Open Access Article
Open Access ArticleUtilization of 2-nitrophenols in annulations with aryl isothiocyanates towards the synthesis of 2-aminobenzoxazoles†
Thanh D. Nguyen‡
 ab,
Danh T. Tran‡
ab,
Danh T. Tran‡ ab,
Tan N. Huynh‡
ab,
Tan N. Huynh‡ ab,
Thang M. Ly
ab,
Thang M. Ly ab and
Tung T. Nguyen
ab and
Tung T. Nguyen *ab
*ab
aFaculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam. E-mail: tungtn@hcmut.edu.vn
bVietnam National University Ho Chi Minh City, Linh Trung Ward, Ho Chi Minh City, Vietnam
First published on 15th June 2023
Abstract
A method for the annulation of 2-nitrophenols with aryl isothiocyanates is reported. The reactions proceeded in the presence of an iron(III) acetylacetonate catalyst, elemental sulfur, NaOH as a base, and DMSO as a solvent. Derivatives of 2-aminobenzoxazoles bearing nitro, cyano, acetyl, sulfone, secondary amine, and pyrrolyl groups were successfully isolated.
Introduction
The replacement of 2-aminophenols by 2-nitrophenols in annulations has attracted prominent attention over the last decade.1 Since anilines are typically obtained from the reduction of their nitro upstreams, the direct utilization of 2-nitrophenols would arguably help avoid this extra step, thus increasing the atom efficiency. Yet precedent methods for the utilization of 2-nitrophenols have exorbitantly focused on furnishing carbon-based C2-substituted benzoxazoles.2 As such, diversification by using 2-nitrophenols in annulations still requires more examples.2-Aminophenols are excellent substrates to couple with aryl isothiocyanates, affording 2-aminobenzoxazoles (Scheme 1).3 Given the importance of the aforesaid N,O-heterocycles in bio-related studies,4 substantial studies have been devoted to extension of substrate scope.5 Notably, 2-aminophenols attached with varied functionalities are neither commercially available nor viably prepared. Herein we would like to report a method for synthesis of 2-aminobenzoxazoles from annulation of 2-nitrophenols and aryl isothiocyanates. Tolerance of an array of functionalities on 2-nitrophenols including acetyl, methylsulfonyl, cyano, amines, and pyrrolyl groups was observed. Our method marks a rare example for using nitroarenes to replace the related anilines in synthesis of 2-aminobenzoxazoles (Scheme 1).
Results and discussion
Encouraged by the previous reports,2d,f we started our investigation on the annulation of 2-nitrophenol 1a and phenyl isothiocyanate 2a facilitated by Fe/S cluster. Effects of reaction conditions on the yield of the target product 3aa were thoroughly studied,6 and some of the notable results are shown in Table 1. While the use of iron powder did not furnish 3aa, a 28% yield of 3aa was obtained in the presence of catalytic amount of FeCl3·6H2O (entry 2). A nearly identical yield was obtained when anhydrous FeCl3 was used (entry 3). Among the tested iron salts, Fe(acac)3 was the superior (entry 4).6 Decreasing the amount of Fe(acac)3 slowed down the annulation (entry 5). The reaction did not progress in the absence of Fe(acac)3. The yield of 3aa was slightly improved if the temperature was boosted to 100 °C (entry 6). The amount of elemental sulfur was pivotal to affording a reasonable yield of 3aa (entry 7). The choice of base should also be considered. While the use of DMAP did not improve the yield of 3aa (entry 8), a nearly quantitative yield of 3aa was obtained in the presence of NaOH (entry 9). It should be noted that omitting base still afforded 3aa, albeit in a moderate yield (entry 10). The reaction could be tolerated in DMF solvent (entry 11).| Entry | [Fe] | Base | Yield of 3aa (%) |
|---|---|---|---|
| a 1a (0.1 mmol), 2a (0.2 mmol), [Fe] (0.02 mmol), elemental sulfur (0.2 mmol), base (0.2 mmol), DMSO (0.5 mL), 80 °C, 3 h. Yields are GC yields.b Fe(acac)3 (0.01 mmol).c 100 °C.d Elemental sulfur (0.1 mmol).e DMF (0.5 mL) instead of DMSO. | |||
| 1 | Fe powder | DABCO | 0 |
| 2 | FeCl3·6H2O | DABCO | 28 |
| 3 | FeCl3 | DABCO | 30 |
| 4 | Fe(acac)3 | DABCO | 73 |
| 5b | Fe(acac)3 | DABCO | 57 |
| 6c | Fe(acac)3 | DABCO | 81 |
| 7c,d | Fe(acac)3 | DABCO | 67 |
| 8c | Fe(acac)3 | DMAP | 85 |
| 9c | Fe(acac)3 | NaOH | 92 |
| 10c | Fe(acac)3 | — | 55 |
| 11c,e | Fe(acac)3 | NaOH | 79 |
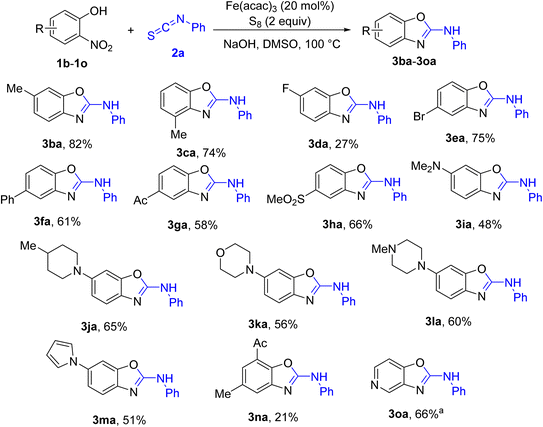
Scope of aryl isothiocyanates was next studied. The results are shown in Scheme 2. In comparison with a related report from our group,5g significant differences regarding electronic properties of aryl isothiocyanates were observed. Low to moderate yields of the annulation products were obtained in case very electron-poor aryl isothiocyanates were attempted (3ad, 3af). Such effect was not detected if 2-aminophenol was used to couple with aryl isothiocyanates.5g The reaction conditions were tolerant of cyano functionality (3ai).
We then turned our attention to extending the scope of 2-nitrophenols, as this is the major improvement compared with previous methods.5 The results are shown in Scheme 3. To our expectation, an array of 2-aminobenzoxazoles bearing useful functionalities has been successfully isolated. Steric effect on the nitrogen atom of 2-nitrophenol was somewhat minor (3ca). In contrast to the previous report,5f 5-fluoro-2-nitrophenol was not compatible with reaction conditions (3da). Meanwhile, 2-nitrophenols bearing acetyl and methylsulfonyl substituents were competent substrates (3ga, 3ha). As substitution of fluorine by amines was feasible,7 2-nitrophenols attached with secondary amines and pyrrole were prepared (1i–1m), then treated with the standard conditions. The ensuing annulations were somewhat successful (3ia–3ma). These molecules were hardly obtained from methods starting with 2-aminophenols.5 2-Aminobenzoxazole derived from 4-hydroxy-3-nitropyridine was obtained in moderate yield (3oa), somewhat indicating that heteroaryl-based 2-nitrophenols were also competent substrates. Attempt to use 2-nitroaniline to couple with phenyl isothiocyanate was not successful at this moment.
Some control experiments were then performed (Scheme 4). An attempt to couple 4-nitrophenol 4a with 2a was unsuccessful (eqn (1)). The result implied the importance of ortho effect if somehow a Fe/S cluster is formed.2d,f The addition of a radical quencher such as 1,1-diphenylethylene did not inhibit the main annulation (eqn (2)), suggesting that a radical-based mechanism was unlikely. Based on these results, a possible mechanism is proposed (Scheme 5). The formation of the Fe/S cluster I would be crucial for the mechanism.2d,f Complexation of phenoxide 5 (obtained from deprotonation of 1a), 2a, and I would yield II, followed by elimination of oxygenated sulfur compounds to afford III.8 Intramolecular addition followed by an extrusion of nitrone IV would regenerate the active cluster I.2d Reduction of IV in the presence of elemental sulfur would yield the target 3aa.
Conclusion
In conclusion, we have developed a method for annulation of 2-nitrophenols and aryl isothiocyanates to yield 2-aminobenzoxazoles. The successes relied on the generation of Fe/S cluster by using Fe(acac)3 catalyst and elemental sulfur. Tolerance of a wide range of useful functionalities is presented. Our method would offer a unique, direct tactic to obtain complex 2-aminobenzoxazoles for relevant studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Vietnam National University Ho Chi Minh City (VNU-HCM) for financial support via project no. NCM2019-20-01. Partial experiments were carried out on instruments provided via project “Laboratory of Advanced Materials”.Notes and references
- For recent review of using nitroarenes as N source: H. Zhu and T. G. Driver, Synthesis, 2022, 54, 3142 CrossRef CAS
.
-
(a) A. Hari, C. Karan, W. C. Rodrigues and B. L. Miller, J. Org. Chem., 2001, 66, 991 CrossRef CAS PubMed
; (b) M. Wu, X. Hu, J. Liu, Y. Liao and G.-J. Deng, Org. Lett., 2012, 14, 2722 CrossRef CAS PubMed
; (c) L. Tang, Z. Yang, T. Sun, D. Zhang, X. Ma, W. Rao and Y. Zhou, Adv. Synth. Catal., 2018, 360, 3055 CrossRef CAS
; (d) L. A. Nguyen, T. T. T. Nguyen, Q. A. Ngo and T. B. Nguyen, Org. Biomol. Chem., 2021, 19, 6015 RSC
; (e) R. R. Putta, S. Chun, S. H. Choi, S. B. Lee, D.-C. Oh and S. Hong, J. Org. Chem., 2020, 85, 15396 CrossRef CAS PubMed
; (f) T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, J. Am. Chem. Soc., 2013, 135, 118 CrossRef CAS PubMed
.
- For reviews:
(a) J. Sharma, P. Mishra and J. Bhadoria, Results Chem., 2022, 4, 100670 CrossRef CAS
; (b) M. Kadagathur, A. S. Shaikh, G. S. Jadhav, D. K. Sigalapalli, N. Shankaraiah and N. D. Tangellamudi, ChemistrySelect, 2021, 6, 2621 CrossRef CAS
.
-
(a) J. C. Harmange, M. M. Weiss, J. Julie Germain, A. J. Polverino, G. Borg, J. Bready, D. Chen, D. Choquette, A. Coxon, T. DeMelfi, L. DiPietro, N. Doerr, J. Estrada, J. Flynn, R. F. Graceffa, S. P. Harriman, S. Kaufman, D. S. La, A. Long, M. W. Martin, S. Neervannan, V. F. Patel, M. Potashman, K. Regal, P. M. Roveto, M. L. Schrag, C. Starnes, A. Tasker, Y. Teffera, L. Wang, R. D. White, D. A. Whittington and R. Zanon, J. Med. Chem., 2008, 51, 1649 CrossRef CAS
; (b) S. K. Sahoo, S. Maddipatla, S. N. R. Gajula, M. N. Ahmad, G. Kaul, S. Nanduri, R. Sonti, A. Dasgupta, S. Chopra and V. M. Yaddanapudi, Bioorg. Med. Chem., 2022, 64, 116777 CrossRef PubMed
.
-
(a) C. L. Cioffi, J. J. Lansing and H. Yüksel, J. Org. Chem., 2010, 75, 7942 CrossRef CAS PubMed
; (b) B. Liu, M. Yin, H. Gao, W. Wu and H. Jiang, J. Org. Chem., 2013, 78, 3009 CrossRef CAS PubMed
; (c) J. Liu and J. M. Hoover, Org. Lett., 2019, 21, 4510 CrossRef CAS PubMed
; (d) M. Kasthuri, H. S. Babu, K. S. Kumar, C. Sudhakar and P. V. N. Kumar, Synlett, 2015, 26, 897 CrossRef CAS
; (e) X. Zhang, X. Jia, J. Wang and X. Fan, Green Chem., 2011, 13, 413 RSC
; (f) J. Zhang, L. Chen, Y. Dong, J. Yang and Y. Wu, Org. Biomol. Chem., 2020, 18, 7425 RSC
; (g) D. T. Tran, T. N. Huynh, P. C. Nguyen, N. T. S. Phan and T. T. Nguyen, Tetrahedron Lett., 2023, 122, 154510 CrossRef CAS
.
- Please see the ESI† for full optimization.
- Z. Wrobél and A. Kwast, Synthesis, 2007, 2, 259 CrossRef
.
- T. B. Nguyen, K. Pasturaud, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2015, 17, 2562 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02873a |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2023 |