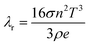Open Access Article
Open Access ArticleUltralight elastic Al2O3 nanorod-graphene aerogel for pressure sensing and thermal superinsulation†
Fengqi Liu,
Yonggang Jiang *,
Junzong Feng,
Liangjun Li and
Jian Feng
*,
Junzong Feng,
Liangjun Li and
Jian Feng *
*
Science and Technology on Advanced Ceramic Fibers and Composites Laboratory, College of Aerospace Science and Technology, National University of Defense Technology, Changsha 410073, P. R. China. E-mail: jygemail@nudt.edu.cn; fengj@nudt.edu.cn
First published on 18th May 2023
Abstract
Novel nanorod aerogels have gained tremendous attention owing to their unique structure. However, the intrinsic brittleness of ceramics still severely limits their further functionalization and application. Here, based on the self-assembly between one-dimensional (1D) Al2O3 nanorods and two-dimensional (2D) graphene sheets, lamellar binary Al2O3 nanorod-graphene aerogels (ANGAs) were prepared by the bidirectional freeze-drying technique. Thanks to the synergistic effect of rigid Al2O3 nanorods and high specific extinction coefficient elastic graphene, the ANGAs not only exhibit robust structure and variable resistance under pressure, but also possess superior thermal insulation properties compared to pure Al2O3 nanorod aerogels. Therefore, a series of fascinating features such as ultra-low density (3.13–8.26 mg cm−3), enhanced compressive strength (6 times higher than graphene aerogel), excellent pressure sensing durability (500 cycles at 40% strain) and ultra-low thermal conductivity (0.0196 W m−1 K−1 at 25 °C and 0.0702 W m−1 K−1 at 1000 °C) are integrated in ANGAs. The present work provides fresh insight into the fabrication of ultralight thermal superinsulating aerogels and the functionalization of ceramic aerogels.
1. Introduction
Among the wide range of aerogels, ceramic aerogels distinguish themselves by their low density, high porosity and excellent heat resistance, and demonstrate great potential for applications in the fields of thermal insulation, catalysis, adsorption, etc.1–3 However, their further practical applications are severely limited by their intrinsic brittleness due to the point-to-point contact between the particle building units. The addition of strengthening phases, such as fibers,4,5 whiskers6,7 or polymers,8,9 can improve the mechanical properties of ceramic aerogels, but brings a significant increase in density (over 0.2 g cm−3), and the addition of polymers sacrifices the temperature resistance of ceramic aerogels. To overcome these problems, flexible ceramic fibers are used as basic assembly units for building fiber aerogels with excellent elasticity.10–12 However, the thermal conductivity of the fiber aerogel increases significantly at high temperatures because the macroporous structure of the fibrous skeleton is not conducive to suppressing the radiative heat transfer at elevated temperatures.13,14Different from nanoparticle or nanofiber microstructures, the emerging nanorod aerogels stand out with its unique structure and excellent properties.15 Nanorods with high aspect ratio not only can form a continuous microstructure instead of inefficient connections between particles, but also can introduce nanoscale pores that facilitate thermal insulation. So far, there are few reports on novel nanorod aerogels, and the only reports are focused on Al2O3 nanorod aerogels (ANAs). Zhang et al.16 prepared low-density (0.146 g cm−3) Si-doped Al2O3 nanorod aerogels with a compressive strength of 1.50 MPa and a thermal conductivity of 0.089 W m−1 K−1 at 1200 °C. Our previous work developed carbon-coated Al2O3 nanorod aerogels with densities as low as 0.086 g cm−3, which exhibited enhanced compressive strength (2.98 MPa) and thermal insulation properties (0.065 W m−1 K−1 at 1200 °C).15 However, the intrinsic brittleness of the Al2O3 nanorod aerogels were not fundamentally improved by either the fiber-reinforced or the carbon-coated strategy,17 and brittle fracture still occurred at large strains, which is not favorable for thermal insulation applications in structures with variable dimensions. Therefore, the fabrication of elastic Al2O3 nanorod aerogels remains a great challenge.
Graphene, as an advanced 2D material with high mechanical strength, large specific surface area and excellent electrical conductivity, is considered an attractive candidate for the construction of multifunctional three-dimensional (3D) aerogels.18,19 Although graphene has high thermal conductivity within layers,20 directionally aligned graphene sheet layers can effectively block the heat transfer between layers and thus obtained graphene aerogels (GAs) can exhibit low thermal conductivity.21 In addition, graphene, which has excellent electrical conductivity, is also an ideal material for sensor applications.22 Nevertheless, pure graphene aerogels generally exhibit poor mechanical properties and severe volume shrinkage due to the weak van der Waals interactions between the building blocks,23 so components such as carbon nanotubes,24 polymers25 and ceramics26 are incorporated to enhance the graphene backbone strength and promote efficient load transfer. The reinforced graphene aerogels then can acquire excellent mechanical properties with reasonable structural design, such as chemical vapor deposition (CVD),27 directional freezing28 and 3D printing.29 However, although the carbon nanotube-modified graphene aerogels feature high elasticity, the maximum compressive stretch and elastic modulus are not significantly improved due to the softness of the carbon skeleton, which results in a significant reduction in the detection range of pressure sensing. Hybridization of polymers with graphene can impart enhanced elasticity and compressive strength to the aerogels, but the obtained hybrid aerogels are difficult to apply for high temperature insulation due to the mismatch of heat resistance.30
In recent years, high temperature resistant ceramic component has been used to composite with graphene to obtain robust aerogels. Zhang et al.31 constructed hybrid aerogels with high compressive and strong mechanical properties by depositing Al2O3 nanolayers on graphene backbone, which can achieve strains up to 80% and a three-fold increase in maximum compressive strength compared to pure graphene aerogels. However, the high cost and relatively low efficiency of the atomic layer deposition (ALD) method limit its mass production. Xu et al.32 developed a versatile method for building elastic aerogels by combining graphene with ceramic fibers, but as mentioned above, the micron-sized holes introduced by the fibers have negative effects on the thermal insulation properties of the aerogels. Additionally, Yin et al.33 also attempted to mechanically incorporate oxide particles (TiO2, CeO2 and Fe2O3) into graphene, but the inevitable aggregation of particles leads to defect generation and performance degradation. Hence, new types of ceramic additives need to be investigated for the simple preparation of elastic ceramic-graphene aerogels for high temperature thermal insulation and pressure sensing.
Here, laminated binary Al2O3 nanorods-graphene aerogels (ANGAs) based on intermolecular interactions and bidirectional freeze-drying techniques were successfully prepared. The advantageous complementary effect between rigid Al2O3 nanorods and flexible graphene sheets confers the desired properties to ANGAs. As reinforcing phase, Al2O3 nanorods were tightly adsorbed onto graphene sheets by hydrogen bonding, resulting in substantially elevated compressive strength of ANGAs compared to graphene aerogels (GAs). The flexible graphene sheets are interconnected to form a robust elastic conductive backbone, which overcomes the brittleness of Al2O3 ceramics and enables ANGAs to exhibit excellent pressure sensing properties. More importantly, the graphene sheets with high specific extinction coefficient not only confine the motion of gas molecules within the layer gap, but also suppress the radiative heat transfer at high temperatures. Consequently, the obtained ANGAs exhibit thermal superinsulation properties over the full temperature range from low to high temperatures. The successful synthesis of ANGAs provides a new strategy for the preparation of multifunctional elastic ceramic-based aerogels.
2. Experiments and methods
2.1. Materials
Al2O3 nanorods were prepared based on previous work with a diameter of about 10–20 nm and a length of 500–1000 nm.15 Graphene oxide (GO) dispersion (10–50 μm in diameter) with a concentration of 5 mg mL−1 was purchased from Suzhou Tanfeng Graphene Technology Co., Ltd. Polyvinyl alcohol (PVA) (98–99% alcohol solubility, Mw = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were obtained from Macklin Co., Ltd. 184 Silicone Elastomer (PDMS) were bought from Dow Corning Co., Ltd. The ultrapure water machine in laboratory provided deionized water.
000) were obtained from Macklin Co., Ltd. 184 Silicone Elastomer (PDMS) were bought from Dow Corning Co., Ltd. The ultrapure water machine in laboratory provided deionized water.
2.2. Preparation of ANGAs, GAs and ANAs
A wedge-shaped PDMS with an angle of 30° was combined with a copper rod whose bottom was placed in liquid nitrogen to form a bidirectional freezing platform. A homogeneous mixture of 5 mL of graphene dispersion, 5 mL of PVA solution (0.1 wt%) and a certain amount of Al2O3 nanorod sols was obtained after stirring at 500 rpm for 1 h. The beaker with the mixed solution was placed on the bidirectional freezing platform, and the Al2O3 nanorod-graphene aerogels (ANGAs) were obtained after 48 h freeze-drying process and 2 h treatment in argon atmosphere at 800 °C. The mass ratio of Al2O3 sols to the mixed solution (graphene dispersion and PVA solution) was determined as the Al2O3 content, and the samples with 2%, 4%, 6%, 8% and 10% Al2O3 content were named as ANGA-2, ANGA-4, ANGA-6, ANGA-8 and ANGA-10, respectively. The graphene aerogels (GAs) were prepared by the same procedure as ANGAs, except that Al2O3 nanorod sols are not added. Pure Al2O3 nanorod aerogels (ANAs) were prepared based on sample ANGA-10, except that the graphene dispersion was replaced by an equal amount of water.2.3. Construction and characterization of pressure sensors based on ANGAs
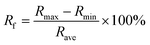
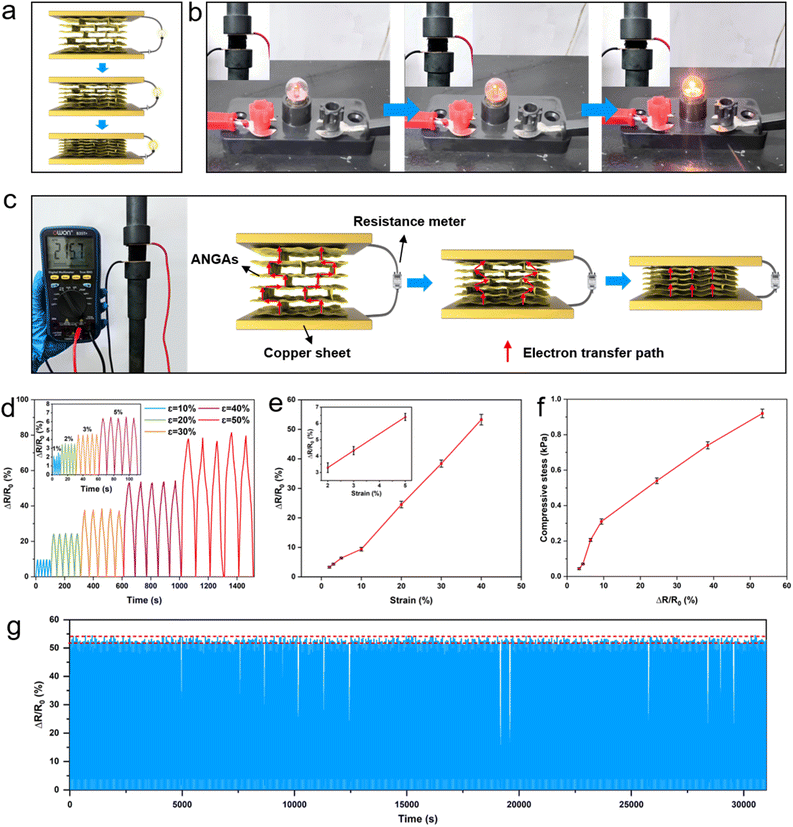
All pressure sensing tests were based on sample ANGA-8. Two copper sheets with dimensions of 5 × 5 × 0.1 cm are connected to the resistance meter (B35, Owon) through two wires, respectively. ANGAs of size 3 × 3 × 3 cm are placed between the copper sheets. The copper sheets were attached to the probe of the mechanical testing machine for programmed control of the strain of the sample with a loading/unloading speed of 20 mm min−1. The resistance of the sample in the initial state (0% strain) is defined as R0, and the resistance of the sample after compression is defined as Rt. The change in resistance (ΔR) is the difference between R0 and Rt. ΔR/R0 is defined as the sensor response. We define the sensor response fluctuation as:where Rf is the response fluctuation (%), Rmax is the maximum value of sensor response, Rmin is the is the minimum value of sensor response, and Rave is the is the average value of sensor response.
2.4. Characterization
A ZEISS scanning electron microscope (Sigma 300, Germany) was applied to obtain SEM images and EDS mappings. XRD spectra were recorded with an alpha diffractometer (Panalytical, The Netherlands) at Cu-Kα radiation (40 kV, 40 mA). FL4204GL universal testing machine (Fuller Test Technology) was used to evaluate the mechanical properties of the samples at a motion rate of 1 mm min−1. Thermogravimetric-differential scanning calorimetry (TG-DTA) was tested by a NETZSCH thermogravimetric analyzer (STA 449F3, Germany) (argon flow rate of 50 mL min−1 and heating rate of 10 °C min−1). Sample surface temperature changes were monitored by a M200A infrared camera (InfiRay). The room temperature thermal conductivity (air atmosphere) in the direction perpendicular to the laminate structure was tested by Hotdisk (TPS2000s, Switzerland). The high temperature thermal conductivity (argon atmosphere) of the sample was measured in an argon atmosphere by TPS2000s. Non-covalent interactions between graphene and Al2O3 nanorods were simulated by density functional theory (DFT) through the CP2K quantum chemistry package.3. Results and discussion
3.1. Fabrication and characterization of ANGAs
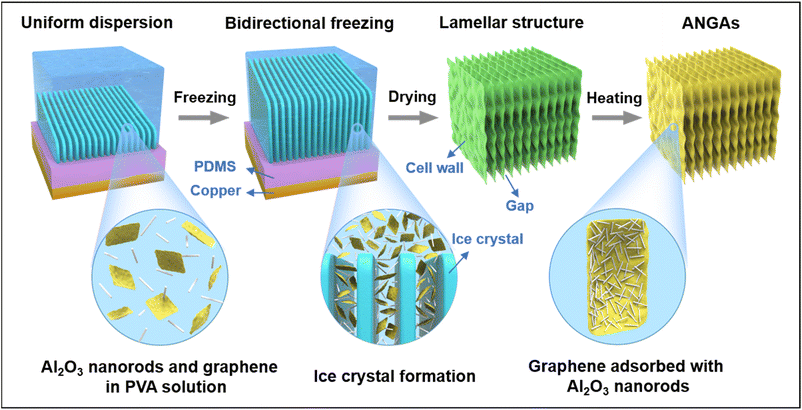
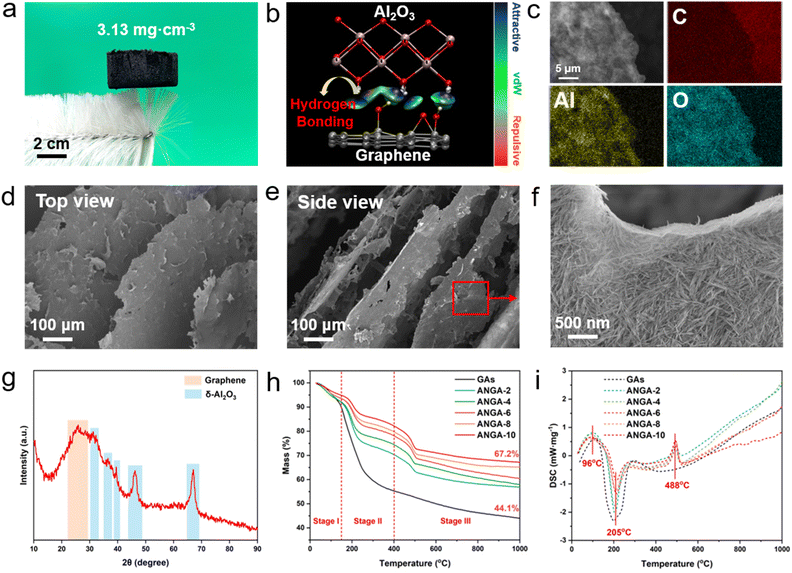
In order to obtain ANGAs with excellent thermal insulation and pressure sensing properties, the following principles need to be taken into account: (i) establishing interactions between the Al2O3 nanorods and the graphene sheets so that the two can be uniformly and tightly bounded, rather than unevenly dispersed by mechanical mixing; (ii) forming stable chemical crosslinks between graphene sheets for building a robust elastic backbone; (iii) orienting the graphene sheets to minimize layer-to-layer heat transfer, thus reducing the thermal conductivity.The preparation process of ANGAs is shown in Fig. 1a. After homogeneous dispersion of Al2O3 nanorods and graphene in polyvinyl alcohol (PVA) solution, elastic ANGAs with layer-like structure were obtained after bi-directional freezing, vacuum drying and high temperature annealing. By adjusting the content of Al2O3 nanorods, ultralight ANGAs with low densities ranging from 3.13–8.26 mg cm−3 could be obtained, which can be evidenced by samples standing stably on top of a brush (Fig. 2a). Benefiting from the abundant hydroxyl groups on the surface of Al2O3 nanorods as well as graphene oxide (GO), strong hydrogen bondings can be formed between the two, which is proved by the calculation of non-covalent bonded intermolecular interactions (Fig. 2b). The shifts of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Al–O peaks in the FTIR spectra after the combination of Al2O3 nanorods and GO also indicate the formation of hydrogen bondings (Fig. S1†). In addition, as shown in the mapping image (Fig. 2c), the C, Al and O elements are homogeneously dispersed on the graphene sheets. This is because the Al2O3 can be trapped by the target sites (–OH) on the graphene surface under the induction of hydrogen bonding, and then uniformly distributed on the graphene sheet surface to self-assemble into nanoceramic-enhanced graphene heterostructures. While PVA acts as a chemical cross-linking agent to firmly weld the graphene structural units together after annealing, thus forming a stable skeleton.34,35 To obtain the desired anisotropic graphene structure, the bidirectional freezing technique was employed for the preparation of ANGAs. The temperature gradient from the bidirectional freezing platform in two directions makes the ice crystals grow in a lamellar pattern and the graphene sheets adsorbed with Al2O3 nanorods are extruded into the ice crystal interstices. As shown in Fig. 2d and e, the directionally aligned lamellar ANGAs were successfully prepared after the sublimation of the ice crystals and annealing. As can be seen in enlarged SEM image (Fig. 2f), the rigid Al2O3 nanorods, similar to steel rebars in cement, serve as reinforcement for the graphene nanosheets at the nanoscale, which provides a structural basis for the enhanced compressive strength of ANGAs. The enlarged SEM, TEM images and EDS mappings of the ANGAs also indicate that the submicron Al2O3 nanorods are closely attached to the large-size graphene sheets, and the distribution of Al and C elements further proves the composition of the ANGAs (Fig. S2–S4†).
O and Al–O peaks in the FTIR spectra after the combination of Al2O3 nanorods and GO also indicate the formation of hydrogen bondings (Fig. S1†). In addition, as shown in the mapping image (Fig. 2c), the C, Al and O elements are homogeneously dispersed on the graphene sheets. This is because the Al2O3 can be trapped by the target sites (–OH) on the graphene surface under the induction of hydrogen bonding, and then uniformly distributed on the graphene sheet surface to self-assemble into nanoceramic-enhanced graphene heterostructures. While PVA acts as a chemical cross-linking agent to firmly weld the graphene structural units together after annealing, thus forming a stable skeleton.34,35 To obtain the desired anisotropic graphene structure, the bidirectional freezing technique was employed for the preparation of ANGAs. The temperature gradient from the bidirectional freezing platform in two directions makes the ice crystals grow in a lamellar pattern and the graphene sheets adsorbed with Al2O3 nanorods are extruded into the ice crystal interstices. As shown in Fig. 2d and e, the directionally aligned lamellar ANGAs were successfully prepared after the sublimation of the ice crystals and annealing. As can be seen in enlarged SEM image (Fig. 2f), the rigid Al2O3 nanorods, similar to steel rebars in cement, serve as reinforcement for the graphene nanosheets at the nanoscale, which provides a structural basis for the enhanced compressive strength of ANGAs. The enlarged SEM, TEM images and EDS mappings of the ANGAs also indicate that the submicron Al2O3 nanorods are closely attached to the large-size graphene sheets, and the distribution of Al and C elements further proves the composition of the ANGAs (Fig. S2–S4†).
The crystalline phase composition of ANGAs was also explored by XRD. As seen in Fig. 2g, nanorods are composed of δ-Al2O3, which is transformed from the original boehmite phase after heat treatment,36 and graphene was evidenced by the broad peak at around 25°. The thermal decomposition processes of ANGAs with different Al2O3 nanorod contents and graphene aerogels (GAs) during calcination were further analyzed by TG-DSC (Fig. 2h and i). At temperatures below 150 °C (Stage I), the weight loss of the samples was mainly caused by the removal of adsorbed moisture, which was proved by the exothermic peak at 96 °C in the DSC curves. In Stage II (150–400 °C), the significant weight loss is mainly attributed to the pyrolysis of oxygen-containing functional groups in graphene (–OH, –COOH, –COC–) and the production of CO, CO2 and water, etc.37 The TG-DSC curves of ANGAs and GAs in Stage III (above 400 °C) are distinctly different. For GAs, the removal of the more stable functional groups and the decomposition of organic carbon leads to the weight loss in Stage III.38 In contrast, for ANGAs, a significant exothermic peak was observed at 488 °C due to the crystalline phase transition of Al2O3 nanorods from boehmite to γ-Al2O3.36 Notably, thanks to the excellent heat resistance of Al2O3, the sample residue increased remarkably with the increase of Al2O3 nanorods content from 44.1% for GAs to 67.2% for ANGA-10, indicating that the addition of Al2O3 nanorods could significantly improve the thermal stability of the aerogels.
3.2. Mechanical properties of ANGAs
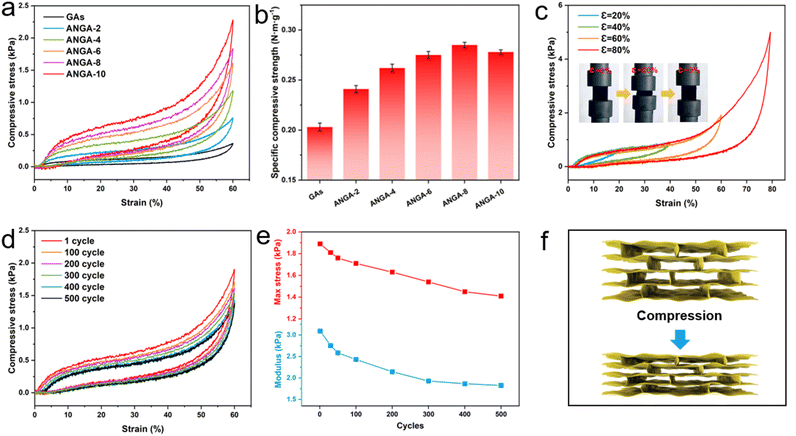
Ceramics are known to be intrinsically brittle and the previously reported Al2O3 nanorod aerogels are also subject to structural collapse under large deformation.15,16 However, the situation changes for ANGAs, where the rigid Al2O3 nanorods exhibit excellent elasticity after bonding with the graphene backbone and the mechanical properties of graphene are further enhanced by Al2O3 nanorods. The compression process of ANGAs undergoes three typical stages, including the elastic deformation stage (less than 10% strain), the plateau stage (10–40% strain) and the densification stage (over than 40% strain). It can be seen from the stress–strain curves of GAs and ANGAs that the compressive strength of the samples at 60% strain increases rapidly by more than 6 times with the increase of Al2O3 nanorods content, from 0.36 kPa for GAs to 2.29 kPa for ANGAs-10 (Fig. 3a). Since the increase in Al2O3 content also brings about an increase in density, the specific compressive strength is employed to reasonably evaluate the variation in compressive performance. As can be seen from Fig. 3b, the specific compressive strength of the samples increased by more than 40% from 0.203 N m g−1 for GAs to 0.286 N m g−1 for ANGA-8. With the further increase in Al2O3 nanorods, the specific compression strength of the sample decreased (0.278 N m g−1 for ANGA-10), so the compression performance of ANGA-8 was further investigated.Thanks to the interplay of Al2O3 nanorods and graphene, ANGA-8 was able to recover to its initial state even at a large strain of 80% and had a compressive strength of 4.98 kPa at 80% strain (Fig. 3c), indicating that it could remain elastic under a pressure of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times of its own weight. In addition, ANGA-8 demonstrates outstanding compression durability, with only 2.8% plastic deformation after 500 compression cycles (60% strain) (Fig. 3d). Meanwhile, the maximum compression strength decreased from 1.89 kPa to 1.41 kPa, accompanied by a drop in elastic modulus from 3.10 kPa to 1.83 kPa (Fig. 3e). The excellent elasticity of ANGAs and the greatly elevated compressive strength are mainly attributed to the synergistic effect of Al2O3 nanorods and graphene framework. Compared with the random structure, the ordered lamellar structure obtained by bidirectional freezing facilitates the formation of more effective force support points. At the macroscopic scale, the elastic bridging layers between the lamellae are able to transfer and dissipate the load efficiently through folding and bending after the graphene skeleton is subjected to pressure (Fig. 3f). At the nanoscale, the rigid Al2O3 nanorods act as reinforcing agents and tightly attach to the flexible graphene sheets through intermolecular interactions. The rigid nanorods can support and resist deformation when the graphene sheet is deformed, thus enhancing the compressive strength. In addition, Al2O3 nanorod-reinforced graphene units are able to constitute a robust 3D network structure under the bonding effect of PVA. Thus, the 1D nanorods and 2D graphene combine rigidity and flexibility, leading to the birth of ANGAs with excellent mechanical properties.
000 times of its own weight. In addition, ANGA-8 demonstrates outstanding compression durability, with only 2.8% plastic deformation after 500 compression cycles (60% strain) (Fig. 3d). Meanwhile, the maximum compression strength decreased from 1.89 kPa to 1.41 kPa, accompanied by a drop in elastic modulus from 3.10 kPa to 1.83 kPa (Fig. 3e). The excellent elasticity of ANGAs and the greatly elevated compressive strength are mainly attributed to the synergistic effect of Al2O3 nanorods and graphene framework. Compared with the random structure, the ordered lamellar structure obtained by bidirectional freezing facilitates the formation of more effective force support points. At the macroscopic scale, the elastic bridging layers between the lamellae are able to transfer and dissipate the load efficiently through folding and bending after the graphene skeleton is subjected to pressure (Fig. 3f). At the nanoscale, the rigid Al2O3 nanorods act as reinforcing agents and tightly attach to the flexible graphene sheets through intermolecular interactions. The rigid nanorods can support and resist deformation when the graphene sheet is deformed, thus enhancing the compressive strength. In addition, Al2O3 nanorod-reinforced graphene units are able to constitute a robust 3D network structure under the bonding effect of PVA. Thus, the 1D nanorods and 2D graphene combine rigidity and flexibility, leading to the birth of ANGAs with excellent mechanical properties.
3.3. Pressure sensing properties of ANGAs
Graphene aerogels are considered to be promising pressure sensors due to their excellent electrical conductivity and elasticity, but pure graphene has a limited pressure detection range due to its low compressive strength and proneness to deformation, which seriously hinders its application. Here, ANGAs not only inherit the properties of graphene aerogels, such as electrical conductivity and elasticity, but also feature enhanced compressive strength, and thus can effectively extend the upper limit of pressure detection. The conductive network formed by interconnecting Al2O3 nanorod-graphene structural units experiences a change in resistance under pressure, as evidenced by changes in the brightness of the light bulb (Fig. 4a and b), together with a robust elastic backbone, providing a structural basis for pressure sensing.As shown in Fig. 4c, ANGAs are placed between two copper sheets connected with wires to form the pressure sensor. The strains of the ANGAs are precisely controlled by a programmable mechanical tester, and the resistance of the ANGAs is measured by a resistance meter. During the loading process, the porosity of the ANGAs decreases and the lamellar pore walls are moved closer to each other, which is more conducive to rapid electron transfer in the aerogel network, thus exhibiting a lower resistance. After unloading, the resistance returns to the initial state. As shown in Fig. 4d, each compression cycle curve is symmetrical, suggesting that the resistance can recover quickly during unloading as well. It can be found that the response stability of ANGAs at 1% strain is poor, with a response fluctuation of 34.29%. Compared with pure GAs, the ANGAs with the addition of non-conductive Al2O3 nanorod have higher resistance, which causes the samples to be less sensitive to minor resistance changes caused by small strains. As the strain continued to increase, the stability of the sensor response increased and the response fluctuations decreased to 3.41% for 2% strain, 3.07% for 3% strain, and 2.46% for 5% strain. Also, the response fluctuations at 10%, 20%, 30%, and 40% strain were all below 3%, with fluctuations of 1.09%, 1.83%, 2.97%, and 2.22%, respectively. When the strain is 50%, the sensor response fluctuation rises to 6.38% in 5 cycles. Therefore, ANGAs have excellent sensing stability in the strain range of 2% to 40%. It can be observed from Fig. 4e that the average sensor response at 2%, 3%, 5%, 10%, 20%, 30% and 40% are 3.29%, 4.34%, 6.4%, 9.3%, 24.5%, 38.4% and 53.3%, respectively. In order to establish the relationship between the sensor response and the loading pressure, the pressure values at different strains were identified according to Fig. 3a. As can be observed in Fig. 4f, the variation of resistance increases with increasing pressure and the corresponding pressure value (0.044–0.92 kPa) subjected to the sensor can be determined by resistance change from the curve. The detection pressure of ANGAs at 2% strain is 0.044 kPa, which is also the pressure sensing sensitivity of ANGAs. Notably, the upper pressure detection limit increased dramatically by up to 6 times from 0.14 kPa for GAs to 0.92 kPa for ANGAs after coupling with Al2O3 nanorods. Considering the relatively large fluctuations at 50% strain, the cyclic durability of the ANGAs was further tested at 40% strain. Thanks to the excellent structural stability of the aerogels, no significant resistance decay was observed in the 500 cycles. In addition, the response fluctuation of ANGAs in 500 cycles is 4.43%, which is slightly higher compared to the fluctuation in 5 cycles (2.22%), but still at a relatively low level. All of the above results indicate that ANGAs can be used as reliable pressure sensors in practical applications.
3.4. Thermal insulation performance of ANGAs
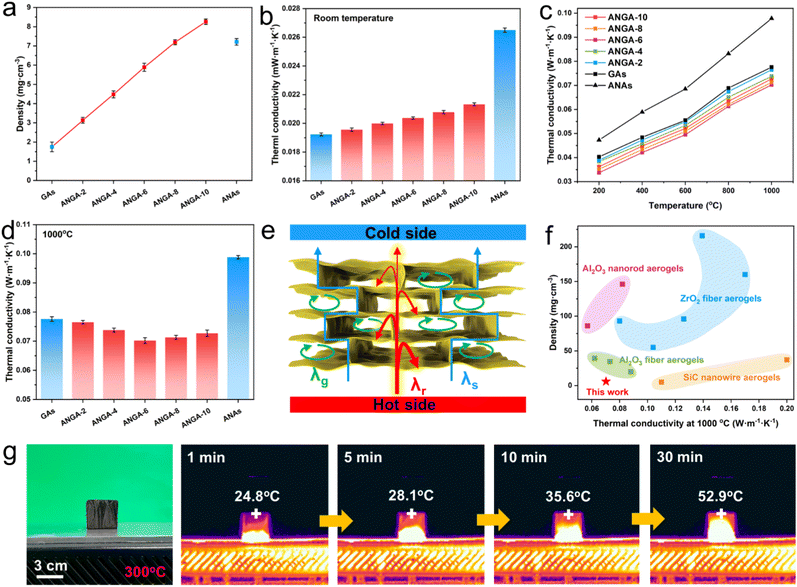
Even though nanorods can form a nanoporous structure that is more conducive to thermal insulation than fibrous aerogels, the ANAs still lead to a rapid increase in thermal conductivity at high temperatures.39 In addition, for the ordered laminar structured Al2O3 nanorod aerogels obtained by directional freeze-drying, gas molecules can still pass through the nanopores between the nanorods for heat transfer between the lamellae. Therefore, the thermal insulation properties of Al2O3 nanorod aerogels still need to be further improved.Generally speaking, for porous materials with pore size less than 1 mm, the total thermal conductivity is considered to be the integrated effect of solid thermal conductivity (λs), gas thermal conductivity (λg) and radiative thermal conductivity (λr).40 At room temperature, λr can usually be neglected due to the small contribution, i.e., the thermal conductivity of the material can be simplified to the sum of λs and λg.15 The density and room temperature thermal conductivity of GAs, ANAs, and ANGAs are shown in Fig. 5a and b. The density of the aerogels increases from 1.75 mg cm−3 for GAs, 3.13 mg cm−3 for ANGA-2 to 8.26 mg cm−3 for ANGA-10 as the proportion of nanorods increases. The thermal conductivity of the samples follows the same trend as the density, increasing from 0.0192 W m−1 K−1 for GAs, 0.0196 W m−1 K−1 for ANGA-2 to 0.0213 W m−1 K−1 for ANGA-10, which is due to the growth of λs caused by the higher density. However, the thermal conductivity of ANAs (0.0265 W m−1 K−1) is still 24.4% higher than that of ANGA-10, even though ANAs have a lower density (7.21 mg cm−3). For ANAs, the overlapped Al2O3 nanorods form a porous skeleton (Fig. S4†), so that gas molecules can pass through the pores for heat transfer. For ANGAs, the layered graphene sheets can fill the pores between the nanorods, confining the movement of gas molecules within the gaps of the lamellas. Consequently, the heat transfer through the cell wall is substantially suppressed, which substantially reduces the λg. Besides, the ultralow density and narrow bridging between the layers also reduce the λs. Therefore, the extremely low λs and λg together contribute to the super-insulation performance of ANGAs, which even possess a lower thermal conductivity than that of air (0.024 W m−1 K−1).41
Compared with the polymer reinforced phase, ceramic reinforced graphene aerogel can be used for high-temperature insulation due to its excellent thermostability, so the high-temperature insulation properties of ANGAs have been further investigated (Fig. 5c and d). As predicted, the high-temperature thermal conductivity of ANGAs was dramatically reduced after the incorporation of graphene compared to ANAs, and the difference becomes more pronounced with increasing temperature. At 1000 °C, the thermal conductivity decreased by 26.4% from 0.0988 W m−1 K−1 for ANAs to 0.0727 W m−1 K−1 for ANGA-10. According to classical heat transfer theory, λr can be represented as:42
According to above equation, the increase in density is beneficial to limit the high temperature radiative heat transfer. At Al2O3 nanorod content below 6% (ANGA-6), the decrease in λr due to the elevated density can compensate for the increase in λs, thus leading to a reduction in total thermal conductivity. As the density of aerogel rises further, the increase in λs outweighs the decrease in λr, resulting in an increase in thermal conductivity instead. Overall, the lamellar ANGAs with high specific extinction coefficient can significantly weaken the λr, and the ultra-low density and the confined lamellar pore structure can effectively suppress the λs and λg, respectively, so that the ANGAs exhibit superinsulation performance at both high and low temperatures (Fig. 5e). Remarkably, ANGAs show great advantages in density and high-temperature thermal conductivity compared with previously reported ultralight ceramic aerogels (Fig. 5f), including SiC nanowire aerogels (900 °C),44,45 ZrO2 fiber aerogels,14,46–49 Al2O3 fiber aerogels50–52 and Al2O3 nanorod aerogels.15,16 In order to demonstrate the thermal insulation performance of ANGAs more visually, a cube ANGA-6 with a diameter of 3 cm was placed on a heating platform of 300 °C (Fig. 5g). The temperature of the upper surface of the sample increased slowly from 24.8 °C at 1 min, 28.1 °C at 5 min, 35.6 °C at 10 min to 52.9 °C at 30 min, illustrating its great potential as a thermal insulator.
4. Conclusions
In conclusion, high performance ultralight elastic ANGAs were successfully prepared by combining Al2O3 nanorods and graphene. This win–win strategy not only overcomes the brittleness of Al2O3 nanorod aerogels but also substantially improves the compressive strength of graphene aerogels. The robust backbone enabled the ANGAs to exhibit compressive durability and pressure sensing stability over 500 cycles at 40% strain. In addition, thanks to the graphene with excellent light-shielding capability and the laminar structure obtained by bidirectional freezing, the ANGAs obtained ultra-low thermal conductivity of 0.0196 W m−1 K−1 and 0.0702 W m−1 K−1 at 25 °C and 1000 °C, respectively. We believe that this work paves the way for the construction of multifunctional elastic ceramic-based aerogels.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the financial support from Key R&D Program of Hunan Province (grant number 2022GK2027). All authors contributed to the completion of the manuscript and the final version of the manuscript has been approved by all the authors.Notes and references
- X. Xu, S. Fu, J. Guo, H. Li, Y. Huang and X. Duan, Mater. Today, 2021, 42, 162–177 CrossRef CAS.
- Z. Xiong, Y. Zhu, Z. Wang, Y. Chen and H. Yu, Adv. Funct. Mater., 2021, 2106978 Search PubMed.
- L. Zhu, L. Zong, X. Wu, M. Li, H. Wang, J. You and C. Li, ACS Nano, 2018, 12, 4462–4468 CrossRef CAS PubMed.
- F. Peng, Y. Jiang, J. Feng, H. Cai, J. Feng and L. Li, Chem. Eng. J., 2021, 411, 128402 CrossRef CAS.
- F. Peng, Y. Jiang, J. Feng, F. Liu, J. Feng and L. Li, J. Eur. Ceram. Soc., 2022, 42, 6684–6702 CrossRef CAS.
- X. Hou, R. Zhang and D. Fang, Ceram. Int., 2017, 43, 9547–9551 CrossRef CAS.
- X. Zhang, T. Zhang, Z. Yi, L. Yan, S. Liu, X. Yao, A. Guo, J. Liu and F. Hou, Ceram. Int., 2020, 46, 28561–28568 CrossRef CAS.
- H. Maleki, L. Durães and A. Portugal, Microporous Mesoporous Mater., 2014, 197, 116–129 CrossRef CAS.
- J. P. Randall, M. A. B. Meador and S. C. Jana, J. Mater. Chem. A, 2013, 1, 6642 RSC.
- Y. Si, X. Wang, L. Dou, J. Yu and B. Ding, Sci. Adv., 2018, 4, eaas8925 CrossRef PubMed.
- Z. An, C. Ye, R. Zhang and P. Zhou, Ceram. Int., 2019, 45, 22793–22801 CrossRef CAS.
- X. Zhang, F. Wang, L. Dou, X. Cheng, Y. Si, J. Yu and B. Ding, ACS Nano, 2020, acsnano.0c06423 Search PubMed.
- S. Fu, D. Liu, Y. Deng, J. Guo, H. Zhao, J. Zhou, P. Zhang, H. Yu, S. Dang, J. Zhang, H. Li and X. Xu, Nano Res., 2023, 16, 5047–5055 Search PubMed.
- J. Guo, S. Fu, Y. Deng, X. Xu, S. Laima, D. Liu, P. Zhang, J. Zhou, H. Zhao, H. Yu, S. Dang, J. Zhang, Y. Zhao, H. Li and X. Duan, Nature, 2022, 606, 909–916 CrossRef CAS PubMed.
- F. Liu, C. He, Y. Jiang, Y. Yang, F. Peng, L. Liu, J. Men, J. Feng, L. Li, G. Tang and J. Feng, Chem. Eng. J., 2023, 455, 140502 CrossRef CAS.
- E. Zhang, W. Zhang, T. lv, J. Li, J. Dai, F. Zhang, Y. Zhao, J. Yang, W. Li and H. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 20548–20558 CrossRef CAS PubMed.
- F. Liu, Y. Jiang, F. Peng, J. Feng, L. Li and J. Feng, Chem. Eng. J., 2023, 141721 CrossRef CAS.
- X. Xu, Q. Zhang, Y. Yu, W. Chen, H. Hu and H. Li, Adv. Mater., 2016, 28, 9223–9230 CrossRef CAS PubMed.
- Q. Zhang, X. Xu, D. Lin, W. Chen, G. Xiong, Y. Yu, T. S. Fisher and H. Li, Adv. Mater., 2016, 28, 2229–2237 CrossRef CAS PubMed.
- F. An, X. Li, P. Min, H. Li, Z. Dai and Z.-Z. Yu, Carbon, 2018, 126, 119–127 CrossRef CAS.
- Y. Xie, S. Xu, Z. Xu, H. Wu, C. Deng and X. Wang, Carbon, 2016, 98, 381–390 CrossRef CAS.
- R. Zhang, R. Hu, X. Li, Z. Zhen, Z. Xu, N. Li, L. He and H. Zhu, Adv. Funct. Mater., 2018, 28, 1705879 CrossRef.
- Y. Xia, C. Gao and W. Gao, J. Polym. Sci., 2022, 60, 2239–2261 CrossRef CAS.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- L. Guan, L. Zhao, Y. Wan and L. Tang, Nanoscale, 2018, 10, 14788–14811 RSC.
- J. Fan, S. Hui, T. P. Bailey, A. Page, C. Uher and F. Yuan, J. Mater. Chem. A, 2019, 7, 1574–1584 RSC.
- Y. Ma, M. Yu, J. Liu, X. Li and S. Li, ACS Appl. Mater. Interfaces, 2017, 9, 27127–27134 CrossRef CAS PubMed.
- H.-L. Gao, Y.-B. Zhu, L.-B. Mao, F.-C. Wang, X.-S. Luo, Y.-Y. Liu, Y. Lu, Z. Pan, J. Ge, W. Shen, Y.-R. Zheng, L. Xu, L.-J. Wang, W.-H. Xu, H.-A. Wu and S.-H. Yu, Nat. Commun., 2016, 7, 12920 CrossRef CAS PubMed.
- Q. Zhang, F. Zhang, S. P. Madarametla, H. Li, C. Zhou and D. Lin, Small, 2016, 12, 1681–1814 CrossRef.
- L. Zhou and Z. Xu, J. Hazard. Mater., 2020, 388, 121804 CrossRef CAS PubMed.
- Q. Zhang, D. Lin, B. Deng, X. Xu, Q. Nian, S. Jin, K. D. Leedy, H. Li and G. J. Cheng, Adv. Mater., 2017, 29, 1605506 CrossRef PubMed.
- X. Meng, X. Peng, Y. Wei, S. Ramakrishna, Y. Sun and Y. Dai, Chem. Eng. J., 2022, 437, 135444 CrossRef CAS.
- H. Yin, S. Zhao, J. Wan, H. Tang, L. Chang, L. He, H. Zhao, Y. Gao and Z. Tang, Adv. Mater., 2013, 25, 6270–6276 CrossRef CAS PubMed.
- J.-Y. Hong, B. M. Bak, J. J. Wie, J. Kong and H. S. Park, Adv. Funct. Mater., 2015, 25, 1053–1062 CrossRef CAS.
- S. Ye, Y. Liu and J. Feng, ACS Appl. Mater. Interfaces, 2017, 9, 22456–22464 CrossRef CAS PubMed.
- F. Peng, Y. Jiang, J. Feng, J. Feng and L. Li, J. Am. Ceram. Soc., 2022, 105, 2288–2299 CrossRef CAS.
- J. Shen, Y. Hu, M. Shi, X. Lu, C. Qin, C. Li and M. Ye, Chem. Mater., 2009, 21, 3514–3520 CrossRef CAS.
- F. Farivar, P. L. Yap, K. Hassan, T. T. Tung, D. N. H. Tran, A. J. Pollard and D. Losic, Carbon, 2021, 179, 505–513 CrossRef CAS.
- W. Zou, X. Wang, Y. Wu, L. Zou, G. Zu, D. Chen and J. Shen, Ceram. Int., 2019, 45, 644–650 CrossRef CAS.
- L. W. Hrubesh and R. W. Pekala, J. Mater. Res., 1994, 9, 731–738 CrossRef CAS.
- X. Zhang, X. Cheng, Y. Si, J. Yu and B. Ding, ACS Nano, 2022, 16, 5487–5495 CrossRef CAS PubMed.
- T. Xie, Y.-L. He and Z.-J. Hu, Int. J. Heat Mass Transfer, 2013, 58, 540–552 CrossRef CAS.
- G. H. Tang, Y. Zhao and J. F. Guo, Int. J. Heat Mass Transfer, 2016, 99, 192–200 CrossRef CAS.
- L. Su, H. Wang, M. Niu, X. Fan, M. Ma, Z. Shi and S.-W. Guo, ACS Nano, 2018, 12, 3103–3111 CrossRef CAS PubMed.
- B. Li, X. Yuan, Y. Gao, Y. Wang, J. Liao, Z. Rao, B. Mao and H. Huang, Mater. Res. Express, 2019, 6, 045030 CrossRef.
- X. Zhang, B. Wang, N. Wu, C. Han, C. Wu and Y. Wang, J. Eur. Ceram. Soc., 2020, 40, 1877–1885 CrossRef CAS.
- G. Zu, J. Shen, W. Wang, L. Zou, Y. Lian, Z. Zhang, B. Liu and F. Zhang, Chem. Mater., 2014, 26, 5761–5772 CrossRef CAS.
- T. Wang, Q. Yu and J. Kong, Int. J. Appl. Ceram. Technol., 2018, 15, 472–478 CrossRef CAS.
- T. Wang, Q. Yu, J. Kong and C. Wong, Ceram. Int., 2017, 43, 9296–9302 CrossRef CAS.
- R. Liu, X. Dong, S. Xie, T. Jia, Y. Xue, J. Liu, W. Jing and A. Guo, Chem. Eng. J., 2019, 360, 464–472 CrossRef CAS.
- L. Li, C. Jia, Y. Liu, B. Fang, W. Zhu, X. Li, L. Schaefer, Z. Li, F. Zhang, X. Feng, N. Hussain, X. Xi, D. Wang, Y. Lin, X. Wei and H. Wu, Mater. Today, 2022, 54, 72–82 CrossRef CAS.
- B. Zhang, Y. Liu, Q. Wu, M. Zhou, D. Su, H. Ji and X. Li, J. Eur. Ceram. Soc., 2022, 42, 5995–6004 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01070h |
| This journal is © The Royal Society of Chemistry 2023 |