth, T. Schober, S. Koniev, A. Hrebonkin, A. Nesterov-Mueller, I. V. Komarov and A. S. Ulrich, Angew. Chem., Int. Ed., 2021, 60, 21789–21794 CrossRef CAS.
- A. G. Lvov, V. Z. Shirinian, V. V. Kachala, A. M. Kavun, I. V. Zavarzin and M. M. Krayushkin, Org. Lett., 2014, 16, 4532–4535 CrossRef CAS.
- A. G. Lvov, A. M. Kavun, V. V. Kachala, K. A. Lyssenko and V. Z. Shirinian, Org. Biomol. Chem., 2019, 17, 4990–5000 RSC.
- J. Fan, T. Wang, C. Li, R. Wang, X. Lei, Y. Liang and Z. Zhang, Org. Lett., 2017, 19, 5984–5987 CrossRef CAS PubMed.
- A. V. Yadykov, A. M. Scherbakov, V. V. Trofimova, A. G. Lvov, A. I. Markosyan, I. V. Zavarzin and V. Z. Shirinian, Org. Lett., 2019, 21, 9608–9612 CrossRef CAS PubMed.
- F. Zhang, Z. Zhang, L. Deng, H. Guo, T. Xia, W. Mao and J. Zhang, Org. Lett., 2023, 25, 872–876 CrossRef CAS PubMed.
- K. Starčević, G. Karminski-Zamola, I. Piantanida, M. Žinić, L. Šuman and M. Kralj, J. Am. Chem. Soc., 2005, 127, 1074–1075 CrossRef.
- B. Reisinger, N. Kuzmanovic, P. Löffler, R. Merkl, B. König and R. Sterner, Angew. Chem., Int. Ed., 2014, 53, 595–598 CrossRef CAS PubMed.
- M. P. O'Hagan, J. Ramos-Soriano, S. Haldar, S. Sheikh, J. C. Morales, A. J. Mulholland and M. C. Galan, Chem. Commun., 2020, 56, 5186–5189 RSC.
- U. Al-Atar, R. Fernandes, B. Johnsen, D. Baillie and N. R. Branda, J. Am. Chem. Soc., 2009, 131, 15966–15967 CrossRef CAS PubMed.
- D. Wilson and N. R. Branda, Angew. Chem., Int. Ed., 2012, 51, 5431–5434 CrossRef CAS.
- D. Kim, A. Aktalay, N. Jensen, K. Uno, M. L. Bossi, V. N. Belov and S. W. Hell, J. Am. Chem. Soc., 2022, 144, 14235–14247 CrossRef CAS PubMed.
- R. Göstl and S. Hecht, Chem.–Eur. J., 2015, 21, 4422–4427 CrossRef PubMed.
- M. Irie and M. Mohri, J. Org. Chem., 1988, 53, 803–808 CrossRef CAS.
- X. Mei, J. Wang, Z. Zhou, S. Wu, L. Huang, Z. Lin and Q. Ling, J. Mater. Chem. C, 2017, 5, 2135–2141 RSC.
- G. B. Kistiakowsky and W. R. Smith, J. Am. Chem. Soc., 1934, 56, 638–642 CrossRef CAS.
- K. Imato, A. Sasaki, A. Ishii, T. Hino, N. Kaneda, K. Ohira, I. Imae and Y. Ooyama, J. Org. Chem., 2022, 87, 15762–15770 CrossRef CAS PubMed.
- C. O. Parker and P. E. Spoerri, Nature, 1950, 166, 603 CrossRef.
- F. B. Mallory and C. W. Mallory, Org. React., 1984, 1–456 CAS.
- Y. Shi, S. K. Mellerup, K. Yuan, G.-F. Hu, F. Sauriol, T. Peng, N. Wang, P. Chen and S. Wang, Chem. Sci., 2018, 9, 3844–3855 RSC.
- K. A. Muszkat and E. Fischer, J. Chem. Soc. B, 1967, 662–678 RSC.
- W. M. Moore, D. D. Morgan and F. R. Stermitz, J. Am. Chem. Soc., 1963, 85, 829–830 CrossRef CAS.
- M. Irie, T. Lifka, K. Uchida, S. Kobatake and Y. Shindo, Chem. Commun., 1999, 747–750 RSC.
- M. Herder, B. M. Schmidt, L. Grubert, M. Pätzel, J. Schwarz and S. Hecht, J. Am. Chem. Soc., 2015, 137, 2738–2747 CrossRef CAS PubMed.
- M. Takeshita, T. Hirowatari and A. Takedomi, Tetrahedron Lett., 2016, 57, 3565–3567 CrossRef CAS.
- X. Guo, J. Zhou, M. A. Siegler, A. E. Bragg and H. E. Katz, Angew. Chem., Int. Ed., 2015, 54, 4782–4786 CrossRef CAS PubMed.
- E. M. Glebov, N. V. Ruban, I. P. Pozdnyakov, V. P. Grivin, V. F. Plyusnin, A. G. Lvov, A. V. Zakharov and V. Z. Shirinian, J. Phys. Chem. A, 2018, 122, 7107–7117 CrossRef CAS PubMed.
- A. V. Zakharov, E. B. Gaeva, A. G. Lvov, A. V. Metelitsa and V. Z. Shirinian, J. Org. Chem., 2017, 82, 8651–8661 CrossRef CAS PubMed.
- S. Fukumoto, T. Nakashima and T. Kawai, Angew. Chem., Int. Ed., 2011, 50, 1565–1568 CrossRef CAS PubMed.
- H. Ogawa, K. Takagi, T. Ubukata, A. Okamoto, N. Yonezawa, S. Delbaere and Y. Yokoyama, Chem. Commun., 2012, 48, 11838–11840 RSC.
- V. Z. Shirinian, A. G. Lvov, M. M. Krayushkin, E. D. Lubuzh and B. V. Nabatov, J. Org. Chem., 2014, 79, 3440–3451 CrossRef CAS PubMed.
- T. Nakashima, R. Fujii and T. Kawai, Chem.–Eur. J., 2011, 17, 10951–10957 CrossRef CAS PubMed.
- A. V. Zakharov, A. V. Yadykov, A. G. Lvov, E. A. Mitina and V. Z. Shirinian, Org. Biomol. Chem., 2020, 18, 3098–3103 RSC.
Footnotes |
| † A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2023-l3sjk). |
| ‡ Electronic supplementary information (ESI) available. CCDC 2255492–2255496 and 2257444. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc02165c |
| This journal is © The Royal Society of Chemistry 2023 |