Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel nanocomposite containing zinc ferrite nanoparticles embedded in carboxymethylcellulose hydrogel plus carbon nitride nanosheets with multifunctional bioactivity
Fatemeh Ganjalia,
Mostafa Ghafori Goraba,
Hooman Aghamirza Moghim Aliabadi b,
Saman Rahmatic,
Reza Ahangari Cohand,
Reza Eivazzadeh-Keihan*d,
Ali Maleki
b,
Saman Rahmatic,
Reza Ahangari Cohand,
Reza Eivazzadeh-Keihan*d,
Ali Maleki *a,
Hossein Ghafuri
*a,
Hossein Ghafuri a and
Mohammad Mahdavi
a and
Mohammad Mahdavi *e
*e
aCatalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir; Fax: +98-21-73021584; Tel: +98-21-73228313
bAdvanced Chemical Studies Lab, Department of Chemistry, K. N. Toosi University of Technology, Tehran, Iran
cProtein Chemistry Laboratory, Department of Medical Biotechnology, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
dNanobiotechnology Department, New Technologies Research Group, Pasteur Institute of Iran, Tehran, Iran. E-mail: reza.tab_chemist@yahoo.com
eEndocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran. E-mail: momahdavi@sina.tums.ac.ir
First published on 19th July 2023
Abstract
A novel and biologically active nanobiocomposite is synthesized based on carbon nitride nanosheet (g-C3N4) based carboxymethylcellulose hydrogels with embedded zinc ferrite nanoparticles. Physical-chemical aspects, morphological properties, and their multifunctional biological properties have been considered in the process of evaluation of the synthesized structure. The hydrogels' compressive strength and compressive modulus are 1.98 ± 0.03 MPa and 3.46 ± 0.05 MPa, respectively. Regarding the biological response, it is shown that the nanobiocomposite is non-toxic and biocompatible, and hemocompatible (with Hu02 cells). In addition, the developed material offers a suitable antibacterial activity for both Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli).
1. Introduction
Hydrogels represent cross-linked three-dimensional polymers, which can be obtained from natural or synthetic sources. The remarkable characteristic attributed to hydrogels is the ability to absorb water or liquids with biological properties without changing their structures.1–3 Hydrogels were first reported in 1960 by Wichterle and Lím.4 The abovementioned characteristics of hydrogels have led to their widespread use in a variety of biomedical fields, including tissue regeneration,5,6 contact lenses,7 wound dressings,8 expansion of stem cells,9 tissue engineering,10,11 drug delivery,12 functional coatings,13 or as antibacterial14 and antimicrobial15 materials. A variety of synthetic polymers have been used to develop hydrogels, including polyethylene glycol,16 polyvinyl alcohol,17 polyacrylamide,18 and polyacrylic acid.19 In addition, various natural polymers have also been used for this purpose, such as alginate, starch, gelatin, cellulose, chitosan, and their derivatives.20 Natural polymers with specific properties such as non-toxicity, biocompatibility, biodegradability, and hydrophilicity are particularly important in the biological and biomedical fields.20–22 Among natural polymers, cellulose and, in particular, its modified form, carboxymethylcellulose (CMC), have been the center of attention in the development of hydrogels due to natural abundance, low price, significant mechanical properties and, the simplicity of processing for the preparation of the hydrogels.20,23–25Advanced hydrogels have been further developed by introducing 2D materials such as graphene analogs, allowing the tuning of specific hydrogel properties, including mechanical and electrical ones.26–29 In this regard, graphitic carbon nitride (g-C3N4), as a metal-free polymeric structure with two-dimensional layered morphology, has been particularly applied for various purposes, considering its chemical and thermal stability, wide surface area, biocompatibility, and non-toxicity.30,31 There have been growing reports on the use of g-C3N4 in areas including organic synthesis and catalyst,32 supercapacitors,33 biosensors,34 environmental remediation,35 energy,36 and biomedical application.37 Recently, g-C3N4, especially in the form of g-C3N4 nanosheets (CN), has been functionalized covalently or non-covalently by various molecules to improve its properties and applications.38 For example, vitamin B1 has been attached to CN by the 1,3-dibromopropane linker for the catalysis of quinoxalines.39 Further, melamine conjunction with g-C3N4, allows the formation of molecules with a large volume of NH2 groups.40
Also, metal nanoparticles are finding applications in catalysts,41,42 composites,43–45 sensors and microelectronics,46 semiconductors,47 and biological sciences.48–51 ZnFe2O4 NPs represent a biocompatible nanomaterial with excellent chemical stability, low toxicity, and potential biomedical applications.52,53 In addition, the ability of these nanoparticles to destroy various bacterial and microbial species causes the material to become a valuable component in biocomposites related to medical and environmental fields.54 ZnFe2O4 NPs have been used for the development of hybrid materials with graphene,55 natural and synthetic polymers,56 metal–organic frameworks,57 zeolites,58 and hydrogels.59 Magnesium hydroxide nanoparticles have been used recently as an antibacterial agent in manufacturing nanobiocomposites, including CMC hydrogels and a framework of silk fibroin for wound dressing applications.24
In this research, CN-Pr-Mel/ZnFe2O4/CMC hydrogel nanobiocomposites have been synthesized as a novel structure with potential application in biomedicine (Scheme 1). To synthesize this nanobiocomposite, CN is first functionalized with melamine molecules. ZnFe2O4 nanoparticles with high antibacterial potential are then added to the CN, and subsequently, CMC hydrogel is added to the structure. The structure is evaluated for its antibacterial applications.
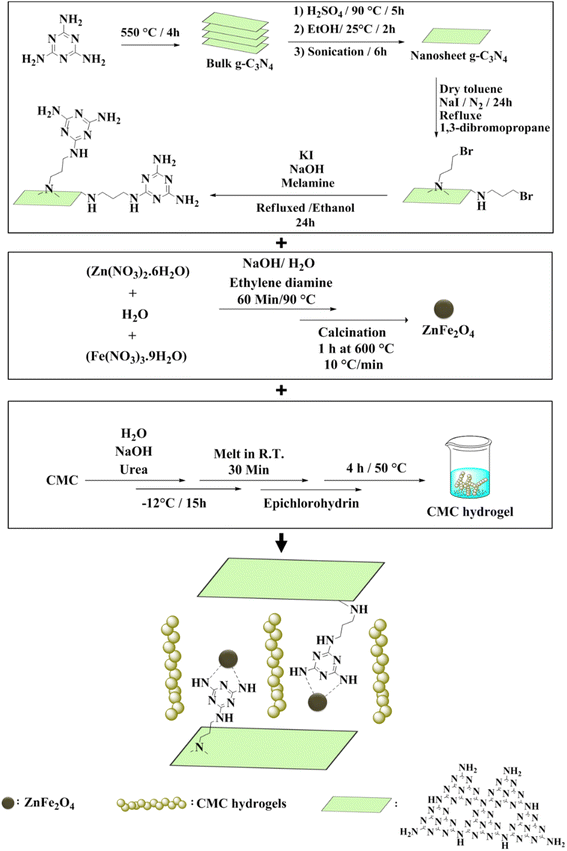 | ||
| Scheme 1 Schematic representation of the CN-Pr-Mel/ZnFe2O4/CMC hydrogel nanobiocomposite synthesis steps. | ||
2. Materials and methods
2.1. Materials and instruments
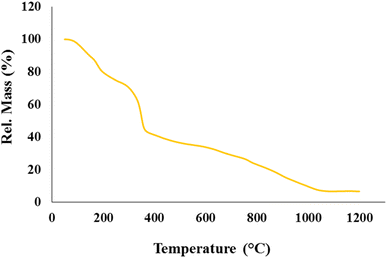
All materials used in this research have high purity and have been purchased from Merck and Flucka. The FT-IR spectrum of the samples is obtained with the help of KBr pellets and through an AVATAR Thermo device. EDX and FE-SEM analyses are performed using Numerix DXP-X10P and MIRA III TESCAN devices. The XRD analysis is performed in the range of 2θ, 5.0° to 80° with a PANalytical X-PERT-PRO MPD apparatus. Thermogravimetric analysis is carried out through an STA504 device. In this regard, the thermal stability of the sample is evaluated in the temperature range of 25 °C to 1200 °C at a temperature rate of 10 °C min−1 in an inert argon atmosphere. Compression mechanical properties of synthesized nanobiocomposite are evaluated according to the method presented by Eivazzadeh-Keihan et al.60 For this purpose, pieces of CN-Pr-Mel/ZnFe2O4/CMC hydrogel nanobiocomposite with approximate 1 × 1 × 1 cm dimensions are prepared. Then, compressive strength and compressive modulus are measured using a universal testing machine (SANTAM-20 model, Iran) with a load cell capacity (0.2 kN) and a crosshead rate of 0.5 mm min−1 at room temperature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Dalton and a degree of substitution of 0.75) is poured into 100.0 mL of the as-prepared solution in the previous step and stirred for 15 minutes at ambient temperature. Then, the lid of the container containing the mixture is closed and placed at −12 °C for 15 hours. After the mentioned time, the components of the container are removed from freezing temperature and placed at room temperature to be melted. In the next step and after melting, the solution is stirred for 30 minutes for a clear appearance. 10% weight of epichlorohydrin solution (ECH) as a cross-linker is poured into the blend and stirred for half an hour to produce a uniform solution. Eventually, after the mixture is passed through a 50 °C oven for 4 hours and a 70 °C freezer for one day, it is freeze-dried for 48 hours and then stored in a cool and dry place.24
000 Dalton and a degree of substitution of 0.75) is poured into 100.0 mL of the as-prepared solution in the previous step and stirred for 15 minutes at ambient temperature. Then, the lid of the container containing the mixture is closed and placed at −12 °C for 15 hours. After the mentioned time, the components of the container are removed from freezing temperature and placed at room temperature to be melted. In the next step and after melting, the solution is stirred for 30 minutes for a clear appearance. 10% weight of epichlorohydrin solution (ECH) as a cross-linker is poured into the blend and stirred for half an hour to produce a uniform solution. Eventually, after the mixture is passed through a 50 °C oven for 4 hours and a 70 °C freezer for one day, it is freeze-dried for 48 hours and then stored in a cool and dry place.243. Results and discussion
3.1. Preparation of the CN-Pr-Mel/ZnFe2O4/CMC hydrogel nanobiocomposite
The main steps have been carried out for preparing CN-Pr-Mel/ZnFe2O4/CMC hydrogel nanobiocomposites. As demonstrated in Scheme 1, the functionalization of exfoliated CN 2D nanosheets with melamine molecules via 1,3-dibromopropane (Pr) linker was accomplished. Then, the ZnFe2O4 nanoparticles, presenting an enhanced antibacterial characteristic, were prepared through a solvothermal method and added to the CN, followed by a CMC hydrogel addition.3.2. Characterization of the CN-Pr-Mel/ZnFe2O4/CMC hydrogel nanobiocomposite
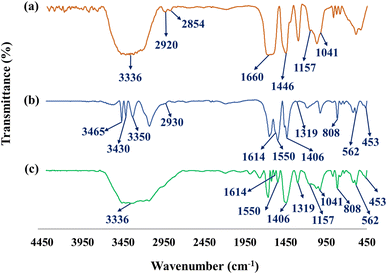
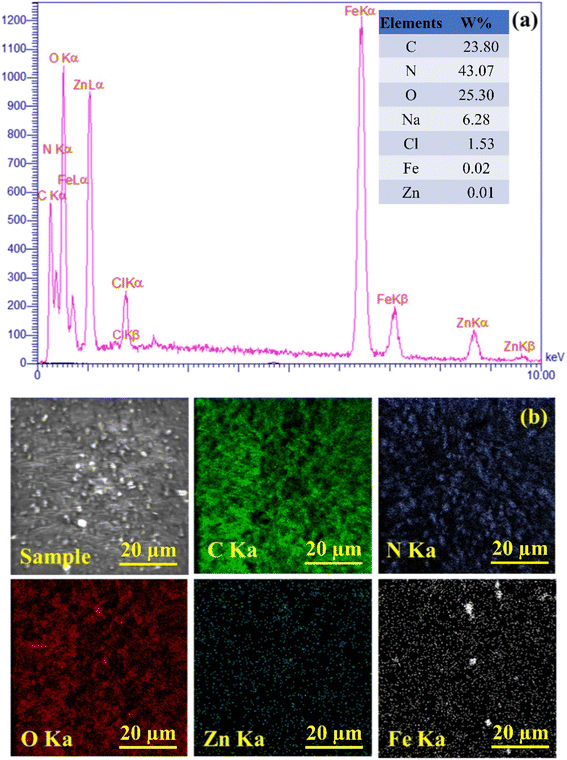
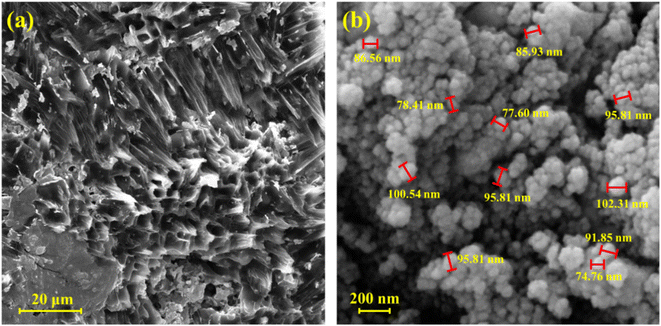
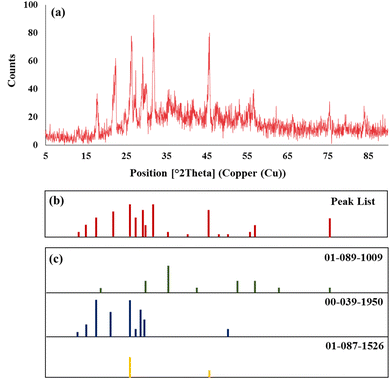
The main properties of the CN-Pr-Mel/ZnFe2O4/CMC hydrogels have been studied employing techniques including FT-IR, EDS, XRD, TGA, and FE-SEM. Functional groups, chemical bonds, structural elements, crystalline structure, thermal stability, and morphology are evaluated in this regard. Further, the mechanical properties of this new structure are also estimated.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration in the CN and melamine structure. In addition, the stretching vibration of the heterocyclic C–N can be recognized by the peaks at 1406 cm−1 and 1319 cm−1.39,67 The peak at 808 cm−1 also indicates the breathing vibration of the tri-s-triazine groups of the CN structure.39 It should be noted that concerning the CN functionalization process, the small peak in the range of 2800 cm−1 to 3000 cm−1 can be related to the stretching vibration of the C–H group of the 1,3-dibromopropane linker.62 Finally, peaks in the range from 3000 cm−1 to 3500 cm−1 can be related to the stretching vibration of N–H in melamine and CN.39,67 Fig. 1c shows the FT-IR spectrum of the final nanobiocomposite, and the main peaks related to hydrogels and CN-Pr-Mel/ZnFe2O4 nanocomposite can also be observed in this spectrum, which is proof of the correct formation of the desired structure.
N stretching vibration in the CN and melamine structure. In addition, the stretching vibration of the heterocyclic C–N can be recognized by the peaks at 1406 cm−1 and 1319 cm−1.39,67 The peak at 808 cm−1 also indicates the breathing vibration of the tri-s-triazine groups of the CN structure.39 It should be noted that concerning the CN functionalization process, the small peak in the range of 2800 cm−1 to 3000 cm−1 can be related to the stretching vibration of the C–H group of the 1,3-dibromopropane linker.62 Finally, peaks in the range from 3000 cm−1 to 3500 cm−1 can be related to the stretching vibration of N–H in melamine and CN.39,67 Fig. 1c shows the FT-IR spectrum of the final nanobiocomposite, and the main peaks related to hydrogels and CN-Pr-Mel/ZnFe2O4 nanocomposite can also be observed in this spectrum, which is proof of the correct formation of the desired structure.
3.3. Bio-application of the designed CN-Pr-Mel/ZnFe2O4/CMC hydrogel
| Agents | MICmean ± SD (MBCmean ± SD) for three independent tests | |
|---|---|---|
| S. aureus | E. coli | |
| CN-Pr-Mel/ZnFe2O4/CMC hydrogel | 1000 ± 1.0 | 500 ± 1.0 |
| Penicillin | 1.4 ± 0.1 | 6.8 ± 0.4 |
| Streptomycin | 12.49 ± 0.0 | 3.1 ± 0.6 |
4. Conclusions
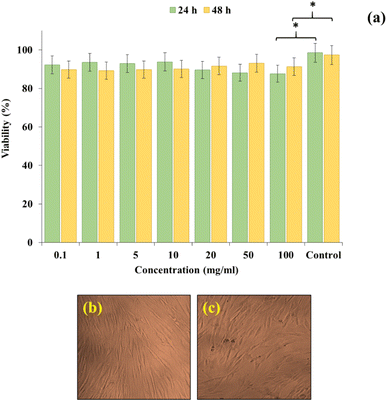
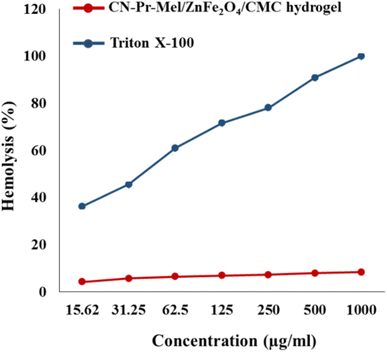
CN-Pr-Mel/ZnFe2O4/CMC hydrogels have been synthesized and evaluated for biomedical applications. To synthesize this nanobiocomposite, CN is first functionalized with melamine molecules. ZnFe2O4 nanoparticles with high antibacterial potential are then added to the CN, and subsequently, CMC hydrogel is added to the structure. The application of this structure for antibacterial applications is evaluated. In biological analysis, cell viability at 100 mg mL−1 is 87.6% after 24 h, showing biocompatibility with Hu02 cells. Further, the hemolytic effect of the structure is below 9% at the concentration of 1000 μg mL−1, also compatible with blood. Concerning the antibacterial activity, the MIC of the nanobiocomposite in S. aureus and E. coli is 500 μg mL−1 and 1000 μg mL−1, respectively, demonstrating the antibacterial activity.Conflicts of interest
The authors listed in this article have no conflict of interest.Acknowledgements
All authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology.References
- R. Eivazzadeh-Keihan, F. Ganjali, H. A. M. Aliabadi, A. Maleki, S. Pouri, M. Mahdavi, A. E. Shalan and S. Lanceros-Méndez, J. Nanostruct. Chem., 2022, 1–11 CAS.
- R. Eivazzadeh-Keihan, L. Choopani, H. A. M. Aliabadi, F. Ganjali, A. Kashtiaray, A. Maleki, R. A. Cohan, M. S. Bani, S. Komijani and M. M. Ahadian, Mater. Chem. Phys., 2022, 126347 CrossRef CAS.
- F. Ganjali, R. Eivazzadeh-Keihan, H. Aghamirza Moghim Aliabadi, A. Maleki, S. Pouri, R. Ahangari Cohan, S. M. Hashemi and M. Mahdavi, J. Inorg. Organomet. Polym. Mater., 2022, 32, 4057–4069 CrossRef CAS.
- W. Otto and L. Drahoslav, Nature, 1960, 185, 117–118 CrossRef.
- K. Yue, Y. Liu, B. Byambaa, V. Singh, W. Liu, X. Li, Y. Sun, Y. S. Zhang, A. Tamayol and P. Zhang, Bioeng. Transl. Med., 2018, 3, 37–48 CrossRef CAS PubMed.
- Q. Feng, K. Wei, S. Lin, Z. Xu, Y. Sun, P. Shi, G. Li and L. Bian, Biomaterials, 2016, 101, 217–228 CrossRef CAS PubMed.
- C. Katsoulos, L. Karageorgiadis, N. Vasileiou, T. Mousafeiropoulos and G. Asimellis, Ophthalmic Physiol. Opt., 2009, 29, 321–329 CrossRef PubMed.
- R. Eivazzadeh-Keihan, H. Bahreinizad, Z. Amiri, H. A. M. Aliabadi, M. Salimi-Bani, A. Nakisa, F. Davoodi, B. Tahmasebi, F. Ahmadpour and F. Radinekiyan, TrAC, Trends Anal. Chem., 2021, 141, 116291 CrossRef CAS.
- V. V. Rao, M. K. Vu, H. Ma, A. R. Killaars and K. S. Anseth, Bioeng. Transl. Med., 2019, 4, 51–60 CrossRef CAS PubMed.
- M. Liu, X. Zeng, C. Ma, H. Yi, Z. Ali, X. Mou, S. Li, Y. Deng and N. He, Bone Res., 2017, 17014 CrossRef CAS PubMed.
- S. Naahidi, M. Jafari, M. Logan, Y. Wang, Y. Yuan, H. Bae, B. Dixon and P. Chen, Biotechnol. Adv., 2017, 35, 530–544 CrossRef CAS.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 1–17 Search PubMed.
- Y. Nakayama, K. Ji-Youn, S. Nishi, H. Ueno and T. Matsuda, J. Biomed. Mater. Res., 2001, 57, 559–566 CrossRef CAS PubMed.
- S. Li, S. Dong, W. Xu, S. Tu, L. Yan, C. Zhao, J. Ding and X. Chen, Adv. Sci., 2018, 5, 1700527 CrossRef.
- K. Yang, Q. Han, B. Chen, Y. Zheng, K. Zhang, Q. Li and J. Wang, Int. J. Nanomed., 2018, 13, 2217 CrossRef CAS PubMed.
- A. K. Whitehead, H. H. Barnett, M. E. Caldorera-Moore and J. J. Newman, Regener. Biomater., 2018, 5, 167–175 CrossRef CAS PubMed.
- T. Gao, M. Jiang, X. Liu, G. You, W. Wang, Z. Sun, A. Ma and J. Chen, Polymers, 2019, 11, 171 CrossRef PubMed.
- G. Sharifzadeh, H. Hezaveh, I. I. Muhamad, S. Hashim and N. Khairuddin, Mater. Sci. Eng., C, 2020, 110, 110609 CrossRef CAS PubMed.
- J.-O. Jeong, J.-S. Park, E. J. Kim, S.-I. Jeong, J. Y. Lee and Y.-M. Lim, Int. J. Mol. Sci., 2020, 21, 187 CrossRef CAS.
- C. Chang and L. J. C. p. Zhang, Carbohydr. Polym., 2011, 84, 40–53 CrossRef CAS.
- R. Eivazzadeh-Keihan, Z. Sadat, H. Aghamirza Moghim Aliabadi, F. Ganjali, A. Kashtiaray, M. Salimi Bani, S. Komijani, M. M. Ahadian, R. Ahangari Cohan and A. Maleki, Sci. Rep., 2022, 12, 1–13 CrossRef PubMed.
- R. Eivazzadeh-Keihan, Z. Pajoum, H. A. M. Aliabadi, F. Ganjali, A. Kashtiaray, M. S. Bani, F. Lalebeigi, E. Z. Ziabari, A. Maleki and M. M. Heravi, Carbohydr. Polym., 2023, 300, 120246 CrossRef CAS PubMed.
- L.-H. Fu, C. Qi, M.-G. Ma and P. Wan, J. Mater. Chem. B, 2019, 7, 1541–1562 RSC.
- R. Eivazzadeh-Keihan, F. Khalili, N. Khosropour, H. A. M. Aliabadi, F. Radinekiyan, S. Sukhtezari, A. Maleki, H. Madanchi, M. R. Hamblin, M. Mahdavi, S. M. A. Haramshahi, A. E. Shalan and S. Lanceros-Mendez, ACS Appl. Mater. Interfaces, 2021, 13, 33840–33849 CrossRef CAS.
- R. Eivazzadeh-Keihan, M. G. Gorab, H. A. M. Aliabadi, E. B. Noruzi, A. Kashtiaray, M. S. Bani, A. Etminan, H. Mirzahoseini, R. A. Cohan and A. Maleki, Cellulose, 2023, 1–16 Search PubMed.
- F. Ma, C.-W. Yuan, J.-N. Liu, J.-H. Cao and D.-Y. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 19902–19912 CrossRef CAS PubMed.
- H. Dai, Y. Huang and H. J. C. p. Huang, Carbohydr. Polym., 2018, 185, 1–11 CrossRef PubMed.
- A. Ahmed, M. B. K. Niazi, Z. Jahan, T. Ahmad, A. Hussain, E. Pervaiz, H. A. Janjua and Z. J. E. P. J. Hussain, Eur. Polym. J., 2020, 130, 109650 CrossRef CAS.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. Madanchi, H. A. M. Aliabadi and A. J. C. P. Maleki, Carbohydr. Polym., 2020, 248, 116802 CrossRef CAS PubMed.
- R. Mohammadi, H. Alamgholiloo, B. Gholipour, S. Rostamnia, S. Khaksar, M. Farajzadeh and M. Shokouhimehr, J. Photochem. Photobiol., A, 2020, 402, 112786 CrossRef CAS.
- H. Mohtasham, B. Gholipour, S. Rostamnia, A. Ghiasi-Moaser, M. Farajzadeh, N. Nouruzi, H. W. Jang, R. S. Varma and M. Shokouhimehr, Colloids Surf., A, 2021, 614, 126187 CrossRef CAS.
- J. Wen, J. Xie, X. Chen and X. J. A. s. s. Li, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS.
- X. Chang, X. Zhai, S. Sun, D. Gu, L. Dong, Y. Yin and Y. J. N. Zhu, Nanotechnology, 2017, 28, 135705 CrossRef PubMed.
- J. Zou, S. Wu, Y. Liu, Y. Sun, Y. Cao, J.-P. Hsu, A. T. S. Wee and J. J. C. Jiang, Carbon, 2018, 130, 652–663 CrossRef CAS.
- S. Patnaik, D. P. Sahoo and K. Parida, Renewable Sustainable Energy Rev., 2018, 82, 1297–1312 CrossRef CAS.
- Y. Ren, D. Zeng and W.-J. Ong, Chin. J. Catal., 2019, 40, 289–319 CrossRef CAS.
- H. Taheri, M. A. Unal, M. Sevim, C. Gurcan, O. Ekim, A. Ceylan, Z. Syrgiannis, K. C. Christoforidis, S. Bosi and O. J. S. Ozgenç, Small, 2020, 16, 1904619 CrossRef CAS PubMed.
- M. Majdoub, Z. Anfar and A. Amedlous, ACS Nano, 2020, 14, 12390–12469 CrossRef CAS PubMed.
- A. Rashidizadeh, H. Ghafuri, H. R. Esmaili Zand and N. Goodarzi, ACS Omega, 2019, 4, 12544–12554 CrossRef CAS PubMed.
- Z. Alirezvani, M. G. Dekamin, F. Davoodi and E. Valiey, ChemistrySelect, 2018, 3, 10450–10463 CrossRef CAS.
- R. Eivazzadeh-Keihan, R. Taheri-Ledari, N. Khosropour, S. Dalvand, A. Maleki, S. M. Mousavi-Khoshdel and H. Sohrabi, Colloids Surf., A, 2020, 587, 124335 CrossRef CAS.
- F. Ganjali, A. Kashtiaray, S. Zarei-Shokat, R. Taheri-Ledari and A. Maleki, Nanoscale Adv., 2022, 4, 1263–1307 RSC.
- R. Eivazzadeh-Keihan, N. Bahrami, F. Radinekiyan, A. Maleki and M. Mahdavi, Mater. Res. Express, 2021, 8, 026102 CrossRef CAS.
- M. Forouzandeh-Malati, F. Ganjali, E. Zamiri, S. Zarei-Shokat, F. Jalali, M. Padervand, R. Taheri-Ledari and A. Maleki, Langmuir, 2022, 38, 13728–13743 CrossRef CAS PubMed.
- F. Hassanzadeh-Afruzi, F. Esmailzadeh, S. Asgharnasl, F. Ganjali, R. Taheri-Ledari and A. Maleki, Sep. Purif. Technol., 2022, 291, 120956 CrossRef CAS.
- A. Gautam, P. Komal, P. Gautam, A. Sharma, N. Kumar and J. P. Jung, Metals, 2021, 11, 329 CrossRef CAS.
- V. Biju, T. Itoh, A. Anas, A. Sujith and M. Ishikawa, Anal. Bioanal. Chem., 2008, 391, 2469–2495 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, S. Asgharnasl, M. S. Bani, F. Radinekiyan, A. Maleki, M. Mahdavi, P. Babaniamansour, H. Bahreinizad, A. E. Shalan and S. J. L. Lanceros-Méndez, Langmuir, 2021, 37, 8847–8854 CrossRef CAS.
- R. Eivazzadeh-Keihan, H. Dogari, F. Ahmadpour, H. A. M. Aliabadi, F. Radinekiyan, A. Maleki, L. S. Fard, B. Tahmasebi, M. F. P. Mojdehi and M. J. S. R. Mahdavi, Sci. Rep., 2021, 11, 13428 CrossRef CAS.
- R. Eivazzadeh-Keihan, E. B. Noruzi, F. Radinekiyan, M. S. Bani, A. Maleki, B. Shaabani and M. J. C. Haghpanahi, ChemistryOpen, 2020, 9, 735–742 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, S. Rahmati, Z. Sadat, F. Ganjali, H. A. M. Aliabadi, A. Kashtiaray and A. Maleki, Mater. Today Commun., 2023, 105461 CrossRef CAS.
- A. Manohar, V. Vijayakanth and K. H. Kim, J. Alloys Compd., 2021, 886, 161276 CrossRef CAS.
- M. Amiri, T. Gholami, O. Amiri, A. Pardakhti, M. Ahmadi, A. Akbari, A. Amanatfard and M. Salavati-Niasari, J. Alloys Compd., 2020, 849, 156604 CrossRef CAS.
- B. Surendra, H. Nagaswarupa, M. Hemashree and J. Khanum, Chem. Phys. Lett., 2020, 739, 136980 CrossRef CAS.
- R. Eyvazzadeh-Keihan, N. Bahrami, R. Taheri-Ledari and A. Maleki, Diamond Relat. Mater., 2020, 102, 107661 CrossRef CAS.
- R. Eivazzadeh-Keihan, F. Radinekiyan, S. Asgharnasl, A. Maleki and H. Bahreinizad, J. Mater. Res. Technol., 2020, 9, 12244–12259 CrossRef CAS.
- J. Chen, B. Zhang, L. Qi, Y. Pei, R. Nie, P. Heintz, X. Luan, Z. Bao, Q. Yang, Q. Y. Q. Ren, Z. Zhang and W. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 23002–23009 CrossRef CAS.
- D. Xu, H. Lv and B. Liu, Front. Chem., 2018, 6, 550 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, F. Khalili, H. A. M. Aliabadi, A. Maleki, H. Madanchi, E. Z. Ziabari and M. Bani, Int. J. Biol. Macromol., 2020, 162, 1959–1971 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. A. M. Aliabadi, S. Sukhtezari, B. Tahmasebi, A. Maleki and H. Madanchi, Sci. Rep., 2021, 11, 650 CrossRef CAS PubMed.
- Y. Zheng, L. Lin, X. Ye, F. Guo and X. Wang, Angew. Chem., 2014, 126, 12120–12124 CrossRef.
- Z. Tajik, H. Ghafuri, N. Ghanbari and P. Hanifehnejad, Sci. Rep., 2021, 11, 19792 CrossRef PubMed.
- T. Etemadinia, A. Allahrasani and B. Barikbin, Polym. Bull., 2019, 76, 6089–6109 CrossRef CAS.
- R. Eivazzadeh-Keihan, Z. Pajoum, H. A. M. Aliabadi, A. Mohammadi, A. Kashtiaray, M. S. Bani, B. Pishva, A. Maleki, M. M. Heravi and M. Mahdavi, RSC Adv., 2023, 13, 8540–8550 RSC.
- A. Mohammadi, R. Eivazzadeh-Keihan, H. A. M. Aliabadi, A. Kashtiaray, R. A. Cohan, M. S. Bani, S. Komijani, A. Etminan, A. Maleki and M. Mahdavi, J. Biotechnol., 2023, 367, 71–80 CrossRef CAS PubMed.
- M. Bayindir Bilgic, N. T. Lacin, H. Berber and B. Mansuroglu, Mater. Tech., 2019, 34, 386–393 CrossRef CAS.
- Y. Zhao, D. Yu, H. Zhou, Y. Tian and O. Yanagisawa, J. Mater. Sci., 2005, 40, 2645–2647 CrossRef CAS.
- B. Surendra, H. Nagaswarupa, M. Hemashree and J. Khanum, Chem. Phys. Lett., 2020, 739, 136980 CrossRef CAS.
- R. Eyvazzadeh-Keihan, N. Bahrami, R. Taheri-Ledari and A. Maleki, Diamond Relat. Mater., 2020, 102, 107661 CrossRef CAS.
- R. Eivazzadeh-Keihan, F. Radinekiyan, S. Asgharnasl, A. Maleki and H. Bahreinizad, J. Mater. Res. Technol., 2020, 9, 12244–12259 CrossRef CAS.
- F. Yang, G. Li, Y.-G. He, F.-X. Ren and G.-x. Wang, Carbohydr. Polym., 2009, 78, 95–99 CrossRef CAS.
- R. Eivazzadeh-Keihan, M. Ghafori Gorab, H. Aghamirza Moghim Aliabadi, M. Mahdavi, A. R. Akbarzadeh, A. Maleki and H. Ghafuri, Sci. Rep., 2021, 11, 20310 CrossRef CAS PubMed.
- B. H. Suryanto, T. Fang, S. Cheong, R. D. Tilley and C. Zhao, J. Mater. Chem. A, 2018, 6, 4686–4694 RSC.
| This journal is © The Royal Society of Chemistry 2023 |