Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sporothioethers: deactivated alkyl citrates from the fungus Hypomontagnella monticulosa†
Henrike Heinemann a,
Kevin Becker
a,
Kevin Becker a,
Hedda Schrey
a,
Hedda Schrey bc,
Haoxuan Zengbc,
Marc Stadler
bc,
Haoxuan Zengbc,
Marc Stadler bc and
Russell J. Cox
bc and
Russell J. Cox *a
*a
aOCI, BMWZ, Leibniz University of Hannover, Schneiderberg 38, 30167, Hannover, Germany. E-mail: russell.cox@oci.uni-hannover.de
bDepartment Microbial Drugs, Helmholtz Centre for Infection Research (HZI), Inhoffenstraße 7, 38124 Braunschweig, Germany
cInstitute of Microbiology, Technische Universität Braunschweig, Spielmannstraße 7, 38106 Braunschweig, Germany
First published on 11th October 2023
Abstract
Submerged cultivation of Hypomontagnella monticulosa MUCL 54604 resulted in formation of a stereoisomeric mixture of new sulfur-containing sporothriolide derivatives named sporothioethers A and B. The presence of the 2-hydroxy-3-mercaptopropanoic acid moiety attenuates the antimicrobial activity in comparison to the precursor sporothriolide suggesting a detoxification mechanism. However, moderate effects on biofilms of Candida albicans and Staphylococcus aureus were observed for sporothriolide and sporothioethers A and B at concentrations below their MICs.
Alkyl citrates are a structurally broad class of natural products requiring an alkyl citrate synthase (ACS) for their biosynthesis.1 Examples of fungal alkyl citrates (Scheme 1A) include byssochlamic acid 1,2,3 the potent squalene synthase inhibitor squalestatin S1 2,4,5 and piliformic acid 3.6 We recently fully characterised the biosynthetic pathway to sporothriolide 8 that is an alkyl citrate isolated from Hypomontagnella spp.7–9 The spo biosynthetic gene cluster (BGC) is responsible for production of 8 (Scheme 1B).10,11 Decanoyl-CoA, produced by a dedicated fatty acid synthase (FAS), is used by the ACS SpoE to form the alkyl citrate 4. Subsequent enzymatic dehydration to 5, decarboxylation to 6, hydroxylation to 7 and lactonization then forms the furofurandione sporothriolide 8 (Scheme 1B). Spontaneous Diels–Alder (DA) reaction with the polyene trienylfuranol A 9, then results in formation of the sporochartines 10–13 (Scheme 1B). Sporothriolide 8 itself possesses useful antifungal activity.9 For example it has been shown to protect pepper seedlings from the plant pathogen Botrytis cinerea.12 Sporochartines A-D 10–13, in turn, are potent cytotoxins active against human cancer cell lines.11
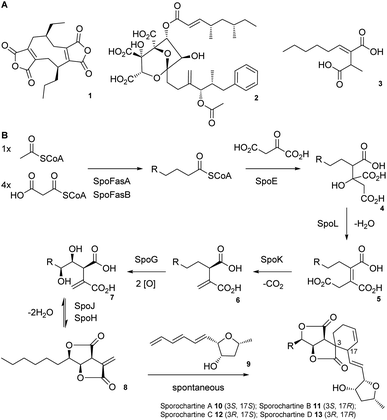 | ||
| Scheme 1 A) Structures of alkyl citrate metabolites from fungi; (B) biosynthesis of sporothriolide 8 and sporochartines 10–13. R = C6H14. | ||
Here, we report the isolation, synthesis, structure elucidation, and biological testing of two new sulphur-containing sporothriolide derivatives, termed sporothioether A 14 and B 15 (Fig. 1) from H. monticulosa MUCL 54604.10
In previous work, sporothriolide 8 was isolated from extracts of H. monticulosa MUCL 54604 after cultivation in PDB medium.10 However, when H. monticulosa was cultivated in DPY medium, sporothriolide 8 was not detected. DPY was therefore previously used as a non-producing condition during transcriptomic analysis of the spo BGC.10 However, analysis of the transcriptomic data did not reveal significant down-regulation of the spo genes in DPY media, and LCMS analysis of the culture extract revealed the presence of a new metabolite under these conditions. Purification of the new metabolite using preparative reversed-phase chromatography (ESI, Fig. S1†) resulted in isolation of an inseparable 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of isomers 14 and 15 (as indicated by 1H NMR integrals, ESI, Fig. S5†). A measured m/z of 361.1316 [M + H]+ (ESI, Fig. S2†) suggested a molecular formula of C16H24O7S for each compound. Methylation with TMS-diazomethane led to formation of a 3
2 mixture of isomers 14 and 15 (as indicated by 1H NMR integrals, ESI, Fig. S5†). A measured m/z of 361.1316 [M + H]+ (ESI, Fig. S2†) suggested a molecular formula of C16H24O7S for each compound. Methylation with TMS-diazomethane led to formation of a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of the corresponding methyl esters 16 and 17 (m/z 373 [M − H]−), indicating the presence of a carboxyl group in 14 and 15 (ESI, Fig. S3†).
2 mixture of the corresponding methyl esters 16 and 17 (m/z 373 [M − H]−), indicating the presence of a carboxyl group in 14 and 15 (ESI, Fig. S3†).
In the 1H-NMR and HSQC spectra of the 14 and 15 mixture (Table S1†) peaks for a methyl group (δH 0.93 ppm), seven methylenes (δH 1.34, 1.35, 1.41, 1.50, 1.81, 3.00/(3.08; 2.88), 3.06/(3.24; 2.96)), four methines (δH 3.66/3.17, 3.84/3.79, 4.63/4.71, 5.14/5.28) and a hydroxymethine (δH, 4.36/4.34) were observed. All peaks were doubled in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. The 13C data revealed two sets of 16 carbons. Three of the 16 carbons of each set are carbonyls (δC 176.4/176.3, 174.6/177.5, 176.1/177.0), seven are methylene carbons (δC 23.5, 26.3, 29.8, 29.8/34.3, 30.0, 32.7, 38.4/37.1), one is a methyl carbon (δC 14.2) and five are methines (δC 44.8/45.6, 45.0/47.7, 72.3, 80.4/81.3, 82.5/83.6).
2 ratio. The 13C data revealed two sets of 16 carbons. Three of the 16 carbons of each set are carbonyls (δC 176.4/176.3, 174.6/177.5, 176.1/177.0), seven are methylene carbons (δC 23.5, 26.3, 29.8, 29.8/34.3, 30.0, 32.7, 38.4/37.1), one is a methyl carbon (δC 14.2) and five are methines (δC 44.8/45.6, 45.0/47.7, 72.3, 80.4/81.3, 82.5/83.6).
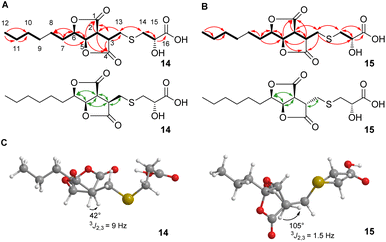
2D NMR spectra (ESI, Fig. S7–S10†) showed the core structures of 14 and 15 to be the sporothriolide scaffold (Fig. 1A and B). In addition to this core structure, both new compounds harbour a 2-hydroxy-3-mercaptopropanoic acid moiety attached to C-13 of the core via a thioether bond. This linkage was supported by the HMBC correlation between CH2-14 and C-13 (Fig. 1A and B). The presence of the thioether was additionally confirmed by the distinctive 1H/13C chemical shifts of C-13 (14, δH 3.06 and δC 29.8; 15, δH 3.24/2.96 and δC 34.3) and C-14 (14, δH 3.0 and δC 38.4; 15, δH 3.08/2.98 and δC 37.1). However, the stereochemistry at position C-3 differs in sporothioether A 14 and B 15. ROESY correlations (Fig. 1, A, B) showed that H-3, H-2, H-5 and H-6 are all syn in 14, but in 15 H-3 is anti to H-2, H-5 and H-6, as no ROESY correlation is observed. An energy-minimised model structure of the anti diastereomer 15 suggested a dihedral angle of 105° for H-3/H-2 consistent with the observed 3J2,3 value of 1.5 Hz (Fig. 1C). In contrast, an energy-minimised model of diastereomer 14 has a dihedral angle of 42° consistent with the observed 3J2,3 value of 9 Hz (Fig. 1C). Assuming the same absolute stereochemistry at C-2, C-5 and C-6 as in the parent compound sporothriolide 8, we conclude that in syn diastereomer 14, C-3 is R-configured, and in anti diastereomer 15, C-3 is S-configured.
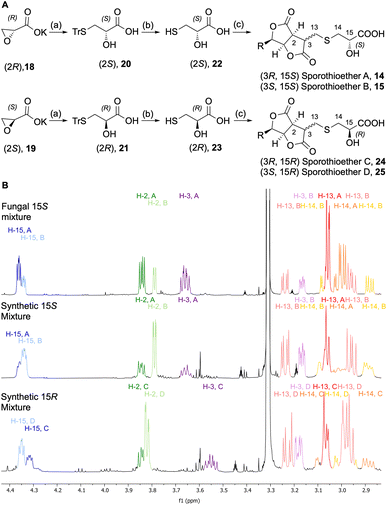
In order to determine the configuration of the stereocenter at C-15 we initially attempted to form Mosher's esters of the 15-OH.13 However, this approach led to inconclusive results due to the complexity of the spectra of the resulting mixed diastereomers. In an alternative strategy, 15S and 15R sporothioether derivates were chemically synthesized for comparison by NMR spectroscopy (Scheme 2A).
Commercially available oxirane carboxylate enantiomers 18 and 19 were subjected to regioselective ring opening over two steps by treatment with triphenylmethanethiol to give intermediates 20 and 21, followed by acidic deprotection to give 22 and 23. Sporothriolide 8 was purified from H. monticulosa and reacted under basic conditions with either 22 or 23. The resulting sporothioethers were purified by reversed-phase chromatography as inseparable mixtures of diastereomers in each case.
Analysis of the 1H NMR data (Scheme 2B) of the synthetic products revealed that both contain a mixture of sporothioether stereoisomers, as indicated by two sets of signals with an integral ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The major compound in both mixtures is 3S and the minor component is 3R. This was indicated by the 3J2,3 value of 1.6 Hz for the 3S configured sporothioethers. For the 3R configured stereoisomers 3J2,3 values of ∼9 Hz were observed, the same as for the natural 3R sporothioether 14. 1H NMR chemical shifts of the synthetic (15S, 3RS)-sporothioether mixture matched the signals of the fungal sporothioether mixture 14 and 15, whereas the signals of the synthetic (15R, 3RS)-sporothioether mixture 24 and 25 differed (Scheme 2B). Therefore, sporothioether A 14, the major compound in the natural mixture, is identified as (3R, 15S) and the minor compound sporothioether B 15 is identified as (3S, 15S). The non-natural synthetic diastereomers were now named sporothioether C 24 (3R, 15R) and sporothioether D 25 (3S, 15R).
2. The major compound in both mixtures is 3S and the minor component is 3R. This was indicated by the 3J2,3 value of 1.6 Hz for the 3S configured sporothioethers. For the 3R configured stereoisomers 3J2,3 values of ∼9 Hz were observed, the same as for the natural 3R sporothioether 14. 1H NMR chemical shifts of the synthetic (15S, 3RS)-sporothioether mixture matched the signals of the fungal sporothioether mixture 14 and 15, whereas the signals of the synthetic (15R, 3RS)-sporothioether mixture 24 and 25 differed (Scheme 2B). Therefore, sporothioether A 14, the major compound in the natural mixture, is identified as (3R, 15S) and the minor compound sporothioether B 15 is identified as (3S, 15S). The non-natural synthetic diastereomers were now named sporothioether C 24 (3R, 15R) and sporothioether D 25 (3S, 15R).
It seems likely that the sporothioethers are biosynthesised by addition of 2S-2-hydroxy-3-mercaptopropanoic acid 22 to sporothriolide 8 itself in vivo. Formation of an epimeric mixture at C-3 may indicate that this is not an enzymatic process. To test this hypothesis sporothriolide 8 was incubated with 3-mercaptopropionic acid 26 under physiological conditions in phosphate buffer at pH 7.5. LCMS analysis revealed the formation of a new compound with an m/z of 343 [M − H]−, which corresponds to the mass of the expected product 30 and 31 (ESI, scheme S1†).
The inseparable mixtures of 14 and 15, and 24 and 25, were assayed for biological activity (see ESI†). In cytotoxicity assays no activity was detected for the sporothioethers against KB-3-1 and L929 cell lines in the tested range (37–0.63 μg mL−1). Similarly, in previous studies no cytotoxic activity was detected for sporothriolide 8 itself.9 Both, the sporothioether mixture 14 and 15 and sporothriolide 8, were tested in biofilm inhibition and dispersion assays against Stapylococcus aureus and Candida albicans (Table 1). Sporothriolide 8 displayed significant activity against the formation of S. aureus biofilms at subtoxic concentrations (MIC (S. aureus) > 66.6 μg mL−1), meanwhile only weak inhibitory effects on preformed biofilm of S. aureus were observed.
| Test organism | 14 + 15 mixture | 8 | |
|---|---|---|---|
| a Microporenic acid A, 74 ± 12 (250 μg mL−1), 75 ± 6 (7.8 μg mL−1), 42 ± 7 (3.9 μg mL−1).b Farnesol: 75 ± 9 (250 μg mL−1), 51 ± 7 (31.3 μg mL−1), 38 ± 10.c Microporenic acid A: 64 ± 7 (250 μg mL−1), 41 ± 15 (15.6 μg mL−1); SD: standard deviation; —no inhibition. | |||
| Biofilm inhibition [% ± SD] | S. aureus (DSM 1104)a | 77 ± 9 (250 μg mL−1) | 75 ± 8 (250 μg mL−1) |
| 24 ± 9 (62.5 μg mL−1) | 55 ± 9 (3.9 μg mL−1) | ||
| 37 ± 10 (2 μg mL−1) | |||
| C. albicans (DSM 11225)b | 58 ± 8 (250 μg mL−1) | — | |
| 40 ± 10 (31.3 μg mL−1) | |||
| Biofilm dispersion [% ± SD] | S. aureus (DSM 1104)c | 77 ± 9 (250 μg mL−1) | 53 ± 6 (250 μg mL−1) |
| 24 ± 9 (62.5 μg mL−1) | 36 ± 5 (62.5 μg mL−1) | ||
In addition, the sporothioether mixture 14 and 15 displayed moderate inhibitory activity against biofilm formation of C. albicans (Table 1). In antimicrobial assays the mixtures of 14 and 15 and 24 and 25 were tested against selected bacteria and fungi in the same assays that were used previously to assess the bioactivity of sporothriolide 8.9 The minimum inhibitory concentrations (MIC, Table 2) showed that sporothriolide 8 possesses moderate antimicrobial activity against the tested microorganisms as reported previously,9 but sporothioethers 14 and 15 were inactive against most organisms and had significantly attenuated effects vs. Mucor hiemalis and Schizosaccharomyces pombe. Sporothioethers 24 and 25 were inactive against all tested microorganisms.
| Test organism | MIC [μg ml−1] | |||
|---|---|---|---|---|
| 14 and 15 mix | 24 and 25 mix | 8 (ref. 9) | Ref. | |
| a Nystatin.b Ciprobay.c Oxytetracyclin hydrochloride.d Kanamycin.e Gentamycin; – no inhibition; nt, not tested. The cell density was adjusted to 8 × 106 cells per ml. | ||||
| Schizosaccharomyces pombe (DSM 70572) | 66.6 | — | 8.3 | 4.2a |
| Pichia anomala (DSM 6766) | — | — | 33.3 | 8.3a |
| Mucor hiemalis (DSM 2656) | 66.6 | — | 4.2 | 4.2a |
| Candida albicans (DSM 1665) | — | — | 16.6 | 8.3a |
| Rhodotorula glutinis (DSM 10134) | — | — | 16.6 | 2.1a |
| Acinetobacter baumannii (DSM 30008) | — | — | nt | 0.26b |
| Escherichia coli (DSM 1116) | — | — | — | 1.7c |
| Bacillus subtilis (DSM 10) | — | — | — | 8.3c |
| Mycobacterium smegmatis (ATCC 700084) | — | — | nt | 1.7d |
| Staphylococcus aureus (DSM 346) | — | — | — | 1.7c |
| Pseudomonas aeruginosa (PA 14) | — | — | — | 0.42e |
| Chromobacterium violaceum (DSM 30191) | — | — | nt | 0.42c |
The sporothioethers 14 and 15 appear to arise by spontaneous addition of 2S-2-hydroxy-3-mercaptopropionate to sporothriolide. This is supported by observation of facile addition of 3-mercaptopropionate to 8 in the absence of biological catalysts. This is also consistent with the S-configuration of the 2-hydroxy-3-mercaptopropanoic acid moiety in other natural compounds, such as berkeleylactone A 28,14 sumularin C 30,15 and thiopleurotin 32 (Fig. 2).16
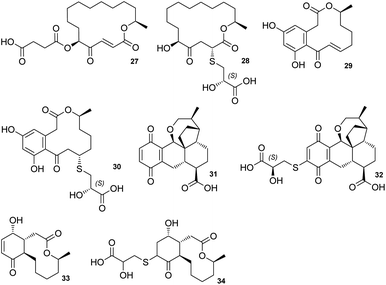 | ||
| Fig. 2 Structures of metabolites containing the 2-hydroxy-3-mercaptopropanoic acid moiety and their congeners. | ||
For the latter compound, cysteine was identified as the origin for the sidechain 22 and this is also likely to be the case for the sporothioethers.16,17 In our hands the 3-epimeric forms of the sporothioethers were inseparable. However, it is clear that material isolated from biological and synthetic sources contains differing proportions of the epimers (Scheme 2B). This may be explained by preferential extraction, or preferential degradation, of one epimer from the biological source.
Some of the 2-hydroxy-3-mercaptopropanoic acid moiety-bearing natural compounds have potent bioactivities, whereas others are significantly less toxic in comparison to their originating compounds. For example, sumalarin C 30 is a potent cytotoxin vs. tumor cell lines, comparable to its congener 10,11-dehydrocurvularin 29 (Fig. 2).15,18 Berkeleylactone A 28 displays strong antibacterial effects against Gram-positive bacteria, even though it is denoted as pro-drug of antibiotic A26771B 27. Here it is hypothesised that the sulfur side chain disrupts the γ-keto-α,β-unsaturated carboxyl, which is thought to be important for bioactivity of the macrolide antibiotic 27 (Fig. 2).19,20
In contrast, thiopleurotinic acid A 32, derived from the cytotoxic and antibacterial compound dihydropleurotinic acid 31 does not possess bioactivity (Fig. 2).16 Another case in which the addition of 2-hydroxy-3-mercaptopropanoic acid 22 is reported as a detoxification mechanism in fungi is antimicrobial compound Sch-642305 33. It is converted into a sulfur derivative 34 (Fig. 2) harboring the 2-hydroxy-3-mercaptopropanoic acid moiety in Aspergillus niger, losing its bioactivity in the process.21 Our results are also consistent with the hypothesis that addition of 2-hydroxy-3-mercaptopropanoic acid may be a self-resistance mechanism, as sporothioethers A 14 and B 15 display significantly reduced antifungal activity than the parent compound 8.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft priority program “Taxon-Omics: New Approaches for Discovering and Naming Biodiversity” (SPP 1991), specifically CO 1328/4-2 and CO 1328/4-1 and by personal PhD stipend from the “Drug Discovery and Cheminformatics for New Anti-Infectives (iCA)” to HZ, and is financially supported by the Ministry for Science & Culture of the German State of Lower Saxony (MWK no. 21—78904-63-5/19). HH gratefully acknowledges funding from the Leibniz University Hannover. The publication of this article was partially funded by the Open Access Fund of the Leibniz University of Hannover.Notes and references
- E. Kuhnert, J. C. Navarro-Muñoz, K. Becker, M. Stadler, J. Collemare and R. J. Cox, Stud. Mycol., 2021, 99, 1–43 CrossRef.
- K. Williams, A. J. Szwalbe, N. P. Mulholland, J. L. Vincent, A. M. Bailey, C. L. Willis, T. J. Simpson and R. J. Cox, Angew. Chem., Int. Ed., 2016, 55, 6784–6788 CrossRef CAS.
- D. H. R. Barton and J. K. Sutherland, J. Chem. Soc., 1963, 1769–1772 Search PubMed.
- B. Bonsch, V. Belt, C. Bartel, N. Duensing, M. Koziol, C. M. Lazarus, A. M. Bailey, T. J. Simpson and R. J. Cox, Chem. Commun., 2016, 52, 6777–6780 RSC.
- N. Liu, Y. S. Hung, S. S. Gao, L. Hang, Y. Zou, Y. H. Chooi and Y. Tang, Org. Lett., 2017, 19, 3560–3563 CrossRef CAS PubMed.
- N. C. J. E. Chesters and D. O'Hagan, J. Chem. Soc., Perkin Trans., 1997, 1, 827–834 RSC.
- K. Becker and M. Stadler, J. Antibiot., 2021, 74, 1–23 CrossRef CAS PubMed.
- S. E. Helaly, B. Thongbai and M. Stadler, Nat. Prod. Rep., 2018, 35, 992–1014 RSC.
- F. Surup, E. Kuhnert, E. Lehmann, S. Heitkämper, K. D. Hyde, J. Fournier and M. Stadler, Mycology, 2014, 5, 110–119 Search PubMed.
- D. S. Tian, E. Kuhnert, J. Ouazzani, D. Wibberg, J. Kalinowski and R. J. Cox, Chem. Sci., 2020, 11, 12477–12484 RSC.
- C. Leman-Loubière, G. Le Goff, C. Debitus and J. Ouazzani, Front. Mar. Sci., 2017, 4, 1–9 Search PubMed.
- K. Krohn, K. Ludewig, H. J. Aust, S. Draeger and B. Schulz, J. Antibiot., 1994, 47, 113–118 CrossRef CAS PubMed.
- T. R. Hoye, C. S. Jeffrey and F. Shao, Nat. Protoc., 2007, 2, 2451–2458 CrossRef CAS PubMed.
- A. A. Stierle, D. B. Stierle, D. Decato, N. D. Priestley, J. B. Alverson, J. Hoody, K. McGrath and D. Klepacki, J. Nat. Prod., 2017, 80, 1150–1160 CrossRef CAS PubMed.
- L. H. Meng, X. M. Li, C. T. Lv, C. S. Li, G. M. Xu, C. G. Huang and B. G. Wang, J. Nat. Prod., 2013, 76, 2145–2149 CrossRef CAS.
- B. Sandargo, B. Thongbai, M. Stadler and F. Surup, J. Nat. Prod., 2018, 81, 286–291 CrossRef CAS.
- B. Ferko, M. Zeman, M. Formica, S. Veselý, J. Doháňošová, J. Moncol, P. Olejníková, D. Berkeš, P. Jakubec, D. J. Dixon and O. Caletková, J. Org. Chem., 2019, 84, 7159–7165 CrossRef CAS.
- M. V. De Castro, L. P. Ióca, D. E. Williams, B. Z. Costa, C. M. Mizuno, M. F. C. Santos, K. De Jesus, É. L. F. Ferreira, M. H. R. Seleghim, L. D. Sette, E. R. Pereira Filho, A. G. Ferreira, N. S. Gonçalves, R. A. Santos, R. J. Andersen and R. G. S. Berlinck, J. Nat. Prod., 2016, 79, 1668–1678 CrossRef CAS.
- J.-M. Zhang, X. Liu, Q. Wei, C. Ma, D. Li and Y. Zou, Nat. Commun., 2022, 13, 225 CrossRef PubMed.
- Y. Zhang, J. Bai, L. Zhang, C. Zhang, B. Liu and Y. Hu, Angew. Chem., Int. Ed., 2021, 60, 6639–6645 CrossRef CAS PubMed.
- E. Adelin, M. T. Martin, M. F. Bricot, S. Cortial, P. Retailleau and J. Ouazzani, Phytochemistry, 2012, 84, 135–140 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06542a |
| This journal is © The Royal Society of Chemistry 2023 |