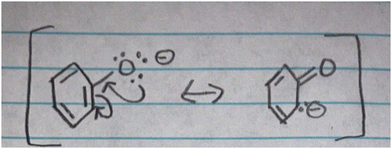Organic chemistry students’ use of stability in mental models on acid and base strength†
Betül
Demirdöğen
 *a,
Isaiah
Nelsen
*a,
Isaiah
Nelsen
 b and
Scott E.
Lewis
b and
Scott E.
Lewis
 b
b
aZonguldak Bülent Ecevit University, Ereğli College of Education, Department of Mathematics and Science Education, Zonguldak, Turkey. E-mail: betuldemirdogen@gmail.com
bDepartment of Chemistry, University of South Florida, USA
First published on 2nd June 2023
Abstract
The Brønsted–Lowry acid–base model is fundamental when discussing acid and base strength in organic chemistry as many of the reactions include a competing proton transfer reaction. This model requires evaluating chemical stability via a consideration of electronic granularity. The purpose of this study is to identify students’ mental models on acid and base strength in terms of granularity and stability. Fourteen students enrolled in organic chemistry participated in this case study. Data were collected through semi-structured interviews including total case comparison tasks on stability, acidity, and basicity. Analysis of data revealed that there were four groups of students differentiated by their reasoning: (1) acid and base strength through structure without association to stability, (2) acid and base strength through electronics without association to stability, (3) acid strength associated with electronically centered stability, and (4) acid and base strength associated with electronically centered stability. This characterization can support teaching and research to promote reasoning that leads to a more consistent mental model across acid and base strength.
Introduction
The chemistry of acids and bases is foundational in understanding much of the content in organic chemistry (Boothe et al., 2023) such as methods of activation, organic synthesis, and classes of reactions (American Chemical Society, 2015; Stoyanovich, Gandhi and Flynn, 2015) and in performing well at related items in exams (Raker et al., 2013). In line with this focus on the chemistry of acids and bases in curricular documents regarding organic chemistry, empirical research on students’ prediction of reaction products and reasoning on mechanism problems also indicated that a working knowledge of acid–base chemistry is essential (Bhattacharyya and Bodner, 2005; Anderson and Bodner, 2008; Grove et al., 2012; Bhattacharyya, 2014; Graulich, 2015).The centrality of acid–base chemistry in organic chemistry directed researchers to describe essential learning outcomes for acids and bases (Stoyanovich et al., 2015). This study identified twenty-five organic (e.g., SN1 and aldol condensation) and three biochemical reactions (e.g., Krebs cycle) that necessitate a Brønsted–Lowry acid–base reaction step at some point. Three-quarters of the reactions required identifying the most acidic or basic site in a molecule and one-third of the reactions required determining the stronger/weaker acid or base using relative base stabilities and relevant factors (e.g., resonance and inductive effects). That is, understanding acid and base strength is vital in reasoning about reaction mechanisms. However, students may have difficulties in enacting a scientific mental model of acid strength. They tend to relate acid strength with intrinsic (e.g., atom identity), explicit (e.g., number of specific atoms), and implicit (e.g., polarity) properties (Bhattacharyya, 2006; McClary and Talanquer, 2011a; McClary and Bretz, 2012; Tümay, 2016; Shah et al., 2018). In addition, when determining the stability of conjugate bases, student responses were influenced by one factor as opposed to a series of related factors (Bhattacharyya, 2006; McClary and Talanquer, 2011a; McClary and Bretz, 2012; Tümay, 2016; Shah et al., 2018). Despite the emphasis researchers have placed on understanding student knowledge on acid strength in organic chemistry, base strength has not been a specific topic of interest and has been researched only in tandem with acid strength (e.g., Cooper et al., 2016; Petterson et al., 2020). Considering the importance of acid and base strength in organic chemistry (Bhattacharyya and Bodner, 2005; Stoyanovich et al., 2015), research on how students conceptualize not only acid but also base strength can aid student success in determining reaction processes. Therefore, this study aimed to reveal students’ mental models on acid and base strength.
Mental models on acid and base strength
Mental modeling is a theory on knowledge organization through which researchers can “explain human cognitive processes of understanding reality, translating reality into internal representations” (Park and Gittelman, 1995, p. 303). The human cognitive system is able to construct mental models that are refined in order to interpret lived experiences (Coll and Treagust, 2003; Clement and Rea-Ramirez, 2008). When constructing a mental model for a system (i.e., real or imaginary situation, event or process), individuals generate mental entities, which represent their perception about the entities including established properties and relationships (Johnson-Laird, 1983; Nersessian, 2008). Individuals may exclude critical entities and their associated properties when forming mental models. When learning science, these characteristics of the mental model result in alternative explanations in relation to scientific knowledge, which can generate misconceptions (Coll and Treagust, 2003; Lin and Chiu, 2007). Although mental models can have limiting characteristics, they are functional to students allowing them to explain, predict, and reason when problem solving (Gentner, 2002). When functioning with mental models, students might retrieve the mental model from their long-term memory (i.e., permanent mental model) or generate a new method on the spot to solve the problem (i.e., temporal mental model) (van der Veer et al., 1999; Gentner, 2002; Vosniadou, 2002). That is, mental models are dynamic in nature and may change with accessible information through remembering (McClary and Talanquer, 2011a), stimulation by the features of the task (Osman and Stavy, 2006), and available implicit cognitive resources (e.g., prior knowledge and intuitive heuristics, Greca and Moreira, 2000). Examination and identification of students’ mental models requires elicitation—making this internal representation external. Elicited mental models (or expressed mental models) (Gilbert and Boulter, 1998) are the external representations of the corresponding mental model. Expressed mental models are accessible through action, speech, and writing (Gilbert et al., 2000). In this study, students’ mental models were identified through their speech and drawing for specific tasks, which were used to infer their reasoning on stability, acidity, and basicity (Gilbert et al., 2000). In doing so, we aimed to reveal students’ mental models on acid and base strength. Considering the existence of three scientific acid–base models (i.e., Arrhenius, Brønsted–Lowry, and Lewis), analysis of mental models unavoidably requires defining the context of the tasks (Tümay, 2016). The tasks in this study required students to compare relative acidity and basicity of organic compounds in water, stimulating them to consider the stability of bases and conjugate bases, two emergent properties in the Brønsted Lowry model.Review of the literature on students’ conceptualizations, mental models or alternative conceptions/misconceptions/difficulties on acid strength identified six categories of students considering their expressed mental models. Students in the first category retained an empirical definition of acid that relies on sour taste, red litmus paper, and pH, and they carried over their definition when predicting relative acid strength (Tümay, 2016). In the second category, acidity is viewed as an intrinsic property (McClary and Talanquer, 2011a, 2011b; McClary and Bretz, 2012; Bretz and McClary, 2015; Shah et al., 2018) where explicit features (i.e., structural) such as atom type (e.g., O) or functional groups (e.g., –COOH) are associated with acid strength. For students in the third category, acids are seen as hydrogen ion (H+) donors (Arrhenius model) when dissolved in water; hence acid strength is related to the degree of ionization influenced by bond strength and polarity (Bhattacharyya, 2006; Tümay, 2016). In the fourth category, students model substances that lose hydrogens or protons relying on intrinsic (e.g., atom identity), explicit (e.g., number of specific atoms), and implicit (e.g., polarity) properties (McClary and Talanquer, 2011a, 2011b). Students in the fifth category identify proton loss based on the stability of the conjugate base influenced by implicit properties (i.e., atom size, atom electronegativity, resonance/delocalization, inductive effect, and hybridization) (Brønsted–Lowry model) (Bhattacharyya, 2006; McClary and Talanquer, 2011a, 2011b; McClary and Bretz, 2012; Bretz and McClary, 2015; Tümay, 2016; Shah et al., 2018). In the sixth category, students focus on electron acceptance capacity, leading students to take into consideration the number of lone electron pairs or empty orbitals (Lewis model) (McClary and Talanquer, 2011a, 2011b). Mental models, however, are not always consistent and the features present in a task or the nature of the task can result in the existence of multiple mental models across tasks (McClary and Talanquer, 2011a). Our understanding on students’ mental models on acid strength has reached a certain level through the empirical evidence available in literature, which helps us to improve the instruction and to advance research on acid strength. However, our understanding on students’ mental models on base strength is very limited. Therefore, we intended to identify students’ mental models on acid and base strength in terms of granularity and stability.
As of late, granularity has been a relatively prominent term among chemistry education researchers to describe levels in activities and entities in chemical phenomena (Bodé et al., 2019; Deng and Flynn, 2021; Talanquer, 2022). Phenomenological, structural, electronic, and energetics are the fundamental granularity levels (Bodé et al., 2019; Deng and Flynn, 2021). However, different granularity exists depending on context and need (Machamer et al., 2000; Bodé et al., 2019; Deng and Flynn, 2021). The structural level includes descriptions of structural features of molecules and atoms (Deng and Flynn, 2021). For instance, when comparing the plausibility of alternative mechanisms for given reactants, steric hindrance and number of alpha-carbon substituents would be relative structural granularities in students’ explanations. In acid–base chemistry, atom size was proposed as structural granularity that students would enact in their reasoning (Deng and Flynn, 2021). The electronic level captures descriptions of electronic features of molecules and atoms (Deng and Flynn, 2021). For instance, electronegativity and formal charge could be utilized when explaining plausibility of alternative reaction mechanisms (Deng and Flynn, 2021) while delocalization and inductive effects are fundamental in acid–base chemistry. The energetic level includes descriptions of the energetics such as thermodynamic and kinetic considerations. For instance, the stabilities of conjugate acids/bases to reason about the direction of an acid–base equilibrium or activation energy to justify the plausibility of alternative reaction mechanisms are relevant energetic features. The phenomenological level captures descriptions of chemical phenomena, an emergent property, which is the result of entities including their properties and activities (Machamer et al., 2000) using structural, electronic, and energetic features. Within the given context, structural (i.e., size) and electronic (i.e., spreading of charge) features could be used to explain the phenomenon “stability”, which can then be utilized to predict other phenomena (i.e., acid and base strength).
Invoking stability in reasoning about acid and base strength
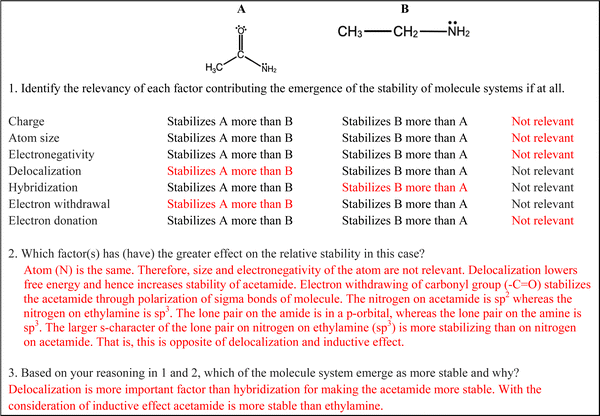
The Brønsted–Lowry model for acid and bases led to both the emergence of conjugate acid–base pair concept and the consideration of acid–base reactions as competing proton transfer reactions, which in turn influence acid strength (McClary and Talanquer, 2011a; Tümay, 2016). During an acid–base reaction in a solvent, the acid donates a proton in a forward reaction resulting in the formation of a conjugate base. The conjugate base accepts the proton in a backward reaction. In this competing proton transfer reaction; the relative stability of all species determines the direction and extent of this dynamic process, which form the phenomenon of acid and base strength. A strong acid readily donates its proton to a base in the forward reaction if the resulting negatively charged conjugate base is stable. The stability of the conjugate base is supported by low charge density, which is influenced by multiple factors. Electronegativity of the atom influences the degree to which a charge is localized. Atom size, resonance (delocalization), and electron withdrawal–donation (induction) affect spreading of charge. Hybridization (orbital) of the atom determines to what degree electrons are held closer to the nucleus. All factors should be considered concurrently when determining how factors contribute to the stability of the conjugate base. The more stable a conjugate base is, the weaker base it is and the stronger the originating acid.Considering the mechanism in an acid–base reaction in the Brønsted–Lowry model, stability is an important phenomenon that should be considered when determining not only acid strength but also base strength. From this perspective, both stability and acid–base strength are conceived as emergent in nature since they stem from entities of the substance, activities in the substance, and weighing all these entities and activities in the given task (McClary and Talanquer, 2011a; Tümay, 2016). In this study, we aimed to reveal to what degree students invoke stability in their expressed mental models when predicting acid and base strength. To increase the accessibility of the results, we will present how an idealized response should look like for one of the tasks (Acidity 1). This idealized response was also reviewed by two organic chemistry instructors at the setting. Please see ESI† for idealized responses for all the tasks. In Acidity 1, students were asked to compare the relative acidity of acetic acid (CH3COOH) and ethanol (CH3CH2OH) in water. An idealized student response should include the following reasoning:
• The conjugate bases of these acids are acetate ion (CH3COO−) and ethoxide ion (CH3CH2O−).
• Size and electronegativity of atom: atoms with negative charges are oxygen in both conjugate bases. Since atoms are the same therefore size and electronegativity are not relevant.
• Resonance/delocalization: spreading charge lowers free energy and increases stability for acetate ion, which in turn increases the acidity of acetic acid.
• Inductive effect: electron withdrawing of carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stabilizes the acetate ion through polarization of sigma bonds in the molecule. Therefore, induction increases stability for acetate ion, which in turn increases the acidity of acetic acid.
O) stabilizes the acetate ion through polarization of sigma bonds in the molecule. Therefore, induction increases stability for acetate ion, which in turn increases the acidity of acetic acid.
• Hybridization/orbital – The negatively charged oxygen on acetic acid is sp2 whereas the oxygen on ethanol is sp3. The larger s-character on the oxygen on acetate ion stabilizes the negative charge on the oxygen of acetate relative to the oxygen of ethoxide. Therefore, induction increases stability for acetate ion, which in turn increases the acidity of acetic acid.
Methodology
Research question
To explore students’ reasoning on acid and base strength, this study was guided by the following research question: What are students’ mental models on acid and base strength in terms of granularity and inclusiveness of stability?Research design
Given the tacit nature of mental models, this research is qualitative-interpretive (Marshall and Rossman, 2011). Among the qualitative strategies, case study guided the design, data collection, and data analysis of this study. Yin (2009) valued this type of research method when “a how or why question is being asked about a contemporary set of events, over which the investigator has little or no control” (p. 13). Since the researchers had no control on students’ use of mental model other than asking questions to reveal them, students’ reasoning process as a case provided in-depth information about characterization of students’ mental models on acid and base strength. Moreover, this case study was designed to expand the theories of mental model and granularity, which are the aims of case study (Yin, 2009). There are several types of case studies depending on the intent of the case analysis (Creswell, 2007; Yin, 2009). This case study is descriptive—the focus was to describe students’ reasoning on acid and base strength considering granularity and inclusiveness of stability.Participants
Fourteen students participated in the study at a large and research-intensive university in the southeastern U.S. in Fall 2022 after obtaining approval from the university's institutional review board. Seven students from each Organic Chemistry class (I and II) were recruited by announcement. We informed students orally and through the reading of a consent form about the purpose of research, their rights, and how the data are processed. The students then submitted their written consent forms. Pseudonyms were used to ensure confidentiality. Student quotes were edited to remove verbal utterances (e.g., um) and repeated phrases to improve clarity. Students’ participation was compensated with a 20 dollar gift card. Content coverage in the courses were determined via conversations with the course instructors and a review of course materials. Acids and bases are covered in Organic Chemistry I; more specifically, how the stability of a conjugate base relates to acid strength and factors that influence stability (e.g., type of atom, delocalization, induction, orbital). These ideas are also reviewed at the beginning of Organic Chemistry II. Organic Chemistry II emphasizes basicity of amines and requires utilization of acid strength in the context of reaction mechanism (ACS, 2015; Stoyanovich et al., 2015). Assessments include items that measured students’ abilities to compare acid strength, compare base strength, and use relative strength in predicting the position of acid–base equilibrium reactions. Students were interviewed right after their completion of learning and assessment of acid and base strength in both courses. Since students taking either of the two sequential Organic Chemistry classes had experience in learning concepts of stability, acidity, and basicity, they were the subjects of interest.Data collection
This study collected data using semi-structured interviews in the form of case comparison tasks. Case comparison tasks have been previously used to identify all relevant variables to the task (Alfieri et al., 2013) in chemistry education literature (e.g., Kranz et al., 2023). While preparing and organizing semi-structured interviews, we considered not only students’ learning experiences during organic chemistry classes but also fundamental concepts required to understand stability, acidity, and basicity. Each student was given six case comparison tasks presented in two tasks for each of the three concepts of interest in this study (i.e., stability, acidity, and basicity, Table 1). Each student was asked to compare the cases in terms of the given concept and provide an explanation for their answers. The cases were selected from the organic chemistry textbook used in the setting (Klein, 2017) and another available textbook (Solomons and Fryhle, 2012). The original study design was to investigate the students’ conceptions of resonance, so cases were selected where resonance was present. In addition, there was an attempt to select cases that were considered to be relatively familiar to students (e.g., acetamide vs. ethyl amine) and less familiar (e.g., propoxide vs. propene-1-olate) to gain more insight on students’ reasoning. An additional goal of this investigation was to examine the impact of representations on students’ explanations. Two different interview protocols (i.e., Protocol A and Protocol B) were used that had the same content and ordering of questions but differed in how resonance was represented. Students were randomly assigned to Protocol A or B. The impact of resonance representations will be the subject of a future investigation and not discussed further herein. Complete interview protocols can be found in the ESI.†| Tasks | Chemistry content | Representations at protocols | Task description used in this study | |
|---|---|---|---|---|
| A | B | |||
| Stability 1 | Phenolate vs. cyclohexanolate | Single Lewis | Single Lewis | Negatively charged ions |
| Acidity 1 | Acetic acid vs. ethanol | Resonance structures of conjugate base | Resonance hybrid structure of conjugate base | Conjugate base with resonance |
| Basicity 1 | Acetamide vs. ethyl amine | Resonance hybrid structure of base | Resonance hybrid structures of base | Base with resonance |
| Stability 2 | Allylic carbocation vs. non-allylic carbocation | Single Lewis structures | Single Lewis structures | Positively charged ions |
| Acidity 2 | Allylic hydrogen vs. non-allylic hydrogen on a substance | Single Lewis structure | Single Lewis structure | Acid with single Lewis structure |
| Basicity 2 | Propoxide vs. propene-1-olate | Single Lewis structures | Single Lewis structures | Base with single Lewis structures |
Data analysis
Interview questions included “How do you define stability and how do you use your definition to explain which one is more stable?” “How do you determine which substance is more acidic?” and “How does your answer relate to your definition of acid strength?” We focused on these parts of the interview transcriptions where students provide their reasoning at each case comparison task on stability, acidity, and basicity since mental models are meant to support understanding, reasoning, and prediction when solving problems (Gentner, 2002).We identified how students communicated their mental models that guided their reasoning on each task through their speech and drawings peculiar to the task. This helped us code their expressed mental models. Considering the nature of mental models, researchers coded students’ mental models on stability, acid strength, and base strength in an inductive manner. For instance, if students defined stability in relation to the degree to which electrons are delocalized and focused on this when reasoning which substance is more stable, we coded this mental model on stability as “delocalization”. Examples for coding the mental model at each task are presented in Table 2 and a detailed analysis is included in ESI.† Once the coding scheme was complete, two researchers independently coded each student's mental model at every case comparison task. All discrepancies in coding were discussed to reach a consensus code assignment.
| Task | Mental model | Excerpt |
|---|---|---|
| Stability | Delocalization | Resonance is basically how much room the electron could take up and the different positions that it could exist in to be stabilized (S10) |
| Identity | I think it's vynillic. But I guess my point is,…The carbon is it's next to a double bond…. So and I think that that increases stability…. I don't know why…. I just remember the fact. (S2) | |
| Octet | …the most stable one doesn't need more ions or it doesn't need more bonds or anything like that, we can find the way it is. (S4) | |
| Acidity | Stability of conjugate base | …the least stable base those are the most acidic structure. (S3) |
| Electronegative atom | …there are two oxygens on the ace acetate ion and then on the ethoxide ion, there's only one oxygen…. Oxygen is very electro negative, more electronegative than carbon, and there are more carbons in ethoxide than acetate. (S9) | |
| Polarity and octet | Acetic acid would be more acidic than the ethanol. This is because there's more oxygens and this is going back to like if you look at a periodic table and like how it is layed out versus like polarity and everything. Since there's more oxygens and then this one has more Hs, this one's going to be more acidic because it's just like, it's just like the difference in the polarity. That it's [referring to acetic acid], like, higher, I would think, because that, like, shows, it's, like, willing to take, like, stuff in order to fill, like those octet to make those charges. (S12) | |
| Basicity | Atom identity | If this [O in acetamide] takes an H somewhere, it's going to become an always making it more acidic, but we need more basic. I'm assuming this [ethyl amine] is basic (S14) |
| Stability of base | …the acetamide I think would be a weaker base because it's resonance stabilized rather than the ethyl amine, which is not resonance stabilized, making it a stronger base (S2) | |
| Neutralization/removing charge | From more basic I would say the propoxide because if you do resonance with the propane-1-olate, you can make it where that double bond will go to that oxygen and then to fulfill like it's charge. So there would be no there'd be no charge, whereas with the propoxide, there's nowhere to do resonance. So it will always have that negative charge. (S12) |
Another focus of this study is to reveal the granularity observed in students’ mental model on acid and base strength. Granularity levels were characterized as structural or electronic descriptions (Bodé et al., 2019; Deng and Flynn, 2021) as they are the most relevant levels in the phenomena under investigation and different granularity exists depending on context and need (Machamer et al., 2000; Bodé et al., 2019; Deng and Flynn, 2021). Electronic and structural granularity were defined inductively within the context of this study. Structural granularity refers to students’ descriptions of features of molecules, atoms, and ions (Deng and Flynn, 2021). In the context of acid and base strength, structural granularity differs from what is proposed in the literature since teaching of acid and base strength in organic chemistry heavily relies on stability (Stoyanovich et al., 2015). Moreover, reasoning about stability requires utilization of structural (e.g., lone pairs, charges, and connectivity of those) and electronic (e.g., delocalization and polarization) granularity levels. For instance, explicit structural features are utilized by students when asked to compare the stability of two ions of which one has the structure (i.e., allylic lone pair) that enables the delocalization of electrons using structural representations (i.e., Lewis). Based on inductive coding, structural granularity was defined as atom identity, atom count, functional group identity, bond type, bond count, electron count, charge, resonance specific representational features, and connectivity of atoms, ions, and molecules.
Electronic level granularity was also conceptualized with the context of this study in mind. We defined electronic granularity as electronic activities and the emergent properties relevant to the phenomenon (e.g., acid and base strength). When assessing electronic granularity, we focused on the instances where students refer to electronic activities in the ion or molecule and the resulting effect. Delocalization of electrons, spreading charge, electron withdrawal, electron donation, bearing charge, and polarization were the codes that emerged during coding for electronic granularity. Detailed information describing each code is presented in the ESI.† Granularity level was coded to consensus using the same procedures as described in mental models.
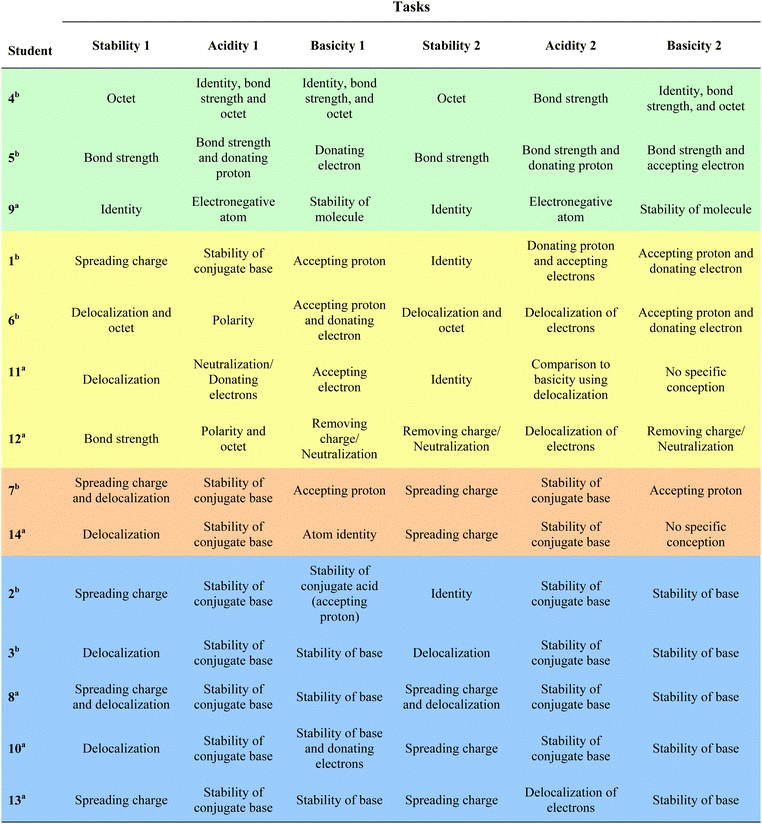
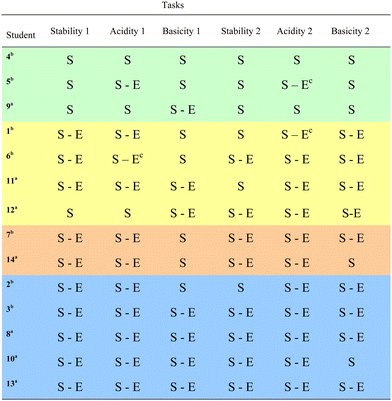
Following the completion of mental models and granularity analyses, an inductive, constant comparative analysis was conducted. A summary table for students’ mental models on each case comparison task (Table 3) and a table for levels of granularity in their explanations (Table 4) were prepared. The tables were examined for similarities, and differences in participants’ mental models across all tasks to gain an insight about their reasoning. This examination identified the existence of groups that are dissimilar from other groups both in the way they described stability (i.e., structural vs. electronic) and in the degree they associate stability to predict acid and base strength. Four groups were identified represented by the differing colors in Tables 3 and 4. These four groups of students are the main findings of this study and are characterized in Fig. 1 and detailed in the Results section.
| S indicates use of structural granularity. S–E indicates use of structural and electronic granularity.a Indicates students in Organic Chemistry I.b Indicates students in Organic Chemistry II.c Indicates use of electron withdrawal as electronic granularity without mentioning delocalization and/or spreading charge. |
|---|
|
Results
Analysis of data revealed that there were four groups of students indicating various reasoning when they predicted acid and base strength. These groups are distinguished in Fig. 1 on two dimensions where one dimension indicates granularity in students’ explanations and the other corresponds to inclusiveness of stability when predicting acid and base strength. The following descriptions represent the four groups: (1) acid and base strength through structure without association to stability, (2) acid and base strength through electronics without association to stability, (3) acid strength associated to electronically centered stability, and (4) acid and base strength associated to electronically centered stability.Group 1: acid and base strength through structure without association to stability
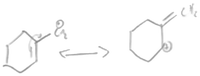
One group of students focused on structural level granularity (S4, S5, S9), highlighting features like atom count and bond type. The origin of their structurally focused mental model rested in two approaches: either electronic features were mentioned in passing with a far heavier focus on structural features, or the mental models remained strictly structural (Table 4).When defining stability, S5 chose a situational definition, explaining, “stability is like the ability to hold the bond together so that the atoms will not go away.” This definition was apparent as the student relied on bond strength with a focus on atom identity and bond type when determining the stability of each molecule. S4 showed a similar affinity for this mental model, utilizing the octet rule and more specifically examining the bond type in each molecule to determine stability. When asked to determine the more stable molecule the student reasoned, “I'd say phenolate. It's more stable because of the double bonds.” Akin to S5 and S9, this student's mental model is contingent on bonds from a structural perspective; this definition of stability can be described as a rule in which more bonds equal greater stability.
As these students (S4, S5, and S9) progressed to the topic of acidity, these students did not connect stability to acid strength. Upon being asked to determine the more acidic molecule in Acidity 1, S9 responded “Honestly, I just think I remember the CH3COO being more negatively charged than the ethoxide ion, but I am not a hundred percent sure.” S9 then emphasized the number of oxygens, highlighting the element's electronegative nature as her mental model. S4 and S5 were able to remain relatively consistent in their mental models without invoking stability. Initially, both students cited the differences in bond strength as the causal factor for determining acid strength. Interestingly, S5 also used resonance, a concept grounded in electronic granularity; however, this student inappropriately applied this principle. From S5's perspective, the existence of resonance within a system induces a weaker bond and results in a better hydrogen donor. Although resonance was mentioned, the mental model used was based on a structural framework (i.e., bond strength). S5's reliance on structural features was even more apparent in Acidity 2, where no resonance features were provided. The student again emphasized bond strength, but this time focused on each bond's proximity to oxygen. S4 established a connection between bond strength and acidity, but her reasoning was inconsistent as she first utilized atom identity and then upon further questioning settled on the explanation “because of its full of octet.”
Basicity appeared to generate many of the same problems that each student experienced when differentiating acidity. S4 again utilized structural features in her mental model to argue bond strength's role in determining base strength. In Basicity 1, she reasoned “Acetamide also has double bonds in its structure and double bonds are also stronger… as well, like the charges as well, like, this one was a plus charge, and then it only can have three bonds to it. So, like in terms of like stability, those are more stable…” Despite the mention of stability, it is important to note that the student displayed no conception of how this stability influences the basicity of a molecule even after being prompted by the interviewer. A similar phenomenon was witnessed in Basicity 2, when S9 introduced the idea of stability, yet showed little to no understanding of its implications. S9 clarified “the pi bonds just means it's super stable. But I don't know if the pi bonds has anything to do with basicity.” S9 experienced a similar issue with Basicity 1, recognizing the presence of resonance but unable to explain the connection between resonance and basicity. S5, however, formulated a new mental model for base strength involving electron donation/acceptance. In Basicity 1, she relied on electron count, a concept grounded in structural granularity since lone pairs and bonding electrons are explicitly represented on the structures given. Attempting to stay consistent, S5 approached Basicity 2 with a similar electron donation/acceptance mental model. Nonetheless, the student realized the shortcomings of this model and reverted back to his bond strength mental model seen in acidity, confessing “So the weaker the bond I mean, if it is weaker, the oxygen is less likely to receive the electron, but I am not too sure. I still don't know.”
Group 2: acid and base strength through electronics without association to stability
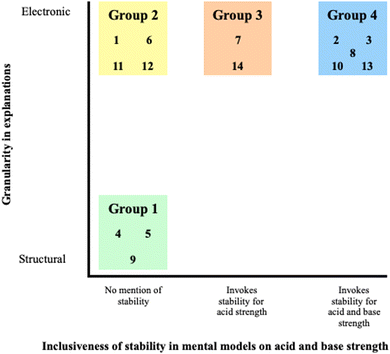
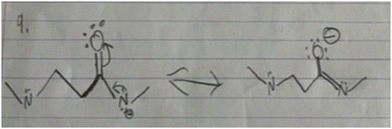
Four of the students (S1, S6, S11, S12) within this study utilized a mental model contingent on electronics when determining acidity and basicity whereas explanations of students in group 1 included more structurally focused granularity. However, within their model, the association between electronics and stability was not established when analyzing acid and base strength.In establishing the means by which stability is determined within Stability 1 and 2, each student provided congruent reasoning using primarily electronic granularity as the central focus (Tables 3 and 4). The two mental models employed by these students were the delocalization of electrons and the spreading of the charge. Structural granularity such as bond type, atom count, and connectivity occasionally operated as substitutions for their electronic counterparts. With Stability 1, S1 and S6 expressed that an increased ability for electron delocalization results in greater stability. From here, they both recognized the charge present on the oxygen and recalled that the existence of a charge generates instability. Attempting to connect this idea with their mental model of delocalization, both students attempted to illustrate the delocalization being described, but after multiple drafts, both students were unable to move the charge as shown by S6 in Fig. 2. S1 acknowledged, “I know that there is a way to move this down. I just don't know what it is.” S11 was also unfamiliar with the utility of delocalization in resonance, describing her knowledge on the subject more as a memorized concept explaining, “I just know that. Okay, I just have that in my head that like the more resonance, the more stable.” When all three of these students were asked to carry out a similar task in Stability 2, two out of the three students (S1 and S11) were unable to use electronics and isolated connectivity as their main argument. S6 likewise began with noticing that the positive charge retained an allylic nature (connectivity) however expounded on this observation with electronic granularity explaining that “it can do resonance and the resonance will make it more stable.” S12 followed a more structural mental model when assigning stability, using bond type as the determining factor. In Stability 1, S12 cited the presence of a double bond as the greatest indication of stability, however in Stability 2 she cited delocalization.
Each student in this group did not carry over their electronically centered mental models of stability in determining acid strength. Although stability was not employed when determining acidity, each student was still able to exhibit a mental model loosely grounded in electronic granularity (Table 4). Three students (S1, S6, and S12) incorporated some mode of electronegativity into their mental model of acid strength referencing the idea of polarity several times. S6 highlighted her mental model of polarity stating “So when an atom is electronegative, they become, their electron cloud they're not even anymore… when the cloud is bigger on one side than the atom is gonna have more charge. And then that charge will make the molecule more acidic or basic.” Likewise, in Acidity 2 S1 denotes hydrogen's proximity to an electronegative atom as the determining factor in acid strength. S12 referred to a polarity mental model as well yet provided an explanation by connecting the concept with resonance. This student hypothesized “if there's like a cloud around that whole like structure, for example, then say it's like then the clouds are going to be bigger towards the oxygen showing that there's more like resonance over there.” The only outlier within this session of the interview was S1's decision to use the stability of the conjugate base as a mental model for Acidity 1 as expressed by her “the more stable one would be the more acidic”. She was able to utilize both structural (bond type) and electronic granularity (delocalization) to describe how a more stable conjugate base results in a more acidic molecule, however this was not used in Acidity 2.
This set of students also attempted to use electronic granularity to the base strength questions. For instance, in Basicity 1, two students (S1 and S6) defined bases through an accepting proton mental model, electing to determine base strength through electron count. As S1 described “It has a… lone pair which means it has more of a potential to accept an extra hydrogen into it's because it has the NH2 plus the lone pair which means okay, there's more space to accept the hydrogen.” S11 followed a similar mental model for Basicity 1 by recognizing a positive charge and arguing that this impacts electron acceptance, which is an indication of a mental model on base congruent with Lewis definition. A considerably different narrative arises for S1 and S11 in Basicity 2 where both students showed frustration and settled on the explanation that the additional double bond was the deciding factor in base strength. Nevertheless, two students were still able to find ways to incorporate their mental model of electronics. For S6 in Basicity 2, the explanation was that propoxide's ability to “do resonance” resulted in an increased ability to accept protons. S12 provided a consistent line of reasoning for both Basicity 1 and 2, utilizing resonance and charge neutralization to explain differences in base strength. Students in this group provided no description of how electronics corresponds to stability and the impact of stability on basicity or acidity.
Group 3: acid strength associated to electronically centered stability
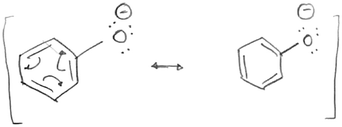
Two students utilized their mental models of electronically centered stability when predicting acid strength but not base strength (S7 and S14), which contrasts with the previous groups. In group 3, students compared acid strength using the stability of the conjugate base while holding electronic granularity as the determining factor in their mental models on stability (Tables 3 and 4). Nevertheless, they were not able to use the stability of the base when differentiating base strength between two molecules.Students in this group denoted stability as the degree to which charge is spread through the delocalization of electrons. In their explanations, charge as structural granularity and electronic granularity including spreading charge and delocalization of electrons were evident. For instance, when comparing the stability of two negatively charged ions (Stability 1), S7 expressed that although charge destabilizes an atom “resonance contributes to stability” explaining: “I think it's because the charge can be more evenly distributed across the atoms and that's why it's more stable”. The student depicted this phenomenon in Fig. 3 and verbally, “but the electrons on the oxygen, they could resonate down to form like, a double bond.” Similarly, S14 elucidated how delocalization aids in the spreading of charge on the allylic cation presented in Stability 2. The student reasoned “…because the carbon is lacking the electrons…it will want to move there to make it more stable. the carbon…by the resonance again…but then the plus sign will move to here”
The students stayed consistent in their framework and implemented their electronic mental models of stability when predicting acid strength in two cases (Table 4). They used delocalization of electrons as electronic granularity in both cases whereas different structural granularity existed in each case comparison. For instance, in Acidity 1 where students were given conjugate bases with resonance to compare acidity of acetic acid and ethanol, S14 explained “…I think there's maybe conjugate base tells me why it's more acidic…it's even shown [referring resonance structures of acetate ion], like, showing me how the electrons are moving…But then it's just a resonance again, making it more stable”. Even in Acidity 2 where conjugate base structures were not provided, each student was still able to use the same mental model when predicting acid strength of two hydrogens in a molecule. S7 explained why the hydrogen closest to the carbonyl group was more acidic reasoning “I'm thinking like if…that hydrogen leaves that nitrogen will be left with…a negative charge. And that can be like resonance stabilized onto the oxygen”. The student further elaborated on why delocalization does not occur for another hydrogen through structural granularity, more specifically connectivity.
These two students differed in terms of their mental models on basicity and neither cited stability (Table 3). S7's mental model on basicity relied on the Brønsted–Lowry definition. The student was also able to recognize the resonance present within the structures yet was not able to associate how this impacted stability and proton acceptance. In Basicity 1 where only structural granularity was observed, she explained “I will define a base as being a proton acceptor, and I determined that it was acetamide over ethyl amine because when I looked at the resonance structures, I saw that the second and third structures both have a negative oxygen”. Defining a base and predicting base strength seemed to be more challenging for S14 due to the inconsistency in her mental model. She elected identity as the approach when comparing the base strengths’ of acetamide and ethylamine. During the interview she explained “It's usually I think there was like a structure. As I said here NH2, oxygen [referring to OH], there was like and a halide. And it usually goes this way. And this is more acidic going to more acidic side [referring to order beginning from NH2 and ending with halide]”. For this student, acid is an intrinsic property, which can be inferred from atom identity. Relying on this, she reasoned that acetamide is more acidic because of oxygen, which makes it less basic. When the student was asked why propoxide was more basic than propene-1-olate (Basicity 2), she explained, “the difference is just the pi bond there. But I'm not sure how it helps with that. But I'm not sure if I have learned this before, if I should have. I don't know”.
Group 4: acid and base strength associated to electronically centered stability
Five students employed electronically centered stability as a framework for predicting both acid strength and base strength (S2, S3, S8, S10 and S13), differing from group 3 in their extension of the stability model to the basicity prompts. Each of these student's mental model of acid strength was related to the stability of the conjugate base while base strength was determined by the stability of base as well.These students related stability to the degree to which the charges on negatively and positively charged ions were dispersed and/or how the electrons delocalized. (Stability 1 and Stability 2 in Table 3). For instance, S10 defined stability as “how much room the electron could take up and the different positions that it could exist in to be stabilized” indicating a focus on delocalization. S13 elected an alternative approach, claiming stability was related to the spreading of the charge, stating, “I can…disperse…Just the negative.” One student's mental model (S8) included not only delocalization but also the spreading of charge, equating stability to the movement of the pi bond and dispersal of charges as depicted in Fig. 4. Students utilized functional group identity (aromatic ring, S2 and S8) or connectivity (allylic, S3 and S13) or both (S10) as structural granularity to enact how delocalization and/or spreading of charge stabilizes ions in the tasks. There was only one student (S2) who defined stability in relation to identity when comparing the stability of two positively charged ions (Stability 2). This student reasoned, “The carbon is it's next to a double bond and I think that that increases stability.” S2 could not provide an explanation when questioned further, as evidenced in his statement “I don't know why…I just remember the fact.”
All students, apart from S13, were consistent in their mental models of acid strength, implementing their knowledge on the stability of the conjugate base in both acidity tasks (Table 3). S8 explained his mental model by emphasizing the most stable negative charge upon deprotonation. This student depicted his model in Fig. 5. S13 was distinct from the others in his approach to Acidity 2 in which delocalization was emphasized, reasoning “I would say that…it's like the blue hydrogen just because…It has the ability to do resonance with the oxygen or not with oxygen, but rather with the source of electrons that are right here”. S2 followed a similar mental model in Acidity 1. Regardless of their mental models, resonance specific representational features in Acidity 1 and connectivity in Acidity 2 were the common structural granularities in all students’ reasoning. Nevertheless, in terms of electronic granularity, students selected a wider variety of features. Delocalization was the shared electronic granularity for all students in both tasks; however, only some students were able to activate spreading charge in both acidity tasks (S2 and S8) while others utilized it specifically for Acidity 1 (S3 and S13) where resonance specific representations were provided. Moreover, one of the students in this group (S3) also included electron withdrawal in her explanations for both cases. She expressed “induction would be greater than this one [referring to ethoxide] because this [referring to acetate] has two Os to the carbon and this [referring to ethoxide] only has one oxygen.”
With the exception of S2, all students utilized their electronic centered stability mental models when reasoning for base strength in two cases (Table 3). For instance, S3 focused on stability to explain why acetamide was less basic, reasoning “…the acetamide I think would be a weaker base because it's resonance stabilized”. Moreover, S10 expounded, “it is more basic. If it's less stable” when comparing bases with single Lewis structures (Basicity 2). S2 applied the stability mental model in an alternative manner, determining the stability of the conjugate acid rather than that of the base. Relying on Brønsted–Lowry definition of a base, he elaborated “…I put a…theoretical hydrogen…on the oxygen [referring to the one in acetamide] and then one on the nitrogen of the ethyl amine and…I determined which one of those two would be more stable.” While S2 was able to determine base strength through stability, the granularity remained strictly structural in his reasoning. (i.e., atom identity, electron count, and charge). In the context of electronic granularity, students’ explanations showed partiality toward electron delocalization (excluding S10 in Basicity 2). For instance, S13 explained “the pi bonds move, that source of electrons moves around the structure, not around, but rather from the one oxygen, the top oxygen to the right nitrogen”. Spreading of charge was also enacted by one student in Basicity 2 (S2) while it was observed in two students’ reasoning (S8 and S10) in Basicity 1. Although used less frequently, other types of electronic granularity (i.e., polarization, electron withdrawal, and bearing charge) existed in students’ explanations for Basicity 1 when resonance specific representations were provided. For instance, S3 focused on atom identity and its effect on polarization reasoning “…this has a nitrogen [referring to acetamide] as well, but oxygen has a higher electronegativity…which decides the polarity”.
Discussion
Teaching on acid and base strength in organic chemistry heavily relies on a consideration of stability (Stoyanovich et al., 2015). Building on this statement, the results from this study lead to three assertions. First, students in this study can be demarcated by whether or not they associated acid and base strength with chemical stability. Second, students that employ electronic-based stability displayed a more consistent mental model across acid and base strength (group 4) than students that focused on structural features (group 1) or did not associate stability to electronic features (group 2). Third, evaluating base strength is more challenging than acid strength even among students who enacted electronic-based stability for acids (group 3).First, students are different from each other when reasoning on acid and base strength in relation to the degree to which they consider stability. Students in groups 1 and 2 did not associate the stability of bases and conjugate bases when comparing relative basicity and acidity. Among those, students in group 2 gravitated towards electronics (e.g., bond polarity, electronegativity, and donating electrons) in their processes. This could be explained by students’ enactment of various mental models consistent with different scientific models on acids and bases. Arrhenius (i.e., gives hydrogen when dissolved in water) and Lewis models (i.e., electron transfer) could stimulate students’ reliance on electronics when predicting acid and base strength (Bhattacharyya, 2006; McClary and Talanquer, 2011a; Tümay, 2016). Although students have spent a considerable amount of time in Organic Chemistry I on the factors affecting acid and base strength, lack of explicit focus on the factors in relation to Brønsted–Lowry model (De Vos and Pilot, 2001; Furio-Mas et al., 2005) might lead students to enact different models of acids and bases. Group 1 students did not associate stability with acid and base strength; instead, they gave prominence to structural features (e.g., atom identity, electron count, and bond type) that are accessed easily (Shah and Oppenheimer, 2008; Heckler, 2011) when predicting acid and base strength. The tendency to use surface similarity (e.g., functional group identity) and overgeneralization (e.g., bond strength or electronegativity or octet) could explain this group of students’ reasoning (Talanquer, 2014). This tendency may hinder students’ understanding of scientific concepts, which can result in missing relevant scientific concepts and in generating conflicting responses (Tümay, 2016). Either electronics or structural centered, most of the time, students’ uses of models were triggered by the features of the task (Osman and Stavy, 2006; McClary and Talanquer, 2011a), which is accompanied by the use of relational heuristics (e.g., McClary and Talanquer, 2011a, 2011b; Tümay, 2016). For instance, S4 enacted a hybrid mental model on acid strength including identity, citing the COOH functional group, bond strength, comparing C to O versus CH2 and CH3, and octet, citing a full valence, when explaining why acetic acid is more acidic (Acidity 1, Table 3). However, she expressed a single mental model where bond strength is central for Acidity 2 (Table 3). She explained that “And basically when you see a lot of CH bonds you know it's basic because their structure they are weak bonds, whereas the C double bonds O, wouldn't be a shorter bond and I assume acid…the stronger.”
Second, students that enact electronic-based stability displayed a more consistent mental model across acid (group 3 and 4) and base strength (group 4) than students who utilized alternative methods (groups 1 and 2). That is, the stability of conjugate base was their major focus for acid strength while the stability of base was considered for base strength across all tasks. Consistent use of stability in relative acidity and basicity is more aligned with the scientific assumptions on acid and base strength in the Brønsted–Lowry model (McClary and Talanquer, 2011a; Tümay, 2016). Electronic-based stability could trigger the retrieval of a mental model of acid and base strength from long-term memory that is based on foundational principles (Gentner, 2002; Vosniadou, 2002; McClary and Talanquer, 2011a) and support students’ reasoning on relative acid and base strength (Gentner, 2002). Another factor that explains why students with electronic-centered stability are more consistent in their mental models across relative acidity and basicity could be related to the type of reasoning. Invoking stability could help students to apply analytical reasoning (i.e., Type 2) instead of heuristic reasoning (i.e., Type 1) (McClary and Talanquer, 2011a; Talanquer, 2014). Considering stability for acid and base strength is the relevant knowledge in Brønsted–Lowry model (McClary and Talanquer, 2011a; Tümay, 2016) and strong relevant knowledge in a topic could support analytical reasoning (Evans, 2008). Analytical reasoning may result in more consistent and scientific mental models (group 3 and 4) whereas heuristic reasoning (group 1 and 2) could lead to conflicting responses (Tümay, 2016). Students with electronic-centered stability searched for the following cues that help to determine how these influence relative acidity and basicity; factors effecting polarizability and bearing charge (i.e., size and electronegativity of atom), and delocalization. However, students stopped searching for inductive effects and hybridization, which were evidenced as more difficult for students (McClary and Talanquer, 2011a). Students’ stopping the search for all relevant factors might be related to their difficulty in conceptualizing acid strength as an emergent property (Tümay, 2016).
Third, base strength is more challenging than acid strength even for students who expressed electronic-centered stability. That is, the stability of the conjugate base was considered by half of the students when predicting acid strength whereas fewer students utilized stability of the base when comparing relative basicity. One explanation may be the lack of emphasis on base strength relative to acid strength in the curriculum. The organic chemistry textbook used at the setting of this study (Klein, 2017) describes acid strength in relation to several factors influencing the stability of conjugate base extensively through explanations and worked examples, whereas base strength is not as thoroughly explored. Base strength is also mentioned in relation to nucleophilicity and addressed in amines instead of a phenomenon by itself. Another reason for why base strength is more challenging than acid strength could be the switch in framing of stability in the judgment making process. In judging acid strength, one must identify the most stable conjugate base, while in judging base strength one must identify the least stable base. Switching this frame of reference may be difficult for some students and may require explicit modeling.
Implications
To help students understand chemical stability and the role of stability in acidity, and basicity, chemistry instruction could benefit from scaffolding the emergent nature of stability (Wilensky and Resnick, 1999) and how stability relates to acid and base strength (Kranz et al., 2023). To emphasize the emergent nature, instructors should use the terms “emergence” and “system” explicitly since chemical systems are emergent in nature including entities and their properties and activities, in increasing complexity that results in novel properties (Machamer et al., 2000). It is also important that students should be informed about the acid–base model that is used when comparing relative acidity and basicity. Since the Brønsted–Lowry model is the fundamental model in organic chemistry (Stoyanovich et al., 2015), chemistry instructors could emphasize this model at first by introducing examples as “an acid–base reaction system” instead of “an acid–base reaction”. Then, students can be prompted with a question such as “How do acidic and basic properties emerge in an acid–base reaction system?” Finally, the instructor can focus on relative acid and base strength. Student discussions can be directed toward the relative stability of all species in determining the direction and extent of this dynamic process, which forms the basis of acid and base strength. Moreover, “delocalization” should be used as an alternative to “resonance” when discussing chemical stability. Although the term resonance has been used since its introduction in 1950s, electron delocalization describes the physical reality more than the term resonance (Kerber, 2006). In summary, instruction may benefit by focusing on three sequential learning outcomes: (1) explicit teaching of stability, (2) explicit mapping of basicity onto the construct of stability, and (3) mapping of acidity onto the construct of stability of the conjugate base.As explicit teaching of stability requires comprehension of the emergent nature of stability, we proposed an example scaffold based on Deng and Flynn's model (2021). The scaffold, shown in Fig. 6, uses three prompts: evaluating the relevance of each factor contributing to emergence of stability for two molecule systems (correct responses are shown in red), comparing the relative effect of relevant factors (i.e., entities and activities) for two systems, and reasoning about stability emerged from the interaction of all entities and activities in the molecule system. Chemistry instructors could model how to reason on emergence of stability in different molecule systems.
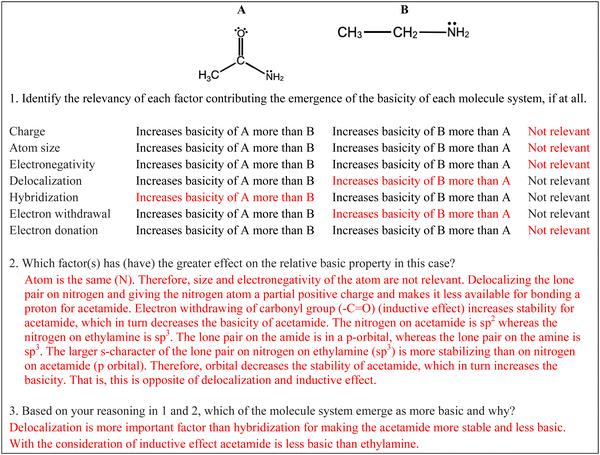
Following a scaffold on chemical stability, a similar scaffold can be used regarding relative basicity (Fig. 7) also based on the Deng and Flynn's model (2021). Before utilizing the scaffolding prompts, the chemistry instructor could emphasize how relative stability determines relative base strength within a competing proton transfer system. Student responses to prompts 2 and 3 in Fig. 7 can elucidate the extent the students’ employ stability considerations in making basicity predictions. Various worked examples the emergent property of basicity that requires consideration of different type and number of factors might help students build a more conceptual understanding.
Finally, chemistry instructors could map acidity onto the construct of stability of the conjugate base. Before mapping, chemistry instructors could emphasize why stability is the determining factor for the emergent property of acidity in the Brønsted–Lowry model. A similar scaffold can be developed for relative acid strength with students rating the conjugate base for two molecule systems. Responses to prompts 2 and 3 can then explore how students invoke chemical stability of the conjugate bases and how this rating relates to relative acid strength. The explicit teaching of the emergent nature of stability, acidity, and basicity and the use of scaffolds may prevent students’ reliance on heuristics driven by their mental models that can be triggered by the tasks and hence help students to form a better comprehension of these phenomena.
This study has been one of the first attempts investigating students’ understanding of base strength to the best of the researchers’ knowledge. In this study, students had difficulties in explaining how the relative stability of bases relates to the relative base strength. Additional research can adopt the prompts presented in Fig. 6 and 7 to utilize in a larger scale study where the focus is on students understanding of various factors that influence the stability of bases and their strength. The results from a study of this form can offer additional insight into the challenges students may face with base strength.
Limitations
There were several limitations inherent to this study. First, the conclusions are limited to the students at the research institution where the study was conducted. However, we attempted to expand the theories of mental model (Park and Gittelman, 1995) and granularity (Luisi, 2002; Talanquer, 2022), which is one of the aims of the case study (Yin, 2009). This study provided evidence for the applicability of those models to stability and basicity since available literature utilized those when investigating acid strength (e.g., Tümay, 2016) and acid–base reactions (e.g., Deng and Flynn, 2021). Researchers can benefit from using the theories of mental model and granularity to reveal whether conclusions reached in this study are valid in their institutions. Also, the conclusions can be a foundation to devise questions for a larger scale study where students’ reasoning on acid and base strength are investigated. Second, limitations rest in the exploratory nature of this study. We did not prepare interviews to identify whether students invoke stability in their mental models on acid and base strength. However, through follow-up questions, we were able to capture to what degree students relate stability with relative acidity and basicity. Further research could benefit from questions that intentionally reveal students’ consideration of stability. Finally, it is unknown the extent that assessments in the course promoted students’ use of electronic granularity or stability in making predictions of relative acid or base strength. Future research that investigates the extent to which students use these features during their assessments would inform instruction and assessment design.Conclusion
Four groups of students emerged, differentiated by their reasoning on acid and base strength: (1) acid and base strength through structure, (2) acid and base strength through electronics, (3) acid strength associated with electronically centered stability, and (4) acid and base strength associated with electronically centered stability. Among those groups, a more consistent mental model across acid and base strength was observed in students that enact electronic-based stability. In addition, students employing electronic-based stability for acids experienced difficulty in relating their mental model of stability to base strength.Conflicts of interest
The authors report no conflicts of interest for this work.Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. 2142324. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. Author BD would like to thank The Scientific and Technological Research Council of Turkey for their support (TÜBITAK 2219) and Zonguldak Bülent Ecevit University. The authors wish to acknowledge Ayesha Farheen, Dianna Kim, Jessica D. Young, and Pallavi Nayyar for feedback on project development. The authors also acknowledge the students who participated in the interviews and instructors for feedback on the instructional setting.References
- Alfieri L., Nokes-Malach T. J. and Schunn C. D., (2013), Learning through case comparisons: a Meta-analytic review, Educational Psychology, 48, 87–113.
- American Chemical Society, (2015), Committee on professional training. Organic chemistry supplement, https://www.acs. org/content/dam/acsorg/about/governance/committees/training/acsapproved/degreeprogram/organic-chemistry- supplement.pdf (accessed January 2023).
- Anderson T. L. and Bodner G. M., (2008), What can we do about ‘Parker’? A case study of a good student who didn't ‘get’ organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101.
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: how graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7(4), 240–247.
- Bhattacharyya G., (2014), Trials and tribulations: Student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bodé N. E., Deng J. M. and Flynn A. B., (2019), Getting past the rules and to the WHY: Causal mechanistic arguments when judging the plausibility of organic reaction mechanisms, J. Chem. Educ., 96(6), 1068–1082.
- Boothe J. R., Zotos E. K. and Shultz G. V., (2023), Analysis of post-secondary instructors’ pedagogical content knowledge of organic acid–base chemistry using content representations, Chem. Educ. Res. Pract., 24(2), 577–598.
- Bretz S. L. and McClary L., (2015), Students’ understandings of acid strength: How meaningful is reliability when measuring alternative conceptions? J. Chem. Educ., 92(2), 212–219.
- Clement J. J. and Rea-Ramirez M. A., (2008), Model based learning and instruction in science, New York: Springer.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40(5), 464–486.
- Cooper M. M., Kouyoumdjian H. and Underwood S. M., (2016), Investigating students’ reasoning about acid–base reactions, J. Chem. Educ., 93(10), 1703–1712.
- Creswell J. W., (2007), Qualitative inquiry and research design: Choosing among five traditions, London, UK: Sage.
- De Vos W. and Pilot A., (2001), Acids and bases in layers: the stratal structure of an ancient topic, J. Chem. Educ., 78(4), 494–499.
- Deng J. M. and Flynn A. B., (2021), Reasoning, granularity, and comparisons in students’ arguments on two organic chemistry items, Chem. Educ. Res. Pract., 22(3), 749–771.
- Evans J. S. B., (2008), Dual-processing accounts of reasoning, judgment, and social cognition, Annu. Rev. Psychol., 59, 255–278.
- Furio-Mas C., Calatayud M. L., Guisasola J. and Furio-Gomez C., (2005), How are the concepts and theories of acid–base reactions presented? Chemistry in textbooks and as presented by teachers, Int. J. Sci. Educ., 27(11), 1337–1358.
- Gentner D., (2002), Mental models, psychology of, in Smelser N. J. and Bates P. B. (ed.), International encyclopedia of the social and behavioral sciences, Amsterdam, Netherlands: Elsevier Science, pp. 9683–9687.
- Gilbert J. K. and Boulter C. J., (1998), Learning science through models and modeling, in Fraser B. J. and Tobin K. G. (ed.), International handbook of science education, Amsterdam: Kluwer Academic Publishers, pp. 53–66.
- Gilbert J. K., Boulter C. J. and Rutherford M., (2000), Explanations with models in science education, in Gilbert J. K. and Boulter C. J. (ed.), Developing models in science education, Dordrecht: Springer, pp. 193–208.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Greca I. M. and Moreira M. A., (2000), Mental models, conceptual models, and modelling, Int. J. Sci. Educ., 22(1), 1–11.
- Grove N. P., Cooper M. M. and Cox E. L., (2012), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89(7), 850–853.
- Heckler A. F., (2011), The role of automatic, bottom-up processes: In the ubiquitous patterns of incorrect answers to science questions, in Mestre J. P. and Ross B. H. (ed.), Psychology of learning and motivation, San Diego, CA: Academic Press, vol. 55, pp. 227–267.
- Johnson-Laird P. N., (1983), Mental models: Towards a cognitive science of language, inference, and consciousness, Harvard University Press, No. 6.
- Kerber R. C., (2006), If it's resonance, what is resonating? J. Chem. Educ., 83(2), 223.
- Klein D., (2017), Organic chemistry, 4th edn, New York, NY: Wiley.
- Kranz D., Schween M. and Graulich N., (2023), Patterns of reasoning–exploring the interplay of students’ work with a scaffold and their conceptual knowledge in organic chemistry, Chem. Educ. Res. Practice, 24(2), 453–477.
- Lin J. W. and Chiu M. H., (2007), Exploring the characteristics and diverse sources of students’ mental models of acids and bases, Int. J. Sci. Educ., 29(6), 771–803.
- Luisi P. L., (2002), Emergence in chemistry: Chemistry as the embodiment of emergence, Found. Chem., 4(3), 183–200.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about mechanisms, Philosophy Sci., 67(1), 1–25.
- Marshall G. B. and Rossman C., (2011), Designing qualitative research, 5th edn, London: Sage.
- McClary L. M. and Bretz S. L., (2012), Development and assessment of a diagnostic tool to identify organic chemistry students’ alternative conceptions related to acid strength, Int. J. Sci. Educ., 34(15), 2317–2341.
- McClary L. and Talanquer V., (2011a), College chemistry students’ mental models of acids and acid strength, J. Res. Sci. Teach., 48(4), 396–413.
- McClary L. and Talanquer V., (2011b), Heuristic reasoning in chemistry: Making decisions about acid strength, Int. J. Sci. Educ., 33(10), 1433–1454.
- Nersessian N. J., (2008), Creating scientific concepts, Cambridge: MIT Press.
- Osman M. and Stavy R., (2006), Development of intuitive rules: Evaluating the application of the dual-system framework to understanding children's intuitive reasoning, Psychonomic Bull. Rev., 13(6), 935–953.
- Park O. C. and Gittelman S. S., (1995), Dynamic characteristics of mental models and dynamic visual displays, Instructional Sci., 23(5), 303–320.
- Petterson M. N., Watts F. M., Snyder-White E. P., Archer S. R., Shultz G. V. and Finkenstaedt-Quinn S. A., (2020), Eliciting student thinking about acid–base reactions via app and paper–pencil based problem solving, Chem. Educ. Res. Pract., 21(3), 878–892.
- Raker J., Holme T. and Murphy K., (2013), The ACS exams institute undergraduate chemistry anchoring concepts content map II: Organic chemistry, J. Chem. Educ., 90(11), 1443–1445.
- Shah A. K. and Oppenheimer D. M., (2008), Heuristics made easy: An effort-reduction framework, Psychol. Bull., 134(2), 207–222.
- Shah L., Rodriguez C. A., Bartoli M. and Rushton G. T., (2018), Analysing the impact of a discussion-oriented curriculum on first-year general chemistry students' conceptions of relative acidity, Chem. Educ. Res. Pract., 19(2), 543–557.
- Solomons T. G. and Fryhle C. B., (2012), Organic chemistry, 7th edn, New York, NY: Wiley.
- Stoyanovich C., Gandhi A. and Flynn A. B., (2015), Acid–base learning outcomes for students in an introductory organic chemistry course, J. Chem. Educ., 92(2), 220–229.
- Talanquer V., (2014), Chemistry education: Ten heuristics to tame, J. Chem. Educ., 91(8), 1091–1097.
- Talanquer V., (2022), The complexity of reasoning about and with chemical representations, JACS Au, 2(12), 2658–2669.
- Tümay H., (2016), Emergence, learning difficulties, and misconceptions in chemistry undergraduate students’ conceptualizations of acid strength, Sci. Educ., 25, 21–46.
- van der Veer G. C., Kok E. and Bajo T., (1999), Conceptualizing mental representations of mechanics: a method to investigate representational change, in Kayser D. and Vosniadou S. (ed.), Modeling changes in understanding: Case studies in physical reasoning, Oxford: Elsevier, pp. 44–60.
- Vosniadou S., (2002), Mental models in conceptual development, in Magnani L. and Nersessian N. J. (ed.), Model-based reasoning, Boston, MA: Springer, pp. 353–68.
- Wilensky U. and Resnick M., (1999), Thinking in levels: a dynamic systems approach to making sense of the world, J. Sci. Educ. Technol., 8(1), 3–19.
- Yin R. K., (2009), Case study research: Design and methods, 4th edn, Thousand Oaks, CA: Sage.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3rp00049d |
| This journal is © The Royal Society of Chemistry 2023 |