Open Access Article
Open Access ArticleFluorinated hydrogel nanoparticles with regulable fluorine contents and T2 relaxation times as 19F MRI contrast agents†
Ziwei Duana,
Changjiang Liua,
Junjie Tanga,
Ruling Zhanga,
Danfeng Pengb,
Ruitao Lub,
Zong Cao*a and
Dalin Wu *a
*a
aSchool of Biomedical Engineering, Shenzhen Campus of Sun Yat-Sen University, Shenzhen, 518107, China. E-mail: wudlin6@mail.sysu.edu.cn; caozhong@mail.sysu.edu.cn
bShenzhen International Institute for Biomedical Research, Shenzhen, 518109, China
First published on 25th July 2023
Abstract
Medical imaging contrast agents that are able to provide detailed biological information have attracted increasing attention. Among the new emerging imaging contrast agents, 19F magnetic resonance imaging contrast agents (19F MRI CAs) are extremely promising for their weak background disturbing signal from the body. However, to prepare 19F MRI CAs with a long T2 relaxation time and excellent biocompatibility in a simple and highly effective strategy is still a challenge. Herein, we report a new type of 19F MRI hydrogel nanocontrast agents (19F MRI HNCAs) synthesized by a surfactant-free emulsion polymerization with commercial fluorinated monomers. The T2 relaxation time of 19F MRI HNCA-1 was found to be 25–40 ms, guaranteeing its good imaging ability in vitro. In addition, according to an investigation into the relationship between the fluorine content and 19F MRI signal intensity, the 19F MRI signal intensity was not only determined by the fluorine content in 19F MRI HNCAs but also by the hydration microenvironment around the fluorine atoms. Moreover, 19F MRI HNCAs demonstrated excellent biocompatibility and imaging capability inside cells. The primary exploration demonstrated that 19F MRI HNCAs as a new type of 19F MRI contrast agent hold potential for imaging lesion sites and tracking cells in vivo by 19F MRI technology.
Introduction
Magnetic resonance imaging (MRI) has gained increasing attention in modern medical diagnosis, because it can allow collecting high-quality information of soft tissues without the use of harmful radioactive nuclides.1–3 Traditionally, MRI technology depends on the local differences in proton spin densities and the relaxation rates of water protons in vivo. However, the above-mentioned two parameters do not have significant differences between detecting sites and healthy tissues in the body, resulting in a failure to obtain sufficient contrast or provide precise information. As a result, contrast agents (CAs) are normally introduced in MRI examination to enhance the image contrast by regulating the relaxation properties of the neighboring water protons.4,5 At present, the normally introduced 1H MRI CAs in clinical use are paramagnetic and superparamagnetic meta ion-based species, such as gadolinium chelates and iron oxide nanoparticles.6,7 Although a significant enhancement of MRI performance by applying paramagnetic and superparamagnetic meta ion-based compounds has been achieved, the 1H MRI CAs still possess some limitations, especially their application security,8 which should be seriously considered. For example, gadolinium-based CAs are able to cause nephrogenic systemic fibrosis in patients with impaired kidney function according to a recent symmetrically study.9,10To further improve the safety of MRI, researchers have started to develop other types of MRI CAs based on heteronuclear atoms, such as fluorine.11,12 Different from 1H MRI CAs that influence the relaxation properties of nearby water protons, the fluorine atoms in 19F MRI CAs are able to be visualized directly by MRI equipment.13 In addition, 19F has a 100% natural abundance and a gyromagnetic ratio close to hydrogen (83% compared with hydrogen), giving 19F MRI excellent imaging resolution.14 Another advantage of 19F MRI is the images have a higher imaging contrast due to the absence of 19F atoms in the detecting domains.15 In addition, the signal intensity in 19F MRI is proportional to the fluorine content, enabling the quantitative application of 19F MRI.16 19F MRI is able to provide more insightful information of soft tissues in vivo over 1H MRI since the fluorine atoms in vivo are mainly embedded in the solid matrices of teeth and bones in vivo.17 In principle, any species containing fluorine atoms, including small molecules,18,19 macromolecules,20 and nano-objectives,21 has potential as 19F MRI CAs.
Perfluorocarbons (PFCs), because of their high fluorine content, have been widely investigated as 19F MRI CAs.22 In practice, PFCs are not the best molecules as 19F MRI CAs, because of their serious accumulation in the liver and spleen.23,24 In addition, the low boiling point of PFCs prohibits this type of 19F MRI CAs from achieving long-term storage in vitro and circulation in vivo, even though the PFC emulsions are stabilized by robust surfactants.25,26 The fluorine content in 19F MRI CAs is not the only parameter determining the imaging result of 19F MRI CAs. For high-efficiency 19F MRI CAs, fluorine atoms should hold very excellent local mobility in 19F MRI CAs under hydration condition, which can ensure a T2 relaxation time of 19F MRI CAs above 10 ms to guarantee sufficient signal collecting intensity during measurement.27 It is well known that the strong hydrophobicity of fluorine atoms causes them to aggregate in 19F MRI CAs, further lose their local mobility, and shorten the T2 relaxation time under hydration conditions.28 In order to solve this critical problem, various strategies have been explored by researchers, such as copolymerizing fluorine-based monomers with various hydrophilic monomers to create a hydration environment around fluorine atoms,29,30 synthesizing hydrophilic monomers appending fluorine atoms,31 and developing smart “On–Off” signal amplifying 19F MRI CAs echoing physiological triggers.32,33 Changkui Fu et al. recently reported water-soluble fluoropolymers with sulfoxide side-china groups as low-fouling 19F MRI CAs (T2 = 373–431 ms). Their results demonstrated that introducing water-soluble groups around fluorine atoms in 19F MRI CAs is an impactful strategy to prolong the T2 relaxation time and enhance the imaging efficacy in vitro and in vivo.34 More directly, Jinhao Gao et al. applied water-soluble fluorine-containing ionic liquids (T2 = 4.4 s) as fluorine markers in 19F MRI. They encapsulated a fluorine-containing ionic liquid inside porous silica nanoparticles and the ionic liquids were sealed by pH-responsive polymers on the surface of the porous silica nanoparticles. Their hybrid system acted as a pH-responsive “Off–On” 19F MRI CA.35 Apart from the mobility of fluorine atoms in the 19F MRI CAs, the magnetically equivalency of the fluorine nuclei is another determinative factor in the 19F MRI CAs performance, because un-equivalent fluorine nuclei can result in blurry MR images.36
In order to achieve high performance in 19F MRI, the influence of the topological structures (linear, block, hyperbranched, star-like) and nanostructures (micelles, vesicles, and worm-like micelles) of 19F MRI CAs on the imaging performance have also been explored in depth,37–43 because the mobility of the fluorine atoms in 19F MRI CAs is determined by the topological structures, while the uptake and accumulation behavior of the 19F MRI CAs inside disease lesions are influenced by their nanostructures.44,45 For example, Kristofer J. Thurecht et al. explored the influence of the topology structure on the T2 relaxation times and imaging performance of 19F MRI CAs by synthesizing a series of hyperbranched polymeric scaffolds. The longest T2 relaxation time of 71 ms could be obtained by incorporating hydrophilic oligo(ethyl glycol) (Mw = 400) in their hyperbranched 19F MRI CAs.29 In the last two decades, polymerization-induced self-assembly (PISA) as a heterogeneous polymerization technology has been developed as an extraordinary powerful method to fabricate different nanostructures of amphiphiles, and accordingly, PISA has been also received increasing attention in 19F MRI CAs preparation. Wei Zhao et al. first prepared poly(oligo(ethylene glycol) methyl ether acrylate-co-2,2,2-trifluoroethyl acrylate) (poly(OEGA-co-TFEA)) as a macro-RAFT chain transferring agent (macro-CTA). After that, AIBN initiated the PISA of styrene and 3-vinyl benzaldehyde with macro-CTA in isopropanol to form 19F MRI CAs with micelles, worm-like micelles, and polymersomes nanostructures. The results revealed that the 19F MRI CAs with a worm-like nanostructure had the best cell-uptake behavior compared with the others. The T2 relaxation times of all the 19F MRI CAs were from 176 ms to 179 ms in D2O. This work indicated the great potential of utilizing PISA to prepare 19F MRI CAs and to adjust their morphologies.46
The imaging performance of the newly synthesized 19F MRI CAs have been improved considerably in the last two decades. However, most of the high-performance 19F MRI CAs developed to date require many synthetic steps and tedious purification procedures in their preparation process, which prohibits most of the synthesized 19F MRI CAs from practical application in clinical use. Thus, exploring facile strategies to fabricate high-performance 19F MRI CAs with acceptable preparation procedures still requires attention and effort. Some research has revealed that to achieve high-resolution in vivo 19F MRI, a very high concentration of 19F MRI CAs (above 50 mM) is normally administrated,47–49 which indicates that prolonging the circulation time of the 19F MRI CAs in vessels in vivo and promoting their accumulation inside the imaging lesion in vivo are pivotal as well. Thus, fabricating high-performance 19F MRI CAs with a long T2 relaxation time and long circulation time in vivo and with excellent biocompatibility by a simple, efficient, and economical strategy demands increasing attention from researchers.
Herein, we present a new type of 19F MRI hydrogel nanoparticle contrast agents (19F MRI HNCAs) synthesized by a surfactant-free emulsion polymerization of the commercial fluorinated monomers (Fig. 1). We hypothesize that the hydration state in hydrogel nanoparticles is able to render the fluorine atoms with enough mobility, allowing the T2 relaxation times of the 19F MRI HNCAs to be over 10 ms. Our explorations demonstrated a T2 relaxation time of the 19F MRI HNCAs of 25–40 ms at concentrations ranging from 5–40 mg mL−1 under a magnetic field strength of 9.4 T. They displayed excellent 19F NMR intensity in D2O/H2O (1/9, v/v) and were able to image well in vitro. In addition, the 19F MRI HNCAs demonstrated excellent biocompatibility and great colloid stability in aqueous solution (PBS buffer) for more than one year at room temperature. Most essentially, we found that the 19F NMR intensity and imaging efficiency mainly depended on the mobility of fluorine atoms rather than the fluorine content inside the 19F MRI HNCAs. Increasing the fluorine content in the 19F MRI HNCA blindly without considering the mobility of the fluorine atoms resulted in an attenuation of the 19F NMR signal intensity and imaging capability. The present research results have great significance in the design and preparation of other advanced 19F MRI CAs.
Experimental section
Materials
Methacryloyl chloride (MAC) (95%), 2,2,2-trifluoroethyl methacrylate (98%), tertiary-butyl acrylate (TBA) (99%), 2,2,2-trifluoroethyl methacrylate (TFMA) (98%), sodium 4-vinylbenzenesulfonate (NaVBS) (90%), potassium persulfate (KPS) (99.9%), trifluoroacetic acid (TFAA) (99%), and n-hydroxysuccinimide (NHS) (95%) were purchased from Shanghai Acmec Biochemical. Bis-(2-hydroxyethyl) disulfide (BHDSH) (>90%) was a product of Shanghai Aladdin Biochemical Technology. Triethylamine (99.5%), methanol (≧99.9%), 3,6-diamino-9-[2-(methoxycarbonyl)phenyl]-xanthylium chloride (Rhodamine-123) (98%), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCL) (≧97%) were products of Anhui Senrise Technology. Deuterium oxide (D2O) (99.9%) and chloroform-d (CDCl3) (99.8%) were purchased from Shanghai Macklin Biochemical Technology. Dichloromethane (DCM) (≧98%), formic acid (FA) (≧88%), ethyl acetate (EA) (99.5%), and petroleum ether (PE) (≧99.9%) were purchased from Guangzhou Brand. Dimethyl sulfoxide (DMSO) (99.9%) was obtained from Beijing Innochem Technology. Tertiary-butyl acrylate and 2,2,2-trifluoroethyl methacrylate were passed through Al2O3 to remove the stabilizer before usage. Phosphotungstate negative staining solution (PNSS) (2%, pH 7.0) was purchased from Leagene Biotechnology. Dulbecco's modified eagle medium (DMEM) with 4.5 g L−1 D-glucose, L-glutamine, 110 mg mL−1 sodium, fetal bovine serum (FBS), and antibiotic–antimycotic (AA) were purchased from Thermo Fisher Scientific. Phosphate-buffered saline (PBS) was purchased from Beijing Solarbio Science & Technology. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Shanghai Aladdin Biochemical Technology. DAPI staining solution (DAPI) (98%) (2 μg mL−1) was a product of Wuhan Servicebio Technology. Paraformaldehyde (PFA) was purchased from biosharp.Synthesis of disulfide dimethacrylate (2,2′-DED)
2,2′-DED was synthesized according to the literature.50 BHDSH (1.5425 g, 10 mmol) and triethylamine (4.1699 mL, 30 mmol) were dissolved in DCM (30 mL) in a two-necked flask equipped with argon (Ar). After the mixture was cooled down by immersion in an ice bath, MAC (2.6135 g, 25 mmol) was added dropwise. The reaction continued under an Ar atmosphere at room temperature for 24 h. The reaction was extracted by DCM and washed with water and brine, dried, and concentrated under vacuum. The crude product was purified by silica gel column chromatography (EA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE = 1
PE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) to give a lightly yellow colored liquid 1.8920 g (72% yield). 1H NMR (CDCl3, 400 MHz, ppm): 6.13 (2H, s, 2H), 5.59 (2H, q, 2H), 4.41 (4H, t, 4H), 2.98 (4H, t, 4H), 1.94 (6H, s, 6H). The NMR spectra was provided in the ESI (Fig. S6†).
15) to give a lightly yellow colored liquid 1.8920 g (72% yield). 1H NMR (CDCl3, 400 MHz, ppm): 6.13 (2H, s, 2H), 5.59 (2H, q, 2H), 4.41 (4H, t, 4H), 2.98 (4H, t, 4H), 1.94 (6H, s, 6H). The NMR spectra was provided in the ESI (Fig. S6†).
Synthesis of 19F MRI HNCAs-1
The polymeric nanoparticles (PNs) were synthesized by a surfactant-free emulsion polymerization. NaVBS (40 mg) and KPS (20 mg) were dissolved in a 40 mL mixture of H2O and methanol (90/10, v/v). TBA, TFMA, and 2,2′-DED were then added into above solution. Then, oxygen was removed by bubbling argon through the solution for 20 min. The polymerization was carried out under an argon atmosphere at 70 °C for 18 h. The produced PNs were washed three times with ethanol and finally with H2O. 19F MRI HNCAs-1 were finally prepared by hydrolyzing the tert-butyl groups of the PNs in a mixture of TFAA and FA (80/20, v/v). The product was finally dialyzed against ddH2O for 48 h with changing the ddH2O three times.Synthesis of rhodamine-123 modified 19F MRI HNCAs-1 (19F MRI HNCAs-1 Rh-123)
19F MRI HNCAs-1 (100 mg) were first dispersed in anhydrous DMSO (2 mL) in a two-necked flask under an argon atmosphere. Then, EDC·HCL (1 mg, 0.005216 mmol) and NHS (600 μg, 0.005214 mmol) were added into the above DMSO solution. The flask was immersed in an ice bath. After 30 min, rhodamine-123 (200 μg, 0.0005252 mmol) was added. The mixture was allowed to react at room temperature in the dark for 36 h. The rhodamine-123 modified 19F MRI HNCAs-1 were washed three times with ethanol and finally with DMSO. The obtained 19F MRI HNCAs-1 Rh-123 were dispersed in DMSO and stored in the refrigerator at −4 °C.Transmission electron microscopy (TEM) analysis
TEM measurements were carried out using an HT7800 SEM instrument (Hitachi High-Tech Scientific Solutions, Japan). The operating pressure of electricity was set to 120 kV. The sample was stained by PNSS as a contrast agent before the measurements.Dynamic light scattering (DLS) and zeta potential measurements
DLS and zeta potential measurements were carried out using a Zetasizer Nano-ZS90 instrument (Malvern Instruments, Malvern, U.K.) equipped with a 4.0 mV He–Ne laser operating at 633 nm and a detection angle of 173°. The measuring temperature was set as 25 °C. The number-weighted hydrodynamic diameter was obtained from analysis of the autocorrelation functions using the method of cumulants. Three measurements were made for each sample with 60 s equilibrium time before each measurement. The concentration of the objectives was 5 mg mL−1 in PBS.19F Nuclear magnetic resonance (19F NMR)
19F NMR experiments were carried out on a Bruker AVANCE III HD Ascend 400 MHz spectrometer using a 12 μs pulse width, relaxation delay of 1 s, acquisition time of 0.72 s, and 32 scans at 25 °C. All the chemical shifts are given herein in ppm.Spin–lattice relaxation times (T1)
The T1 times were measured on a Bruker AVANCE III HD Ascend 400 MHz spectrometer using the standard inversion-recovery pulse sequence at 25 °C. The samples were dissolved in a mixture of D2O/PBS (10/90, v/v) with a certain concentration. For each measurement, the relaxation delay was 1 s and the number of scans was 8.Spin–spin relaxation times (T2)
The T2 times of the 19F HNCAs were measured using the Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence at 25 °C. The 19F MRI HNCAs were dispersed in a mixture of D2O/PBS (10/90, v/v) with a certain concentration. The relaxation delay was 2 s, and the number of scans was 32. For each measurement, the echo times were set from 10 to 1200 s, and 14 points were collected. The decay in amplitude of the spin echo could be described by a single-exponential function, which allowed calculating the T2 relaxation times.19F MRI imaging
Images of phantoms containing the 19F MRI HNCAs solutions were acquired on a Bruker BioSpec 94/20 USR MRI at 9.4 T. The 19F MRI HNCAs solutions (concentrations from 0 mg mL−1 to 40 mg mL−1 in PBS) were loaded in 2 mL Schering bottles. 1H MRI images were acquired using the RARE sequence (field of view = 4 cm, slice thickness = 20 mm, TE = 8.5 ms, TR = 1000 ms, number of averages = 32, FOV = 40 × 40 mm2, matrix = 32 × 32). 19F MRI images were acquired using the FLASH sequence (field of view = 4 cm, slice thickness = 20 mm, TE = 1.3 ms, TR = 800 ms, number of averages = 32, FOV = 40 × 40 mm2, matrix = 32 × 32).Cytotoxicity studies
To quantify a potential impact on cell viability, the MTT assay was applied. Cells were cultured with 19F MRI HNCAs (concentrations from 0 mg mL−1 to 10 mg mL−1) in a 96-well plate in a final volume of 200 μL for 24 h. The MTT solution (20 μL) was added into each well and incubated for 4 h. The liquid in each well of the plate was suction removed, and washed with PBS once. The DMSO (150 μL) was added into each well of the plate, and subsequently incubated on a shaker for 5 min. Finally, the absorbance of the cells was measured using a multifunctional microplate detector (BioTek Instrument, U.S.) at 490 nm.Confocal microscopy analysis
Imaging was conducted to identify if the 19F MRI HNCAs-1 were internalized by the cells. Cells were seeded at a density of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per dish onto a confocal dish. Next, 5 mg mL−1 rhodamine-123 modified 19F MRI HNCAs were added and incubated for 4 h. The cells were fixed for 15 min in 4% PFA, before being rinsed for 3 × 5 min in PBS. Subsequently, DAPI was added to the fixed cells and incubated for 10 min, before being rinsed for 3 × 5 min in PBS. Images were acquired using an Olympus FV3000 laser confocal microscope and were analyzed using XV Viewer software.
000 cells per dish onto a confocal dish. Next, 5 mg mL−1 rhodamine-123 modified 19F MRI HNCAs were added and incubated for 4 h. The cells were fixed for 15 min in 4% PFA, before being rinsed for 3 × 5 min in PBS. Subsequently, DAPI was added to the fixed cells and incubated for 10 min, before being rinsed for 3 × 5 min in PBS. Images were acquired using an Olympus FV3000 laser confocal microscope and were analyzed using XV Viewer software.
Biosafety
The biosafety of the 19F MRI HNCAs-1 was assessed through hematoxylin and eosin (H&E) staining and biochemical index analysis at 5 days after the intravenous injection of the 19F MRI HNCAs-1 (100 μL, 15 mg mL−1) and PBS (blank) and intratumoral injection of the 19F MRI HNCAs-1 (50 μL, 15 mg mL−1) and PBS (blank) in to BALB/c mice. The mice were subsequently killed and the major organs, including heart, liver, spleen, lung, and kidney, were collected, and fixed in 4% PFA solution and embedded in paraffin. Embedded tissue specimens were sectioned and photographed by a microscope. Also, 500 μL blood samples from the mice were collected in 1.5 mL centrifuge tubes, followed by centrifugation at 3000 rpm for 10 min at 4 °C, and the upper serum was collected for biochemical analysis. The serum biochemistry tests included aspartate transaminase (AST), albumin (ALB), total protein (TP), urea (UREA), creatinine (CRE), and cholesterol (CHO). All the experiments concerning the mice samples were approved by the Institutional Animal Care and Use Committee, Sun Yat-sen University (Approval No.: SYSU-IACUC-2020-B1108). We also state that informed consent was obtained for any experimentation with human subjects.19F MRI signal detection inside 4T1 cells
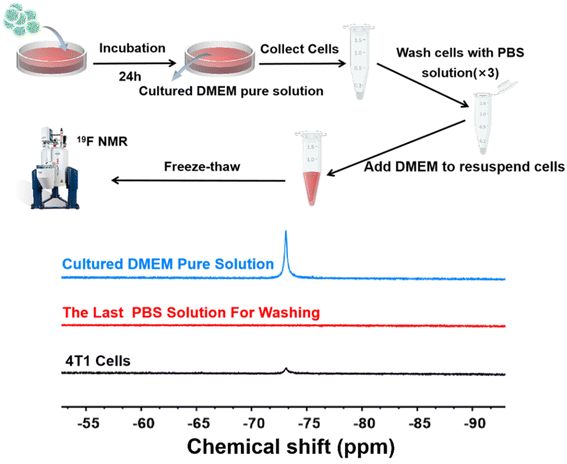
After incubating 19F MRI HNCAs-1 (concentration: 10 mg mL−1 in DMEM) with 4T1 cells at 37 °C for 24 h, the 4T1 cells (∼6 × 106) were trypsinized and collected into a 1.5 mL centrifuge tube. Subsequently, the 4T1 cells were centrifuged and washed three times by pure PBS solution. The washed cells were resuspended in 300 μL clean DMEM and then subjected to five freeze–thawing cycles. Finally, the cells were centrifuged and the supernatants were collected for 19F NMR measurement. The cultured DMEM pure solution (the co-culture medium) and the last PBS solution that was used to wash the 4T1 cells were characterized by 19F NMR.Results and discussion
19F MRI HNCAs synthesis and characterization
The detailed synthesis procedure and constitution of the 19F MRI HNCAs are presented in Fig. 1 and the Experimental section. The surfactant-free emulsion co-polymerization of tertiary-butyl acrylate (TBA) and trifluoroethyl methacrylate (TFMA) was applied to synthesize the 19F MRI HNCAs in this work, because of its simplicity, scale-up potential, and potential for diameter manipulation. TFMA with magnetically equivalent fluorine atoms was chosen as the fluorine marker. By turning the feed ratio of TBA and TFMA during the polymerization, the fluorine content inside the 19F MRI HNCAs and the 19F NMR intensity can be easily regulated in principle. During the polymerization, 1% (mole ratio to total monomers) crosslinking agent ((2,2′-dithiodiethanol) diacrylate (2,2′-DED)) (Fig. S6†) was added to maintain the morphology of the 19F MRI HNCAs in aqueous solution. In order to verify the validity of this synthesis protocol, 19F MRI HNCAs-1 with a monomer mole ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (TBA
1 (TBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TFMA = 9
TFMA = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were first synthesized. Around 30 g polymeric nanoparticles (PNs) (the precursor of the 19F MRI HNCAs-1) could be prepared by this approach every time.
1) were first synthesized. Around 30 g polymeric nanoparticles (PNs) (the precursor of the 19F MRI HNCAs-1) could be prepared by this approach every time.
Fig. 2 presents the dynamic light scattering (DLS) results, showing the PNs-1 with a diameter of 96 ± 26 nm (Fig. 2A, black line). The zeta potential value of PNs-1 was −37.7 ± 0.3 mV (Fig. 2B, black column) indicating the sufficient negative charge on the surface and their good dispersity in aqueous solution. The TEM image demonstrated the PNs-1 had a spherical structure (Fig. 2C). In order to synthesize the 19F MRI HNCAs-1, the tert-butyl groups inside the PNs-1 were deprotected in the presence of trifluoroacetic acid (TFAA) and formic acid (FA). According to the DLS results (Fig. 2A, red line), the 19F MRI HNCAs-1 had a diameter of 230 ± 73 nm. The increase in the diameter was caused by the swelling of the hydrophilic poly(acrylic acid)s in the 19F MRI HNCAs-1. Inside 19F MRI HNCAs-1, the hydrophilic poly(acrylic acid)s were able to enhance the hydration effect for the nearby fluorine atoms. The zeta potential value of the 19F MRI HNCAs-1 was −22.4 ± 0.4 mV, as shown in Fig. 2B (red column), demonstrating that the negative charges (SO32−) were not located on surface of the 19F MRI HNCAs-1, which could be caused by the increased flexibility of the polymer chains and the migration of the negative charges (SO32−) into the core of the 19F MRI HNCAs-1. The TEM image in Fig. 2D presented the round morphology of the 19F MRI HNCAs-1, clearly demonstrating that using 1% crosslinking agent (2,2′-DED) was enough to maintain the integrity of the 19F MRI HNCAs-1 during the hydrolysis reaction. This is important as nanosized objectives are apt to aggregate in aqueous solution to reduce their total surface energy, especially in the presence of ions. The 19F MRI HNCAs-1 could maintain their stable hydrodynamic diameter values and PDI for at least 30 days in PBS buffer at room temperature, as shown in Fig. S1,† demonstrating that the 19F MRI HNCAs-1 could be stored for a long time.
Magnetic resonance properties of the 19F MRI HNCAs
As discussed in the introduction, the mobility of fluorine atoms is restricted by the hydrophobic interaction among fluorine atoms and tert-butyl groups and dipole–dipole interactions among different fluorine atoms in PNs. We hypothesized that the mobility of fluorine atoms in PNs-1 could be optimized by deprotecting the hydrophobic tert-butyl groups, and accordingly, the 19F NMR intensity of the 19F MRI HNCAs-1 could be promoted, because the left hydrophilic carboxylic groups could enhance the hydration condition around the fluorine atoms. As shown in Fig. 3A, the 19F NMR intensity of the PNs-1 at a chemical shift of −73 ppm in D2O/H2O (1/9, v/v) was negligible, while the 19F NMR intensity (chemical shift of −73 ppm) increased remarkably after removing the tert-butyl groups for 19F MRI HNCAs-1 in D2O/H2O (1/9, v/v) at the same concentration of nanoobjects (10 mg mL−1). This encouraging result verified our above hypothesis directly. Compared with the 1H MRI technology, one of the greatest advantages of 19F MRI is that the signal intensity is proportional to the fluorine content in 19F MRI CAs. As shown in Fig. 3B and S2,† the signal-to-noise ratio (SNR) was linearly dependent on the concentration of 19F MRI HNCAs-1 in aqueous solution (2.5–40 mg mL−1), indicating the 19F MRI HNCAs-1 are suitable for quantitative MRI measurements. Because the carboxylic acid groups can undergo protonation and deprotonation when the pH values of the solution changes, this process may change the hydration environment of fluorine atoms and influence the imaging property of 19F MRI HNCAs-1. Accordingly, 19F NMR measurements were also carried out at varying pH values from 3.0 to 11.0. As shown in Fig. S3,† the 19F signal of 19F MRI HNCAs-1 maintained a consistent intensity at this pH range, demonstrating the imaging performance of 19F MRI HNCAs-1 was not influenced by the pH of the surroundings.Beside the mobility of fluorine atoms, the fluorine content in 19F MRI CAs also influence the 19F NMR intensity and the 19F MRI imaging behavior.44 Thus, by adjusting the feed mole ratio of monomer TBA and TFMA in the polymerization process, another four 19F MRI HNCAs samples with different fluorine contents were synthesized by applying the same procedure and conditions as well. Compared with the 19F MRI HNCAs-1, the fluorine contents of the newly synthesized 19F MRI HNCAs were enhanced with increasing the feed ratio of TFMA in the polymerization. The detailed monomer ratios and the newly synthesized 19F MRI HNCAs characteristics are presented in Table S1.† The diameters of the newly synthesized 19F MRI HNCAs were between 180 nm and 250 nm. As shown in Fig. 4A, before deprotecting the tert-butyl groups, the 19F NMR intensities of the newly synthesized 19F MRI HNCAs did not appear at a chemical shift of −72.9 ppm, while the 19F NMR intensities appeared again at a chemical shift of −72.9 ppm after the tert-butyl groups were removed, which was consistent with the result of the 19F MRI HNCAs-1. In principle, 19F MRI CAs with a higher fluorine content should display a higher 19F NMR intensity. However, for our 19F MRI HNCAs, 19F MRI HNCAs-1, which had the lowest fluorine content, displayed the strongest 19F NMR intensity at the same fluorine concentration of 0.7 mg mL−1 and aqueous medium volume of 0.5 mL during the 19F NMR intensity measurements (Fig. 4A). This could be attributed to the fluorine atoms easily suffering from self-aggregation due to their strong dipole–dipole interactions and super hydrophobicity in aqueous solution. Compared with the other four newly prepared 19F MRI HNCAs, the low fluorine content inside 19F MRI HNCAs-1 resulted in the least self-aggregation and the greatest mobility of fluorine atoms, which finally caused it to display the strongest 19F NMR intensity.
For our research in this article, because 19F MRI HNCAs-1 had the best 19F NMR performance in aqueous solution compared with the others, the 19F MRI HNCAs-1 were chosen for further study. It is well known that calcium ions existing in the cell nutrition medium and bodily fluids in vivo can complex with carboxylic acid to form supramolecular crosslinking points,51 which can slow down the chain segments movement of poly(acrylate acid)s. Because abundant carboxylic acids are located inside the 19F MRI HNCAs-1, we speculated that the calcium ions probably are able to change the fluorine atoms nearby microstructure and influence the 19F NMR intensity of the 19F MRI HNCAs-1. As shown in Fig. S4,† the 19F NMR intensity of the 19F MRI HNCAs-1 in aqueous solution containing calcium ions with a concentration of 6 mg mL−1 was almost the same with that in a calcium ions-free aqueous solution, which demonstrated that the formation of supramolecular crosslinking points between carboxylic acids and calcium ions surrounding fluorine atoms could not prohibit the mobilities of the fluorine atoms and decrease the 19F NMR intensity of the 19F MRI HNCAs-1.
In aqueous solution, a longer T2 relaxation time of 19F MRI CAs is essential for generating high-resolution 19F MRI images in vitro and in vivo. Normally, an ideal 19F MRI CA should have a T2 relaxation time longer than 10 ms for achieving excellent performance in in vivo imaging.27 Therefore, the T1 and T2 relaxation times of the 19F MRI HNCAs-1 were measured using the inversion-recovery and Carr–Purcell–Meiboom–Gill (CPMG) pulse sequences, respectively. The T2 relaxation time of 19F MRI HNCAs-1 at concentrations from 5 mg mL−1 to 10 mg mL−1 were all over 40 ms without drastic change, indicating that the fluorine atoms maintained their constant mobility in this concentration range, as shown in Fig. 4B. The T2 relaxation time longer than 10 ms for 19F MRI HNCAs-1 was attributed to the good mobility of fluorine atoms inside the hydration microenvironment caused by the nearby hydrophilic carboxyl groups. However, the T2 relaxation time decreased to around 25 ms when the concentration of the 19F MRI HNCAs-1 increased to 20 mg mL−1, while the T2 relaxation times of around 25 ms were stably maintained in the concentration range of 20–40 mg mL−1. Obviously, 19F MRI HNCAs-1 had T2 relaxation times longer than 10 ms in the concentration range of 5–40 mg mL−1, demonstrating the 19F MRI HNCAs-1 have great potential for use in imaging. The T1 relaxation times of 19F MRI HNCAs-1 in the concentration range of 5–40 mg mL−1 were maintained as 450–500 ms without any obvious concentration dependency.
19F MRI imaging behavior of the 19F MRI HNCAs-1 in vitro
As discussed above, the 19F MRI HNCAs-1 displayed an excellent 19F NMR intensity and long T2 relaxation time in aqueous solution as shown in Fig. 4, thus the 19F MRI HNCAs-1 should be able to perform well in 19F MRI in vitro in principle. As shown in Fig. 5, hot-spot images of the 19F MRI HNCAs-1 aqueous solution at concentrations of 5–40 mg mL−1 were successfully obtained. Moreover, the brightness of the hot-spot images was linearly dependent on the concentration of the 19F MRI HNCAs-1. In comparison, the aqueous solution of the PNs-1 (before deprotecting the tert groups) did not show an 19F MRI intensity in the same concentration range and measuring condition. The in vitro hot-spot imaging results were agreement with the 19F NMR outcome in Fig. 3A, showing that removing the hydrophobic tert-butyl groups was able to endow the 19F MRI HNCAs-1 with a strong fluorine atoms mobility and 19F NMR intensity. | ||
| Fig. 5 1H/19F MRI performances of the 19F MRI HNCAs-1 and PNs-1 aqueous solution at concentrations of 5–40 mg mL−1 in vitro. | ||
Cell-uptake behavior and biocompatibility of the 19F MRI HNCAs-1
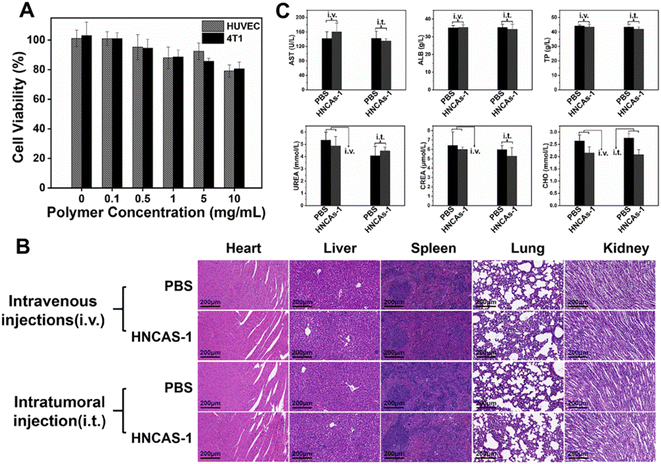
The successful imaging behaviors of the 19F MRI HNCAs-1 aqueous solution in vitro encouraged us to explore their application potential at the cellular level. The cell-uptake behavior of the 19F MRI HNCAs-1 was first investigated by flow cytometry in both red and green channels. In order to trace the 19F MRI HNCAs-1 by flow cytometry, the 19F MRI HNCAs-1 were first modified by rhodamine-123 with EDC·HCl and NHS. As shown in Fig. S5,† the fluorescence emission at 530 nm in the fluorescence spectrum demonstrated the successful modification of rhodamine-123 on the 19F MRI HNCAs-1. As shown in Fig. 6A, at 1 h post incubation of the 19F MRI HNCAs-1 with 4T1 cells (breast cancer cell), the fluorescence histograms demonstrated that the 19F MRI HNCAs-1 had been endocytosed by 4T1 already. The fast uptake behavior of the 19F MRI HNCAs-1 by 4T1 cells could probably be attributed to the extreme hydrophilicity of the carboxylic acid groups located on the surface of the 19F MRI HNCAs-1. The uptake amount of the 19F MRI HNCAs-1 by 4T1 cells reached a maximum at 4 h post incubation. After that, the amount of 19F MRI HNCAs-1 inside the 4T1 cells started to decrease due to the metabolism process inside the 4T1 cells. The mean fluorescence intensities of the 4T1 cell exploration outcomes were in agreement with the above fluorescence histograms results, as shown in Fig. 6B. In addition, in order to confirm the cell-uptake behavior, CLSM was used to visualize the presence of rhodamine-123-modified 19F MRI HNCAs-1 inside the 4T1 cells. As shown in Fig. 6C, the Z-slices through the cellular equator presented obvious blue and green fluorescence signals, largely within the cytoplasm of the 4T1 cancer cells, demonstrating the successful cellular uptake at 4 h post incubation of the 19F MRI HNCAs-1 with 4T1 cells. The successful uptake behavior demonstrated that the 19F MRI HNCAs-1 hold potential in imaging species and metabolic processes inside cells.As practicable 19F MRI CAs, the biocompatibility and toxicity of the 19F MRI HNCAs-1 for cells and mice are determining factors. The cytotoxicity of the 19F MRI HNCAs-1 was first investigated against 4T1 cells and HUVEC (human umbilical vein endothelial cells) by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. As shown in Fig. 7A, the 19F MRI HNCAs-1 at concentrations of 0.1 to 10 mg mL−1 showed cell viability ratios above 80% after 24 h incubation, demonstrating the 19F MRI HNCAs-1 had excellent biocompatibility for both cancer and mammalian cells. In order to systematically access the biocompatibility and safety in mice, 100 μL 19F MRI HNCAs-1 with a concentration of 15 mg mL−1 was administrated into mice via lateral tail vein injection and intratumoral injection. At 5 days post-injection of the 19F MRI HNCAs-1, the important organs (heart, liver, spleen, lung, and kidney) of the mice were harvested and subjected to histological examination. The H&E staining images of the main organs indicated that they maintained their typical physiological structures without any tissue damage, as shown in Fig. 7B. In addition, the main physiology indices of blood, including albumin (ALB), aspartate transaminase (AST), creatinine (CRE), total protein (TP), cholesterol (CHOL), and urea (UREA), were at similar levels to the PBS control group, both maintaining a healthy level, as shown in Fig. 7C. All the above exploration results demonstrated that the 19F MRI HNCAs-1 possess satisfactory biosafety.
19F MRI capability of the 19F MRI HNCAs-1 inside 4T1 cells
The 19F MRI HNCAs-1 displayed excellent 19F NMR intensity, 19F MRI performance in vitro, highly efficient cell-uptake behavior, and excellent biosafety against cells and mice. As a result, we hypothesized that the 19F MRI HNCAs-1 would be able to image inside cells. To test this hypothesis, the19 F MRI HNCAs-1 were co-cultured with 4T1 cells for 24 h. The detailed purification processes are shown in Fig. 8 and described in the Experimental section. As shown in Fig. 8, the cultured DMEM pure solution showed 19F NMR intensity (blue line) demonstrating that not all the 19F MRI HNCAs-1 could be phagocytosed by 4T1 cells during the co-culture. In order to confirm that the 19F MRI HNCAs-1 could image inside 4T1 cells after being phagocytosed, the co-cultured 4T1 cells were carefully washed with PBS three times to wash away any free 19F MRI HNCAs-1 outside the 4T1 cells. The 19F NMR signal at a chemical shift of −73 ppm of the PBS washing solution (the third-time of washing solution) was not detectable (red line), demonstrating the free 19F MRI HNCAs-1 outside the 4T1 cells had been fully washed away. After the freeze–thawing processes, the remaining 4T1 cells were transferred and characterized by 19F NMR in DMEM. The appearance of the 19F NMR signal at a chemical shift of −73 ppm (black line) indicated that the 19F MRI HNCAs-1 inside 4T1 cells still maintained their 19F MRI capability. The above results demonstrated again that the 19F MRI HNCAs-1 could be endocytosed by 4T1 cells. More importantly, the 19F NMR intensity of the endocytosed 19F HNCAs-1 inside 4T1 was still retained.Conclusion
We developed a simple and high-performance surfactant-free emulsion polymerization strategy to synthesize 19F MRI CAs utilizing commercial fluorinated monomers. The research results demonstrated that donating a good hydration microenvironment surrounding the fluorine atoms could promote the mobility of the fluorine atoms and enhance the 19F MR imaging performance of the 19F MRI CAs. However, an intensive 19F NMR intensity could not be achieved by just increasing the amount of fluorinated monomer during the synthesis process or the fluorine content in the 19F MRI HNCAs. The 19F MRI HNCAs-1 displayed long-term storage stability, robust functional stability, great biosafety in vivo, and excellent 19F MR imaging capability in vitro. For that reason, we believe that the 19F MRI HNCAs-1 could serve as a powerful 19F MRI contrast agent for not only the precise imaging of lesion sites (such as tumor, inflammation) but also could be used for cell tracking in vivo non-invasively. Additionally, the strategy used to prepare the 19F MRI CAs in this article has a certain referential significance for the preparation of new types of 19F MRI CAs.Conflicts of interest
The authors declare they have no competing financial interests.Acknowledgements
Dalin Wu acknowledges the financial support of the National Natural Science Foundation of China (22075327) and the Natural Science Foundation of Guangdong Province (2022A1515011392). In addition, Zheng Hu was acknowledged for her constructive suggestion on running the cell experiments.References
- P. Debbage and W. Jaschke, Histochem. Cell Biol., 2008, 130, 845–875 CrossRef CAS PubMed.
- E. T. Ahrens and J. W. M. Bulte, Nat. Rev. Immunol., 2013, 13, 755–763 CrossRef CAS PubMed.
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS PubMed.
- D. Hao, T. Ai, F. Goerner, X. Hu, V. M. Runge and M. Tweedle, J. Magn. Reson. Imaging, 2012, 36, 1060–1071 CrossRef PubMed.
- J. L. Major and T. J. Meade, Acc. Chem. Res., 2009, 42, 893–903 CrossRef CAS PubMed.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS.
- H. B. Na, I. C. Song and T. Hyeon, Adv. Mater., 2009, 21, 2133–2148 CrossRef CAS.
- M.-F. Bellin, Eur. J. Radiol., 2006, 60, 314–323 CrossRef PubMed.
- J. T. Heverhagen, G. A. Krombach and E. Gizewski, Rofo, 2014, 186, 661–669 CrossRef CAS PubMed.
- P. S. N. Rowe, L. V. Zelenchuk, J. S. Laurence, P. Lee, W. M. Brooks and E. T. McCarthy, Am. J. Physiol., 2015, 309, F764–F769 CAS.
- J. C. Knight, P. G. Edwards and S. J. Paisey, RSC Adv., 2011, 1, 1415–1425 RSC.
- E. T. Ahrens, B. M. Helfer, C. F. O'Hanlon and C. Schirda, Magn. Reson. Med., 2014, 72, 1696–1701 CrossRef CAS.
- A. Mali, E. L. Kaijzel, H. J. Lamb and L. J. Cruz, J. Controlled Release, 2021, 338, 870–889 CrossRef CAS PubMed.
- E. T. Ahrens and J. Zhong, NMR Biomed., 2013, 26, 860–871 CrossRef CAS PubMed.
- E. T. Ahrens, R. Flores, H. Xu and P. A. Morel, Nat. Biotechnol., 2005, 23, 983–987 CrossRef CAS PubMed.
- M. Srinivas, P. A. Morel, L. A. Ernst, D. H. Laidlaw and E. T. Ahrens, Magn. Reson. Med., 2007, 58, 725–734 CrossRef CAS PubMed.
- J.-X. Yu, R. R. Hallac, S. Chiguru and R. P. Mason, Prog. Nucl. Magn. Reson. Spectrosc., 2013, 70, 25–49 CrossRef CAS PubMed.
- G. D. Kenny, K. P. Shaw, S. Sivachelvam, A. J. P. White, R. M. Botnar and R. T. M. de Rosales, J. Fluorine Chem., 2016, 184, 58–64 CrossRef CAS PubMed.
- J. Zhang, Y. Yuan, Y. Li, H. Yang, H. Zhang, S. Chen, X. Zhou, Z. Yang and Z.-X. Jiang, J. Org. Chem., 2020, 85, 6778–6787 CrossRef CAS PubMed.
- Z.-X. Jiang and Y. B. Yu, J. Org. Chem., 2010, 75, 2044–2049 CrossRef CAS.
- M. Ogawa, S. Nitahara, H. Aoki, S. Ito, M. Narazaki and T. Matsuda, Macromol. Chem. Phys., 2010, 211, 1602–1609 CrossRef CAS.
- J. Ruiz-Cabello, B. P. Barnett, P. A. Bottomley and J. W. M. Bulte, NMR Biomed., 2011, 24, 114–129 CrossRef CAS PubMed.
- I. Tirotta, V. Dichiarante, C. Pigliacelli, G. Cavallo, G. Terraneo, F. B. Bombelli, P. Metrangolo and G. Resnati, Chem. Rev., 2015, 115, 1106–1129 CrossRef CAS PubMed.
- R. Holman, O. Lorton, P. C. Guillemin, S. Desgranges, C. Contino-Pépin and R. Salomir, Front. Chem., 2022, 9, 810029 CrossRef.
- I. N. Kuznetsova, Pharmaceutical Chemistry Journal, 2003, 37, 415–420 CrossRef CAS.
- Y. Huang, A. M. Vezeridis, J. Wang, Z. Wang, M. Thompson, R. F. Mattrey and N. C. Gianneschi, J. Am. Chem. Soc., 2017, 139, 15–18 CrossRef CAS PubMed.
- D. Jirak, A. Galisova, K. Kolouchova, D. Babuka and M. Hruby, Magn. Reson. Mater. Phys., Biol. Med., 2019, 32, 173–185 CrossRef CAS PubMed.
- Y. Wang, X. Tan, A. Usman, Y. Zhang, M. Sawczyk, P. Král, C. Zhang and A. K. Whittaker, ACS Macro Lett., 2022, 11, 1195–1201 CrossRef CAS PubMed.
- K. J. Thurecht, I. Blakey, H. Peng, O. Squires, S. Hsu, C. Alexander and A. K. Whittaker, J. Am. Chem. Soc., 2010, 132, 5336–5337 CrossRef CAS.
- B. E. Rolfe, I. Blakey, O. Squires, H. Peng, N. R. B. Boase, C. Alexander, P. G. Parsons, G. M. Boyle, A. K. Whittaker and K. J. Thurecht, J. Am. Chem. Soc., 2014, 136, 2413–2419 CrossRef CAS PubMed.
- Z. Guo, M. Gao, M. Song, Y. Li, D. Zhang, D. Xu, L. You, L. Wang, R. Zhuang, X. Su, T. Liu, J. Du and X. Zhang, Adv. Mater., 2016, 28, 5898–5906 CrossRef CAS PubMed.
- Y. Yuan, S. Ge, H. Sun, X. Dong, H. Zhao, L. An, J. Zhang, J. Wang, B. Hu and G. Liang, ACS Nano, 2015, 9, 5117–5124 CrossRef CAS PubMed.
- H. Lin, X. Tang, A. Li and J. Gao, Adv. Mater., 2021, 33, 2005657 CrossRef CAS.
- C. Fu, B. Demir, S. Alcantara, V. Kumar, F. Han, H. G. Kelly, X. Tan, Y. Yu, W. Xu, J. Zhao, C. Zhang, H. Peng, C. Boyer, T. M. Woodruff, S. J. Kent, D. J. Searles and A. K. Whittaker, Angew. Chem., Int. Ed., 2020, 59, 4729–4735 CrossRef CAS PubMed.
- X. Zhu, X. Tang, H. Lin, S. Shi, H. Xiong, Q. Zhou, A. Li, Q. Wang, X. Chen and J. Gao, Chem, 2020, 6, 1134–1148 CAS.
- K. L. Peterson, K. Srivastava and V. C. Pierre, Front. Chem., 2018, 6, 160–181 CrossRef PubMed.
- C. Zhang, Y. Zhou, Q. Liu, S. Li, S. Perrier and Y. Zhao, Macromolecules, 2011, 44, 2034–2049 CrossRef CAS.
- K. Wang, H. Peng, K. J. Thurecht, S. Puttick and A. K. Whittaker, Polym. Chem., 2014, 5, 1760–1771 RSC.
- K. Wang, H. Peng, K. J. Thurecht, S. Puttick and A. K. Whittaker, Biomacromolecules, 2015, 16, 2827–2839 CrossRef CAS PubMed.
- S. Li, J. Han and C. Gao, Polym. Chem., 2013, 4, 1774–1787 RSC.
- O. Munkhbat, M. Canakci, S. Zheng, W. Hu, B. Osborne, A. A. Bogdanov and S. Thayumanavan, Biomacromolecules, 2019, 20, 790–800 CrossRef CAS.
- N. J. Warren and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 10174–10185 CrossRef CAS PubMed.
- X. Tang, X. Gong, A. Li, H. Lin, C. Peng, X. Zhang, X. Chen and J. Gao, Nano Lett., 2020, 20, 363–371 CrossRef CAS PubMed.
- Y. Mo, C. Huang, C. Liu, Z. Duan, J. Liu and D. Wu, Macromol. Rapid Commun., 2023, 2200744 CrossRef PubMed.
- D. Janasik and T. Krawczyk, Chem. - Eur. J., 2022, 28, e202102556 CAS.
- W. Zhao, H. T. Ta, C. Zhang and A. K. Whittaker, Biomacromolecules, 2017, 18, 1145–1156 CrossRef CAS PubMed.
- C. Zhang, S. S. Moonshi, Y. Han, S. Puttick, H. Peng, B. J. A. Magoling, J. C. Reid, S. Bernardi, D. J. Searles, P. Král and A. K. Whittaker, Macromolecules, 2017, 50, 5953–5963 CrossRef CAS.
- C. Zhang, S. S. Moonshi, W. Wang, H. T. Ta, Y. Han, F. Y. Han, H. Peng, P. Král, B. E. Rolfe, J. J. Gooding, K. Gaus and A. K. Whittaker, ACS Nano, 2018, 12, 9162–9176 CrossRef CAS.
- X. Tang, A. Li, C. Zuo, X. Liu, X. Luo, L. Chen, L. Li, H. Lin and J. Gao, ACS Nano, 2023, 17, 5014–5024 CrossRef CAS PubMed.
- C. Miao, F. Li, Y. Zuo, R. Wang and Y. Xiong, RSC Adv., 2016, 6, 3013–3019 RSC.
- M. B. Gindele, K. K. Malaszuk, C. Peter and D. Gebauer, Langmuir, 2022, 38, 14409–14421 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02827e |
| This journal is © The Royal Society of Chemistry 2023 |