Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterisation of new silicon–perfluoropropenyl compounds†
Lulu Alluhaibi *a,
Alan Brisdon
*a,
Alan Brisdon b,
Sylwia Klejna
b,
Sylwia Klejna a and
Abeer Muneerb
a and
Abeer Muneerb
aAcademic Centre for Materials and Nanotechnology, AGH University of Science and Technology, ul. Kawiory 30, 30-055 Kraków, Poland. E-mail: lulu.alluhaibi@gmail.com
bSchool of Chemistry, The University of Manchester, Manchester M13 9PL, UK
First published on 3rd May 2023
Abstract
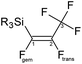
Novel, stable silicon–pentafluoropropane compounds have been synthesised from the direct reaction of hydrofluorocarbons Z-CFH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3 (Z-HFC-1225ye) with nBuLi, followed by appropriate silicon-halide. The compounds have been characterized by multinuclear NMR studies (19F, 1H, 29Si and 13C), DFT studies and structural confirmation was obtained by X-ray diffraction. Based on the outcome of treating synthetic silicon–pentafluoropropene compounds with different nucleophilic sources (nBuLi, tBuLi, MeLi, and PhLi) and computed for this reaction DFT energetics, it is clear that the C–Ftrans bond is more active than C–Fgem (Fgem and Ftrans are labelled with respect to Si). This provides a route for efficient modification of pentafluoropropene group, that can be a crucial step in developing pharmaceuticals that include propenyl or vinyl groups, addressing the demand for medicines based on long carbonic chains.
CFCF3 (Z-HFC-1225ye) with nBuLi, followed by appropriate silicon-halide. The compounds have been characterized by multinuclear NMR studies (19F, 1H, 29Si and 13C), DFT studies and structural confirmation was obtained by X-ray diffraction. Based on the outcome of treating synthetic silicon–pentafluoropropene compounds with different nucleophilic sources (nBuLi, tBuLi, MeLi, and PhLi) and computed for this reaction DFT energetics, it is clear that the C–Ftrans bond is more active than C–Fgem (Fgem and Ftrans are labelled with respect to Si). This provides a route for efficient modification of pentafluoropropene group, that can be a crucial step in developing pharmaceuticals that include propenyl or vinyl groups, addressing the demand for medicines based on long carbonic chains.
Introduction
Fluorine plays an important role in the medical field, particularly in pharmacological developments ranging from perfluorinated fluids used as artificial blood and fluoropolymers used in grafts, through applications in drug delivery and in improving the metabolic stability of new medications.1 It has been found that at least one fluorine moiety is present in 37% of all active small molecule pharmaceutical ingredients that have been approved by the FDA in 2020. Furthermore, between 2011 and 2020, a 26% increase in fluorine-containing pharmaceuticals in all pharmaceuticals approved by the FDA has been noted.2Due to the importance of fluorocarbon fragments in pharmaceuticals, a number of studies covered the methods of attaching the fluorocarbon fragment into organic compounds3 or transition-metal complexes,4 as well as C–F bond activation have been reported.5 Unfortunately, there is a lack of studies of pentafluoropropene group (CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) comparing to analogues perfluorocarbon groups, such as trifluoromethyl CF3 and trifluoroethene (CF
CFCF3) comparing to analogues perfluorocarbon groups, such as trifluoromethyl CF3 and trifluoroethene (CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2).6 Therefore, this paper focuses on new silicon-based perfluoropropenyl compounds, which would be suitable for transferring that fluorocarbon fragment via a Hiyama cross coupling reaction into suitable organic substrates. Although these transfers have already been done for tin-containing compounds,7 the silicon analogues would be preferred because the majority of silicon compounds are non-toxic and commercially available.8 We synthesized a series of silicon–pentafluoropropene compounds in E configuration with the general formula presented in Fig. 1 and Table 1. The obtained compounds have been fully characterized by multinuclear NMR studies (19F, 1H, 29Si and 13C). The second part of this paper focuses on the investigation of the C–F bond activation through treating synthetic silicon–pentafluoropropene compounds with different nucleophilic sources (nBuLi, tBuLi, MeLi, and PhLi). The DFT energetics have been also computed for this reaction.
CF2).6 Therefore, this paper focuses on new silicon-based perfluoropropenyl compounds, which would be suitable for transferring that fluorocarbon fragment via a Hiyama cross coupling reaction into suitable organic substrates. Although these transfers have already been done for tin-containing compounds,7 the silicon analogues would be preferred because the majority of silicon compounds are non-toxic and commercially available.8 We synthesized a series of silicon–pentafluoropropene compounds in E configuration with the general formula presented in Fig. 1 and Table 1. The obtained compounds have been fully characterized by multinuclear NMR studies (19F, 1H, 29Si and 13C). The second part of this paper focuses on the investigation of the C–F bond activation through treating synthetic silicon–pentafluoropropene compounds with different nucleophilic sources (nBuLi, tBuLi, MeLi, and PhLi). The DFT energetics have been also computed for this reaction.
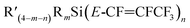 compounds with 19F{1H} NMR data (376 MHz, CDCl3, 291 K), (Fgem and Ftrans are labelled with respect to Si)
compounds with 19F{1H} NMR data (376 MHz, CDCl3, 291 K), (Fgem and Ftrans are labelled with respect to Si)
| Compound | δ CF3 | δ Fgem | δ Ftrans |
|---|---|---|---|
(Et)3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) CFCF3) (a.1) |
−68.13 ppm (d.d) | −137.16 ppm (q.d) | −141.20 ppm (q.d) |
| 3J CF3 Ftrans = 13.8 Hz | 4J Fgem CF3 = 6.5 Hz | 3J Ftrans CF3 = 13.8 Hz | |
| 4J CF3 Fgem = 6.5 Hz | 3J Fgem Ftrans = 13.0 Hz | 3J Ftrans Fgem = 13.5 Hz | |
(Bu)3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.2) CFCF3) (a.2) |
−67.91 ppm (d.d) | −136.57 ppm (q.d) | −141.33 ppm (q.d) |
| 3J CF3 Ftrans = 13.7 Hz | 4J Fgem CF3 = 6.2 Hz | 3J Ftrans CF3 = 13.8 Hz | |
| 4J CF3 Fgem = 6.4 Hz | 3J Fgem Ftrans = 11.9 Hz | 3J Ftrans Fgem = 11.8 Hz | |
ClCH2(Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.3) CFCF3) (a.3) |
−67.83 ppm (d.d) | −139.02 ppm (m) | −139.14 ppm (m) |
| 3J CF3 Ftrans = 13.3 Hz | |||
| 4J CF3 Fgem = 6.2 Hz | |||
nBu(Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.4) CFCF3) (a.4) |
−67.62 ppm (d.d) | −137.26 ppm (q.d) | −141.89 ppm (q.d) |
| 3J CF3 Ftrans = 13.7 Hz | 4J Fgem CF3 = 6.8 Hz | 3J Ftrans CF3 = 13.2 Hz | |
| 4J CF3 Fgem = 6.5 Hz | 3J Fgem Ftrans = 12.2 Hz | 3J Ftrans Fgem = 12.8 Hz | |
Ph(Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.5) CFCF3) (a.5) |
−67.36 ppm (d.d) | −136.30 ppm (q.d) | −140.89 ppm (q.d) |
| 3J CF3 Ftrans = 13.2 Hz | 4J Fgem CF3 = 6.2 Hz | 3J Ftrans CF3 = 13.2 Hz | |
| 4J CF3 Fgem = 6.3 Hz | 3J Fgem Ftrans = 12.9 Hz | 3J Ftrans Fgem = 12.9 Hz | |
Me(Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.6) CFCF3) (a.6) |
−67.06 ppm (d.d) | −134.12 ppm (q.d) | −138.22 ppm (q.d) |
| 3J CF3 Ftrans = 13.7 Hz | 4J Fgem CF3 = 6.1 Hz | 3J Ftrans CF3 = 13.0 Hz | |
| 4J CF3 Fgem = 6.2 Hz | 3J Fgem Ftrans = 12.2 Hz | 3J Ftrans Fgem = 12.5 Hz | |
(Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7) CFCF3)2 (a.7) |
−68.83 ppm (d.d) | −137.75 ppm (q.d) | −140.36 ppm (m) |
| 3J CF3 Ftrans = 13.2 Hz | 4J Fgem CF3 = 5.8 Hz | ||
| 4J CF3 Fgem = 5.4 Hz | 3J Fgem Ftrans = 12.7 Hz | ||
(iPr)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) CFCF3)2 (a.8) |
−68.97 ppm (d.d) | −136.95 ppm (q.d) | −138.54 ppm (m) |
| 3J CF3 Ftrans = 14.1 Hz | 4J Fgem CF3 = 4.8 Hz | ||
| 4J CF3 Fgem = 5.8 Hz | 3J Fgem Ftrans = 12.7 Hz | ||
(Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) CFCF3)2 (a.9) |
−67.95 ppm (d.d.d) | −133.95 ppm (q.d) | −136.94 ppm (m) |
| 3J CF3 Ftrans = 13.0 Hz | 4J Fgem CF3 = 5.5 Hz | ||
| 4J CF3 Fgem = 4.5 Hz | 3J Fgem Ftrans = 13.1 Hz | ||
| J CF3 Fexternal = 3.9 Hz | |||
PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)3 (a.10) CFCF3)3 (a.10) |
−69.31 ppm (broad d) | −131.04 ppm (q.d) | −141.08 ppm (m) |
| 3J CF3 Ftrans = 13.4 Hz | 4J Fgem CF3 = 5.9 Hz | ||
| 3J Fgem Ftrans = 13.5 Hz | |||
Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)4 (a.11) CFCF3)4 (a.11) |
−70.05 ppm (d.m) | −127.32 ppm (q.d) | −145.45 ppm (m) |
| 3J CF3 Ftrans = 16.1 Hz | 4J Fgem CF3 = 6.5 Hz | ||
| 3J Ftrans Fgem = 13.0 Hz |
Results and discussion
Synthesis of silicon–perfluoropropenyl compounds
Based on the previously published method by Brisdon et al.9,10 1,1,3,3,3-pentafluoropropene (CF3CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFH) – known commercially as Z-HFC-1225ye – was used as a starting material to generate the intermediate Z-perfluoropropenyl lithium (CF3CF
CFH) – known commercially as Z-HFC-1225ye – was used as a starting material to generate the intermediate Z-perfluoropropenyl lithium (CF3CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFLi), followed by reaction with
CFLi), followed by reaction with 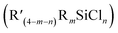 to prepare
to prepare 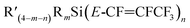 , as outlined in Scheme 1.
, as outlined in Scheme 1.
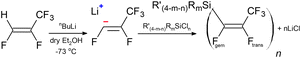 | ||
Scheme 1 The general synthesis of silicon–perfluoropropenyl compounds 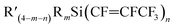 , (n = 1, 2, 3, 4; m = 1, 2, 3) and R = Me, Et, Bu, iPr, Ph; R′ = ClCH2, nBu, Me, Ph. , (n = 1, 2, 3, 4; m = 1, 2, 3) and R = Me, Et, Bu, iPr, Ph; R′ = ClCH2, nBu, Me, Ph. | ||
The 19F{1H} spectra of all of the silicon–perfluoropropenyl compounds produced the anticipated results: 3 signals with a relative intensity ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and correlated with the expectations of the perfluoropropenyl fragment. Similarly to the published main-group9 and transition-metal perfluoropropenyl complexes,10 the CF3 signal appeared between −65 ppm to −70 ppm with a higher intensity than Fgem and Ftrans making its assignment straightforward.
1, and correlated with the expectations of the perfluoropropenyl fragment. Similarly to the published main-group9 and transition-metal perfluoropropenyl complexes,10 the CF3 signal appeared between −65 ppm to −70 ppm with a higher intensity than Fgem and Ftrans making its assignment straightforward.
In addition, the signal produced, on average, coupling constants of around 13 Hz between CF3 and Ftrans and around 6 Hz between CF3 and Fgem. The signals for Fgem and Ftrans were observed around (−127 to −137) ppm and (−136 to −145) ppm, respectively, and displayed mutual coupling with the CF3 nuclei and each other. Interestingly, in the di-, tri- and tetra-perfluoropropenyl substituted compounds (Table 1), the CF3 signal was observed sometime as a doublet of doublets of doublets. The presence of an additional coupling to the CF3 group is thought to have occurred from a fluorine atom through space, or through the bonds, in addition to the coupling from Fgem and Ftrans. This was similar to the coupling patterns that had been observed for [(COD)Pt(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2].10 The 19F{1H} shows an instance of additional coupling in Si(E-CF
CFCF3)2].10 The 19F{1H} shows an instance of additional coupling in Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)4 (a.11), wherein the CF3 signal was observed as a doublet of multiplets instead of a doublet of doublets as expected if coupling only occurred to Fgem and Ftrans.
CFCF3)4 (a.11), wherein the CF3 signal was observed as a doublet of multiplets instead of a doublet of doublets as expected if coupling only occurred to Fgem and Ftrans.
The 13C{1H} NMR spectra of the perfluoropropenyl part of the compounds were as expected, (see ESI†) wherein the C3 signal was observed as a quartet of doublets of doublets due to the coupling to three equivalent F nuclei of the CF3 group through one bond, coupling to Ftrans through two bonds and to Fgem through three bonds and the coupling constants were found to be ca. 270, 37, and 10 Hz respectively. The C2 signal appeared as a doublet of quartet of doublets due to the coupling to Ftrans, the three equivalent CF3 fluorines and Fgem, and the coupling constants were found to be ca. 270, 39, and 20 Hz respectively. The C1 signal also appeared as a doublet of quartet of doublets, the coupling constants were found to be ca. 287, 4.7, and 1.7 Hz respectively.
The 29Si{1H} NMR spectra of the mono-perfluoropropenyl substituted compounds exhibited a significant coupling with Fgem nucleus, resulting in doublet splitting patterns, with coupling constant of between 20 to 30 Hz, which agrees with the coupling constant of the Si satellites in the 19F{H} spectrum of Fgem. For comparison, di-, tri- and tetra-substituted-perfluoropropenyl compounds exhibited multiplet splitting patterns as expected.
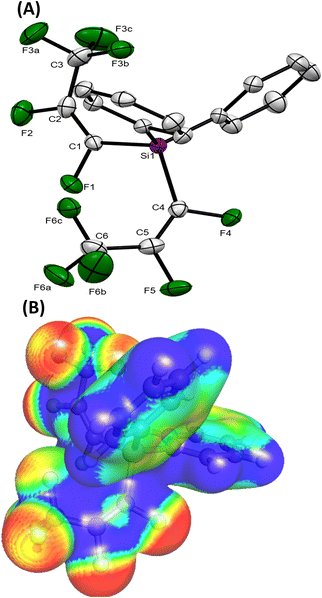
The majority of the silicon–perfluoropropenyl compounds were liquids, which limited the ability for structural characterisation by single crystal X-ray diffraction. However, (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) was a solid and attempts to grow single crystals were successful. The crystallographic data for the obtained crystal presented in Fig. 2 (see ESI, Tables S11 and S12†) confirmed that (Ph)2Si(E-CF
CFCF3)2 (a.9) was a solid and attempts to grow single crystals were successful. The crystallographic data for the obtained crystal presented in Fig. 2 (see ESI, Tables S11 and S12†) confirmed that (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) was di-substituted, which correlated with the findings from the multinuclear NMR studies (Table 1). The bond lengths and angles for the perfluoropropenyl part of the compound showed similar data to the reported crystallographic values for the transition-metals perfluoropropenyl compounds.9,10
CFCF3)2 (a.9) was di-substituted, which correlated with the findings from the multinuclear NMR studies (Table 1). The bond lengths and angles for the perfluoropropenyl part of the compound showed similar data to the reported crystallographic values for the transition-metals perfluoropropenyl compounds.9,10
X-ray crystallography and DFT studies
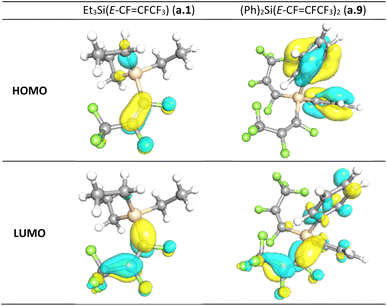
For all compounds, the geometry optimization and electronic structure calculations were performed with DFT. For Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9), the reproduction of the observed solid-state distances by calculations of gas phase geometry using parameters that excluded diffuse functions from the basis set was imperfect, it was pleasing to see that many trends of the observed geometry were reproduced. As illustrated in Table 2, the differences between the observed bond lengths and calculated values were ca. 0.02 Å. The HOMO and LUMO (highest occupied molecular orbital and lowest unoccupied molecular orbital) orbitals are localized mainly on the carbons of the perfluoropropenyl group of the molecule in all cases except compounds with Ph group present, in which these orbitals are more spatially separated (see Fig. 3 and ESI-Table S10†). Thus, in the case of (Me)2PhSi(E-CF
CFCF3)2 (a.9), the reproduction of the observed solid-state distances by calculations of gas phase geometry using parameters that excluded diffuse functions from the basis set was imperfect, it was pleasing to see that many trends of the observed geometry were reproduced. As illustrated in Table 2, the differences between the observed bond lengths and calculated values were ca. 0.02 Å. The HOMO and LUMO (highest occupied molecular orbital and lowest unoccupied molecular orbital) orbitals are localized mainly on the carbons of the perfluoropropenyl group of the molecule in all cases except compounds with Ph group present, in which these orbitals are more spatially separated (see Fig. 3 and ESI-Table S10†). Thus, in the case of (Me)2PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.5), (Ph)2MeSi(E-CF
CFCF3) (a.5), (Ph)2MeSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.6) and (Ph)2Si(E-CF
CFCF3) (a.6) and (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) HOMO is localized on the Ph group, whereas the LUMO mainly on the CF
CFCF3)2 (a.9) HOMO is localized on the Ph group, whereas the LUMO mainly on the CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3 part. The localization of the LUMO on the perfluoropropenyl group in all studied compounds indicates the electrophilic character of this group. Further, the calculations of the relative charges on the carbons of the perfluoropropenyl group were also performed. The positive charges on C2 were found to be higher than those on C1, (see Table 3). This suggested the likelihood for preferential nucleophilic attack at the C2 of the perfluoropropenyl group. This charge distribution appears to be consistent irrespective of the other groups coordinated to the silicon centre. For all of the compounds for which calculations were performed, as illustrated in Table 3, Me2Si(E-CF
CFCF3 part. The localization of the LUMO on the perfluoropropenyl group in all studied compounds indicates the electrophilic character of this group. Further, the calculations of the relative charges on the carbons of the perfluoropropenyl group were also performed. The positive charges on C2 were found to be higher than those on C1, (see Table 3). This suggested the likelihood for preferential nucleophilic attack at the C2 of the perfluoropropenyl group. This charge distribution appears to be consistent irrespective of the other groups coordinated to the silicon centre. For all of the compounds for which calculations were performed, as illustrated in Table 3, Me2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7), Ph2Si(E-CF
CFCF3)2 (a.7), Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) and PhSi(E-CF
CFCF3)2 (a.9) and PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)3 (a.10) the C2 centre is more positive than C1. The electrostatic potential maps computed for (a.1), (a.2), (a.3), (a.5), (a.6), (a.7), (a.8), and (a.9) confirm this trend (see ESI Table S13†).
CFCF3)3 (a.10) the C2 centre is more positive than C1. The electrostatic potential maps computed for (a.1), (a.2), (a.3), (a.5), (a.6), (a.7), (a.8), and (a.9) confirm this trend (see ESI Table S13†).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) from the crystallographic data (solid phase) with estimated standard deviations in parentheses; and the calculated with DFT bond lengths (Å) for (Ph)2Si(E-CF
CFCF3)2 (a.9) from the crystallographic data (solid phase) with estimated standard deviations in parentheses; and the calculated with DFT bond lengths (Å) for (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) in the gas phase
CFCF3)2 (a.9) in the gas phase
| Solid phase | Gas phase | ||
|---|---|---|---|
| Atoms | Bond length Å | Atoms | Bond length Å |
| Si1–C1 | 1.895(2) | Si3–C26 | 1.921 |
| C1–C2 | 1.323(3) | C26–C33 | 1.317 |
| C2–C3 | 1.491(4) | C33–C35 | 1.499 |
| F1–C1 | 1.362(2) | F34–C26 | 1.341 |
| F2–C2 | 1.342(3) | F36–C33 | 1.324 |
| F3a—C3 | 1.330(3) | F37–C35 | 1.320 |
| F3b—C3 | 1.326(3) | F38–C35 | 1.316 |
| F3c—C3 | 1.319(3) | F39–C35 | 1.319 |
| Si1–C4 | 1.906(2) | Si3–C23 | 1.925 |
| C4–C5 | 1.319(3) | C23–C24 | 1.317 |
| C5–C6 | 1.484(4) | C24–C28 | 1.497 |
| F4–C4 | 1.365(3) | F25–C23 | 1.340 |
| F5–C5 | 1.349(3) | F27–C24 | 1.323 |
| F6a—C6 | 1.334(3) | F29–C28 | 1.320 |
| F6b—C6 | 1.314(3) | F31–C28 | 1.316 |
| F6c—C6 | 1.329(3) | F30–C28 | 1.319 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7), Ph2Si(E-CF
CFCF3)2 (a.7), Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9), and PhSi(E-CF
CFCF3)2 (a.9), and PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)3 (a.10)
CFCF3)3 (a.10)
Next, calculations of thermodynamic reaction energies between 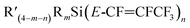 compounds and nucleophilic reagent R′′Li, according to reaction summarized in Scheme 2, were conducted. As an example, DFT reaction energetics for Et3Si(E-CF
compounds and nucleophilic reagent R′′Li, according to reaction summarized in Scheme 2, were conducted. As an example, DFT reaction energetics for Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) are presented in Table 4. For the energetics of the rest of the studied compounds in reaction with R′′Li see ESI-Table S10.† These calculations revealed that the nucleophilic attack at C2 position giving Z-isomer as a product is energetically more favourable in all studied cases. Generally, the preference of R′′ to attack the C2 position over C1 position increases in the order of: Ph < Me < nBu < tBu. Furthermore, the reactivity of nucleophiles in the reaction producing Z-isomer increases in the following order: Ph < tBu ≤ Me < nBu, except for (Me)2Si(E-CF
CFCF3) (a.1) are presented in Table 4. For the energetics of the rest of the studied compounds in reaction with R′′Li see ESI-Table S10.† These calculations revealed that the nucleophilic attack at C2 position giving Z-isomer as a product is energetically more favourable in all studied cases. Generally, the preference of R′′ to attack the C2 position over C1 position increases in the order of: Ph < Me < nBu < tBu. Furthermore, the reactivity of nucleophiles in the reaction producing Z-isomer increases in the following order: Ph < tBu ≤ Me < nBu, except for (Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7), where using tBuLi is energetically the most favourable.
CFCF3)2 (a.7), where using tBuLi is energetically the most favourable.
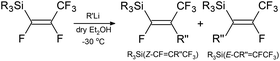 | ||
| Scheme 2 The general reaction of silicon–perfluoropropenyl compounds with R′′Li (R = Me, Et, nBu, iPr, Ph; R′′ = nBu, tBu, Me, Ph). | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) giving Z-/E-isomer, respectively
CFCF3) (a.1) giving Z-/E-isomer, respectively
| Nucleophile R′Li | ΔE [kcal mol−1] for product | |
|---|---|---|
R3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR′CF3)n CR′CF3)n |
R3Si(E-CR′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)n CFCF3)n |
|
| nBuLi | −76.35 | −70.27 |
| MeLi | −73.55 | −67.31 |
| tBuLi | −72.86 | −59.34 |
| PhLi | −69.99 | −66.70 |
C–F activation via nucleophilic attack
The outcome from DFT calculation suggests that C–Ftrans bond has a higher tendency to be replaced than C–Fgem bond. This theory has been tested by treating the silicon–perfluoropropenyl compounds with different nucleophilic sources (nBuLi, tBuLi, MeLi, and PhLi), as shown in Scheme 2. In most of the reactions studied (see Table 6), 19F{1H} spectra showed four peaks, and the J values confirmed the existence of a mixture of two compounds for the reaction between R3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) and the organolithium reagents. For example, the reaction of Et3Si(E-CF
CFCF3) and the organolithium reagents. For example, the reaction of Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) and nBuLi, the 19F{1H} NMR spectrum showed a total of four peaks, two in the CF3 region and two others. Based on their integration values they could be divided into two sets of peaks with relative intensities of 3
CFCF3) (a.1) and nBuLi, the 19F{1H} NMR spectrum showed a total of four peaks, two in the CF3 region and two others. Based on their integration values they could be divided into two sets of peaks with relative intensities of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For the first pair of peaks (Z-isomer in Scheme 2), a mutual J coupling of approximately 8 Hz was measured, while for the E-isomer, the mutual J coupling was slightly larger (around 10 Hz). In both cases the 19F and 19F{1H} NMR spectra exhibited the same splitting patterns, which excluded the probability of couplings to H. The 29Si{1H} NMR spectrum showed two peaks with similar intensity ratios as those found in the 19F{1H} spectra. Each signal was a doublet with J values of approximately 30 Hz and 9 Hz.
1. For the first pair of peaks (Z-isomer in Scheme 2), a mutual J coupling of approximately 8 Hz was measured, while for the E-isomer, the mutual J coupling was slightly larger (around 10 Hz). In both cases the 19F and 19F{1H} NMR spectra exhibited the same splitting patterns, which excluded the probability of couplings to H. The 29Si{1H} NMR spectrum showed two peaks with similar intensity ratios as those found in the 19F{1H} spectra. Each signal was a doublet with J values of approximately 30 Hz and 9 Hz.
The elemental analysis data for the resulting mixture from treatment of Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) with nBuLi was C: 54.55% and H: 8.23%. These values are close to the values calculated for (a.1) in which one of the fluorines has been replaced by a nBu group, which are C: 54.92% and H: 8.51% (see Table 5). This suggests that the two compounds observed are isomers, rather than two different products.
CFCF3) (a.1) with nBuLi was C: 54.55% and H: 8.23%. These values are close to the values calculated for (a.1) in which one of the fluorines has been replaced by a nBu group, which are C: 54.92% and H: 8.51% (see Table 5). This suggests that the two compounds observed are isomers, rather than two different products.
| Compound | Molecular formula | % weight (theory) | % weight (found) |
|---|---|---|---|
| (a.1) | C9H15F5Si | C, 43.89; H, 6.14 | C, 43.20; H, 5.95 |
| (a.1) + nBuLi | C13H24F4Si | C, 54.90; H, 8.51 | C, 54.55; H, 8.23 |
| (a.1) + tBuLi | C13H24F4Si | C, 54.90; H, 8.51 | C, 54.89; H, 8.03 |
| (a.1) + MeLi | C10H18F4Si | C, 49.56; H, 7.49 | C, 49.73; H, 7.12 |
| (a.1) + PhLi | C15H20F4Si | C, 59.19; H, 6.63 | C, 59.63; H, 6.91 |
For some reactions the 19F{1H} and 29Si{1H} spectra confirmed that only one compound had been generated, as was the case for the reaction between Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) and tBuLi. In this case, the mutual F–F coupling in the 19F{1H} spectrum was around 7 Hz, while the Si–F coupling observed in the 29Si{1H} spectrum was 32.9 Hz. These values are similar to those observed for the more intense of the two sets of signals in the mixture that resulted from the reaction between Et3Si(E-CF
CFCF3) (a.1) and tBuLi. In this case, the mutual F–F coupling in the 19F{1H} spectrum was around 7 Hz, while the Si–F coupling observed in the 29Si{1H} spectrum was 32.9 Hz. These values are similar to those observed for the more intense of the two sets of signals in the mixture that resulted from the reaction between Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) and nBuLi.
CFCF3) (a.1) and nBuLi.
When compared to similar systems (see Fig. 4), a range of coupling constants between CF3 and F are observed, but generally the CF3–Ftrans coupling constants are bigger (>10 Hz) than the coupling constants between CF3 and Fgem (<10 Hz). The 4J(CF3–F) coupling between substituents on the same side of the double bond are between 15–20 Hz. The 19F{1H} NMR data for the major product formed in the reaction of nBuLi with Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) and the only product formed when tBuLi was used, had F–F coupling constants of ca. 8.0 and 7.0 Hz respectively. This suggests that the first compound in the mixture occurred as a result of the Ftrans substitution by the nBu group leaving Fgem to couple with the CF3 signal. This substitution produced Et3Si(Z-CF
CFCF3) (a.1) and the only product formed when tBuLi was used, had F–F coupling constants of ca. 8.0 and 7.0 Hz respectively. This suggests that the first compound in the mixture occurred as a result of the Ftrans substitution by the nBu group leaving Fgem to couple with the CF3 signal. This substitution produced Et3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3) (12Z), see Scheme 2 and Table 6, which correlated with the 29Si{1H} NMR data, where the coupling between Si and F was approximately 30 Hz. This is similar to the coupling between Si and Fgem in Et3Si(E-CF
CnBuCF3) (12Z), see Scheme 2 and Table 6, which correlated with the 29Si{1H} NMR data, where the coupling between Si and F was approximately 30 Hz. This is similar to the coupling between Si and Fgem in Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1). On the other hand, the low abundance product was Et3Si(E-CnBu
CFCF3) (a.1). On the other hand, the low abundance product was Et3Si(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (12E) which likely results from substitution of Fgem with the nBu group. This is consistent with both the coupling between CF3 and F, which is ca. 11.0 Hz, in agreement with b.4,5 b.5
CFCF3) (12E) which likely results from substitution of Fgem with the nBu group. This is consistent with both the coupling between CF3 and F, which is ca. 11.0 Hz, in agreement with b.4,5 b.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and b.6
6 and b.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (see Fig. 4 and Table 6) and the smaller Si–F coupling since the fluorine is now further from the silicon centre. The fluorine NMR data also indicated that it was unlikely for the second compound to be formed with the cis geometry. The results of the 13C{1H} NMR spectra also correlate well with the suggested interpretation of the other NMR data. For the major product of the reaction involving nBuLi, expansions of the signals for the perfluoropropenyl carbons are shown in Fig. 5, while the corresponding signals for the minor product are shown in Fig. 6. For the major species, the carbon of the CF3 couples to the other fluorine with J = 24.3 Hz, whereas in the minor product the coupling between the carbon nucleus of the CF3 and the other F is 50.7 Hz, which indicates that the CF3 is separated from the F by fewer bonds in the minor species (E-isomer) compared with the major product (Z-isomer). Similarly, the coupling between the carbon directly bonded to the unique fluorine atom exhibits a larger quartet coupling in E-isomer (39.5 Hz) than in Z-isomer (6.6 Hz).
8 (see Fig. 4 and Table 6) and the smaller Si–F coupling since the fluorine is now further from the silicon centre. The fluorine NMR data also indicated that it was unlikely for the second compound to be formed with the cis geometry. The results of the 13C{1H} NMR spectra also correlate well with the suggested interpretation of the other NMR data. For the major product of the reaction involving nBuLi, expansions of the signals for the perfluoropropenyl carbons are shown in Fig. 5, while the corresponding signals for the minor product are shown in Fig. 6. For the major species, the carbon of the CF3 couples to the other fluorine with J = 24.3 Hz, whereas in the minor product the coupling between the carbon nucleus of the CF3 and the other F is 50.7 Hz, which indicates that the CF3 is separated from the F by fewer bonds in the minor species (E-isomer) compared with the major product (Z-isomer). Similarly, the coupling between the carbon directly bonded to the unique fluorine atom exhibits a larger quartet coupling in E-isomer (39.5 Hz) than in Z-isomer (6.6 Hz).
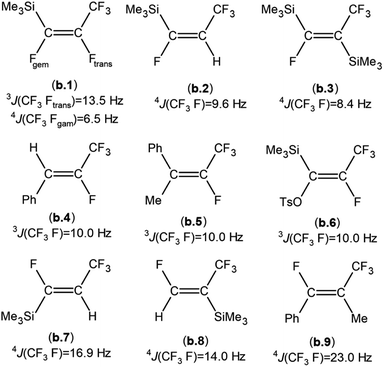 | ||
| Fig. 4 Some examples of published compounds formally derived from substitution of a fluorine atom from a sp2 hybridised carbon atom of a perfluoropropenyl group: (b.1),1 (b.2),11 (b.3),11 (b.4),5 (b.5),6 (b.6),8 (b.7),11 (b.8),11 (b.9),5 (TsO = CH3C6H4SO2). | ||
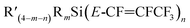 and nucleophilic sources with product ratio (given as %), and 19F{1H}, 29Si{1H} NMR data (CDCl3)
and nucleophilic sources with product ratio (given as %), and 19F{1H}, 29Si{1H} NMR data (CDCl3)
| Reactant 1 | Reactant 2 | Ratio | Result | δ CF3 | δ F | δ Si |
|---|---|---|---|---|---|---|
Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) CFCF3) (a.1) |
nBuLi | 88 | Et3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3) (12Z) CnBuCF3) (12Z) |
−60.28 ppm, d, 4J = 8.6 Hz | −99.31 ppm, q, 4J = 8.2 Hz | 6.20 ppm, d, 2J = 31.2 Hz |
| 12 | Et3Si(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (12E) CFCF3) (12E) |
−67.34 ppm, d, 3J = 10.6 Hz | −106.75 ppm, 3J = 10.9 Hz | 4.96 ppm, d, 3J = 9.4 Hz | ||
| tBuLi | 100 | Et3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3) (13Z) CtBuCF3) (13Z) |
−55.85 ppm, d, 4J = 7.4 Hz | −87.06 ppm, q, 4J = 7.1 Hz | 8.70 ppm, d, 2J = 32.8 Hz | |
| MeLi | 79 | Et3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3) (14Z) CMeCF3) (14Z) |
−62.50 ppm, d, 4J = 8.6 Hz | −98.47 ppm, q, 4J = 8.7 Hz | 6.15 ppm, d, 2J = 30.7 Hz | |
| 21 | Et3Si(E-CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (14E) CFCF3) (14E) |
−67.07 ppm, d, 3J = 10.3 Hz | −105.47 ppm, q, 3J = 10.3 Hz | 8.95 ppm, d, 3J = 6.8 Hz | ||
| PhLi | 77 | Et3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPhCF3) (15Z) CPhCF3) (15Z) |
−58.61 ppm, d, 4J = 9.0 Hz | −93.33 ppm, q, 4J = 9.2 Hz | 7.90 ppm, d, 2J = 30.3 Hz | |
| 23 | Et3Si(E-CPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (15E) CFCF3) (15E) |
−67.77 ppm, d, 3J = 10.6 Hz | −100.27 ppm, q, 3J = 10.5 Hz | 5.73 ppm, d, 3J = 5.7 Hz | ||
nBu3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)(a.2) CFCF3)(a.2) |
nBuLi | 88 | nBu3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3) (16Z) CnBuCF3) (16Z) |
−60.18 ppm, d, 4J = 8.4 Hz | −98.95 ppm, q, 4J = 8.5 Hz | 2.1 ppm, d, 2J = 31.7 Hz |
| 12 | nBu3Si(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (16E) CFCF3) (16E) |
−67.22 ppm, d, 3J = 10.6 Hz | −106.90 ppm, q, 3J = 10.9 Hz | 0.93 ppm, d, 3J = 9.3 Hz | ||
| tBuLi | 100 | nBu3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3) (17Z) CtBuCF3) (17Z) |
−55.55 ppm, d, 4J = 7.1 Hz | −86.66 ppm, q, 3J = 7.2 Hz | 2.15 ppm, d, 2J = 31.1 Hz | |
| MeLi | 83 | nBu3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3) (18Z) CMeCF3) (18Z) |
−62.42 ppm, d, 4J = 8.4 Hz | −98.11 ppm, q, 4J = 8.5 Hz | 2.0 ppm, d, 2J = 31.0 Hz | |
| 17 | nBu3Si(E-CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (18E) CFCF3) (18E) |
−67.06 ppm, d, 3J = 10.6 Hz | −105.64 ppm, q, 3J = 10.5 Hz | 0.90 ppm, d, 3J = 8.7 Hz | ||
| PhLi | 69 | nBu3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPhCF3) (19Z) CPhCF3) (19Z) |
−58.51 ppm, d, 4J = 9.1 Hz | −93.02 ppm, q, 4J = 9.0 Hz | 3.71 ppm, d, 2J = 30.6 Hz | |
| 31 | nBu3Si(E-CPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (19E) CFCF3) (19E) |
−67.67 ppm, d, 3J = 10.8 Hz | −100.34 ppm, q, 3J = 10.8 Hz | −0.01 ppm, d, 3J = 5.4 Hz | ||
nBuMe2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.4) CFCF3) (a.4) |
nBuLi | 57 | nBuMe2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3) (20Z) CnBuCF3) (20Z) |
−59.74 ppm, d, 4J = 8.5 Hz | −100.36 ppm, q, 4J = 8.6 Hz | 3.71 ppm, d, 2J = 30.6 Hz |
| 43 | nBuMe2Si(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (20E) CFCF3) (20E) |
−66.88 ppm, d, 3J = 10.5 Hz | −108.49 ppm, q, 3J = 10.4 Hz | −0.01 ppm, d, 3J = 5.4 Hz | ||
| tBuLi | 100 | nBuMe2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3) (21Z) CtBuCF3) (21Z) |
−55.20 ppm, d, 4J = 7.3 Hz | −88.24 ppm, q, 4J = 7.1 Hz | −1.85 ppm, d, 2J = 37.2 Hz | |
| MeLi | 40 | nBuMe2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3) (22Z) CMeCF3) (22Z) |
−61.59 ppm, d, 4J = 8.3 Hz | −99.39 ppm, q, 4J = 8.5 Hz | −21.95 ppm, d, 2J = 37.0 Hz | |
| 60 | nBuMe2Si(E-CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (22E) CFCF3) (22E) |
−66.65 ppm, d, 3J = 10.2 Hz | −107.12 ppm, q, 3J = 10.5 Hz | 7.25 ppm, d, 3J = 5.6 Hz | ||
Me2PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.5) CFCF3) (a.5) |
nBuLi | 55 | Me2PhSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3) (23Z) CnBuCF3) (23Z) |
−59.38 ppm, d, 4J = 8.2 Hz | −99.54 ppm, q, 4J = 8.6 Hz | −7.7 ppm, d, 2J = 37.8 Hz |
| 45 | Me2PhSi(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (23E) CFCF3) (23E) |
−66.48 ppm, d, 3J = 10.0 Hz | −107.00 ppm, q, 3J = 10.2 Hz | −6.22 ppm, d, 3J = 11.5 Hz | ||
| tBuLi | 62 | Me2PhSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3) (24Z) CtBuCF3) (24Z) |
−59.17 ppm, d, 4J = 8.2 Hz | −99.55 ppm, q, 4J = 9.0 Hz | −2.45 ppm, d, 2J = 38.0 Hz | |
| 38 | Me2PhSi(E-CtBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (24E) CFCF3) (24E) |
−66.27 ppm, d, 3J = 10.3 Hz | −106.72 ppm, q, 3J = 10.9 Hz | −1.14 ppm, d, 3J = 6.6 Hz | ||
| MeLi | 72 | Me2PhSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3) (25Z) CMeCF3) (25Z) |
−60.17 ppm, d, 4J = 8.2 Hz | −98.95 ppm, q, 4J = 8.7 Hz | 6.10 ppm, d, 2J = 37.4 Hz | |
| 28 | Me2PhSi(E-CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (25E) CFCF3) (25E) |
−67.20 ppm, d, 3J = 10.5 Hz | −106.91 ppm, q, 3J = 10.9 Hz | 1.88 ppm, d, 3J = 5.6 Hz | ||
Ph2MeSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.6) CFCF3) (a.6) |
nBuLi | 70 | Ph2MeSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3) (26Z) CnBuCF3) (26Z) |
−59.31 ppm, d, 4J = 8.3 Hz | −97.03 ppm, q, 4J = 8.5 Hz | −12.60 ppm, d, 2J = 38.0 Hz |
| 30 | Ph2MeSi(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (26E) CFCF3) (26E) |
−66.65 ppm, d, 3J = 10.4 Hz | −105.47 ppm, q, 3J = 10.3 Hz | −9.34 ppm, d, 3J = 12.9 Hz | ||
| tBuLi | 100 | Ph2MeSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3) (27Z) CtBuCF3) (27Z) |
−54.77 ppm, d, 4J = 6.7 Hz | −84.52 ppm, q, 4J = 6.7 Hz | −10.03 ppm, d, 2J = 37.5 Hz | |
| MeLi | 52 | Ph2MeSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3) (28Z) CMeCF3) (28Z) |
−61.46 ppm, d, 4J = 7.9 Hz | −96.13 ppm, q, 4J = 7.7 Hz | −21.91 ppm, d, 2J = 37.3 Hz | |
| 48 | Ph2MeSi(E-CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (28E) CFCF3) (28E) |
−66.37 ppm, d, 3J = 10.4 Hz | −103.41 ppm, q, 3J = 10.7 Hz | −10.99 ppm, d, 3J = 5.3 Hz | ||
| PhLi | 100 | Ph2MeSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPhCF3) (29Z) CPhCF3) (29Z) |
−61.13 ppm, d, 4J = 9.6 Hz | −79.91 ppm, q, 4J = 9.5 Hz | −22.29 ppm, d, 2J = 36.3 Hz | |
Me2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7) CFCF3)2 (a.7) |
nBuLi | 50 | Me2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3)2 (30Z) CnBuCF3)2 (30Z) |
−59.71 ppm, d, 4J = 8.4 Hz | −100.32 ppm, q, 3J = 8.5 Hz | −21.93 ppm, m |
| 50 | Me2Si(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (30E) CFCF3)2 (30E) |
−66.79 ppm, d, 3J = 10.5 Hz | −108.51 ppm, q, 4J = 10.0 Hz | −0.57 ppm, m | ||
| tBuLi | 100 | Me2Si(Z-CtBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (31Z) CFCF3)2 (31Z) |
−55.18 ppm, d, 4J = 7.2 Hz | −88.23 ppm, q, 4J = 7.5 Hz | 1.80 ppm, m | |
| MeLi | 40 | Me2Si(Z-CMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (32Z) CFCF3)2 (32Z) |
−62.05 ppm, d, 4J = 8.6 Hz | −99.44 ppm, q, 4J = 8.5 Hz | −22.76 ppm, m | |
| 60 | Me2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3)2 (32E) CMeCF3)2 (32E) |
−66.75 ppm, d, 3J = 10.3 Hz | −107.19 ppm, q, 3J = 10.4 Hz | −21.94 ppm, m | ||
iPr2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) CFCF3)2 (a.8) |
nBuLi | 100 | iPr2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3)2 (33Z) CnBuCF3)2 (33Z) |
−61.98 ppm, d, 4J = 8.6 Hz | −101.26 ppm, q, 4J = 8.4 Hz | −3.27 ppm, m |
| tBuLi | 100 | iPr2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3)2 (34Z) CtBuCF3)2 (34Z) |
−56.61 ppm, d, 4J = 6.5 Hz | −89.0 ppm, q, 4J = 5.9 Hz | −12.80 ppm, m | |
| MeLi | 100 | iPr2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3)2 (35Z) CMeCF3)2 (35Z) |
−67.53 ppm, m | −139.77 ppm, m | −13.16 ppm, m | |
Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) CFCF3)2 (a.9) |
nBuLi | 77 | Ph2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3)2 (36Z) CnBuCF3)2 (36Z) |
−59.93 ppm, d, 4J = 7.7 Hz | −97.61 ppm, q, 4J = 8.0 Hz | −11.95 ppm, m |
| 23 | Ph2Si(E-CnBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (36E) CFCF3)2 (36E) |
−67.02 ppm, d, 3J = 10.5 Hz | −105.09 ppm, q, 3J = 10.8 Hz | −9.25 ppm, m | ||
| PhLi | 100 | Ph2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPhCF3)2 (37Z) CPhCF3)2 (37Z) |
−57.84 ppm, d, 4J = 8.4 Hz | −90.35 ppm, q, 4J = 8.8 Hz | −21.80 ppm, m |
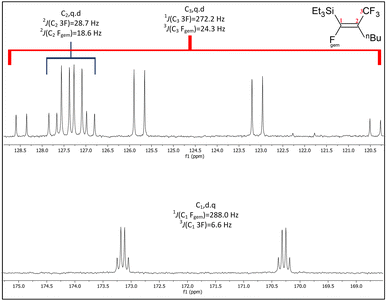 | ||
Fig. 5 Expansions of C1 to C3 signals in the 13C{1H} NMR spectrum for the major product from the reaction of Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) with nBuLi, (100 MHz, CDCl3, 298 K). CFCF3) (a.1) with nBuLi, (100 MHz, CDCl3, 298 K). | ||
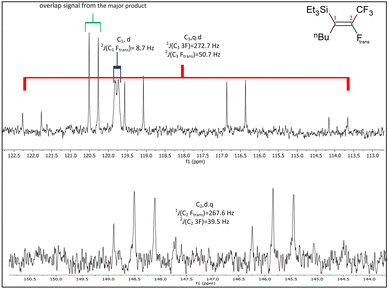 | ||
Fig. 6 Expansions of C1 to C3 signals in the 13C{1H} NMR spectrum for the minor product from the reaction of Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) with nBuLi, (100 MHz, CDCl3, 298 K). CFCF3) (a.1) with nBuLi, (100 MHz, CDCl3, 298 K). | ||
The 13C{1H} NMR spectrum for the single product formed in the reaction with tBuLi, shown in Fig. 7, is very similar to that observed for Z-isomer, both in terms of the J coupling and splitting patterns. This suggests that the only product formed when using tBuLi is (Et)3Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3) (13Z). According to the DFT study of Ph2Si(E-CF
CtBuCF3) (13Z). According to the DFT study of Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2, of the carbons in the perfluoropropenyl group C2 is energetically the most likely site for attack by the incoming nucleophile. Therefore, substitution of Ftrans is the most likely result of nucleophilic attack, while attack at C1 to give the Fgem substituted compound is less favoured. This is consistent with the observation of a small amount of (Et)3Si(E-C(nBu)
CFCF3)2, of the carbons in the perfluoropropenyl group C2 is energetically the most likely site for attack by the incoming nucleophile. Therefore, substitution of Ftrans is the most likely result of nucleophilic attack, while attack at C1 to give the Fgem substituted compound is less favoured. This is consistent with the observation of a small amount of (Et)3Si(E-C(nBu)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (13E). However, in case of bigger group such as tBu,10 the steric hindrance prevents any attack on C1.
CFCF3) (13E). However, in case of bigger group such as tBu,10 the steric hindrance prevents any attack on C1.
 | ||
Fig. 7 Expansions of C1 to C3 signals in the 13C{1H} NMR spectrum for the product from the reaction of Et3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) with tBuLi, (100 MHz, CDCl3, 298 K). CFCF3) (a.1) with tBuLi, (100 MHz, CDCl3, 298 K). | ||
In Table 6, a summary of all successful attempts to substitute one fluorine atom with an organic group, by reaction with organolithium compounds is listed. PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)3 (a.10) and Si(E-CF
CFCF3)3 (a.10) and Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)4 (a.11) were found not to result in substitution and therefore characterization was not possible. In these cases treatment with nBuLi and tBuLi resulted in 19F{1H} NMR spectra that showed a number of signals in the CF3 area, which were no longer present after work up of the reaction. Reactions with MeLi and PhLi resulted in 19F{1H} NMR spectra which showed that there was no reaction. The reason that these two substrates reacted differently could be based on steric or electronic factors, or both. For example the large size of the tBu group could affect the ability to add to the presumably already sterically crowded PhSi(E-CF
CFCF3)4 (a.11) were found not to result in substitution and therefore characterization was not possible. In these cases treatment with nBuLi and tBuLi resulted in 19F{1H} NMR spectra that showed a number of signals in the CF3 area, which were no longer present after work up of the reaction. Reactions with MeLi and PhLi resulted in 19F{1H} NMR spectra which showed that there was no reaction. The reason that these two substrates reacted differently could be based on steric or electronic factors, or both. For example the large size of the tBu group could affect the ability to add to the presumably already sterically crowded PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)3 (a.10) and Si(E-CF
CFCF3)3 (a.10) and Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)4 (a.11) molecules, while MeLi and PhLi are considered to be less reactive compared to nBuLi and tBuLi, consistent with DFT calculations. Moreover, the presence of a large number of sterically demanding perfluoropropenyl groups attached to the silicon centre make these substitution reactions less likely, instead allowing for alternative reactions, that result in breaking the Si perfluoropropenyl bond and producing small volatile fluorocarbon molecules which could be easily removed on work up.
CFCF3)4 (a.11) molecules, while MeLi and PhLi are considered to be less reactive compared to nBuLi and tBuLi, consistent with DFT calculations. Moreover, the presence of a large number of sterically demanding perfluoropropenyl groups attached to the silicon centre make these substitution reactions less likely, instead allowing for alternative reactions, that result in breaking the Si perfluoropropenyl bond and producing small volatile fluorocarbon molecules which could be easily removed on work up.
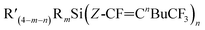 and
and 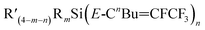 in varying proportions. Most substrates resulted in the major products being the Z-isomers, such as was the case for (Et)3Si(E-CF
in varying proportions. Most substrates resulted in the major products being the Z-isomers, such as was the case for (Et)3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1), (nBu)3Si(E-CF
CFCF3) (a.1), (nBu)3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.2), (Ph)2MeSi(E-CF
CFCF3) (a.2), (Ph)2MeSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.6) and (Ph)2Si(E-CF
CFCF3) (a.6) and (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9). However, for nBu(Me)2Si(E-CF
CFCF3)2 (a.9). However, for nBu(Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.4) and (Me)2Si(E-CF
CFCF3) (a.4) and (Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7) similar proportions of the E- and Z-isomeric products were formed. By contrast, (iPr)2Si(E-CF
CFCF3)2 (a.7) similar proportions of the E- and Z-isomeric products were formed. By contrast, (iPr)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) gave exclusively the Z isomer (iPr)2Si(Z-CF
CFCF3)2 (a.8) gave exclusively the Z isomer (iPr)2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3)2 (33Z). This variance in the proportion of E and Z isomers formed could be due to steric hindrance due to the sizes of the R groups attached to Si. For example, in the case of the most sterically demanding substituent, iPr, it was found that (iPr)2Si(E-CF
CnBuCF3)2 (33Z). This variance in the proportion of E and Z isomers formed could be due to steric hindrance due to the sizes of the R groups attached to Si. For example, in the case of the most sterically demanding substituent, iPr, it was found that (iPr)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) gave only the isomer (iPr)2Si(Z-CF
CFCF3)2 (a.8) gave only the isomer (iPr)2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CnBuCF3)2 (33Z). As the groups become smaller the proportion of Z isomer decreases and the E-isomeric product increases. For example: (iPr)2Si(E-CF
CnBuCF3)2 (33Z). As the groups become smaller the proportion of Z isomer decreases and the E-isomeric product increases. For example: (iPr)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) gave 100% of the Z-isomeric product, (Ph)2Si(E-CF
CFCF3)2 (a.8) gave 100% of the Z-isomeric product, (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) gave 77% Z-isomer and (Me)2Si(E-CF
CFCF3)2 (a.9) gave 77% Z-isomer and (Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7) 50% Z-isomer.
CFCF3)2 (a.7) 50% Z-isomer.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1), (nBu)3Si(E-CF
CFCF3) (a.1), (nBu)3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.2), (nBu)Me2Si(E-CF
CFCF3) (a.2), (nBu)Me2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.4) (Ph)2MeSi(E-CF
CFCF3) (a.4) (Ph)2MeSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.6), (Me)2Si(E-CF
CFCF3) (a.6), (Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7), and (iPr)2Si(E-CF
CFCF3)2 (a.7), and (iPr)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) all reactions resulted in exclusive formation of the Z-isomeric product. However, based on the NMR data reaction with (Me)2PhSi(E-CF
CFCF3)2 (a.8) all reactions resulted in exclusive formation of the Z-isomeric product. However, based on the NMR data reaction with (Me)2PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.5) gave a mixture of both (Me)2PhSi(Z-CF
CFCF3) (a.5) gave a mixture of both (Me)2PhSi(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBuCF3) (24Z) and (Me)2PhSi(E-CtBu
CtBuCF3) (24Z) and (Me)2PhSi(E-CtBu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (24E) in the ratio 62
CFCF3) (24E) in the ratio 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38. In the case of the reaction of tBuLi with (Ph)2Si(E-CF
38. In the case of the reaction of tBuLi with (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) the 19F{1H} NMR data suggested fragmentation, due to the observation of many signals around the CF3 region in the 19F{1H} NMR spectrum of the crude reaction sample. However, these signals disappeared after the reaction had been worked up and it is suggested that they are therefore small volatile fluorocarbon species.
CFCF3)2 (a.9) the 19F{1H} NMR data suggested fragmentation, due to the observation of many signals around the CF3 region in the 19F{1H} NMR spectrum of the crude reaction sample. However, these signals disappeared after the reaction had been worked up and it is suggested that they are therefore small volatile fluorocarbon species.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) and (nBu)3Si(E-CF
CFCF3) (a.1) and (nBu)3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.2) gave a mixture of both products with a high proportion of Z-isomers. Smaller differences in the proportions of E- and Z-isomers were found in the mixtures that came from reacting MeLi with nBu(Me)2Si(E-CF
CFCF3) (a.2) gave a mixture of both products with a high proportion of Z-isomers. Smaller differences in the proportions of E- and Z-isomers were found in the mixtures that came from reacting MeLi with nBu(Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.4), (Ph)2MeSi(E-CF
CFCF3) (a.4), (Ph)2MeSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.6) and (Me)2Si(E-CF
CFCF3) (a.6) and (Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7). However, by analysis of the 19F{1H} NMR spectrum, only for (iPr)2Si(E-CF
CFCF3)2 (a.7). However, by analysis of the 19F{1H} NMR spectrum, only for (iPr)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) was 100% of the Z product obtained, (iPr)2Si(Z-CF
CFCF3)2 (a.8) was 100% of the Z product obtained, (iPr)2Si(Z-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMeCF3)2 (25Z). Finally, (Ph)2Si(E-CF
CMeCF3)2 (25Z). Finally, (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) did not show any reaction, even after extending the reaction time to 5 days and the amount of MeLi added has been increased.
CFCF3)2 (a.9) did not show any reaction, even after extending the reaction time to 5 days and the amount of MeLi added has been increased.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.1) and (nBu)3Si(E-CF
CFCF3) (a.1) and (nBu)3Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.2) gave a mixture of the two isomeric products, with a high proportion of the Z-isomers. The reactions with (Ph)2MeSi(E-CF
CFCF3) (a.2) gave a mixture of the two isomeric products, with a high proportion of the Z-isomers. The reactions with (Ph)2MeSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.6) and (Ph)2Si(E-CF
CFCF3) (a.6) and (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) gave the single Z-isomer exclusively. Unfortunately, the substitution of F with the Ph group was unsuccessful for nBu(Me)2Si(E-CF
CFCF3)2 (a.9) gave the single Z-isomer exclusively. Unfortunately, the substitution of F with the Ph group was unsuccessful for nBu(Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.4), (Me)2PhSi(E-CF
CFCF3) (a.4), (Me)2PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3) (a.5), (Me)2Si(E-CF
CFCF3) (a.5), (Me)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7) and (iPr)2Si(E-CF
CFCF3)2 (a.7) and (iPr)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.8) according to multinuclear NMR spectroscopy.
CFCF3)2 (a.8) according to multinuclear NMR spectroscopy.Experimental
Materials and methods
All reagents and solvents were purchased from Sigma-Aldrich (purity 97–98%) and used without purification. Non-chlorinated solvents were dried over sodium wire for at least 24 h prior to use. Z-HFC-1225ye was kindly donated by Mexichem Fluor. NMR spectra were recorded at 20 °C on a Bruker Avance III 400 MHz spectrometer operating at 400.00, 100.61, 376.46, and 79 MHz for 1H, 13C, 19F, and 29Si respectively using CDCl3 as solvent. Chemical shift values are quoted relative to TMS and CFCl3 in parts per million (ppm) on the δ scale and coupling constant (J) values are reported in Hz. Elemental analysis was conducted by the University of Manchester's School of Chemistry Micro-Analytical service. Single crystal was grown by slow evaporation of a chloroform solution and X-ray structures were obtained using SuperNova diffractometers using Mo Kα radiation (λ = 0.71073 Å). All the raw data frames were reduced and corrections were applied for Lorentz, polarisation and absorption using the multi-scan methods with CrysAlisPro.12Computational methods
The X-ray structural data were solved by direct methods, with full-matrix least-squares refinement of F2 using: Olex2,13 Shelx14 and Shelxtl15 programs. Mercury16 was used to generate the graphical representations. The geometry of Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) was optimised using hybrid Density Functional Theory (DFT) at the B3LYP/6-31G(d,p) level;17,18 using the GAMESS software19 to calculate the bond lengths of (Ph)2Si(E-CF
CFCF3)2 (a.9) was optimised using hybrid Density Functional Theory (DFT) at the B3LYP/6-31G(d,p) level;17,18 using the GAMESS software19 to calculate the bond lengths of (Ph)2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) in gas phase and the Mulliken charges for Me2Si(E-CF
CFCF3)2 (a.9) in gas phase and the Mulliken charges for Me2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.7), Ph2Si(E-CF
CFCF3)2 (a.7), Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9), PhSi(E-CF
CFCF3)2 (a.9), PhSi(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)3 (a.10), and Si(E-CF
CFCF3)3 (a.10), and Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)4 (a.11). The electronic structure of (a.1, a.2, a.4, a.5, a.6, a.7, a.8, a.9) compounds and reaction energetics for nucleophilic attack was obtained with B3-LYP/TZVPP20 using TURBOMOLE V7.3 2018 suite of quantum chemical programs.21
CFCF3)4 (a.11). The electronic structure of (a.1, a.2, a.4, a.5, a.6, a.7, a.8, a.9) compounds and reaction energetics for nucleophilic attack was obtained with B3-LYP/TZVPP20 using TURBOMOLE V7.3 2018 suite of quantum chemical programs.21
Synthesis of silicon–perfluoropropenyl compounds 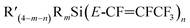
Was prepared with same procedure described in10 but on different scales (see ESI, Scheme S1†). In a three-necked round-bottom flask under a positive pressure of nitrogen cooled to between −75 to −80 °C were placed dry diethyl ether (150 mL) and one equivalent of Z-HFC-1225ye. One equivalent of nBuLi (2.5 M solution in hexanes) was added slowly so as to maintain the temperature below −78 °C. The solution was stirred for 1 h to ensure formation of perfluoropropenyl lithium. In the next step, a solution of the appropriate silicon-halide was added slowly. The mixture was left to stir and warm slowly to room temperature overnight. Hexane (25 mL) was added to the reaction mixture and the resulting solution was filtered through a pad of Celite, and solvent was removed using a rotary evaporator.
Reactions between silicon–perfluoropropenyl compounds 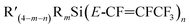 and nucleophilic sources
and nucleophilic sources
A solution of dry THF (150 mL) and 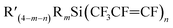 was placed in a three-necked round-bottom flask under a positive pressure of nitrogen. The solution was cooled to −30 °C, and then RLi (solution in hexanes) was added slowly. The mixture was slowly warmed to room temperature and left stirring for 24 hours. The reaction was worked up with hexanes (10 mL), followed by filtration through Celite, and solvent was removed using a rotary evaporator (see ESI, Scheme S2†).
was placed in a three-necked round-bottom flask under a positive pressure of nitrogen. The solution was cooled to −30 °C, and then RLi (solution in hexanes) was added slowly. The mixture was slowly warmed to room temperature and left stirring for 24 hours. The reaction was worked up with hexanes (10 mL), followed by filtration through Celite, and solvent was removed using a rotary evaporator (see ESI, Scheme S2†).
Conclusions
Derived from (HFC-1225 ye), eleven new and stable silicon–perfluoropropenyl compounds have been successfully prepared, and fully characterised by multinuclear NMR spectroscopy. The compounds formed are generally liquids at room temperature, except Ph2Si(E-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF3)2 (a.9) which was solid and structural confirmation was obtained by X-ray diffraction studies.
CFCF3)2 (a.9) which was solid and structural confirmation was obtained by X-ray diffraction studies.
The investigation of silicon–perfluoropropenyl compounds was extended to study the substitution reactions using a wide range of organolithium nucleophilic sources: nBuLi, tBuLi, MeLi, and PhLi, leading to twenty-six new compounds. Two types of products were identified: one where carbolithiation had occurred at C1 and one at C2, leading to two isomers of the 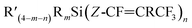 and
and 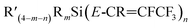 formula, respectively. The outcomes of these reactions were rationalised based on steric arguments. Bulky groups around the silicon centre or in the incoming nucleophile (e.g. nBuLi vs. tBuLi) led to a greater proportion of the Z-isomer. Due to uneven charges on the carbons of the pentafluoropropene group, where C2 attached to Ftrans has higher positive charge than C1 attached to Fgem, the nucleophilic attack preferred C2–Ftrans to generate Z-isomer. The calculated reaction energetics between silicon–perfluoropropenyl compounds and organolithium reagents, confirmed that the Z-isomer is energetically more favoured product.
formula, respectively. The outcomes of these reactions were rationalised based on steric arguments. Bulky groups around the silicon centre or in the incoming nucleophile (e.g. nBuLi vs. tBuLi) led to a greater proportion of the Z-isomer. Due to uneven charges on the carbons of the pentafluoropropene group, where C2 attached to Ftrans has higher positive charge than C1 attached to Fgem, the nucleophilic attack preferred C2–Ftrans to generate Z-isomer. The calculated reaction energetics between silicon–perfluoropropenyl compounds and organolithium reagents, confirmed that the Z-isomer is energetically more favoured product.
Author contributions
L. Alluhaibi: conceptualization, investigation, methodology, writing – original draft, and writing – review & editing; A. Brisdon: supervision; S. Klejna: investigation, visualization, writing – review & editing; A. Muneer: formal analysis.Conflicts of interest
The authors declare there are no conflicts of interest.Acknowledgements
This research was partly supported by program “Excellence Initiative-Research University” for the AGH University of Science and Technology. L. Alluhaibi would like to acknowledge the financial support of the Saudi Arabian Government (King Abdullah Scholarship Program). S. Klejna was supported by the National Science Centre Poland under grant agreement UMO-2019/35/D/ST5/02964. S. Klejna acknowledge PLGrid and Cyfronet for providing computer facilities and support.References
- R. B. Larichev, V. A. Petrov, G. J. Grier, M. J. Nappa, W. J. Marshall, A. A. Marchione and R. J. Dooley, Org. Process Res. Dev., 2014, 18, 1060–1066 CrossRef CAS.
- P. J. Morgan, G. C. Saunders, S. A. Macgregor, A. C. Marr and P. Licence, Organometallics, 2022, 41, 883–891 CrossRef CAS PubMed.
- H. J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander and M. Stahl, ChemBioChem, 2004, 5, 637–643 CrossRef PubMed.
- P. J. Morgan, M. W. D. Hanson-Heine, H. P. Thomas, G. C. Saunders, A. C. Marr and P. Licence, Organometallics, 2020, 39, 2116–2124 CrossRef CAS.
- T. A. Gray, W. R. Dolbier and K. Onnishi, Synthesis, 1987, 10, 956–958 Search PubMed.
- W. Dmowski, J. Fluorine Chem., 1984, 26, 223–241 CrossRef CAS.
- D. O'Hagan, J. Fluorine Chem., 2010, 131, 1071–1081 CrossRef.
- K. Funabiki, T. Ohtsuki, T. Ishihara and H. Yamanaka, J. Chem. Soc., Perkin Trans. 1, 1998, 2413–2423 RSC.
- A. K. Brisdon, R. G. Pritchard and A. Thomas, Organometallics, 2012, 31, 1341–1348 CrossRef CAS.
- L. M. Alluhaibi, A. K. Brisdon and R. G. Pritchard, J. Fluorine Chem., 2017, 203, 146–154 CrossRef CAS.
- R. N. Haszeldine, C. R. Pool and A. E. Tipping, J. Chem. Soc., Perkin Trans. 1, 1974, 2293–2296 RSC.
- P. W. Betteridge, J. R. Carruthers, R. I. Cooper, K. Prout and D. J. Watkin, J. Appl. Crystallogr., 2003, 36, 1487 CrossRef CAS.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- T. R. Schneider and G. M. Sheldrick, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2002, 58, 1772–1779 CrossRef PubMed.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeersch, E. Zurek and G. R. Hutchison, J. Cheminf., 2012, 4, 17 CAS.
- A. Schäfer, H. Horn and R. Ahlrichs, J. Chem. Phys., 1992, 97, 2571–2577 CrossRef.
- R. Ahlrichs, M. Bär, M. Häser, H. Horn and C. Kölmel, Chem. Phys. Lett., 1989, 162, 165–169 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2240893. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra01353g |
| This journal is © The Royal Society of Chemistry 2023 |