Open Access Article
Open Access ArticleA municipal wastewater treatment plant “drinking beer” for reduction of cost and carbon emission
Yifan Lianga,
Zuchao Huanga,
Zengrui Pana,
Xubo Zhangb,
Meng Xub,
Yunchang Shenc and
Jun Li *a
*a
aKey Laboratory of Microbial Technology for Industrial Pollution Control of Zhejiang Province, College of Environment, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: tanweilijun@zjut.edu.cn
bDeqing Hengfeng Wastewater Treatment Co. Ltd, Huzhou 313200, China
cHuzhou Deqing Ming Kang Biological Co. Ltd, Huzhou 313200, China
First published on 5th July 2023
Abstract
In wastewater treatment plants (WWTPs), external carbon sources are often required due to low C/N influent. However, the use of external carbon sources can increase treatment costs and cause large carbon emissions. Beer wastewater, which contains a substantial amount of carbon, is often treated separately in China, consuming significant energy and cost. However, most studies using beer wastewater as an external carbon source are still on a laboratory scale. To address this issue, this study proposes using beer wastewater as an external carbon source in an actual WWTP to reduce operating costs and carbon emissions while achieving a win–win situation. The denitrification rate of beer wastewater was found to be higher 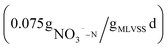 than that of sodium acetate
than that of sodium acetate 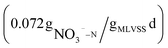 , resulting in improved treatment efficiency of the WWTP. Specifically, COD, BOD5, TN, NH4+–N and TP increased by 3.4%, 1.6%, 10.8%, 1.1%, and 1.7%, respectively. Additionally, the treatment cost and carbon emission per 10
, resulting in improved treatment efficiency of the WWTP. Specifically, COD, BOD5, TN, NH4+–N and TP increased by 3.4%, 1.6%, 10.8%, 1.1%, and 1.7%, respectively. Additionally, the treatment cost and carbon emission per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of wastewater treated were reduced by 537.31 yuan and 2.27 t CO2, respectively. These results indicate that beer wastewater has significant utilization potential and provide a reference for using different types of production wastewater in WWTPs. This study's findings demonstrate the feasibility of implementing this approach in an actual WWTP setting.
000 tons of wastewater treated were reduced by 537.31 yuan and 2.27 t CO2, respectively. These results indicate that beer wastewater has significant utilization potential and provide a reference for using different types of production wastewater in WWTPs. This study's findings demonstrate the feasibility of implementing this approach in an actual WWTP setting.
1 Introduction
In recent years, the Chinese government has placed great emphasis on improving the capacity and number of urban wastewater treatment plants (WWTPs) in the country.1 However, despite this progress, the operational performance and energy efficiency of most WWTPs in China still lag behind those of developed countries.2 To ensure sustainable and stable operation of WWTPs, it is crucial to find ways to improve their performance and reduce energy consumption.3 Additionally, given that carbon emissions from China's wastewater treatment account for 1–2% of the total social carbon emissions,4 reducing carbon emissions from WWTPs has become a pressing concern, especially in light of China's carbon peaking and carbon neutrality goals.One key issue that WWTPs in China face is the need for an external carbon source due to low carbon nitrogen ratio (C/N) influent, particularly in the southern regions of the country, and this is closely related to denitrification efficiency.5–7 Despite efforts to conserve carbon sources by not setting up primary sedimentation tanks, insufficient carbon sources remain a significant challenge. Thus, it is crucial to reduce the use of external carbon sources and improve their utilization rate. To address this issue, researchers have explored denitrification of wastewater under low C/N ratio conditions.8,9 However, increasing the nitrate cycle rate has been found to have limited impact on improving denitrification efficiency under such circumstances.10 Some researchers have proposed new processes and optimized existing ones. For example, step feed,11 simultaneous nitrification and denitrification (SND),12 and endogenous denitrification (ED)13 technologies have been developed, which have improved the utilization efficiency of carbon sources in influent, resulting in improved denitrification rates.14 Autotrophic denitrification using iron and hydrogen as electron donors and inorganic carbon source has also been studied.15,16 However, these new process is not yet widely applicable in WWTPs due to technological limitations. Despite ongoing efforts to develop new methods, adding an external carbon source remains a common approach to improving the nitrogen removal rate in WWTPs.
The most commonly used external carbon source in China's WWTPs is commercial carbon source, particularly sodium acetate (NaAC) and glucose (GLU).17 However, such sources have several drawbacks, including hidden safety risks and high costs. Different types of commercial carbon source also result in varying nitrogen removal rates.18 Moreover, the need for supporting facilities such as measurement and management further increases the operation and management cost.19 Methanol, another external carbon source, poses safety risks due to its flammability, necessitating additional safety measures during transportation and storage.20 Researchers have explored alternative sources of external carbon, such as biodegradable polymers (BDPs) and natural materials based on cellulose, to reduce costs and increase environmental friendliness. However, BDPs are more expensive,6,21 and methane is susceptible to oxygen concentrations.22 Although natural cellulose-based sources have been studied as potential alternatives,23 the initial and saturated carbon release rates vary significantly, and the effect after addition is unstable, making them unsuitable for widespread application.24 Therefore, a low-cost, high-performance, and environmentally friendly external carbon source is necessary. Production wastewater may be a satisfactory alternative to commercial carbon sources. Further research is needed to determine the feasibility and effectiveness of production wastewater as an external carbon source in WWTPs, but it holds promise as a potential solution to the challenges associated with existing external carbon sources.
Studies on using production effluents, such as beer effluents, as external carbon sources have mostly been conducted on a laboratory scale. Na et al. explored the influence of the dosage of beer wastewater on nitrogen and phosphorus removal in a laboratory-scale AAO reactor,25 and Mielcarek et al. explored the influence of beer wastewater on microorganisms in a laboratory-scale anaerobic sequencing batch reactor.26 However, laboratory scale experiments have their limitations, such as the inability to simulate the change of water quality and quantity in the actual WWTP, the actual situation of the operation method cannot be applied, and the cost accounting and carbon emission accounting cannot be carried out. The production wastewater containing carbon produced in the process of agricultural products and food production has high organic concentration and good biodegradability.8 Quan et al. used hydrolyzed molasses (the percentage of biodegradable substrates was 47.5%) and methanol as external carbon source in sequencing batch reactor (SBR) to treated urban wastewater, and the nitrogen removal rate was 91.6 ± 1.6%, which was better than methanol (85.3 ± 2.0%).27 Fernandez Nava et al. achieved the denitrification of wastewater with nitrate content of 2500 mg L−1 by using sugar, beverage and dairy plant wastewater as the only external carbon source in SBR.28 Studies have shown that carbon-containing industrial wastewater can be used as an external carbon source for WWTPs and has great potential for practical use. However, relevant policies in China limit its practical application, which also leads to less research on the practical application of production wastewater in WWTPs.29
In recent years, China has implemented environmental protection policies that require industrial wastewater to be treated to meet specific standards before it is transferred to WWTPs via sewage pipes. However, some industrial wastewater, which contains high concentrations of organic matter, has high biodegradability, low toxicity, and few side effects, also undergoes pretreatment before being transferred to WWTPs. This pretreatment process reduces the influent C/N ratio of WWTPs, which wastes a significant amount of available carbon sources and increases treatment costs for both industrial enterprises and WWTPs. To address this issue, the Ministry of Ecology and Environment and the State Administration for Market Regulation of China issued an amendment to the Discharge Standard of Pollutants for Beer Industry (GB 19821-2005) in December 2020.30 This amendment clarified that the wastewater of alcohol enterprises, including beer wastewater, can be used as a high-quality external carbon source for WWTPs. This can promote the stable operation of WWTPs and reduce their operating costs and carbon emissions. However, despite this policy change, there are still limited reports on the practical application of beer wastewater as an external carbon source in actual WWTPs. In light of this, it is important to investigate the potential of beer wastewater as an external carbon source and assess its economic and environmental impact when used in WWTPs. This knowledge can help inform future policies that encourage the use of production wastewater as a carbon source in WWTPs.
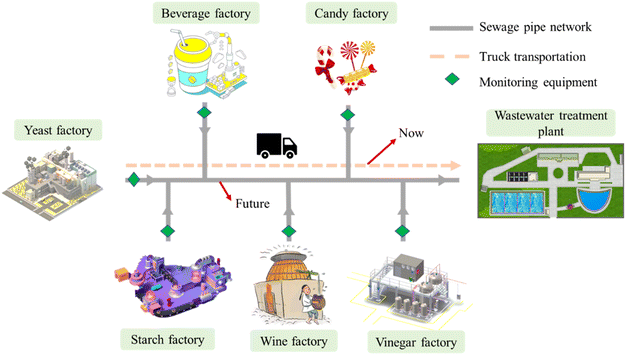
This paper proposes a system that uses beer wastewater as an external carbon source and applies it to an actual WWTP to reduce the operating costs and carbon emissions of the WWTP. The study analyzes the data of the WWTP in 2017 (before adding beer wastewater) and 2021 (after adding beer wastewater) to investigate the change in treatment effect before and after using beer wastewater as an external carbon source, the denitrification rate of activated sludge in beer wastewater as a carbon source, the economic cost analysis of the system, and carbon emission changes of beer wastewater as an external carbon source. Based on these analyses, it is predicted that production wastewater (wastewater with high carbon content that can be used as an additional carbon source, such as sugar wastewater, wine wastewater and vinegar wastewater) will be widely used as a carbon source in WWTPs in the future.
2. Materials and methods
2.1 Overview of wastewater treatment plant and adding method of beer wastewater
The WWTP in Huzhou City, Zhejiang Province, China, was commissioned in February 2002 and has a designed capacity of treating 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 of wastewater per day, with industrial and domestic wastewater accounting for 40% and 60%, respectively. The WWTP employs an anaerobic–anoxic–oxic (AAO) process, as depicted in Fig. 1. In China, due to the low C/N ratio of influent, an initial sedimentation tank is generally not employed to prevent the loss of particulate organic matter and increase the content of influent organic matter. However, the WWTP still faces the challenge of insufficient influent carbon source and needs to add an external carbon source.
000 m3 of wastewater per day, with industrial and domestic wastewater accounting for 40% and 60%, respectively. The WWTP employs an anaerobic–anoxic–oxic (AAO) process, as depicted in Fig. 1. In China, due to the low C/N ratio of influent, an initial sedimentation tank is generally not employed to prevent the loss of particulate organic matter and increase the content of influent organic matter. However, the WWTP still faces the challenge of insufficient influent carbon source and needs to add an external carbon source.
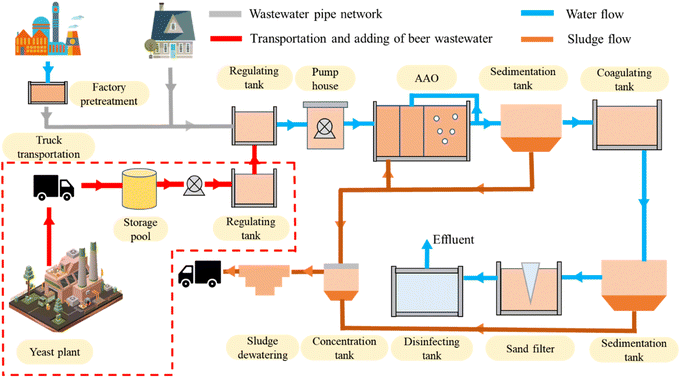 | ||
| Fig. 1 Main process flow of the wastewater treatment plant, and the transportation and adding method of beer wastewater. | ||
The yeast plant uses brewery wastewater to produce yeast. After precipitation, the sediment from the beer wastewater is used as a raw material for yeast production, and the upper beer wastewater becomes the production wastewater. According to the Discharge Standard of Pollutants for Beer Industry (GB 19821-2005) in China, the yeast plant is required to treat the production wastewater by itself or outsource it to relevant companies to make it meet the discharge standard before it can be discharged into the sewage pipe network. However, this approach can be expensive. The water quality of the beer wastewater, including pH, chemical oxygen demand (COD), biochemical oxygen demand (BOD5), total nitrogen (TN), ammonia nitrogen (NH4+–N), and total phosphorus (TP), is summarized in Table 1.
| Water quality parameters | Concentration (mg L−1) or range |
|---|---|
| COD | 134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570–208 570–208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 789 789 |
| BOD5/COD | 0.76 ± 0.08 |
| TN | 1056–2195 |
| NH4+–N | 400–786 |
| TP | 371–1301 |
| pH | 4.37–4.64 |
To reduce costs and promote a win–win situation, the WWTP has entered into a partnership with the yeast plant, using the beer wastewater as an external carbon source. The transportation and dosing method of the beer wastewater to the WWTP is illustrated in the red dotted line in Fig. 1. The production wastewater is transported by truck from the yeast plant to the WWTP and stored in a storage tank. Because the influent carbon source decreases at night, the beer wastewater is transported to the regulating tank by pump at night and added to the influent regulating tank through the overflow. The dosage is approximately one ton of beer wastewater per ten thousand tons of wastewater, which translates to a beer wastewater to water intake ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Therefore, pH adjustment and pretreatment are not required. Moreover, to prevent TN in beer wastewater from affecting denitrification, the beer wastewater is mixed with influent water.
000. Therefore, pH adjustment and pretreatment are not required. Moreover, to prevent TN in beer wastewater from affecting denitrification, the beer wastewater is mixed with influent water.
2.2 Analysis method
The influent water sample of the WWTP was taken from the influent water of the wastewater pipe network into the regulating tank, and the effluent water sample was taken from the effluent water of the disinfection tank. The parameters COD, BOD5, NH4+–N, NO3−–N, TP, TN, mixed liquid suspension (MLSS) and mixed liquid volatile suspension (MLVSS) were measured according to standard methods.31 Dissolved oxygen, pH and temperature were measured using a German WTW Multi 3420.2.3 Method of measuring denitrification rate
Three sets of rectors (R1, R2 and R3) were set up to compare denitrification rate when NaAC and beer wastewater were used as carbon source. Sludge is taken from the biochemical tank of the WWTP, and prior to the experiment, the sludge was aerated for 3 h to consume the residual and internal carbon source, and then nitrogen blowing to remove oxygen. Sludge volume in rectors was 500 ml, and MLSS and MLVSS of the sludge were 3941 and 2068 mg L−1, respectively. Electromagnetic agitator stirring to ensure adequate reaction during the experiment. NaNO3 was added to the three reactors to ensure that the initial nitrate nitrogen concentration was 30 mg L−1, R1 and R2 were added with NaAC solution (2 ml, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1), beer wastewater (2 ml), respectively, and R3 added pure water (2 ml) as the blank group. Experiments were run for two hours, respectively on 0, 1, 3, 5, 10, 15, 20, 30, 40, 60, 90 and 120 minutes in R1, R2 and R3 extracted the same volume water samples to measure the concentration of NO3−–N.
000 mg L−1), beer wastewater (2 ml), respectively, and R3 added pure water (2 ml) as the blank group. Experiments were run for two hours, respectively on 0, 1, 3, 5, 10, 15, 20, 30, 40, 60, 90 and 120 minutes in R1, R2 and R3 extracted the same volume water samples to measure the concentration of NO3−–N.
2.4 Carbon emission calculation
The carbon emissions from the beer wastewater treatment station (BWTS) and the WWPT were calculated according to the Technical Specification for Low-carbon Operation Evaluation of Sewage Treatment Plant (T/CAEPI 49-2022) (in Chinese).32 The emission of CH4 generated in the direct emission process are calculated according to formula (1).
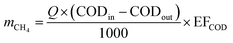 | (1) |
The emission of N2O generated in the direct emission process were calculated in a similar way to COD, through formula (2).
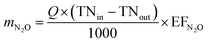 | (2) |
The carbon emission from indirect emission process mainly includes the emission from chemicals and electricity consumption. It is calculated using formula (3).
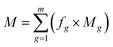 | (3) |
Indirect emissions from truck transport cannot be ignored. According to the Standard for Carbon Emission Calculation of Urban Sewage Treatment and Sludge Treatment and Disposal Project (T/CABEE 040-2022) (in Chinese).35 the carbon emissions generated by the transportation part are based on formula (4). It is known that the distance to transport the wastewater from the yeast plant to the WWTP is 2.6 km, and the carrying vehicle is heavy diesel truck (with a load of 30 t), each carrying 20 t wastewater, 40 times a year.
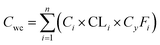 | (4) |
Wastewater can produce CH4 emissions in sewage pipe network, which are often ignored in carbon emissions accounting. And this part is calculated according to formula (5).
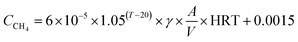 | (5) |
3. Results and discussion
3.1 Performance of the system
The effects of adding beer wastewater on the pollutant treatment efficiency of the WWTP were investigated. The treatment efficiency of COD, BOD, TP, TN, and NH4+–N before and after adding beer wastewater is shown in Fig. 2. Monthly data was obtained by averaging the influent and effluent data for the whole month. The influent data of the WWTP in 2021 was the original influent data before mixing beer wastewater. As shown in Fig. 2(a), the influent COD exhibited a noticeable seasonal fluctuation, with a decrease starting in July and rising again during winter. Similarly, the influent BOD, TP, TN, and NH4+–N exhibited a decreasing trend in July and began to rise during winter (Fig. 2(b)–(d)). These seasonal trends were attributed to various factors, including seasonal and holiday events, such as the Spring Festival, which affect both residents' activities and factory production. In addition, Zhang et al. highlighted that the influent characteristics of WWTPs in China vary depending on the region and season.36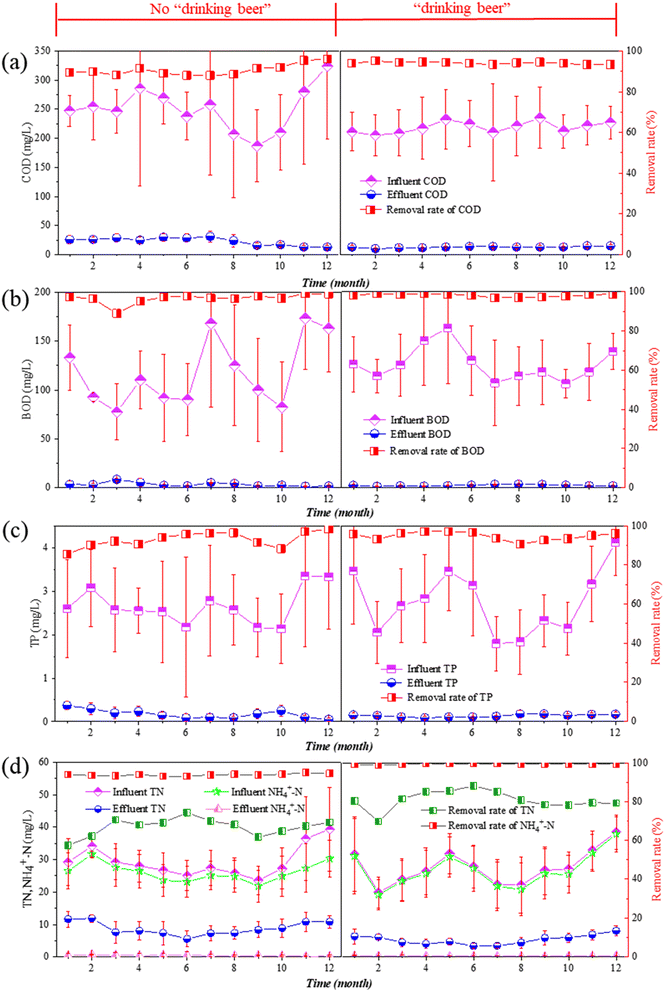 | ||
| Fig. 2 The variations of (a) COD; (b) BOD; (c) TP; (d) TN and NH4+–N before (2017) and after (2021) adding beer wastewater. | ||
In Fig. 2(a), the influent COD concentration before and after adding beer wastewater was 250.5 ± 37.8 (in 2017) and 218.8 ± 9.7 mg L−1 (in 2021), and the effluent COD concentration was 23.2 ± 6.6 and 13.1 ± 1.4 mg L−1, reaching the average removal rate of 90.6% and 94.0%, respectively. The removal efficiency of COD is obviously improved. Of the same change, the average removal rate of BOD, TP, TN and NH4+–N (Fig. 2(b)–(d)) increased from 96.6, 93.1, 70.1 and 98.2% to 98.2, 94.8, 80.9 and 99.3%. In addition, the effluent concentration of TP, TN and NH4+–N decreased from 0.2 ± 0.1, 8.8 ± 2.0 and 0.5 ± 1.1 mg L−1 to 0.1 ± 0.03, 5.2 ± 1.5 and 0.2 ± 0.1 mg L−1, which is an impressive improvement. Previous studies have demonstrated that carbon-rich wastewater, such as kitchen waste and agricultural food waste, can improve nitrogen and phosphorus removal.37,38 In conclusion, when the beer wastewater was used as an external carbon source in the WWTP, the treatment effect is significantly improved.
3.2 Denitrification rate
To investigate the denitrification effect of beer wastewater as carbon source, the denitrification rates of NaAC (R1) and beer wastewater (R2) as carbon source were measured respectively, and the results were shown in Fig. 3. The NO3−–N concentration in the blank group (R3) remained stable over time, indicating that the experiment was not affected by residual carbon source and internal carbon source, as shown in Fig. 3. Conversely, the NO3−–N concentration in R1 and R2 presents a linear transformation over time, and the linear fitting equation is shown in the Fig. 3. The slope of R2 was smaller than that of R1, indicating that the NO3−–N degradation rate is faster when beer wastewater is used as carbon source. The denitrification rates of R1 and R2 are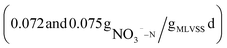 , respectively, after calculation. The results showed that the denitrification rate with beer wastewater as carbon source was slightly higher than that with sodium acetate as carbon source, which is consistent with previous research.39 In previous studies, Ali Mahmoud et al. used fermentation filtrates such as oil and whey powder, methanol and acetate (control) as carbon source respectively to conduct denitrification experiments, and the results showed that the specific denitrification rates of all fermentation liquids were higher than those of methanol and acetate.40 Moreover, Eunji Kim et al. found that food waste recycling wastewater, as an external carbon source, could conduct efficient and stable denitrification.9 These studies imply that high-carbon production effluents may have higher denitrification rates than commercial carbon source. In conclusion, beer wastewater as a carbon source is superior to NaAC in denitrification rate.
, respectively, after calculation. The results showed that the denitrification rate with beer wastewater as carbon source was slightly higher than that with sodium acetate as carbon source, which is consistent with previous research.39 In previous studies, Ali Mahmoud et al. used fermentation filtrates such as oil and whey powder, methanol and acetate (control) as carbon source respectively to conduct denitrification experiments, and the results showed that the specific denitrification rates of all fermentation liquids were higher than those of methanol and acetate.40 Moreover, Eunji Kim et al. found that food waste recycling wastewater, as an external carbon source, could conduct efficient and stable denitrification.9 These studies imply that high-carbon production effluents may have higher denitrification rates than commercial carbon source. In conclusion, beer wastewater as a carbon source is superior to NaAC in denitrification rate.
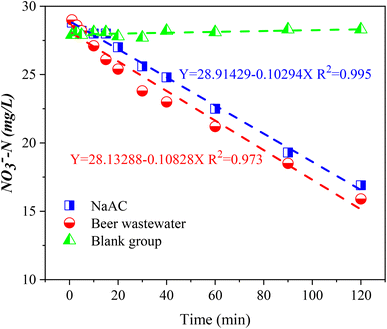 | ||
| Fig. 3 Denitrification rate of activated sludge with sodium acetate and beer wastewater as carbon source. | ||
3.3 Cost of system
In order to assess the cost reduction resulting from the use of beer wastewater as a carbon source, three major factors were analysed, including the cost of commercial carbon sources at the wastewater treatment plant (WWTP), original processing cost of the yeast plant, and transportation cost of beer wastewater.Fig. 4(a) displays the average amount and cost of external carbon source used per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of water treated per month before and after using beer wastewater as carbon source in the WWTP. The commercial carbon source used in the WWTP were GLU and NaAC, with GLU used before the application of beer wastewater and NaAC used in emergencies after the application of beer wastewater, mainly due to the reasons caused by the resumption of factory work after holidays or the cleaning of factory equipment. The fluctuations in chemicals usage were related to change in influent water quality. The average dosage of GLU per 10
000 tons of water treated per month before and after using beer wastewater as carbon source in the WWTP. The commercial carbon source used in the WWTP were GLU and NaAC, with GLU used before the application of beer wastewater and NaAC used in emergencies after the application of beer wastewater, mainly due to the reasons caused by the resumption of factory work after holidays or the cleaning of factory equipment. The fluctuations in chemicals usage were related to change in influent water quality. The average dosage of GLU per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of water treated before using beer wastewater was 493.21 ± 441.70 kg, while the average amount of NaAC per 10
000 tons of water treated before using beer wastewater was 493.21 ± 441.70 kg, while the average amount of NaAC per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of water treated after using beer wastewater was 35.68 ± 61.32 kg. This suggests that the amount of chemical usage significantly decreased after the application of beer wastewater as an additional carbon source.
000 tons of water treated after using beer wastewater was 35.68 ± 61.32 kg. This suggests that the amount of chemical usage significantly decreased after the application of beer wastewater as an additional carbon source.
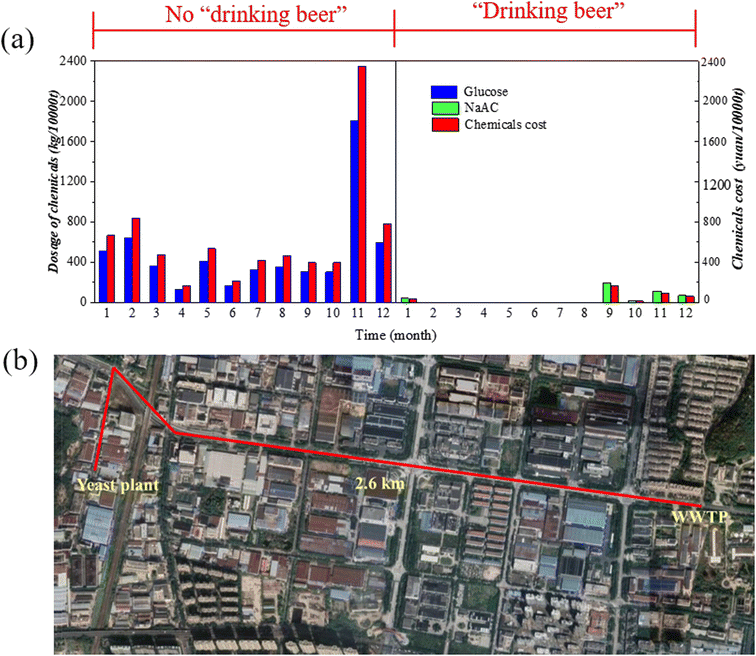 | ||
| Fig. 4 Changes in costs: (a) consumption and expenditure of commercial carbon source; (b) transportation route and distance. | ||
The market price for GLU and NaAC are 1300 and 850 yuan per ton respectively. The total annual cost of carbon sources was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 078
078![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 yuan before using beer wastewater, and it decreased to 48
428 yuan before using beer wastewater, and it decreased to 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 501 yuan after using beer wastewater. Because the yeast plant and the wastewater treatment plant cooperate is a win–win situation, the yeast plant saves the cost of treating the wastewater, and the sewage plant saves the cost of buying commercial carbon sources. Therefore, the beer wastewater is provided free by the yeast plant to the wastewater plant, and the wastewater plant does not charge the yeast plant for treating the wastewater. Consequently, the commercial carbon source cost of the WWTP decreased from 641.17 yuan to 103.86 yuan per 10
501 yuan after using beer wastewater. Because the yeast plant and the wastewater treatment plant cooperate is a win–win situation, the yeast plant saves the cost of treating the wastewater, and the sewage plant saves the cost of buying commercial carbon sources. Therefore, the beer wastewater is provided free by the yeast plant to the wastewater plant, and the wastewater plant does not charge the yeast plant for treating the wastewater. Consequently, the commercial carbon source cost of the WWTP decreased from 641.17 yuan to 103.86 yuan per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of water treated, representing an 83.8% decrease, and the annual savings amounted to 1
000 tons of water treated, representing an 83.8% decrease, and the annual savings amounted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 029
029![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927 yuan.
927 yuan.
Fig. 4(b) illustrates the transportation route and distance of yeast plant wastewater, which is transported by truck from the yeast plant to the WWTP. As a result, the total amount of wastewater transported by the yeast plant to the WWTP throughout the year is about 800 tons, with each transportation volume being 20 tons at a cost of 200 yuan per transportation. The total transportation cost of yeast wastewater is 8000 yuan, and the cost is 10 yuan per ton. The cost of the yeast plant's own treatment, including investment in wastewater treatment facilities and depreciation charges of 12.50 yuan per ton and running costs of 4 yuan per ton, is approximately 16.50 yuan per ton. Thus, the wastewater treatment cost of the yeast plant decreased by 6.50 yuan per ton.
In conclusion, the application of beer wastewater as a carbon source has significant economic advantages due to the reduced cost of commercial carbon sources at the WWTP and the decreased transportation cost of yeast wastewater. The total cost was reduced by 543.81 yuan, demonstrating the economic feasibility of utilizing beer wastewater as a carbon source.
3.4 Carbon emission
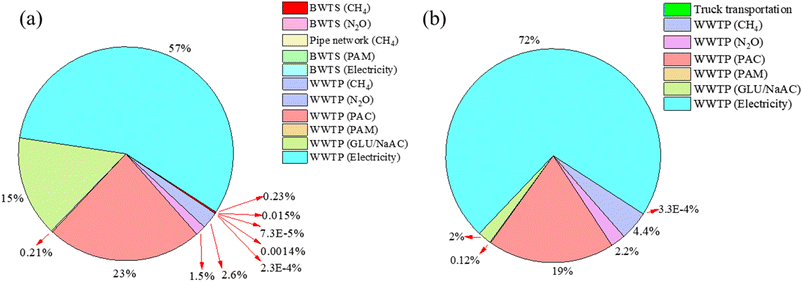
It is necessary to calculate the carbon emissions of WWTP, which can directly show the emission reduction results.41 A study was conducted to assess the changes in carbon emissions before and after the use of beer wastewater as an external carbon source in the WWTP (Fig. 5).In 2017, prior to the implementation of beer wastewater as an external carbon source, the carbon emissions included direct emissions of CH4 and N2O from the biological wastewater treatment system (BWTS) (data on chemicals and electricity consumption in treating beer wastewater were obtained from a brewery in Qingdao, China), indirect emissions from chemical and electricity consumption, CH4 emissions from pipeline transportation of the wastewater, direct emissions of CH4 and N2O from the WWTP, and indirect emissions from chemical and electricity consumption. In contrast, in 2021, after the implementation of beer wastewater as an external carbon source, the carbon emissions included those from trucking the beer wastewater and direct and indirect emissions from the WWTP.
According to Table 2, the total annual carbon emissions from the BWTS and WWTP were 8734.89 t CO2 in 2017. However, after the implementation of beer wastewater as an external carbon source, the total annual carbon emissions decreased to 4484.51 t CO2 in 2021, which reflects a reduction of 51.3%. Notably, the total emissions decreased by 4250.38 t CO2, which was largely attributed to the reduced use of chemicals in the WWTP.5 Specifically, the use of an external carbon source in the WWTP reduced the carbon emissions caused by it from 15.21% to 2.04%. This resulted in a reduction of 1236 t CO2, which accounted for 29.1% of the total reduction.
| Carbon emission classification | 2017 | 2021 | ||||
|---|---|---|---|---|---|---|
| Annual (kg CO2) | Proportion (%) | Annual (kg CO2) | Proportion (%) | |||
| Beer wastewater treatment station | Direct | CH4 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 363.62 363.62 |
0.23 | — | — |
| N2O | 185.08 | 0.0021 | — | — | ||
| Indirect | PAM | 9.61 | 0.00011 | — | — | |
| Electricity | 1950.47 | 0.022 | — | — | ||
| Pipe network | — | CH4 | 30.25 | 0.00035 | — | — |
| Truck transportation | — | — | — | — | 14.60 | 0.00033 |
| Wastewater treatment plant | Direct | CH4 | 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569.80 569.80 |
2.57 | 199![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063.86 063.86 |
4.42 |
| N2O | 134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 769.77 769.77 |
1.54 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622.48 622.48 |
2.22 | ||
| Indirect | PAC | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 048![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045.00 045.00 |
23.47 | 855![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651.60 651.60 |
19.08 | |
| PAM | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226.50 226.50 |
0.21 | 5362.50 | 0.12 | ||
| GLU/NaAC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 327![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296.00 296.00 |
15.21 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296.00 296.00 |
2.04 | ||
| Electricity | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 959 959![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441.07 441.07 |
56.83 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 234 234![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500.75 500.75 |
72.13 | ||
| Total | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734 734![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886.66 886.66 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 484 484![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511.66 511.66 |
||||
The carbon emissions from electricity consumption increased from 56.8% to 72.1% of the total carbon emissions, mainly because of the reduction in total carbon emissions. However, the total carbon emissions from electricity consumption decreased by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724
724![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 941 t CO2, and this was related to equipment upgrades at the WWTP. In 2017, the total carbon emissions from the BWTS were 22
941 t CO2, and this was related to equipment upgrades at the WWTP. In 2017, the total carbon emissions from the BWTS were 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508.8 tons, primarily due to CH4 emissions from the treatment process (90.5%). In contrast, in 2021, the WWTP still produced carbon emissions for beer wastewater treatment, but it reduced the use of chemicals, which greatly reduced the carbon emissions from chemical use. The increase in carbon emissions from truck transportation was only 0.0146 t CO2.
508.8 tons, primarily due to CH4 emissions from the treatment process (90.5%). In contrast, in 2021, the WWTP still produced carbon emissions for beer wastewater treatment, but it reduced the use of chemicals, which greatly reduced the carbon emissions from chemical use. The increase in carbon emissions from truck transportation was only 0.0146 t CO2.
The total amount of water treated in 2017 and 2021 was 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643
643![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 tons and 16
300 tons and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 735
735![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 tons, respectively. This difference accounted for the higher direct carbon emissions from the WWTP in 2017 compared to those in 2021. Furthermore, it can be determined that the carbon emissions per 10
190 tons, respectively. This difference accounted for the higher direct carbon emissions from the WWTP in 2017 compared to those in 2021. Furthermore, it can be determined that the carbon emissions per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of water treated by the WWTP decreased from 4.95 t CO2/10,000 t in 2017 to 2.68 t CO2/10
000 tons of water treated by the WWTP decreased from 4.95 t CO2/10,000 t in 2017 to 2.68 t CO2/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t in 2021. This represents a reduction of 2.27 t CO2/10
000 t in 2021. This represents a reduction of 2.27 t CO2/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t, or 45.8%.
000 t, or 45.8%.
In conclusion, the implementation of beer wastewater as an external carbon source in the WWTP significantly reduced the use of chemicals and carbon emissions. The findings of this study suggest that this practice can serve as a promising method for reducing carbon emissions in WWTPs.
3.5 Vision of the future
Fig. 6 illustrates that production wastewater, including beer wastewater, can serve as an additional carbon source in WWTPs. Various types of production wastewater, such as sugar wastewater, brewing wastewater, and vinegar wastewater, have been proven to be applicable as an additional carbon source in laboratory-scale studies, as demonstrated by Fernandez-Nava et al., who proved that sugar factory wastewater, beverage factory wastewater and dairy factory wastewater can be used as additional carbon source for WWTPs.28 Tang et al. also showed that food wastewater could be used as an additional carbon source for WWTPs.37 In addition, the cost of transporting beer wastewater will increase dramatically with the increase of distance, and the cheapest and most efficient solution is to use the current drainage network, which will be the future direction.The Amendment to Discharge Standard of Pollutants for Beer Industry, recently issued by China, indicates that China is beginning to recognize the significant value of beer wastewater as an additional carbon source for WWTPs. As regulations improve, production wastewater that can be used will be discharged directly into the sewage pipe network without pretreatment and under monitored conditions, leading to an increasing availability of production wastewater for WWTPs.
4. Conclusions
Previous studies on using beer wastewater as an external carbon source have primarily focused on laboratory-scale experiments, with limited research on its application in actual wastewater treatment plants. Additionally, there has been little examination of the costs and carbon emissions associated with this approach.In this study, a method of applying beer wastewater to an actual WWTP was proposed, the treatment efficiency, carbon emission, cost and denitrification rate using beer wastewater as an external carbon source are analyzed. The results showed that the treatment efficiency of COD, BOD, TN, NH4+–N and TP increased by 3.4, 1.6, 10.8, 1.1 and 1.7%, respectively. Additionally, this approach reduces treatment cost and carbon emission by more than 1 million yuan and 4250.38 t CO2 per year, respectively. Furthermore, the denitrification rate of beer wastewater as carbon source is 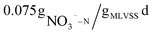 , slightly faster than NaAC
, slightly faster than NaAC 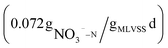 .
.
In conclusion, beer wastewater has significant potential as a carbon source for wastewater treatment, leading to improved treatment efficiency and substantial economic and environmental benefits. In the future, the development and utilization of production wastewater is likely to become an important trend. Therefore, policies should be developed that implement different treatment methods for different type of wastewater, avoiding one-size-fits-all policies, in order to promote the rational use and redistribution of resources. Such efforts will contribute to China's carbon peak and carbon-neutral goals and further reduce the costs of WWTP treatment. In addition, the effects of beer wastewater on aeration rate, excess sludge production and microbial community in WWTPs need to be further explored.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the Zhejiang Key Research and Development Program (No. 2021C03171), Construction Science and Technology Project (ZJZX-202108116CZ) and National Natural Sciences Foundation of China (No. 51478433).References
- Q. H. Zhang, W. N. Yang, H. H. Ngo, W. S. Guo, P. K. Jin, M. Dzakpasu, S. J. Yang, Q. Wang, X. C. Wang and D. Ao, Current status of urban wastewater treatment plants in China, Environ. Int., 2016, 92–93, 11–22 CrossRef CAS PubMed.
- J. Y. Lu, X. M. Wang, H. Q. Liu, H. Q. Yu and W. W. Li, Optimizing operation of municipal wastewater treatment plants in China: the remaining barriers and future implications, Environ. Int., 2019, 129, 273–278 CrossRef PubMed.
- J. Zhang, Y. Shao, H. Wang, G. Liu, L. Qi, X. Xu and S. Liu, Current operation state of wastewater treatment plants in urban China, Environ. Res., 2021, 195, 110843 CrossRef CAS PubMed.
- X. Song, J. Lin, J. Liu, H. Gong, H. Fan, L. Zhang, Y. Wei, Q. Sui and Y. Peng, The R&D and practice of key technologies for sewage treatment plants facing the future, Acta Sci. Circumstantiae, 2022, 42(4), 1–6 CAS.
- L. Li, X. Wang, J. Miao, A. Abulimiti, X. Jing and N. Ren, Carbon neutrality of wastewater treatment-a systematic concept beyond the plant boundary, Environ. Sci. Ecotechnology, 2022, 11, 100180 CrossRef CAS PubMed.
- Z. Xu, X. Dai and X. Chai, Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes, Sci. Total Environ., 2018, 634, 195–204 CrossRef CAS PubMed.
- F. Sun, S. Wu, J. Liu, B. Li, Y. Chen and W. Wu, Denitrification capacity of a landfilled refuse in response to the variations of COD/NO3−-N in the injected leachate, Bioresour. Technol., 2012, 103(1), 109–115 CrossRef CAS PubMed.
- J. Yan, F. Luo, L. Wu, Y. Ou, C. Gong, T. Hao, L. Huang, Y. Chen, J. Long, T. Xiao and H. Zhang, Cost-effective desulfurization of acid mine drainage with food waste as an external carbon source: a pilot-scale and long-term study, J. Cleaner Prod., 2022, 361, 130174 CrossRef.
- E. Kim, S. G. Shin, M. A. H. Jannat, J. V. Tongco and S. Hwang, Use of food waste-recycling wastewater as an alternative carbon source for denitrification process: a full-scale study, Bioresour. Technol., 2017, 245, 1016–1021 CrossRef CAS PubMed.
- L. Pelaz, A. Gomez, A. Letona, G. Garralon and M. Fdz-Polanco, Nitrogen removal in domestic wastewater. Effect of nitrate recycling and COD/N ratio, Chemosphere, 2018, 212, 8–14 CrossRef CAS PubMed.
- S. Ge, Y. Peng, S. Wang, J. Guo, B. Ma, L. Zhang and X. Cao, Enhanced nutrient removal in a modified step feed process treating municipal wastewater with different inflow distribution ratios and nutrient ratios, Bioresour. Technol., 2010, 101(23), 9012–9019 CrossRef CAS PubMed.
- J. Guo, L. Zhang, W. Chen, F. Ma, H. Liu and Y. Tian, The regulation and control strategies of a sequencing batch reactor for simultaneous nitrification and denitrification at different temperatures, Bioresour. Technol., 2013, 133, 59–67 CrossRef CAS PubMed.
- J. Zhao, X. Wang, X. Li, S. Jia and Y. Peng, Combining partial nitrification and post endogenous denitrification in an EBPR system for deep-level nutrient removal from low carbon/nitrogen (C/N) domestic wastewater, Chemosphere, 2018, 210, 19–28 CrossRef CAS PubMed.
- X. Gao, T. Zhang, B. Wang, Z. Xu, L. Zhang and Y. Peng, Advanced nitrogen removal of low C/N ratio sewage in an anaerobic/aerobic/anoxic process through enhanced post-endogenous denitrification, Chemosphere, 2020, 252, 126624 CrossRef CAS PubMed.
- Y. Liang, Z. Pan, H. Feng, X. Cheng, T. Guo, A. Yan and J. Li, Biofilm coupled micro-electrolysis of waste iron shavings enhanced iron and hydrogen autotrophic denitrification and phosphate accumulation for wastewater treatment, J. Environ. Chem. Eng., 2022, 10(6), 108959 CrossRef CAS.
- Y. Liang, Z. Pan, J. Sheng, Y. Ni and J. Li, Fe–C wrapped in polyurethane sponge cubes tied in anoxic zones to enrich multiple denitrifying bacteria enhancing wastewater nitrogen removal, J. Water Process. Eng., 2023, 51, 103384 CrossRef.
- X. Fu, R. Hou, P. Yang, S. Qian, Z. Feng, Z. Chen, F. Wang, R. Yuan, H. Chen and B. Zhou, Application of external carbon source in heterotrophic denitrification of domestic sewage: a review, Sci. Total Environ., 2022, 817, 153061 CrossRef CAS PubMed.
- H. B. Chen, D. B. Wang, X.-m. Li, Q. Yang and G.-m. Zeng, Enhancement of post-anoxic denitrification for biological nutrient removal: effect of different carbon sources, Environ. Sci. Pollut. Res., 2015, 22(8), 5887–5894 CrossRef CAS PubMed.
- I. Bodik, A. Blstakova, S. Sedlacek and M. Hutnan, Biodiesel waste as source of organic carbon for municipal WWTP denitrification, Bioresour. Technol., 2009, 100(8), 2452–2456 CrossRef CAS PubMed.
- E. Torresi, M. E. Casas, F. Polesel, B. G. Plosz, M. Christensson and K. Bester, Impact of external carbon dose on the removal of micropollutants using methanol and ethanol in post-denitrifying Moving Bed Biofilm Reactors, Water Res., 2017, 108, 95–105 CrossRef CAS PubMed.
- Z. Xu, X. Dai and X. Chai, Biological denitrification using PHBV polymer as solid carbon source and biofilm carrier, Biochem. Eng. J., 2019, 146, 186–193 CrossRef CAS.
- R. C. Ma, Y. X. Chu, J. Wang, C. Wang, M. B. Leigh, Y. Chen and R. He, Stable-isotopic and metagenomic analyses reveal metabolic and microbial link of aerobic methane oxidation coupled to denitrification at different O2 levels, Sci. Total Environ., 2021, 764, 142901 CrossRef CAS PubMed.
- Z. Luo, S. Li, X. Zhu and G. Ji, Carbon source effects on nitrogen transformation processes and the quantitative molecular mechanism in long-term flooded constructed wetlands, Ecol. Eng., 2018, 123, 19–29 CrossRef.
- Q. Hang, H. Wang, Z. Chu, B. Ye, C. Li and Z. Hou, Application of plant carbon source for denitrification by constructed wetland and bioreactor: review of recent development, Environ. Sci. Pollut. Res., 2016, 23(9), 8260–8274 CrossRef CAS PubMed.
- D. C. Na, Y. Zhang, F. J. Liu, L. N. Wang, C. Y. Sun and Y. F. Li, Study on Beer Wastewater as External Carbon Source in the A(2)/O Process for Enhancing Nitrogen and Phosphorus Removal, International Conference on Materials Science and Engineering Application (ICMSEA), Wuhan, Peoples R China, 2016, pp. 32–35 Search PubMed.
- A. Mielcarek, J. Rodziewicz, W. Janczukowicz, T. Dulski, S. Ciesielski and A. Thornton, Denitrification aided by waste beer in anaerobic sequencing batch biofilm reactor (AnSBBR), Ecol. Eng., 2016, 95, 384–389 CrossRef.
- Z. X. Quan, Y. S. Jin, C. R. Yin, J. J. Lee and S. T. Lee, Hydrolyzed molasses as an external carbon source in biological nitrogen removal, Bioresour. Technol., 2005, 96(15), 1690–1695 CrossRef CAS PubMed.
- Y. Fernandez-Nava, E. Maranon, J. Soons and L. Castrillon, Denitrification of high nitrate concentration wastewater using alternative carbon sources, J. Hazard. Mater., 2010, 173(1–3), 682–688 CrossRef CAS PubMed.
- S. Park, H. K. Kim, D. H. Kim, G. M. Lee, J. Yoon, H. Choi, M. Kim, K. Han, Y. Kim and H. M. Chung, The effectiveness of injected carbon sources in enhancing the denitrifying processes in groundwater with high nitrate concentrations, Process Saf. Environ. Prot., 2019, 131, 205–211 CrossRef CAS.
- Ministry of Ecology and Environment and the State Administration for Market Regulation of China, Discharge Standard of Pollutants for Beer industry, (GB 19821—2005) modification list, (in Chinese), https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/swrwpfbz/200601/t20060101_69272.shtml Search PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 21st edn, 2005 Search PubMed.
- China Association of Environment Protection Industry, Technical Specification for Low-carbon Operation Evaluation of Sewage Treatment Plant, (T/CAEPI 49-2022) (in Chinese), http://www.caepi.org.cn/epasp/website/webgl/webglController/view?xh=1654504685430037732352 Search PubMed.
- X. Zhou, F. Yang, F. Yang, D. Feng, T. Pan and H. Liao, Analyzing greenhouse gas emissions from municipal wastewater treatment plants using pollutants parameter normalizing method : a case study of Beijing, J. Cleaner Prod., 2022, 376, 134093 CrossRef CAS.
- T. Xie and C. Wang, Greenhouse gas emissions from wastewater treatment plants, J. Tsinghua Univ., Sci. Technol., 2012, 52(4), 473–477 Search PubMed.
- China Association of Building Energy Efficiency, Standard for Carbon Emission Calculation of Urban Sewage Treatment and Sludge Treatment and Disposal Project, (T/CABEE 040-2022) (in Chinese), http://www.ttbz.org.cn/StandardManage/BuyDetail/73855/ Search PubMed.
- J. Zhang, H. Wang, Y. Shao, G. h. Liu, L. Qi, W. Dang, J. Yuan, Y. Li and Z. Xia, Analysis on common problems of the wastewater treatment industry in urban China, Chemosphere, 2022, 291, 130875 Search PubMed.
- J. Tang, X. C. Wang, Y. Hu, Y. Pu, J. Huang, H. H. Ngo, Y. Zeng and Y. Li, Nutrients removal performance and sludge properties using anaerobic fermentation slurry from food waste as an external carbon source for wastewater treatment, Bioresour. Technol., 2019, 271, 125–135 CrossRef CAS PubMed.
- F. J. Fernandez, M. C. Castro, J. Villasenor and L. Rodriguez, Agro-food wastewaters as external carbon source to enhance biological phosphorus removal, Chem. Eng. J., 2011, 166(2), 559–567 CrossRef CAS.
- T. Xie, Z. Zeng and L. Li, Achieving partial denitrification using organic matter in brewery wastewater as carbon source, Bioresour. Technol., 2022, 349, 126849 CrossRef CAS PubMed.
- A. Mahmoud, R. A. Hamza and E. Elbeshbishy, Enhancement of denitrification efficiency using municipal and industrial waste fermentation liquids as external carbon sources, Sci. Total Environ., 2022, 816, 151578 CrossRef CAS PubMed.
- R. Kadam, K. Khanthong, B. Park, H. Jun and J. Park, Realizable wastewater treatment process for carbon neutrality and energy sustainability: A review, J. Environ. Manage., 2023, 328, 116927 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |