Exploring students’ dominant approaches to handling epistemic uncertainty when engaging in argument from evidence†
Mary Tess
Urbanek
 a,
Benjamin
Moritz
b and
Alena
Moon
a,
Benjamin
Moritz
b and
Alena
Moon
 *a
*a
aUniversity of Nebraska-Lincoln, Department of Chemistry, Lincoln, NE, USA. E-mail: amoon3@unl.edu
bBenedictine College, Department of Chemistry, Atchison, KS, USA
First published on 24th May 2023
Abstract
While uncertainty is inherent to doing science, it is often excluded from science instruction, especially postsecondary chemistry instruction. There are a variety of barriers to infusing uncertainty into the postsecondary chemistry classroom, including ensuring productive struggle with uncertainty, evaluating student engagement with uncertainty, and facilitating engagement in a way that fits within the postsecondary chemistry context. In this study, we aimed to address these difficulties by designing an argumentation task that enables the direct observation of students interacting with epistemic uncertainty. This task was administered as a written assignment to a large-enrollment, second-semester general chemistry course. Student responses were analyzed to generate a rubric that captures the varied ways students grapple with epistemic uncertainty. In accordance with previous literature, we observed students not engaging with the uncertainty (e.g., generating vague, incomprehensible arguments) and selectively engage with the uncertainty (e.g., use data selectively to avoid uncertainty). However, we also observed the qualitatively distinct approaches students utilized to productively manage epistemic uncertainty. Importantly, we believe that these ways of productively handling uncertainty translate to the kinds of scientific reasoning, personal decision making, and socioscientific reasoning that these learners will continue to engage in. Therefore, this work has implications for supporting students’ scientific argumentation by offering instructors a practical way to engage their students with uncertainty and a model to interpret and respond to their students.
Introduction and rationale
Engaging in argumentation has been historically difficult to facilitate in chemistry courses for numerous reasons. Students struggle to coordinate multiple pieces of evidence to justify a claim as well as manage the uncertainty that is an inherent to making knowledge claims (J. Osborne and Patterson, 2011; J. Osborne, 2013; Novak and Treagust, 2018; Chen et al., 2019). This uncertainty is challenging for the student as well as the instructor, as many instructors are unsure how to help students navigate this uncertainty and even argue that the uncertainty itself is not conducive to learning (Manz and Suárez, 2018; Chen et al., 2019; Chen, 2020; Puig and Evagorou, 2023; Suh et al., 2023). Therefore, most argumentation practices that take place in science classrooms tend to focus on having students develop claims and construct explanations about well-understood phenomenon that lack this uncertainty (McNeill et al., 2006; Berland and Reiser, 2009; Reiser et al., 2012). While this approach has been productive in helping students learn what kinds of evidence are necessary to generate claims and explanations, it is limited in helping students handle the uncertainty involved in generating tentative claims (Lee et al., 2014). In this study, we engage postsecondary chemistry students in a written argumentation task that requires them to grapple with said uncertainty. From the students’ constructed responses, we can gain insight into the different ways that students productively handle this uncertainty and how these responses can translate to real scientific and socioscientific issues.Background
Uncertainty in science
Uncertainty is an inherent aspect of science, and is integrated into the methodologies, culture, and conversations surrounding science (Abd-El-Khalick and Lederman, 2000; Metz, 2004; Allchin, 2012; J. Osborne, 2013). This uncertainty can present itself in a number of ways to scientists, from considering experimental design objectives to articulating findings and presenting evidence in a logical and convincing way. However, this uncertainty also presents itself in more nuanced ways. As Manz and Suárez (2018) state, “Uncertainty is endemic to science, and scientific practices are largely concerned with managing uncertainty and drawing conclusions in light of it… Uncertainty manifests as nonobvious decisions about what to do, and as pushback, both from scientists’ empirical work and the audience they are seeking to convince.”To provide students with the opportunity to engage authentically with science, uncertainty must be integrated into the classroom and curriculum (Buck et al., 2014; Lee et al., 2014). Giving students the opportunity to engage with uncertainty in our classroom exposes them to “the forms of uncertainty that scientists experience, and that in turn drive practices such as argumentation, explanation, and investigation in scientific activity” (Manz and Suárez, 2018). The National Research Council has explicitly outlined expectations regarding student's ability to handle uncertainty and conflicting information in the classroom. They assert that students should be able to (1) recognize when data conflicts with initially held ideas or expectations, (2) recognize limitations of conclusions drawn from data, and (3) be able to revise their initial understanding to incorporate new information (National Research Council, 2012). These standards set the expectation that as students progress through their science education, they should be developing skills to manage uncertainty (National Research Council, 2012).
Despite the explicit statement that students should be progressing in their understanding of managing uncertainty, science instruction in the classroom has continued to treat science as an entity that is final and completely certain (Osborne, 2014). Treating science as a final answer can be detrimental to perceptions of science, as it removes the ability for students to view science as an approach to knowledge generation. Therefore, it is key for instructors to expose students to this uncertainty and help them productively grapple with it while engaging in science practices (Manz, 2018; Chen and Qiao, 2020; Chen and Techawitthayachinda, 2021; Chen, 2022; Watkins and Manz, 2022).
Not only does this uncertainty exist within the science educational context, it also permeates outside the classroom as well. Socio-scientific issues (SSI) often carry a sense of uncertainty to them, as they involve both scientific and personal concerns (Evagorou et al., 2012; Alred and Dauer, 2020; Kelp et al., 2022). These issues often require students to evaluate data that conflicts with other pieces of evidence, which can further escalate their level of uncertainty (Evagorou et al., 2012; Novak and Treagust, 2018; Bolger et al., 2021). It thus becomes crucial for students to not only manage uncertainty within the classroom, but also in the public space. For example, during the COVID-19 pandemic, citizens had to assess the credibility of data sources, consider ethical and personal concerns, and evaluate scientific knowledge to make decisions about wearing masks and getting vaccinated (Kelp et al., 2022; Puig and Evagorou, 2023). Generating responsible citizens requires science education to help students foster an understanding of uncertainty and ways to manage it when generating an argument (Osborne, 2013, 2014; Manz, 2018).
There are several types of uncertainty that students may encounter while engaging in their science courses. One type of uncertainty that students must learn to grapple with is epistemic uncertainty. Epistemic uncertainty is defined as, “learners' subjective awareness and knowing of incomplete skills or abilities about how to explain a phenomenon, derive trends from muddled data, interpret and represent raw data as evidence, and put forth scientific arguments” (Chen and Qiao, 2020). Learning to productively manage epistemic uncertainty is a key competency for science students to develop, as without it they are limited in their ability to engage in science practices and make informed decisions about the world around them (Manz, 2015, 2018; Manz and Suárez, 2018; Chen et al., 2019; Chen and Qiao, 2020; Chen and Techawitthayachinda, 2021; Chen, 2022; Watkins and Manz, 2022).
How students respond to uncertainty
One way in which uncertainty is often embedded into the classroom is through the implementation of argumentation in assessments and activities. This has been heavily explored in the K-12 science curriculum (Evagorou et al., 2012; Buck et al., 2014; Lee et al., 2014; Novak and Treagust, 2018; Chen et al., 2019). Findings from this research have shown that managing uncertainty is far from a trivial task for students. Typically, managing uncertainty requires the student to be able to coordinate multiple pieces of evidence and data to form one cohesive claim. This is especially difficult when the evidence supports different claims. For example, Novak and Treagust (2018) found that when students were presented with conflicting data and asked to generate an argument, the students would write a separate claim for each set of data. Since the evidence conflicted, the students would develop arguments that contained multiple, contradictory claims within it.Other literature has shown that students tend to rely on personal reasoning to navigate uncertainty rather than scientific knowledge (Chinn and Brewer, 1998; Evagorou et al., 2012; Mehl et al., 2020; Puig and Evagorou, 2023). For example, Puig and Evagorou (2023) found that pre-service teachers often relied on their own personal experiences to evaluate the credibility of a news headline about COVID-19 instead of the scientific knowledge they were learning in the course. These findings were consistent with Evagorou et al.'s (2012) investigation of how 12 to 13 year-old students in England generated claims about a particular SSI involving squirrel populations. They found that a student's previous experiences and personal beliefs offered a lens through which they interpreted data.
Chinn and Brewer (1993) have extensively studied how students interact with anomalous data in science education. They have identified seven unique ways that students will respond to anomalies. Only one of these ways leads students to revising their theory to fully incorporate the new evidence. They have found that students can ignore the data, reinterpret the data in such a way that it aligns with their current understanding, or provide an explanation that asserts the conflicting evidence is not relevant to their theory. However, in most of these responses we can see that students avoid engaging with the conflicting evidence to maintain their pre-instructional theory.
There are several factors that influence the way in which a student interacts with conflicting evidence (Chinn and Brewer, 1993, 1998; Sundstrom et al., 2020; Phillips et al., 2021). Students’ understanding of the nature of science and uncertainty plays a role in determining their actions. For example, Phillips and colleagues (2018) found that students in a physics lab that viewed the purpose of lab as being a confirmatory experience (i.e., that evidence collected in the lab was meant to confirm what they had been taught in lecture) typically avoided engaging with conflicting data. Additionally, students who hold epistemic ideas that learning science involves memorizing facts rather than making sense of the world will often avoid engaging with conflicting evidence when presented with it (Chinn and Brewer, 1993, 1998). Other influencing factors include how strongly a student holds onto an initial theory, what prior experience and knowledge a student has, and how the student views the data. For conflicting evidence to be meaningful to students, they must view it as valid and inexplainable by their current understanding (Nussbaum and Novick, 1982; Chinn and Brewer, 1998). Additionally, when a new explanation is presented to the student, they must view it as an accessible and plausible explanation for the phenomenon (Nussbaum and Novick, 1982).
Uncertainty in post-secondary science classrooms
How students engage with uncertainty in the post-secondary science classroom has not been as extensively explored as in K-12 studies. Additionally, these post-secondary studies tend to be focused on how students manage the uncertainty that is prompted during laboratory experiences (Walker et al., 2019; Sundstrom et al., 2020; Bolger et al., 2021; Phillips et al., 2021). For example, Bolger and colleagues (2021) found that students enrolled in a model-based biology inquiry lab were often unsure how to proceed when exposed to evidence that conflicted with an initial model. Some students ignored the conflicting evidence while others attributed that evidence to being collected incorrectly. Other students engaged with the data and worked to revise their model to incorporate new evidence into their model. The results from this study showed that students were afforded the opportunity to engage in sense-making when presented with the uncertainty, even if that uncertainty was not resolved.Argument-Driven Inquiry labs (ADI) have also given insight into how students engage with uncertainty in the post-secondary context (Walker et al., 2019). In these labs, students work in small groups to collect data and construct arguments about a particular concept. Once lab groups have constructed an argument, they then share their findings, evidence, and justifications to other groups. After presenting their own argument and hearing counterarguments, the students work together to revise their arguments. However, Walker et al. (2019) found that students struggle to revise their arguments during these labs. They completed semi-structured interviews with students in an ADI lab course to gain insight into students’ perceptions of argumentation competencies. These interviews showed that students had unique ways of interpreting what it meant to change their claim. For example, some students believed that changing a claim required new evidence to be collected, or that existing evidence had to be “manipulated” to align with a new claim. Very few students believed that changing a claim could be justified by the reinterpretation of existing evidence.
Previous research has shown that managing uncertainty is a challenging process for students across both K-12 and post-secondary contexts (Evagorou et al., 2012; Novak and Treagust, 2018; Chen et al., 2019; Walker et al., 2019; Bolger et al., 2021; Phillips et al., 2021). Across these studies, many students were unable to meaningfully reckon with uncertainty while engaging in science practices and instruction (Walker et al., 2019; Bolger et al., 2021; Phillips et al., 2021). Given the stress that recent educational efforts have placed on students authentically engaging in science in the classroom, we must continue to provide students with the opportunity to engage in uncertainty in argumentation (National Research Council, 2012; Buck et al., 2014; Lee et al., 2014). Additionally, we must provide students with instruction that equips them to deal with this uncertainty. To do so, we need to know the ways in which students can productively engage with uncertainty in a science practice context. This guides our research question for this study:
What are the dominant approaches students use to handle uncertainty when engaging in argumentation?
Methods
Overview
A task was developed specifically to engage students with uncertainty during argumentation. The task was then administered as a written assignment in a large-enrollment general chemistry course. Response process interviews were conducted following the administration in class to establish the validity of responses generated from the task (i.e., task prompted desired thinking) and validity of our observations (i.e., our observations of students’ reasoning aligned with the reasoning they voiced using). Response process interviews and written responses were analyzed to characterize the dominant approaches students use when interacting with uncertainty.Task design
Evidence-centered design (ECD) enables researchers to design assessments to observe student engagement in complex constructs (e.g., uncertainty-infused argumentation) (Mislevy et al., 2003, 2004; Pellegrino et al., 2016). A key component of ECD is developing a task that enables observation and evidence gathering of the target competency (Mislevy et al., 2003, 2004; Pellegrino et al., 2016). A full outline of how we used ECD principles to develop our task can be found in the online ESI,† Part A. In this study, the target competency is handling epistemic uncertainty in argumentation. To enable observation of this, we designed a task that required the learners to consider data sets that contained evidence that supported conflicting claims. In this way, the uncertainty was embedded within the task; learners were prompted to grapple with the uncertainty in order to make assertions about the data. Additionally, the task has no correct answer, putting the onus on the student to justify their assertion with the evidence provided.The goal of the task was to select optimal conditions to dehydrate onion slices based on three different onion qualities. There were three conditions the students considered: the temperature of dehydration, the thickness of the onion slice, and whether the onion slices should be treated with salt before dehydration. The three onion qualities—moisture content, browning, and flavor loss—were all measured with a data set that characterized the quality across the conditions (Mitra et al., 2014). Moisture content data showed the mass of water per mass of dry solid for the varying conditions. The browning and flavor loss data showed rate constants for the reactions responsible for the phenomenon across all possible conditions.
After reviewing each set of data, the students are probed to discuss the information they extracted and provide reasoning for why they think the trends may be occurring. Once the students have reviewed the data for each of these onion qualities, they construct an argument that explains what they believe are the best conditions to dehydrate the onions at and why. A figure depicting the task components is shown in Fig. 1.
The uncertainty for the students lies in the data itself. The different onion qualities indicate different conditions as being optimal. For example, the moisture content of the onion is optimal at high temperatures and thin thicknesses, but the other qualities of the onion are optimized at low temperatures and thick thicknesses. Students must reconcile this conflict when crafting their final argument. A full copy of the task can be found in the ESI,† Part B.
Data collection
Data was collected from a General Chemistry II course for non-majors at a large, Midwestern, research-intensive institution in the Spring of 2022. In total, there were five sections of lecture taught by three different instructors. Grades in the course were determined by four exams, weekly homework, and the American Chemical Society's General Chemistry Exam.The task was administered in the course electronically as a regular homework assignment and points were awarded for completion. Because the task was administered as a homework assignment, it was open for a week's time. IRB approval was obtained and students submitted a consent form prior to completing the assignment. In total, 487 students consented to having their responses utilized for research purposes. During the period in which the assignment was open, the researcher held office hours for students to attend if they had any questions regarding the assignment. Additionally, students were given contact information for the researcher to ask any questions they had. Once the assignment concluded, six students were recruited for response process interviews. Recruitment for the interviews was completed via the homework assignment, where students volunteered to be contacted to schedule an interview. Participants were compensated with a $20 gift card for participating in the interview.
Data analysis – rubric development
The goal of the rubric was to characterize the type of reasoning patterns students utilized when constructing an argument with conflicting data. In order to accomplish this, students’ written responses were initially sorted into groups based on what the student selected as the optimal conditions. This was based on the assumption that if students were selecting the same optimal conditions, it was possible that they were utilizing similar reasoning patterns. Once the responses had been sorted into condition categories, the first two authors independently open-coded the responses. This coding consisted of looking for what evidence the student incorporated into their response, what evidence was omitted, and if there were any misinterpretations in the student's response. To identify patterns in the responses, we individually summarized the key characteristics in each student's explanation.Once we had open-coded several responses, we met to compare our summaries and look for patterns within our notes that could help differentiate student reasoning patterns. To do this, we grouped students who omitted similar evidence, had similar misinterpretations, or prioritized similar variables together. Once the groups were sorted, we built descriptions that characterized the unique reasoning features present in the students' arguments. From these descriptions, we organized a preliminary rubric. To ensure that this preliminary rubric was appropriate, we pulled a new set of data that had the same conditions and tried applying the rubric to it. We then iterated through testing the rubric by coding new sets of data, discussing our coding, and refining the rubric until it reached stability.
A full breakdown of the IRR process, as well as each iteration of the rubric, can be found in the ESI,† Part C. We first began by independently coding fifty responses that neither researcher had seen before. Once these had been coded, we determined that constraining the code options based off the condition set the student selected did not always lead to truly capturing the type of reasoning the student employed. Therefore, we combined all the codes into one rubric.
We then pulled a new set of fifty student responses and coded them independently. For this round of coding, we calculated a Cohen's Kappa value of 0.558, which indicated a weak level of agreement (Watts and Finkenstaedt-Quinn, 2021). We discussed each disagreement until consensus was reached. We saw that the main reason for the disagreement came from confusion about what role the student's condition choice played in assigning a reasoning code.
Once this source of disagreement had been discussed, a new set of fifty responses were coded. This time, we calculated a Cohen's Kappa value of 0.631, which indicated a weak to moderate agreement (Watts and Finkenstaedt-Quinn, 2021). As we discussed our disagreements, we decided to make two changes to the rubric. First, we decided to combine all the separate misinterpretation codes into one code for two reasons. First, we did not gain any insight into their reasoning by parsing out the misunderstandings. Additionally, it was difficult to determine the source of the misinterpretation(s) the student had. We also decided to remove the “No Justification of Thickness or Temperature” code, as it only described omission rather than reasoning students were employing to grapple with uncertainty.
Once the rubric had been changed, a new set of fifty responses were coded by each researcher. We calculated a Cohen's Kappa value of 0.799 for this set of codes, which indicated a strong level of agreement (Watts and Finkenstaedt-Quinn, 2021). Once we had established our IRR, all remaining student responses were coded with the finalized rubric.
Results
Overview
From the developed rubric, we observed that students engaged with the epistemic uncertainty in the task in various ways. Some students faced challenges when trying to interpret the data and were not able to generate a claim based on the data. Other students left out discussion of the conflict in their response to avoid having to address the uncertainty. However, several students noticed that the data conflicted on the best onion slice and attempted to address that conflict in their response. In the space below, we discuss results from the response process interviews that illustrate the validity of our rubric. Then, the different codes and engagement levels are described. We also illustrate the connections between condition sets and the type of reasoning that the students utilize below.Response process interviews
The goal with the response process interviews was to verify that our interpretation of the students’ response to the assignment aligned with the students’ intentions. In total, we completed six interviews. For these semi-structured interviews, we pulled the student's response for the assignment and had them talk through their responses for each question. This interview structure allowed us to gain further insight into the reasoning the students utilized to justify their response, which later helped us to develop the rubric. We could see this when we interviewed Leek, who originally selected 50 °C, 3 mm, treated onion slices for their best onions. Leek's written response to the assignment explained why they believed these were the best conditions.“When considering the MC [moisture content, ESI,† Part B, Fig. 2] graph, the content in the 3 mm onions is only one and a half units higher for the 50- and 60-degrees onions than the 70 degrees onions. However, the rate of browning for the 70-degree onions is significantly higher than that of the 50-degree 3 mm onions. The 50-degree onions take the cake with their incredibly low rate of thiosulfinate loss, a solid 0.6 on the table for 3 mm treated onions. Overall, I believe that the following parameters will make for the best quality onions with the longest shelf life.” (Leek's written response)
In Leek's written response, we can see that they are selecting a temperature that would give good values for the browning and flavoring of the onion. However, it is not completely clear how they have decided on their thickness selection. During Leek's interview, they were asked to explain how they decided their condition set:
“For the one millimeter thick onions, I noticed that the rate constants were extremely high. At this point, I was very sure that the one millimeter onions weren’t going to be a viable solution to drying the onions because it failed in the browning charts [ESI,† Part B, Table 1]. And it also failed over here [ESI,† Part B, Table 2]. So, I started looking back and forth between the three and five millimeters slices. And at this point, I think I kind of like, made a guess to go with a certain middle ground… like three millimeters… because for treated samples it was lower for the flavor and for the browning constants.” (Leek's response process interview)
Here we can see that Leek further elaborates on their reasoning for selecting 3 mm for their thickness. They expressed that they worried that selecting the 1 mm thickness, which is the most favorable for the moisture content, would create issues for the browning and flavor loss rates. Therefore, they decide that selecting an average value is the best way to address the conflict. Leek's interview helped us to realize that a student could utilize multiple types of reasoning to construct their argument for the best conditions and allowed us to further clarify the patterns we were seeing in the student responses.
Reasoning category 1: no engagement
In this study, the no engagement category includes reasoning that prohibits students from fully engaging with the data, and thus uncertainty, in the task. There are two examples of this type of reasoning. The first occurs when a student has some key misunderstanding of the data or task. In order to earn this code, the student's misinterpretation had to act as a barrier for the student engaging with the uncertainty in the task. This type of reasoning was captured in the misinterpretation code. There were three common misinterpretations we saw when analyzing the data. First, a student may have some misunderstanding about what they are trying to accomplish in the task. The overarching goal of the assignment was to select the set of conditions that led to the highest quality onion. However, some students specified when they selected their conditions that they would lead to the lowest quality onion. For example, Chive picked a condition set of 70 °C, 1 mm, untreated onions because it led to the least preservation for the slices. This goes against the goal of the assignment, thus removing the uncertainty in their argument.“I chose these conditions because at 70 [°C] there was less water left and the smaller it was the faster it got dehydrated, I said not to add salt because salt preserves food and we don’t want to preserve the onions.” (Chive)
The other common misinterpretation pertained to the meaning of the data. This included misinterpreting the moisture content bar graph, such as saying the higher the values were, the lower the moisture content was. It also included when a student misinterpreted the meaning of the rate constant, such as when a response said that a higher rate constant meant a lower overall rate. This is shown in Pearl's response, who selected 70 degrees, 1 mm, untreated onions.
“I think this because it had the highest rates for both browning and moisture content. This means that it will brown slower and lose [flavoring] slower. The moisture content was also low but not the lowest. The lowest is the 1 mm treated at 70 degrees. I still picked these parameters because it had a big difference in rates for browning and flavoring.” (Pearl)
Because Pearl believed that a higher rate constant meant a slower rate, they believed they were picking a set of conditions that benefitted all three onion qualities. This removed the conflict in the data, thus removing the uncertainty that they would have had to engage with otherwise.
Another example of no engagement occurred when a student's reasoning was vague. A student was assigned a vague code when the student selected a variable (temperature, thickness, or treatment) to dehydrate the onion instead of a condition set (such as 70 degrees, 1 mm, treated). The vague reasoning code was also used to capture responses that did not offer any insight into the student's reasoning for selecting a condition set. In these situations, students typically just restated the goal of the assignment and did not cite any evidence to support their final conclusion.
Reasoning category 2: selective engagement
We define selective engagement as reasoning that students utilize that did not acknowledge or address the uncertainty. Students did this in one of two ways. The first way was by not discussing one of the onion qualities. By doing this, students avoided discussing the evidence that challenged their condition set. This type of reasoning was captured with the neglected moisture content and neglected browning and flavoring codes. We can see this play out in Cipollini's reasoning for selecting 50 °C, 5 mm, and treated onion slices.“These parameters are the best because the rate of browning was the second lowest at 0.74. The rate of thiosulphinate loss was the lowest with all of these parameters at 0.2.” (Cipollini)
The condition set that Cipollini chose had one of the highest moisture contents for the onion, which was not ideal. However, we do not have evidence to suggest that Cipollini recognized this as a limitation for their condition set.
The second way students can exhibit selective engagement is by taking a compartmentalized approach in their argument. This type of reasoning occurs when a student considers how a particular condition will affect an onion quality favorably but does not discuss any of the negative aspects of selecting a particular condition. This reasoning differs from the neglecting codes in that the student will discuss all three qualities of the onion in their response. Shallot employed this reasoning to justify the conditions 70 °C, 5 mm, treated onions.
“Higher temp = lower moisture content, Thicker cut = less browning, [treatment] = less thiosulphinate [flavor] loss.” (Shallot)
Here we can see that Shallot does not discuss any of the drawbacks of selecting the high temperature or high thickness slices. Additionally, they are thinking about how a particular condition will affect only one quality, instead of considering the effect each condition will have on each of the onion's qualities. By doing this, Shallot effectively avoided discussing the conflicting data in their response.
Reasoning category 3: full engagement
The final reasoning category captured students who recognized that the data conflicted on the best onion slice and explicitly addressed said conflict in their argument. These students recognized the limitations of their selection while discussing the benefits of their choice. By doing this in their written responses, these students were able to fully engage with the uncertainty that was embedded in the task. One way that students exhibited this type of reasoning was by choosing to prioritize certain onion qualities over others. For example, some students decided to select conditions that they believed minimized the moisture content of the onion, even if it meant sacrificing the other two qualities. Students who used this reasoning pattern were coded with a prioritizing moisture content code. This was the type of reasoning that Scallion used to justify their selection condition set of 70 degrees, 1 mm, treated onions.“I believe these are the best parameters to dehydrate the onions because treated 1 mm slices at 70 °C retain only approximately 2 kg water per kg dry solid, which allows for the onions to have a longer shelf life and preserve a higher quality. Even though it has a 0.346 rate constant for browning and a 7.0 rate of thiosulphinate loss, I would prioritize a longer shelf life since it creates more time to sell the units.” (Scallion)
The data the students considered in the task indicated very similar conditions as being ideal for the browning and flavoring of the onion. Therefore, students who selected conditions to prioritize those qualities at the expense of moisture content received a 2 out of 3 code. This was the type of reasoning that Allium utilized in their response.
“Going off the data, it checks off 2 out of the 3 boxes that we are looking for in terms of highest quality. For moisture content, it does not provide the best moisture and is in fact one of the lowest. However, when examining the browning constant rate, it has a rate of 0.074, which is among the lowest and one of the longest to brown. Finally, for the factor that gives onions their flavor, it has a loss rate of 0.2, which is the best of any data presented on the table.” (Allium)
Some students try to handle the conflict by selecting conditions to try to make up for where a previous condition lacked. For example, some students selected a higher thickness, which benefitted browning and flavoring, to make up for the negative effects selecting a higher temperature caused on those qualities. Responses that utilized this type of reasoning were coded with the balancing positive and negative code. This is the type of reasoning that Hagyma used.
“While the high temperature does increase the rate of browning and thiosulphinate loss, it also removes significantly more moisture than lower temperatures, making the final product more shelf stable. Using a thicker slice and salt treatment will help to counteract the browning and thiosulphinate loss.” (Hagyma)
Some students will take this a step further and consider how much a particular condition choice affects one of the onion's qualities. For example, the moisture content figure the students considered shows a large decrease in moisture between 60 and 70 °C (ESI,† Part B). Some students used this drastic decrease in moisture content between the two temperatures as evidence to further justify their condition selection. When students considered this difference between data points in their reasoning, they earned a variance code. Basila considered the differences between data points as justification in their argument.
“Based on Fig. 2 [ESI,† Part B, Fig. 2], going from just 60 degrees to 70 makes a large difference in the moisture content for all groups in every thickness and treatment. Because a lower moisture content is better for the shelf life and quality of product, it is very important that we choose 70 degrees. While Tables 1 and 2 [ESI,† Part B, Tables 1 and 2] do show a higher rate of browning and thiosulphinate loss for higher temperatures of dehydrating, the difference of moisture content in Fig. 2 [ESI,† Part B, Fig. 2] just shows way too big of a difference to use 50 or 60 degrees for dehydration.” (Basila)
Another way students approached addressing the conflicting data was to select conditions that gave an average value for all three onion qualities. In these situations, students opted to not favor a particular quality over another, thus receiving a middle ground code. Vidalia took this approach to decide on a condition set.
“I believe that the best way [is] to do a 3 mm treated onion slice for the dried onions because it is a happy medium of flavor and dehydration. If we went with the 1 mm sample it wouldn’t be as flavorful while having slightly less dehydration than 3 [mm]. If we went with 5 mm, the flavor would be very good but it would also have a shorter browning time than 3 mm.” (Vidalia)
Some students used a combination of the previously outlined reasoning codes to get to their final response. In these situations, students would use one type of reasoning to come to their decision about the onion's temperature, but another type of reasoning to decide on their thickness. The first combination that was present in this data set were students who both prioritized moisture content and took a middle ground approach. We can see this in Pyaaz's reasoning for selecting treated onion slices that were dehydrated at 70 °C and were 3 mm thick.
“The onions should be dehydrated at 70 degrees Celsius due to these onions significantly scoring better on the moisture test, whilst lagging slightly further behind in the other quality parameters. The onions should be cut to be 3 mm thick, because of the significant improvement in dehydration, [but] with thinner onion slices the rates of spoilage and flavor degradation are higher.” (Pyaaz)
The other combination of reasoning patterns students used was combining 2 out of 3 reasoning with middle ground reasoning. This code is similar to the prioritizing MC/middle ground code in that the students prioritize one quality with one choice and take a middle ground for another. The difference between the two codes lies in what qualities the student decides to prioritize more. We can see this reasoning play out in Bulb's response below.
“I believe 60 °C is the best temperature because it is a moderate temperature and at too high of a temperature, the browning and thiosulphinate loss rates are too high, and at too low of a temperature, the moisture content is too high. In addition, it is best to treat the onions because for all three quality parameters, the treated onions were better than the untreated. Lastly, 5 mm is the best thickness because, although it was the worst thickness based on moisture content, it was the best thickness based on browning and thiosulphinate loss rates, which are more direct predictors of spoilage rate than moisture content.” (Bulb)
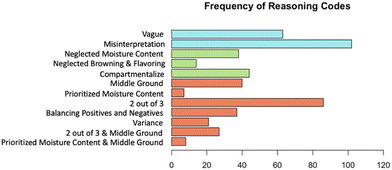
A breakdown of the frequency of each reasoning pattern can be seen in Fig. 2. The most common reasoning patterns came from students who had misinterpretations within their analysis. The second most common reasoning is students who prioritized the browning and flavoring, which was captured by the two out of three code.
Fig. 3 shows the number of students who did not engage, selectively engaged, or fully engaged with the uncertainty. We can see that a majority of students fully engaged with the uncertainty in the task and demonstrated understanding of the limitations and benefits of selecting their particular condition set. The second highest frequency reasoning was students who unproductively engaged with the uncertainty, with many students having a misinterpretation that made it difficult to engage with the data. Finally, nearly 100 students omitted discussion of the conflict, as seen by the frequency of selective engagement in Fig. 3.
 | ||
| Fig. 3 Frequency of the engagement levels students utilized in their responses. The colors in this figure correspond to the codes in Fig. 2. | ||
Connecting reasoning patterns, engagement, and condition sets
To gain insight into how the students' reasoning and condition selection aligned, we generated a Sankey diagram that mapped certain conditions to the reasoning patterns shown in the rubric (Fig. 4). From this diagram, we can see that every condition set had some students who had vague reasoning or misinterpreted the data or assignment.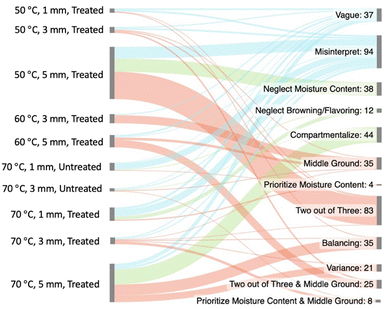 | ||
| Fig. 4 Sankey diagram depicting condition choices and the various reasoning patterns students utilized to justify their decision. Condition sets that had less than ten students select it are omitted from the diagram. The colors in this diagram correspond to the colors in Fig. 3. | ||
Additionally, we can see that students engaged with the uncertainty to varying degrees to arrive at their condition selection. For example, some students who selected 50 °C, 5 mm, treated slices did not engage with the uncertainty because of having some misinterpretations about the data or task. Other students in this condition set selectively engaged with the uncertainty by omitting discussion of the onion's moisture content, and others productively engaged with the uncertainty to earn a two out of three code.
We can see that there are common reasoning patterns within each condition set. For example, students who opted for 60 °C, 3 mm, and treated slices typically arrived at that answer by taking a middle ground approach. Another condition set that has common reasoning patterns is the 70 °C, 5 mm, treated slices. Here we see that students typically arrived at this answer by taking a compartmentalized approach to the data, trying to strike a balance between the onion qualities, or considering the variance between data points.
We can also see that students may decide on different condition sets even if they utilize the same reasoning patterns. For example, we can see that students who used 2 out of 3 and middle ground reasoning often came to two different conclusions for the best conditions. Some students selected 60 °C, 5 mm, treated slices, while other students selected 50 °C, 3 mm, treated slices. The difference in the condition set selection depended on when the student utilized the middle ground reasoning and when they use the 2 out of 3 reasoning. When students select 60 °C, 5 mm, treated slices, they typically take a middle ground approach to justify the temperature and a 2 out of 3 approach to justify the thickness. When students select 50 °C, 3 mm, treated, slices, they typically take the 2 out of 3 approach for the temperature and the middle ground approach for thickness. While either condition can be reached with the same type of reasoning, the way in which the student employs the reasoning to come to a final decision may differ.
Discussion and implications
In this study, students revealed a variety of strategies for handling the uncertainty posed by conflicting data in this task. Like other studies have shown, there was a subset of strategies that avoided or ignored the conflict altogether (Chinn and Brewer, 1993; Walker et al., 2019; Novak and Treagust, 2018; Bolger et al., 2021; Phillips et al., 2021). However, students who employed these strategies still demonstrated productive reasoning. For example, the compartmentalize reasoners productively identified the optimal conditions for each parameter through interpreting the data. For this subset of reasoners, in follow-up interviews, we found that even simple prompting (i.e., “walk us through your final argument one more time”) resulted in students revisiting the data and their argument to generate more comprehensive interpretations. This translates into the classroom context, as it shows that instructors simply prompting students to revisit the data or explain their analysis again could help students more productively manage uncertainty.There was a substantial subset of strategies that were rather unproductive, where students misunderstood components of the task or generated vague reasoning. In this study, we viewed these forms of reasoning as barriers to meaningful participation in the argumentation and data analysis. This allows us to consider how instructors could offer feedback to students that will empower the learner to engage more meaningfully. For misunderstandings, this feedback can simply and directly correct the specific misunderstanding or prompt the learner to revisit the data. Rather than interacting with the vague reasoning as though it is strongly held or representative of misunderstandings, we believe that simply prompting the learner to try again by messaging that their reasoning did not make sense could guide the learner to more productive reasoning. Further, rather than messaging to the student that their answer was wrong or incorrect, which are not useful epistemic criteria for argumentation, it offers an epistemic criterion for argumentation as the reason for revision (i.e., the reasoning did not make sense).
The primary novel contribution of these findings is the variety of ways students grappled with the conflict and resulting uncertainty. Critically, these students recognized the conflict and reckoned with it. This contrasts with findings about conflicting data in which students can dismiss, ignore, and rationalize away conflict (Evagorou et al., 2012; Novak and Treagust, 2018; Walker et al., 2019; Bolger et al., 2021; Phillips et al., 2021). We view some of these kinds of reasoning as transferrable to socioscientific argumentation (e.g., 2 out of 3, middle ground). For example, a reasoner could prioritize lowering global temperatures and ocean health over energy demands to argue for limiting CO2 emissions using 2 out of 3 reasoning (Chen et al., 2019). We further want to highlight ‘variance’ reasoning because these learners demonstrated deeper analysis than their peers. Where their peers considered all three trends in the data, these students attended to the magnitude of the trends. Specifically, they identified differences between data points to make comparisons between data sets. We view this level of analysis as a target mode to guide learners to. In follow-up interviews, we saw learners move towards this deeper analysis with relatively simple prompts: “what is your justification for prioritizing these two qualities?”
The ways of reasoning revealed in this study can enable tailored and specific feedback that will support learners in developing data analysis skills. Further, this task utilizes a relatively neutral context that can be implemented in a variety of chemistry classes to engage students with conflict and uncertainty. We have administered this task as a stand-alone assignment and paired with peer review (Berg and Moon, 2022). Both forms of administration have their advantages. Preliminary evidence from our research program is revealing that peer review can facilitate the prompting to deeper analysis that we have highlighted as a major implication for practice above. Finally, we believe that administering this task can offer epistemological messaging that represents scientific reasoning as using evidence that all people are entitled to engage in, rather than representing scientific reasoning as arriving at the correct answer and only accessible by those who do so (Chen et al., 2019).
Limitations
There are multiple limitations with this study. The primary data source used herein was written responses, which represent a static snapshot of student reasoning. This manifests in the results as distinct categories of reasoning that can be used by a student to engage with this problem. The reality is that these reasonings can be used rather dynamically. Indeed, follow-up interviews confirm that these reasonings change and develop throughout the interview. The claim made in this article is limited to asserting the existence of these reasonings. No claims can be made about how these reasonings are related to one another or develop over time. Ongoing work in our research group aims to elucidate and support these latter claims.We believe this task can fit in a wide variety of chemistry coursework, but it is important to recognize that it is a reading and writing heavy task. The task was administered at an institution at which most students speak English as their first language. So there remains an open question about the accessibility of this task for learners whose native language is not English. Future work and future users of the task should consider their unique students and what modifications to the task may best meet their students’ needs.
Conclusions
In this study, we identified qualitatively different ways that chemistry students grapple with uncertainty in their argumentation. A number of students demonstrated misunderstandings or vague reasoning that impeded their interaction with the data to generate arguments. Some students engaged selectively with the data to circumvent the conflict that was causing uncertainty. Most students used a variety of ways to deal with the uncertainty, including using external justifications to prioritize variables and making careful comparisons within the data to accommodate the conflict. These findings can be used by instructors and researchers to interpret and support students in learning to manage uncertainty productively.Author contributions
MTU: conceptualization, task design, data collection, data analysis, writing. BM: rubric development and data analysis. AM: conceptualization, oversight of data collection and analysis, writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge Amelie Cole for help with rubric development and interrater reliability, and the other members of the Moon group for their insight in this project. We would also like to thank the student participants for engaging so thoughtfully in the task.Notes and references
- Abd-El-Khalick F. and Lederman N. G., (2000), Improving science teachers’ conceptions of nature of science: a critical review of the literature, Int. J. Sci. Educ., 22(7), 665–701 DOI:10.1080/09500690050044044.
- Allchin D., (2012), Teaching the nature of science through scientific errors, Sci. Educ., 96(5), 904–926 DOI:10.1002/sce.21019.
- Alred A. R. and Dauer J. M., (2020), Understanding Factors Related to Undergraduate Student Decision-Making about a Complex Socio-scientific Issue: Mountain Lion Management, EURASIA J. Math., Sci. Technol. Educ., 16(2), em1821 DOI:10.29333/ejmste/113757.
- Berg S. A. and Moon A., (2022), Prompting hypothetical social comparisons to support chemistry students' data analysis and interpretations, Chem. Educ. Res. Pract., 23(1), 124–136.
- Berland L. K. and Reiser B. J., (2009), Making sense of argumentation and explanation, Sci. Educ., 93(1), 26–55 DOI:10.1002/sce.20286.
- Bolger M. S., Osness J. B., Gouvea J. S. and Cooper A. C., (2021), Supporting scientific practice through model-based inquiry: a students’-eye view of grappling with data, uncertainty, and community in a laboratory experience, CBE Life Sci. Educ., 20(4), 1–22 DOI:10.1187/cbe.21-05-0128.
- Buck Z. E., Lee H. S. and Flores J., (2014), I Am Sure There May Be a Planet There: student articulation of uncertainty in argumentation tasks, Int. J. Sci. Educ., 36(14), 2391–2420 DOI:10.1080/09500693.2014.924641.
- Chen Y. C., (2020), Dialogic Pathways to Manage Uncertainty for Productive Engagement in Scientific Argumentation: A Longitudinal Case Study Grounded in an Ethnographic Perspective, Sci. Educ., 29(2), 331–375 DOI:10.1007/s11191-020-00111-z.
- Chen Y. C., (2022), Epistemic uncertainty and the support of productive struggle during scientific modeling for knowledge co-development, J. Res. Sci. Teach., 59(3), 383–422 DOI:10.1002/tea.21732.
- Chen Y. C. and Qiao X., (2020), Using students’ epistemic uncertainty as a pedagogical resource to develop knowledge in argumentation, Int. J. Sci. Educ., 42(13), 2145–2180 DOI:10.1080/09500693.2020.1813349.
- Chen Y. C. and Techawitthayachinda R., (2021), Developing deep learning in science classrooms: tactics to manage epistemic uncertainty during whole-class discussion, J. Res. Sci. Teach., 58(8), 1083–1116 DOI:10.1002/tea.21693.
- Chen Y. C., Benus M. J. and Hernandez J., (2019), Managing uncertainty in scientific argumentation, Sci. Educ., 103(5), 1235–1276 DOI:10.1002/sce.21527.
- Chinn C. A. and Brewer W. F., (1993), The Role of Anomalous Data in Knowledge Acquisition: A Theoretical Framework and Implications for Science Instruction, Rev. Educ. Res., 63(1), 1 DOI:10.2307/1170558.
- Chinn C. A. and Brewer W. F., (1998), An Empirical Test of a Taxonomy of Responses to Anomalous Data in Science. J. Res. Sci. Teach., 35(6), 623–654 DOI:10.1002/(SICI)1098-2736(199808)35:6
![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) 623::AID-TEA3
623::AID-TEA3![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) 3.0.CO;2-O.
3.0.CO;2-O. - Evagorou M., Jimenez-Aleixandre M. P. and Osborne J., (2012), “Should We Kill the Grey Squirrels?” A Study Exploring Students’ Justifications and Decision-Making, Int. J. Sci. Educ., 34(3), 401–428 DOI:10.1080/09500693.2011.619211.
- Kelp N. C., Witt J. K. and Sivakumar G., (2022), To Vaccinate or Not? The Role Played by Uncertainty Communication on Public Understanding and Behavior Regarding COVID-19, Sci. Commun., 44(2), 223–239 DOI:10.1177/10755470211063628.
- Lee H. S., Liu O. L., Pallant A., Roohr K. C., Pryputniewicz S. and Buck Z. E., (2014), Assessment of uncertainty-infused scientific argumentation, J. Res. Sci. Teach., 51(5), 581–605 DOI:10.1002/tea.21147.
- Manz E., (2015), Resistance and the Development of Scientific Practice: Designing the Mangle Into Science Instruction, Cognition Instruct., 33(2), 89–124 DOI:10.1080/07370008.2014.1000490.
- Manz E., (2018), Designing for Analyzing Productive Uncertainty in Science Investigations, in Kay J. and Luckin R. (ed.), Rethinking Learning in the Digital Age: Making the Learning Science Count, 13th International Conference of the Learning Sciences (ICLS) 2018, vol. 1, London UK: International Society of the Learning Sciences.
- Manz E. and Suárez E., (2018), Supporting teachers to negotiate uncertainty for science, students, and teaching, Sci. Educ., 102(4), 771–795 DOI:10.1002/sce.21343.
- McNeill K. L., Lizotte D. J., Krajcik J. and Marx R. W., (2006), Supporting Students’ Construction of Scientific Explanations by Fading Scaffolds in Instructional Materials, J. Learn. Sci., 15(2), 153–191.
- Mehl C. E., Jin H. and Llort K. F., (2020), Student Decision Making in a Scenario-based Investigation of an Ecosystem, Eurasia J. Math., Sci. Technol. Educ., 16(1), 1–14 DOI:10.29333/ejmste/112278.
- Metz K. E., (2004), Children's understanding of scientific inquiry: their conceptualization of uncertainty in investigations of their own design, Cognition Instruction, 22(2), 219–290 DOI:10.1207/s1532690xci2202_3.
- Mislevy R. J., Steinberg L. S. and Almond R. G., (2003), On the Structure of Educational Assessments.
- Mislevy R. J., Almond R. G. and Lukas J. F., (2004), A Brief Introduction to Evidence-Centered Design CSE Report 632.
- Mitra J., Shrivastava S. L., Rao P. S., (2014), Non-enzymatic browning and flavour kinetics of vacuum dried onion slices, Int. Agrophys., 29(1), 91–100.
- National Research Council (2012), A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas, Washington, DC: The National Academies Press DOI:10.17226/13165.
- Novak A. M. and Treagust D. F., (2018), Adjusting claims as new evidence emerges: Do students incorporate new evidence into their scientific explanations? J. Res. Sci. Teach., 55(4), 526–549 DOI:10.1002/tea.21429.
- Nussbaum J. and Novick S., (1982), Alternative Frameworks, Conceptual Conflict and Accommodation: Toward a Principled Teaching Strategy, Instr. Sci., 11, 183–200 DOI:10.1007/BF00414279.
- Osborne J., (2013), The 21st century challenge for science education: assessing scientific reasoning, Thinking Skills Creativity, 10, 265–279 DOI:10.1016/j.tsc.2013.07.006.
- Osborne J., (2014), Teaching Scientific Practices: Meeting the Challenge of Change, J. Sci. Teacher Educ., 25(2), 177–196 DOI:10.1007/s10972-014-9384-1.
- Osborne J. F. and Patterson A., (2011), Scientific argument and explanation: a necessary distinction? Sci. Educ., 95(4), 627–638 DOI:10.1002/sce.20438.
- Pellegrino J. W., DiBello L. V. and Goldman S. R., (2016), A Framework for Conceptualizing and Evaluating the Validity of Instructionally Relevant Assessments, Educ. Psychologist, 51(1), 59–81 DOI:10.1080/00461520.2016.1145550.
- Phillips A. M. L., Watkins J. and Hammer D., (2018), Beyond “asking questions”: problematizing as a disciplinary activity, J. Res. Sci. Teach., 55(7), 982–998 DOI:10.1002/tea.21477.
- Phillips A. M. L., Sundstrom M., Wu D. G. and Holmes N. G., (2021), Not engaging with problems in the lab: students’ navigation of conflicting data and models. Phys. Rev. Phys. Educ. Res., 17(2), 020112.
- Puig B. and Evagorou M., (2023), COVID-19: A Context to Promote Critical Thinking and Argumentation in Secondary and University Students, in Brain, decision making and Mental Health, Springer International Publishing AG, pp. 219–236 DOI:10.1007/978-3-031-15959-6.
- Reiser B. J., Berland L. K. and Kenyon L., (2012), Engaging Students in the Scientific Practices of Explanation and Argumentation: Understanding A Framework for K-12 Science Education, Science, 79(4), 6–11.
- Suh J. K., Hand B., Dursun J. E., Lammert C. and Fulmer G., (2023), Characterizing adaptive teaching expertise: teacher profiles based on epistemic orientation and knowledge of epistemic tools, Sci. Educ., 1–28 DOI:10.1002/sce.21796.
- Sundstrom M., Phillips A. M. and Holmes N. G., (2020), Problematizing in inquiry-based labs: How students respond to unexpected results, Phys. Educ. Res. Conf. Proc., 539–544 DOI:10.1119/perc.2020.pr.Sundstrom.
- Walker J. P., van Duzor A. G. and Lower M. A., (2019), Facilitating Argumentation in the Laboratory: The Challenges of Claim Change and Justification by Theory, J. Chem. Educ., 96(3), 435–444 DOI:10.1021/acs.jchemed.8b00745.
- Watkins J. and Manz E., (2022), Characterizing pedagogical decision points in sense-making conversations motivated by scientific uncertainty, Sci. Educ., 106(6), 1408–1441 DOI:10.1002/sce.21747.
- Watts F. M. and Finkenstaedt-Quinn S. A., (2021), The Current State of Methods for Establishing Reliability in Qualitative Chemistry Education Research Articles, Chem. Educ. Res. Pract., 22(3), 565–578 10.1039/D1RP00007A.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3rp00035d |
| This journal is © The Royal Society of Chemistry 2023 |