What resources do high school students activate to link energetic and structural changes in chemical reactions? – A qualitative study
Benjamin
Pölloth
 *,
Dominik
Diekemper
*,
Dominik
Diekemper
 and
Stefan
Schwarzer
and
Stefan
Schwarzer

Eberhard Karls Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: benjamin.poelloth@uni-tuebingen.de
First published on 29th May 2023
Abstract
Recent progress in elucidating chemical reactions allows to explain chemistry by the potential energy of the involved chemical structures. Nevertheless, from an educational point of view, empirical results indicate that students often do not connect the core idea of energy with other chemical concepts. From a resource-oriented perspective, students do not draw on a coherent concept of chemistry to solve a problem but rather activate diverse cognitive resources, crucially depending on the context. It is thus of interest which resources high school students activate to reflect on energetic aspects of a chemical reaction. In this study, 38 German high school students in 16 focus groups were asked to explain kinetic and thermodynamic aspects of the reaction between hydrogen and chlorine. The unguided focus group phase and the following semistructured qualitative interview were analysed by qualitative content analysis. Results show that students have a diverse network of cognitive resources on energetic aspects. However, this network's structure seems to be dominated by terminology and a few prominent ideas such as activation energy. In contrast, students seldom drew connections between bond-making and energy release. Many students mainly argued on a macroscopic level and relied heavily on technical terms. If they argued on the sub-microscopic scale, however, they often focussed on the whole system rather than on specific molecules and their structure. Hence, students interpreted concepts like activation energy or reaction coordinate diagrams on the system level leading to unproductive reasoning. Overall, it seems that students seldom activate resources on molecular structures to argue about energetic changes in chemical reactions. Also, they rarely refer to the fundamental principle of energy minimisation to reason about the driving force of reactions. These results suggest that chemical reactions should be explained already in high schools on a molecular level providing a more explicit reference to energy as a function of chemical structures.
Introduction
How does chemistry answer its fundamental question of why chemical reactions occur? Reviewing the history of Organic Chemistry (OC) offers an exemplary answer. For a long time, OC was known as a strange and mysterious subject (Ridd, 2008). Knowledge in chemistry was mainly based on trial and error. Only in the 1930s, this changed due to ground breaking research, for example, by Ingold and others (Barton, 1996). They enhanced the scientific character of OC by consequently applying physical principles to chemical reactions and revolutionised the way of chemical reasoning (Saltzman, 1986): Chemical reactivity was primarily rationalised by the energy† of relevant (intermediate) products or transition states. Unfortunately, these energies were not directly accessible and had to be derived indirectly, e.g. through kinetic experiments (Hughes et al., 1936; Pölloth et al., 2022). Thus, chemists attempted to make assumptions about chemical reactions solely based on the structure of a molecule. Therefore, fundamental concepts like electro- and nucleophilicity were developed to translate chemical structures into energetic statements. Using these concepts, reactivity in OC could be rationalised indirectly by the chemical structure of the particles involved, as shown in the inner triangle of Fig. 1 (Goodwin, 2007; Graulich and Caspari, 2019).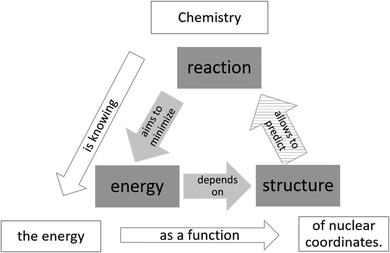 | ||
| Fig. 1 Scheme of the scientific connections between reaction, energy and structure based on Goodwin (2007) and Jensen (2007). | ||
This understanding has been revolutionised again in the last decades through the rise of computational power and the establishment of modern quantum chemical methods (Neese et al., 2019; Seeman and Tantillo, 2022). They allow to calculate the energy of almost arbitrary chemical structures directly and led to novel insights into many reactions, mechanisms and concepts. A prime example is the revaluation of the role of non-covalent interactions (NCI) in chemical reactions (Rösel and Schreiner, 2022). The detailed analysis of calculated energies has implications for interpretations in many areas of chemistry, such as the explanation of the factors leading to asymmetric reactions: Enantioselectivity was commonly attributed to the steric hindrance discriminating the reaction of one enantiomer. In contrast, recent studies suggest that it is primarily the stabilising contribution of attractive NCIs to potential energy that selectively accelerates the reaction of the other enantiomer (Eschmann et al., 2021; Pölloth et al., 2021). This is just one example of the manifold consequences for the scientific understanding of chemistry resulting from the novel insights into potential energy. Hence, chemistry itself may be described as depicted in the outer triangle of Fig. 1: “Chemistry is knowing the energy as a function of nuclear coordinates.” (Jensen, 2007)
Also, for teaching and learning chemistry in high schools, energy is defined as a core idea, for example, by the National Research Council (2012), in the Anchoring Concept Content Map of the American Chemical Society (Holme et al., 2015) or the German Educational Standards for Chemistry (“Bildungsstandards”, Kultusministerkonferenz, 2004, 2020). Nevertheless, empirical studies indicate a lack of conceptual understanding among students in this field (Cooper and Klymkowsky, 2013; Abell and Bretz, 2018). Especially the connection of energy to other core concepts of chemistry – like reactivity or structure – seems to be relatively weak (Bernholt et al., 2020; Podschuweit and Bernholt, 2020). A wide variety of “misconceptions”, for example, regarding chemical bonds, are reported (Hunter et al., 2022). However, it remains unclear which mental resources students activate when asked to reason about energy changes in chemical reactions (Macrie-Shuck and Talanquer, 2020). In terms of a contemporary scientific chemical understanding, as depicted in Fig. 1, the extent to which students use ideas about the dependence of energy and chemical structure is of particular interest.
Theoretical framework
In their review of “Research on the Teaching and Learning of Chemical Bonding”, Hunter et al. (2022) annotate that most of the reviewed studies interpret students’ alternative conceptions as “strongly held, stable cognitive structures”. They argue that this point of view on student conceptions may impact the results drastically and conclude:“[W]e suggest alternative theoretical assumptions for examining students’ ideas about bonding (e.g., the knowledge-in-pieces perspective or resources perspective) […] may enable us to identify more targeted approaches to supporting students’ learning of bonding concepts.”
These conclusions might also be valid for the broader field of energy in chemical reactions. Hence, in the next section, an alternative framework to describe students’ conceptions is outlined.
Conceptual knowledge and resources framework
Empirical studies indicate that many students leave chemistry classes without a “big picture” of chemistry (Cooper et al., 2017). The acquired knowledge tends to be fragmented into small, disconnected parts that often cannot be transferred onto other problems. Bruner (1995) proposes that “to understand something is to sense the simpler structure that underlies a range of instances.” Consequently, the coherence of the knowledge network is a crucial factor for students’ success (Podschuweit and Bernholt, 2020). Assuming knowledge is constructed in the learners’ minds (Bodner, 1986), learning entails connecting new knowledge elements to existing mental structures (Cooper, 2014). Hence, it is central to know how students think (Gulacar et al., 2022). On almost every topic, students have specific ideas, e.g. from media, everyday life experiences, intuition or previous schooling (Barke, 2006). Accordingly, “misconceptions” of students have been broadly analysed and ordered since the 1970s (diSessa, 2014a; Hunter et al., 2022). For some time, these misconceptions were seen as stable concepts in learners' minds that need to be deconstructed (Lamichhane et al., 2018). However, a deficit-oriented approach to students’ conceptions may impede the productive use of these conceptions for teaching (Heeg et al., 2021). Therefore, the theoretical framework about student conception began to change; a multitude of theories on “conceptual change” arose (Gropengießer and Marohn, 2018). From this perspective, student conceptions are not primarily seen as barriers to learning but as essential building blocks for new knowledge. Cognitive conflicts are triggered to change and improve these concepts. There is no unifying theory of conceptual change. However, often students’ ideas are still interpreted as stable and coherent conceptions (diSessa, 2014a). In contrast, diSessa (2014a) argues that “[s]tudents do not just lack knowledge they think differently than experts”. Hence, students’ ideas cannot be described as a coherent network that includes a few wrong ideas. Moreover, knowledge “consists of many quasi-independent elements” in a complex loosely-connected network (diSessa, 2014a, 2018). Hammer et al. (2005) propose to call these mental components rather generally “resources”. These resources may originate, for example, from learning, everyday experiences or intuition. Several of these resources are activated whenever a student needs to apply knowledge – for example, to solve a scientific problem. However, students will not always activate the same resources. Instead, problem context strongly influences which resources are activated (Hammer et al., 2005; diSessa, 2014a). Thus, in this framework, learning is not seen as “the formation of a cognitive object, but rather as a cognitive state the learner enters” (Hammer et al., 2005). Consequently, the question of whether a resource is correct or incorrect becomes less critical. Rather, the question is whether the activated resource is productive or unproductive in solving a specific problem.The above described change of perspectives also impacts research on learning. As long as student ideas are seen as stable conceptions, they simply have to be collected and classified. These collections help teachers to identify the misconceptions in class and to plan interventions to overcome them. In contrast, if the context determines which resources are activated, the focus of research must also change. Student reasoning should be analysed on a fine-grained scale in the context of a specific problem – for example, in clinical interviews or observation studies of learning processes (diSessa, 2014b). The identified resources students activate, then, must be interpreted as a small segment of a very complex network. Therefore, the focus of this study is to identify resources students activate to reason on energy and chemical structure in the context of a specific chemical reaction. The analysis attempts to take the network structure of these ideas seriously.
The energy concept in high school chemistry
To adequately analyse and interpret students’ resources on energy, it is crucial to outline the level of understanding expected as a benchmark for high school students. The introductory section presents a recent theoretical-chemical perspective on the role of energy. However, for high school education, these ideas must be reduced to their essentials (Streller et al., 2019). The way and extent energetic aspects of chemical reactions are explained in high school education differs dramatically between various educational contexts. All of the students in the sample attended an academic-track high school in the German federal state Baden-Württemberg. Therefore, the currently valid curriculum for this educational context is analysed in detail.Energy is defined as one of the core ideas in chemistry for the German university entrance qualification at high schools (Kultusministerkonferenz, 2020). Accordingly, energetic aspects of chemical reactions are discussed repeatedly throughout the curriculum in Baden-Württemberg. Already in the first years of high school chemistry education, students are expected to compare exothermic and endothermic reactions and to explain “the supply of energy as a prerequisite for starting chemical reactions” (“Bildungsplan Gymnasium”, Ministerium für Kultus, Jugend und Sport Baden-Württemberg, 2022). The impact of catalysts on the activation energy as well as the reaction energy of combustion reactions are discussed in grades 8–10 (age 14–16). In this context, most commonly, reaction coordinate diagrams are introduced. In grades 11–12, the knowledge about energetic aspects is deepened through the implementation of calorimetry, entropy and the Gibbs-Helmholtz equation. Students are expected to discuss the “limits of the energetic point of view” using examples like metastable states. In summary, several important relations between energy and chemical reactions are discussed. In contrast, aspects of the dependence of energy and chemical structure are not explicitly mentioned.
As mentioned above, energy teaching can also be approached from another perspective. One example are the core ideas for K-12 education as proposed by the National Research Council (2012). They argue that “energy is best understood at the microscopic scale, at which it can be modeled as either motions of particles or as stored in force fields (electric, magnetic, gravitational) that mediate interactions between particles.” From this point of view, the relationship of energy and chemical structure is pronounced more explicitly, as the following quote exemplifies: “A stable molecule has less energy, by an amount known as the binding energy, than the same set of atoms separated.”
As a last perspective, a very brief view into university-level chemistry education may be interesting. The main discipline handling energetic aspects is physical chemistry (PC), which is traditionally divided into kinetics and thermodynamics (Atkins et al., 2022). This study focuses on some central aspects of these sub-disciplines that are also found in high school curricula. Activation (free) energy plays a central role in explaining chemical kinetics; central aspects in thermodynamics are, e.g. the energy balance and the driving force of chemical reactions. The different aspects are merged in potential energy surfaces (Kaliakin et al., 2015) that may be reduced to reaction coordinate diagrams (RCDs).
Empirical findings on students’ understanding of energy
After giving a few insights into different aspects of energy in chemistry curricula, this section aims to provide an overview of relevant empirical studies. First, students’ understanding of energy in general is briefly introduced before focusing on potential energy. Then, empirical results on students’ understanding of energy and structure are reported with an emphasis on chemical bonding. Finally, students’ understanding of the connections between energy and chemical reactions is discussed primarily in the context of reaction coordinate diagrams and reaction mechanisms. Due to the multitude of topics related to energy in chemistry, the literature review herein does not claim to be exhaustive.Aim of the study and research questions
The empirical findings mentioned above illustrate several shortcomings in students’ conceptual understanding of energy in chemical contexts. An explanation may involve missing cross-connections of energy and other fundamental ideas of chemistry. From a resource-based perspective, this suggests that energetic resources are rarely activated for reasoning about chemistry. This study aims to investigate in detail which mental resources high school students activate to explain energetics of a chemical reaction. The investigation focuses on central aspects of kinetics and thermodynamics students are supposed to learn about in high school. It is of particular interest to what extent students relate energetic and structural changes. Therefore, the following research questions will be explored:(1) Which cognitive resources do students activate to explain the activation energy of a chemical reaction?
(2) How do students explain the release of heat during a chemical reaction?
(3) How do students rationalise the driving force of a reaction?
(4) How do students use and interpret reaction coordinate diagrams to explain relationships of energy, structure and reaction?
Methodology
For the analysis of student resources, diSessa (2014b) proposed investigations in a small-grained, qualitative empirical setting with a well-defined context. Therefore, in-depth analyses of students’ reasoning are needed. Qualitative interviews provide a suitable methodological framework for such an investigation (Bernard, 2013; Mey and Mruck, 2020). Details on the process of developing and testing the method are provided in the next paragraph before the final interview guide, the sample, and data gathering and analysis are described. Finally, the trustworthiness of the method will be discussed.Development and piloting of the empirical method
The first draft of the interview guide was constructed based on the following three principles. (1) Findings from the chemistry education research literature form the basis of the guide. Therefore, a few prompts from other studies were adapted for the present interview guide (Becker and Cooper, 2014; Atkinson et al., 2020; Macrie-Shuck and Talanquer, 2020; Atkinson and Bretz, 2021). (2) The guide is embedded in authentic problem-solving processes. Choosing an appropriate context is critical for analysing resources as their activation depends on the context (Hammer et al., 2005; Podschuweit and Bernholt, 2020). A typical context in learning chemistry is explaining a demonstrated chemical reaction. A suitable reaction should be relatively simple, involving only a few atoms with a manageable number of degrees of freedom. Therefore, the reaction of elementary hydrogen and chlorine to hydrogen chloride is used as context. (3) The main areas of physical chemistry (PC) structure the research questions and the interview guide. The field of activation energy represents kinetics; the energy balance and the driving force are attributed to thermodynamics (Atkins et al., 2022).Following these guidelines, a first draft of the interview guide was written and discussed with experts in science education from different universities. In a pilot study, four qualitative interviews were conducted by one of the first two authors of this paper. The respective other author observed the interviews. After each interview, the two authors provided feedback on interview techniques and evaluated the guide. These insights helped to improve the objectivity and quality of the interview guide iteratively. In the first draft, the interviewer firmly directed the course of the discussion. The strict interview procedure hindered students from following and expressing their ideas. Hence, an independent preparation and presentation time was implemented. These changes led to a significantly better elaboration of the student's thoughts at the beginning of the interviews. Unfortunately, in this setup, students’ thoughts during the preparation time were not available for analysis. Thus, in a second revision, groups of two to three students were interviewed. At the beginning of the interview, students were allowed to discuss relatively uninfluenced in such focus groups (Przyborski and Riegler, 2020). The final interview guide was tested with other two groups of four students.
Procedure of the empirical study
First, the focus group watches a short video clip showing the reaction of chlorine and hydrogen gas. In the video, the two gases are mixed in a syringe, a piezo igniter generates a spark, and the reaction mixture explodes. The explosion is re-shown in slow motion as well as with a thermal imager (Fig. 2). Hence, the students get the following work assignment:“A tutoring student (grade 10) performed the experiment that you just saw in the video in his chemistry lesson. He did not understand anything at all and asks you to explain what happened during the reaction. The following questions are of particular interest to him:
(a) What is the spark at the beginning of the reaction for?
(b) Why does the reaction mixture turn hot?
(c) Why does a reaction occur at all?
Try to explain as detailed as possible and include graphic elements.”
After a maximum of 10 minutes of preparation, the students present their explanations to the tutoring student represented by the interviewer. Then, the interviewer asks further questions on topics or terms mentioned by the students in their presentation. Finally, the interviewer addresses the topics from the interview guide that the students did not mention before. The complete interview guide is presented in Table 1 (Appendix).
Survey study
A detailed description of the sample may help to increase the transferability of the reported results. Hence, participants were asked to complete a short survey. The questionnaire included questions on sociodemographic data (age, gender identity) and performance in chemistry (grade, experience in chemistry). Scientific interest was measured with a scale developed by Pawek (2009) based on Engeln (2004). To keep the survey as short as possible, only five of the original seven items were used as a scale and one of these items was carefully updated (“scientific shows on TV” was changed to “scientific shows on TV or YouTube”). An excellent Cronbach's alpha value of α = 0.89 indicates the reliability of the scale. The scale for the self-concept of ability in chemistry comprised six items from a literature scale (Pawek, 2009; Budke et al., 2019; Budke, 2019) and yielded a Cronbach's alpha value of α = 0.88. The scales were validated in the aforementioned literature studies, however, the content validity of the slightly adapted scales was not further investigated in the context of this study. Therefore, the results from this survey should not be further interpreted quantitatively.Sample
Semistructured group interviews were conducted with 38 high school students in 16 groups from 5 school classes. The students visited grade 11–13 in an academic track high school (“Gymnasium” or “berufliches Gymnasium”). Gender identity was evenly distributed across the sample; 19 students identified as female, 17 as male, and two did not specify their gender identity. All the students chose chemistry as a main subject. In Fig. 3, the survey results of the students participating in the study are depicted. While the results are reported for each student individually, a colour scheme is used to indicate the focus group the students participated in. This colour code will be used throughout the manuscript. The students were able to choose their focus groups independently. The distribution of the chemistry grades in the last report card was extensive on the German grading scale from 1 (very good) to 6 (insufficient). The medium of the grades was 2.5, with a high standard deviation of 1.5. While 13 of the students achieved the best grade of “1”, six students in the study reported a deficient grade of “5”. The scientific interest is broadly distributed with a mean of 1.8 ± 1.1 on a scale from 0 (high interest) to 4 (no interest). For the self-concept of ability, a mean of 1.6 and a wide distribution with a standard deviation of ± 0.9 was found. Despite this broad sample, the focus groups themselves were relatively homogeneous in terms of performance and interest. Only a few groups, e.g. groups Green, Cyan, or Red, were composed of students with very different levels of interest and self-concept.Data gathering and analysis
The authors conducted the interviews in an out-of-school laboratory in summer 2022. Entire school classes visited the laboratory. The Ethics Committee of the University of Tuebingen approved the study setup. Participation in the interview study was voluntary; all participants approved informed consent. In case of underage participants, additional consent from the parents was obtained. The audio data were transcribed following the rules of Dresing and Pehl (2018); further analysis was performed using MaxQDA (VERBI, 2022). Each student in a focus group was assigned a number (1, 2, 3). Hence, qualitative data analysis was performed following the method of Kuckartz (2016). The main content categories were derived deductively from the interview guide. The first and second authors independently coded one-third of the material (13 students in 5 group interviews). The intercoder reliability was found to be acceptable with K = 0.64. The authors discussed coding differences and specified or merged unclear categories. The revised coding scheme yielded a good intercoder reliability of K = 0.81. The subcategories were formed inductively. The first and the second author of this paper coded five interviews independently. Approximately 75% of the inductively found subcategories were consistent for both coders. Differences existed mainly regarding the grain size of the categories. The authors discussed the remaining issues and agreed on preliminary subcategories. The first author coded the remaining material. Only a few subcategories were added based on the remaining material. The final subcategories were reviewed for consistency by the second author. The entire category system with anchoring examples is presented in Table 2 (Appendix).Trustworthiness of the results
In qualitative research, special attention should be paid to the trustworthiness of results based on the quality criteria credibility, transferability, dependability, confirmability and reflexivity (Korstjens and Moser, 2018). The credibility of the results in this study was strengthened through the method triangulation described above combining unguided focus groups and guided interviews. This method triangulation proofed successful as students often only briefly mentioned several concepts or ideas in the unguided focus group phase. Relying only on these statements may have led to misinterpretation or bias through the researchers’ expectation. The guided interviews therefore enabled the researchers to further inquire how the high school students understood a particular word or how elaborate their knowledge of a mentioned concept was.Confirmability was enhanced through investigator triangulation in conducting the interviews and in analysing the data. Relevant parameter like the interrater reliability coefficient are reported above. To improve transferability, a detailed, “thick” description of the context of the interviews and descriptive data on the participants are added. The diversity of the sample, e.g. in terms of self-concept of ability or scientific interest, further increases transferability. Dependability is ensured through the detailed description of the method as well as insights into the development of the interview guide and the piloting process. Reflexivity was fostered by peer feedback during the pilot interviews, which helped to unravel research relationships with participants. Furthermore, the researchers reflected critically on their own biases and how these would influence the research.
Results and discussion
In the following, the results are presented organised by research questions (RQ). Specific results are discussed directly, whereas this section's last paragraph aims to discuss the main results comprehensively.RQ1: students’ reasoning on the “spark”
The first guiding question focused on the “spark” igniting the reaction mixture and thus on a kinetic aspect of a reaction. The results of the qualitative content analysis of the interviews are illustrated in Fig. 4. It aims to make the diverse argumentation patterns of students visible. Fig. 4 is best read starting from the green box (“The spark…”), which marks the start of students’ argumentation. Following the lines of one colour allows the reader to follow the argumentation of one specific focus group in the unguided phase (solid lines) and their answers in the following guided interview (dashed lines). All main categories displayed in the figure are explained in more detail below, anchoring examples can be found in Table 2 (Appendix). The categories were ordered top down by the levels of Johnstone's triangle. The sub-microscopic level was further subdivided into the perspective on the whole system and the second “zoom” into molecular structures. This division is rationalized below. Arguments on dynamic aspects of reactions can be found on the left side of the figure and static aspects on the right side (Caspari et al., 2018). | ||
| Fig. 4 Students’ reasoning on the question (green box) what “the spark at the beginning of the reaction is for”. Each focus group is represented by one colour as described and named in Fig. 3. Categories were deduced from a qualitative content analysis of the interviews. Anchoring examples for each category are found in the appendix. Font sizes reflect the frequency of the corresponding subcategory among the focus groups. Solid lines represent the argumentation patterns of students during the unguided phase of the focus groups in chronological order. The final argument of the students is marked with a dot. Categories that were addressed only in the semistructured interview phase are connected with dashed lines. | ||
• “This spark […]isthe activation energy“ (eight students from six focus groups with similar statements)
• “We need the sparkasactivation energy” (four students)
• “So we needed the spark […]forthe activation energy” (seven students)
• Three students related the activation energy immediately to RCDs
Ten groups explained that the activation energy is needed to “start the reaction”; including all groups that equated “spark” and “activation energy”. Five groups already finished their explanation of the spark's function at this level.
Several groups used metaphors, e.g. the activation energy “is needed at the beginning to nudge the reaction” (Green), or “[y]ou cannot drive a car without a key. And the spark is the key that makes that whole thing run” (Orange).
Scientifically (Gibbs’) activation energy describes the “energy difference between the transition state of an elementary reaction and the ground state of the reactants for that step” (Perrin et al., 2022). Hence, it is the energy that (one or two) specific particles need to react. Generally, this energy is needed for bond-breaking. In contrast, many students understood activation energy as the energy added to the system (“the spark is the activation energy”). While this may sound like hair-splitting, the conflation of “activation energy” and energy added to the system leads to several argumentative dead ends, as shown in the following.
“Even if a reaction occurs voluntarily but just much too slow, it can be possible that you first have to add energy.” (Cyan)
Only two groups argued with reaction rates in the unguided phase. For other groups, the question of acceleration versus starting the reaction seemed to be controversial:
“I: What exactly is activation energy needed for in the reaction? […]
1: Okay, to start the reaction, isn’t it? […]
3: And to accelerate it, too.
2: No.
3: No? Okay.
I: So only to start it?
3: I didn’t think so.
2: I think so, but I’m not sure.
1: Yeah, yeah.
2: The activation!” (Green)
As an argument for why the activation energy starts or accelerates the reaction, most of the focus groups stated rather unspecifically that it brings “the energy that is needed for every reaction” (Red) or to “cross the threshold” (Grey). These explanations were mainly general, without a specific reference to the reaction of hydrogen and chlorine.
Also, several groups referred to other ideas like the spark “mix[es] hydrogen and chlorine gas” (Turquoise), “increases the pressure” (Brown) or makes “hydrogen chloride explode” (Turquoise). Only single groups mentioned these ideas, and they do not seem to be very productive. Therefore, they are not discussed in detail here and are mainly omitted in Fig. 4.
“2: Yes, the spark makes them react extremely quickly, because they move so fast and collide with each other, and that's always the thing.” (Turquoise)
The “spark” would thus induce the reaction of all particles at the same time. While this would explain why the reaction occurs at higher temperatures with a higher rate, there is no reference to how the very short-term spark could ignite the reaction. Only group Lime referred to this problem and gave a productive explanation:
“2: […] you only have a very small portion of gas, which is heated very hot. This releases energy and practically triggers a chain reaction. […]
1: And then, when the first two molecules, let's say, react, energy is also released and stimulates another one.
2: Yes, exactly. Then it pushes on and on. This is only an initial nudge. […]
3: Like dominoes!” (Lime)
“[Energy needs to be added] to have enough energy so that the two particles, the Cl and the H atom, can meet in such a way um, that I reach the transition state. The transition state is necessary, um, because that is the correct arrangement of the two particles in relation to each other, so that the bonds can meet at all and um, the bonds can be formed at all.” (Yellow)
This explanation relies on the structure of the particles. However, group Yellow indicates energy is needed for bond-making instead of bond-breaking. The idea of exothermic bond-breaking is a well-known conception of students (Galley, 2004; Nahum et al., 2007; Zohar and Levy, 2019; Hunter et al., 2022). Although the argument was scientifically incorrect here, students used it in a productive manner.
RQ2: students’ reasoning on the “origin of heat”
While the first question focussed on kinetic aspects, the second guiding question is suited in thermodynamics. Fig. 5 is structured analogous to Fig. 4 by the arguments of the focus groups on the question of where “the heat origins from” (green box). The other boxes describe the main categories from the qualitative content analysis of the interviews ordered top-down by means of the Johnstone triangle. The subdivision into sub-microscopic system and molecular structure is explained above. | ||
| Fig. 5 Students’ reasoning on the question (green box) “why the reaction mixture turns hot”. Each focus group is represented by one colour as described and named in Fig. 3. Categories were deduced from a qualitative content analysis of the interviews. Anchoring examples for each category are found in the appendix. Font sizes reflect the frequency of the corresponding subcategory among the focus groups. Solid lines represent the argumentation patterns of students during the unguided phase of the focus groups in chronological order. The final argument of the students is marked with a dot. Categories that were addressed only in the guided interview phase are connected with dashed lines. | ||
“And it gets hot simply because energy is released in an exothermic reaction.” (Green)
Almost all student groups related the heat increase to a release of energy during the reaction. Most groups used the term “exothermic”. Most of the time, “exothermic” was employed as a causal factor for heat: “Yes, so the reaction mixture becomes hotbecauseit is an exothermic reaction” (Grey). Only three students vice versa, deduced the description “exothermic” from the observation of the reaction: “the reaction product gets warm um and that simplymeansthat the reaction is exothermic” (Purple). Half of the groups did not go into further detail on this question. It seems that for most of them, the origin of the heat was already comprehensively explained by referring to the term “exothermic”; one group made it explicit, stating, “That is really stupid, that question” (Lime). Hence, the extent of student discussion on this guiding question is much smaller than for the other questions.
• Five groups said it was “in the reactants” (Turquoise) without further explanation.
• Four groups argued with the chemical or inner energy of the reactants: “It was stored chemically in the reactants. In other words, chemical energy.” (Purple)
• Five groups argued with a lower energy of products. “And the particles, that is hydrogen and chlorine, have a higher energetic potential than hydrogen chloride.” (Lime)
• Three groups argued that all the energy released was only the energy brought into the reaction by the spark. They did not relate the energy changes to a chemical reaction at all.
RQ3: students’ reasoning on “why a reaction occurs at all”
The last guiding question focused on the driving force of chemical reactions. The term “driving force” itself was omitted in the interview – not least because “force” is a misleading term in the context of energy (Perrin et al., 2022). Again, students’ arguments on why “a reaction occurs” (green box) are clustered in Fig. 6. As described below, for this question, only very few students argued on the submicroscopic level at all. Hence, some related arguments on particles or bonds were summarized in umbrella categories (“properties of…”). The ideas are discussed in detail below. | ||
| Fig. 6 Students’ reasoning on the question (green box) “why a reaction occurs at all”. Each focus group is represented by one colour as described and named in Fig. 3. Categories were deduced from a qualitative content analysis of the interviews. Anchoring examples for each category are found in the appendix. Font sizes reflect the frequency of the corresponding subcategory among the focus groups. Solid lines represent the argumentation patterns of students during the unguided phase of the focus groups in chronological order. The final argument of the students is marked with a dot. Categories that were addressed only in the guided interview phase are connected with dashed lines. | ||
“1: Okay, and why does a reaction take place at all?
2: Um, because…/through…/
1: (unsure)/the activation energy two substances react with each other…?” (Salmon)
Again, activation energy seems to be a domain that is primarily activated in the context of chemical reactions. The driving force, in contrast, does not seem to be a central theme for high school students. One group made it explicit:
“[This] is actually interesting because when we used to look at experiments in school when you see a reaction you see straight away that it's a reaction and [you do] not [ask] why the reaction is happening.” (Green)
“1: The reason for this is simply that eight electrons are very stable for the compound and that's what you have here. Seven and one add up to eight. And that is exactly why HCl is more stable than the starting materials.
2: And that's the background, why this happens voluntarily. Or why it happens AT ALL.” (Purple)
Many groups elaborated thoroughly on the octet rule in that context. Even relatively weak students were able to explain the rule. This may be rationalized as in German high schools the octet rule is often already taught in the first years of chemistry (e.g., Ministerium für Kultus, Jugend und Sport Baden-Württemberg, 2022). Nevertheless, in the reaction discussed herein already the two reactants Cl2 and H2 have a full valence shell. Thus, the octet rule cannot explain the driving force of this reaction. During the interview, all student groups were confronted with this cognitive conflict. The focus groups handled that intervention in different manners. Some groups denied the fact that molecular hydrogen and chlorine have a full valence shall:
“I: Let's take Cl2. Does that have a full outer shell?
1: Yeah, no, in this case not. It only has seven [electrons].
I: But it is Cl 2 . […]
1: Well, if I have seven valence electrons, I don't want to react with something that also has seven valence electrons. That wouldn't do me much good.
2: I want to react with something that has one.” (Grey)
This argumentation reflects a poor understanding of the octet rule, especially regarding covalent bonds. Other focus groups discovered the problem themselves:
“2: [A]t the beginning both have not fulfilled their octet rule um or of course they/the molecules have each fulfilled their octet rule. So H2, for example, has now/shares one each, so they both have two. And so does chlorine. […]
1: Both are aiming for the eight electrons. And can only achieve that by forming a bond together.
2: Yeah, no, you have to think about it, the thing is: both already have a stable bond in the definition of the octet rule.
1: Yeah, but they can be even more stable.
I: So, the cause of the reaction would be, in your opinion? […]
1: I would say that if there aren't eight electrons, a substance is very reactive because it strives for a stable state.” (Purple)
In this group, student 2 could correct their argumentation and clearly outlined why the reactants already satisfy the octet rule. Student 2 explained that idea several times to student 1. Nonetheless, student 1 chose to stick to their explanation with the octet rule and ignored the argumentation of student 2. Hence, this focus group and several other groups were unable to use the octet rule productively during the whole interview.
“3: Maybe it [the activation energy] does something to the atom or molecule or something, that they combine or react with each other, which they wouldn't normally do. […] Maybe they get mixed up somehow, or something, and then there's room for the Cl2to combine with the H2, for example. Because otherwise they would both be full, or what do you call that?
2: Yes, the valence shell.” (Magenta)
Despite the visible lack of conceptual knowledge, the octet rule gave students an idea about the activation of a reaction and a rough idea of the driving force for an elementary step of the total reaction. This example shows how scientifically vague ideas can become productive resources for learning chemistry. Thus, the problem is not the octet rule itself but rather the lack of a meta-perspective on it, as Joki et al. (2015) outline: “The more widely understood problem of teaching chemical bonding is not merely the octet framework, but involves the balance between coarse and categorical mnemonics and explanatory schemes.”
“Reactions always happen […] voluntarily if at the end of the reaction there is an energetically favourable state for the substances. And that is the case here, because obviously HCl is energetically more favourable than when you, um, hang around alone. Not alone, but in the elements.” (Gold)
Furthermore, four of these groups discussed entropy and free enthalpy. “[T]he simplest possibility, but that would not be a logical reason, would be to calculate […]. With free enthalpy you can calculate that it runs quasi spontaneously or not. […] But that is not satisfying” (Cyan). The argument on free enthalpy is scientifically accurate and is the basis for describing reactions, e.g. in Theoretical Chemistry. Nevertheless, the mathematical argument felt insufficient to students.
RQ4: students’ reasoning with reaction coordinate diagrams
All 16 groups were able to draw a reaction coordinate diagram for exothermic reactions. Half of the groups drew the diagram spontaneously; the other half after they were explicitly asked for a graphical representation during the interview. Six groups discussed RCDs even as a first association during the preparation time:“2: Can you draw this, um, diagram with the graph with the activation energy?” (Orange)
Starting and ending point of the diagram were interpreted as reactants and products by 15 groups. Some groups were referring to the reactants on the macroscopic level, “hydrogen gas and chlorine gas” (Red); five groups used Lewis structures to depict reactants and products. However, it cannot be clearly distinguished in most cases if students referred to the macroscopic level or the structure of the reactants and products. Two groups furthermore directly equated the start- and endpoint of the graph and the start and end of the reaction. Only two groups specified that the energies of reactants and products were represented – which is in line with the interpretation of the y-axis as energy. Most groups quickly connected the “hill” to the activation energy; two students associated the hill with the concept of catalysis.
“So, it is exactly the point where this spark comes. That means that nothing has reacted yet.” (Gold)
In contrast, another group said, “That's where the reaction is strongest, I think.” (Red) and other two groups argued that “from this point […] the reaction runs on its own” (Grey). Only three groups were able to explain the summit with structural arguments. Two of these groups mentioned the term transition state.
Similarly, one arbitrary point on the reaction coordinate curve meant to students either the energy of the system during the reaction (five groups), a specific point of time after the activation of the reaction (six groups) or a point, when there is a mixture of reactants and products (five groups). Four groups, however, referred to intermediate products:
“So maybe a lot of intermediate products? So, an intermediate product that changes somehow?” (Black)
“Um, the question is whether the diagram refers to the entire system or to a single particle. If it refers to a single particle, then you can't [assign the arbitrary point on the curve], because this intermediate form between 2 HCl and Cl2and H2, um, at least in my opinion, cannot be represented. But if it was the entire system, then you could say yes, for example, there is a fifty-fifty mixture or something.” (Lime)
In the students’ interpretation, an RCD shows the change of a system's energy (y-axis) over the reaction time (x-axis). Unfortunately, this perspective is hardly productive in explaining chemical reactions. Moreover, it implies odd and inconsistent ideas about reaction processes. Group Blue is an excellent example to illustrate the problem. As reported above, they were the only group in the sample that connected activation energy with bond-breaking and released energy with bond-making. They showed a rough idea on a possible reaction mechanism and a solid understanding of reaction processes. Nevertheless, their assumptions on RCDs do not allow any other conclusion than the following:
“Well, up to that point [the summit], the energy is added so that the molecules can split and from that point on, the chlorine and hydrogen [atoms] react with each other.” (Blue)
In a nutshell, this implies that first all molecules in the reaction mixture would split into single atoms increasing the energy of the system. Only after that, the exothermic recombination process starts and heat is released.
The interpretation of the x-axis as a timeline is not only problematic for the specific subject of RCDs. Overall, it suggests that many students do not primarily attribute changes in potential energy to changes of chemical structure but rather to arbitrary aspects, such as time. Consequently, it becomes hard to reason about the driving force of a reaction. Almost all groups in the sample interpreted RCDs similarly. The uniformity of answers may indicate that RCDs are generally taught incorrectly. In that case, it could be appropriate to entitle the described interpretations of RCDs as “home-made misconceptions” (Barke, 2006) that are mainly provoked by teaching in school itself.
Summary discussion of the main findings
Out of the manifold results, several aspects were observed throughout all topics. These main findings are discussed herein together.Students have a diverse network of resources on energetic aspects of chemical reactions
In the analysis, a wide variety of mental resources was found that students activated for reasoning about energetic aspects of a reaction. Even focus groups composed of students with relatively low self-concepts of ability were able to use resources from different areas to explain chemistry. One example is the group Black which had shallow and incorrect conceptions of RCDs. Being asked about the meaning of an intermediate point on the curve, they used everyday logic to suspect that there are “intermediate products somehow that keep on changing somehow” (Black). Even so this explanation is very vague, it is a piece of knowledge that can provide a starting point to build productive explanations of reaction processes (Hammer et al., 2005).However, some concepts are very dominant in students’ mental networks while others are only loosely connected. For example, showing a “spark” seems to immediately activate resources or terms related to the concept of “activation energy”. For some students, “activation energy” appears to be the central concept in almost every context of chemical energy. Similarly, the concept of catalysis is often predominantly associated with exothermic reactions. In contrast, the question of “why a reaction happens” hardly seems to activate any idea related to energy.
Students often refer to terms rather than concepts
Terms like “exothermic”, “activation energy”, or representations like RCDs are used predominantly as explanations. They seem to be the primary tools used in school for explaining energetics. Moreover, many students confuse the cause and representation of phenomena. The description “exothermic” or the graphical representation of an RCD must be derived from experimental observations. In contrast, students use these representations to rationalise the experimental observations as black boxes. Some of these terms and representations are primarily connected to other words and not to concepts. Vinner (1997) calls these terms pseudo-conceptual. Similar results can also be found, e.g. in Organic Chemistry (Crandell et al., 2020).Students often focus on a sub-microscopic system rather than on specific molecules
Research indicates a missing link between energy changes on the macroscopic level and the sub-microscopic level (Cooper and Klymkowsky, 2013). Also in this study, many groups mainly focused on the macroscopic, symbolic, or terminological level. Nonetheless, it should be emphasised that many focus groups were able to argue on the sub-microscopic level – at least when explicitly asked to do so. The findings of this study suggest a further problem with reasoning on the sub-microscopic level: students seem to mainly argue with the whole system, rather than with molecules and their structures. For example, the relation of energy changes and changes in particle speed seems to be clear to many students. However, it is somehow difficult for them to draw the conclusions about how this would affect a specific molecule, lead to a change of structure and yield a product with a different arrangement of atoms. This phenomenon can be observed across all research questions. Especially the interpretation of activation energy or RCDs on a system level does not allow a meaningful discussion as outlined in detail above.Students rarely connect changes in energy with changes in structures
As described in the introduction, the central paradigm of chemistry is to explain chemical reactions by the energy of the relevant structures (Goodwin, 2007; Nahum et al., 2007). Almost every energy change in a chemical reaction is caused by a change of chemical structures – mainly by bond-breaking and making. However, it seems that only very few students attribute energetic to structural changes (Greenbowe and Meltzer, 2003). This connection is rarely made even when students know that energy is needed to break bonds. The problem becomes even more prominent for bond-making, which is almost never linked to the release of energy. As in other studies (Galley, 2004; Zohar and Levy, 2019; Hunter et al., 2022), also the idea of exothermic bond-breaking can be found in the sample. This indicates a lack of conceptual understanding of chemical bonds as the energetically most favourable state of two atoms.Students seldom refer to energy minimisation to reason on chemical reactivity
Closely related to the findings described in the last paragraph is reasoning about the driving force of reactions. The idea of energy minimisation appears to be intangible for many students. Furthermore, the above-described lacking connection between bond-making and energy release impedes productive reasoning on energy minimisation. However, minimising energy is the fundamental driving force for processes in nature. Hence, it should be surprising that students refer to this central idea much less than to other, more specific concepts. Again, it may be seen that students’ mental network is not strictly hierarchically structured. Rather, it seems to be connected around student- and context-specific main nodes, that are strongly influenced by the way energy usually is taught in schools.Limitations
As with any qualitative study, the insights gained in this work are not representative. Results may vary for other school systems, countries or school classes. The interviews were conducted in an out-of-school laboratory. The visit of the laboratory was organized in a way to guarantee comparable conditions for all participants at the study. However, due to practical reasons students performed the interviews at different times during their visit. Thus, it cannot be entirely excluded that fatigue effects or contents of the out-of-school laboratory influenced the interviews. The ideas in the focus groups were often developed in mutual exchange, or other students' ideas were picked up. Sometimes, it was technically challenging to differentiate students’ voices unambiguously for every statement. To avoid misattribution, quotes throughout the manuscript are only assigned to focus groups, not individual students. All students chose chemistry as a major subject. The results, therefore, represent rather experienced learners. However, students learn the basics of chemical energetics early in high school chemistry. It would be desirable to analyse which factors in learning chemistry influence the activation of different resources. This study does not allow to distinguish to what extent the connections between the mental resources originate from chemistry lessons, everyday logic, or other influences. The results of the questionnaires reported solely serve to describe the sample. They should not be used for any further quantitative analysis.Implications and conclusions
From these findings, several implications for research and teaching can be derived.A resource-based framework is helpful to analyse and utilise students’ ideas
Students activate diverse resources to solve problems rather than drawing on a coherent concept (Hammer et al., 2005; diSessa, 2018). This perspective may be productive for practitioners as well as for researchers.For teachers, it can be helpful to be aware of the rich and diverse character of students’ ideas. They provide the starting point for further elaboration on the network structure of students’ thinking. For example, students predominantly connect added energy and particle speed (Macrie-Shuck and Talanquer, 2020). This idea is scientifically correct – however, it did not prove productive for many groups to explain activation energy because they did not consider the structure of the involved particles. Nevertheless, the resource of particle speed is a suitable starting point to further elaborate on activation energy. The simple question “But why do particles need this energy in a collision?” helped many students in the sample to activate resources on bond-breaking. Hence, this allowed them to strengthen the mental connection between energetics and chemical structure. This approach is even applicable to scientifically wrong arguments like the octet rule as the driving force of the reaction. As shown above, this perspective may enable students to understand that energy is needed to break bonds. The discussion of the octet rule as a description of energetically favourable states - rather than as a cause itself – may allow new mental connections to be made.
For researchers, the orientation towards this framework helps to better describe students’ ideas and learning (Podschuweit and Bernholt, 2020; Hunter et al., 2022). The present work focuses on the analysis and description of student resources. For future research, it would be interesting to analyse, how far these resources are coherently structured or context-dependent. Macrie-Shuck and Talanquer (2020), for example, characterised students’ ideas on energy transfer “as alternative constructions generated ‘on the fly’ to make sense of the phenomena under analysis” rather than as “stable alternative conceptions”. A re-analysis of the data material from this interview study, focussing on individual students’ arguments, could provide further insights into the extent to which students’ concepts are coherently activated throughout the different guiding questions.
The perception of the fluid nature of students’ conception requires new approaches to analyse learning. diSessa (2014b) proposes a detailed analysis of learning processes. Therefore, the various resources students activate have to be analysed in more detail. Due to the high context dependency of the activated resources, this demands a multitude of studies in diverse settings. Subsequently, “microgenetic studies” on learning processes should be conducted to make processes of conceptual change visible. The positive experiences using focus groups in this study may inspire other study designs.
The level of a sub-microscopic system and the level of molecular structures should be clearly distinguished
The Johnstone triangle (Johnstone, 1991) is one of the most central and helpful tools for teaching chemistry. The distinction of the macro-, micro- and sub-microscopic level helps to analyse students’ thinking and problems and to plan meaningful learning arrangements. However, Taber (2013) describes that the Johnstone triangle is not used in a “canonical form”. The results herein illustrate how an unspecific reference to the sub-microscopic level may hinder productive learning. The conflation of the level of a sub-microscopic system with the level of molecular structure implies problematic ideas, e.g. on activation energy or RCDs. For teaching chemistry, it may thus be necessary to clearly distinguish whether terms, representations and concepts refer to a sub-microscopic system or to molecular structures. This becomes especially prominent for the example of the term activation energy that is solely meaningful on the level of specific molecules and their structures. It must be clearly distinguished from added energy (macroscopic level) that leads to the acceleration of particles in a sub-microscopic system. Phrases like “The activation energy is added” should be strictly omitted. Also, RCDs describe energy changes for the manipulation of structural properties of specific particles. Teachers should directly problematise the prevalent interpretation of RCDs on a sub-microscopic system level. In this regard, it may be helpful to introduce the reaction coordinate for a simple elementary reaction as a function of bond lengths.This clear distinction may help students to improve their “systems thinking”. Orgill et al. (2019) describe that the basis of systems thinking is “visualizing the interconnections and relationships between the parts in the system” before “examining how systems-level phenomena emerge from interactions between the system's parts”. Results like this study might suggest that a prerequisite for systems thinking is students’ awareness of different levels of consideration (parts vs. systems). For future research, it may be interesting to analyze to what extent high school students can distinguish between these different levels.
Teaching and learning chemistry in high schools should emphasise the connections between chemical reactions, energy and structure
As outlined in the introduction, the energy of the emerging structures controls chemical reactions (see Fig. 1). The findings herein indicate a significant lack of these connections in students’ mental networks: Students rarely refer to the minimisation of energy to reason about the driving force of reactions. Questions on the energy of reaction do not seem to primarily activate resources on chemical structures. Activation energy does not immediately activate resources on bond-breaking. The different bond energies of reactants and products rarely explain energy changes in chemical reactions. These examples indicate that the core idea of energy is poorly connected to other chemical core concepts.Researchers have been demanding a novel teaching approach for chemical bonding: traditionally, the introduction of chemical bonds focuses on different bonding “types”. Nahum et al. (2007) propose to rely on attractive and repulsive electrostatic forces to rationalise chemical bonds. This approach has already been implemented successfully, e.g. in middle schools (Joki et al., 2015). The results herein urge for the implementation of these perspectives also in the broader context of energy in chemistry education. Quantum mechanics provide the contemporary scientific basis for these views (Nahum et al., 2007; Seeman and Tantillo, 2022). The definition of chemistry given by Jensen (2007) may be translated into high school-suited core ideas in agreement with other suggestions (Nahum et al., 2007; National Research Council, 2012; Cooper and Klymkowsky, 2013), as follows:
“Chemistry is knowing the energy…”
• Chemical processes (at constant temperature and pressure) happen spontaneously if the Gibbs energy of the system is minimised (Atkins et al., 2022). For pedagogical reasons, the umbrella term “energy” may arguably be used to introduce a simplified version of this concept. Nevertheless, the fundamental role of entropy should not be neglected in high school chemistry.
• A chemical reaction is a rearrangement of atoms to minimise the potential energy of a system (Cooper and Klymkowsky, 2013).
“…the energy as a function of nuclear coordinates.” (Jensen, 2007)
• All atoms§ interact through attractive and repulsive electrostatic forces; thus, each spatial arrangement of atoms corresponds to a well-defined potential energy (Israelachvili, 2011; National Research Council, 2012; Holme et al., 2015).
• In a chemical bond, attractive and repulsive forces are in equilibrium; chemical bonds describe the distance between two nuclei with minimal potential energy (Nahum et al., 2007).
• In molecules (or other stable chemical compounds) attractive and repulsive forces are in equilibrium; molecules are spatial arrangements of atoms with a local minimum of potential energy.
While these principles are already well-known in parts of the chemistry education research literature, the results of this study urge for further research how these principles can be better understood by high school students. This may be realised through “design-based studies” (Barab and Squire, 2004; diSessa, 2014b). Therefore, new approaches and techniques to teach and learn the presented core ideas about energy need to be developed, including innovative teaching methods, appropriate experiments, animations or quantum mechanics-based learning approaches. Such interventions should be situated in a variety of contexts. Studying the learning processes with these tools in detail may provide new insights into how students activate and refine their mental resources.
The results of this study highlight the potential of students’ mental resources on energy as a foundation for further learning. At the same time, they also uncover weakly developed connections, especially between energy changes and structural changes, and regarding the fundamental principle of energy minimisation. A more explicit focus on these connections in chemistry teaching may help students to activate even more targeted productive resources to reason about chemical reactions.
Conflicts of interest
There are no conflicts of interest to declare.Appendices
Appendix 1. Full interview guide
Work assignment for the focus groups (translated to English):| Student statement | Prompt |
|---|---|
| □ “Energy” | □ You mentioned the terms __________________ Can you explain what you mean by that? |
| □ “Reaction” | □ How do these terms relate to each other? |
| □ No energy diagram drawn | □ In school, we often draw energy diagrams for exothermic reactions. Can you explain this to me? |
| □ Hint: They kind of look like a mountain and valley (drawing in the air). Do you know what I mean? | |
| □ 3. Hint: (present unlabelled diagram) This is what I mean. But I think the labels are still missing. | |
| □ Incomplete energy diagram | □ What is plotted on the axes? |
| □ And what does this represent (pointing to reactants/products/peaks)? | |
| □ Once energy diagram is complete | □ What is this drawing supposed to say? |
| □ Can you also draw the particles from the reaction equation in the diagram? | |
| □ (point to any point on the declining curve) Does this point then also stand for anything? | |
| □ Term activation energy | □ What exactly do I need the activation energy for in this reaction? |
| □ What do the reactants need activation energy for? | |
| □ Activation energy is needed to break the bond | □ Why does it cost energy to split a bond? |
| □ What actually holds the atoms together in such a bond? | |
| □ Slowly: If two chlorine atoms are really far away from each other and I bring them together. How does the energy change then? | |
| □ Energy is released | □ Energy can't just be created like that. Where was the energy before the reaction? |
| □ Reaction is exothermic | □ But why does the energy change at all if I connect the atoms differently? |
| □ “Inner energy” | □ What do you mean by the term ___________ energy? (Take up students’ term) |
| □ “Chemical energy” | □ Why do reactants and product have different ___________ energies at all? |
| □ What does it depend on how much energy a particular compound has? | |
| □ More stable product | □ What should we understand by the stability of a compound? |
| □ Stability of the compounds | □ How is stability related to energy? |
| □ Why is HCl more stable than the reactants? | |
| □ Octet rule | □ What is the advantage of such an electron octet? |
| □ Full outer shell | □ But both the reactants have a full outer shell. Why is still energy released during the reaction? |
| □ Particles “want”, “prefer” or similar | □ An atom or molecule does not really have a will. What do you mean by “it wants”? |
| □ What does it depend on whether and how the particles rearrange in a reaction? | |
“Observe the reaction shown in the video. (Hint: It is the following reaction: H 2 + Cl2 → 2 HCl.)
A tutor student (10th grade) performed the experiment you just saw in the video in his chemistry lesson. He did not understand anything at all and asks you to explain what happened during the reaction. The following questions are of particular interest to him:
(a) What is the spark at the beginning of the reaction for?
(b) Why does the reaction mixture turn hot?
(c) Why does a reaction occur at all?
You have about 10 minutes to prepare a suitable explanation for the tutor. Discuss as a team how you would answer the questions. Try to explain as detailed as possible and include graphic elements. You can start with the explanation as soon as you are done with the preparation.”
Appendix 2. Category system of the qualitative data analysis
| Category | Anchoring example | Number of focus groups |
|---|---|---|
| a Two groups (Turquoise and Brown) mentioned the term “catalyst” once and disjointed of their argumentation. They were omitted in Fig. 4. b For clarity, these connections are mainly omitted in Fig. 4. | ||
| The function of the spark | ||
| term “activation energy” | This spark at the beginning that is the activation energy. (Green) | 14 |
| starts reaction | One could see that the reaction only takes place afterwards. That means you need it so that the reaction takes place at all. (Gold) | 9 |
| overcoming thresholds | You have to think of it as a threshold that has to be crossed and then it happens by itself. (Grey) | 4 |
| provides energy for the reaction | And the activation energy is necessary for a reaction to take place. Yes, exactly. And that's why you have the spark, because it brings in the necessary energy to make this curve swing upwards, so that the reaction then runs on its own. (Salmon) | 8 |
| accelerates reaction | And if a reaction is also voluntary, but just much too slow, it is still possible that you first have to add energy. (Cyan) | 7 |
| catalyses reaction | Yes, maybe somehow this spark serves as a catalyst, so that it doesn’t need so much energy. That the reaction takes place or something, I don’t know. (Plum) | 5a |
| accelerates particles | For example, in the form of heat, I can imagine that the warmer a system is, the faster the particles move. And you need a certain speed and orientation so that when they meet, they react with each other. Maybe it's simply this. In the form of activation energy. That you practically have enough energy when the particles meet that they then really react with each other. (Cyan) | 10 |
| bond-making and breaking | Maybe they are somehow mixed up or something, and then there is room again for the Cl 2 to combine with the H 2 , for example. (Magenta) | 4 |
| bond-breaking | So, you practically have these bonds and then you add energy, the activation energy. That is, […] these bonds […] practically oscillate too much, that is, the bonds break, that is, I then have four atoms so to say. (Salmon) | 8 |
| overcoming stable states | Oh, yes, that the substances react with each other is because they are chemically stable or something, and then they don’t actually want to react with each other, and then you have to force them to react, and then you add energy. (Plum) | 5 |
| reaching of a transition state | There is practically a transition state here and this transition state must be overcome so that I can get to my products later. And in order to achieve this and to have enough energy, the two particles, the Cl and the H atom, need to collide in such a way that I reach the transition state. (Yellow) | 2 |
| other | Comprises arguments such as enables electron flow; increases pressure; checking apparatus; mixing of reactants; exothermic reactions always need activation energy; makes hydrogen chloride explode; or no idea. | 6b |
| The heat origins from | ||
| added energy | Erm, yes, so the energy was released with the spark, with electricity, I suppose. And that was only generated by pressing this button. (Red) | 3 |
| released energy | From then on, energy is released in the form of heat. (Purple) | 15 |
| exothermic reaction | Yes, the reaction mixture becomes hot because it is an exothermic reaction […]. That's why it gets hot. (Grey) | 11 |
| particle movement | Well, um, something gets hot at the particle level, as far as I know, when particles are moving very fast. (Turquoise) | 4 |
| the reactants | Um maybe in the reactants somehow? But maybe in a different state? (Black) | 5 |
| Only arguments that did not mention inner, potential or chemical energy. | ||
| inner energy of the reactants | It was stored chemically in the substances. So chemical energy. (Purple) | 4 |
| the formation of the product | So, you can practically assign an energetic potential to a particle. And the particles, practically hydrogen and chlorine, have a higher energetic potential than hydrogen chloride. […] (Lime) | 5 |
| bond-making | Yes, the fact that they connect somehow creates energy. (Magenta) | 4 |
| bond-breaking | Well, I would say the bonds, um, when they are broken, then energy is simply released. (Lime) | 4 |
| A reaction occurs at all due to | ||
| pressure | Yes, exactly (laughter). The two gases each have um, free-floating particles that are not bound to each other. And because they come into this syringe, […] they are pressed together in such a way that they rub against each other, and this could of course also cause the heat. And then, because they have this attraction and it becomes too tight, the whole thing explodes, so that the particles can float around freely again and no longer have to be in a confined space. (Red) | 3 |
| specific properties of reactants | And the reaction takes place because the hydrogen is combustible, I think. (Red) | 7 |
| Other mentioned properties comprise “explosive” and “reactive” | ||
| added energy | And, why the reaction takes place at all is because a spark is added to this gas. And only then can it start. So, if the gas would just lie there in its pure form, gaseous, then nothing would happen. (Brown) | 5 |
| the octet rule | The third task, why the reaction takes place in the first place, is that if you look at the periodic table, you can see from the main groups how many electrons the respective […] element has. And in the case of the reaction, that would be one valence electron for hydrogen and seven valence electrons for chlorine, and reactions take place more frequently when the reacting substances together have eight electrons in the outermost shell. The reason for this is simply that eight electrons are very stable […] for the compound and that is what we have here. Seven and one add up to eight. And that is exactly why HCl is more stable than the starting materials. (Purple) | 11 |
| striving for stability | This means that two relatively unstable substances react to form a more stable one. (Purple) | 2 |
| energetically favourable state | I would actually justify it again with the energetically more favourable position. You simply say that all molecules always strive for the most energetically favourable state possible. In this case, it is clearly more favourable in the form of hydrogen chloride. (Lime) | 4 |
| free enthalpy | 2: No, not entropy, enthalpy. […] | 4 |
| 3: Free enthalpy. […] With the Gibbs-Helmholtz equation? (laughs) […] | ||
| 1: The reaction is exergonic. | ||
| 2: Yes, no, then just give him the table and say: Here, look at it. (Lime) | ||
| Comprises argumentations on exergonic reactions or on entropy and enthalpy, even if term “free enthalpy” is not mentioned. | ||
| properties of particles | You have a minimal charge difference. And the charge difference is then sufficient so that, like a magnet, plus and minus […] attract each other, so that they also attract each other. (Orange) | 5 |
| Other properties mentioned were electrophilicity, polarity, and oxidation state | ||
| properties of bonds | And maybe also because that's a, I don't know, um, a practical bond, a practical state for the molecule that we//like that/yes.//(Gold) | 3 |
Author contributions
Benjamin Pölloth: conceptualisation, investigation, methodology, formal analysis (lead), funding acquisition, project administration, writing – original draft preparation. Dominik Diekemper: investigation, formal analysis (supporting), writing – review & editing; Stefan Schwarzer: supervision, funding acquisition, writing – review & editing.Acknowledgements
We thank all students participating in the empirical study, the FCI (Fonds der Chemischen Industrie) for financial support and Alexander Fritz and Malín Moya Llasat for the transcription of the interviews.Notes and references
- Abell T. N. and Bretz S. L., (2018), Dissolving Salts in Water: Students’ Particulate Explanations of Temperature Changes, J. Chem. Educ., 95(4), 504–511.
- Andrade V., Shwartz Y., Freire S. and Baptista M., (2022), Students' mechanistic reasoning in practice: Enabling functions of drawing, gestures and talk, Sci. Ed., 106(1), 199–225.
- Atkins P. W., de Paula J. and Keeler J. J., (2022), Physikalische Chemie, Weinheim: Wiley-VCH.
- Atkinson M. B. and Bretz S. L., (2021), Measuring Changes in Undergraduate Chemistry Students’ Reasoning with Reaction Coordinate Diagrams: A Longitudinal, Multi-institution Study, J. Chem. Educ., 98(4), 1064–1076.
- Atkinson M. B., Popova M., Croisant M., Reed D. J. and Bretz S. L., (2020), Development of the Reaction Coordinate Diagram Inventory: Measuring Student Thinking and Confidence, J. Chem. Educ., 97(7), 1841–1851.
- Atkinson M. B., Croisant M. and Bretz S. L., (2021), Investigating first-year undergraduate chemistry students’ reasoning with reaction coordinate diagrams when choosing among particulate-level reaction mechanisms, Chem. Educ. Res. Pract., 22(1), 199–213.
- Barab S. and Squire K., (2004), Design-Based Research: Putting a Stake in the Ground, J. Learn. Sci., 13(1), 1–14.
- Barke H.-D., (2006), Chemiedidaktik: Diagnose und Korrektur von Schülervorstellungen, Berlin, Heidelberg: Springer.
- Barton D. H. R., (1996), Ingold, Robinson, Winstein, Woodward, and I, Bull. Hist. Chem., 19, 43–47.
- Becker N. M. and Cooper M. M., (2014), College chemistry students' understanding of potential energy in the context of atomic-molecular interactions, J. Res. Sci. Teach., 51(6), 789–808.
- Bernard H. R., (2013), Social research methods: Qualitative and quantitative methods, Thousand Oaks, Calif.: SAGE Publications.
- Bernholt S., Höft L. and Parchmann I., (2020), Die Entwicklung fachlicher Basiskonzepte im Chemieunterricht – Findet ein kumulativer Aufbau im Kompetenzbereich Fachwissen statt? [Central scientific concepts in chemistry education—Does a cumulative development of conceptual competences take place?], Unterrichtswiss, 48(1), 35–59.
- Bodner G. M., (1986), Constructivism: A theory of knowledge, J. Chem. Educ., 63(10), 873–878.
- Bruner J. S., (1995), On learning mathematics, Math. Teach., 88, 330–335.
- Budke M., (2019), Dissertation, Universität Osnabrück.
- Budke M., Parchmann I. and Beeken M., (2019), Empirical Study on the Effects of Stationary and Mobile Student Laboratories: How Successful Are Mobile Student Laboratories in Comparison to Stationary Ones at Universities?, J. Chem. Educ., 96(1), 12–24.
- Caspari I., Kranz D. and Graulich N., (2018), Resolving the complexity of organic chemistry students' reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19(4), 1117–1141.
- Cooper M. M., (2014), Evidence-based reform of teaching and learning, Anal. Bioanal. Chem., 406(1), 1–4.
- Cooper M. M. and Klymkowsky M. W., (2013), The trouble with chemical energy: why understanding bond energies requires an interdisciplinary systems approach, CBE Life Sci. Educ., 12(2), 306–312.
- Cooper M. M., Posey L. A. and Underwood S. M., (2017), Core Ideas and Topics: Building Up or Drilling Down?, J. Chem. Educ., 94(5), 541–548.
- Crandell O. M., Kouyoumdjian H., Underwood S. M. and Cooper M. M., (2019), Reasoning about Reactions in Organic Chemistry: Starting It in General Chemistry, J. Chem. Educ., 96(2), 213–226.
- Crandell O. M., Lockhart M. A. and Cooper M. M., (2020), Arrows on the Page Are Not a Good Gauge: Evidence for the Importance of Causal Mechanistic Explanations about Nucleophilic Substitution in Organic Chemistry, J. Chem. Educ., 97(2), 313–327.
- diSessa A. A., (2014a), A History of Conceptual Change Research, in Sawyer R. K. (ed.), The Cambridge Handbook of the Learning Sciences, Cambridge: Cambridge University Press, pp. 88–108.
- diSessa A. A., (2014b), The construction of causal schemes: learning mechanisms at the knowledge level, Cogn. Sci., 38(5), 795–850.
- diSessa A. A., (2018), A Friendly Introduction to “Knowledge in Pieces”: Modeling Types of Knowledge and Their Roles in Learning, in Kaiser G., Forgasz H., Graven M., Kuzniak A., Simmt E. and Xu B. (ed.), Invited Lectures from the 13th International Congress on Mathematical Education, Cham: Springer International Publishing, pp. 65–84.
- Dresing T. and Pehl T. (ed.), (2018), Praxisbuch Interview, Transkription & Analyse: Anleitungen und Regelsysteme für qualitativ Forschende, Marburg: Eigenverlag.
- Eckhard J., Rodemer M., Bernholt S. and Graulich N., (2022), What Do University Students Truly Learn When Watching Tutorial Videos in Organic Chemistry? An Exploratory Study Focusing on Mechanistic Reasoning, J. Chem. Educ., 99(6), 2231–2244.
- Engeln K., (2004), Schülerlabors: authentische, aktivierende Lernumgebungen als Möglichkeit, Interesse an Naturwissenschaften und Technik zu wecken, Berlin, Kiel: Logos.
- Eschmann C., Song L. and Schreiner P. R., (2021), London Dispersion Interactions Rather than Steric Hindrance Determine the Enantioselectivity of the Corey-Bakshi-Shibata Reduction, Angew. Chem., Int. Ed., 60(9), 4823–4832.
- Galley W. C., (2004), Exothermic Bond Breaking: A Persistent Misconception, J. Chem. Educ., 81(4), 523–525.
- Goodwin W., (2007), Scientific Understanding after the Ingold Revolution in Organic Chemistry, Philos. Sci., 74(3), 386–408.
- Graulich N. and Caspari I., Bridging the Gap Between Philosophy of Science and Student Mechanistic Reasoning, (2019), in Schultz M., Schmid S. and Lawrie G. A. (ed.), Research and Practice in Chemistry Education, Singapore: Springer Singapore, pp. 109–121.
- Greenbowe T. and Meltzer D., (2003), Student learning of thermochemical concepts in the context of solution calorimetry, Int. J. Sci. Educ., 25(7), 779–800.
- Gropengießer H. and Marohn A., (2018), Schülervorstellungen und Conceptual Change, in Krüger D., Parchmann I. and Schecker H. (ed.), Theorien in der naturwissenschaftsdidaktischen Forschung, Berlin, Heidelberg: Springer, pp. 49–67.
- Gulacar O., Vernoy B., Tran E., Wu A., Huie E. Z., Santos E. V., Wadhwa A., Sathe R. and Milkey A., (2022), Investigating Differences in Experts’ Chemistry Knowledge Structures and Comparing Them to Those of General Chemistry Students, J. Chem. Educ., 99(8), 2950–2963.
- Hammer D., Elby A., Scherr R. E. and Redish E. F., (2005), Resources, framing, and transfer, in Mestre J. (ed.), Transfer of Learning from a Modern Multidisciplinary Perspective, Greenwich, CT: Information Age Publishing, pp. 89–120.
- Heeg J., Bittorf R. M. and Schanze S., (2021), Erforschung potenzieller Entwicklungsverläufe diagnostischer Fähigkeiten angehender Chemielehrkräfte hinsichtlich Lernendenvorstellungen – Die Bedeutung individueller Vorstellungen über Lernendenvorstellungen [Investigating Possible Development Processes of Preservice Chemistry Teachers’ Diagnostic Skills Regarding Learners’ Conceptions—The Influence of Individual Conceptions About Learners’ Conceptions], ZfDN, (27), 17–44.
- Holme T., Luxford C. and Murphy K., (2015), Updating the General Chemistry Anchoring Concepts Content Map, J. Chem. Educ., 92(6), 1115–1116.
- Hughes E. D., Ingold C. K. and Shapiro U. G., (1936), 50. Mechanism of substitution at a saturated carbon atom. part VI. hydrolysis of iso propyl bromide, J. Chem. Soc., 225–236.
- Hunter K. H., Rodriguez J.-M. G. and Becker N. M., (2022), A Review of Research on the Teaching and Learning of Chemical Bonding, J. Chem. Educ., 99(7), 2451–2464.
- Israelachvili J. N., (2011), Intermolecular and surface forces, Amsterdam, Boston, Paris: Elsevier.
- Jensen F., (2007), Introduction to computational chemistry, Chichester England, Hoboken NJ: John Wiley & Sons.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comp. Assist. Learn., 7(2), 75–83.
- Joki J., Lavonen J., Juuti K. and Aksela M., (2015), Coulombic interaction in Finnish middle school chemistry: a systemic perspective on students' conceptual structure of chemical bonding, Chem. Educ. Res. Pract., 16(4), 901–917.
- Kaliakin D. S., Zaari R. R. and Varganov S. A., (2015), 3D Printed Potential and Free Energy Surfaces for Teaching Fundamental Concepts in Physical Chemistry, J. Chem. Educ., 92(12), 2106–2112.
- Korstjens I. and Moser A., (2018), Series: Practical guidance to qualitative research. Part 4: Trustworthiness and publishing, Eur. J. General Pract., 24(1), 120–124.
- Kuckartz U., (2016), Qualitative Inhaltsanalyse. Methoden, Praxis, Computerunterstützung, Weinheim: Beltz.
- Kultusministerkonferenz, (2004), Bildungsstandards im Fach Chemie für den Mittleren Schulabschluss.
- Kultusministerkonferenz, (2020), Bildungsstandards im Fach Chemie für die Allgemeine Hochschulreife.
- Lamichhane R., Reck C. and Maltese A. V., (2018), Undergraduate chemistry students’ misconceptions about reaction coordinate diagrams, Chem. Educ. Res. Pract., 19(3), 834–845.
- Macrie-Shuck M. and Talanquer V., (2020), Exploring Students’ Explanations of Energy Transfer and Transformation, J. Chem. Educ., 97(12), 4225–4234.
- Maskill H. (ed.), (2006), The Investigation of Organic Reactions and Their Mechanisms, Oxford, UK: Blackwell Publishing Ltd.
- Mey G. and Mruck K. (ed.), (2020), Handbuch Qualitative Forschung in der Psychologie, Wiesbaden: Springer Fachmedien Wiesbaden.
- Ministerium für Kultus, Jugend und Sport Baden-Württemberg, Bildungsplan des Gymnasiums Chemie (überarbeitet 2022), 2022.
- Minshall B. L. and Yezierski E. J., (2021), Data-driven activity reform: employing design research to improve scaffolding and concept development, Chem. Educ. Res. Pract., 22(1), 136–145.
- Nahum T. L., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a new teaching approach for the chemical bonding concept aligned with current scientific and pedagogical knowledge, Sci. Ed., 91(4), 579–603.
- National Research Council, (2012), A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas, Washington, D.C.: National Academies Press.
- Neese F., Atanasov M., Bistoni G., Maganas D. and Ye S., (2019), Chemistry and Quantum Mechanics in 2019: Give Us Insight and Numbers, J. Am. Chem. Soc., 141(7), 2814–2824.
- Neumann K., Viering T., Boone W. J. and Fischer H. E., (2013), Towards a learning progression of energy, J. Res. Sci. Teach., 50(2), 162–188.
- Opitz S. T., Neumann K., Bernholt S. and Harms U., (2019), Students’ Energy Understanding Across Biology, Chemistry, and Physics Contexts, Res. Sci. Educ., 49(2), 521–541.
- Orgill M., York S. and MacKellar J., (2019), Introduction to Systems Thinking for the Chemistry Education Community, J. Chem. Educ., 96(12), 2720–2729.
- Park M. and Liu X., (2016), Assessing Understanding of the Energy Concept in Different Science Disciplines, Sci. Educ., 100(3), 483–516.
- Pawek C., (2009), Dissertation, Christian-Albrechts-Universität Kiel.
- Perrin C. L., Agranat I., Bagno A., Braslavsky S. E., Fernandes P. A., Gal J.-F., Lloyd-Jones G. C., Mayr H., Murdoch J. R., Nudelman N. S., Radom L., Rappoport Z., Ruasse M.-F., Siehl H.-U., Takeuchi Y., Tidwell T. T., Uggerud E. and Williams I. H., (2022), Glossary of terms used in physical organic chemistry (IUPAC Recommendations 2021), Pure Appl. Chem., 94(4), 353–534.
- Podschuweit S. and Bernholt S., (2020), Investigating Network Coherence to Assess Students’ Conceptual Understanding of Energy, Educ. Sci., 10(4), 103.
- Pölloth B., Häfner M. and Schwarzer S., (2022), Gleichzeitig oder nacheinander? – Mit historischen Einsichten und Experimenten Reaktionswege der nukleophilen Substitution entdecken [At the same time or one after the other? – Exploring reaction paths of nucleophilic substitution reactions using historic insights and experiments], CHEMKON, 29(2), 77–83.
- Pölloth B., Sibi M. P. and Zipse H., (2021), The Size-Accelerated Kinetic Resolution of Secondary Alcohols, Angew. Chem., Int. Ed., 60(2), 774–778.
- Popova M. and Bretz S. L., (2018a), “It's Only the Major Product That We Care About in Organic Chemistry”: An Analysis of Students’ Annotations of Reaction Coordinate Diagrams, J. Chem. Educ., 95(7), 1086–1093.
- Popova M. and Bretz S. L., (2018b), Organic chemistry students’ challenges with coherence formation between reactions and reaction coordinate diagrams, Chem. Educ. Res. Pract., 19(3), 732–745.
- Popova M. and Bretz S. L., (2018c), Organic chemistry students’ interpretations of the surface features of reaction coordinate diagrams, Chem. Educ. Res. Pract., 19(3), 919–931.
- Przyborski A. and Riegler J., (2020), Gruppendiskussion und Fokusgruppen, in Mey G. and Mruck K. (ed.), Handbuch Qualitative Forschung in der Psychologie, Wiesbaden: Springer Fachmedien Wiesbaden, pp. 396–408.
- Ridd J. H., (2008), Organic pioneer, Chem. World, 50–53.
- Rösel S. and Schreiner P. R., (2022), Computational Chemistry as a Conceptual Game Changer: Understanding the Role of London Dispersion in Hexaphenylethane Derivatives (Gomberg Systems), Isr. J. Chem., 62(1–2), e202200002.
- Saltzman M. D., (1986), The development of physical organic chemistry in the United States and the United Kingdom: 1919-1939, parallels and contrasts, J. Chem. Educ., 63(7), 588–593.
- Seeman J. I. and Tantillo D. J., (2022), Understanding chemistry: from “heuristic (soft) explanations and reasoning by analogy” to “quantum chemistry”, Chem. Sci., 13(39), 11461–11486.
- Streller S., Bolte C., Dietz D. and La Noto Diega R., (2019), Chemiedidaktik an Fallbeispielen, Berlin, Heidelberg: Springer.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- VERBI, (2022), MAXQDA, Berlin: Consult. Sozialforschung GmbH.
- Vinner S., (1997), The Pseudo-Conceptual and the Pseudo-Analytical Thought Processes in Mathematics Learning, Educ. Stud. Math., 34, 97–129.
- Zohar A. R. and Levy S. T., (2019), Students' reasoning about chemical bonding: The lacuna of repulsion, J. Res. Sci. Teach., 56(7), 881–904.
Footnotes |
| † While it is scientifically more appropriate to distinguish between energy, enthalpy, free enthalpy and Gibb's energy, we chose to use the term “energy” as an umbrella term throughout the manuscript. This may be rationalised by the knowledge of high school students attending this study. |
| ‡ It should be noted that in the case of the radical chain reaction investigated herein, it may be appropriate to talk about the start of a chain reaction even on the systems’ level. The activation energy for the homolytic bond-breaking is too high to happen without adding energy at standard conditions. |
| § As Jensen (2007) outlines, due to the Born-Oppenheimer approximation, “the word nucleus and atom are often used interchangeably”. However, one should be aware that an atom in a molecule is not well-defined. For high school purposes, it may be appropriate to simply refer to atoms. |
| This journal is © The Royal Society of Chemistry 2023 |



