Open Access Article
Open Access ArticleNanocapsules of unprecedented internal volume seamed by calcium ions†
Kanishka
Sikligar
a,
Steven P.
Kelley
 a,
Durgesh V.
Wagle
b,
Piyuni
Ishtaweera
a,
Gary A.
Baker
a,
Durgesh V.
Wagle
b,
Piyuni
Ishtaweera
a,
Gary A.
Baker
 *a and
Jerry L.
Atwood
*a and
Jerry L.
Atwood
 *a
*a
aDepartment of Chemistry, University of Missouri – Columbia, 601 S College Avenue, Columbia, MO – 65211, USA. E-mail: AtwoodJ@missouri.edu; bakergar@missouri.edu
bDepartment of Chemistry and Physics, Florida Gulf Coast University, 10501 FGCU Blvd. S., Fort Myers, FL – 33965, USA
First published on 3rd July 2023
Abstract
The inception of an unprecedented class of voluminous Platonic solids displaying hierarchical geometry based on pyrogallol[4]arene moieties seamed by divalent calcium ion is described. Single-crystal X-ray structural determination has established the highly conserved geometry of two original Ca2+-seamed nanocapsules to be essentially cubic in shape with C-ethylpyrogallol[4]arene units located along the twelve edges of the cube which are then bridged by metallic polyatomic cations ([Ca4Cl]7+ or [Ca(HCO2)Na4]5+) at the six cube faces. The accessible volume of the nanocapsules is ca. 3500 Å3 and 2500 Å3 and is completely isolated from the exterior of the capsules. These remarkable nanocapsule discoveries cast a spotlight on a marginalized area of synthetic materials chemistry and encourage future exploration of diversiform supramolecular assemblies, networks, and capsules built on calcium, with clear benefits deriving from the intrinsic biocompatibility of calcium. Finally, a proof-of-concept is demonstrated for fluorescent reporter encapsulation and sustained release from the calcium-seamed nanocapsules, suggesting their potential as delivery vehicles for drugs, nutrients, preservatives, or antioxidants.
The development of molecular capsule research began in 1995 with reports by Rebek et al. of “tennis ball” assemblies.1–3 These hydrogen-bonded, self-assembled dimers enclose a volume sufficient to host a methanol molecule (Venclosed ≈ 100 Å3). In 1997, MacGillivray and Atwood synthesized and characterized the hexameric [C-methylresorcin[4]arene]6(H2O)8, a molecular capsule held together by 60 hydrogen bonds and having a Venclosed of ∼1400 Å3.4 This nanocapsule possessed an overall geometry of the Archimedean solid referred to as a snub cube.5 Subsequently, it was discovered that C-alkylpyrogallol[4]arenes in a range of nonaqueous solvents afforded hexameric capsules held together by 72 hydrogen bonds.6–8 In 2005, McKinlay et al. found that 24 Cu2+ ions stripped 48 H+ ions from the [C-propan-3-olpyrogallol[4]arene]6 structure to yield Cu24[C-propan-3-olpyrogallol[4]arene]6.9 Consequently, a range of M24[C-alkylpyrogallol[4]arene]6 (hexameric) and M8[C-alkylpyrogallol[4]arene]2 (dimeric) nanocapsules were demonstrated, both in the solid state and in solution, for M = Zn,10,11 Cu,12,13 Ni,13,14 Co,15,16 Ga,17,18 In,19 Fe,20 Mn,21,22 V,23 and U.24 Furthermore, very recently C-butylpyrogallol[4]arene formed an anion-based self-assembly leading to the formation of hexameric nonocapsules that are ion-paired with cations.25 Although the 24 years of continuous research since the discovery of the hexameric capsule have produced numerous variants of the known hexameric and dimeric structures, almost no examples exist for alternate geometries beyond these two. Solution studies have pointed to the existence of larger geometries, but these have never been isolated.26 Even nanocapsules built on less usual metals (e.g., alkali or alkaline Earth metals, mixed-valence) or mixed macrocycles, for example Ga12[C-alkylpyrogallol[4]arene]6,17 (Fe2+)16(Fe3+)16[C-alkylpyrogallol[4]arene]6,20 (Mn3+)4(Mn2+)21[C-alkylpyrogallol[4]arene]6,22 Zn18[(C-alkylpyrogallol[3](resorcinarene[1]))]6,27 and Zn7[(C-alkylpyrogallol[3](resorcinarene[1]))]2,28 generally represent iterations of earlier-established geometries.
Recently, solvothermal methods have emerged as synthetic tools to prepare varied nanocages,29,30 including nanocapsules incorporating C-alkylpyrogallol[4]arenes.31–41 Initially, the solvothermal method was employed in the synthesis of discrete nanocapsules with metal ions like magnesium39,40 and as a tool to alter the geometries of the existing nanocapsules.27,28 Followingly, the solvothermal method was used to functionalize nanocapsules.34,42 For instance, the magnesium nanocapsule was functionalized externally (altering the ligand to C-propan-3-olpyrogallol[4]arene; PgC3OH from C-propylpyrogallol[4]arene; PgC3) and internally (coordinating six proline molecules endohedrally).42 Very recently, solvothermal methods were employed in synthesizing hierarchical self-assemblies from nanocapsules of Dy,41 Gd,41 and Pr,37 advocating the use of solvothermal methods in obtaining diverse43 and controlled self-assemblies.
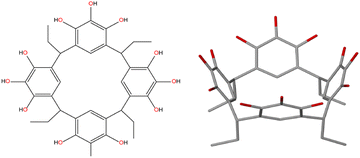
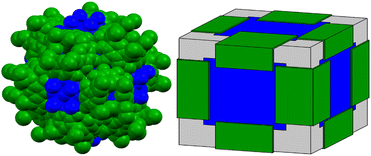
Calcium-based metal–organic frameworks are known44 however, zero-dimensional calcium-seamed nanocapsules have never been isolated. Such Ca2+-seamed nanocapsules may prove interesting as benign catalytic centers and find biomedical application, given the high natural abundance (3.4% of Earth's core) and biocompatibility of calcium. Calcium ion was recently shown by the Dauenhauer group to accelerate glycosidic ether scission in cellulose,45 with Ca2+ acting primarily to stabilize charged transition states and secondarily in the disruption of native cellulose hydrogen bonding. In another example, Rösch et al. reported that β-diketiminate-ligated low-valent Ca+ can mediate dinitrogen reduction, assisted by potassium as the terminal reductant.46 These developments have inspired efforts in our group to expand the chemistry of pyrogallol[4]arene-based nanocapsules to include calcium as the bridging species. Remarkably, a solvothermal treatment of CaCl2 with C-ethylpyrogallol[4]arene (PgC2, Fig. 1) afforded completely unexpected dodecamers comprising giant cube-shaped nanocapsules compositionally described by [(Ca4Cl)6][(C-ethyl-pyrogallol[4]arene)12].
The ligand C-ethylpyrogallol[4]arene (PgC2) 1 was prepared by condensation between pyrogallol and propionaldehyde.401 is a cup-shaped molecule displaying twelve hydroxyl groups positioned at the rim of the cup (Fig. 1). Firstly, a series of experiments were carried out assembling 1 and anhydrous calcium chloride. The ligand 1 when mixed with anhydrous calcium chloride in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) DMF
1 (v/v) DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (MeOH) solution, followed by sonication (60 min), and heating of the reaction flask at 125 °C (24 h) afforded golden plate-like crystals of calcium-seamed nanocapsule 2 [(Ca4Cl)6][(PgC2)12], which were structurally characterized by single crystal X-ray diffraction (SCXRD) using a synchrotron light source. We attempted to isolate more examples of this new family of capsules using other Ca2+ salts. Mixing ligand 1 with calcium bromide hydrate in 6 mL DMF, followed by sonication (60 min), and heating of the reaction flask at 125 °C (96 h) afforded golden plate-like crystals of an isostructural capsule, this one seamed by a polyatomic ion composed of Ca2+, Na+, and formate (HCO2−) (nanocapsule 3 [Ca32(CaNa4(HCO2)5)6(OH)8(DMF)24(OH2)54(PgC2)12]). The source of Na+ ions in 3 is unknown; attempts to reproduce it by deliberately adding Na+ and HCO2− have only led to the isolation of known crystalline forms of Ca(HCO2)2. Subsequent attempts to synthesize a capsule using CaBr2 in the absence of Na+ and HCO2− have repeatedly led to the isolation of a homogeneous crystalline phase with a unit cell of comparable volume to 2 and 3 as determined by SCXRD (tetragonal P, a = b = 38.26(2) Å, c = 39.92(2) Å, α = β = γ = 90°; V = 58
methanol (MeOH) solution, followed by sonication (60 min), and heating of the reaction flask at 125 °C (24 h) afforded golden plate-like crystals of calcium-seamed nanocapsule 2 [(Ca4Cl)6][(PgC2)12], which were structurally characterized by single crystal X-ray diffraction (SCXRD) using a synchrotron light source. We attempted to isolate more examples of this new family of capsules using other Ca2+ salts. Mixing ligand 1 with calcium bromide hydrate in 6 mL DMF, followed by sonication (60 min), and heating of the reaction flask at 125 °C (96 h) afforded golden plate-like crystals of an isostructural capsule, this one seamed by a polyatomic ion composed of Ca2+, Na+, and formate (HCO2−) (nanocapsule 3 [Ca32(CaNa4(HCO2)5)6(OH)8(DMF)24(OH2)54(PgC2)12]). The source of Na+ ions in 3 is unknown; attempts to reproduce it by deliberately adding Na+ and HCO2− have only led to the isolation of known crystalline forms of Ca(HCO2)2. Subsequent attempts to synthesize a capsule using CaBr2 in the absence of Na+ and HCO2− have repeatedly led to the isolation of a homogeneous crystalline phase with a unit cell of comparable volume to 2 and 3 as determined by SCXRD (tetragonal P, a = b = 38.26(2) Å, c = 39.92(2) Å, α = β = γ = 90°; V = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 436(2) Å3). The crystal structure has not yet been solved, as all SCXRD measurements on these crystals have failed to produce a data set of sufficient resolution to be solved by small molecule methods. Regardless, the isolation of 2 and 3 indicate that the unusual cubic geometry is reproducible, with as-yet undiscovered variations likely to exist (Fig. 2).
436(2) Å3). The crystal structure has not yet been solved, as all SCXRD measurements on these crystals have failed to produce a data set of sufficient resolution to be solved by small molecule methods. Regardless, the isolation of 2 and 3 indicate that the unusual cubic geometry is reproducible, with as-yet undiscovered variations likely to exist (Fig. 2).
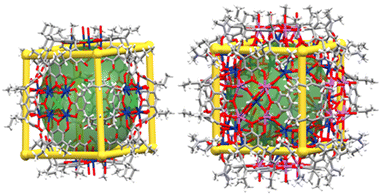
Crystal structure analysis shows that both capsules have 12 PgC2 moieties on the edges of the cube which are bridged across the faces of the cube by polyatomic ions containing four metal ions in a cross-shaped arrangement (Fig. 3 and S6, S7†). In 2 these consist of discrete, planar groups of 4 Ca2+ ions bridged to a single central Cl− (Fig. S8†); similar arrangements have been reported with bridging Cl−, F−, and O2− ions.47–49 In capsule 3, the PgC2 ligands coordinate to Na+ ions which are bridged to a central Ca2+ ion in an analogous shape (Fig. S9†). The cubic faces and PgC2 moieties in 3 are further cross linked by tetranuclear clusters of Ca2+ ions bridged by formate (Fig. S10†). The interiors of the capsules are completely sealed from the exterior environments of the capsule. The accessible internal volumes are estimated at 3500 Å3 and 2500 Å3 for 2 and 3, respectively. The smaller internal volume of 3 is due to the fact that the polyatomic ion occupies part of the internal capsule; otherwise, the two capsules are nearly identical in size. Both capsules pack through assorted noncovalent interactions between edges or corners of the cubes, leaving large regions outside the capsules filled with disordered solvent molecules.
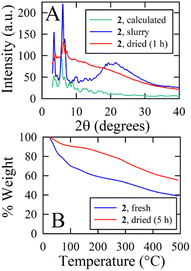
As the crystal structures indicate that the nanocapsule interiors are totally isolated from the exterior, these nanocapsules could potentially be used to make large, discrete, and isolated volumes for host–guest applications. Nanocapsule 2 could be crystallized reproducibly and was used to examine the stability of these capsules. Powder X-ray diffraction (PXRD) on a bulk sample of 2 covered in mother liquor and crystals of 2 allowed to dry in ambient air for 1 h are given in Fig. 4A. The diffraction pattern of the fresh slurry at ambient temperature matches reasonably well to a theoretical pattern predicted from atomic coordinates. The main differences are a number of smaller or missing peaks in the theoretical pattern which likely correspond to atoms removed from the model by the solvent mask. Allowing 2 to dry in air for 1 h results in the loss of all but the lowest angle diffraction peaks; this indicates that much of the crystalline order is lost, most likely due to evaporation of the interstitial solvent, but the structure retains order at its longest length scales, corresponding to the capsules themselves. Thermogravimetric analysis (TGA) shows that both fresh and dried samples of bulk 2 immediately lose mass on heating in a step that stabilizes at ca. 100 °C (Fig. 4B). For the fresh sample, this step results in the loss of 30.3% of the sample mass; for comparison, the calculated density of 2 with solvent omitted (0.713 g cm−3) is 34.3% lower than the density of 1.07 g cm−3 calculated for [Mg24(C-propylpyrogallol[4]arene)6(proline)6(DMF)2(Hs2O)30]42 for which the solvent is modeled. The dried sample of 2 loses 9.5% of its mass. The mass loss of the wet sample is triple that of the dried sample, which is similar to the relative sizes of the interstitial voids which are 2.4 times the size of the encapsulated voids (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 Å3vs. 7132 Å3 per unit cell, respectively). Together, the PXRD and TGA data indicate that, although the crystals are ultimately unstable to solvent loss even at room temperature, the capsules are apparently stable and are able to prevent loss of the encapsulated solvent until after the loss of the interstitial solvent.
425 Å3vs. 7132 Å3 per unit cell, respectively). Together, the PXRD and TGA data indicate that, although the crystals are ultimately unstable to solvent loss even at room temperature, the capsules are apparently stable and are able to prevent loss of the encapsulated solvent until after the loss of the interstitial solvent.
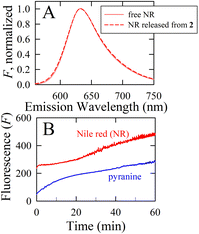
The possibility for hosting functional cargo by entrapping it within the interior environment of calcium-seamed nanocapsules during nanocapsular assembly and crystallization was investigated by synthesizing capsule 2 in the presence of the fluorescent probes Nile red and pyranine (8-hydroxypyrene-1,3,6-trisulfonate). A comparison reveals that the fluorescence spectrum of Nile red (NR) released from 2 upon dissolution in DMSO perfectly matched that of free NR in DMSO (Fig. 5A), both showing a fluorescence maximum at 633 nm. The pyranine was similarly unperturbed by encapsulation within calcium-seamed 2 (data not shown). These experiments indicate that fluorescent cargo can be entrapped within 2 and subsequently released intact.
Dissolution experiments were carried out to obtain the release profiles of NR and pyranine from 2. Fig. 5B displays the time release behavior of the entrapped fluorescent reporters using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) dimethylformamide (DMF)
1 (v/v) dimethylformamide (DMF)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH as the release medium. The calcium-seamed capsules do not dissolve in this medium. After dispersing crystals of NR- or pyranine-containing 2 into 1
MeOH as the release medium. The calcium-seamed capsules do not dissolve in this medium. After dispersing crystals of NR- or pyranine-containing 2 into 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF
1 DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, the fluorescent reporter is gradually released by diffusion through the crystalline matrix and into the surrounding medium. The time-resolved fluorescence intensity reveals sustained release of the entrapped fluorescent reporters over the course of an hour. Interestingly, the release profiles differ markedly for NR and pyranine. NR, a fairly small (∼300 Da), uncharged, hydrophobic molecule, exhibits significant initial “burst” release, followed by slower additional release over time. The release behavior for pyranine exhibits a much more steady release over the course of an hour. Although both fluorescent reporters are expected to display guest-to-wall binding to nanocapsules within 2, attributable to π stacking and CH⋯π interactions, the marked difference in release behavior predominantly reflects the highly charged (trianionic) nature but also the larger size (∼500 Da) of pyranine. That is, the different time release profiles reflect differences in the guest chemistry, suggesting disparate guest populations and selectivity in binding strength. Although details of the mechanism of entrapment and kinetic release profiles are beyond the scope of this study, these results provide proof-of-concept for solute entrapment and cargo release from the interior of these voluminous calcium-seamed nanocapsule assemblies, suggesting these synthetic hosts might act as controlled delivery vehicles for other guests such as (bio)pharmaceuticals, nutrients, preservatives, or antioxidants. These prospects are currently under exploration within our laboratories.
MeOH, the fluorescent reporter is gradually released by diffusion through the crystalline matrix and into the surrounding medium. The time-resolved fluorescence intensity reveals sustained release of the entrapped fluorescent reporters over the course of an hour. Interestingly, the release profiles differ markedly for NR and pyranine. NR, a fairly small (∼300 Da), uncharged, hydrophobic molecule, exhibits significant initial “burst” release, followed by slower additional release over time. The release behavior for pyranine exhibits a much more steady release over the course of an hour. Although both fluorescent reporters are expected to display guest-to-wall binding to nanocapsules within 2, attributable to π stacking and CH⋯π interactions, the marked difference in release behavior predominantly reflects the highly charged (trianionic) nature but also the larger size (∼500 Da) of pyranine. That is, the different time release profiles reflect differences in the guest chemistry, suggesting disparate guest populations and selectivity in binding strength. Although details of the mechanism of entrapment and kinetic release profiles are beyond the scope of this study, these results provide proof-of-concept for solute entrapment and cargo release from the interior of these voluminous calcium-seamed nanocapsule assemblies, suggesting these synthetic hosts might act as controlled delivery vehicles for other guests such as (bio)pharmaceuticals, nutrients, preservatives, or antioxidants. These prospects are currently under exploration within our laboratories.
Conclusions
In summary, we have discovered a new family of nanocapsules self-assembled from pyrogallol[4]arene building blocks and calcium ions. These two structures show conserved geometry based on a Platonic solid (cube) but differ from all previous pyrogallol[4]arene nanocapsules in the number of PgC moieties, the role of the polyatomic metal ions in completely blocking the interior, and the high variability in the number, type, and connectivity of the seaming metal ions (and their counterions). These capsules possess a completely sealed and exceptionally large interior which our preliminary investigations suggest is stabilized against solvent loss more so than the interstitial space, further expanding the applicability of pyrogallol[4]arene capsules in the creation of discrete, fully-encapsulated nanoscale chambers. The complexity of the seaming polyatomic ions adds variability to the growing range of applications that nanocapsules have as high-nuclearity metal–organic clusters. We attribute the scarcity of examples of these capsules to the complexity of the polyatomic ions which play a critical role in capsule assembly but also the unpredictability in assembly, plus the fact that the structural characterization of supramolecules of this size is beyond the capabilities of many in-house single-crystal diffractometers. Nevertheless, we are confident that these examples represent the first of a diverse family of colossal nanocapsules, and we continue to search for the next examples.Data availability
Crystallographic data in the ESI† have been deposited at the joint Cambridge Crystallographic Data Centre (CCDC 2: 1955663; 3: 2071418).Author contributions
All authors approve the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Advanced Light Source, which is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy. We also thank Dr David Stalla and the MU's Electron Microscopy Core “Excellence in Electron Microscopy Award” for assistance with SEM-EDS analysis.Notes and references
- R. Wyler, J. de Mendoza and J. Rebek Jr, Angew. Chem., Int. Ed., 1993, 32, 1699–1701 CrossRef
.
- N. Branda, R. Wyler and J. Rebek, Science, 1994, 263, 1267–1268 CrossRef CAS PubMed
.
- R. S. Meissner, J. Rebek and J. de Mendoza, Science, 1995, 270, 1485–1488 CrossRef CAS PubMed
.
- L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469–472 CrossRef CAS
.
- L. R. MacGillivray and J. L. Atwood, Angew. Chem., Int. Ed., 1999, 38, 1019–1034 CrossRef
.
- T. Gerkensmeier, Eur. J. Org. Chem., 1999, 9, 2257–2262 CrossRef
.
- J. L. Atwood, L. J. Barbour and L. A. Jerga, Chem. Commun., 2001, 22, 2376–2377 RSC
.
- G. W. V. Cave, J. Antesberger, L. J. Barbour, R. M. McKinlay and J. L. Atwood, Angew. Chem., Int. Ed., 2004, 43, 5263–5266 CrossRef CAS PubMed
.
- R. M. McKinlay, G. W. V. Cave and J. L. Atwood, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5944–5948 CrossRef CAS PubMed
.
- A. S. Rathnayake, C. L. Barnes and J. L. Atwood, Cryst. Growth Des., 2017, 17, 4501–4503 CrossRef CAS
.
- N. P. Power, S. J. Dalgarno and J. L. Atwood, New J. Chem., 2007, 31, 17–20 RSC
.
- S. J. Dalgarno, N. P. Power, J. E. Warren and J. L. Atwood, Chem. Commun., 2008, 13, 1539–1541 RSC
.
- H. Kumari, A. V. Mossine, S. R. Kline, C. L. Dennis, D. A. Fowler, S. J. Teat, C. L. Barnes, C. A. Deakyne and J. L. Atwood, Angew. Chem., Int. Ed., 2012, 51, 1452–1454 CrossRef CAS PubMed
.
- H. Kumari, C. L. Dennis, A. V. Mossine, C. A. Deakyne and J. L. Atwood, ACS Nano, 2012, 6, 272–275 CrossRef CAS PubMed
.
- J. L. Atwood, E. K. Brechin, S. J. Dalgarno, R. Inglis, L. F. Jones, A. Mossine, M. J. Paterson, N. P. Power and S. J. Teat, Chem. Commun., 2010, 46, 3484–3486 RSC
.
- A. S. Rathnayake, K. A. Feaster, J. White, C. L. Barnes, S. J. Teat and J. L. Atwood, Cryst. Growth Des., 2016, 16(7), 3562–3564 CrossRef CAS
.
- R. M. McKinlay, P. K. Thallapally and J. L. Atwood, Chem. Commun., 2006, 28, 2956–2958 RSC
.
- R. L. McKinlay, P. K. Thallapally, G. W. V. Cave and J. L. Atwood, Angew. Chem., Int. Ed., 2005, 44, 5733–5736 CrossRef CAS PubMed
.
- D. V. Wagle, S. P. Kelley, G. A. Baker, K. Sikligar and J. L. Atwood, Angew. Chem., Int. Ed., 2020, 59, 8062–8065 CrossRef CAS PubMed
.
- A. S. Rathnayake, H. W. L. Fraser, E. K. Brechin, S. J. Dalgarno, J. E. Baumeister, J. White, P. Rungthanaphatsophon, J. R. Walensky, S. P. Kelley, C. L. Barnes and J. L. Atwood, J. Am. Chem. Soc., 2018, 140(46), 15611–15615 CrossRef CAS PubMed
.
- A. S. Rathnayake, H. W. L. Frazer, E. K. Brechin, S. J. Dalgarno, J. E. Baumeister, P. Rungthanaphatsophon, J. R. Walensky, C. L. Barnes and J. L. Atwood, J. Am. Chem. Soc., 2018, 140(40), 13022–13027 CrossRef CAS PubMed
.
- A. S. Rathnayake, H. W. L. Fraser, E. K. Brechin, S. J. Dalgarno, J. E. Baumeister, J. White, P. Rungthanaphatsophon, J. R. Walensky, C. L. Barnes, S. J. Teat and J. L. Atwood, Nat. Commun., 2018, 9, 2119–2125 CrossRef PubMed
.
- K. Su, M. Wu, D. Yuan and M. Hong, Nat. Commun., 2018, 9, 4941 CrossRef PubMed
.
- L. Mei, P. Ren, Q. Y. Wu, Y. B. Ke, J. S. Geng, K. Liu, X. Q. Xing, Z. W. Huang, K. Q. Hu, Y. I. Liu, L. Y. Yuan, G. Mo, Z. H. Wu, J. K. Gibson, Z. F. Chai and W. Q. Shi, J. Am. Chem. Soc., 2020, 142, 16538–16545 CrossRef CAS PubMed
.
- M. Chwastek, P. Cmoch and A. Szumna, J. Am. Chem. Soc., 2022, 144, 5350–5358 CrossRef CAS PubMed
.
- H. Kumari, S. R. Kline, D. A. Fowler, A. V. Mossine, C. A. Deakyne and J. L. Atwood, Chem. Commun., 2014, 50, 109–111 RSC
.
- C. Zhang, K. Sikligar, R. S. Patil, C. L. Barnes, G. A. Baker and J. A. Atwood, Chem. Commun., 2018, 54, 635–637 RSC
.
- C. Zhang, K. Sikligar, R. S. Patil, C. L. Barnes, S. J. Teat and J. L. Atwood, Chem. Commun., 2017, 53, 9613–9615 RSC
.
- V. López, G. R. Pérez, A. Arregui, E. Mateo-Marti, L. Bañares, J. N. Martin-Gago, J. M. Soler, J. Gómez-Herrero and F. Zamora, ACS Nano, 2009, 3, 3352–3357 CrossRef PubMed
.
- Z. Zhang, Y. Chen, X. Xu, J. Zhang, G. Xiang, W. He and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 429–433 CrossRef CAS PubMed
.
- X. Hu, S. Feng, J. Du, L. Shao, J. Lang, C. Zhang, S. P. Kelley, J. Lin, S. J. Dalgarno, D. A. Atwood and J. L. Atwood, Chem. Sci., 2020, 11, 12547–12552 RSC
.
- X. Hu, J. Chai, C. Zhang, J. Lang, S. P. Kelley, S. Feng, B. Liu, D. A. Atwood and J. L. Atwood, J. Am. Chem. Soc., 2019, 141, 9151–9154 CrossRef CAS PubMed
.
- C. Zhang, F. Wang, R. S. Patil, C. L. Barnes, T. Li and J. L. Atwood, Chem.–Eur. J., 2018, 24, 14335–14340 CrossRef CAS PubMed
.
- C. Zhang, R. S. Patil, C. L. Barnes and J. L. Atwood, Cryst. Growth Des., 2017, 17, 4541–4543 CrossRef CAS
.
- C. Zhang, R. S. Patil, C. Lui, C. L. Barnes and J. L. Atwood, J. Am. Chem. Soc., 2017, 139, 2920–2923 CrossRef CAS PubMed
.
- L. Shao, B. Hua, X. Hu, D. Stalla, S. P. Kelley and J. L. Atwood, J. Am. Chem. Soc., 2020, 142(16), 7270–7275 CrossRef CAS PubMed
.
- K. Sikligar, S. P. Kelley, L. Shao, G. A. Baker and J. L. Atwood, Cryst. Growth Des., 2021, 21(4), 1891–1897 CrossRef CAS
.
- K. Su, M. Wu, D. Yuan and M. Hong, Nat. Commun., 2018, 9, 4941–4946 CrossRef PubMed
.
- C. Zhang, R. S. Patil, T. Li, C. L. Barnes and J. L. Atwood, Chem. Commun., 2017, 53, 4312–4315 RSC
.
- C. Zhang, R. S. Patil, T. Li, C. L. Barnes, S. J. Teat and J. L. Atwood, Chem.–Eur. J., 2017, 23, 8520–8524 CrossRef CAS PubMed
.
- K. Su, K. Su, M. Wu, W. Wang, M. Zhou, D. Yuan and M. Hong, Science, 2018, 61, 664–669 CAS
.
- C. Zhang, R. S. Patil, C. L. Barnes and J. L. Atwood, Chem. Commun., 2017, 53, 12144–12147 RSC
.
- K. Sikligar, S. P. Kelley, G. A. Baker and J. L. Atwood, Cryst. Growth Des., 2022, 22(5), 2806–2811 CrossRef CAS
.
- S. Xian, Y. Lin, H. Wang and J. Li, Small, 2021, 17, 2005165 CrossRef CAS PubMed
.
- G. G. Facas, V. Maliekkal, C. Zhu, M. Neurock and P. J. Dauenhauer, JACS Au, 2021, 1(3), 272–281 CrossRef CAS PubMed
.
- B. Röche, T. X. Gentner, J. Langer, C. Färber, J. Eyselein, L. Zhao, C. Ding, G. Frenking and S. Harder, Science, 2021, 371(6534), 1125–1128 CrossRef PubMed
.
- P. Sobota, A. Dr
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) g-Jarz
g-Jarz![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) bek, L. John, J. Utko, J. B. Jerzykiewicz and M. Duczmal, Inorg. Chem., 2009, 48(14), 6584–6593 CrossRef CAS PubMed
bek, L. John, J. Utko, J. B. Jerzykiewicz and M. Duczmal, Inorg. Chem., 2009, 48(14), 6584–6593 CrossRef CAS PubMed .
- M. Almáši, V. Zeleňak, R. Gyepes, L. Zauška and S. Bourrelly, RSC Adv., 2020, 10(54), 32323–32334 RSC
.
- G. B. Deacon, P. C. Junk and J. Moxey, Dalton Trans., 2010, 39(16), 5620–5622 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1955663 and 2071418. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc01629c |
| This journal is © The Royal Society of Chemistry 2023 |