Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design of a robust and strong-acid MOF platform for selective ammonium recovery and proton conductivity†
Genki
Hatakeyama
a,
Hongyao
Zhou
 a,
Takashi
Kikuchi
b,
Masaki
Nishio
a,
Kouki
Oka
a,
Takashi
Kikuchi
b,
Masaki
Nishio
a,
Kouki
Oka
 a,
Masaaki
Sadakiyo
a,
Masaaki
Sadakiyo
 c,
Yusuke
Nishiyama
c,
Yusuke
Nishiyama
 de and
Teppei
Yamada
de and
Teppei
Yamada
 *a
*a
aDivision of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: teppei@chem.s.u-tokyo.ac.jp
bRigaku Corporation, 3-9-12 Matsubaracho, Akishima, Tokyo 196-8666, Japan
cDepartment of Applied Chemistry, Faculty of Science Division I, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
dNano-Crystallography Unit, RIKEN-JEOL Collaboration Center, RIKEN, Yokohama, Kanagawa 230-0045, Japan
eJEOL RESONANCE Inc., Akishima, Tokyo 196-8558, Japan
First published on 1st August 2023
Abstract
Metal–organic frameworks (MOFs) are potential candidates for the platform of the solid acid; however, no MOF has been reported that has both aqueous ammonium stability and a strong acid site. This manuscript reports a highly stable MOF with a cation exchange site synthesized by the reaction between zirconium and mellitic acid under a high concentration of ammonium cations (NH4+). Single-crystal XRD analysis of the MOF revealed the presence of four free carboxyl groups of the mellitic acid ligand, and the high first association constant (pKa1) of one of the carboxyl groups acts as a monovalent ion-exchanging site. NH4+ in the MOF can be reversibly exchanged with proton (H+), sodium (Na+), and potassium (K+) cations in an aqueous solution. Moreover, the uniform nanospace of the MOF provides the acid site for selective NH4+ recovery from the aqueous mixture of NH4+ and Na+, which could solve the global nitrogen cycle problem. The solid acid nature of the MOF also results in the proton conductivity reaching 1.34 × 10−3 S cm−1 at 55 °C by ion exchange from NH4+ to H+.
1. Introduction
Metal–organic frameworks (MOFs or porous coordination polymers, PCPs) have achieved great progress in the last two decades.1–8 Due to the designability and uniform nanopore structure, MOFs show high selectivity for gaseous molecules, which is advantageous for various applications such as gas storage/separation, catalysis, and drug delivery systems.9–17 Recently, ion-conductive MOFs have been receiving more attention in accordance with the increasing demand for the development of solid-state electrolytes and energy storage materials.18–21 Historically, the acidic points of solid materials have played important roles in ion-conductive materials by providing dissociable ions at the acidic point. These solid acids have been utilized not only as ion conductors but also as ion-exchange materials and solid acid catalysts in industrial processes.22–25Many MOFs are reported to show ionic conductivity, and proton conductive MOFs, in particular, have been intensely studied.26–28 However, the number of studies on MOFs as ion-exchange materials is still limited. Some MOFs are reported to adsorb heavy metal ions such as lead, arsenic, selenium, mercury, silver, and palladium,29–34 while no MOFs have been reported that can execute reversible ion exchange for small monovalent cations like ammonium (NH4+), sodium (Na+), and potassium (K+) in water. The significant barrier for the solid-acid MOF is the poor stability in water.35 Early MOFs were very susceptible to decomposition in water or water vapor. Later, many MOFs with improved chemical stability were reported. For example, UiO-66 (ref. 36) shows outstanding strength thanks to the strong metal–ligand interaction between the zirconium cation and the carboxy group of terephthalic acid. The combination of the uniform nanopore and ion-exchanging sites of MOFs could expand the scope for the new solid materials endowed with high ionic selectivity and high chemical stability.
Ionic selectivity is vital in the field of radioactive waste treatment or dialysis.37–40 In addition, the selective capture of NH4+ could solve the global nitrogen cycle problem. Today, 1.8 billion tons of ammonia are produced annually by the Haber–Bosch process,41 and the accumulation of ammonia in the soil and sea has become a serious issue.42,43 The recovery and reuse of NH4+ in wastewater can reduce the risk of environmental damage. However, the Na+ present in wastewater at a high concentration competes with NH4+ in the ion-exchanging process.44 Therefore, the selective recovery of NH4+ requires an ion-exchange site on the uniform nanoporous frameworks. Highly regulated nanopores of MOFs could be suitable for selective ion capture; however, MOFs with the combination of permanent porosity, high chemical stability and reversible ion-exchanging ability have yet to be achieved.
This paper reports a novel MOF consisting of zirconium and mellitic acid incorporating ammonium cations, hereafter called Zr–mel–NH4. This MOF shows high water durability and has a strong-acid point, which can undergo reversible ion exchange with proton (H+), NH4+, Na+, and K+ cations in an aqueous solution. Furthermore, a high proton conductivity of 1.34 × 10−3 S cm−1 was achieved by ion-exchanging from NH4+ to H+. Moreover, the uniform pore of the MOF provides selective NH4+ adsorption from the mixture of NH4+ and Na+.
2. Results and discussion
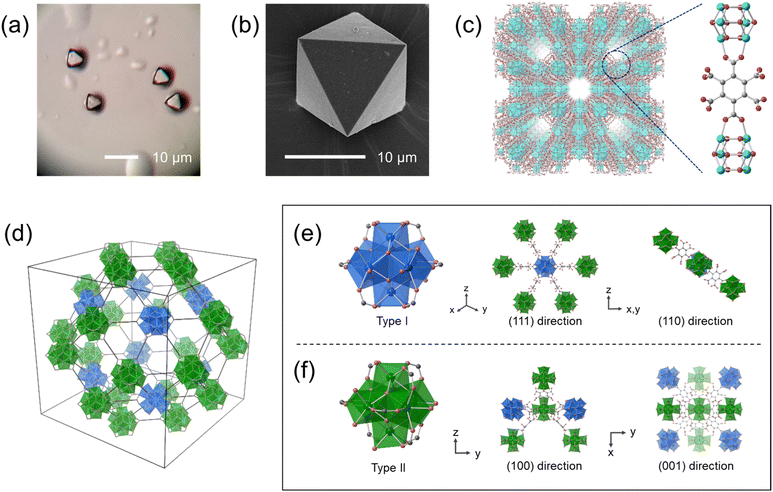
Hydrothermal synthesis of ZrOCl2·8H2O (8.3 mM), mellitic acid (0.13 M) with a high concentration of ammonium chloride (2.3 M) and acetic acid (8.7 M) in an aqueous solution produced white crystalline powders. Optical microscope and SEM images show that the crystals have an octahedral shape with a size of 5 to 15 μm (Fig. 1a and b and S1†). The ratio of carbon to nitrogen was found to be 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol
1 (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) by elemental analysis (Table S1†), which suggests that Zr–mel–NH4 contains one NH4+ per mellitic acid. The chemical formula was estimated to be Zr6O4(OH)4(L–NH4)3.6(CH3CO2)2.4·36H2O (L = C12H3O12).
mol) by elemental analysis (Table S1†), which suggests that Zr–mel–NH4 contains one NH4+ per mellitic acid. The chemical formula was estimated to be Zr6O4(OH)4(L–NH4)3.6(CH3CO2)2.4·36H2O (L = C12H3O12).
We confirmed the structure of Zr–mel–NH4 by single-crystal X-ray diffraction analysis (Fig. 1c and Table S2†). The MOF contains Zr6Ox(OH)8−x clusters bridged by two carboxy groups of the mellitic acid, and the other four carboxy groups remain uncoordinated. The calculated formula agrees with the result of the elemental analysis. Zr–mel–NH4 has an Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group with a = 41.547(2) Å, which is about twice the cell constant of UiO-66 (a = 20.7004(2) Å).36 The large cell constant originates from the long-range superlattice structure. The Zr6O4(OH)4 clusters in UiO-66 are connected to the neighboring 12 clusters by the linker. In contrast, Zr–mel–NH4 is composed of two types of Zr clusters, type I and type II (Fig. 1d and S2†). The type I cluster connects to six linkers, and the type II cluster is coordinated by eight linkers (Fig. 1e and f). The absence of the linkers caused the periodic lack of Zr clusters and resulted in the superlattice structure.
m space group with a = 41.547(2) Å, which is about twice the cell constant of UiO-66 (a = 20.7004(2) Å).36 The large cell constant originates from the long-range superlattice structure. The Zr6O4(OH)4 clusters in UiO-66 are connected to the neighboring 12 clusters by the linker. In contrast, Zr–mel–NH4 is composed of two types of Zr clusters, type I and type II (Fig. 1d and S2†). The type I cluster connects to six linkers, and the type II cluster is coordinated by eight linkers (Fig. 1e and f). The absence of the linkers caused the periodic lack of Zr clusters and resulted in the superlattice structure.
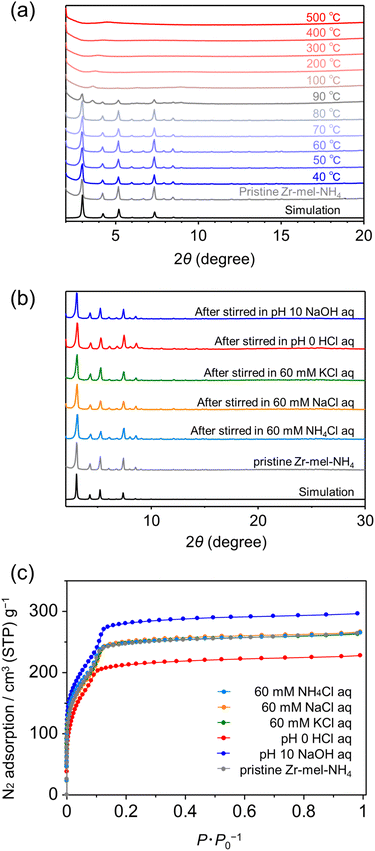
We examined the thermal stability by thermogravimetric analysis of Zr–mel–NH4 and powder XRD patterns of the MOF after heating. The TG curve showed a two-step weight loss at 25 °C and 300 °C corresponding to water elimination and the decomposition of the organic linker in MOFs, respectively (Fig. S3†). PXRD patterns of the Zr–mel–NH4 after thermal treatment showed the structural change starting at 90 °C, and the structure became amorphous at 100 °C (Fig. 2a). These results suggest that the structure of Zr–mel–NH4 is collapsed by dehydration. The chemical stability of the Zr–mel–NH4 was evaluated from the powder XRD patterns after soaking in various aqueous solutions. The PXRD patterns of Zr–mel–NH4 after immersion in hydrochloric acid (HCl, pH 0) and sodium hydroxide (NaOH, pH 10) solutions are unchanged from that of the pristine Zr–mel–NH4 (Fig. 2b), showing that the structures are highly stable under both acidic and basic conditions. Zr–mel–NH4 also has durability with 60 mM NH4Cl, NaCl, and KCl aqueous solutions, and the octahedral shape of the MOF crystals was maintained after immersion in these solutions (Fig. S1†). The BET surface area of the Zr–mel–NH4 was evaluated by nitrogen (N2) gas adsorption analysis (Fig. 2c). Pristine Zr–mel–NH4 showed N2 uptake up to 0.2P × P0−1, and the BET surface area was determined to be 876 m2 g−1, which is comparable to that of UiO-66 and its derivatives (Table S3†).45 Saito–Foley pore size analysis46,47 shows that a uniform pore with a cavity size of 7 Å is present in the Zr–mel–NH4 (Fig. S4†), which agrees with the cavity size expected from the single-crystal XRD analysis. The MOFs maintained their permanent porosities after soaking in NH4Cl, NaCl, and KCl salt solutions (Fig. 2c). It is to be noted that the degradation of porosity is observed for the MOF soaked in HCl, and the capacity was recovered by the additional ion exchange with NH4Cl (Fig. S5†). The degradation of the porosity was derived from the collapse of the MOF by the elimination of H2O, and the collapse of the MOF was accelerated by the Zr–mel–H. The PXRD patterns are unchanged after the N2 gas adsorption experiment (Fig. S6†). As discussed below, the NH4 cations in the MOFs soaked in HCl, NaCl, and KCl are thought to be fully replaced by H, Na, and K, respectively.
UiO-66 is widely known for its high stability in water due to the strong coordination bonds with the high-valence zirconium. Therefore, we have attempted to use UiO-66–SO3Na, UiO-66–(COOH)2, and zirconium-sulfoterephthalate MOF,48 and all of them have acid groups capable of trapping NH4+. However, these MOFs were unstable in an aqueous NH4+ solution, probably due to the reaction with trace NH3 (Fig. S7–S9†). The high stability of Zr–mel–NH4 with NH4+ arises from the synthesis procedure where NH4Cl is added to the reaction mixture so that only the MOFs stable with NH4+ can survive and maintain their framework in the reaction.
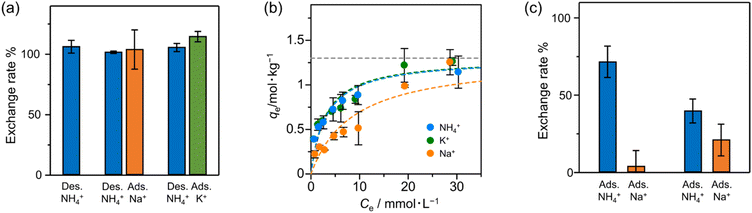
We investigated the ion-exchanging properties of Zr–mel–NH4 with H+, Na+ and K+ (Fig. 3a). The amount of desorbed NH4+ and the amount of adsorbed exchanging ions were evaluated by ion chromatography, and the result shows that nearly 100% of NH4+ in Zr–mel–NH4 was replaced by H+, Na+, and K+, which is hereafter called Zr–mel–X (X = H, Na, and K) (Table S1†). The existence of NH4+, Na+, and K+ in Zr–mel–X was confirmed by SEM-EDX analysis (Fig. S10†). Then, the ion-exchange capability of the Zr–mel–H with NH4+, Na+, and K+ was studied. We confirmed that an equimolar amount of the H+ on the linker of Zr–mel–H was replaced by NH4+, Na+, and K+, respectively (Fig. S11†). The ion exchange between NH4+ and H+ was repeated three times, and the reversibility was confirmed by PXRD patterns and SEM image (Fig. S12–S14†).
To investigate the affinity between Zr–mel–H and each of the cations, the ion-exchange experiments were performed at the initial concentrations of 1, 2, 3, 5, 7, 10, 20, and 30 mmol L−1. The Langmuir adsorption isotherms of Zr–mel–H with NH4+, Na+, and K+ show greater adsorption amounts with NH4+ and K+ than with Na+ below 10 mmol L−1 (Fig. 3b). The Langmuir adsorption equation is as follows:
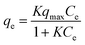 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na+ = 30
Na+ = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30/mM). Notably, 76% of H+ on the linkers was exchanged with NH4+, whereas only 4% of the H+ was exchanged with Na+. Moreover, the exchanging rate of Zr–mel–H with NH4+ remains higher than that with Na+, even at a higher concentration of Na+ than NH4+ (NH4+
30/mM). Notably, 76% of H+ on the linkers was exchanged with NH4+, whereas only 4% of the H+ was exchanged with Na+. Moreover, the exchanging rate of Zr–mel–H with NH4+ remains higher than that with Na+, even at a higher concentration of Na+ than NH4+ (NH4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na+ = 30
Na+ = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120/mM). This high selectivity for NH4+ is an advantage for the ammonia recovery from wastewater containing a large amount of Na+. Note that the concentration of K+ in wastewater is generally lower than both NH4+ and Na+, and the little selectivity between NH4+ and K+ should pose less of a problem compared to the competitive adsorption of Na+ (Fig. S16†).49 The adsorption affinity in water between the guest ions and MOFs is related to the hydration state of the ions. The cations are strongly hydrated in water and become dehydrated when adsorbed by the MOF. The lower dehydration energy required for soft cations like NH4+ compared to hard cations like Na+50–54 results in a greater tendency of NH4+ to be dehydrated and adsorbed inside the MOF. This selectivity is unique to inorganic ion-exchange materials with regulated nanopore structure,55 in contrast to the conventional ion-exchanging polymer resins that accommodate the cations under hydrated states.
120/mM). This high selectivity for NH4+ is an advantage for the ammonia recovery from wastewater containing a large amount of Na+. Note that the concentration of K+ in wastewater is generally lower than both NH4+ and Na+, and the little selectivity between NH4+ and K+ should pose less of a problem compared to the competitive adsorption of Na+ (Fig. S16†).49 The adsorption affinity in water between the guest ions and MOFs is related to the hydration state of the ions. The cations are strongly hydrated in water and become dehydrated when adsorbed by the MOF. The lower dehydration energy required for soft cations like NH4+ compared to hard cations like Na+50–54 results in a greater tendency of NH4+ to be dehydrated and adsorbed inside the MOF. This selectivity is unique to inorganic ion-exchange materials with regulated nanopore structure,55 in contrast to the conventional ion-exchanging polymer resins that accommodate the cations under hydrated states.
The number of acid points, spatially interconnected pores, and ligand defects in solid acids is associated with high proton conductivity.48,56 For investigating the relation between proton conductivity and water uptake, the water vapor adsorption analysis of Zr–mel–NH4 and Zr–mel–H was executed. From Fig. 4a, Zr–mel–NH4 and Zr–mel–H adsorb 487 and 405 cm3·(STP) per g at 90% RH, corresponding to 74 and 59 equivalents of water, respectively. Hysteresis between the adsorption and desorption steps was observed in the RH range of 0–50%. Zr–mel–H showed a rise in water adsorption at a higher humidity than that of Zr–mel–NH4.
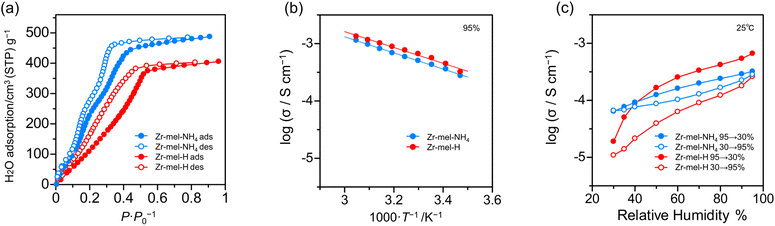 | ||
| Fig. 4 (a) The water vapor adsorption isotherms of Zr–mel–NH4 and Zr–mel–H. (b) Temperature dependency and (c) humidity dependency of proton conductivity of Zr–mel–NH4 and Zr–mel–H. | ||
The powders of Zr–mel–NH4 and Zr–mel–H were pelletized to perform an AC-impedance analysis (Fig. S17†), and the ionic conductivity (σ) was evaluated from the fitting curves of the Nyquist plots (Fig. S18†). The σ of Zr–mel–NH4 and Zr–mel–H was measured from 15 to 55 °C at 95% RH (Fig. S19†). The σ of Zr–mel–NH4 and Zr–mel–H were 1.14 × 10−3 S cm−1 and 1.34 × 10−3 S cm−1 at 55 °C, respectively (Fig. 4b), which is comparable to the σ of a proton conductive MOF with a sulfuric group as the acid point.57–59 The activation energy of Zr–mel–NH4 and Zr–mel–H shows the same value of 0.12 eV, which indicates that proton transport takes place in a Grotthuss mechanism. The ion exchange from NH4+ to H+ increases the acidity of the acid point and conductivity. Humidity dependency of the resistance was examined by variable humidity impedance measurement from 30 to 95% RH at 25 °C (Fig. S20 and S21†). Two cycles of humidity change were performed to confirm the repeatability. The σ of Zr–mel–NH4 and Zr–mel–H were 2.83 × 10−4 S cm−1 and 3.58 × 10−4 S cm−1 at 95% RH, respectively (Fig. 4c and S22†). The hystereses were observed to be similar to water adsorption isotherms, and their reversibility was also confirmed. Protonated samples have larger hysteresis of conductivity than Zr–mel–NH4. This implies that higher water pressure is required to adsorb the water in Zr–mel–H, which is in good agreement with the results of the water vapor adsorption analysis. Zr–mel–NH4 and Zr–mel–H maintained their structure after being placed under proton conductivity measurement conditions (Fig. S23 and S24†).
3. Conclusions
In summary, we report the first MOF consisting of zirconium and mellitic acid that can reversibly exchange monovalent cations such as H+, NH4+, Na+, and K+ in water. Single-crystal XRD analysis revealed that Zr–mel–NH4 has the structure of UiO-66 with periodic defects and four uncoordinated carboxy groups on the linkers. Elemental analysis indicates that one of them is exchanged with H+ and acts as an ion-exchange site. The MOF maintains its structure and permanent porosity stably in acid (pH 0), alkaline (pH 10), and aqueous NH4Cl, NaCl, and KCl solutions from PXRD patterns, nitrogen adsorption isotherms, and SEM observations. Zr–mel–NH4 exhibits reversible ion-exchange between NH4+ and H+, Na+, and K+. The ion-exchange site within the uniform nanoporous structure provides selective NH4+ recovery from a mixture of aqueous NH4+ and Na+ solution, in accordance with the difference in the hydration state of NH4+ and Na+. Furthermore, by ion-exchanging from NH4+ to H+, the proton conductivity reached ca. 10−3 S cm−1 due to the increasing proton donor property. These achievements suggest that Zr–mel–NH4 could be applied as a solid acid platform in various applications.Author contributions
Teppei Yamada designed the research. Genki Hatakeyama executed all synthesis and measurements and wrote the draft of the manuscript. Takashi Kikuchi executed the SCXRD analysis. Masaaki Sadakiyo conducted the powder XRD measurement and analysis. Yusuke Nishiyama discussed the proton conduction mechanism. Masaki Nishio and Kouki Oka designed the synthetic conditions. Hongyao Zhou and Teppei Yamada revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI 21H00017 (Hydrogenomics), 21K04949, 21H05870, 20K21176, 20H02714, Moonshot Research and Development Program “Innovative circular technologies for harmful nitrogen compounds”, JST CREST Grant Number: JPMJCR22O5, the Murata Science Foundation, The Asahi Glass Foundation, Tanikawa Fund Promotion of Thermal Technology, Yamada Science Foundation, The Iwatani Naoji Foundation, and the Canon Foundation. SEM observation, SEM-EDX analysis, and single-crystal X-ray diffraction analysis were supported by the “Advanced Research Infrastructure for Materials and Nanotechnology in Japan (ARIM)” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT).References
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- S. Kitagawa, R. Kitaura and S. I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249–267 CrossRef CAS PubMed.
- Y. V. Kaneti, J. Tang, R. R. Salunkhe, X. Jiang, A. Yu, K. C. W. Wu and Y. Yamauchi, Adv. Mater., 2017, 29, 1604898 CrossRef PubMed.
- L. S. Xie, G. Skorupskii and M. Dincă, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS PubMed.
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC.
- M. Ding, R. W. Flaig, H. L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T. L. Hu, M. O'Keeffe, L. Wang, M. Luo, R. B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939–944 CrossRef CAS PubMed.
- B. E. R. Snyder, A. B. Turkiewicz, H. Furukawa, M. V. Paley, E. O. Velasquez, M. N. Dods and J. R. Long, Nature, 2023, 613, 287–291 CrossRef CAS PubMed.
- Y. Su, K. Otake, J.-J. Zheng, S. Horike, S. Kitagawa and C. Gu, Nature, 2022, 611, 289–294 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- C. De Wu, A. Hu, L. Zhang and W. Lin, J. Am. Chem. Soc., 2005, 127, 8940–8941 CrossRef PubMed.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- G. K. H. Shimizu, J. M. Taylor and S. Kim, Science, 2013, 341, 354–355 CrossRef CAS PubMed.
- J. A. Hurd, R. Vaidhyanathan, V. Thangadurai, C. I. Ratcliffe, I. L. Moudrakovski and G. K. H. Shimizu, Nat. Chem., 2009, 1, 705–710 CrossRef CAS PubMed.
- N. Ma, S. Kosasang, A. Yoshidaa and S. Horike, Chem. Sci., 2021, 12, 5818–5824 RSC.
- S. S. Park, A. J. Rieth, C. H. Hendon and M. Dinca, J. Am. Chem. Soc., 2018, 140, 2016–2019 CrossRef CAS PubMed.
- A. Da and E. Robens, Chemosphere, 2004, 56, 91–106 CrossRef PubMed.
- K. Vaaramaa and J. Lehto, Desalination, 2003, 155, 157–170 CrossRef CAS.
- K. Singh, S. Porada, H. D. de Gier, P. M. Biesheuvel and L. C. P. M. de Smet, Desalination, 2019, 455, 115–134 CrossRef CAS.
- K. Tanabe and W. F. Hölderich, Appl. Catal., A, 1999, 181, 399–434 CrossRef CAS.
- S. Wang, M. Wahiduzzaman, L. Davis, A. Tissot, W. Shepard, J. Marrot, C. Martineau-Corcos, D. Hamdane, G. Maurin, S. Devautour-Vinot and C. Serre, Nat. Commun., 2018, 9, 1–8 CrossRef.
- D. W. Lim and H. Kitagawa, Chem. Soc. Rev., 2021, 50, 6349–6368 RSC.
- M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 9906–9907 CrossRef CAS PubMed.
- Z. Huang, M. Zhao, C. Wang, S. Wang, L. Dai and L. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 41294–41302 CrossRef CAS PubMed.
- L. Rani, J. Kaushal, A. L. Srivastav and P. Mahajan, Environ. Sci. Pollut. Res., 2020, 27, 44771–44796 CrossRef CAS PubMed.
- J. Tang, J. Zhao, S. Wang, L. Zhang, M. Zhao, Z. Huang and Y. Hu, Chem. Eng. J., 2021, 407, 127223 CrossRef CAS.
- L. Liang, Q. Chen, F. Jiang, D. Yuan, J. Qian, G. Lv, H. Xue, L. Liu, H. L. Jiang and M. Hong, J. Mater. Chem. A, 2016, 4, 15370–15374 RSC.
- N. D. Rudd, H. Wang, E. M. A. Fuentes-Fernandez, S. J. Teat, F. Chen, G. Hall, Y. J. Chabal and J. Li, ACS Appl. Mater. Interfaces, 2016, 8, 30294–30303 CrossRef CAS PubMed.
- Y. Peng, H. Huang, Y. Zhang, C. Kang, S. Chen, L. Song, D. Liu and C. Zhong, Nat. Commun., 2018, 9, 187 CrossRef PubMed.
- X. Liu, X. Wang and F. Kapteijn, Chem. Rev., 2020, 120, 8303–8377 CrossRef CAS PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- X. Zhang and Y. Liu, Environ. Sci.: Nano, 2020, 7, 1008–1040 RSC.
- J. Li, X. Wang, G. Zhao, C. Chen, Z. Chai, A. Alsaedi, T. Hayat and X. Wang, Chem. Soc. Rev., 2018, 47, 2322–2356 RSC.
- L. Yue, S. Wang, D. Zhou, H. Zhang, B. Li and L. Wu, Nat. Commun., 2016, 7, 10742 CrossRef CAS PubMed.
- R. M. DuChanois, C. J. Porter, C. Violet, R. Verduzco and M. Elimelech, Adv. Mater., 2021, 33, 1–18 CrossRef PubMed.
- L. Apodaca, Nitrogen Statistics and Information, 2022 Search PubMed.
- W. H. Schlesinger, Nat. Clim. Change, 2009, 1, 112–113 CrossRef.
- W. Steffen, K. Richardson, J. Rockström, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. De Vries, C. A. De Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers and S. Sörlin, Science, 2015, 347, 1259855 CrossRef PubMed.
- E. R. Nightingale, J. Phys. Chem., 1959, 63, 1381–1387 CrossRef CAS.
- Z. Hu, Y. Peng, Z. Kang, Y. Qian and D. Zhao, Inorg. Chem., 2015, 54, 4862–4868 CrossRef CAS PubMed.
- A. Saito and H. C. Foley, AIChE J., 1991, 37, 429–436 CrossRef CAS.
- A. Saito and H. C. Foley, Microporous Mater., 1995, 3, 531–542 CrossRef CAS.
- J. M. Taylor, T. Komatsu, S. Dekura, K. Otsubo, M. Takata and H. Kitagawa, J. Am. Chem. Soc., 2015, 137, 11498–11506 CrossRef CAS PubMed.
- M. Arienzo, E. W. Christen, W. Quayle and A. Kumar, J. Hazard. Mater., 2009, 164, 415–422 CrossRef CAS PubMed.
- A. Clearfield, Chem. Rev., 1988, 88, 125–148 CrossRef CAS.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1993, 87, 2995–2999 RSC.
- M. Abe, Y. Kanzaki and R. Chitrakar, J. Phys. Chem., 1987, 91, 2997–3001 CrossRef CAS.
- Y. Kanzaki, R. Chitrakar and M. Abe, J. Phys. Chem., 1990, 94, 2206–2211 CrossRef CAS.
- T. Ohsaka, M. Abe, Y. Kanzaki, T. Kotani and S. Awano, Mater. Res. Bull., 1999, 34, 1441–1450 CrossRef CAS.
- Y. Jiang, K. Minami, K. Sakurai, A. Takahashi, D. Parajuli, Z. Lei, Z. Zhang and T. Kawamoto, RSC Adv., 2018, 8, 34573–34581 RSC.
- J. M. Taylor, S. Dekura, R. Ikeda and H. Kitagawa, Chem. Mater., 2015, 27, 2286–2289 CrossRef CAS.
- F. Yang, H. Huang, X. Wang, F. Li, Y. Gong, C. Zhong and J. R. Li, Cryst. Growth Des., 2015, 15, 5827–5833 CrossRef CAS.
- X. Y. Dong, J. H. Wang, S. S. Liu, Z. Han, Q. J. Tang, F. F. Li and S. Q. Zang, ACS Appl. Mater. Interfaces, 2018, 10, 38209–38216 CrossRef CAS PubMed.
- X. Chen and G. Li, Inorg. Chem. Front., 2020, 7, 3765–3784 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc02743k |
| This journal is © The Royal Society of Chemistry 2023 |