Open Access Article
Open Access ArticleRemoval of heavy metals from binary and multicomponent adsorption systems using various adsorbents – a systematic review
Jonas Bayuo
 *ab,
Mwemezi J. Rwiza
a,
Mika Sillanpää
c and
Kelvin Mark Mtei
a
*ab,
Mwemezi J. Rwiza
a,
Mika Sillanpää
c and
Kelvin Mark Mtei
a
aSchool of Materials, Energy, Water, and Environmental Sciences (MEWES), The Nelson Mandela African Institution of Science and Technology (NM-AIST), P.O. Box 447, Arusha, Tanzania. E-mail: bayuoj@nm-aist.ac.tz
bDepartment of Science Education, School of Science, Mathematics, and Technology Education (SoSMTE), C. K. Tedam University of Technology and Applied Sciences (CKT-UTAS), Postal Box 24, Navrongo, Upper East Region, Ghana. E-mail: jbayuo@cktutas.edu.gh
cDepartment of Chemical Engineering, School of Mining, Metallurgy and Chemical Engineering, University of Johannesburg, P. O. Box 17011, Doornfontein 2028, South Africa
First published on 28th April 2023
Abstract
The ecosystem and human health are both significantly affected by the occurrence of potentially harmful heavy metals in the aquatic environment. In general, wastewater comprises an array of heavy metals, and the existence of other competing heavy metal ions might affect the adsorptive elimination of one heavy metal ion. Therefore, to fully comprehend the adsorbent's efficiency and practical applications, the abatement of heavy metals in multicomponent systems is important. In the current study, the multicomponent adsorption of heavy metals from different complex mixtures, such as binary, ternary, quaternary, and quinary solutions, utilizing various adsorbents are reviewed in detail. According to the systematic review, the adsorbents made from locally and naturally occurring materials, such as biomass, feedstocks, and industrial and agricultural waste, are effective and promising in removing heavy metals from complex water systems. The systematic study further discovered that numerous studies evaluate the adsorption characteristics of an adsorbent in a multicomponent system using various important independent adsorption parameters. These independent adsorption parameters include reaction time, solution pH, agitation speed, adsorbent dosage, initial metal ion concentration, ionic strength as well as reaction temperature, which were found to significantly affect the multicomponent sorption of heavy metals. Furthermore, through the application of the multicomponent adsorption isotherms, the competitive heavy metals sorption mechanisms were identified and characterized by three primary kinds of interactive effects including synergism, antagonism, and non-interaction. Despite the enormous amount of research and extensive data on the capability of different adsorbents, several significant drawbacks hinder adsorbents from being used practically and economically to remove heavy metal ions from multicomponent systems. As a result, the current systematic review provides insights and perspectives for further studies through the thorough and reliable analysis of the relevant literature on heavy metals removal from multicomponent systems.
1. Introduction
Among the most critical challenges the globe has been dealing with for decades is water contamination. Water bodies all across the world have recently seen significantly higher levels of contamination due to unrestrained industrial, mining, and agricultural operations, as well as human ignorance regarding unplanned waste disposal.1 According to the UN, 80% of all industrial and urban wastewater is discharged untreated into the environment, especially in developing nations.2 However, wastewater is utilized for irrigated agriculture in developing nations due to water scarcity, posing serious health issues to both farmers and consumers while also providing nutrients for cultivated plants.3,4Annually, several heavy metals are released into water sources and the environment due to anthropogenic and natural activities, which is a worldwide concern.5 Fig. 1 shows the detailed main sources of heavy metal pollution in the ecosystem, which include natural and anthropogenic sources. The most prevalent heavy metals discovered in wastewater that must be confiscated using the right technologies before water is recycled or used again are as follows: As(III), Cd(II), Cr(VI), Hg(II), Ni(II), Pb(II), and Cu(II) ions.6 These heavy metals are bio-accumulative, non-biodegradable, and detrimental to plants, animals, human beings, and other living things.7 For instance, they are particularly toxic to the tissues of all living organisms and human organs including the liver, kidney, lungs, and intestines.8 Due to the persistent, non-decaying, toxic, mutagenic, and cancerous nature of heavy metals in humans as shown in Fig. 2, it is crucial to appropriately treat them before releasing them into the environment.9
 | ||
| Fig. 1 Various sources of heavy metals pollution in the ecosystem (this figure has been reproduced from Kanwar et al.10 with permission from Springer, copyright 2020). | ||
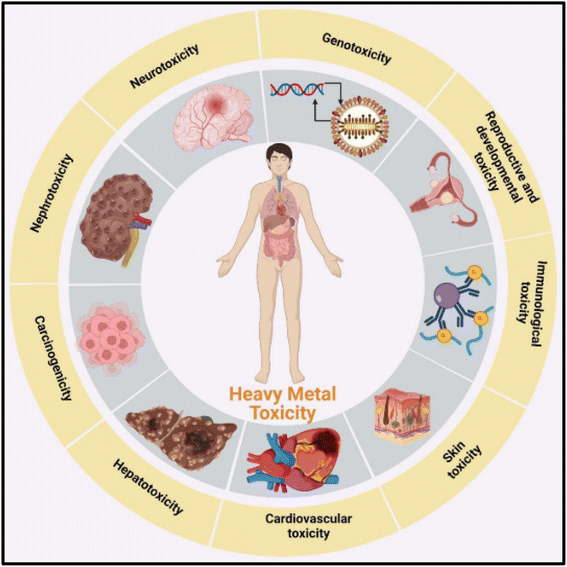 | ||
| Fig. 2 A schematic diagram showing heavy metals toxicity in human organs (this figure has been reproduced from Singh et al.11 with permission from MDPI, copyright 2023). | ||
Numerous traditional treatment methods presented in Fig. 3 have been used, including biological treatment, coagulation, membrane separation procedures, chemical precipitation, ion exchange, enhanced oxidation, and adsorption utilizing commercial activated carbon, to lessen the risks posed by heavy metals and limit their build-up in wastewater.12,13 Due to the high cost involved in the production and regeneration of exhausted commercial activated carbons, the adsorption of heavy metals using commercial activated carbons is not economical for use in wastewater treatment in underdeveloped countries.14
 | ||
| Fig. 3 Conventional and non-conventional technologies for heavy metals removal from aquatic environments (this figure has been reproduced from Nallakukkala et al.15 with permission from MDPI, copyright 2022). | ||
Nevertheless, adsorption, using locally and naturally available adsorbents from different biomass or feedstocks as shown in Fig. 4 is a cheaper and expedient technique for the decontamination of different heavy metals from water and wastewater.16,17 In several studies, inexpensive adsorbents synthesized from industrial and agricultural residues as well as from other sources were employed to achieve maximum heavy metals removal from water systems.18–26 Notwithstanding, most of these studies focused greater attention on the practical application of low-cost adsorbents derived from natural materials for the decontamination of heavy metals from synthetic wastewater typically containing a single component or pollutant. However, real wastewater comprises a mixture of several cations, anions, and compounds instead of a single ion.
 | ||
| Fig. 4 Different biomass or feedstocks used for the production of various adsorbents for heavy metals remediation in water systems (this figure has been reproduced from Wani et al.27 with permission from Springer, copyright 2022). | ||
In recent times, studies on real wastewater treatment are gradually increasing to improve the knowledge of multicomponent adsorption of different pollutants using various adsorbents. For instance, heavy metals usually occur as multicomponent mixtures in the aquatic environment.28 Hence, it is vital to comprehend the interaction of other heavy metals that coexist in a composite solution through the sorption processes in a multicomponent system.29 In multicomponent adsorption, the interaction among the adsorbate molecules and their competition in the solution toward the adsorbent surface is complicated.30 Hence, it is important to elucidate the mechanisms of the multicomponent sorption of adsorbate molecules from the industrial point of view.
Previously, several review articles have been published and most of these studies are based on the removal of heavy metals from single component adsorption systems by using various adsorbents as well as applying conventional isotherm and kinetic models to explain their mechanisms of adsorption.2,31–35 Only a very few reviews focus on heavy metals removal in multicomponent systems and the application of multicomponent adsorption isotherm models in describing sorption mechanisms.36–38 Undoubtedly, broad reviews are useful in comprehending the adsorption of different water pollutants such as heavy metals, dyes, phenols, and pharmaceuticals in multicomponent systems.
To the best of our knowledge, so far, there is no any systematic review article on the removal of heavy metals from binary- and multicomponent adsorption systems using different kinds of adsorbents including both natural and engineered adsorbents. Therefore, this systematic review is focused on assessing the potential applications of different kinds of adsorbents in water purification with an emphasis on the adsorption of toxic heavy metals in multicomponent systems. The experimental operating factors as well as the possible interaction mechanisms among the heavy metals and the adsorbents including synergistic, antagonistic, and non-interaction were also discussed using several multicomponent adsorption isotherm models. Finally, future prospectives and roadmaps are provided with suggestions for future research.
2. Methodology
2.1 Database searching and search strategy
This systematic review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The existing research findings on the removal of heavy metals from binary and multicomponent systems are contextualized and given a detailed overview in this systematic review. A computerized literature search was performed to find articles from the last 10 years regarding heavy metals removal from binary and multicomponent systems in batch adsorption processes. In this review, Web of Science, Scopus, PubMed, ScienceDirect, SpringerLink, and Google Scholar were the primary databases utilized. Each database search was carried out independently, and the selected articles were pooled together later.To search the literary works, several pertinent keywords associated with the review focus were employed including; adsorption, biosorption, binary component, bicomponent, bi-solute, bi-metal, multi-component, multi-solute, multi-metal, equilibrium, isotherm, kinetics, thermodynamics, simultaneous, concurrent, competitive, removal, decontamination, depollution, heavy metals elimination, reduction, abatement, sequestration, adsorption factors/variables/parameters, modeling, optimization, wastewater, aqueous solution, and sorbent materials including natural or unmodified adsorbents, biochar, biosorbents, activated carbon, zeolite-based adsorbents, nano-particles and nano-based adsorbents, composites-based adsorbents, and graphene-based adsorbents. Two or more of the listed keywords were combined with “heavy metals”, which is the most prevalent keyword using Boolean operators (“AND” and “OR”) for searching the relevant literature.
A total of 975 articles were selected and grouped within 5 years intervals with 48% (n = 468) from 2019–2023, 35% (n = 341) from 2014–2018, 12% (n = 117) from 2009–2013, and 5% (n = 49) from 2004–2008. All the articles that were published from 2014–2023 were of interest, however, other articles below the year 2014 and based on historical discoveries and theories formulations were also included to provide complementary information. Mendeley Desktop software was then applied in the management of the gathered articles and citing all the references. Almost all of the selected articles were related to heavy metals adsorption from binary and multicomponent systems using batch experimental approaches. The selected articles were grouped by adsorbent types such as natural or unmodified adsorbents, biochar, biosorbents, activated carbon, zeolite-based adsorbents, nano-particles and nano-based adsorbents, composites-based adsorbents, and graphene-based adsorbents.
2.2 Articles inclusion criteria
The inclusion criteria were applied to judge which article should be included in the review while evaluating the titles and abstracts. Articles that demonstrated a strong relationship between heavy metals removal from binary- and multicomponent adsorption systems using different kinds of sorbent materials including natural or unmodified adsorbents, biochar, biosorbents, activated carbon, zeolite-based adsorbents, nano-particles and nano-based adsorbents, composites-based adsorbents, and graphene-based adsorbents were included. Additionally, studies on equilibrium, isotherm, kinetics, thermodynamics, modeling, and optimization of independent factors influencing heavy metals removal from multicomponent systems were also included. Therefore, after the initial reading of the titles and abstracts as well as the deletion of duplicated papers and noting down the most important information that the review wanted to highlight, a total of 402 articles written in the English Language were selected and exported into the Mendeley Desktop for this systematic review.2.3 Articles quality study and exclusion criteria
During the drafting of the paper, which required careful reading of the contents of all the selected articles, 205 irrelevant papers were further deleted for not meeting the inclusion criteria. Specifically, 19 articles were deleted as they were non-peer-reviewed articles; 10 articles were excluded because of inaccurate descriptions of the research methods; 61 articles were removed because they did focus on the concurrent removal of heavy metals from wastewater or aqueous solutions; 100 articles were omitted as they are very old publications above 10 years but historical works were not excluded. To ascertain the suitability and quality of this review, 15 records including books, letters or short communications to the editors, and pre-print were excluded. According to the PRISMA flowchart shown in Fig. 5, the searching, screening, and filtering produced 197 suitable full-text articles that were ultimately included in this systematic review.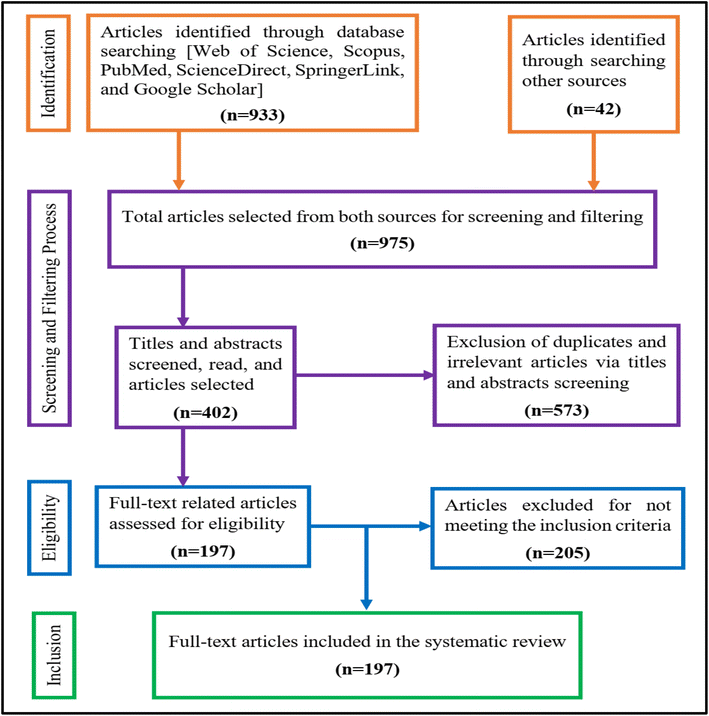 | ||
| Fig. 5 PRISMA flowchart showing a standard four-step protocol for searching, screening, assessing eligibility, and inclusion of full-text articles for the systematic review. | ||
3. Results and discussion
Among the 197 eligible full-text articles selected for this systematic review, 97 of these selected full-text articles based on different types of adsorbents employed for the adsorptive removal of heavy metals from binary and multicomponent sorption systems are presented in Fig. 6. Subsequently, the selected full-text articles were comprehensively reviewed systematically and discussed according to the adsorbent type such as pristine adsorbents [raw or natural or unmodified] (Section 4.1), biochar (Section 4.2), biosorbents (Section 4.3), activated carbon (Section 4.4), zeolite-based adsorbents (Section 4.5), nano-particles and nano-based adsorbents (Section 4.6), composites-based adsorbents (Section 4.7), and graphene-based adsorbents (Section 4.8).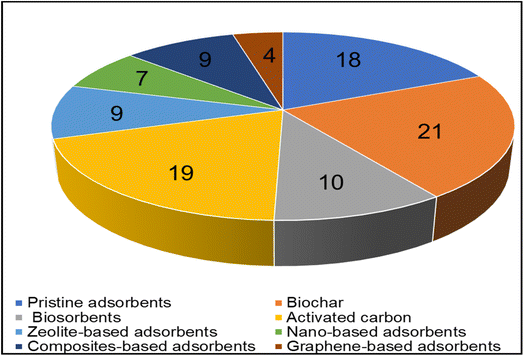 | ||
| Fig. 6 Pie chart showing the number of selected full-text articles based on the different types of adsorbents cited in this article. | ||
3.1 Competitive adsorption of heavy metals from multicomponent systems
The majority of studies in the adsorption field usually deal with the sequestration of heavy metals from single solute systems but real wastewater contains more than one component. Therefore, multicomponent adsorption must be studied to understand the competitive effect of the various inorganic and organic pollutants and the interactions between different pollutants. This is because competitive adsorption reveals the actual interactive behavior of the heavy metals in the multicomponent sorption system. Fig. 7 shows the schematic diagram of the competitive adsorption of adsorbate molecules from aqueous systems onto a sorbent material. | ||
| Fig. 7 Schematic diagram showing competitive adsorption of adsorbate molecules from aqueous systems onto a sorbent material. | ||
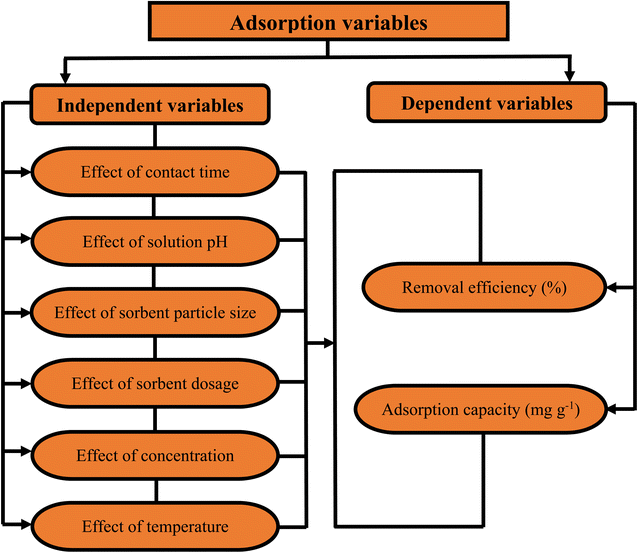
During the competitive sorption processes, several independent parameters significantly affect heavy metals uptake capacity by the promising adsorbents/biosorbents. The effectiveness of these adsorbents/biosorbents is greatly inhibited by the physicochemical properties of the aqueous solution including pH, initial metal ion concentration, temperature, ionic strength, adsorbent/biosorbent particle size, reaction time, and adsorbent/biosorbent dosage. Fig. 8 shows the schematic diagram of the different independent adsorption factors as a function of two dependent variables namely; removal efficiency and adsorption capacity. The impact of the independent adsorption parameters on the decontamination of various heavy metals from single and multi-metal systems are summarized in Table 1.
| Adsorption parameter | Description | Reference |
|---|---|---|
| Reaction time | The rate of heavy metals removal is greatly affected by reaction time and it is significant during the designing and development of water treatment systems. To achieve maximum heavy metals uptake capacity and to know the kinetics of the adsorption process, an equilibrium reaction time is required | 39 |
| Agitation speed | The adsorptive removal of heavy metals could be augmented or declined through agitation. However, a quicker agitation speed, might not sometimes result in higher heavy metals uptake by the sorbent material | 12 |
| Solution pH | The solution pH is an essential parameter in heavy metals sequestration and it influences the adsorbent surface charge. At pH values smaller than that of the pH point of zero charge (pHPZC), the adsorbent surface becomes positively charged, which inhibits and reduces the uptake capacity of heavy metals. At pH values greater than that of pHPZC, the adsorbent surface is negatively charged which enhances heavy metal ions' decontamination from water systems | 40 |
| Adsorbent particle size | The capacity of heavy metals to bind to an adsorbent surface decreases with increasing particle size while increasing with decreasing particle size. To decontaminate heavy metals effectively from aqueous systems through adsorption, the adsorbent surface area upsurges as the particle size reduces. A bigger surface area signifies the availability of many sorption sites on the adsorbent surface for the efficient uptake of heavy metal ions | 41 |
| Adsorbent dosage | The dosage of the sorbent material defines its suitability for a certain amount of heavy metal ions to be adsorbed. Higher amounts of heavy metals are decontaminated from the aqueous systems when more active surface sites are created by upping the adsorbent load. However, one alternative to minimize the adsorbent load required for removing heavy metal ions from single or multicomponent systems is to increase the adsorbent surface area | 42 |
| Initial metal ion concentration | The starting concentration shows dependence on the non-competitive and competitive decontamination of any heavy metal from individual and multi-solute systems. The initial concentration plays a key role in overcoming the resistance to mass transfer between the toxic metal ions and the adsorbent in the solution | 43 |
| Reaction temperature | The uptake ability of heavy metals from aquatic systems strongly depends on the reaction temperature. Therefore, increasing the reaction temperature between the metal ions and the sorbent material in the solution leads to endothermal exothermic adsorption, respectively | 44 |
| Ionic strength | The ionic strength is a very crucial parameter in the decontamination of various heavy metals from aquatic systems because wastewater contains other metal ions or electrolytes. Hence, the coexistence of other cations and anions in wastewater could inhibit the single removal of these heavy metal ions due to electro-statistic interactions between heavy metals and sorbent surfaces | 45 |
More so, in the optimization of the competitive and concurrent removal of more than one heavy metal ion in the coexistence of other cations and anions in multi-solute systems, it is essential to identify the types of interactive effects among the heavy metals. In multicomponent systems, competitive sorption typically takes place, and three primary kinds of effects are possible including synergism, antagonism, and non-interaction.46 The effect of the ionic interactions on the multicomponent sorption system is determined by the ratio of the maximum uptake capacity for one heavy metal ion in the multicomponent system (qmix), to the uptake capacity for the same heavy metal ion present in the single system (q0).47 Mathematically, the interaction factor (Rqe) is expressed in eqn (1).
 | (1) |
| Relation | Meaning | Reference |
|---|---|---|
 |
The adsorptive elimination of one heavy metal is promoted by the coexistence of other heavy metal ions in a multicomponent system suggesting synergism | 49 |
 |
The existence of other cations and anions in the multicomponent system does not influence the individual decontamination of the various heavy metal ions indicating non-interaction | 50 |
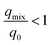 |
The coexistence of other ions significantly inhibited the adsorptive elimination of the individual heavy metal ions from the multicomponent system, which points to antagonism | 37 |
3.2 Multicomponent adsorption system isotherm models
Globally, multicomponent adsorption of water pollutants including heavy metals using various adsorbents has been recognized to be essential and effective. The adsorptive removal of one pollutant may be influenced by the coexistence of other pollutants in the multicomponent system due to adsorbent properties including surface charge, structure, size, porosity, number of active sites, and functional groups present on the sorbent surface.51 In a multicomponent system, the amount of the pollutant removed by the sorbent material may upsurge, reduce or remain unchanged in the existence of the other pollutants.52Adsorbent efficiency and pollutant uptake ability could be quantitatively compared using adsorption isotherm modeling to estimate the adsorption parameters.53 Hence, equilibrium adsorption isotherm data are required in designing adsorption systems.54 Several adsorption isotherm models such as Langmuir, Freundlich, Dubinin–Radushkevich, Temkin, Redlich–Peterson, and Sips as well as kinetic models including pseudo-first-order, pseudo-second-order, Elovich, and Webber–Morris intra-particle diffusion have been employed to explain the interactive effect between pollutants and adsorbents in single component systems.7,21,55–57 However, real wastewater contains a variety of contaminants that cause competition and interaction among the contaminants. The single component isotherm models are usually modified and applied to multicomponent systems since the models used in the single component systems do not apply to multicomponent systems. This is due to the sophisticated mechanism involved in multicomponent adsorption and more in-depth complex models must be utilized.51
These multicomponent adsorption isotherm models are important to establish the relationships between the uptake capacity of one pollutant by the adsorbent and the equilibrium concentration of the other coexisting pollutants in the wastewater. The competitive adsorption isotherm models could determine the type of interactions (as summarized in Table 2) among the various adsorbates and adsorbents in the multicomponent systems at equilibrium.
 | (2) |
The parameters derived from the single component adsorption isotherm model do not describe how the interactions between the different components and the adsorbent in the solution occur. To explain the nature of the sorption process, an interaction term is typically used. This interaction factor reveals the competitive effect between the individual components and adsorbent in the multicomponent system. It is a component's distinctive parameter that is influenced by the concentration of other constituents in the multicomponent system.38
 | (3) |
 | (4) |
 | (5) |
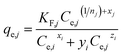 | (6) |
 | (7) |
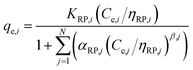 | (8) |
 | (9) |
 | (10) |
 | (11) |
 | (12) |
 | (13) |
4. Competitive decontamination of heavy metals from sorption systems
The possibility of various adsorbents derived from natural, agricultural, and industrial waste materials as well as biosorbents synthesized from microorganisms including algae and bacteria have been reported in several studies. In the present study, the simultaneous adsorptive elimination of heavy metals from multicomponent systems using various sorbent materials as shown in Fig. 9 has been systematically reviewed. This systematic review has categorized the sequestration of various heavy metal ions in the multicomponent systems by these sorbent materials into eight (8) types including natural or unmodified adsorbents, biochar, biosorbents, activated carbon, zeolite, nano-, composite-, and graphene-based adsorbents. In most of the studies, the uptake capacities of the different heavy metals in the binary and multicomponent adsorption systems achieved by these sorbent materials have been reported and discussed in this review to establish the interactive mechanisms among the heavy metals involved. | ||
| Fig. 9 A schematic diagram showing different sorbent materials for the determination of heavy metals from aquatic environments. | ||
4.1 Natural or unmodified (pristine) adsorbents
The application of natural materials as adsorbents have shown that they are versatile and promising for the removal of both inorganic and organic contaminants from aquatic environments. A schematic diagram showing the preparation of unmodified or pristine adsorbents from agro-waste or biomass is displayed in Fig. 10. The simultaneous removal of several heavy metals from multicomponent adsorption systems using natural, raw, unmodified, and pristine adsorbents has been reported and discussed. | ||
| Fig. 10 Schematic illustration of the preparation of unmodified or pristine adsorbents from agro-waste or biomass (this figure has been adapted from Bilal et al.32 with permission from Elsevier, copyright 2022). | ||
Natural banana peels were used for Pb(II) and Cu(II) ions removal from a binary solute system by varying operating variables such as initial concentration, adsorbent dose, pH, and adsorbent particle size in a batch mode through the application of central composite design (CCD).19 The analysis of variance indicates a good fit with the coefficient of determination of 0.998 and 0.988 for Cu(II) and Pb(II), respectively. The removal efficiency of Cu(II) and Pb(II) augmented with increasing adsorbent dose with the sorption rate of Pb(II) being the highest. The optimized operating conditions gave 99.79 and 88.94% removal efficiency for Cu(II) and Pb(II), respectively with 100.00 mg L−1 concentration, pH 5.00, 1.00 g adsorbent load, particle size of 75.00 μm, and desirability of 0.959. The good correlation between the actual and predicted values indicates the validity of the second-order polynomial equation for Cu(II) and Pb(II) ions removal using the banana peels.
More so, the potential of tea pulp in the decontamination of Pb(II) and Cu(II) from single and binary systems was investigated.13 The impact of pH, adsorbent dosage, agitation time, and initial single- and binary-metal concentrations on Pb(II) and Cu(II) removal was conducted using batch technique. At a pH of 5.00–8.00 and pH of 4.00–8.00, Cu(II) and Pb(II) ions optimum uptake capacities were achieved by the adsorbent, respectively. For the single system, the optimum uptake capacities of Pb(II) and Cu(II) attained by the tea pulp were 48.54 and 37.17 mg g−1, respectively, and 43.47 and 28.41 mg g−1 for Pb(II)and Cu(II), correspondingly in the competitive system. The isothermal and kinetic data achieved by tea pulp were well explained by the pseudo-second-order kinetic and Langmuir isotherm models. The study found the tea pulp to be a less-cost bio-adsorbent that could decontaminate Pb(II) and Cu(II) ions effectively from single and bicomponent systems.
Also, the confiscation of Pb(II) and Cu(II) by an adsorbent derived from chili seed in single and multicomponent systems was studied by Forgionny et al.5 In single component systems, it was observed that the uptake capacity of 48.00 mg g−1 for Pb(II) was greater than the Cu(II) uptake capacity of 4.10 mg g−1 as achieved by the chili seed. This suggests that the chili seed has greater selectivity for Pb(II) ions than for Cu(II) ions in the single sorption systems. For multicomponent systems, it was observed that the elimination of Pb(II) and Cu(II) ions by the adsorbent was due to antagonistic interactive effects between Pb(II) and Cu(II) ions. The study revealed that under equal initial concentrations of Pb(II) and Cu(II) and at higher amounts of Pb(II), about 50.00 and 56.00% decline in Pb(II) and Cu(II) removal were observed, correspondingly.
The potentiality of vetiver roots as an adsorbent in removing Ni(II), Cd(II), and Pb(II) from synthetic ternary aqueous systems was carried out.81 Single and ternary metal solutions were prepared and used to perform the batch experiments using optimum operating conditions. Out of five isotherm models tested, the Langmuir isotherm was best fitted to the equilibrium sorption data of Ni(II), Cd(II), and Pb(II) with uptake capacities of 13.55, 10.99, and 17.33 mg g−1, respectively. The impact of competitive sorption was predicted using the modified Langmuir competitive isotherm model. In the mono-metal systems, the highest uptake capacity of Ni(II) and Pb(II) by the biosorbent was decreased by about 3.00 and 33.00 times, respectively in a ternary component system. However, it was later found to improve by about 1.64 times for Cd(II). Based on the findings of this study, it is concluded that the adsorbent could be employed in the elimination of heavy metals.
An adsorbent derived from rice husk was utilized in removing Cu(II), Pb(II), Fe(III), and Cr(III) from multicomponent sorption media.82 The study indicated that at a reaction time of 2.00 h and solution pH of 5.00, the equilibrium removal rate of Cu(II), Pb(II), Fe(III), and Cr(III) was accomplished with Fe(III) ions being the most highly adsorbed. The experimental data of Pb(II), Cr(III), Fe(III), and Cu(II) achieved by the adsorbent well correlated to the pseudo-second-order kinetic model. While the sorption data of Pb(II), Cr(III), Fe(III), and Cu(II) at equilibrium were best represented by the Langmuir model. The study showed the efficacy and affordability of utilizing rice husk-derived adsorbent to simultaneously remove Pb(II), Cr(III), Fe(III), and Cu(II) from aquatic systems.
Similarly, the depollution of Zn(II), Hg(II), and Cd(II) in mono-, binary- and tertiary-metals systems by rice husk ash (RHA) has been investigated.26 Batch tests were performed for both the mono- and multi-metals aqueous media with varying metal ions concentrations to investigate the non- and competitive adsorption characteristics. The study indicated that the decontamination of these metal ions exhibited this trend: Zn(II) > Cd(II) > Hg(II) in the single solute systems. In the binary and tertiary component systems, the sorption process was inhibited by the existence of other ions in the aqueous solutions. It was found that the Freundlich and Langmuir models best fitted the equilibrium data of Zn(II), Cd(II), and Hg(II) in all the sorption systems. The study recommended that there is a need to grasp the mechanisms of competitive sorption of coexisting ions onto RHA for the effective operation of the sorption processes for removing these toxic metal ions from polluted water.
The capability of an adsorbent derived from thiourea spent grated Cocos nucifera (TSGC) was explored for the concurrent depollution of Pb(II), Ni(II), and Cu(II) from ternary water systems.83 The study found that TSGC had a greater affinity for the Cu(II) ions than that of Ni(II) and Pb(II) ions present in the ternary sorption medium. Hence, the uptake capacity of Cu(II) ions by the adsorbent was greater than Ni(II) and Pb(II). The maximum uptake capacity of Ni(II), Pb(II), and Cu(II) attained by the adsorbent was 1.38, 0.32, and 0.24 mmol g−1, respectively. The sorption kinetic data of Cu(II), Ni(II), and Pb(II) best fitted the pseudo-second-order kinetic model. Also, the Langmuir model was well correlated to the sorption data of Ni(II), Pb(II), and Cu(II). The study suggested that TSGC is a promising locally available adsorbent for heavy metals remediation from contaminated water.
Unmodified pomelo peels were applied as a bio-adsorbent to decontaminate Pb(II) ions present in multi-solute systems.84 The study found that 99.90% of the Pb(II) ions were removed using this bio-adsorbent at 210.00 min of agitation time, 20.00 mg L−1 Pb(II) concentration, pH of 3.00, and 50.00 °C temperature. There was no influence of Zn, Cu(II), Mg(II), and Cd(II) on the efficiency of pomelo peels to decontaminate Pb(II) from the liquid phase. The kinetic and equilibrium isotherm data of Pb(II) were observed to obey the Langmuir and pseudo-first-order models, correspondingly. Pomelo peels exhibited the possibility to be employed for the concurrent decontamination of harmful heavy metals coexisting in wastewater.
Likewise, the concurrent sequestration of Cd(II), Ni(II), Zn(II), and Cu(II) from competitive sorption media was examined using orange peel biomass.85 The uptake capacity of the adsorbent was found to be reduced in the binary solute system suggesting an antagonistic effect on the heavy metals removal. There was the existence of a synergistic effect on Cu(II) ions sequestration from the multicomponent system while an antagonistic effect existed during the single decontamination of Zn(II), Cd(II), and Ni(II) ions from the multi-solute medium. The modified Langmuir isotherm model demonstrated good fitness to the equilibrium competitive data of Cd(II), Zn(II), Ni(II), and Cu(II). The results of this study showed that an orange peel-based adsorbent is employable for the multicomponent sequestration of heavy metals found in aqueous media.
An adsorbent was produced from wheat bran and used for removing Cd(II), Pb(II), and Cu(II) from multicomponent media.86 The optimum removal capacity of the adsorbent was found as 901.00, 978.00, and 1136.00 mg g−1 for Cu(II), Cd(II), and Pb(II), respectively. The adsorbent was observed to have the highest affinity towards Pb(II) and Cd(II) ions. The study revealed that there exist synergistic effects among the three heavy metals, in which the adsorptive removal of an individual metal is promoted by the occurrence of other ions. The prepared adsorbent possessed a large specific surface area of 140 m2 g−1 obtained from BET sorption data. Hence, this adsorbent is found to be proficient in treating heavy metals contaminated water.
Also, unmodified peanut shells, sawdust, and activated carbon were used to eliminate Cd(II), Cu(II), and Pb(II) from non- and competitive sorption systems.87 The study found that as the contact time progressed and then approached equilibrium, Cd(II), Pb(II), and Cu(II) ions were eliminated by the activated carbon, peanut shell, and sawdust. Also, the quantity of Pb(II), Cu(II), and Cd(II) ions adsorbed augmented but the removal efficiency thereof decreased as the initial Cd(II), Pb(II), and Cu(II) concentrations increased. The activated carbon exhibited the highest uptake capacity of 5.50, 13.10, and 14.01 mg g−1 for Cd(II), Cu(II), and Pb(II), respectively. While the uptake capacity of Cd(II), Pb(II), and Cu(II) attained by the unmodified peanut shell were 5.07, 9.50, and 49.10 mg g−1, respectively. The untreated sawdust had the lowest sorption capacity of 3.99, 5.30, and 5.50 mg g−1 for Cd(II), Cu(II), and Pb(II) ions, correspondingly. The sorption data of both single and competitive systems was best represented by the pseudo-second-order and Langmuir models. In the multicomponent sorption systems, there existed antagonistic effects among the adsorptive elimination of Pb(II), Cd(II), and Cu(II) ions from the competitive aqueous media.
More so, the elimination of Cd(II), Pb(II), and Cu(II) from unitary, binary, and ternary sorption systems by white pottery clay was conducted.88 The study revealed that the white pottery clay's maximum uptake capacities of Pb(II), Cu(II), and Cd(II) were attained at an equilibrium reaction time of 180.00 min. The kinetic and isothermal data of Pb(II), Cu(II), and Cd(II) were well explained by the pseudo-second-order and Langmuir isotherm models, correspondingly. The Langmuir maximal monolayer uptake capacities attained for Pb(II), Cu(II), and Cd(II) were 159.24, 38.49, and 26.99 mg g−1, respectively. In the Pb(II)–Cu(II) binary sorption medium, the Cu(II) ions exhibited an antagonistic effect on Pb(II) ions elimination by the adsorbent while Cd(II) ions demonstrated very low inhibitory influence on Pb(II) ions decontamination from the Pb(II)–Cd(II) binary medium. The ability of white pottery clay to depollute Cd(II), Pb(II), and Cu(II) ions is significantly reduced as a result of the competitive effect superimposing in the ternary system [Pb(II)–Cu(II)–Cd(II)]. In comparison, the sorption capacity of Pb(II) reduced with rising Cu(II) and Cd(II) initial concentrations. However, the adsorptive elimination of Cu(II) and Cd(II) ions is insignificantly inhibited by Pb(II) initial concentration in the aqueous solution.
Secondary composts (SC) derived from solid wastes of a municipal and wood (W-SC) were synthesized and utilized in removing Cd(II), Ni(II), Zn(II), and Cu(II) from mono- and multi-solutes sorption media.89 It was found that in the mono-metal system, the W-SC had a higher affinity towards the heavy metals than SC with maximum sorption capacity of 42.70, 38.60, 34.90, and 28.70 mg g−1 for Cd(II), Cu(II), Zn(II), and Ni(II), correspondingly. In multi-solute media, the Ni(II) ions uptake rate by both adsorbents was greatly inhibited than that of Cu(II), Zn(II), and Cd(II). The equilibrium sorption data of Cu(II), Cd(II), Ni(II), and Zn(II) was best described by Freundlich and Langmuir models. The study demonstrated that W-SC is very useful for decontaminating several heavy metals from water systems.
In another study, cork stopper particles were utilized as a bio-adsorbent to decontaminate Zn(II) and Cd(II) from monocomponent and bicomponent simulated wastewater.90 The study found that in a single sorption medium, the optimum percentage decontamination of Cd(II) and Zn(II) ions was 82.62 and 90.96%, respectively at 150.00–180.00 min contact time, pH of 5.50–6.00, a biosorbent dosage of 0.85–1.00 g, with 50 mg L−1 initial metal ion concentration. For Zn(II) and Cd(II) ions decontamination from the binary component system, an optimum solution of pH 6.00 was used. In both mono- and bi-solutes sorption systems, the heavy metal ions elimination occurred in this order, Zn(II) > Cd(II). However, in the bi-solute medium, the percentage removal of both heavy metals was observed to be reduced. The kinetic sorption data of Zn(II) and Cd(II) achieved by the cork stopper particles was well explained by the pseudo-first-order kinetic model. Also, the isothermal data of Cd(II) and Zn(II) was best correlated to extended Freundlich and Langmuir models for the non-complex and complex component systems, correspondingly.
The simultaneous depollution of Fe(II) and Cu(II) from binary component sorption media was examined by microcrystalline cellulose (MCC) as an adsorbent.91 The result of the adsorption experiment revealed that MCC could remove more than 70.00% of Fe(II) and Cu(II) indicating that there were sturdy electrostatic attractions among the adsorbent surface and cationic heavy metal ions. Also, the sorption data of Fe(II) and Cu(II) attained by MCC were best represented by the pseudo-second-order kinetic model, demonstrating chemisorption between the polymeric MCC adsorbent and heavy metal molecules. The study concluded that the MCC is a reliable and low-cost sorbent in treating water polluted by Fe(II) and Cu(II) and other water pollutants.
Also, Tekin and Unsal92 conducted a study to investigate the individual and competitive uptake of Ni(II) and Cu(II) onto sepiolite. For the monocomponent systems, the initial mono-metal concentration, mixing speed, and temperature were studied as a function of time to determine the operating conditions where the adsorbents showed a great deal of affinity towards the Ni(II) and Cu(II) ions in the solutions. Among the applied isotherm models, the one-component sorption data did fit the Langmuir isotherm best. The simultaneous and competitive uptake of Cu(II) and Ni(II) was assessed using the extended Langmuir and Freundlich adsorption isotherm models. It was revealed that both isotherm models described the two-component sorption data perfectly with uptake capacities of 222.22 and 256.41 mg g−1 attained by the sepiolite for Ni(II) and Cu(II), correspondingly. Therefore, the single- and binary-sorption results unclosed that the effect of sepiolite on Cu(II) is greater than that of sepiolite on Ni(II).
Bassam et al.93 carried out a study to establish the ease of removing Cd(II), As(III), and Cr(VI) ions competitively from aqueous media using raw rock. The impact of adsorbent dose, agitation time, reaction temperature, pH, and initial concentration on Cd(II), Cr(VI), and As(III) ions removal in the batch mode was undertaken. The kinetic and isothermal data attained by the adsorbent conformed to the pseudo-second-order and Langmuir models, respectively. The optimum monolayer adsorption capacities of the Langmuir isotherm model were found to be 16.36, 17.54, and 15.23 mg g−1 for As(III), Cr(VI), and Cd(II), respectively. The thermodynamic variables suggested that the adsorptive removal of all the investigated heavy metals was exothermic, feasible, and spontaneous. The heavy metal ions desorption showed that this raw rock had excellent recycling capacity. Based on the results, these untreated rocks can be used as inexpensive and environmentally friendly adsorbents to treat water contaminated by heavy metals.
Granular gravel was explored as an adsorbent to decontaminate Cu(II), Zn(II), Ni(II), and Fe(II) ions from single- and multi-solutes.94 It was revealed that the removal rate of Cu(II), Fe(II), Zn(II), and Ni(II) from the single metal system was 98.00, 87.50, 76.05, and 36.38%, respectively. While the removal rate of 7.32, 48.00, 83.00, and 98.30% for Ni(II), Zn(II), Fe(II), and Cu(II), respectively, were attained in the multi-solute medium at a pH of 7.00. The sorption rates of these metal ions using this adsorbent ranged from 0.91–42.86 mg g−1 as well as 0.04–1.63 mg g−1 in the single and mixed metal systems, respectively. The kinetic and isothermal data conformed to the pseudo-second-order and Freundlich models, correspondingly.
4.2 Biochar
In the absence of oxygen and at low temperatures, feedstock or any biomass is pyrolyzed to produce biochar, a solid by-product rich in carbon. Due to electrostatic interactions between heavy metal ions and the negatively charged surface of the biochar as well as ion exchange among the surface electrons and metallic cations, biochar can adsorb heavy metals. A schematic diagram showing the conversion of agro-waste into biochar for the adsorptive removal of heavy metals from aqueous systems is presented in Fig. 11. | ||
| Fig. 11 Schematic diagram showing the conversion of agro-waste into biochar for heavy metals removal from aqueous systems carbon (this figure has been adapted from Wang et al.31 with permission from RSC, copyright 2023). | ||
Ferrihydrite-modified biochar (Fh@BC) was utilized for the simultaneous decontamination of As(III) and Cd(II) ions from binary-metal aqueous systems as well as determine the mechanism of the sorption process.95 The Langmuir model best correlated with equilibrium sorption data with optimum sorption rates of 18.38 and 18.18 mg g−1 for As(III) and Cd(II), respectively. The mechanisms of the simultaneous elimination of As(III) and Cd(II) by Fh@BC were due to cation exchange, complexation, and oxidation. The study showed that Fh@BC is a promising bio-adsorbent for the concurrent decontamination of As(III) and Cd(II) ions present in wastewater.
Likewise, the simultaneous elimination of Cd(II)and As(III) from water systems was achieved by a unique α-FeOOH-treated wheat straw biochar (α-FeOOH@BC).96 The highest removal capacity of α-FeOOH@BC for Cd(II) and As(III) was 78.30 and 62.90 mg g−1, correspondingly, in the single component system. While the maximum adsorption capability of α-FeOOH@BC decreased to 39.30 and 67.20 mg g−1 for Cd(II) and As(III), respectively, in the binary component system. The Langmuir adsorption isotherm and pseudo-second-order models provided good fits for the experimental data of As(III) and Cd(II). Ion exchange and co-precipitation were the two main sorption mechanisms, and the removal of As(III) and Cd(II) ions on α-FeOOH@BC exhibited a competitive effect. The study demonstrated that α-FeOOH@BC can be a useful remediation bio-adsorbent for the simultaneous As(III) and Cd(II) from aquatic environments.
Also, wheat straw was used to produce activated biochar for decontaminating Pb(II) and Cd(II) competitively from aquatic systems.6 At an equilibrium reaction time of 2.00 h, pH of 8.00, and 30.00 mg L−1 metal ion concentration, optimum sorption capacity of 8.85 and 9.03 mg g−1 for Pb(II) and Cd(II) ions, correspondingly, were attained. The Langmuir isotherm and pseudo-second-order models best described the isothermal and kinetic data of Pb(II) and Cd(II), respectively. The optimum monolayer adsorption rates of Pb(II) and Cd(II) ions attained by the biochar at 298 K were 0.60 and 9.30 mg g−1, correspondingly.
Additionally, biochar obtained from anaerobic sludge was utilized to decontaminate Cd(II) and Pb(II) from non- and competitive aqueous systems.97 In a single sorption system, the biochar exhibited a greater removal rate for Pb(II) ions than Cd(II) ions. For the single component system, the optimum removal capacities of 0.55 and 0.75 mmol g−1 were achieved by the biochar for Cd(II) and Pb(II), correspondingly. In the binary-metal systems, the Cd(II) ions decontamination by the biochar was highly inhibited, while Pb(II) ions uptake by the biochar was not significantly interfered with. The sorption data of Pb(II) and Cd(II) at equilibrium was best explained using both Dubinin–Radushkevich and Langmuir models for the single metal system, while the competitive Langmuir adsorption isotherm model well represented Pb(II) and Cd(II) equilibrium data obtained from the bi-solute systems.
Similarly, a corn stalk biochar-supported nanoscale zero-valent iron (nZVI–BC) with diverse ratios of nZVI and BC was used for the concurrent elimination of As(III) and Cd(II) from aquatic media.98 The batch tests results revealed that nZVI–BC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) had the greatest uptake capacity for Cd(II) and As(III) ions and optimum Cd(II)and As(III) sorption capacity was 33.81 and 148.50 mg g−1 achieved at 2.00 and 1.00 h, respectively. More so, the study found that the synergistic effects between As(III) and Cd(II) ions in a competitive system improved the nZVI–BC (1
1) had the greatest uptake capacity for Cd(II) and As(III) ions and optimum Cd(II)and As(III) sorption capacity was 33.81 and 148.50 mg g−1 achieved at 2.00 and 1.00 h, respectively. More so, the study found that the synergistic effects between As(III) and Cd(II) ions in a competitive system improved the nZVI–BC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sorption capacity. In the competitive system, the uptake rate of As(III) and Cd(II) was increased to 158.50 and 179.90 mg g−1, respectively. The experimental data attained for the concurrent abatement of As(III) and Cd(II) from the solution was well correlated to the pseudo-second-order kinetic and Redlich–Peterson isotherm models, respectively. Therefore, nZVI–BC (1
1) sorption capacity. In the competitive system, the uptake rate of As(III) and Cd(II) was increased to 158.50 and 179.90 mg g−1, respectively. The experimental data attained for the concurrent abatement of As(III) and Cd(II) from the solution was well correlated to the pseudo-second-order kinetic and Redlich–Peterson isotherm models, respectively. Therefore, nZVI–BC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is a good choice for the treatment of Cd(II)and As(III) ions in polluted water bodies.
1) is a good choice for the treatment of Cd(II)and As(III) ions in polluted water bodies.
The simultaneous detoxification of Cd(II) and As(V) ions from water systems were studied using ultrasonic and nanoscale TiO2 (TD) biochar.99 The experimental data were well explained by the pseudo-second-order model whereas the equilibrium data fitted well to the Langmuir isotherm model. The maximum uptake capacities of 118.06 and 72.62 mg g−1 were achieved by the adsorbent for As(V) and Cd(II), correspondingly. The removal efficiency of 70.00% accomplished for both Cd(II) and As(V) occurred at optimum pH of 5.00. The presence of both As(V) and Cd(II) ions in the bicomponent system revealed a competitive effect at 100.00 mg L−1 concentration. The study offered a practical technique for the development of the adsorbent, which is a safe adsorbent to simultaneously decontaminate water pollutants, particularly heavy metals.
The chitosan–EDTA treated magnetic biochar (E-CMBC) was employed to remove Cd(II), Ni(II), and Cu(II) in mono-metal and binary-metal sorption media.100 It was observed that the kinetic and equilibrium data of Cd(II), Ni(II), and Cu(II) on E-CMBC were best explained by the Avrami fractional order and the Langmuir models. The optimum uptake capacities were 40.38, 48.73, and 61.95 mg g−1 for Ni(II), Cu(II), and Cd(II), correspondingly. In a binary-metal system, coexisting ions have obvious competitive adsorption behavior on E-CMBC. The maximum sorption capacities of these metal ions were found to be lesser than that in single component systems. The order of the competitive decontamination was: Cu(II) > Ni(II) > Cd(II). Therefore, the study recommended that E-CMBC had the potential to depollute a variety of pollutants from water systems.
The decontamination of Zn(II) and Pb(II) from mono- and bi-solutes sorption media was examined by animal-derived biochar (ADB) was studied.101 The optimum uptake capacity of Zn(II) and Pb(II) onto ADB in the mono-solute system was 2.74 and 3.23 mmol g−1, respectively. While in the bi-solute medium, the uptake capacity of Zn(II) and Pb(II) by the ADS was observed to be 2.31 and 2.7 mmol g−1, respectively. The removal efficiency of Zn(II) and Pb(II) in the mono-solute system was 99.90 and 78.00–80.60%, respectively. In the binary sorption system, the existence of Pb(II) ions altered the removal mechanism of Zn(II) by ADB. The study found that ADB is proficient biochar for the competitive decontamination of Zn(II) and Pb(II) from their aqueous media.
Also, biochar obtained from chicken manure (CM), rice straw (RS), and sewage sludge (SS) was employed to remove Pb(II)and Zn(II) from mono-solute and binary-solute systems.102 The RS biochar was observed to have the highest uptake capacity for Pb(II) and Zn(II) ions from both mono-solute and binary-solute systems. This is because the RS was found to be a highly negatively charged surface, possess enormous aromatic functional groups, and is physically stable. The sorption data obtained from both single- and binary-metal systems were best correlated to both the Freundlich and Langmuir models, respectively. Therefore, this study provided crucial information for using biochar derived from locally available resources for wastewater purification.
More so, the individual and simultaneous sequestration of Pb(II) and Cr(VI) by engineered biochar synthesized from pinewood sawdust were investigated.103 The study revealed that the uptake capacity of Pb(II) and Cr(VI) from the binary sorption medium was found to be greater than that obtained from the single systems at a temperature of 298 K. However, the detoxification of Pb(II) ions from both individual and binary component systems was higher than that of Cr(VI) attained by the biochar. The uptake capacity of Cr(VI) and Pb(II) at the equilibrium was found to be 346.00 and 606.00 mg g−1 in monocomponent systems and 488.00 and 1420.00 mg g−1, correspondingly in the binary system. The study revealed that biochar could be fruitfully employed for the individual detoxification of Pb(II) and Cr(VI) ions from wastewater and in multicomponent systems.
Wu et al.23 produced biochar (BC) and magnetic biochar (MBC) from pomelo peel at different temperatures for the adsorptive elimination of Cu(II), Pb(II), and Zn(II) from single-, binary-, and ternary-metals systems. It was observed that the pyrolysis temperature and magnetization could influence the uptake rate of biochar. The study found that the competitive sorption order of the metals in the ternary-solute system was Pb(II) > Cu(II) > Zn(II). The kinetic and isothermal data best agreed with the pseudo-second-order and Langmuir models. The study showed that pomelo peel biochar is effective in the adsorptive elimination of heavy metals from wastewater where the major contaminant is Pb(II) and maximum uptake capacity could reach about 48.74 mmol g−1. The study also proposed technical support for the effective utilization of the pomelo peels as well as the engineering application of its biochar.
Similarly, biochar derived from raw tea waste (RTW) was produced under different pyrolysis conditions for the elimination of Cd(II), Cu(II), and Pb(II) from single and ternary solute systems.20 However, the biochar produced by pyrolysis of RTW at 900.00 °C (BC 900) demonstrated the highest uptake capacity. The affinity of these metals in the ternary solutions towards the BC 900 followed the pattern: Pb(II) > Cd(II) > Cu(II). While the single metal ions' affinity towards the BC 900 was in the order of Pb(II) > Cu(II) > Cd(II). It was observed that the uptake of Pb(II), Cd(II), and Cu(II) ions from the single and ternary solute systems was best explained using the pseudo-second-order model suggesting chemisorption. The results of the investigation provided new insight into the employability of RTW in water purification.
Also, an alkali-modified biochar prepared from ginkgo leaves was produced by simply one-step pyrolysis and was employed for the concurrent elimination of Cd(II), Cu(II), and Pb(II) from competitive aqueous systems.22 The study found that after the ginkgo leaves were modified with alkali, the biochar exhibited abundant surface functional groups and large porosity, resulting in high adsorption capacities for 589.32, 563.55, and 81.70 mg g−1 for Pb(II), Cd(II), and Cu(II), correspondingly. The isothermal and kinetic data best fitted the Freundlich and pseudo-second-order kinetic model, correspondingly. The alkali-modified biochar could act as a functioning sorbent for heavy metals elimination in actual wastewater because of its superior performance over a wider pH range and its high uptake capacity.
A raw cassava root husk-derived biochar (CRHB) and modified CRHB using ZnO nanoparticles (CRHB-ZnO3) was applied for the simultaneous removal of Cr(VI), Pb(II), Cd(II), and As(III) ions from competitive aqueous solutions by batch mode.104 It was found that at pH 6.00–7.00, 60.00 min contact time, and concentration of 80.00 mg L−1, maximum removal of these heavy metals was achieved. The uptake capacities of these heavy metals by CRHB-ZnO3 were observed to be in this trend: Pb(II) > Cd(II) > As(III) > Cr(VI). The overall maximum uptake capacity achieved by the CRHB and CRHB-ZnO3 during the removal of these four metal ions was observed to be 115.11 and 154.21 mg g−1, correspondingly. The individual uptake capacity by the CRHB-ZnO3 was determined as 44.27, 42.05, 39.52, and 28.37 mg g−1 for Pb(II), Cd(II), As(III), and Cr(VI), correspondingly. The optimum uptake capacity of Pb(II), Cd(II), As(III), and Cr(VI) ions attained by CRHB was found to be 34.47, 32.33, 26.42, and 21.89 mg g−1, respectively. The study also revealed that the pseudo-first-order and pseudo-second-order models best described the kinetic data of the bio-adsorbent. While the sorption data were well correlated to the Langmuir model. The study found that the cassava root husk can be applied as an alternative, inexpensive, non-toxic, and highly suitable adsorbent in removing various toxic cations from aquatic systems.
Peanut shell-derived biochar (PBC) was utilized in removing Pb(II), Cu(II), Ni(II), Cd(II), and Zn(II) ions from single and competitive wastewater.105 The study found that the biochar demonstrated efficient decontamination of these metals from the single component system. Besides, the competitive sorption revealed that the selectivity of the biochar for the heavy metal ions declined according to the following manner Pb(II) > Cu(II) > Cd(II) > Ni(II) > Zn(II), which was found lower than that in the single sorption system. The kinetic and isothermal data of both single and competitive sorption systems were best explained by the Langmuir isotherm and pseudo-second-order models, correspondingly. Therefore, the study indicated that peanut shells-derived biochar is a profitable green adsorbent for water contaminants cleanup.
The single and ternary detoxification of Hg(II), Pb(II), and Zn(II) from wastewater onto a flamboyant char was studied by Sellaoui et al.106 It was found that the flamboyant char is more proficient and suitable for the decontamination of Hg(II) in both mono- and ternary component medium than that of Pb(II) and Zn(II) ions. However, the adsorptive removal of Zn(II) and Pb(II) was observed to be varied based on the existence of the other competitive ions in the ternary system. The existence of other competing heavy metal ions in the ternary component system resulted in the recognition of high antagonistic effects on the sequestration of Zn(II) from the multi-metal sorption system.
In batch and column adsorption tests, the competitive decontamination of Pb(II), Cu(II), and Ni(II) from aquatic systems using date seed biochar was explored.107 The study revealed that the decontamination of Ni(II), Pb(II), and Cu(II) by date seed biochar in the multi-solute systems demonstrates a competitive effect. In all column and batch modes, the uptake capacity of each heavy metal ion was decreased by 48.00–75.00% when compared to single metal systems. Subsequently, Pb(II) > Cu(II) > Ni(II) was the order in which the uptake and affinity towards the adsorbent occurred. The sorption system was well explained by the modified Langmuir model. The date seed biochar in this study demonstrated high potential in removing Pb(II), Ni(II), and Cu(II) competitively from the aqueous media.
Groundnut shells biochar (GB) obtained from different pyrolysis temperatures including GB350 and GB700 was explored in the decontamination of Hg(II), Cd(II), and Pb(II) from single and competitive aqueous media.108 It was revealed that Cd(II) percentage removal by groundnut shell biochar was 99.60%, which is greater than that of Pb(II) and Hg(II). The GB700 was efficient and resulted in 100.00% of Cd(II)removal and Pb(II) ions from the binary component system. Similarly, the percentage of Hg(II) and Pb(II) removal using GB350 was 100.00% except for Cd(II) ions having a removal efficiency of 99.05, 99.46, and 99.69% obtained from the single, binary, and ternary systems, respectively. The Langmuir adsorption isotherm model showed a good fitness to Cd(II), Pb(II), and Hg(II) to the equilibrium data obtained from the mono-, binary, and ternary component systems. The study demonstrated that biochar made from groundnut shells could be employed as a practical and affordable bio-adsorbent in remediating various water contaminants.
An investigation of the competitive reduction of Zn(II), Cu(II), Pb(II), and Fe(II) ions in a quaternary solution by hybrid biochar developed from sugarcane bagasse (SCB) mixed with low-density polyethylene (LDPE) and pure SCB biochar was conducted.18 For the two adsorbents utilized, more than 99.00% removal efficiency was recorded, which implied they can remove heavy metals from the simulated wastewater. The Langmuir isotherm fitted best in each domain and based on the correlation coefficient; the kinetic sorption data best fitted the pseudo-first-order model well.
More so, the simultaneous removal of Zn(II), Fe(II), Cu(II), and Pb(II) from the quaternary system by biochar derived from oil palm (Elaeis guineensis) fibers was conducted.109 The uptake capacity of 16.54, 16.59, 16.65, and 16.67 mg g−1 for Zn(II), Cu(II), Fe(II), and Pb(II) was achieved by the biomass biochar while the hybrid biochar uptake capacity of Pb(II), Zn(II), Fe(II) and Cu(II) was 16.66, 16.54, 16.65 and 16.57 mg g−1, correspondingly. The sorption kinetic and isothermal data conformed to the pseudo-second-order and Elovich isotherm models, respectively.
Also, a study explored the adsorptive removal of Pb(II), Cu(II), and Ni(II) from mono- and multi-solute media by soil amended with plant residue biochar including corn straw, wheat straw, rice husk, and licorice root pulp each at 3.00% w/w.110 For the single component system, the uptake capacities of the metal ions were in the sequence of Pb(II) > Cu(II) > Ni(II), and the Freundlich isotherm fitted well to the sorption data of Cu(II), Ni(II), and Pb(II) at equilibrium. In the competitive system, the coexistence of different metal ions reduced the uptake capacity for each metal ion and the selectivity followed this trend: Ni(II) > Pb(II) > Cu(II). The corn straw biochar (CSB) was found to have the greatest uptake capacity of 0.23, 1.41, and 2.73 mg g−1 for Ni(II) > Pb(II) > Cu(II), correspondingly.
4.3 Biosorbents
The decontamination of various heavy metals from water systems using biosorbents produced from microbial biomasses, agricultural waste, industrial by-products, or natural materials is efficient, cost-effective, and economical due to the possibility of regenerating the biosorbent. Fig. 12 demonstrates the step-by-step procedure for biosorbent preparation from microbial biomass or agro-based waste.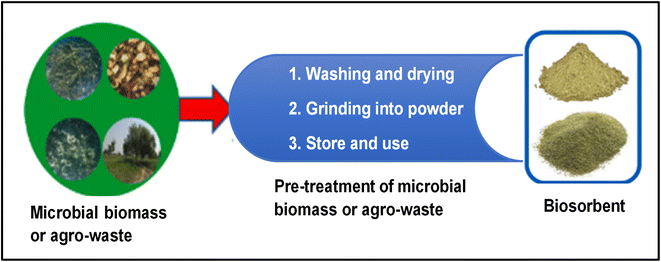 | ||
| Fig. 12 A schematic diagram showing the step-by-step procedure for the preparation of the biosorbent from microbial biomass or agro-based waste (this figure has been adapted from Bilal et al.32 with permission from Elsevier, copyright 2022). | ||
The capability of dry biomass derived from two species of macrophytes was used to decontaminate Pb(II) and Cu(II) from a binary sorption medium.111 The study revealed that maximum removal efficiency was achieved by Pontederia rotundifolia and Salvinia biloba at an agitation time of 120.00 and 240 min, respectively. The biosorbent, Salvinia biloba was suitable in decontaminating Cu(II) from the aquatic media while both the biosorbents could be effective for Pb(II) ions removal. The data of Cu(II) and Pb(II) at equilibrium showed good fitness to the Langmuir isotherm model. The high removal rate, high uptake capacity, and low cost of P. rotundifolia and S. biloba biomass make them suitable for use in biosorption systems to treat wastewater.
Similarly, microalgae waste was modified and applied as an environmentally friendly biosorbent for eradicating Cd(II) and Pb(II) ions from mono- and bicomponent synthetic wastewater.112 The study showed that the kinetic data of Pb(II) and Cd(II) obtained by the biosorbent for both the mono- and bicomponents media agreed well with the pseudo-second-order model. The isothermal data of Pb(II) and Cd(II) also showed good fitness to the Langmuir and Freundlich models, respectively. The Langmuir optimum uptake capacities achieved by the biosorbent were 337.00 and 232.00 mg g−1 for Pb(II) and Cd(II), correspondingly. The results of the binary sorption system indicated that Cd(II) and Pb(II) exhibited antagonistic effects, in which the decontamination of Pb(II) and Cd(II) individually reduces the coexistence of each other. The study showed that the biosorbent has great capability in treating wastewater polluted by heavy metal ions.
Sodium chloride-treated Cystoseira indica as a biosorbent was employed to decontaminate Ni(II) and Co(II) ions simultaneously from polluted water.113 The CCD was employed to elucidate the interactive behavior of the biosorption parameters and optimum operating conditions were observed at 5.90 pH, 0.06 g biosorbent dose, 91.94 mg L−1 Ni(II) concentration, 89.36 mg L−1 Co(II) concentration. The sorption data were best explained by the intra-particle diffusion model at an equilibrium sorption time of 80.00 min. While the extended Freundlich isotherm model showed good representation with the equilibrium data of Ni(II) and Co(II) from the binary component system with an optimum biosorption capacity of 69.99 and 75.21 mg g−1 for Co(II) and Ni(II), correspondingly.
Similarly, a magnetic composite obtained from green macroalgae (Enteromorpha prolifera) as a biosorbent was utilized for Co(II) and Ni(II) depolluting from mono- and binary component systems.114 The study found that the biosorbent had a strong selectivity for both Ni(II) and Co(II) ions with the optimum sorption capacities of 135.00 and 137.00 mg g−1, respectively. The coexistence of alkali and alkali earth metal ions including Na+, K+, Mg(II), and Ca(II) inhibited the decontamination of Ni(II) and Co(II). In comparison with the single sorption system, the sorption capacity of Ni(II) and Co(II) from the binary system was reduced due to the presence of the other ions. The study disclosed that the biosorbent is resourceful in the removal of heavy metals in addition to alkali and alkali earth cations in wastewater simultaneously.
The competitive detoxification of Ni(II) and Cd(II) from aquatic systems was explored using brown algae (Cystoseira indica) as a biosorbent.115 The study revealed that optimum operating conditions for maximum Ni(II)and Cd(II) removal were a biosorbent dosage of 1.00 g L−1, initial concentration of 100.00 mg L−1, and solution pH of 6.00. The kinetic data of Cd(II) and Ni(II) was best fitted to the intra-particle-diffusion kinetic model. While the equilibrium isothermal data of Ni(II) and Cd(II) was better fitted to the extended-Freundlich model with optimum biosorption efficiencies of 55.34 and 18.17 mg g−1 for Ni(II)and Cd(II), correspondingly.
Similarly, the concurrent sorption of Ni(II) and Cd(II) ions in wastewater onto Streptomyces rimosus biomass was conducted.116 The study found that Ni(II) and Cd(II) biosorption from the bi-solute medium attained equilibrium at a shaking time of 3.00 h and the maximum biosorption capacities achieved by the biosorbent were 9.86 and 22.80 mg g−1 for Cd(II) and Ni(II), correspondingly. The sorption data of Cd(II) and Ni(II) showed good representation with the pseudo-second-order and Langmuir models, respectively. The Streptomyces rimosus biomass has been demonstrated to have a high biosorption ability and would be an effective biosorbent for Cd(II) and Ni(II) ions elimination from water systems.
The adsorptive elimination of Cr(III) and Ni(II) from mono- and binary component systems was conducted by using Durvillaea antarctica as a biosorbent.117 The study observed that optimum sorption capacity of 32.85 and 102.72 mg g−1 for Ni(II) and Cr(III) occurred at a pH of 5.00, and a reaction time of 240.00 and 420.00 min, respectively. It was observed that the increasing Ni(II) initial concentration increases its uptake capacity but reduces the uptake capacity of Cr(III) ions. The binary component equilibrium data of Ni(II) and Cr(III) showed the best fit to the Sips model. The competitive biosorption indicated that Cr(III) and Ni(II) ions present in wastewater compete strongly for the same active biosorbent sites.
Also, a study was conducted to decontaminate Cd(II), Cu(II), and Zn(II) from both mono- and ternary aqueous media using phosphogypsum as a biosorbent.118 The uptake capacity of Cu(II), Zn(II), and Cd(II) ions onto the biosorbent upsurged with the pH and the optimum decontamination was attained at pH of 8.00, 9.00, and 9.50, respectively. The sorption rate of Cd(II) attained by the biosorbent was greater than Cu(II) and Zn(II) in both mono- and multi-solute sorption media. Also, in a ternary sorption system, the biosorbent retained its overall uptake capacity with an increment in the uptake of Cu(II) while the uptake capacity of Zn(II) and Cd(II) ions decreased. The experimental data of Cu(II), Zn(II), and Cd(II) were well described by the pseudo-second-order kinetic and Freundlich isotherm models. The study demonstrated that phosphogypsum may be used as a cheap biosorbent for the sequestration of various water contaminants.
The uptake ability of Pb(II), Co(II), Cd(II), Zn(II), Cu(II), and Ni(II) ions from non- and competitive sorption systems by Leucodon sciuroides (LS) mosses as a biosorbent was carried out.119 It was revealed that the optimum uptake efficiency of these heavy metal ions occurred at 30.00 min of agitation time and a pH of 6.00 with Pb(II) and Cu(II) being the most adsorbed by the LS. The equilibrium sorption data of Ni(II), Co(II), and Cu(II) well-fitted to the Langmuir model whereas Pb(II), Zn(II), and Cd(II) equilibrium data conformed to the Freundlich model. Also, the experimental data attained by LS for all six heavy metals correlated well with the pseudo-second-order model. The competitive biosorption showed that the biosorbent can concurrently remove Cu(II) and Pb(II) from complex water systems including the other four heavy metals.
The detoxification of Cd(II), Cu(II), and Ni(II) from mono- and multicomponents sorption medium was conducted by using biogenic vaterite induced by Bacillus subtilis as a biosorbent.120 The study revealed that the sorption data agreed well with the Langmuir in comparison to the Freundlich isotherm model. While the experimental results were best represented using the pseudo-second-order kinetic model. The Langmuir maximum biosorption capacity of Cd(II), Cu(II), and Ni(II) obtained from the single component medium was 270.27, 172.41, and 178.57 mg g−1, respectively. However, in the multi-solute medium, the maximum uptake capacities of 94.34, 30.30, and 175.44 mg g−1 were attained by the adsorbent for Ni(II), Cu(II), and Cd(II), correspondingly. The study observed that in the multi-metal sorption systems, competitive effects occurred amongst the heavy metal ions and with Cu(II) ions uptake by the adsorbent being the least affected by the existence of the other two competing for heavy metal ions.
4.4 Activated carbon
Due to its great adsorption capacity, activated carbon can be used in a variety of applications. It has numerous uses, such as the treatment of industrial effluent, medicine, the discoloration of sugar, and a lot more. The porous nature of activated carbon, which results in a high surface area makes it an effective adsorbent. Fig. 13 is a schematic illustration of the production of activated carbon from biomass. Several studies have employed activated carbon in the remediation of heavy metals from complex water systems as discussed in this section. | ||
| Fig. 13 Schematic illustration of the classical fabrication and activation techniques of activated carbon (this figure has been reproduced from Mariana et al.121 with permission from Elsevier, copyright 2021). | ||
The elimination of As(III) from binary systems onto hybrid granular activated carbon has been investigated using the batch technique.7 The removal of As(III) was found to show dependence on the adsorption factors studied. The equilibrium isothermal and kinetic data attained from both mono- and binary solutes sorption media were best correlated with the Langmuir and pseudo-second-order models, suggesting the mechanism of As(III) removal was due to chemical adsorption. The Langmuir monolayer adsorption capacities of the activated carbon were 205.76 for the mono-solute and 153.09 mg g−1 for binary solute sorption systems, correspondingly. This implies that there existed an antagonistic effect on the removal of As(III) due to the presence of Hg(II) ions in binary sorption media. The thermodynamic variables indicated that the elimination of As(III) ions from both sorption media were endothermic and spontaneous with an increase in randomness at the adsorbate–sorbent interface. The successive regeneration studies showed that the spent activated carbon uptake capacity is renewable after multi-cycles of adsorption–desorption. This suggested that activated carbon has economic prospects and practical industrial applications for As(III) remediation in wastewater.
A response surface methodology (RSM) was utilized to investigate the parameters that influence the competitive uptake capacity of Ni(II) and Cu(II) from binary component systems by activated carbon derived from sewage sludge.122 The uptake efficiencies of Ni(II)and Cu(II) were optimized by the application of CCD under RSM. It was found that the model-predicted data correlated well with the experimental results. While the ANOVA results indicated that the process was mainly influenced by the Ni(II) concentration and adsorbent dose, whereas the remaining factors exhibited very low effects. The best-operating conditions were Ni(II) and Cu(II) concentration, agitation time, adsorbent dosage, and reaction temperature were 40.00 mg L−1, 100.00 min, 4.00 g L−1, and 30.00 °C. Under these optimal settings, the maximum uptake capacities of 4.04 and 7.48 mg g−1, respectively were attained for Cu(II) and Ni(II). From the results of the study, the activated carbon derived from sewage sludge could be utilized beneficially for removing Cu(II) and Ni(II) from aquatic environments.
Similarly, the depollution of Cu(II) and Ni(II) in mono- and bicomponent systems was performed by using activated carbon derived from corn cob.123 The impact of sorption parameters was considered and equilibrium was attained at 240.00 and 100.00 min with uptake capacity of 0.39 and 0.28 mmol g−1 for Cu(II) and Ni(II), correspondingly. It was observed that at higher pH and initial concentration, the sorption process was enhanced and Cu(II) uptake by the adsorbent was not influenced by the coexistence of Ni(II) in the solution. The influence of temperature on Cu(II) and Ni(II) elimination from the monocomponent system was statistically insignificant and statistically significant only during Cu(II) removal from the bicomponent system. The sorption data of the monocomponent system was best explained by Sips and pseudo-second-order models and that of the bicomponent system was best conformed to the extended Langmuir model. The study showed that corn cob-activated carbon is efficient in heavy metals remediation from water systems.
The adsorptive decontamination of Cu(II) and Zn(II) from binary solute systems onto carbonaceous materials obtained by chemical activation and ammoxidation of polish brown coal was investigated.124 The influence of agitation time, temperature, solution pH, and initial concentration as well as the existence of competing ions on the uptake ability of activated carbons, were studied. It was revealed that the sorption capacity upsurges with increasing concentration and the agitation time and optimum removal were found at pH of 8.00 and 6.00 for Cu(II) and Zn(II), correspondingly. The carbonaceous adsorbent derived from ammoxidation was more proficient in the decontamination of Zn(II) and Cu(II) from binary component systems. However, the uptake capacities of Cu(II) and Zn(II) by the carbonaceous adsorbent relative to that of unmodified adsorbent were much smaller. The isothermal and kinetic data obtained from both systems were well represented by the Langmuir and pseudo-second-order models.
More so, the adsorptive decontamination of Cd(II) and Pb(II) ions from mono- and binary component systems with commercial granular activated carbon was conducted in batch mode.125 It was observed that the maximum agitation time was 120.00 min for both heavy metal ions and the optimum pH ranged from 5.50–6.00 and 7.00–8.00 for Pb(II) and Cd(II), correspondingly. The study revealed that the uptake capacity of 9.30 mg g−1 for Pb(II) attained by the activated carbon was higher than Cd(II), which is 9.26 mg g−1. The kinetic data of Pb(II) and Cd(II) showed good fitness to the pseudo-second-order model while both the extended Freundlich and Langmuir models best correlated to the binary component system adsorption data. The optimization results using RSM revealed that initial pH of 6.30, a temperature of 56.80 °C, and a shaking speed of 308.00 rpm as optimum operating conditions.
The depollution of Co(II) and Pb(II) ions from a bicomponent aqueous medium was performed by thiolated sawdust.28 According to the study, the saw dust-activated carbon appeared to be a proficient adsorbent for the concurrent sorption of Co(II) and Pb(II). The Langmuir multicomponent isotherm demonstrated that the existence of Co(II) in the solution reduced the affinity of Pb(II) towards the bio-adsorbent, and so, the effect of the mixtures appeared to be antagonistic. Therefore, the current study emphasized the possibility of sawdust in eliminating heavy metals from mono-solute and multi-solute systems, particularly for small-scale companies.
The elimination of Hg(II) and Zn(II) from the binary system was examined by activated carbon produced from Theobroma cacao pod husk.126 The batch experiment disclosed that the elimination of Hg(II) and Zn(II) simultaneously attained equilibrium rapidly at an agitation time of 90.00 min. The Temkin and Langmuir models well-fitted the equilibrium sorption data of Hg(II) and Zn(II). The sorption kinetic data were best described by four models notably among which are the pseudo-first-order, Elovich, pseudo-second-order, and intraparticle diffusion models. The study indicated that the Theobroma cacao pod husk could be proficient in decontaminating of Hg(II) and Zn(II) from aquatic systems.
Besides, a modified pine bark (as activated carbon) efficiency was tested for the single and simultaneous reduction of Cr(III) and Ni(II) ions from aquatic systems.127 The sorption kinetic data of Cr(III) and Ni(II) obtained by the adsorbent in the single- and binary solute systems was best explained using the pseudo-second-order kinetic model. Also, the isothermal data of Cr(III) and Ni(II) at equilibrium are well correlated with the Langmuir isotherm with monolayer maximum sorption efficiencies of 31.40 and 23.70 mg g−1, correspondingly. In the binary solute system, Ni(II) ions coexistence showed little influence on Cr(III) ions removal but the occurrence of Cr(III) ions decreased Ni(II) ions uptake rate by the adsorbent. In the binary solute medium, the modified Langmuir model well described the sorption data of Cr(III) and Ni(II) achieved by the activated carbon at equilibrium.
The possibility of removing Ni(II), Pb(II), and Zn(II) concurrently from two binary-metal sorption media including Ni(II)–Pb(II) and Ni(II)–Zn(II) onto oxidized activated carbon fiber (ACF-Ox) was conducted.74 The simultaneous sequestration of Pb(II) and Ni(II) ions from the bi-solute sorption medium [Ni(II)–Pb(II)] by the ACF-Ox suggested that the affinity of Pb(II) ions towards ACF-Ox was much greater than that of the Ni(II). However, the concurrent sequestration of Ni(II) and Zn(II) by the ACF-Ox in the bi-solute system [Ni(II)–Zn(II)] indicated that the Ni(II) ions uptake capacity was 1.12 times higher than Zn(II) ions. The competitive sorption data attained by ACF-Ox for Ni(II), Pb(II), and Zn(II) in the binary sorption systems were best fitted to the extended Freundlich isotherm.
A commercial wood-based granular activated carbon (GAC) was acquired and its ability to adsorb Cu(II), Pb(II), and Zn(II) ions in mono- and bicomponents systems was explored.128 The study discovered that the maximum abatement of Cu(II), Zn(II), and Pb(II) from both sorption systems was accomplished at solution pH of 5.50, 4.50, and 6.00, respectively. It was found that the selectivity of these metals towards the GAC was in this trend Pb(II) > Cu(II) > Zn for all the pH ranges studied. The Sips and Langmuir models conformed to the equilibrium data Cu(II), Zn(II), and Pb(II) obtained from the mono-solute system at a pH of 5.00. The Langmuir optimum sorption capacities of Cu(II), Pb(II), and Zn(II) achieved by the activated carbon were 0.094, 0.142, and 0.058 mmol g−1, respectively. The uptake capacities of Zn(II), Cu(II), and Pb(II) at a concentration of 0.01 mmol L−1 were 0.040, 0.085, and 0.130 mmol g−1, respectively. The IAS-Sips and Ideal Adsorbed Solution (IAS)-Langmuir models best represented the bi- and ternary solutes sorption data, respectively. The study added to the body of knowledge on the modeling of binary and ternary sorption systems on GAC, a flexible adsorbent widely utilized in wastewater treatment.
Multicomponent competitive elimination of phenol, Pb(II), Cd(II), and Cr(III) was performed with KOH-activated carbon obtained from mangrove charcoal.129 The study discovered that the optimum removal rates of phenol, Pb(II), Cd(II), and Cr(III) from the multicomponent system were found to be 76.00, 92.00, 47.00, and 31.00%, respectively obtained at optimum pH 5.00, 2.50 g L−1 adsorbent dosage, 120.00 min of reaction time, and 50.00 mg L−1 concentration for phenol and 30.00 mg L−1 of each heavy metal. The optimum uptake rates of phenol, Pb(II), Cr(III), and Cd(II) were 46.00, 32.00, 11.00, and 3.80 mg g−1, respectively. The study observed that the concurrent elimination of phenol, Pb(II), Cr(III), and Cd(II) from the multi-solutes system was fierce due to the comparable sorption mechanisms. The equilibrium data of Pb(II) and Cr(III) showed good fitness to the Langmuir model whereas the sorption data of Cd(II) at equilibrium agreed with the Redlich–Peterson model. Besides, the Freundlich model best explained the phenol sorption data. However, the pseudo-second-order best fitted the kinetic data of phenol, Pb(II), Cd(II), and Cr(III) obtained from the multicomponent system suggesting chemisorption as the adsorption mechanism. The adsorption and desorption for the multicomponent system indicated that HCl could recover the heavy metal ions while NaOH solution was the best eluent for phenol desorption.
The decontamination of Pb(II), Cu(II), Cd(II), Zn(II), and Cr(VI) from both single and multicomponent systems onto wooden commercial activated carbon was conducted.130 The study found that at a pH of 3.00, optimum Cr(VI) removal was accomplished, while a pH of 7.00 resulted in maximum decontamination of Pb(II), Cd(II), Cu(II), and Zn(II) in the single system. The maximum uptake capacities of the heavy metals were observed to exhibit the pattern of Pb(II) > Cr(VI) > Cd(II) > Cu(II) > Zn(II) at a temperature of 25.00 °C in the mono-metal system. For the single component system, the equilibrium sorption data obeyed the Langmuir and pseudo-first-order models. In comparison, the study showed that Cr(VI) and Cu(II) depollution was enhanced by the presence of Cd(II), Zn(II), and Pb(II), while the removal of Zn(II) and Cd(II) was influenced by the coexistence of Pb(II), Cu(II), and Cr(VI) ions in the multicomponent systems. This study offered useful recommendations for the future use of wooden commercial activated carbon in complex wastewater purification including different heavy metals.
The decontamination of Pb(II), Cu(II), Cd(II), and Zn(II) ions as well as Mg(II), Ca(II), and K+ as alkali and alkali earth metal ions in mono- and multi-metals sorption media onto bagasse activated carbon were performed.131 The study revealed that at 60.00 min agitation time, optimum removal of Pb(II), Cd(II), Cu(II), Zn(II), Mg(II), K+, and Ca(II) ions by the bagasse activated carbon was 0.77, 0.64, 0.49, 0.69, 0.47, 0.67 and 0.09 mmol g−1, correspondingly, which was much higher than the untreated bagasse. The kinetic data attained from the monocomponent system obeyed the pseudo-second-order kinetic model. The multicomponent sorption showed that the bagasse-activated carbon had a greater affinity for the heavy metals than the alkali and alkali earth metal ions. At 0.015 g adsorbent load, the optimum Pb(II), Cu(II), Zn(II) and Cd(II) ions removal rate from the wastewater was 94.53, 92.74, 70.46 and 83.30%, respectively while that of Mg(II), Ca(II) and K+ was only 6.64, 4.75, and 0.00%, respectively. This implied that the heavy metals have the chance of being removed selectively from wastewater by the modified bagasse.
The simultaneous removal of Zn(II) and Hg(II) in the coexistence of Mg(II) onto chemically activated bentonite using the batch technique was explored by Mudasir et al.132 At 6.00 pH and 60.00 min reaction time, maximum concurrent removal of Zn(II) and Hg(II) ions in the existence of Mg(II) was accomplished. The uptake capacity, selectivity, and affinity of modified bentonite towards Zn(II) and Hg(II) were higher than unmodified bentonite. The kinetic data achieved by the adsorbent for both Zn(II) and Hg(II) in the coexistence of Mg(II) was well correlated to the pseudo-second-order model. While the equilibrium data of Zn(II) and Hg(II) showed good representation with the Langmuir and Freundlich models. The results demonstrated that the existence of Mg(II) ions in the multicomponent system did not inhibit the uptake of Zn(II) and Hg(II) ions signifying that dithizone-immobilized bentonite had a greater affinity for heavy metals.
The peels of Artocarpus nobilis fruit were used to produce NaOH-activated carbon solutions to concurrently remove Cr(VI) and Cr(III) from aqueous systems.133 The study observed that the best solution pH for Cr(III) and Cr(VI) ions detoxification was found to be pH of 5.00 and 2.00, respectively. At pH of 5.00 and 2.00, the maximum uptake rates of Cr(VI) and Cr(III) ions were 4.94 and 4.89 mg g−1, correspondingly. The kinetic data of Cr(III) and Cr(VI) correlated well with the pseudo-first-order model.
Similarly, untreated and NaOH-modified waste cotton yarn was utilized in removing Pb(II), Cr(III), As(V), and Cd(II) from single- and multi-metal aqueous solutions.134 The study revealed that NaOH-modified cotton yarn adsorbent insignificantly affected the uptake ability of the cations. The uptake rates of Pb(II) were observed to be the greatest in the mono- and multi-sorption media whereas As(V) ions demonstrated the least affinity to the cotton yarn surface. The equilibrium sorption data of Pb(II), Cd(II), Cr(III), and As(V) ions onto cotton yarn was well correlated to the pseudo-second-order and Freundlich models. The study concluded that cotton yarn, a waste product from the textile industry, might potentially be applied as a cheap adsorbent for depolluting various heavy metals from aqueous media.
The elimination of Ni(II) and Cr(VI) from mono- and bi-solutes sorption media by modified diatomite waste as an adsorbent were performed in batch mode.135 In the mono-solute medium, the sorption data of Cr(VI) and Ni(II) achieved by the adsorbent was accurately represented by the Langmuir and Freundlich models. At a solution pH of 3.00 and 8.00, maximum uptake efficiencies of 3.64 and 2.90 mg g−1 for Ni(II) and Cr(VI) were attained. In the bicomponent medium, the coexistence of Ni(II) ions affected Cr(VI) ions removal, but Cr(VI) existence promoted the adsorptive elimination of Ni(II). The kinetic sorption data of Cr(VI) and Ni(II) attained by the adsorbent in the single and binary systems were observed to exhibit good fitness to the pseudo-second-order model. Besides, the extended Freundlich and Langmuir extended multicomponent adsorption isotherm models were found to fit well with the competitive equilibrium data of Ni(II) and Cr(VI). This study provided insights regarding the utilization of modified diatomite wastes for heavy metals elimination from the ecosystem.
Besides, modified argillaceous limestone was used in removing Cd(II), Pb(II), and Ni(II) from the mono- and multicomponent system.136 In the monocomponent system, the decontamination rate of Cd(II), Pb(II), and Ni(II) were much higher than that in the multi-solute media. The study showed that Pb(II) ions uptake capacity by the adsorbent in the competitive system was much higher than the other heavy metal ions. The kinetic and isothermal data of Cd(II), Pb(II), and Ni(II) obtained by the adsorbent demonstrated good fitness to the Langmuir and pseudo-second-order models, respectively. The study uncovered the efficacy of argillaceous limestone in the remediation of aquatic systems for heavy metal ions.
Similarly, raw and activated clay was employed in decontaminating of Cd(II), Zn(II), Pb(II), and Cu(II), ions both individually and simultaneously from wastewater.137 In the single sorption system, the study found the uptake capacity of Pb(II) to be 26.78 and 45.94 mg g−1 for natural and activated clay, correspondingly. For the multicomponent system, the sorption process showed dependence on the initial metal concentration with the uptake rate of 41.71 mg g−1 for Pb(II) being the highest in the binary systems. In multicomponent systems, the removal efficiency of each metal was significantly reduced in the existence of other heavy metal ions. The study indicated that clay can be employed as a viable adsorbent for wastewater treatment.
4.5 Zeolite-based adsorbents
Zeolites are extensively utilized in the petroleum industries as water softeners, and catalysts for the synthesis of laundry detergents, and molecular sieves. Zeolite has a porous and intergranular microstructure with thermal and mechanical stability. Many different forms of zeolites have been employed as adsorbents for removing contaminants, particularly the removal of heavy metals in fields of water treatment, due to their strong ion exchange and adsorption capacities. Fig. 14 and 15, respectively are schematic illustrations of the general synthesis of zeolite and its adsorption characteristics. Several studies have employed zeolite-based adsorbents in the remediation of heavy metals from complex water systems as discussed in this section.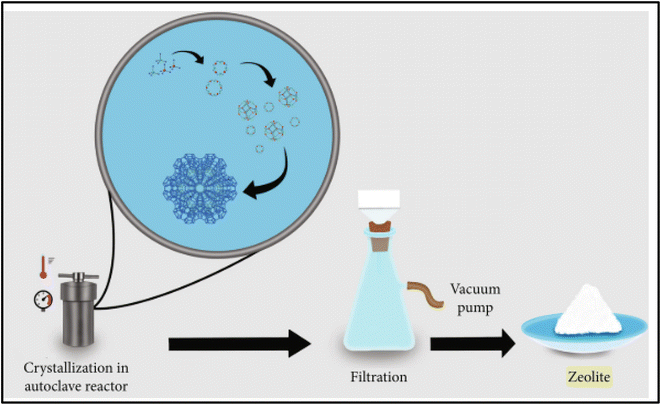 | ||
| Fig. 14 A schematic diagram showing hydrothermal processes for synthesizing zeolite sorbent material for water pollutants removal (this figure has been adapted from De Magalhães et al.138 with permission from Hindawi, copyright 2022). | ||
 | ||
| Fig. 15 A schematic diagram showing zeolite can only adsorb cationic species (b) when modified by a cationic surfactant (a), zeolite can also remove anionic (c) and organic species or both (d) (this figure has been adapted from De Magalhães et al.138 with permission from Hindawi, copyright 2022). | ||
Dahake et al.139 reported on the synthesis of oxime-modified zeolite-A as a promising adsorbent for uranium U(VI) ions sequestration from competitive solute solutions. The influence of several experimental variables was studied using the prepared adsorbent. Maximum U(VI) removal efficiency of 98.00% attained at a pH of 4.00–6.00. The uptake capacities of 4.92 mg g−1 were observed for U(VI) in presence of other competing ions such as Cr(VI), Cd(II), Co(II), Pb(II), and Mn(II) ions. The equilibrium data was found to be best agreed with the Langmuir and pseudo-second-order models. More so, the spent adsorbent could be regenerated and reusable up to eight multicycles of adsorption and desorption with high removal efficiency for each run. It can be inferred from the results that oxime-modified zeolite-A is a capable adsorbent for the recovery of U(VI) ions due to its easy separation, high adsorption, and excellent reusability.
More so, de Morais França et al.46 applied isotherm models to investigate the interactions between zeolite 4A and Cu(II), Ni(II), and Zn(II) with varying concentrations in single- and multicomponent adsorption media. The Freundlich, Langmuir, and Sips models well represented the sorption data of Cu(II) and Ni(II) at equilibrium whereas the Freundlich and Langmuir models correlated well to the isothermal data of Zn(II). The adsorption kinetics was discovered to be fast during Zn(II) and Cu(II) and slow for Ni(II). Hence, higher diffusion values were obtained during the decontamination of Cu(II) and Zn(II) from the sorption systems indicating a strong affinity of the synthesized zeolite 4A for the Zn(II) and Cu(II) in both the mono- and multi-solute sorption media.
Also, zeolite X was employed for the competitive elimination of Cu(II) and Zn(II) from the binary sorption system.140 It was discovered that as Cu(II) initial concentration increased, the uptake capacity of Zn(II) significantly dropped from 175.20 to 7.10 mg g−1. On the contrary, as Zn(II) concentration upsurges, Cu(II) sorption capacity went up from 142.90 to 148.30 mg g−1. It was found that Cu(II) has a greater affinity for and selectivity toward zeolite X in the bicomponent sorption medium. The kinetic data of Zn(II) and Cu(II) showed a good fit for the pseudo-second-order kinetic model. Furthermore, the isothermal data exhibited good fitness to the Langmuir model. According to the study, diatomite-derived zeolite X might be a useful adsorbent for decontaminating different heavy metals from industrial aqueous systems.
The performance of clinoptilolite for removing Zn(II) and Cd(II) ions from wastewater in a batch mode was investigated.14 Zn(II) and Cd(II) concentrations varied between 10.00 and 20.00 mg L−1, and the sorbent loading ranged between 10.00 and 60.00 g L−1 in the aqueous media. The maximum removal rate of both Zn(II)and Cd(II) was reached at 10.00 g L−1 with very low metal ion concentrations. However, the uptake capacity declined when Cd(II) and Zn(II) amounts increased to 200.00 mg L−1. Further experimentations were performed using the maximum concentration of 200.00 mg L−1 while increasing the adsorbent loading to 40.00 and 60.00 g L−1. The findings revealed that maximum percentage removal of more than 50.00%. The clinoptilolite was found to have a greater affinity for Cd(II) ions in a single system. The adsorbent exhibited a better affinity for Zn(II) due to a unique sorption mechanism in the presence of two metals in the binary system with a higher removal rate.
Similarly, clinoptilolite was treated with hexadecyltrimethylammonium bromide (HDTMA-Br) and employed as an adsorbent to decontaminate Cr(VI) and Cu(II) simultaneously from water systems.141 The optimum operating parameters obtained for the concurrent maximum elimination of Cr(VI) and Cu(II) ions from binary solute media were found to be 2.00 g adsorbent dosage, 56.50 mg L−1 Cu(II) concentration, and 10.00 mg L−1 Cr(VI) concentration. It was observed that the existence of Cu(II) in the bi-solute system enhanced the adsorbent surface charge and subsequently increased Cr(VI) ions uptake. The Langmuir model fitted well to the sorption data. While the thermodynamic studies showed that both Cr(VI) and Cu(II) ions uptake by the adsorbent was endothermic and exothermic in nature, respectively.
Furthermore, in mono- and binary solute systems, the comparative decontamination of Cd(II) and Pb(II) by amine-functionalized MCM-48 (NH2–NH–NH–MCM-48) and amine-grafted MCM-48 was studied.142 The study utilized batch adsorption techniques in both single component and multicomponent systems. The effects of the experimental factors were examined, and the findings showed that MCM-48 with an amine attachment demonstrated greater Pb(II) and Cd(II) removal efficiency than MCM-48. The binary metal sorption results indicated that the elimination of the single metal ions is inhibited by the presence of the other heavy metal. The binary component sorption data agreed well with the extended Langmuir model, whilst the sorption data were best explained by the Freundlich and Langmuir models in the single system. The optimum uptake rates of Cd(II) and Pb(II) attained by NH2–NH–NH–MCM-48 were 82.70 and 119.24 mg g−1, respectively, in the single component system. It was discovered that NH2–NH–NH–MCM-48 had a greater affinity for Cd(II) ions in both sorption systems.
On synthesized silica-based Mobil composition of matter no. 41 (MCM-41), Zn(II), and Cr(VI) ions were removed from single- and binary solute systems.143 The Zn(II) and Cr(VI) ions were effectively removed under the optimal working conditions of pH 7.00, 0.10 g L−1 adsorbent load and 2.00 h of contact time. It was found that raising the initial Zn(II) amount caused the uptake capacity of Zn(II) to improve, whereas increasing the Cr(VI) concentration triggered the uptake capacity of Zn(II) to decrease. The elimination of Cr(VI) ions followed a similar pattern as Zn(II) concentration increased. The antagonistic effects on Zn(II) and Cr(IV) removal from the binary metal aqueous solutions were established. The experimental data of Zn(II) obtained from the binary solute system fitted best with the modified Redlich–Peterson isotherm model.
The concurrent decontamination of Cd(II), Zn(II), Cu(II), Co(II), and Pb(II) from aquatic systems by FAU-type zeolites produced from coal fly ash.144 The study revealed that in the co-existence of other heavy metal ions, FAU zeolite derived from coal fly ash could decontaminate heavy metal ions simultaneously in competitive aqueous solutions. The equilibrium sorption data of Pb(II), Cd(II), Co(II), Zn(II), and Cu(II) fitted well to the pseudo-second-order and Langmuir models, correspondingly. The heavy metal ion which was found most adsorbed was Pb(II) having an optimum uptake rate of 109.90 mg g−1 while that of Co(II) was the least with an uptake efficiency of 12.20 mg g−1. The removal efficiency of FAU-type zeolite for the quinary-metals sequestration declined in the trend of Pb(II) > Cu(II) > Cd(II) > Zn(II) > Co(II).
In another study, the competitive elimination of Cd(II), Pb(II), Ni(II), and Cu(II) from quaternary solute systems onto fly ash-based Linde F(K) zeolite was carried out.145 It was found that Cu(II), Pb(II), Ni(II), and Cd(II) removal from the quaternary component systems upsurged with rising pH values, and equilibrium was achieved at a pH of 6.00–7.00. The percentage elimination of these metals was observed to be high at the beginning of the sorption process and then progress slowly until equilibrium was established. The sequence of these heavy metals' uptake capacity at equilibrium was Pb(II) > Cd(II) > Cu(II) > Ni(II). The pseudo-second-order and internal surface diffusion were the best-fitted models, which accurately described the equilibrium sorption data of Pb(II), Ni(II), Cd(II), and Cu(II).
4.6 Nano-particles and nano-based adsorbents
Many contaminants in wastewater, including heavy metals, organic and inorganic solvents, dyes, biological toxins, and pathogens, have been reported to be successfully eliminated by nanomaterials. Nanomaterials are well suited for use in wastewater treatment because of their high surface-to-volume ratio, high sensitivity and reactivity, high adsorption capacity, and simplicity of functionalization. As shown in Fig. 16 and 17, nanomaterials are divided into carbon-based nanoparticles and non-carbon nanomaterials. | ||
| Fig. 16 Carbon-based nanoparticles: fullerene (a), nano-diamond (b), carbon nanotube (c), quantum dots (d) and nanographene (e) for wastewater treatment (this figure has been reproduced from Thakuria and Kataria146 with permission from Springer, copyright 2021). | ||
 | ||
| Fig. 17 Different non-carbon nanomaterials, used for the treatment of heavy metal-contaminated water (this figure has been adapted from Baby et al.147 with permission from MDPI, copyright 2022). | ||
In a study, a magnetic core nanoparticle coated in metallic nanometric silver and functionalized with L-cysteine was employed to remove monomethyl mercury, dimethyl mercury, ethyl mercury, and Hg(II) from aquatic systems simultaneously.148 The results showed that at 30.00 s of agitation time, 400.00 μL of nanoparticles were sufficient to achieve 100.00% sorption performance for all Hg species in the aqueous media at a solution pH of 6.20 and under room temperature. The sorption data obtained for all the Hg species at equilibrium was well explained by the Langmuir isotherm model. The efficacy of this adsorbent in treating wastewater is suggested by its capacity to simultaneously adsorb all species of mercury present in water and achieve full adsorption in just a few seconds.
Magnetic nanoparticles were employed as an adsorbent in removing Cd(II) and Cu(II) concurrently from water systems.149 The study found that the maximum elimination of Cu(II) and Cd(II) ions was accomplished at a pH of 7.00 and 60.00 min shaking time. The sorption kinetic data of Cu(II) and Cd(II) was well fitted to the pseudo-second-order model while the isothermal data best fitted the Langmuir isotherm with optimum uptake efficiencies of 73.50 and 79.40 mg g−1 for Cd(II) and Cu(II), correspondingly. This study indicated that the adsorbent is efficient and reliable to eliminate Cd(II) and Cu(II) from contaminated aquatic systems.
Also, individual and binary sequestration of Ni(II) and Pb(II) from wastewater using polyether sulfone (PES)/chitosan (CS)/Fe3O4–NH2–SH nanofiber was investigated by Jafarnejad et al.150 using RSM. The RSM was employed to determine the impact of the pH of the solution, Fe3O4–NH2–SH, and adsorbent loads on the adsorptive elimination of Ni(II) and Pb(II) ions from wastewater. The optimum predicted uptake capacity of 91.15 and 52.27 mg g−1 was obtained for Ni(II) and Pb(II), respectively, using the following optimum operating settings: pH = 5.50, adsorbent dosage = 1.00 g L−1, and Fe3O4–NH2–SH content = 10.00 wt%. In the binary system, the uptake capacity of these heavy metals declined with the upsurge of multi-metal ion concentration and selectivity followed the order: Pb(II) > Cu(II) > Ni(II) > Co(II) > Zn(II). The kinetic sorption data of Ni(II) and Pb(II) showed good fitness to the double-exponential model. While the isothermal data agreed well with the Langmuir model with optimum uptake efficiencies of 95.30 and 282.40 mg g−1 for Ni(II) and Pb(II) ions, correspondingly. The regeneration capability of the nanofiber adsorbent revealed that the uptake capacity reduced slightly after the fourth adsorption–desorption cycle indicating the suitability of the adsorbent.
Besides, zero-valent iron nanoparticle was employed to remove Cd(II), Cu(II), and Pb(II) simultaneously from water systems.151 The study showed that the competitive decontamination of the Cd(II), Cu(II), and Pb(II) ions was affected by reaction time, pH, concentration as well as the coexistence of other competing ions in the solution. It was revealed that Cu(II) ions were removed faster than Cd(II) and Pb(II) during the first 5.00 min until at about 120.00 min, 95.00–99.00% maximum removal of the studied heavy metals was accomplished. The kinetic and isothermal data of Pb(II), Cu(II), and Cd(II) obtained by the adsorbent from the aqueous systems was best represented using the pseudo-second-order and Langmuir models.
Additionally, magnetically modified mesoporous nanoparticles were utilized for decontaminating Cd(II), Cu(II), and Pb(II) simultaneously from wastewater.152 Through the application of the CCD, it was found that 90.00–105.00% and 90.30–107.00% optimum detoxification of Cd(II), Cu(II), and Pb(II) from real and synthetic wastewaters, respectively was attained by the adsorbent within 10.00 min. The isothermal data correlated well to the Langmuir model with maximum uptake capacities of 238.09, 208.33, and 178.57 mg g−1 for Cd(II), Cu(II), and Pb(II), correspondingly.
Multicomponent adsorptive reduction of Cu(II), Co(II), and Ni(II) by nanostructured layered sodium vanado-silicate (Na–V–Si) adsorbent was carried out in a ternary system.153 The study found that the simultaneous decontamination of Cu(II), Ni(II), and Co(II) was attained within 5.00 min and at a mono-solute concentration of 10.00 mg L−1. The study revealed the sorption capacity and adsorbent affinity were observed to be in the sequence of Cu(II) > Co(II) > Ni(II). More so, the Cu(II) ions had an antagonistic effect on the decontamination of Ni(II)and Co(II) ions in the ternary system. The sorption kinetic data was best explained by pseudo-second-order and pseudo-first-order models. The optimum sorption capacity of the nanostructured layered Na–V–Si was found to be 154.00, 70.70, and 55.40 mg g−1 for Cu(II), Co(II), and Ni(II), correspondingly in the single component systems. The design, operation, and optimization of a practical sorption system for the sequestration of multiple heavy metals from water systems could benefit from the in-depth insights provided by this study.
The concurrent depollution of Cd(II), Pb(II), Cu(II), and Ni(II) from river water has been investigated by Fato et al.154 using ultrafine mesoporous magnetite (Fe3O4) nanoparticles (UFMNPs). The study found that the single elimination of Pb(II), Ni(II), Cu(II), and Cd(II) from the river water by the adsorbent was effective with a maximum removal rate of 98.00, 78.00, 90.00, and 87.00%, respectively. For the multicomponent system, the optimum percentage decontamination of Pb(II), Ni(II), Cu(II), and Cd(II) from the river was found to be 86.00, 54.00, 84.00, and 80.00%, respectively. The study showed that the decontamination efficiency of Pb(II), Ni(II), Cu(II), and Cd(II) from the multi-ion systems was much lower than in the single component system. This study had shown that UFMNPs have a great ability in treating wastewater, particularly for such competitive elimination of several pollutants from aqueous systems.
4.7 Composite-based adsorbents
When it comes to water and wastewater treatment facilities, composites are an excellent option because they require less maintenance and upkeep. Synthetic adsorbents for the removal of hazardous metals from aquatic environments are efficient due to the flexibility with which these sorbent materials can be tuned into any form of composite with desirable surface functional groups. Fig. 18 represents the schematic diagram of different types of composite materials that could be applied in the treatment of various water contaminants including heavy metals, dyes, and pharmaceuticals. | ||
| Fig. 18 A schematic diagram showing different types of composite for the treatment of various water contaminants (this figure has been adapted from Sharma et al.155 with permission from Elsevier, copyright 2022). | ||
A bio-composite adsorbent synthesized from magnetic pine cone gel beads (MPCB) was employed for the individual and simultaneous decontamination of Cu(II) and Cr(VI) from binary sorption media.49 The uptake of Cr(VI) and Cu(II) onto MPCB showed dependence on solution pH, initial Cu(II) and Cr(VI) concentration, and reaction time. The optimum uptake capacity of Cu(II) and Cr(VI) ions attained by the magnetic bio-composite was 69.77 and 132.52 mg g−1, respectively. The sorption data of Cr(VI) and Cu(II) best correlated to the Langmuir pseudo-second-order models with Langmuir maximum sorption capacity of 68.64 and 212.22 mg g−1 for Cu(II) and Cr(VI), respectively. For the binary components system, it was found that both antagonistic and synergistic effects existed. The Cu(II) ions exhibited an antagonism effect on Cr(VI) ions removal while the presence of Cr(V) ions promoted Cu(II) ions decontamination from the binary solutions, pointing to a synergistic effect. The competitive equilibrium sorption data were best explained by the modified competitive Langmuir model. The study discovered that the MPCB is a promising bio-adsorbent and could be employed in the adsorptive elimination of heavy metals from aquatic environments.
The simultaneous and competitive interactive effect between As(V) and As(III) from aqueous media onto chitosan/diatomaceous earth composite (CSD) was also carried out.156 At 60.00 min equilibrium, reaction time, and 0.50 g dosage, maximal uptake capacities of 106.00 and 88.00 mg g−1 were achieved by the adsorbent at pH of 6.00 and 2.00 for As(V) and As(III), correspondingly. The sorption data obtained for As(V) and As(III) at equilibrium was found to be well-fitted to the Langmuir and pseudo-second-order models. The Langmuir optimum uptake rate attained by the adsorbent for As(V) and As(III) was 87.81 mg g−1 and 44.07 mg g−1 at a pH of 6.00, respectively. The study indicated that the synthesized adsorbent demonstrated considerable prospects for usage in the decontamination of arsenic species from aqueous media.
Besides, in a study, ZSM-5 zeolite supporting sulfide nanoscale zero-valent iron (S-nZVI@ZSM-5) composite adsorbent was produced to decontaminate Cr(VI) and As(V) in single and binary systems.157 The adsorbent was found to demonstrate higher uptake capacity for Cr(VI) and As(V) in the single system, and the optimum sorption capacity of Cr(VI) and As(V) was found to be 52.16 and 161.66 mg g−1 at a reaction time of 14.00 and 18.00 h, respectively. In the binary solute medium, the optimum sorption rates of As(V) and Cr(VI) achieved by the adsorbent were lowered by 13.01 and 63.08%, respectively, due to the competitive sorption process, demonstrating that S-nZVI@ZSM-5 exhibited a stronger chemical affinity towards As(V) ions than of Cr(VI). The equilibrium sorption data of As(V) and Cr(VI) attained from both single and binary systems best fitted the pseudo-second-order and Langmuir models.
Also, a jacobsite–biochar nanocomposite (MnFe2O4–BC) was synthesized and applied to eliminate Sb(III) and Cd(II) concurrently from wastewater.158 It was observed that optimum Sb(III) elimination efficiency was greater from the bi-solute medium in comparison to the mono-solute medium. This showed that the presence of Cd(II) promoted Sb(III) ions removal from the binary component systems. The equilibrium data of Sb(III) and Cd(II) attained from the binary solute was best correlated to the Langmuir model with optimum sorption capacities of 237.53 and 181.49 mg g−1, correspondingly. It was established that the produced MnFe2O4–BC nanocomposite could serve as a promising adsorbent for the competitive elimination of Sb(III) and Cd(II) from aqueous phases.
More so, a bentonite–alginate composite (BAC) was employed in eliminating Hg(II)and Pb(II) from both mono- and bicomponents sorption media.159 The study revealed that the adsorbent was more effective for the uptake of Pb(II) ions than Hg(II) ions in the single component system. However, the sorption capacity of these heavy metals decreases in the binary component system suggesting inhibitory effects between the Pb(II) and Hg(II). The equilibrium data of Hg(II) and Pb(II) achieved by the adsorbent in the single metal and binary metal systems best fitted the Hill and competitive Hill isotherm models, respectively.
Likewise, the competitive removal of Cu(II), Zn(II), and Pb(II) by polypyrrole/TiO2 composite was conducted in batch technique.160 The study found that Zn(II) had a greater affinity towards the surface of the adsorbent with a sorption capacity of 77.81 mg g−1. While Cu(II) uptake rate by the adsorbent was highly inhibited by the occurrence of the other competing ions [Zn(II) and Cu(II)] in the aqueous medium. The adsorptive decontamination of the heavy metals was in a pattern of Zn(II) > Pb(II) > Cu(II) in the multi-metal systems. The selectivity of the Zn(II) ions towards the adsorbent over that of Cu(II) and Pb(II) was not known. This study offered some helpful suggestions for developing adsorbents that are selective for particular heavy metals coexisting in wastewater.
The depollution of Cu(II), Pb(II), and Zn(II) from mono- and ternary solute systems using hydroxyapatite–biochar nanocomposite was investigated.161 The kinetic data of Pb(II), Zn(II), and Cu(II) attained by the adsorbent showed good fitness to the pseudo-second-order model. In both mono- and ternary solutes media, the sorption data of Pb(II) attained by the adsorbent obeyed the Langmuir isotherm model whereas the sorption data of Cu(II) and Zn(II) achieved at equilibrium conformed to the Freundlich isotherm model. The study revealed that the adsorbent can be utilized in wastewater purification, especially those polluted with heavy metals.
The detoxification of Cd(II) and Pb(II) from monocomponent and bicomponent media using chitosan functionalized EDTA-silane/MgO as an adsorbent in batch technique was studied.162 The study found that a solution pH of 5.00 and dosage of 0.30 g L−1 were the maximum operating conditions for Pb(II) and Cd(II) removal from the monocomponent medium. At these optimum conditions in the single component system, the optimum uptake rates of Cd(II) and Pb(II) attained by the adsorbent were 1.05 and 2.33 mmol g−1, correspondingly. The results of the binary component system indicated that Cd(II) ions possess synergetic effects on the decontamination of Pb(II) in the competitive system. While the presence uptake by the adsorbent was observed to have an antagonistic effect at Pb(II) high initial concentration. The Pb(II) on Cd(II) experimental data showed good fitness to the pseudo-first-order model. The thermodynamic studies revealed that the decontamination of Pb(II) and Cd(II) was endothermic and spontaneous in nature.
Similarly, the competitive decontamination of Cd(II), Pb(II), Cu(II), and Ni(II) from aquatic systems was studied in the batch system using a nanosized zirconia composite.163 The batch experiments indicated that agitation time, pH, concentration, and coexisting ions affected the concurrent elimination of Pb(II), Ni(II), Cu(II), and Cd(II). It was found that the competitive decontamination of the heavy metals was in the sequence of Pb(II) > Cu(II) > Ni(II) > Cd(II). The sorption data of Pb(II), Cd(II), Cu(II), and Ni(II) showed good fitness to the pseudo-second-order and Langmuir models, respectively. The Langmuir optimum uptake capacities of Ni(II), Cd(II), Cu(II), and Pb(II) by the adsorbent were found to be 0.700, 0.701, 0.615, and 0.620 mmol g−1, correspondingly. The study revealed that the adsorbent is proficient in the uptake of heavy metals from wastewater.
4.8 Graphene-based adsorbents
Due to their exceptional electrical and thermal mobility, huge surface area, high mechanical strength, outstanding corrosion resistance, and configurable surface chemistry, graphene-based materials have recently attracted a lot of interest in the field of water and wastewater treatment. The renewability of the spent adsorbents and adsorbates recovery even after many life cycles is one of the key advantages of graphene-based adsorbents. Fig. 19 shows the synthesis process of graphene-based adsorbent for the decontamination of adsorbate molecules.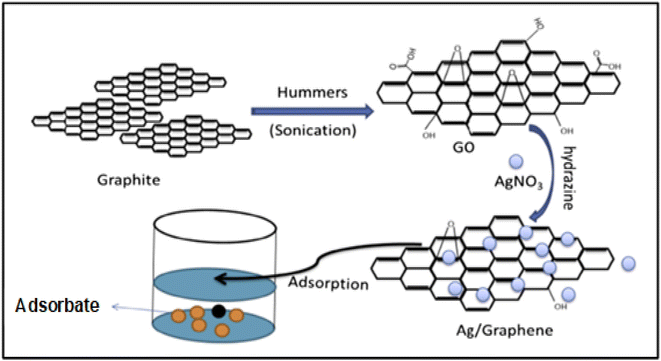 | ||
| Fig. 19 A schematic diagram showing the synthesis process of graphene-based adsorbent for adsorbate molecules removal (this figure has been adapted from Qu et al.164 with permission from Elsevier, copyright 2017). | ||
Divalent cadmium [Cd(II)] and divalent lead [Pb(II)] ions in mono- and binary solutes aqueous media were decontaminated using nickel oxide-decorated reduced graphene oxide.29 The results of the single metal sorption showed that Cd(II) and Pb(II) had sorption rates of about 725.00 and 890.00 mg g−1, respectively. Additionally, the simultaneous sequestration of Cd(II) and Pb(II) from the binary metal systems triggered a rise in Pb(II) uptake rate and decreased the removal capacity of Cd(II). The maximum uptake efficiencies of Pb(II) and Cd(II) attained were 1000.00 and 580.00 mg g−1, correspondingly. This suggested that the elimination of Cd(II) and Pb(II) were influenced by synergistic and antagonistic effects, respectively. The experimental data attained from both single- and binary-metal systems fitted the pseudo-second-order and Langmuir models.
A novel graphene-like biochar (GB)-supported nanoscale zero-valent iron (nZVI) and the underlying mechanisms of synergistic effects between GB and nZVI for the concurrent sorption of As(III) and Cd(II) were investigated.165 It was observed that at pH 4.00 and 7.00, maximum depollution of Cd(II) and As(III) was realized. The study showed that GB has an efficient uptake capacity of 181.50 and 46.40 mg g−1 for As(III) and Cd(II), correspondingly. While nZVI uptake capacity was 363.00 and 92.80 mg g−1 for As(III) and Cd(II), correspondingly. The study indicated a strong synergistic effect between GB and nZVI, which promoted Cd(II) and As(III) ions removal. Therefore, GB and nZVI exhibited high performance and could be employed for the simultaneous depollution of other heavy metal ions from irrigation waters.
To examine the selectivity of Cu(II) ions in mixed multi-metal systems, graphene oxide (GO)-based hybrid membranes (GHMs) were produced.166 The study showed that the Ni(II), Cu(II), and Co(II), were competitively adsorbed onto GHMs and the competitive elimination of Cu(II) in mixed multi-metal solutions demonstrated that Cu(II) ions uptake by the GHMs was higher. According to the sorption data, the adsorptive elimination of Ni(II), Cu(II), and Co(II) was well-described by the diffusion-chemisorption and pseudo-second-order models, respectively. Furthermore, according to the Langmuir model, the uptake rates of Cu(II), Co(II), and Ni(II) on the surface of GHMs were 4.338, 3.339, and 3.160 mg g−1, respectively. These findings demonstrated that GHMs may have potential uses in heavy metals separation and recycling.
A glutathione-functionalized NiFe2O4/graphene oxide composite is employed in the single and competitive detoxification of Hg(II), Pb(II), and Cu(II) from aqueous systems.167 The removal rate of these heavy metal ions showed dependence on pH, agitation time, and concentration. In the single system, the optimum pH of 5.00 was attained for Pb(II) and a pH of 6.00 was optimum for both Hg(II) and Cu(II) ions removal with a removal efficiency of 94.00% at an equilibrium time of 90.00 min. The kinetic data were well explained by both pseudo-second-order and Elovich models as well as the Freundlich isotherm. In the bicomponent system, the percentage removal of Cu(II) and Pb(II) decreased in the presence of Hg(II), Mn(II), and Cd(II) by competition. The study indicated that the adsorbent is efficient in the decontamination various of heavy metals from groundwater samples.
The summaries of other studies on the decontamination of heavy metals from multi-metal sorption systems (binary, ternary, quaternary, and quinary solutions) using various adsorbents are presented in Table 3.
| Adsorbent | Heavy metals | Adsorption system(s) | Interaction effect(s) | Best-fitted adsorption model(s) | Adsorption capacity (mg g−1) | Reference |
|---|---|---|---|---|---|---|
| Sodium dodecyl sulfate | Cd(II), Pb(II), and Zn(II) | Binary | Synergistic and antagonistic | Competitive Langmuir | Pb(II) = 49.37 | 168 |
| Cd(II) = 47.27 | ||||||
| Zn(II) = 44.18 | ||||||
| Modified zeolite | Hg(II) and Pb(II) | Binary | Antagonistic | Pseudo-second-order and Langmuir | Pb(II) = 12.02 | 169 |
| Hg(II) = 6.68 | ||||||
| Iranian scoria | Cd(II) and Cu(II) | Binary | Synergistic | Extended Freundlich and modified Langmuir | Cu(II) = 6.92 | 170 |
| Cd(II) = 2.33 | ||||||
| Modified mesoporous silica | Cd(II) and Ni(II) | Binary | Antagonistic | Pseudo-second-order, Langmuir, and Freundlich | Cd(II) = 156.00 | 171 |
| Ni(II) = 139.70 | ||||||
| Iron dust–zeolite composite | Cd(II) and Ni(II) | Binary | Both synergistic and antagonistic | Pseudo-second-order, Langmuir, and Freundlich | Cd(II) = 78.13 | 172 |
| Ni(II) = 76.33 | ||||||
| Leonardite | Cd(II) and Zn(II) | Binary | Antagonistic | Extended Freundlich | Cd(II) = 23.89 | 173 |
| Zn(II) = 16.86 | ||||||
| Bottom ash | Cd(II) and Zn(II) | Binary | Antagonistic | Extended Langmuir | Zn(II) = 7.72 | 174 |
| Cd(II) = 6.06 | ||||||
| Modified beer lees | Pb(II) and Zn(II) | Binary | Antagonistic | Yoon–Nelson and bed depth service time | Pb(II) = 29.60 | 175 |
| Zn(II) = 5.43 | ||||||
| Termite mound | Pb(II) and Zn(II) | Binary | Antagonistic | Pseudo-second-order and Langmuir | Pb(II) = 11.72 | 176 |
| Zn(II) = 7.62 | ||||||
| Modified chitosan/CoFe2O4 particles | Cu(II) and Pb(II) | Binary | Antagonistic | Pseudo-second-order and Langmuir | Pb(II) = 160.26 | 177 |
| Cu(II) = 139.86 | ||||||
| Fumaria indica biomass | Cu(II) and Pb(II) | Binary | Non-interaction and antagonistic | Freundlich | Pb(II) = 9.91 | 178 |
| Cu(II) = 4.08 | ||||||
| Straw/bentonite-g-poly(acrylic acid-co-acrylamide) resin | Cd(II) and Pb(II) | Binary | Synergism and antagonistic | Pseudo-second-order and Langmuir | Pb(II) = 355.50 | 179 |
| Cd(II) = 315.10 | ||||||
| Industrial chili seeds (Capsicum annuum) waste | Cd(II) and Pb(II) | Binary | Antagonistic | Modified Langmuir | Pb(II) = 8.78 | 180 |
| Cd(II) = 7.32 | ||||||
| Iranian hematite | Cd(II) and Pb(II) | Binary | Antagonistic | Langmuir, extended Freundlich, and extended Langmuir | Pb(II) = 17.86 | 181 |
| Cd(II) = 1.89 | ||||||
| Salix matsudana activated carbon | Cd(II) and Pb(II) | Binary | Antagonistic | Pseudo-second-order and Langmuir | Pb(II) = 31.09 | 182 |
| Cd(II) = 25.32 | ||||||
| Straw-derived biochar | Cd(II) and Co(II) | Binary | Antagonistic | Extended Langmuir | Cd(II) = 193.00 | 183 |
| Co(II) = 89.70 | ||||||
| Sugarcane cellulose | Cu(II), Pb(II), and Zn(II) | Binary | Synergistic | Competitive Langmuir | Zn(II) = 934.60 | 184 |
| Pb(II) = 621.10 | ||||||
| Cu(II) = 462.90 | ||||||
| Modified green tea waste | As(III) and Ni(II) | Binary | Antagonistic | Extended Langmuir and extended Freundlich | As(III) = 0.42 | 185 |
| Ni(II) = 0.31 | ||||||
| Ferrihydrite | As(III) and As(V) | Binary | Antagonistic | Freundlich | As(V) = 14.50 | 186 |
| As(III) = 10.51 | ||||||
| Modified Luffa acutangula peels | Cu(II) and Ni(II) | Binary | Antagonistic | Pseudo-second order, Langmuir and Freundlich | Cu(II) = 22.06 | 187 |
| Ni(II) = 22.72 | ||||||
| Polyamide | Cd(II), Ni(II), and Zn(II) | Binary and ternary | Antagonistic | Pseudo-second-order and Langmuir | Binary = 339.90–399.20 | 188 |
| Ternary = 275.60–314.30 | ||||||
| Sunflower plant biomass-based carbons | Cd(II), Cr(VI) and Ni(II) | Binary and ternary | Antagonistic | Langmuir | Binary = 0.12–0.05 | 189 |
| Ternary = 0.08–0.03 | ||||||
| Carbonaceous nanofibers | Cu(II), Ni(II) and Pb(II) | Binary and ternary | Antagonistic | Langmuir | Binary = 88.62–665.11 | 190 |
| Ternary = 64.56–470.34 | ||||||
| Granular Fe–Mn binary oxide | Cd(II), Cu(II), and Zn(II) | Ternary | Antagonistic | Pseudo-second-order and Langmuir | Cu(II) = 0.124 | 50 |
| Zn(II) = 0.115 | ||||||
| Cd(II) = 0.055 | ||||||
| C-phenylcalix[4]pyrogallolarene material | Cr(III), Cu(II), Ni(II), and Pb(II) | Ternary | Antagonistic | Pseudo-second-order and Langmuir | Pb(II) = 60.97 | 191 |
| Ni(II) = 16.86 | ||||||
| Cr(III) = 14.31 | ||||||
| Cu(II) = 8.14 | ||||||
| Dithizone (DZ) and mesoporous | Cd(II), Cu(II), and Hg(II) | Ternary | Antagonistic | Pseudo-second-order and Langmuir | Cu(II) = 31.79 | 192 |
| Cd(II) = 30.30 | ||||||
| Hg(II) = 25.04 | ||||||
| Bifunctional graft copolymer | Co(II), Ni(II), and Zn(II) | Ternary | Antagonistic | Pseudo-second-order and Langmuir | Ni(II) = 165.00 | 193 |
| Co(II) = 122.00 | ||||||
| Zn(II) = 68.00 | ||||||
| Modified graphene oxide | As(III), Cd(II), and Hg(II) | Ternary | Antagonistic | Pseudo-second-order and Langmuir | Cd(II) = 285.71 | 194 |
| Hg(II) = 227.27 | ||||||
| As(III) = 131.58 | ||||||
| Endoskeleton biomass | Cd(II), Cu(II), and Pb(II) | Ternary | Non-interaction | Pseudo-second-order and Langmuir | Cd(II) = 32.05 | 195 |
| Cu(II) = 14.71 | ||||||
| Pb(II) = 14.67 | ||||||
| Chitosan-pyromellitic dianhydride modified biochar | Cd(II), Cu(II), and Pb(II) | Ternary | Antagonistic | Pseudo-second-order and Langmuir | Cu(II) = 96.11 | 196 |
| Cd(II) = 38.24 | ||||||
| Pb(II) = 13.93 | ||||||
| Olive stones activated carbon | Cd(II), Cu(II), and Ni(II) | Ternary | Both synergistic and antagonistic | Pseudo-second-order, Langmuir, and Sips | Cd(II) = 62.91 | 197 |
| Cu(II) = 17.08 | ||||||
| Ni(II) = 23.46 | ||||||
| Arborvitae leaves | Co(II), Cu(II), and Pb(II) | Ternary | Antagonistic | Langmuir | Pb(II) = 9.32 | 198 |
| Cu(II) = 3.07 | ||||||
| Co(II) = 1.54 | ||||||
| Date stones activated carbon | Cu(II), Ni(II), and Zn(II) | Ternary | Antagonistic | Langmuir | Cu(II) = 18.68 | 199 |
| Ni(II) = 16.12 | ||||||
| Zn(II) = 12.19 | ||||||
| Chicken bone biochar | Cd(II), Cu(II), and Zn(II) | Ternary | Antagonistic | Langmuir | Cu(II) = 108.00 | 200 |
| Cd(II) = 54.00 | ||||||
| Zn(II) = 44.00 | ||||||
| BiPO4/FePO4/CSG nano-adsorbent | Cd(II), Cu(II) Ni(II), and Pb(II) | Quaternary | Antagonistic | Pseudo-second-order, intraparticle diffusion and Langmuir | Cu(II) = 8.65 | 201 |
| Ni(II) = 8.62 | ||||||
| Cd(II) = 8.61 | ||||||
| Pb(II) = 8.54 | ||||||
| Iron oxide-coated gravel | Al(III), Cd(II), Cu(II), Fe(III), and Pb(II) | Quinary | Antagonistic | Pseudo-second-order and Freundlich | Pb(II) = 99.72 | 202 |
| Cu(II) = 99.61 | ||||||
| Cd(II) = 99.51 | ||||||
| Fe(III) = 99.30 | ||||||
| Al(III) = 93.30 | ||||||
| Coal fly ash | Cd(II), Cu(II), Ni(II), Mn(II), and Pb(II) | Quinary | Antagonistic | Pseudo-first-order, pseudo-second-order and Langmuir | Pb(II) = 45.28 | 203 |
| Cu(II) = 32.86 | ||||||
| Cd(II) = 26.93 | ||||||
| Ni(II) = 16.25 | ||||||
| Mn(II) = 14.63 | ||||||
| Sesame straw biochar | Cd(II), Cr(III), Cu(II), Pb(II), and Zn(II) | Quinary | Antagonistic | Freundlich and Langmuir | Pb(II) = 88.00 | 204 |
| Cu(II) = 40.00 | ||||||
| Cr(III) = 21.00 | ||||||
| Zn(II) = 7.00 | ||||||
| Cd(II) = 5.00 |
5. Conclusion and future perspectives
The present study systematically and particularly reviews the multicomponent adsorption of heavy metals from various multicomponent systems such as binary, ternary, quaternary, and quinary solutions using different kinds of adsorbents. This is necessary because the adsorption of a specific heavy metal in complex wastewater may be affected by the other components and the adsorbent properties including surface charge, structure, size, functional groups, porosity, and active sites present on the adsorbent surface. Therefore, it is imperative to review the adsorption of different heavy metals from multicomponent systems using different kinds of adsorbents to elucidate the interactive behaviors among co-existing pollutants by using multicomponent adsorption isotherm models.The systematic review revealed that adsorbents derived from naturally and locally available materials including biomasses, agricultural, and industrial wastes are more promising and efficient in the decontamination of heavy metals from multicomponent systems. It was also discovered that numerous studies evaluate the adsorption characteristics of an adsorbent in a multicomponent system using various important independent adsorption parameters. These independent adsorption parameters include reaction time, solution pH, agitation speed, adsorbent dosage, initial metal ion concentration, ionic strength as well as reaction temperature, which were found to significantly affect the multicomponent adsorption process. Furthermore, through the utilization of the multicomponent sorption isotherm models, the competitive adsorption mechanisms of the heavy metals were found to be characterized by three primary kinds of interactive effects including synergism, antagonism, and non-interaction. Generally, the review conducted on the different kinds of adsorbents indicates that the competitive effects for heavy metal ions become more recognized with the increase in the number of solutes in the aqueous systems where the sorption of one metal ion usually interferes with that of another.
Despite the enormous amount of research and extensive data on the capability of different adsorbents, several significant drawbacks hinder adsorbents from being used practically and economically to remove heavy metal ions from multicomponent systems. As a result, the current systematic review provides the following insights and perspectives for further studies through the thorough and reliable analysis of the relevant literature on heavy metals removal from multicomponent systems.
(1) Due to the non-biodegradable nature of heavy metal ions, they can cause both indirect and direct health-related problems to humans and other lifeforms when released into water resources. Several adsorbents are being used nowadays to remove harmful metal ions from drinking water, wastewater, and groundwater to preserve water systems due to their efficiency. Future studies are therefore recommended to develop non-toxic, secure, high-performing green adsorbents using environmentally friendly processes.
(2) The application of biochar for the adsorptive decontamination of heavy metals from multicomponent systems has been recognized to be very proficient. However, further investigations are required on various types of biochar derived from locally and naturally available materials as well as different synthesis conditions to actually know and select a reliable biochar that could effectively eliminate several heavy metal ions concurrently from the complex wastewater.
(3) In addition, it is important to first assess the effectiveness of adsorbents in a multicomponent adsorption system before deploying them for industrial use. Therefore, choosing the appropriate sorbent material for the type of adsorbate in the aqueous solution is crucial from the perspective of practical applications. Hence, future studies should not only focus on the presence of the adsorbates in the multi-solute system but also their baseline concentration, pH, and other vital independent adsorption factors.
(4) During the field and laboratory studies, several other variables, like cost and time that are significant in industrial and commercial applications are sometimes overlooked. The expenditures should include the cost of producing the adsorbent and byproduct disposal in addition to the cost of the raw materials. According to this systematic review, surface modifications of the adsorbents improved their adsorption capacity but increased the cost of the adsorption process.
(5) There has been limited research on the renewability and reuse of exhausted adsorbent after the adsorption process, and this approach is not financially feasible. However, due to their capacity to both adsorb and desorb heavy metals from aqueous systems, adsorbents can be regarded as dual-functional materials. Particularly in real-scale applications, the regeneration and reusability of adsorbents heavily influence their economic worth and efficiency. Unfortunately, while adsorption is the subject of in-depth analyses in the literature, very few studies address the regeneration of spent adsorbents. Hence, in future studies, attention should be focused on the regeneration of the exhausted adsorbents as well as the recovery of precious metal ions from the sorbent surface after the sorption process to understand the worth of low-cost adsorbents. After the adsorption process, the exhausted adsorbents must be removed from the medium, renewed, reused, and subsequently disposed of appropriately. Therefore, in addition to desorption studies, regeneration capability is crucial when choosing a promising adsorbent for the adsorptive decontamination of heavy metals from single and multicomponent systems.
(6) Future research ought to focus on reducing the antagonistic effects of one heavy metal over other heavy metal ions in multicomponent systems. Additionally, research should be conducted to determine whether there are any synergistic effects of water contaminants in the multicomponent systems using various competitive adsorption isotherm models.
(7) More so, there is limited research on the optimization of competitive sorption process and concurrent removal of more than one heavy metal ion in the coexistence of other cations or anions in multi-solute systems. Therefore, more attention should focus on the optimization of heavy metals removal from multicomponent systems using the design of experiments and statistical packages including Design Expert of Stat-Ease. It is essential to identify optimum operating conditions for the efficient sequestration of heavy metals from multi-solute systems and determine the types of interactive effects among the heavy metals.
Author contributions
Jonas Bayuo: conceptualization, investigation and writing – original draft. Mwemezi Rwiza: reviewing, editing, and supervision. Mika Sillanpää: reviewing and editing. Kelvin Mark Mtei: reviewing, editing and supervision.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors appreciate the effort of the Partnership for Applied Sciences, Engineering, and Technology (PASET) under whose sponsorship this study was successful.References
- J. Bayuo, M. Rwiza and K. Mtei, A comprehensive review on the decontamination of lead(II) from water and wastewater by low-cost biosorbents, RSC Adv., 2022, 12(18), 11233–11254, 10.1039/-D2RA00796G.
- L. Joseph, B. M. Jun, J. R. V. Flora, C. M. Park and Y. Yoon, Removal of heavy metals from water sources in the developing world using low-cost materials: A review, Chemosphere, 2019, 229, 142–159, DOI:10.1016/j.chemosphere.2019.04.198.
- H. Elbasiouny, M. Darwesh, H. Elbeltagy, F. G. Abo-alhamd, A. A. Amer and M. A. Elsegaiy, et al., Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: a review, Environ. Monit. Assess., 2021, 193(7), 1–19, DOI:10.1007/s10661-021-09236-2.
- S. Werner, K. Kätzl, M. Wichern, A. Buerkert, C. Steiner and B. Marschner, Agronomic benefits of biochar as a soil amendment after its use as wastewater filtration medium, Environ. Pollut., 2018, 233, 561–568 CrossRef CAS PubMed.
- A. Forgionny, N. Y. Acelas, R. Ocampo-Pérez, E. Padilla-Ortega, R. Leyva-Ramos and E. Flórez, Understanding mechanisms in the adsorption of lead and copper ions on chili seed waste in single and multicomponent systems: a combined experimental and computational study, Environ. Sci. Pollut. Res., 2021, 28(18), 23204–23219 CrossRef CAS PubMed.
- M. N. Khan, H. Ullah, S. Naeem, J. Uddin, Y. Hamid and W. Ahmad, et al., Remediation of emerging heavy metals from water using natural adsorbent: Adsorption performance and mechanistic insights, Sustainability, 2021, 13(16), 1–17 Search PubMed.
- J. Bayuo, M. J. Rwiza and K. M. Mtei, Non-competitive and competitive detoxification of As(III) ions from single and binary biosorption systems and biosorbent regeneration, Biomass Convers. Biorefin., 2023, 1–28, DOI:10.1007/s13399-022-03734-0.
- H. Kumar, K. L. Maurya, A. K. Gehlaut, D. Singh, S. Maken and A. Gaur, et al., Adsorptive removal of chromium(VI) from aqueous solution using binary bio-polymeric beads made from bagasse, Appl. Water Sci., 2020, 10(1), 1–10, DOI:10.1007/s13201-019-1101-y.
- A. Agarwal, U. Upadhyay, I. Sreedhar, S. A. Singh and C. M. Patel, A review on valorization of biomass in heavy metal removal from wastewater, J. Water Process Eng., 2020, 38, 101602, DOI:10.1016/j.jwpe.-2020.101602.
- V. S. Kanwar, A. Sharma, A. L. Srivastav and L. Rani, Phytoremediation of toxic metals present in soil and water environment: a critical review, Environ. Sci. Pollut. Res., 2020, 27(36), 44835–44860 CrossRef CAS PubMed.
- V. Singh, N. Singh, S. N. Rai, A. Kumar, A. K. Singh and M. P. Singh, et al., Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches, Toxics, 2023, 11(2), 1–15 Search PubMed.
- J. Bayuo, M. Rwiza and K. Mtei, Response surface optimization and modeling in heavy metal removal from wastewater-a critical review, Environ. Monit. Assess., 2022, 194, 1–34, DOI:10.1007/s10661-022-09994-7.
- A. Pasgar, A. Nasiri and N. Javid, Single and competitive adsorption of Cu2+ and Pb2+ by tea pulp from aqueous solutions, Environ. Health Eng. Manage. J., 2022, 9(1), 65–74 CrossRef CAS.
- C. Galletti, M. Dosa, N. Russo and D. Fino, Zn(II) and Cd(II) removal from wastewater using clinoptilolite as adsorbent, Environ. Sci. Pollut. Res., 2021, 28(19), 24355–24361 CrossRef CAS PubMed.
- S. Nallakukkala, A. U. Rehman, D. B. Zaini and B. Lal, Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review, Water, 2022, 14(7), 1–32, DOI:10.3390/w14071171.
- J. Bayuo, M. Rwiza, M. A. Abukari, K. B. Pelig-Ba and K. Mtei, Modeling and optimization of independent factors influencing lead(II) biosorption from aqueous systems: A statistical approach, Sci. Afr., 2022, 16, 1–16, DOI:10.1016/j.sciaf.2022.e01270.
- Y. Dai, Q. Sun, W. Wang, L. Lu, M. Liu and J. Li, et al., Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review, Chemosphere, 2018, 211, 235–253 CrossRef CAS PubMed.
- J. O. Ighalo, S. Ogunniyi, A. G. Adeniyi and C. A. Igwegbe, Competitive adsorption of heavy metals in a quaternary solution by sugarcane bagasse – LDPE hybrid biochar : equilibrium isotherm and kinetics modeling, Chem. Prod. Process Model., 2022, 1–16 Search PubMed.
- F. O. Afolabi, P. Musonge and B. F. Bakare, Bio-sorption of a bi-solute system of copper and lead ions onto banana peels: characterization and optimization, J. Environ. Health Sci. Eng., 2021, 19(1), 613–624 CrossRef CAS PubMed.
- A. Ateş, Y. Mert and M. T. Timko, Evaluation of characteristics of raw tea waste-derived adsorbents for removal of metals from aqueous medium, Biomass Convers. Biorefin., 2021, 0123456789, DOI:10.1007/s13399-021-01721-5.
- J. Bayuo, Decontamination of cadmium(II) from synthetic wastewater onto shea fruit shell biomass, Appl. Water Sci., 2021, 11(5), 1–8, DOI:10.1007/s13201-021-01416-2.
- D. Wang, W. Luo, J. Zhu, T. Wang, Z. Gong and M. Fan, Potential of removing Pb, Cd, and Cu from aqueous solutions using novel modified ginkgo leaves biochar by simply one-step pyrolysis, Biomass Convers. Biorefin., 2021, 0123456789, DOI:10.1007/s13399-021-01732-2.
- Q. Wu, S. Dong, L. Wang and X. Li, Single and competitive adsorption behaviors of Cu2+, Pb2+ and Zn2+ on the biochar and magnetic biochar of pomelo peel in aqueous solution, Water, 2021, 13(6), 1–16 CrossRef.
- E. N. Zare, A. Mudhoo, M. A. Khan, M. Otero, Z. M. A. Bundhoo and C. Navarathna, et al., Water decontamination using bio-based, chemically functionalized, doped, and ionic liquid-enhanced adsorbents: review, Environ. Chem. Lett., 2021, 3075–3114, DOI:10.1007/s10311-021-01207-w.
- J. Bayuo, K. B. Pelig-Ba and M. A. Abukari, Adsorptive removal of chromium(VI) from aqueous solution unto groundnut shell, Appl. Water Sci., 2019, 9(4), 1–11, DOI:10.1007/s13201-019-0987-8.
- A. G. El-Said, N. A. Badawy and S. E. Garamon, Adsorption of Heavy Metal Ions from Aqueous Solutions onto Rice Husk Ash Low-Cost Adsorbent, J. Environ. Anal. Toxicol., 2018, 08(01), 10–15 Search PubMed.
- I. Wani, V. Kushvaha, A. Garg, R. Kumar, S. Naik and P. Sharma, Review on effect of biochar on soil strength: Towards exploring usage of biochar in geo-engineering infrastructure, Biomass Convers. Biorefin., 2022, 1–34, DOI:10.1007/s13399-022-02795-5.
- N. Dhiman, Binary adsorption of [Pb(II) + Co(II)] from aqueous solution using thiolated saw dust, Water Sci. Technol., 2021, 84(9), 2591–2600 CrossRef CAS PubMed.
- Shivangi, S. Bhardwaj and T. Sarkar, Simultaneous removal of cadmium and lead ions from aqueous solutions by nickel oxide-decorated reduced graphene oxides, Int. J. Environ. Sci. Technol., 2022, 19(6), 5595–5610, DOI:10.1007/s13762-021-03510-z.
- C. R. Girish, Simultaneous adsorption of pollutants onto the adsorbent review of interaction mechanism between the pollutants and the adsorbent, Int. J. Eng. Technol., 2018, 7(4), 3613–3622 CAS.
- B. Wang, J. Lan, C. Bo, B. Gong and J. Ou, Adsorption of heavy metal onto biomass-derived activated carbon: A review, RSC Adv., 2023, 13(7), 4275–4302 RSC.
- M. Bilal, I. Ihsanullah, M. Younas and M. Ul Hassan Shah, Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review, Sep. Purif. Technol., 2022, 278, 119510, DOI:10.1016/j.seppur.2021.119510.
- W. S. Chai, J. Y. Cheun, P. S. Kumar, M. Mubashir, Z. Majeed and F. Banat, et al., A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application, J. Cleaner Prod., 2021, 296, 126589, DOI:10.1016/j.jclepro.2021.126589.
- C. Duan, T. Ma, J. Wang and Y. Zhou, Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review, J. Water Process Eng., 2020, 37(130), 101339, DOI:10.1016/j.jwpe.-2020.101339.
- S. Wadhawan, A. Jain, J. Nayyar and S. K. Mehta, Role of nanomaterials as adsorbents in heavy metal ion removal from wastewater: A review, J. Water Process Eng., 2020, 33, 101038, DOI:10.1016/j.jwpe.-2019.101038.
- S. A. Mirzaee, N. Jaafarzadeh, S. S. Martinez and Z. Noorimotlagh, Simultaneous adsorption of heavy metals from aqueous matrices by nanocomposites: A first systematic review, Environ. Health Eng. Manage. J., 2022, 9(1), 9–14 CAS.
- C. R. Girish, Multicomponent adsorption and the interaction between the adsorbent and the adsorbate: A review, Int J. Mech. Eng. Technol., 2018, 9(7), 177–188 Search PubMed.
- M. Abdulaziz and S. Musayev, Multicomponent biosorption of heavy metals from aqueous solutions: A review, Pol. J. Environ. Stud., 2017, 26(4), 1433–1441 CrossRef CAS PubMed.
- F. Azadegan, M. E. Bidhendi and A. Badiei, Removal of Hg(II) Ions from Aqueous Environment with the Use of Modified LUS-1 as New Nanostructured Adsorbent, Int. J. Environ. Res., 2019, 13(3), 557–569, DOI:10.1007/s41742-019-00195-8.
- R. Pelalak, Z. Heidari, S. M. Khatami, T. A. Kurniawan, A. Marjani and S. Shirazian, Oak wood ash/GO/Fe3O4 adsorption efficiencies for cadmium and lead removal from aqueous solution: Kinetics, equilibrium and thermodynamic evaluation, Arabian J. Chem., 2021, 14(3), 1–17, DOI:10.1016/j.arabjc.-2021.102991.
- A. Q. Memon, S. Ahmed, Z. A. Bhatti, G. Maitlo, A. K. Shah and S. A. Mazari, et al., Experimental investigations of arsenic adsorption from contaminated water using chemically activated hematite (Fe2O3) iron ore, Environ. Sci. Pollut. Res., 2021, 28(10), 12898–12908 CrossRef CAS PubMed.
- K. Pyrzynska, Removal of cadmium from wastewaters with low-cost adsorbents, J. Environ. Chem. Eng., 2019, 7(1), 102795, DOI:10.1016/j.jece.2018.11.040.
- J. Jjagwe, P. W. Olupot, E. Menya and H. M. Kalibbala, Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review, J. Bioresour. Bioprod., 2021, 6(4), 292–322, DOI:10.1016/j.jobab.2021.03.003.
- S. Iftekhar, D. L. Ramasamy, V. Srivastava, M. B. Asif and M. Sillanpää, Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: a critical review, Chemosphere, 2018, 204, 413–430, DOI:10.1016/j.chemosphere.2018.04.053.
- N. Ferrah, Comparative study of mercury(II) species removal onto naked and modified magnetic chitosan flakes coated ethylenediaminetetraacetic-disodium: kinetic and thermodynamic modeling, Environ. Sci. Pollut. Res., 2018, 25(25), 24923–24938 CrossRef CAS PubMed.
- A. M. de Morais França, F. W. Sousa, A. R. Loiola, F. M. T. de Luna, C. B. Vidal and R. F. do Nascimento, Study of Cu2+, Ni2+, and Zn2+ competitive adsorption on synthetic zeolite: An experimental and theoretical approach, Desalin. Water Treat., 2021, 227, 263–277 CrossRef.
- L. Khalfa, M. Bagane, M. L. Cervera and S. Najjar, Competitive Adsorption of Heavy Metals onto Natural and Activated Clay: Equilibrium, Kinetics, and Modeling, Int. J. Chem. Mol. Eng., 2016, 10(5), 546–552 Search PubMed.
- R. Tovar-gmeza, M. R. Moreno-virgena, J. Moreno-pereza, A. Bonilla-petriciolet, V. Hernandez-montoyaa and C. J. Duran-valleb, IChemE Analysis of synergistic and antagonistic adsorption of heavy metals and acid blue 25 on activated carbon from ternary systems, Chem. Eng. Res. Des., 2014, 93, 755–772 CrossRef.
- M. Touihri, F. Guesmi, C. Hannachi, B. Hamrouni, L. Sellaoui and M. Badawi, et al., Single and simultaneous adsorption of Cr(VI) and Cu(II) on a novel Fe3O4/pine cones gel beads nanocomposite: Experiments, characterization, and isotherms modeling, Chem. Eng. J., 2021, 416, 1–16 CrossRef.
- X. Du, S. Cui, Q. Wang, Q. Han and G. Liu, Non-competitive and competitive adsorption of Zn(II), Cu(II), and Cd(II) by a granular Fe-Mn binary oxide in aqueous solution, Environ. Prog. Sustainable Energy, 2021, 40(4), 1–10 Search PubMed.
- C. R. Girish, Various isotherm models for multicomponent adsorption: A review, Int. J. Civil Eng. Technol., 2017, 8(10), 80–86 Search PubMed.
- V. Uwamariya, Adsorptive Removal of Heavy Metals from Groundwater by Iron Oxide-based adsorbents, Delft University of Technology, Academic Board of the UNESCO-IHE, 2013 Search PubMed.
- S. Aytas, D. A. Turkozu and C. Gok, Biosorption of uranium(VI) by bi-functionalized low-cost biocomposite adsorbent, Desalination, 2011, 280, 354–362 CrossRef CAS.
- A. R. Keshtkar, M. Mohammadi and M. A. Moosavian, Equilibrium biosorption studies of wastewater U(VI), Cu(II) and Ni(II) by the brown alga Cystoseira indica in single, binary, and ternary metal systems, J. Radioanal. Nucl. Chem., 2015, 303(1), 363–376 CrossRef CAS.
- O. A. A. Eletta, F. O. Ayandele and J. O. Ighalo, Adsorption of Pb(II) and Fe(II) by mesoporous composite activated carbon from Tithonia diversifolia stalk and Theobroma cacao pod, Biomass Convers. Biorefin., 2021, 1–10, DOI:10.1007/s13399-021-01699-0.
- S. Kul, Removal of Cu(II) from aqueous solutions using modified sewage sludge ash, Int. J. Environ. Sci. Technol., 2021, 18(12), 3795–3806, DOI:10.1007/s13762-021-03419-7.
- J. Tan, Y. Li, L. Xia, H. Li, S. Song and L. Wu, et al., Enhancement of Cd(II) Adsorption on Microalgae–Montmorillonite Composite, Arabian J. Sci. Eng., 2021, 1–13 Search PubMed.
- R. Tovar-Gómez, D. A. Rivera-Ramírez, V. Hernández-Montoya, A. Bonilla-Petriciolet, C. J. Durán-Valle and M. A. Montes-Morán, Synergic adsorption in the simultaneous removal of acid blue 25 and heavy metals from water using a Ca(PO3)2-modified carbon, J. Hazard. Mater., 2012, 199, 290–300 CrossRef PubMed.
- J. A. V. Butler and C. Ockrent, Studies in Electrocapillarity, J. Phys. Chem., 1930, 34(12), 2841–2859, DOI:10.1021/j150318a015.
- L. Sellaoui, D. Stracke, P. Franco, G. L. Dotto, É. C. Lima and A. B. Lamine, Single and binary adsorption of cobalt and methylene blue on modified chitin: Application of the Hill and exclusive extended Hill models, J. Mol. Liq., 2017, 233, 543–550, DOI:10.1016/j.molliq.2016.10.079.
- S. Suresh, V. C. Srivastava and I. M. Mishra, Equilibrium Modeling of Ternary Adsorption of Phenols onto Modified Activated Carbon, Theor. Found. Chem. Eng., 2018, 52(2), 271–285 CrossRef CAS.
- R. Yang, Gas Separation by Adsorption Processes, Gas Separation & Purification, Butterworths, Boston, MA, 1987 Search PubMed.
- V. K. Rathore, D. K. Dohare and P. Mondal, Competitive adsorption between arsenic and fluoride from binary mixture on chemically treated laterite, J. Environ. Chem. Eng., 2016, 4(2), 2417–2430 CrossRef CAS.
- C. Leodopoulos, D. Doulia, K. Gimouhopoulos and T. M. Triantis, Single and simultaneous adsorption of methyl orange and humic acid onto bentonite, Appl. Clay Sci., 2012, 70, 84–90 CrossRef CAS.
- B. Noroozi and G. A. Sorial, Applicable models for multi-component adsorption of dyes: A review, J. Environ. Sci., 2013, 25(3), 419–429, DOI:10.1016/S1001-0742(12)60194-6.
- X. Luo, Z. Zhang, P. Zhou, Y. Liu, G. Ma and Z. Lei, Synergic adsorption of acid blue 80 and heavy metal ions (Cu2+/Ni2+) onto activated carbon and its mechanisms, J. Ind. Eng. Chem., 2015, 27, 164–174 CrossRef CAS.
- S. K. Papageorgiou, F. K. Katsaros, E. P. Kouvelos and N. K. Kanellopoulos, Prediction of binary adsorption isotherms of Cu2+, Cd2+ and Pb2+ on calcium alginate beads from single adsorption data, J. Hazard. Mater., 2009, 162, 1347–1354, DOI:10.1016/j.jhazmat.2008.06.022.
- L. Zhang, J. Wei, X. Zhao, F. Li, F. Jiang and M. Zhang, et al., Competitive adsorption of strontium and cobalt onto tin antimonate, Chem. Eng. J., 2016, 285, 679–689, DOI:10.1016/j.cej.2015.10.013.
- S. Saha, M. Zubair, M. A. Khosa, S. Song and A. Ullah, Keratin and Chitosan Biosorbents for Wastewater Treatment: A Review, J. Polym. Environ., 2019, 27(7), 1389–1403, DOI:10.1007/s10924-019-01439-6.
- V. C. Srivastava, I. D. Mall and I. M. Mishra, Competitive adsorption of cadmium (II) and nickel (II) metal ions from aqueous solution onto rice husk ash, Chem. Eng. Process., 2009, 48(1), 370–379 CrossRef CAS.
- A. Gupta and C. Balomajumder, Simultaneous adsorption of Cr (VI) and phenol onto tea waste biomass from binary mixture: multicomponent adsorption, thermodynamic and kinetic study, J. Environ. Chem. Eng., 2015, 30(2), 785–796 CrossRef.
- N. Singh, B. Agarwal and C. Balomajumder, Simultaneous treatment of phenol and cyanide containing aqueous solution by adsorption, biotreatment, and simultaneous adsorption and biotreatment (SAB) process, J. Environ. Chem. Eng., 2016, 4(1), 564–575 CrossRef CAS.
- S. Nashtifan, A. Azadmehr and A. Maghsoudi, Comparative and competitive adsorptive removal of Ni2+ and Cu2+ from aqueous solution using iron oxide-vermiculite composite, Appl. Clay Sci., 2017, 140, 38–49 CrossRef.
- M. S. Berber-Mendoza, J. I. Martínez-Costa, R. Leyva-Ramos, H. J. Amezquita Garcia and N. A. Medellín Castillo, Competitive Adsorption of Heavy Metals from Aqueous Solution onto Oxidized Activated Carbon Fiber, Water, Air, Soil Pollut., 2018, 229(8), 1–15 CrossRef CAS.
- L. Zhang, J. Wei, X. Zhao, F. Li, F. Jiang and M. Zhang, et al., Competitive adsorption of strontium and cobalt onto tin antimonite, Chem. Eng. J., 2016, 285, 679–689 CrossRef CAS.
- T. S. Anirudhan and M. Ramachandran, Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): kinetic and competitive adsorption isotherm, Process Saf. Environ. Prot., 2015, 95, 215–225 CrossRef CAS.
- H. E. Reynel-Avila, D. I. Mendoza-Castillo, V. Hernández-Montoya and A. Bonilla-Petriciolet, Multicomponent removal of heavy metals from aqueous solution using low-cost sorbents, Water Prod. Wastewater Treat., 2015, 5(2), 1–9 Search PubMed.
- A. Mirzaei, A. Ebadi and P. Khajavi, Kinetic and equilibrium modeling of single and binary adsorption of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) onto nano-perfluorooctyl alumina, Chem. Eng. J., 2013, 231, 550–560 CrossRef CAS.
- G. McKay and B. Al Duri, Simplified model for the equilibrium adsorption of dyes from mixtures using activated carbon, Chem. Eng. Process., 1987, 22(3), 145–156 CrossRef CAS.
- C. Ramos SN do, A. L. P. Xavier, F. S. Teodoro, M. M. C. Elias, F. J. Gonçalves and L. F. Gil, et al., Modeling mono- and multi-component adsorption of cobalt(II), copper(II), and nickel(II) metal ions from aqueous solution onto a new carboxylated sugarcane bagasse. Part I: Batch adsorption study, Ind. Crops Prod., 2015, 74, 357–371 CrossRef.
- M. Padmaja and K. V. R. Reddy, Heavy metal adsorption in single and ternary systems onto Vetiveria Zizanioides roots, Mater. Today: Proc., 2021, 1–5, DOI:10.1016/j.matpr.2021.05.674.
- P. Vassileva, I. Uzunov, A. Detcheva, U. Snezhanka and D. Voykova, Multi-Component Adsorption of Heavy Metal Ions From Aqueous Solutions Onto Low-Cost Adsorbent Based on Rice Husks, Int. Sci. J. Mechanization Agric. Conserv. Resour., 2019, 181(5), 178–181 Search PubMed.
- N. N. Dilah, M. A. K. M. Hanafiah, K. Khalid, S. C. Ibrahim and A. S. Hamzah, Competitive Adsorption of Cu2+, Pb2+ and Ni2+ on Thiourea Modified Spent Grated Coconut (Cocos Nucifera), Int. J. Eng. Technol., 2018, 7, 148–151 CAS.
- X. L. Yu and Y. He, Optimal ranges of variables for effective adsorption of lead(II) by the agricultural waste pomelo (Citrus grandis) peels using Doehlert designs, Sci. Rep., 2018, 8(1), 1–9, DOI:10.1038/s41598-018-19227-y.
- G. Sandoval-Flores, S. Alvarado-Reyna, L. G. Elvir-Padilla, D. I. Mendoza-Castillo, H. E. Reynel-Avila and A. Bonilla-Petriciolet, Kinetics, Thermodynamics, and Competitive Adsorption of Heavy Metals from Water Using Orange Biomass, Water Environ. Res., 2018, 90(12), 2114–2125 CrossRef CAS PubMed.
- Y. Zheng, H. Ye, G. Zhao, H. Rao, H. Liu and F. Liu, et al., Competitive adsorption and correlative mechanism of heavy metal ions using ploy(cellulose/humic acid/acrylic acid) in multi-element aqueous medium, Polym. Bull., 2021, 78(5), 2523–2535, DOI:10.1007/s00289-020-03223-2.
- X. Liu, X. Xu, X. Dong and J. Park, Competitive adsorption of heavy metal ions from aqueous solutions onto activated carbon and agricultural waste materials, Pol. J. Environ. Stud., 2020, 29(1), 749–761 CrossRef CAS PubMed.
- G. Li, J. Zhang, J. Liu, C. Sun and Z. Yan, Adsorption characteristics of white pottery clay towards Pb(II), Cu(II), and Cd(II), Arabian J. Geosci., 2020, 13(13), 1–15 Search PubMed.
- L. Liu, X. Guo, S. Wang, L. Li, Y. Zeng and G. Liu, Effects of wood vinegar on properties and mechanism of heavy metal competitive adsorption on secondary fermentation-based composts, Ecotoxicol. Environ. Saf., 2018, 150(35), 270–279, DOI:10.1016/j.ecoenv.2017.12.037.
- S. E. Ebrahime and S. H. Alsaade, Competitive Adsorption of Cd (II) and Zn (II) in Single and Binary Systems from Aqueous Solutions onto Cork Stopper Particles, Assoc. Arab Univ. J. Eng. Sci., 2019, 26(1), 17–27 Search PubMed.
- M. Abdullah, L. C. Abdullah, T. S. Y. Choong, S. N. A. M. Jamil, R. A. Majid and A. A. Adeyi, et al., Simultaneous adsorption of heavy metal ions (Cu2+ and Fe2+) from binary solutions by microcrystalline cellulose (MCC): Initial concentration effect, pH and kinetics studies, AIP Conf. Proc., 2022, 2454, 1–6 Search PubMed.
- B. Tekin and A. Unsal, Adsorption Isotherms for Removal of Heavy Metal Ions (Copper and Nickel) from Aqueous Solutions in Single and Binary Adsorption Processes, Gazi Univ. J. Sci., 2022, 36(2), 495–509 Search PubMed.
- R. Bassam, M. El Alouani, J. Maissara, N. Jarmouni, M. Belhabra and M. El Mahi Chbihi, et al., Investigation of competitive adsorption and desorption of heavy metals from aqueous solution using raw rock: Characterization kinetic, isotherm, and thermodynamic, Mater. Today: Proc., 2021, 52, 158–165 Search PubMed.
- B. Sizirici, I. Yildiz, A. AlYammahi, F. Obaidalla, M. AlMehairbi and S. AlKhajeh, et al., Adsorptive removal capacity of gravel for metal cations in the absence/presence of competitive adsorption, Environ. Sci. Pollut. Res., 2018, 25(8), 7530–7540 CrossRef CAS PubMed.
- X. Tian, Q. Xie, G. Chai and G. Li, Simultaneous adsorption of As(III) and Cd(II) by ferrihydrite-modified biochar in aqueous solution and their mutual effects, Sci. Rep., 2022, 12(1), 1–11, DOI:10.1038/s41598-022-09648-1.
- S. Zhu, T. Qu, M. K. Irshad and J. Shang, Simultaneous removal of Cd(II) and As(III) from co-contaminated aqueous solution by α-FeOOH modified biochar, Biochar, 2020, 2(1), 81–92, DOI:10.1007/s42773-020-00040-8.
- B. J. Ni, Q. S. Huang, C. Wang, T. Y. Ni, J. Sun and W. Wei, Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge, Chemosphere, 2019, 219, 351–357, DOI:10.1016/j.chemosphere.2018.12.053.
- D. Yang, L. Wang, Z. Li, X. Tang, M. He and S. Yang, et al., Simultaneous adsorption of Cd(II) and As(III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems, Sci. Total Environ., 2020, 708, 134823, DOI:10.1016/j.scitotenv.2019.134823.
- M. Luo, H. Lin, Y. He, B. Li, Y. Dong and L. Wang, Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar, Bioresour. Technol., 2019, 284, 333–339, DOI:10.1016/j.biortech.2019.03.108.
- L. Zheng, Y. Gao, J. Du, W. Zhang, Y. Huang and Q. Zhao, et al., Single and binary adsorption behaviour and mechanisms of Cd(II), Cu(II) and Ni(II) onto modified biochar in aqueous solutions, Processes, 2021, 9(10), 1–12 Search PubMed.
- C. Xue, L. Zhu, S. Lei, M. Liu, C. Hong and L. Che, et al., Lead competition alters the zinc adsorption mechanism on animal-derived biochar, Sci. Total Environ., 2020, 713, 1–7, DOI:10.1016/j.scitotenv.2019.-136395.
- M. Zhao, Y. Dai, M. Zhang, C. Feng, B. Qin and W. Zhang, et al., Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies, Sci. Total Environ., 2020, 717, 1–11, DOI:10.1016/j.scitotenv.2020.136894.
- L. Sellaoui, H. Wang, M. Badawi, A. Bonilla-Petriciolet and Z. Chen, Synergistic adsorption of Pb2+ and Cr(VI) on an engineered biochar highlighted by statistical physical modeling, J. Mol. Liq., 2020, 312, 1–5 CrossRef.
- P. T. Tho, H. T. Van, L. H. Nguyen, T. K. Hoang, T. N. Ha Tran and T. T. Nguyen, et al., Enhanced simultaneous adsorption of As(III), Cd(II), Pb(II) and Cr(VI) ions from aqueous solution using cassava root husk-derived biochar loaded with ZnO nanoparticles, RSC Adv., 2021, 11(31), 18881–18897 RSC.
- R. Shan, Y. Shi, J. Gu, Y. Wang and H. Yuan, Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems, Chin. J. Chem. Eng., 2020, 28(5), 1375–1383, DOI:10.1016/j.cjche.2020.02.012.
- L. Sellaoui, D. I. Mendoza-Castillo, H. E. Reynel-Ávila, B. A. Ávila-Camacho, L. L. Díaz-Muñoz and H. Ghalla, et al., Understanding the adsorption of Pb2+, Hg2+ and Zn2+ from aqueous solution on a lignocellulosic biomass char using advanced statistical physics models and density functional theory simulations, Chem. Eng. J., 2019, 365, 305–316 CrossRef CAS.
- Z. Mahdi, Q. J. Yu and A. El Hanandeh, Competitive adsorption of heavy metal ions (Pb2+, Cu2+, and Ni2+) onto date seed biochar: batch and fixed bed experiments, Sep. Sci. Technol., 2019, 54(6), 888–901, DOI:10.1080/01496395.2018.1523192.
- S. J. Cobbina, A. B. Duwiejuah and A. K. Quainoo, Single and simultaneous adsorption of heavy metals onto groundnut shell biochar produced under fast and slow pyrolysis, Int. J. Environ. Sci. Technol., 2019, 16(7), 81–90, DOI:10.1007/s13762-018-1910-9.
- J. O. Ighalo, A. G. Adeniyi, O. A. A. Eletta and L. T. Arowoyele, Competitive adsorption of Pb(II), Cu(II), Fe(II) and Zn(II) from aqueous media using biochar from oil palm (Elaeis guineensis) fibers: a kinetic and equilibrium study, Indian Chem. Eng., 2020, 1–11, DOI:10.1080/00194506.2020.1787870.
- H. R. Boostani, M. Najafi-Ghiri and A. G. Hardie, Single and competitive adsorption isotherms of some heavy metals onto a light textured calcareous soil amended with agricultural wastes-biochars, Arch. Agron. Soil Sci., 2019, 65(3), 360–373, DOI:10.1080/03650340.2018.1503651.
- F. de Freitas, L. D. Battirola and R. L. T. de Andrade, Adsorption of Cu2+ and Pb2+ Ions by Pontederia rotundifolia (L.f.) (Pontederiaceae) and Salvinia biloba Raddi (Salviniaceae) Biomass, Water, Air, Soil Pollut., 2018, 229(11), 1–12 CrossRef CAS.
- X. Sun, H. Huang, Y. Zhu, Y. Du, L. Yao and X. Jiang, et al., Adsorption of Pb2+ and Cd2+ onto Spirulina platensis harvested by polyacrylamide in single and binary solution systems, Colloids Surf., A, 2019, 583, 123926, DOI:10.1016/j.colsurfa.2019.123926.
- M. Khajavian, A. Hallajsani and P. Ghelichi, Optimizing binary biosorption of cobalt and nickel ions on brown algae using a central composite design, Int. J. Environ. Sci. Technol., 2020, 17(12), 4759–4774, DOI:10.1007/s13762-020-02761-6.
- Q. Q. Zhong, Y. Q. Zhao, L. Shen, B. Hao, X. Xu and B. Y. Gao, et al., Single and Binary Competitive Adsorption of Cobalt and Nickel onto Novel Magnetic Composites Derived from Green Macroalgae, Environ. Eng. Sci., 2020, 37(3), 188–200 CrossRef CAS.
- M. Khajavian, D. A. Wood, A. Hallajsani and N. Majidian, Simultaneous biosorption of nickel and cadmium by the brown algae Cystoseria indica characterized by isotherm and kinetic models, Appl. Biol. Chem., 2019, 62(1), 1–12, DOI:10.1186/s13765-019-0477-6.
- R. Yous, F. Mohellebi, H. Cherifi and A. Amrane, Competitive biosorption of heavy metals from aqueous solutions onto Streptomyces rimosus, Korean J. Chem. Eng., 2018, 35(4), 890–899 CrossRef CAS.
- J. R. Guarín-Romero, P. Rodríguez-Estupiñán, L. Giraldo and J. C. Moreno-Piraján, Simple and Competitive Adsorption Study of Nickel(II) and Chromium(III) on the Surface of the Brown Algae Durvillaea antarctica Biomass, ACS Omega., 2019, 4(19), 18147–18158 CrossRef PubMed.
- A. Es-Said, H. Nafai, L. E. Hamdaoui, A. Bouhaouss and R. Bchitou, Adsorptivity and selectivity of heavy metals Cd(II), Cu(II), and Zn(II) toward phosphogypsum, Desalin. Water Treat., 2020, 197, 291–299 CrossRef CAS.
- O. Ucarli, O. T. Yayintas, M. S. Engin, S. Cay, G. Saglikoglu and S. Yilmaz, Investigation of Competitive and Noncompetitive Adsorption of Some Heavy Metals Ions on Leucodon sciuroides (Hedw.) Schwägr, Langmuir, 2020, 36(28), 8265–8271 CrossRef CAS PubMed.
- R. Liu and B. Lian, Non-competitive and competitive adsorption of Cd2+, Ni2+, and Cu2+ by biogenic vaterite, Sci. Total Environ., 2019, 659(1), 122–130, DOI:10.1016/j.scitotenv.2018.12.199.
- M. Mariana, A. K. Abdul, E. M. Mistar, E. B. Yahya, T. Alfatah and M. Danish, et al., Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption, J. Water Process Eng., 2021, 43, 102221, DOI:10.1016/j.jwpe.2021.102221.
- O. Khelifi, A. M. Affoune, M. Nacef, M. L. Chelaghmia and H. Laksaci, Response Surface Modeling and Optimization of Ni(II) and Cu(II) Ions Competitive Adsorption Capacity by Sewage Sludge Activated Carbon, Arabian J. Sci. Eng., 2022, 47(5), 5797–5809, DOI:10.1007/s13369-021-05534-6.
- N. F. Campos, G. A. J. C. Guedes, L. P. S. Oliveira, B. M. V. Gama, D. C. S. Sales and J. M. Rodríguez-Díaz, et al., Competitive adsorption between Cu2+ and Ni2+ on corn cob activated carbon and the difference of thermal effects on mono and bicomponent systems, J. Environ. Chem. Eng., 2020, 8(5), 1–47, DOI:10.1016/j.jece.-2020.104232.
- J. Kazmierczak-razna, A. Zioła-frankowska, P. Nowicki, M. Frankowski, R. Wolski and R. Pietrzak, Removal of heavy metal ions from one- and two-component solutions via adsorption on N-doped activated carbon, Materials, 2021, 14(22), 1–17 CrossRef PubMed.
- M. Kavand, P. Eslami and L. Razeh, The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems, J. Water Process Eng., 2020, 34, 1–8, DOI:10.1016/j.jwpe.2020.101151.
- P. P. Ndibewu, C. M. Kede, P. G. Tchieta, H. Z. Poumve and A. N. Tchakounte, Simultaneous adsorption of mercury (II) and zinc (II) ions from aqueous solution onto activated carbons derived from a lowland bioresource waste, J. Appl. Surf. Interfaces, 2019, 5(1), 21–30 Search PubMed , http://creativecommons.org/licenses/by/4.0/.
- A. L. Arim, G. Guzzo, M. J. Quina and L. M. Gando-Ferreira, Single and binary sorption of Cr(III) and Ni(II) onto modified pine bark, Environ. Sci. Pollut. Res., 2018, 25(28), 28039–28049 CrossRef CAS PubMed.
- P. Loganathan, W. G. Shim, D. P. Sounthararajah, M. Kalaruban, T. Nur and S. Vigneswaran, Modelling equilibrium adsorption of single, binary, and ternary combinations of Cu, Pb, and Zn onto granular activated carbon, Environ. Sci. Pollut. Res., 2018, 25(17), 16664–16675 CrossRef CAS PubMed.
- M. F. Sabbagh and M. H. Al-malack, Highly competitive multicomponent adsorption of organic and heavy metals using activated mangrove charcoal, Desalin. Water Treat., 2021, 242, 162–177, DOI:10.5004/dwt.2021.27851.
- C. Suo, K. Du, R. Yuan, H. Chen, F. Wang and B. Zhou, Adsorption study of heavy metal ions from aqueous solution by activated carbon in single and mixed system, Desalin. Water Treat., 2020, 183, 1–10 CrossRef.
- S. Huang, S. Jin, Y. Wang, J. Liu, J. Yu and D. Liu, Selective adsorption of heavy metal ions from aqueous solution by modified bagasse, Chem. Ecol., 2020, 1–16, DOI:10.1080/02757540.2020.1787998.
- M. Mudasir, R. A. Baskara, A. Suratman, K. S. Yunita, R. Perdana and W. Puspitasari, Simultaneous Adsorption of Zn(II) and Hg(II) Ions on Selective Adsorbent of Dithizone-Immobilized Bentonite in the Presence of Mg(II) Ion, J. Environ. Chem. Eng., 2020, 8(4), 1–9, DOI:10.1016/j.jece.2020.104002.
- A. P. G. M. V. Samaraweera, N. Priyantha, W. S. S. Gunathilake, P. A. Kotabewatta and T. P. K. Kulasooriya, Biosorption of Cr(III) and Cr(VI) species on NaOH-modified peel of Artocarpus nobilis fruit. 1. Investigation of kinetics, Appl. Water Sci., 2020, 10(5), 1–11, DOI:10.1007/s13201-020-01187-2.
- S. Mihajlović, M. Vukčević, B. Pejić, A. P. Grujić and M. Ristić, Application of waste cotton yarn as adsorbent of heavy metal ions from single and mixed solutions, Environ. Sci. Pollut. Res., 2020, 27(28), 35769–35781 CrossRef PubMed.
- H. Sha, Y. Wu and Y. Fan, Utilization of industrial waste as a novel adsorbent: Mono/competitive adsorption of chromium(VI) and nickel(II) using diatomite waste modified by EDTA, Appl. Organomet. Chem., 2018, 32(1), 1–15 CrossRef.
- S. He, Y. Li, L. Weng, J. Wang, J. He and Y. Liu, et al., Competitive adsorption of Cd2+, Pb2+ and Ni2+ onto Fe3+-modified argillaceous limestone: Influence of pH, ionic strength and natural organic matters, Sci. Total Environ., 2018, 637, 69–78, DOI:10.1016/j.scitotenv.2018.04.300.
- L. Khalfa, A. Sdiri, M. Bagane and M. L. Cervera, Multi-element modeling of heavy metals competitive removal from aqueous solution by raw and activated clay from the Aleg formation (Southern Tunisia), Int. J. Environ. Sci. Technol., 2020, 17(4), 2123–2140, DOI:10.1007/s13762-019-02614-x.
- L. F. De Magalhães, G. R. Da Silva and A. E. C. Peres, Zeolite Application in Wastewater Treatment, Adsorpt. Sci. Technol., 2022, 2022, 1–26, DOI:10.1155/2022/4544104.
- R. Dahake, P. Tiwari and A. Bansiwal, Multicycle adsorption and desorption for recovery of U(VI) from aqueous solution using oxime modified zeolite-A, J. Radioanal. Nucl. Chem., 2021, 327(1), 133–142, DOI:10.1007/s10967-020-07482-1.
- G. Yao, X. Zhang, Z. Sun and S. Zheng, High adsorption selectivity of zeolite X in the binary ionic system of Cu(II) and Zn(II), J. Porous Mater., 2019, 26(4), 1197–1207, DOI:10.1007/s10934-019-00721-1.
- S. R. Zekavat, F. Raouf and S. S. A. Talesh, Simultaneous adsorption of Cu2+ and Cr(VI) using HDTMA-modified zeolite: Isotherm, kinetic, mechanism, and thermodynamic studies, Water Sci. Technol., 2020, 82(9), 1808–1824 CrossRef CAS PubMed.
- H. Vatandoust, H. Younesi, Z. Mehraban, A. Heidari and H. Khakpour, Comparative Adsorption of Cd (II) and Pb (II) by MCM-48 and Amine-Grafted MCM-48 in Single and Binary Component Systems, Water Conserv. Sci. Eng., 2021, 6(2), 67–78 CrossRef.
- S. K. Toor, J. P. Kushwaha and V. K. Sangal, Single and Binary Adsorption of Zn (II) and Cr (VI) Heavy Metals onto Synthesized Silica -Based MCM-41, ChemistrySelect, 2019, 4(9), 2576–2584 CrossRef CAS.
- I. V. Joseph, L. Tosheva and A. M. Doyle, Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash, J. Environ. Chem. Eng., 2020, 8(4), 1–9, DOI:10.1016/j.jece.2020.103895.
- T. Cheng, C. Chen, R. Tang, C. H. Han and Y. Tian, Competitive adsorption of Cu, Ni, Pb, and Cd from aqueous solution onto fly ash-based linde F(K) Zeolite, Iran. J. Chem. Chem. Eng., 2018, 37(1), 61–72 CAS.
- A. Thakuria and B. Kataria, Nanoparticle-based methodologies for targeted drug delivery- an insight, J. Nanopart. Res., 2021, 1–30, DOI:10.1007/s11051-021-05190-9.
- R. Baby, M. Z. Hussein, A. H. Abdullah and Z. Zainal, Nanomaterials for the Treatment of Heavy Metal Contaminated Water, Polymers, 2022, 14(3), 1–17 CrossRef PubMed.
- Y. Vicente-Martínez, M. Caravaca and A. Soto-Meca, Simultaneous adsorption of mercury species from aquatic environments using magnetic nanoparticles coated with nanomeric silver functionalized with L-Cysteine, Chemosphere, 2021, 282, 1–10, DOI:10.1016/j.chemosphere.2021.131128.
- A. Esrafili, S. Bagheri, M. Kermani, M. Gholami and M. Moslemzadeh, Simultaneous adsorption of heavy metal ions (Cu2+ and Cd2+) from aqueous solutions by magnetic silica nanoparticles (Fe3O4@SiO2) modified using EDTA, Desalin. Water Treat., 2019, 158, 207–215 CrossRef CAS.
- M. Jafarnejad, M. D. Asli, F. A. Taromi and M. Manoochehri, Synthesis of multi-functionalized Fe3O4-NH2-SH nanofiber based on chitosan for single and simultaneous adsorption of Pb(II) and Ni(II) from aqueous system, Int. J. Biol. Macromol., 2020, 48, 201–217, DOI:10.1016/j.ijbiomac.2020.01.017.
- M. M. Eglal and A. S. Ramamurthy, Competitive Adsorption and Oxidation Behavior of Heavy Metals on nZVI Coated with TEOS, Water Environ. Res., 2015, 87(11), 2018–2026 CrossRef CAS PubMed.
- N. Kanani, M. Bayat, F. Shemirani, J. B. Ghasemi, Z. Bahrami and A. Badiei, Synthesis of magnetically modified mesoporous nanoparticles and their application in simultaneous determination of Pb(II), Cd(II) and Cu(II), Res. Chem. Intermed., 2018, 44(3), 1689–1709 CrossRef CAS.
- X. Zhang and Y. Liu, Concurrent removal of Cu(II), Co(II) and Ni(II) from wastewater by nanostructured layered sodium vanadosilicate: Competitive adsorption kinetics and mechanisms, J. Environ. Chem. Eng., 2021, 9(5), 1–8, DOI:10.1016/j.jece.2021.105945.
- F. P. Fato, D. W. Li, L. J. Zhao, K. Qiu and Y. T. Long, Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles, ACS Omega, 2019, 4(4), 7543–7549 CrossRef CAS PubMed.
- A. Sharma, D. Mangla, Shehnaz and S. A. Chaudhry, Recent advances in magnetic composites as adsorbents for wastewater remediation, J. Environ. Manage., 2022, 306, 114483 CrossRef CAS PubMed.
- S. S. Salih, A. Mahdi, M. Kadhom and T. K. Ghosh, Competitive adsorption of As(III) and As(V) onto chitosan/diatomaceous earth adsorbent, J. Environ. Chem. Eng., 2019, 7(5), 103407, DOI:10.1016/j.jece.2019.103407.
- C. Zhou, C. Han, X. Min and T. Yang, Simultaneous adsorption of As(V) and Cr(VI) by zeolite supporting sulfide nanoscale zero-valent iron: Competitive reaction, affinity and removal mechanism, J. Mol. Liq., 2021, 338, 1–11, DOI:10.1016/j.molliq.2021.116619.
- Y. Y. Wang, H. Y. Ji, H. H. Lu, Y. X. Liu, R. Q. Yang and L. L. He, et al., Simultaneous removal of Sb(III) and Cd(II) in water by adsorption onto a MnFe2O4-biochar nanocomposite, RSC Adv., 2018, 8(6), 3264–3273 RSC.
- T. C. Nguyen, P. Loganathan and T. V. Nguyen, Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash, Environ. Sci. Pollut. Res., 2018, 25(21), 20430–20438, DOI:10.1007/s11356-017-9610-4.
- J. Chen, M. Yu, C. Wang, J. Feng and W. Yan, Insight into the Synergistic Effect on Selective Adsorption for Heavy Metal Ions by a Polypyrrole/TiO2 Composite, Langmuir, 2018, 34(34), 10187–10196 CrossRef CAS PubMed.
- Y. Y. Wang, Y. X. Liu, H. H. Lu, R. Q. Yang and S. M. Yang, Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions, J. Solid State Chem., 2018, 261, 53–56, DOI:10.1016/j.jssc.2018.02.010.
- A. Shahbazi, N. N. Marnani and Z. Salahshoor, Synergistic and antagonistic effects in simultaneous adsorption of Pb(II) and Cd(II) from aqueous solutions onto chitosan functionalized EDTA-silane/MgO, Biocatal. Agric. Biotechnol., 2019, 22(2), 101398, DOI:10.1016/j.bcab.2019.101398.
- J. Yang, Y. Chu, Z. Li and Y. Zhang, Effective removal of heavy metals by nanosized hydrous zirconia composite hydrogel and adsorption behavior study, Environ. Sci. Pollut. Res., 2018, 25(33), 33464–33477 CrossRef CAS PubMed.
- Z. Qu, L. Fang, D. Chen, H. Xu and N. Yan, Effective and regenerable Ag/graphene adsorbent for Hg(II) removal from aqueous solution, Fuel, 2017, 203, 128–134, DOI:10.1016/j.fuel.2017.04.105.
- K. Liu, F. Li, J. Cui, S. Yang and L. Fang, Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms, J. Hazard. Mater., 2020, 395, 1–13, DOI:10.1016/j.jhazmat.2020.122623.
- W. Dou, J. Liu and M. Li, Competitive adsorption of Cu2+ in Cu2+, Co2+, and Ni2+ mixed multi-metal solution onto graphene oxide (GO)-based hybrid membranes, J. Mol. Liq., 2021, 322, 1–15, DOI:10.1016/j.molliq.2020.114516.
- P. Khorshidi, R. H. S. M. Shirazi, M. Miralinaghi, E. Moniri and S. Saadi, Adsorptive removal of mercury (II), copper (II), and lead (II) ions from aqueous solutions using glutathione-functionalized NiFe2O4/graphene oxide composite, Res. Chem. Intermed., 2020, 46(7), 3607–3627 CrossRef CAS.
- X. Li, S. He, C. Feng, Y. Zhu, Y. Pang and J. Hou, et al., Non-competitive and competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto SDS in process of micellar-enhanced ultrafiltration, Sustain, 2018, 10(1), 1–12 CAS.
- H. Shirzadi and A. Nezamzadeh-Ejhieh, An efficient modified zeolite for simultaneous removal of Pb(II) and Hg(II) from aqueous solution, J. Mol. Liq., 2017, 230(II), 221–229, DOI:10.1016/j.molliq.2017.01.029.
- S. Seyfi, A. R. Azadmehr and A. Maghsoudi, Comparative and competitive adsorption of Cu(II) and Cd(II) using scoria: Equilibrium, kinetic and thermodynamic studies, Chem. Res. Chin. Univ., 2017, 33(3), 471–478 CrossRef CAS.
- A. K. Thakur, G. M. Nisola, L. A. Limjuco, K. J. Parohinog, R. E. C. Torrejos and V. K. Shahi, et al., Polyethylenimine-modified mesoporous silica adsorbent for simultaneous removal of Cd(II) and Ni(II) from aqueous solution, J. Ind. Eng. Chem., 2017, 49, 133–144, DOI:10.1016/j.jiec.2017.01.019.
- Z. Ahali Abadeh and M. Irannajad, Removal of Ni and Cd ions from aqueous solution using iron dust-zeolite composite: Analysis by thermodynamic, kinetic and isotherm studies, Chem. Res. Chin. Univ., 2017, 33(2), 318–326 CrossRef.
- A. Terdputtakun, O. Arqueropanyo, P. Sooksamiti, S. Janhom and W. Naksata, Adsorption isotherm models and error analysis for single and binary adsorption of Cd(II) and Zn(II) using leonardite as adsorbent, Environ. Earth Sci., 2017, 76(22), 1–11 CAS.
- H. Sukpreabprom, A. Oanong, W. Naksata, P. Sooksamiti and S. Janhom, Single and binary adsorption of Cd (II) and Zn (II) ions from aqueous solutions onto bottom ash, Korean J. Chem. Eng., 2015, 32(5), 896–902 CrossRef CAS.
- Y. Dong and H. Lin, Competitive adsorption of Pb(II) and Zn(II) from aqueous solution by modified beer lees in a fixed bed column, Process Saf. Environ. Prot., 2017, 111(II), 263–269, DOI:10.1016/-j.psep.2017.06.016.
- N. Abdus-Salam and M. O. Bello, Kinetics, thermodynamics and competitive adsorption of lead and zinc ions onto termite mound, Int. J. Environ. Sci. Technol., 2015, 12(11), 3417–3426 CrossRef CAS.
- C. Fan, K. Li, J. Li, D. Ying, Y. Wang and J. Jia, Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles, J. Hazard. Mater., 2017, 326, 211–220, DOI:10.1016/j.jhazmat.2016.12.036.
- M. Iqbal and R. A. Khera, Adsorption of copper and lead in single and binary metal system onto Fumaria indica biomass, Chem Int., 2015, 1(3), 157b–163b CAS , http://bosaljournals.com/chemint/.
- Z. Wang, W. Zhou and L. Zhu, Mono-/competitive adsorption of cadmium(II) and lead(II) using straw/bentonite-g-poly(acrylic acid-co-acrylamide) resin, Polym. Bull., 2020, 77(7), 3795–3811, DOI:10.1007/s-00289-019-02939-0.
- N. A. Medellin-Castillo, E. Padilla-Ortega, M. C. Regules-Martínez, R. Leyva-Ramos, R. Ocampo-Pérez and C. Carranza-Alvarez, Single and competitive adsorption of Cd(II) and Pb(II) ions from aqueous solutions onto industrial chili seeds (Capsicum annuum) waste, Sustainable Environ. Res., 2017, 27(2), 61–69, DOI:10.1016/j.serj.2017.01.004.
- N. Khorshidi and A. R. Azadmehr, Competitive adsorption of Cd (II) and Pb (II) ions from aqueous solution onto iranian hematite (Sangan mine): Optimum condition and adsorption isotherm study, Desalin. Water Treat., 2017, 8, 106–119 CrossRef.
- Y. Shu, K. Li, J. Song, B. Li and C. Tang, Single and competitive adsorption of Cd(II) and Pb(II) from aqueous solution by activated carbon prepared with Salix matsudana Kiodz, Water Sci. Technol., 2016, 74(12), 2751–2761 CrossRef CAS PubMed.
- M. Pipíška, B. M. Richveisová, V. Frišták, M. Horník, L. Remenárová and R. Stiller, et al., Sorption separation of cobalt and cadmium by straw-derived biochar: a radiometric study, J. Radioanal. Nucl. Chem., 2017, 311(1), 85–97 CrossRef.
- F. Wang, Y. Pan, P. Cai, T. Guo and H. Xiao, Single and binary adsorption of heavy metal ions from aqueous solutions using sugarcane cellulose-based adsorbent, Bioresour. Technol., 2017, 241, 482–490, DOI:10.1016/j.biortech.2017.05.162.
- S. Yang, Y. Wu, A. Aierken, M. Zhang, P. Fang and Y. Fan, et al., Mono/competitive adsorption of Arsenic(III) and Nickel(II) using modified green tea waste, J. Taiwan Inst. Chem. Eng., 2016, 60, 213–221, DOI:10.1016/j.jtice.2015.07.007.
- P. Qi and T. Pichler, Competitive Adsorption of As(III) and As(V) by Ferrihydrite: Equilibrium, Kinetics, and Surface Complexation, Water, Air, Soil Pollut., 2016, 227(10), 1–9, DOI:10.1007/s11270-016-3091-9.
- R. Ahmad and S. Haseeb, Competitive adsorption of Cu2+ and Ni2+ on Luffa acutangula modified Tetraethoxysilane (LAP-TS) from the aqueous solution: Thermodynamic and isotherm studies, Groundw. Sustain. Dev., 2015, 1, 146–154 CrossRef.
- I. M. El-Naggar, S. A. Ahmed, N. Shehata, E. S. Sheneshen, M. Fathy and A. Shehata, A novel approach for the removal of lead (II) ion from wastewater using Kaolinite/Smectite natural composite adsorbent, Appl. Water Sci., 2019, 9(1), 1–13, DOI:10.1007/s13201-018-0845-0.
- M. Jain, V. K. Garg, K. Kadirvelu and M. Sillanpää, Adsorption of heavy metals from multi-metal aqueous solution by sunflower plant biomass-based carbons, Int. J. Environ. Sci. Technol., 2016, 13(2), 493–500 CrossRef CAS.
- C. Ding, W. Cheng, X. Wang, Z. Y. Wu, Y. Sun and C. Chen, et al., Competitive sorption of Pb(II), Cu(II) and Ni(II) on carbonaceous nanofibers: A spectroscopic and modeling approach, J. Hazard. Mater., 2016, 313(II), 253–261, DOI:10.1016/j.jhazmat.2016.04.002.
- Jumina, Y. Priastomo, H. R. Setiawan, Mutmainah, Y. S. Kurniawan and K. Ohto, Simultaneous removal of lead(II), chromium(III) and copper(II) heavy metal ions through an adsorption process using C-phenylcalix[4]pyrogallolarene material, J. Environ. Chem. Eng., 2020, 8(4), 103971, DOI:10.1016/j.jece.2020.103971.
- S. Nazerdeylami and R. Zare-Dorabei, Simultaneous adsorption of Hg2+, Cd2+ and Cu2+ ions from aqueous solution with mesoporous silica/DZ and conditions optimize with experimental design: Kinetic and isothermal studies, Micro Nano Lett., 2019, 14(8), 823–827 CrossRef CAS.
- J. Ray, S. Jana, S. K. Bhanja and T. Tripathy, Efficient removal of Co(II), Ni(II), and Zn(II) metal ions from binary and ternary solutions using a pH responsive bifunctional graft copolymer, Colloid Polym. Sci., 2018, 296(8), 1275–1291 CrossRef CAS.
- M. Alimohammady, M. Jahangiri, F. Kiani and H. Tahermansouri, Highly efficient simultaneous adsorption of Cd(II), Hg(II) and As(III) ions from aqueous solutions by modification of graphene oxide with 3-amino pyrazole: Central composite design optimization, New J. Chem., 2017, 41(17), 8905–8919, 10.1039/C7NJ01450c.
- N. Rahbar, H. Yazdanpanah, Z. Ramezani, M. R. Shushizadeh, M. Enayat and M. Mansourzadeh, Comparative and competitive adsorption of Cu(II), Cd(II) and Pb(II) onto Sepia pharaonis endoskeleton biomass from aqueous solutions, Water Environ. J., 2018, 32(2), 209–216 CrossRef CAS.
- J. Deng, Y. Liu, S. Liu, G. Zeng, X. Tan and B. Huang, et al., Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar, J. Colloid Interface Sci., 2017, 506(II), 355–364, DOI:10.1016/j.jcis.2017.07.069.
- T. Bohli, A. Ouederni and I. Villaescusa, Simultaneous adsorption behavior of heavy metals onto microporous olive stones activated carbon: analysis of metal interactions, Euro-Mediterr. J. Environ. Integr., 2017, 2(1), 1–15 CrossRef.
- J. Shi, Z. Fang, Z. Zhao, T. Sun and Z. Liang, Comparative study on Pb(II), Cu(II), and Co(II) ions adsorption from aqueous solutions by arborvitae leaves, Desalin. Water Treat., 2016, 57(10), 4732–4739 CAS.
- F. Bouhamed, Z. Elouear, J. Bouzid and B. Ouddane, Multi-component adsorption of copper, nickel, and zinc from aqueous solutions onto activated carbon prepared from date stones, Environ. Sci. Pollut. Res., 2016, 23(16), 15801–15806, DOI:10.1007/s11356-015-4400-3.
- J. H. Park, J. S. Cho, Y. S. Ok, S. H. Kim, S. W. Kang and I. W. Choi, et al., Competitive adsorption and selectivity sequence of heavy metals by chicken bone-derived biochar: Batch and column experiment, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2015, 50(11), 1194–1204 CrossRef CAS PubMed.
- S. Kheirandish, M. Ghaedi, K. Dashtian, R. Jannesar, M. Montazerozohori and F. Pourebrahim, et al., Simultaneous removal of Cd(II), Ni(II), Pb(II) and Cu(II) ions via their complexation with HBANSA based on a combined ultrasound-assisted and cloud point adsorption method using CSG-BiPO4/FePO4 as novel adsorbent: FAAS detection and optimization process, J. Colloid Interface Sci., 2017, 500, 241–252, DOI:10.1016/j.jcis.2017.03.070.
- B. Sizirici and I. Yildiz, Simultaneous adsorption of divalent and trivalent metal cations by iron oxide-coated gravel, Int. J. Environ. Sci. Technol., 2018, 15(12), 2647–2656, DOI:10.1007/s13762-018-1644-8.
- K. He, Y. Chen, Z. Tang and Y. Hu, Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash, Environ. Sci. Pollut. Res., 2016, 23(3), 2778–2788 CrossRef CAS PubMed.
- J. H. Park, Y. S. Ok, S. H. Kim, J. S. Cho, J. S. Heo and R. D. Delaune, et al., Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions, Chemosphere, 2016, 142, 77–83, DOI:10.1016/j.chemosphere.2015.05.093.
| This journal is © The Royal Society of Chemistry 2023 |