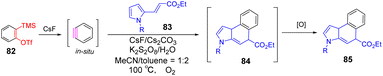Open Access Article
Open Access ArticleAdvances in the synthesis of nitrogen-containing heterocyclic compounds by in situ benzyne cycloaddition
Hui Yub and
Feng Xu *a
*a
aSchool of Mathematics and Information Science, Guiyang University, Guiyang, Guizhou 550005, P. R. China. E-mail: fengxuyh@163.com
bDepartment of Pharmacy, Shizhen College of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou 550200, China. E-mail: 332375217@qq.com
First published on 13th March 2023
Abstract
Nitrogen-containing heterocyclic compounds are prevalent in various natural products, medicines, agrochemicals, and organic functional materials. Among strategies to prepare nitrogen-containing heterocyclic compounds, pathways involving benzyne intermediates are attractive given that they can readily assemble highly diverse heterocyclic compounds in a step-economical manner under transition-metal-free conditions. The synthesis of nitrogen-containing heterocyclic compounds from benzyne intermediates offers an alternative strategy to the conventional metal-catalyzed activation approaches. In the past years, chemists have witnessed the revival of benzyne chemistry, mainly attributed to the wide application of various novel benzyne precursors. The cycloaddition of benzynes is a powerful tool for the synthesis of nitrogen-containing heterocyclic compounds, which can be constructed by [n + 2] cyclization of benzyne intermediates in situ generated from benzyne precursors under mild reaction conditions. This review focuses on the application of cycloaddition reactions involving in situ benzynes in the construction of various nitrogen-containing heterocyclic compounds.
1 Introduction
Nitrogen-containing compounds have been fundamental for the construction diversity of molecules in the chemical sciences and pharmaceuticals. Among them, nitrogen-containing heterocyclic molecules have a wide range of applications in medicinal chemistry and exhibit several biological functions, such as antibacterial, anticancer, and antimicrobial activities (Scheme 1a).1–4 Among the many methods for preparing nitrogen-containing heterocyclic compounds, utilizing benzyne intermediates is one of the most attractive strategies, which can effectively construct complex and diverse benzazepine derivatives under mild conditions efficiently and economically. In particular, the research on cyclic isomerization of tethered triynes to generate benzyne species under heating conditions has made benzyne chemistry a field that has flourished in recent years.5–7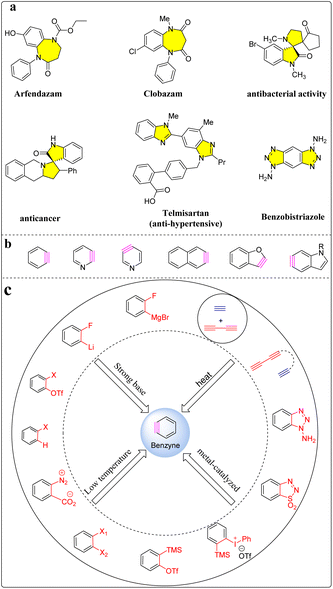 | ||
| Scheme 1 (a) Some examples of functional aromatics, (b) general benzyne species, (c) preparation strategy of benzyne species. | ||
As one of the classic intermediates, benzyne is widely used in the synthesis of functionalized aromatic compounds.8 Due to the unique structure of the benzyne intermediate, it is easily captured by other reagents. This capture can establish many new reaction types, and results in the research of benzyne chemistry have always attracted the attention of the field of organic synthesis. Benzyne species are often referred to as 1,2-dehydrobenzene or other aromatic heterocyclic analogs (naphthalene, benzofuran, pyridine, and indoleyne, etc.), and their varieties are diverse (Scheme 1b).9,10
In 1902, Stoermer and Kahlert et al. reported the existence of the benzene species by treating 3-bromobenzofuran with a strong base in ethanol solution,11 the usual strategy for the preparation of benzyne intermediates is to remove two adjacent atoms or groups from the benzene ring. Due to the instability, benzyne intermediate must be generated in situ from appropriate precursors under suitable reaction conditions, including thermo-synthesis, oxidation, deprotonation, halogen–metal exchange, C–Si bond cleavage by fluoride source or decarboxylation by transition metal-catalyzed (Scheme 1c).12–16 Type benzyne precursors include aryl-halides,17,18 aromatics-diazo-2-carboxylates,19,20 1,2,3-benzothiadiazole 1,1-dioxide,21 1-aminobenzotriazol,22,23 and o-silanoyl trifluoroester,24 all of which have a significant feature that a good leaving group is present on the aromatic ring. Although this method can effectively prepare benzyne intermediates, it usually requires strong bases or additives and harsh reaction conditions. These unfavorable factors limit the application range of substrates, and strong bases are prone to nucleophile reactions with benzyne intermediates, reducing the reaction efficiency of benzyne intermediates. With the development of the cycloaddition reaction, the benzyne species can also be obtained from the thermal activation of tethered triynes by hexadehydro-Diels–Alder cycloaddition (HDDA), which can build functionalized aromatic products with diverse structures.6,25–29
Selecting suitable precursors, reaction conditions, and benzene trapping agents was crucial for effectively utilizing benzene intermediates. The cycloaddition reaction of benzyne can achieve 100% atomic economy, which provides an ideal way to efficiently utilize benzyne precursors to synthesize various cyclization products. Cyclization requires good LUMO–HUMO orbital matching, and the benzene intermediate has a low LUMO, which is easy for various dienophiles to undergo [4 + 2], [2 + 2] cycloaddition, and [3 + 2] dipolar cycloaddition.30,31 In the past decade, various benzyl precursor cyclization methods have been developed to synthesize nitrogen-containing heterocyclic compounds with potential applications providing a new route.
In the present minireview, we focus on benzene reactions from various precursors that can react with various dienes under mild conditions to form nitrogen-containing heterocyclic compounds. The application of HDDA in the synthesis of nitrogen-containing heterocycles is also reviewed.
2 Construction of nitrogen-containing heterocyclic compounds from in situ benzyne species by cyclization
2.1 General strategy for the preparation of benzyne intermediates
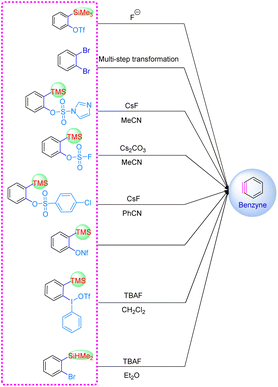
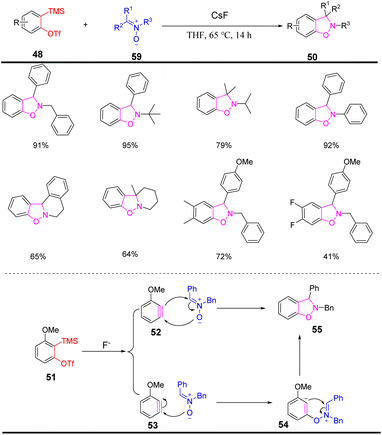
The high reactivity of the benzyne intermediate is due to its unique carbon–carbon triple bond, that is, the existence of an sp2 hybrid orbital in the carbon–carbon triple bond, resulting in very easy functionalization. Up to now, the structure of benzyne species has been confirmed from a microscopic point of view using infrared spectroscopy, nuclear magnetic resonance detection, low-temperature scanning tunneling microscopy, and atomic force microscopy.32–35 In 1983, Kobayashi et al. utilized o-trimethylsilyl aryl trifluoromethanesulfonate to generate benzyne intermediates induced by fluoride ions.24 In 2000, Fu et al. reported a novel phenylyne precursor [2-(hydroxydimethylsilyl)phenyliodoium trifluoromethanesulfonate], which can produce benzyne intermediates under mild conditions, which avoided carcinogen hexamethylphosphoramide (HMPA) in the preparation.36 2-(Trimethylsilyl)phenyl trifluoromethanesulfonate has unique advantages in organic synthesis as a benzyne precursor, but in its synthesis process, toxic, expensive, and highly corrosive trifluoromethanesulfonic anhydride will be used. Therefore, the development of relatively green trimethylsilylphenyl precursors is of great significance for the practical application of benzyne species.37–44 For example: 2-(trimethylsilyl)phenyliodoium trifluoromethanesulfonate, 2-(trimethylsilyl)phenylimidazolyl sulfonate, 2-(trimethylsilyl)phenylnonafluorobutane sulfonate, 2-(trimethylsilyl)phenyl thiofluoride, 2-(trimethylsilyl)phenyl bromide, 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate, etc. (Scheme 2). These simple and environmentally friendly methods allowed obtaining the cycloaddition products of benzene intermediates.2.2 [2 + 2] Cycloaddition reaction
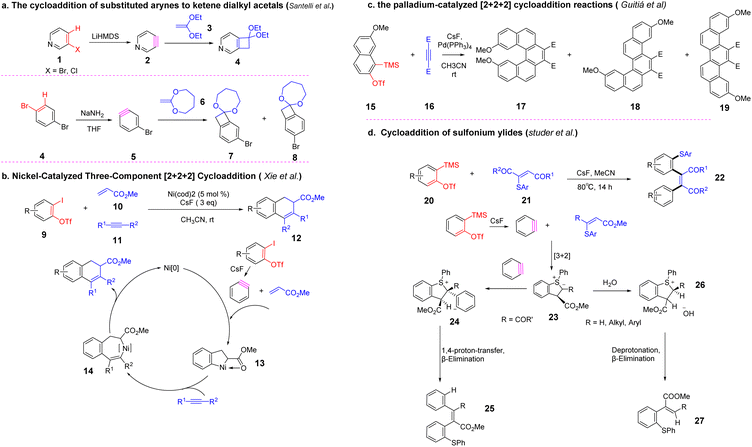
The [2 + 2] cycloaddition reaction is a valuable synthesis toolbox. Various novel compounds can be constructed by combining different mono-enes or mono-acetylenes with benzynes, such as benzocyclobutenes, benzene fused rings, pentahelicene, and bifunctional products (Scheme 3)45–49. Subsequently, the use of benzyne intermediates by [2 + 2] cyclization for the construction of nitrogen-containing compounds was developed. In 2009, Hsung et al. reported an enamide-benzyne-[2 + 2] cycloaddition, using potassium fluoride as the fluorine source, a benzyne species was formed in situ in 1,4-dioxane, and then reacted with enamide to obtain the [2 + 2] product50 (Scheme 4). The [2 + 2] cycloaddition products might further undergo ring-opening to obtain intramolecular [4 + 2] cycloadducts with high stereoselectivity, which enriches the construction methods of nitrogen-containing nitrogen heterocycles.In 2012, the in situ formation of benzyne intermediate from enamines as starting materials and the total synthesis of p-(±)-chelidonine and (±)-norchelidonine via cyclization reported by Hsung et al.51 The synthesis method highlights the power of enamines as a building block and the importance of benzyne chemistry, especially the intermolecular [2 + 2] cyclization of benzyne intermediates with enamines as a critical step in initiating this synthesis pathway (Scheme 5).
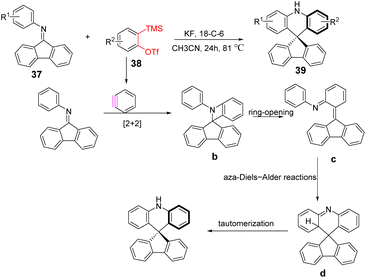
He et al. developed a novel strategy for the preparation of spiroacridines via a [2 + 2] cycloaddition/ring-opening sequence of benzynes with N-arylimines (Scheme 6). Initially, the benzyne undergoes a [2 + 2] cycloaddition with imine to generate azetidine intermediate b. This four-membered ring undergoes rapid intramolecular ring opening by nucleophilic attack on the N-ortho position of the N-aryl ring to form quinomethide imine intermediate. The subsequent intramolecular aza-Diels–Alder reaction c leads to the formation of d, followed by fluorine anion-assisted tautomerization to access the final product. The reaction provides a powerful tool for the synthesis of valuable nitrogen-containing polyaromatic skeletons.
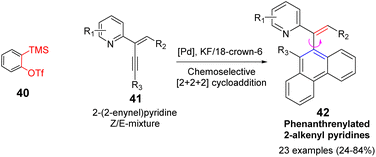
Wang et al. established a palladium-catalyzed chemical and stereoselective [2 + 2 + 2] cyclization reaction of 2-(2-alkenyl) pyridine with alkynes in 2021 (Scheme 7).52 Palladium catalyst is crucial for the chemical selectivity control and product selectivity of Z/E mixture of 2-(2-enynyl)pyridines. A wide range of phenanthrenylated 2-alkenylpyridines can be achieved via this strategy, which contains a chiral axis between the olefin and the phenanthrene ring, with good yield (84% yield) and excellent E-selectivity.
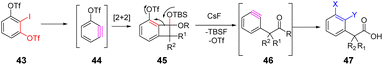
Li et al. developed a novel method for the synthesis of 3-trifluoromethoxyaryne intermediates. Starting from the [2 + 2] cycloaddition, a chemically selective ring-opening method was carefully designed to allow the formation of corresponding 2,3-aryne intermediates.53 This strategy is universal, efficient, transition metal-free, and can be used for various combinations of aromatic reagents and drug synthesis. The regioselectivity of the reaction is affected by steric hindrance, which preferentially supports pronuclear transformation (Scheme 8).
At present, there are few examples of [2 + 2] cyclizations involving benzynes to construct nitrogen-containing heterocycles have been reported, which are often accompanied by other cyclization reactions that can be used as an initiated step to construct more structurally complex compounds by 2 + 2 cyclization.
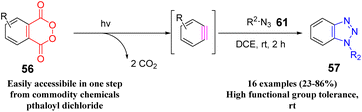
In 2015, Shi et al. reported the in situ generation of benzyne from phthaloyl peroxides by photolysis in the presence of 1,2-dichloroethane to construct benzo[d][1,2,3]triazole by [3 + 2] cycloaddition with azide.55 (Scheme 10.) The method could be well applied to various organic azides and phthaloyl peroxides, and the corresponding benzotriazole derivatives can be obtained at room temperature, and the yield is moderate to good.
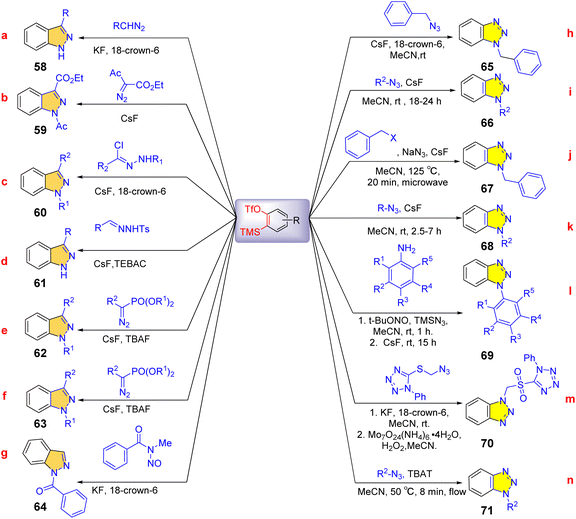
Benzotriazole, indazole scaffolds are antibacterial, antifungal, and antiviral drugs with significant potency.56–60 Therefore, the simple synthesis of rapidly and scalable widely functionalized benzotriazoles, indazole has important practical significance. Yamamoto et al. established a simple, efficient, and versatile method for the synthesis of N-unsubstituted indazoles and 1-aryl indazoles by 1,3-dipolar cycloaddition of benzene with diazomethane derivatives. Under very mild conditions, various indazoles can be obtained, and the yield are from good to high (Scheme 11a).61 Subsequently, polysubstituted indazoles were obtained by the [3 + 2] cycloaddition of various diazo compounds with o-(trimethylsilyl)aryl triflates at room temperature in the presence of CsF and provided a straightforward and effective method for obtaining indazole skeleton. These reactions involve a dipolar cycloaddition of in situ generated diazo compounds and arynes (Scheme 11b–d).62–64 3-Alkyl/aryl 1 h-indazoles and 3-alkyl/aryl 3H-indazole-3-phosphonates can be synthesized by 1,3-dipolar cycloaddition of α-substituted α-diazomethylphosphonates with arynes under simple reaction conditions. The product distribution was controlled by the phosphoryl group (Scheme 11e and f).65 A novel cascade cyclization reaction of N-alkyl-N-nitrosamides with benzyne has been developed under transition metal-free conditions at room temperature via benzyne δ-insertion and C(sp3)–H bond functionalization strategies. It provided functionalized indazoles with high yield and regioselectivity. The protocol also demonstrates a wide range of substrates (Scheme 11g).66
In 2008, Feringa et al. reported that fluorine-promoted elimination of o-(trimethylsilyl)aryl trifluoroesters to form benzyne species that can undergo [3 + 2] cycloaddition with various azides to generate substituted benzotriazoles.67 The advantages of mild reaction conditions and easy availability of raw materials make it a classic ‘click’ reaction of azides and benzynes (Scheme 11h and i).68 Subsequently, in the presence of CsF, various simple and efficient preparation methods for benzotriazole have been developed, including microwave promotion and multi-step reactions (Scheme 11j–m).69–72 A metal-free flow synthesis of benzotriazole was reported by Heretsch et al. (Scheme 11n)73 Benzotriazoles can be formed by [3 + 2] cycloaddition in a few minutes using azides with in situ generated benzyne compounds. The thermal strain of dangerous azides and the accumulation of reaction intermediates are minimized by a short reaction time in the flow, improving process safety.
Hosoya et al. established a method for synthesizing novel benzotriazole derivatives based on the intramolecular cycloaddition of containing azide groups.74 Intramolecular azide aromatic cycloaddition from common raw materials obtained a unique cyclo-fused benzotriazole. This method can be applied to the construction of a unique benzotriazole library. Mechanistic studies have shown that the intermolecular [3 + 2] cycloaddition between 3-alkoxy and azides results in a high stereoselectivity due to the inductive electron-withdrawing effect of alkoxy groups (Scheme 12).
In 2016, the [3 + 2] cyclic addition of aminoquinone derivatives and benzyne intermediates to obtain carbazolquinone compounds with important biological and pharmaceutical significance developed by He et al.(Scheme 13.)75 The tandem reaction metal-free is achieved by the continuous formation of C–C and C–N bonds, and the nucleophilic attack of this reaction is carbon arylation of aminoquinones rather than nitrogen arylation. Control experiments and mechanistic studies showed that initially, the carbon of the enamine moiety undergoes nucleophilic addition to the in situ formation of benzyne, resulting in the formation of C-arylated zwitterionic intermediates 83. Then the nucleophilic aryl anions could be added to imine nitrogen and oxidized to obtain the cycle product 84 (path b). The first step, nucleophilic addition, for asymmetric alkynes, determines regional selectivity. The synthesis method can obtain Morson quinolone A and Konignanone B. These studies indicate that this method has potential scalability and flexibility in forming molecular diversity.
Meanwhile, Sha et al. reported that benzo[e]indole compounds were synthesized by intermolecular Diels–Alder reaction of benzyne intermediate with 2-vinylpyrrole at 45–86% yield (Scheme 14).76 Utilizing diphenylmethyl (CH2Ph2) as a protective group, the benzyne intermediate selectively reacts only with the exposed alkenyl group. Due to the wide range of substrates and the avoidance of metal catalysts in this method, it shows potential application value for synthesizing heterocyclic compounds.
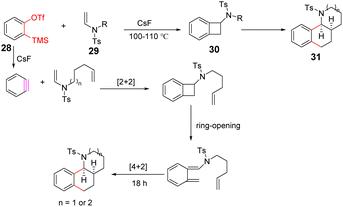
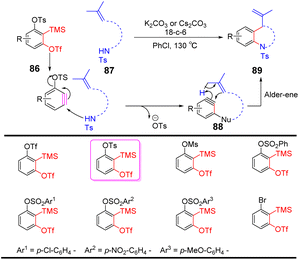
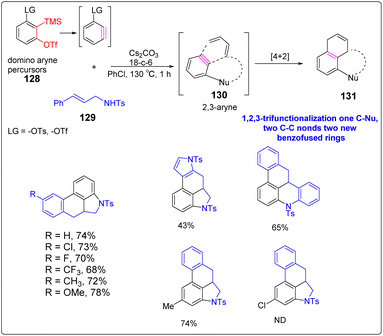
Li et al. successfully developed an efficient, transition metal-free, broad-spectrum domino aryne annulation approach by ucleophilic-ene cascade domino pathway, which can construct a variety of benzo-fused N-heterocycles and generate substituted arenes.77 An essential factor for the success of this domino transformation depends on the ability of the second leaving groups to dissociate on the 1,2-benzdiyne equivalents. –OTs were employed as the second leaving group to obtain 77% cyclization yield, while other leaving groups (LGs, i.e., OTf, sulfonyloxy groups, bromide) were ineffective in the cyclization process. The strategy provides new avenues for benzyne cyclization reactions (Scheme 15).
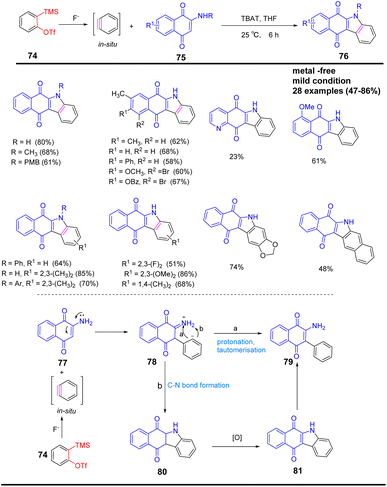
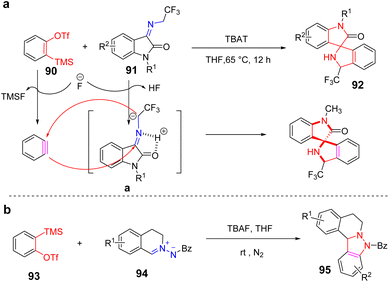
Same year, Ko et al. red an efficient synthesis of spiro[oxindole-3,2′-pyrrolidine] derivatives containing quaternary carbon centers by [3 + 2] cycloaddition under mild reaction conditions using TBAT as fluorine sources78 (Scheme 16a). In the conversion process, imine 91 is converted to azomethine ylide a by abstraction of the indicated hydrogen, which is acidic upon treatment with a weak base such as fluoride. The azomethine ylide intermediate a undergoes a [3 + 2] cyclization reaction with the in situ generated benzyne to give the final product 92. In 2019, Wu et al. established a [3 + 2] cyclization strategy for the synthesis of N-substituted indazolo[3,2-a]isoquinolines from benzenes and C, N-cyclic azomethine imines. This method can yield indazoline[3,2-a]isoquinolines with good anti-cancer cell proliferation activity in a one-step reaction under mild conditions (Scheme 16b).79.
 | ||
| Scheme 16 1,3-Dipolar cycloaddition reactions with benzynes. (a) Synthesis of spiro[oxindole-3,2′-pyrrolidine] derivatives, (b) synthesis of N-substituted indazolo[3,2-a]isoquinolines skeleton. | ||
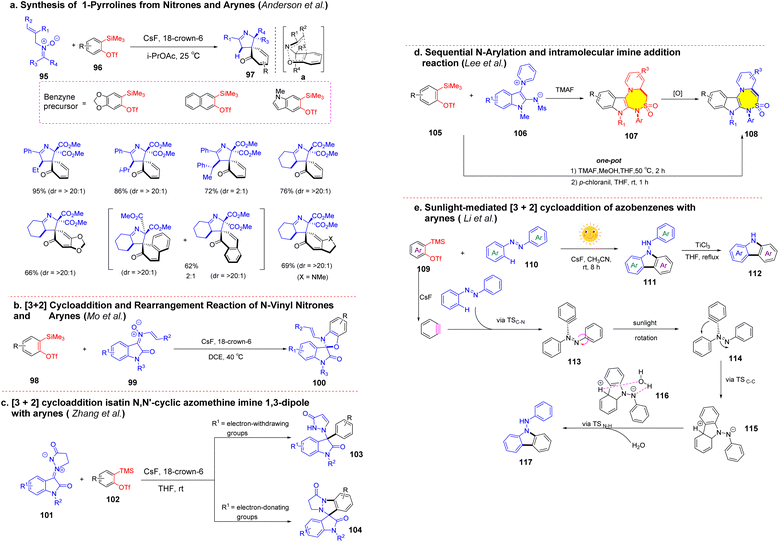
In recent years, the synthesis method of various nitrogen-containing heterocyclic compounds by [3 + 2] cycloaddition of benzynes was established. Anderson et al. developed a cycloaddition rearrangement cascade reaction to access 1-pyrrolines from nitrones and arynes in 2020 (Scheme 17a).80 The reaction provides an altered approach for producing spiropyrrolidines that cannot be obtained through the nitrile pyrrole cycloaddition mechanism and contains unsaturated functional groups that can be further derived by stereoselective functionalization. This cascade reaction is a new pathway in the synthesis of nitrogen-containing heterocyclic compounds via [3,3′]-sigmatropic rearrangement. In 2022, Mo et al. reported similar reactions. Various spirooxindole-benzo[d]oxazoles were prepared by [3 + 2] cycloaddition and selective rearrangement of N-vinyl oxindole nitro compounds and benzynes compounds under transition-metal-free conditions in good to excellent yields (Scheme 17b).81 Zhang et al. developed a [3 + 2] cyclization/arylation of isatin N,N′-cyclic azomethine imine 1,3-dipole with in situ generated benzyne to access 3,3-disubstituted oxindole scaffolds in moderate yields. The method could form various biologically active disubstituted oxindoles with simple and mild reaction advantages, practical and one-pot (Scheme 17c).82 Lee et al. reported that 1,4-thiadiazepine was obtained by sequential N-arylation and intramolecular imine addition of indoloazomethine with 2-(trimethylsilyl)aryl triflates in the presence of TMAF and MeOH.83 Substrates with simple and easy-to-access structures could successfully convert into indolizine compounds in quantitative yield (Scheme 17d). In 2021, Li et al. reported an efficient strategy for the preparation of carbazole via [3 + 2] cycloaddition of azobenzene and arynes at room temperature in the presence of sunlight. The reaction was carried out catalyst-free and showed good functional group compatibility. DFT calculations show that trace H2O plays a crucial role in the formation of the product, the O–H bond cloud promotes aromatization by losing a proton, and the nitrogen anion takes H+ from H2O to realize the protonation reaction forming the final product (Scheme 17e).84
These reactions demonstrate that under relatively green reaction conditions, structurally diverse nitrogen-containing heterocyclic compounds can be constructed by combining suitable benzyne precursors with structurally simple 1,3-dipole dienes. These methods are atomically economical, without involving transition metals, and have the potential for industrial applications.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cross-coupling with nickel as a catalyst. This method is suitable for the in situ formation of various benzynes from o-(trimethylsilyl) aryl triflates to obtain 9,10-dihydrophenanthrenes containing substituted aromatics. It is easy to synthesize stilbene natural products by this method.87 (Scheme 18c).
1 cross-coupling with nickel as a catalyst. This method is suitable for the in situ formation of various benzynes from o-(trimethylsilyl) aryl triflates to obtain 9,10-dihydrophenanthrenes containing substituted aromatics. It is easy to synthesize stilbene natural products by this method.87 (Scheme 18c).
In 2019, with further study of this type of reaction, a new strategy has been successively reported for preparing multifunctional azacyclic compounds from simple and available feedstock.88 The reaction designed a structurally unique domino benzyne intermediate, which efficiently prepared a more complex benzoheterocycle after a continuous nucleophilic reaction and an intramolecular Diels–Alder reaction with styrene as a structural unit. The approach constructed one C–N bond and two C–C bonds in a metal-free catalyst and had excellent chemical selectivity. (Scheme 19).
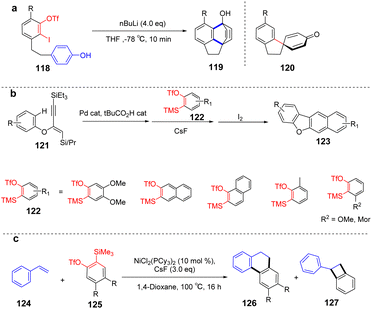
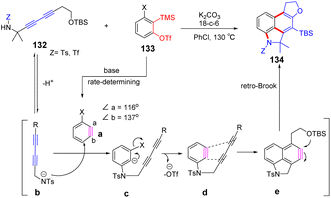
Subsequently, a rapid preparation of naphthalene derivatives reaction from linear 1,3-diyne with benzyne intermediates developed by Hoye et al.89 Benzyne intermediate a can be generated in situ by using K2CO3 as a base, and density functional theory (DFT) shows that the bond angle of carbon–carbon triple bond on intermediate a is distorted. It can be inferred that the sulfonamide group on the linear diyne will preferentially attack a to form intermediate c. Because trifluoromethanesulfonate has a good leaving ability, intermediate d will be rapidly converted to new benzyne intermediate e. Subsequently, the newly formed benzyne intermediate is tripped by linear 1,3-diyne, and an intramolecular [4 + 2] cyclization reaction occurs to obtain the new benzyne intermediate. Finally, the –OTBS is more easily trapped by e, which can produce the final product 134 by continuous nucleophilic addition and Brook rearrangement (Scheme 20).
Two flexible cascade reactions to prepare multiple functionalized heterocyclic compounds were developed. The approach relies on an easily accessible electron-rich-aza-Diels–Alder cycloaddition reaction and a subsequent aromatization step. The strategy has a broad substrate and allows for the rapid total synthesis of the natural product benzo[c]phenanthridine alkaloid nornitidine (Scheme 21a).90
In 2019, Portilla et al. developed a general synthetic strategy for various E-2-arylideneaminopyrroles via benzyne-promoted azo-Diels–Alder cycloaddition, which is key intermediates for nature product pyrrolo[2,3-c]isoquinolines scaffold. There is a strong correlation between the viscosity and fluorescence intensity of these compounds in different solvents, a property that may be useful in fluorescent molecular rotor (FMR) applications (Scheme 21b).91 Other non-commonly benzyne precursors have been reported in cyclization reaction applications. The photolysis of phtaloyl peroxide by laser flash at 266 nm can rapidly remove two CO2 molecules to form benzyne, and N-containing compounds generated via the Diels–Alder reaction of the benzyne intermediate with 1-methylpyrrole-2-carboxylate (Scheme 21c)92 The [4 + 2] cycloaddition reaction of the benzyne precursor (benzenediazonium-2-carboxylate) with azadiene is an effective strategy for the synthesis of aryl-substituted 1,4-dihydroquinolines. These products could be further oxidized to yield 4-arylquinolin-2-ones with biologically active and converted into aryl substituted quinoline compounds (Scheme 21d).93
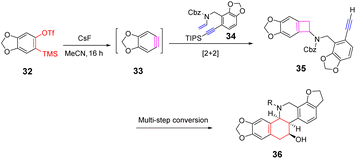
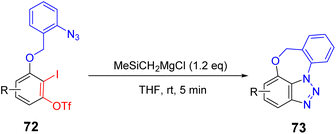
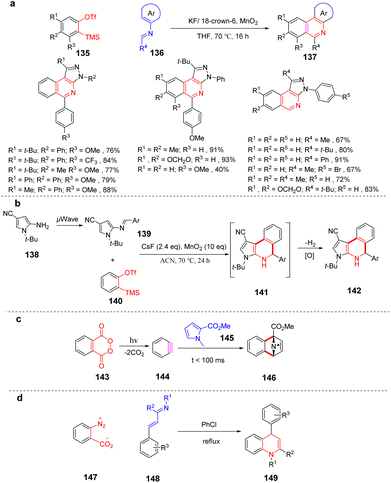
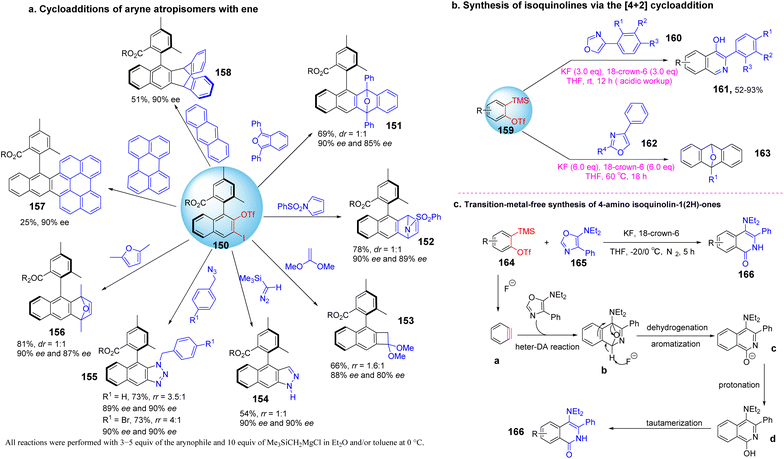
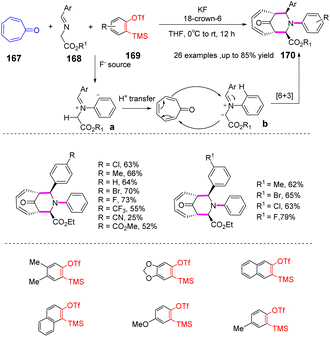
Arynes are widely used as synthetic tools for complex aromatic molecules, such as natural products or large polycyclic aromatic hydrocarbons.94–96 In 2020, Coquerel et al. reported that the active triple bond of enantiomerically enriched aryls could build biaryl stereo-axi compounds.97 The reaction can easily generate benzyne in situ from the corresponding ortho-iodoaryl trifluoride and trimethylsilylmethylmagnesium chloride intermediates and perform normal benzyne cycloaddition reactions in an enantiospecific manner. Cycloaddition of benzyne species with various diene occurs in an enantiospecific, opening up new options for the stereo-controlled synthesis of non-racemic anti-compressive polycyclic compounds in a broad sense (Scheme 22a). In 2022, Raminelli et al. developed potassium fluoride and 18-crown-6 to promote the reaction of various substituted oxazoles with o-(trimethylsilyl)aryl trifluoroesters.98 At room temperature, deserve functionalized isoquinoline compounds were obtained through the [4 + 2] cycloaddition/ring-opening reaction in moderate to good yields. In addition, at a higher temperature (60 °C), bridge oxygen ether compounds can be obtained by the reaction sequence of [4 + 2] cycloaddition, retro-DA, and [4 + 2] cycloaddition. These methods use mild reaction conditions and avoid dangerous aryne precursors and highly toxic reaction conditions (Scheme 22b).
He et al. established a simple and transition metal-free method for the synthesis of 4-amino isoquinolin-1(2H)-ones by multi-step sequence reaction. The strategy involves the tandem Diels–Alder reaction/dehydrogenation, aromatization/tautomerization reaction of benzyne with 4,5-disubstituted oxazoles to access the final product in moderate to excellent yields. The reaction is easy to scale up, and the product can effectively convert into isoquinoline derivatives (Scheme 22c).99
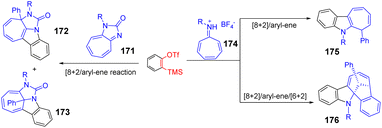
He et al. reported the tandem reaction of [8 + 2]/aryl–olefins between N-heptafluoroethylene and arylacetylene to synthesize cyclohepta[b]indole with biological significance.101 The [6 + 2] cycloaddition was also applied to alkynes species, which further derivatized oxacyclohepta[b]indoles to polycyclic oxhept[b]indoles. The tandem reaction provides a new channel for the synthesis of cyclohepta[b]indoles and oxacyclohepta[b]indoles (Scheme 24).
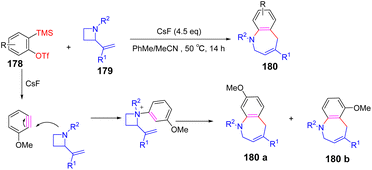
Saito et al. established a novel method to access benzazocine derivatives by the [6 + 2] cycloaddition reaction of 2-vinylazetidines with benzyne without a catalyst102 (Scheme 25). The regioselectivity of aromatic (180a, 180b) can be explained in terms of electronic effects and steric effects of the substituents. The reaction is initiated by the nucleophilic attack of the nitrogen atom of vinylazetidine to benzyne. The zwitterionic species could be protonated to produce an azetidinium salt. The azetidine salt will be rearranged under reaction conditions to provide ring expansion products.
In 2011, Lauten et al. developed a highly stereoscopic and regionally selective intramolecular alder–ene reaction by a benzyne intermediate to obtain a series of aromatic heterocyclic compounds with good yields (Scheme 26).103 Alder-ene reactions are usually synergistic mechanisms, but a reaction pathway involving free radical intermediates has also been proposed. DFT calculations suggest that the reaction may undergo synergistic or step-by-step mechanisms. Control experiments on cis-allyl hydrogen transfer were shown to be carried out in the transition state of trans-allyl hydrogen transfer. This process might be caused by the electron-rich alkene group attacking the electron-poor benzyne intermediate preferentially, resulting in the formation of C–C bonds taking precedence over C–H bonds, which enables regionally selective hydrogen migration and ensuring stereoselectivity and regional selectivity of the reaction.
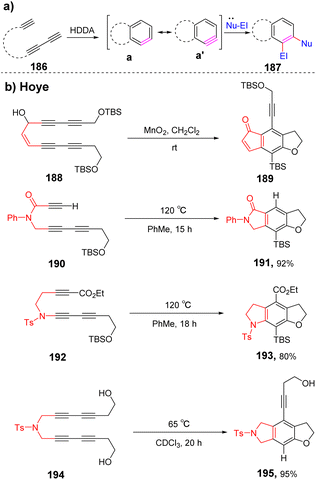 | ||
| Scheme 27 HDDA cycloaromatization reactions, TBS = tert-butyldimethylsilyl, Ts = para-toluenesulfony. (a) The general mode of HDDA reaction, (b) intramolecular trapping reactions of HDDA. | ||
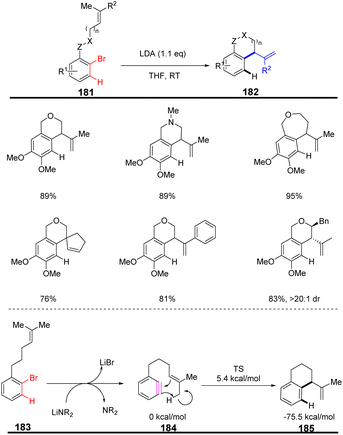
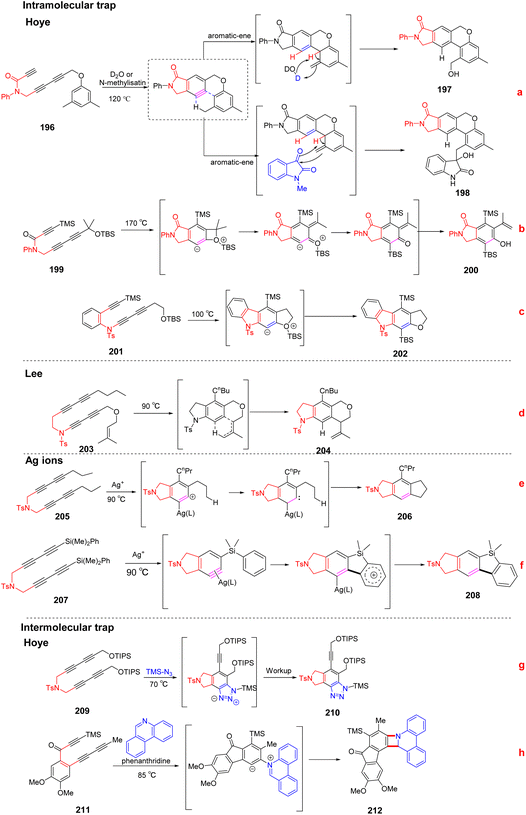
In 2014, Hoye et al. reported that HDDA benzynes were utilized to access the first high-yielding aromatic ene reactions.107 (Scheme 28a) HDDA benzynes intermediates are usually unstable and can react in situ with nucleophiles to obtain N-containing compounds with complex structures. Subsequently, they have conducted in-depth research on HDDA reaction and enriched the methods for obtaining various nitrogen-containing heterocyclic compounds by changing the HDDA benzynes precursors. In 2019, Hoye et al. developed that certain poly-yne substrates could efficiently occur a tandem thermal isomerization by an o-QM intermediate (quinone methides) to construct isoindolinone skeleton110 (Scheme 28b). Experiments and DFT calculations verify that this conversion process may be achieved through multiple reaction intermediates: thermally generated benzyne, strained benzoxetenonium ion, and o-quinone methide base compound. The overall process can be very efficient in obtaining a variety of heterocyclic compounds. A benzenoid ring in fused polycyclic heteroaromatic carbazole (i.e., [2,3]-benzoindole) skeletons can be synthesized in a single step via the HDDA reaction of appropriate benzyne precursors. This strategy allows the generation of highly substituted aromatics111 (Scheme 28c).
Lee et al. developed an efficient intramolecular ene reaction of benzyne intermediate that directly generate the corresponding benzoheterocycle. Alkene donors are tethered to the incipient aryne moiety is one of the most favorable reactions for the ene reaction. In addition, the size of the linker linking the alkene donor to the 1,3-diyne moiety and the substituent pattern on the alkene significantly affect the efficiency of the alkene ring closure process, notable, the linking of two 1,3-diynes or alkenes and heteroatoms in the linker of 1,3-diynes appear to have the most significant impact on the reactivity of various substrates.112 (Scheme 28d).
It has also been reported that transition metal additives can change the reaction route of benzynes, and one of the earliest examples of a metal addition effect was observed with silver ions (Ag+). The current finding is that benzyne is significantly more reactive and selective towards p-nucleophiles in the presence of Ag+ than the corresponding reaction in the absence of Ag+. Ag+ can interact with benzynes to form reactive intermediates that behave like a silver-bound aryl cation or 1,2-carbene-silver carbene (Scheme 28e and f).16,113–115
The construction of benzoheterocyclic compounds by HDDA reaction intermolecular trap has also been reported. Hoye et al. reported that the 1,3-dipolar (trimethylsilyl azide) and heterocyclic dienes (furan or pyrrole) could be used as an intermolecular trap for benzynes produced by the HDDA reaction of triynes to construct nitrogen-containing heterocycles with relatively high structural complexity fast. This strategy has the potential to synthesize diverse scaffolds and can be a candidate method for the discovery and synthesis of complex drugs116 (Scheme 28g). The thermal HDDA cycloisomerization of different benzyne precursors (triyne or tetrayne) yields intermediates that can achieve different modes of intermolecular trap reactions117 (Scheme 28h). The initially formed 1,3-zwitterionic species (i) can collapse intramolecularly, giving a new 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct of heterocycle and benzyne; (ii) can react with an added electrophilic third component, giving functionalized heterocyclic products; or (iii) can react with external protic nucleophiles to generate various three-component assemblies after the collapse of ion pairs generated by protonation of zwitterions.
1 adduct of heterocycle and benzyne; (ii) can react with an added electrophilic third component, giving functionalized heterocyclic products; or (iii) can react with external protic nucleophiles to generate various three-component assemblies after the collapse of ion pairs generated by protonation of zwitterions.
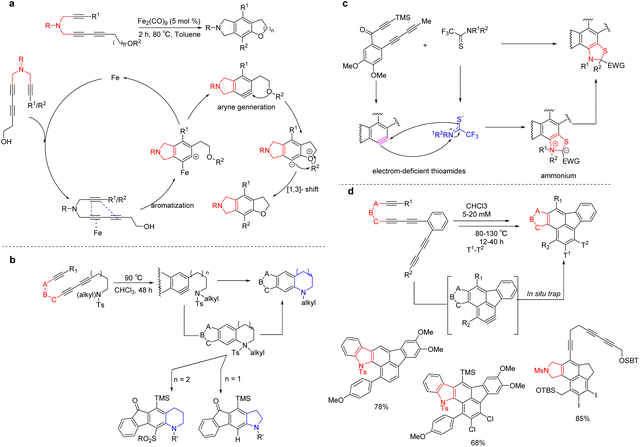
Recently, it has become possible to construct novel nitrogen-containing compounds involving HDDA benzyne intermediates. A facile method for the synthesis of fused isoindolines via the Fe2(CO)9-catalyzed cascade HDDA strategy was developed by Wang et al.118 The main features of this reaction are simple operation, atomic efficiency, time economy, high yield, and wide substrate range. The reaction process mainly undergoes [4 + 2] cycloaddition to generate the benzyne, intramolecular –OH nucleophile trap, [1, 3]-H shift to give the product (Scheme 29a).
Saturated heterocycles [tetrahydroquinolines (n = 2) and indolines (n = 1)] can be synthesized by using the zwitterionic intermediate produced via the reaction of the nitrogen in the tertiary sulfonamide group in the molecule with the benzyne produced by HDDA119(Scheme 29b). The different nitrogen heterocycles and the nature of the substituents present on the zwitterionic nitrogen atoms (alkyl versus aryl) lead to different reaction pathways. Each reaction result can access a new, saturated, benzo-fused piperidine (i.e., a tetrahydroquinoline) or pyrrolidine (i.e., indoline) ring.
The dihydrobenzothiazole could be obtained by reacting thioamides with electron-withdrawing groups on the carbon atoms of thiocarbonyls with benzynes (produced by the hexahydro-Diels–alder cycloisomerization reaction)120(Scheme 29c). The reaction resulted in the formation of stable ammoniates via a rare [3 + 2] cycloaddition reaction. The cycloaddition reactions of different electron-withdrawing groups (including –CF3, –CN, and –CO2Me) in thioamides and various benzyne precursors can obtain the target products smoothly.
Construction of polyacene nitrogen-containing fused aromatics by domino HDDA reaction reported by Hoye et al. in 2018121 (Scheme 29d). A series of staged cycloisomerization reactions can proceed within substrates containing varying numbers of 1,3-diyne units, which each unit separated by a two-atom spacer. The cascade reaction is initiated by a diynophilic alkyne uniquely attached by a three-atom tether. This reagent-free thermal reaction is robust, broad in substrate scope, and yields considerable structural complexity. These methods provide a new channel for the construction of structurally novel and broadly applicable nitrogen-containing compounds.
3 Conclusions
In summary, nitrogen-containing heterocyclic compounds are widely found in natural products, drug molecules, and organic functional materials. Among the many methods for preparing benzoheterocycles, the benzyne species cyclization strategy is an attractive method for constructing benzoheterocyclic molecular skeletons because it can efficiently and economically obtain complex and diverse heterocyclic compounds under mild conditions. Examples of new benzyne precursors and new trapping reagents or reactions will continue to emerge as increasing numbers of investigators contemplate the opportunities this approach provides to in situ forming benzynes. Studies of benzyne cycloaddition reactions have also provided new mechanistic understanding and insights about benzyne reactivity, and many additional such results are embedded in the primary publications cited here. Given the substantial body of evidence represented by the numerous successes described in this review and the enduring relevance of benzynes to access benzoheterocycle compounds, the field is likely to expand as chemists address challenges in sustainability, atom economy, and new patterns of reactivity for benzazepines synthesis. Overall, the recent developments in benzyne chemistry have been primarily driven by the availability of classical precursors. Further progress in this field is expected to open new avenues for the synthesis of nitrogen-containing heterocyclic compounds under mild, environmentally friendly, low-cost, and operationally simple conditions.Conflicts of interest
There are no conflicts to declare.Notes and references
- J. A. Garcia-Lopez and M. F. Greaney, Chem. Soc. Rev., 2016, 45, 6766–6798 RSC.
- I. Takahashi, T. Fujita, N. Shoji and J. Ichikawa, Chem. Commun., 2019, 55, 9267–9270 RSC.
- D. Hamprecht, F. Micheli, G. Tedesco, A. Checchia, D. Donati, M. Petrone, S. Terreni and M. Wood, Bioorg. Med. Chem. Lett., 2007, 17, 428–433 CrossRef CAS PubMed.
- W.-T. Jiaang, Y.-S. Chen, T. Hsu, S.-H. Wu, C.-H. Chien, C.-N. Chang, S.-P. Chang, S.-J. Lee and X. Chen, Bioorg. Med. Chem. Lett., 2005, 15, 687–691 CrossRef CAS PubMed.
- J. He, D. Qiu and Y. Li, Acc. Chem. Res., 2020, 53, 508–519 CrossRef CAS PubMed.
- L. L. Fluegel and T. R. Hoye, Chem. Rev., 2021, 121, 2413–2444 CrossRef CAS PubMed.
- Y. Y. Shinichi Saito, Chem. Rev., 2000, 100, 2901–2915 CrossRef PubMed.
- H. H. Wenk, M. Winkler and W. Sander, Angew. Chem., Int. Ed., 2003, 42, 502–528 CrossRef CAS PubMed.
- A. Yoshimura, A. Saito and V. V. Zhdankin, Chem, 2018, 24, 15156–15166 CrossRef CAS PubMed.
- J. Maier and T. B. Marder, Chem, 2021, 27, 7978–7991 CrossRef CAS PubMed.
- R. v. Stoermer and B. Kahlert, Ber. Dtsch. Chem. Ges., 1902, 35, 1633–1640 CrossRef CAS.
- W. Bachmann and H. Clarke, J. Am. Chem. Soc., 1927, 49, 2089–2098 CrossRef CAS.
- J. Tan and J. Chen, in Comprehensive Aryne Synthetic Chemistry, Elsevier, 2022, pp. 125–221 Search PubMed.
- J. Mortier, Arene chemistry: reaction mechanisms and methods for aromatic compounds, John Wiley & Sons, 2015 Search PubMed.
- P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550–3577 CrossRef CAS PubMed.
- R. Karmakar and D. Lee, Chem. Soc. Rev., 2016, 45, 4459–4470 RSC.
- M. Uchiyama, T. Miyoshi, Y. Kajihara, T. Sakamoto, Y. Otani, T. Ohwada and Y. Kondo, J. Am. Chem. Soc., 2002, 124, 8514–8515 CrossRef CAS PubMed.
- K. C. Caster, C. G. Keck and R. D. Walls, J. Org. Chem., 2001, 66, 2932–2936 CrossRef CAS PubMed.
- M. Stiles, U. Burckhardt and G. Freund, J. Org. Chem., 1967, 32, 3718–3719 CrossRef CAS.
- R. S. Berry, J. Clardy and M. E. Schafer, J. Am. Chem. Soc., 1964, 86, 2738–2739 CrossRef CAS.
- G. Wittig and R. Hoffmann, Org. Synth., 2003, 47, 4 Search PubMed.
- S. Nakazawa, T. Kiyosawa, K. Hirakawa and H. Kato, J. Chem. Soc., Chem. Commun., 1974, 621a RSC.
- S. E. Whitney, M. Winters and B. Rickborn, J. Org. Chem., 1990, 55, 929–935 CrossRef CAS.
- Y. Himeshima, T. Sonoda and H. Kobayashi, Chem. Lett., 1983, 12, 1211–1214 CrossRef.
- Y. Liang, X. Hong, P. Yu and K. Houk, Org. Lett., 2014, 16, 5702–5705 CrossRef CAS PubMed.
- T. Wang, D. Niu and T. R. Hoye, J. Am. Chem. Soc., 2016, 138, 7832–7835 CrossRef CAS PubMed.
- T. R. Hoye, B. Baire, D. Niu, P. H. Willoughby and B. P. Woods, Nature, 2012, 490, 208–212 CrossRef CAS PubMed.
- R. Karmakar and D. Lee, Org. Lett., 2016, 18, 6105–6107 CrossRef CAS PubMed.
- P. Wessig and G. Müller, Chem. Rev., 2008, 108, 2051–2063 CrossRef CAS PubMed.
- F. García, D. Peña, D. Pérez and E. Guitián, Modern Aryne Chemistry, 2021, pp. 27–68 Search PubMed.
- K. A. Spence, J. V. Chari, M. Di Niro, R. B. Susick, N. Ukwitegetse, P. I. Djurovich, M. E. Thompson and N. K. Garg, Chem. Sci., 2022, 13, 5884–5892 RSC.
- A. M. Orendt, J. C. Facelli, J. G. Radziszewski, W. J. Horton, D. M. Grant and J. Michl, J. Am. Chem. Soc., 1996, 118, 846–852 CrossRef CAS.
- R. Warmuth, Chem. Commun., 1998, 59–60 RSC.
- J. G. Radziszewski, B. A. Hess Jr and R. Zahradnik, J. Am. Chem. Soc., 1992, 114, 52–57 CrossRef CAS.
- N. Pavliček, B. Schuler, S. Collazos, N. Moll, D. Pérez, E. Guitián, G. Meyer, D. Peña and L. Gross, Nat. Chem., 2015, 7, 623–628 CrossRef PubMed.
- T. Kitamura, Z. Meng and Y. Fujiwara, Tetrahedron Lett., 2000, 41, 6611–6614 CrossRef CAS.
- T. Kitamura, M. Yamane, K. Inoue, M. Todaka, N. Fukatsu, Z. Meng and Y. Fujiwara, J. Am. Chem. Soc., 1999, 121, 11674–11679 CrossRef CAS.
- S. Kovacs, A. I. Csincsi, T. Z. Nagy, S. Boros, G. Timári and Z. Novak, Org. Lett., 2012, 14, 2022–2025 CrossRef CAS PubMed.
- K. Devaraj, F. J. Ingner, C. Sollert, P. J. Gates, A. Orthaber and L. T. Pilarski, J. Org. Chem., 2019, 84, 5863–5871 CrossRef CAS PubMed.
- B. Michel and M. F. Greaney, Org. Lett., 2014, 16, 2684–2687 CrossRef CAS PubMed.
- T. Ikawa, T. Nishiyama, T. Nosaki, A. Takagi and S. Akai, Org. Lett., 2011, 13, 1730–1733 CrossRef CAS PubMed.
- Q. Chen, H. Yu, Z. Xu, L. Lin, X. Jiang and R. Wang, J. Org. Chem., 2015, 80, 6890–6896 CrossRef CAS PubMed.
- M. Mesgar and O. Daugulis, Org. Lett., 2016, 18, 3910–3913 CrossRef CAS PubMed.
- T. Ikawa, S. Masuda, H. Nakajima and S. Akai, J. Org. Chem., 2017, 82, 4242–4253 CrossRef CAS PubMed.
- Y. Li, C. Mück-Lichtenfeld and A. Studer, Angew. Chem., Int. Ed., 2016, 55, 14435–14438 CrossRef CAS PubMed.
- N. Mariet, M. Ibrahim-Ouali and M. Santelli, Tetrahedron Lett., 2002, 43, 5789–5791 CrossRef CAS.
- P. Maurin, M. Ibrahim-Ouali, J.-L. Parrain and M. Santelli, J. Mol. Struct., 2003, 637, 91–100 CrossRef CAS.
- Z. Qiu and Z. Xie, Angew. Chem., 2009, 121, 5839–5842 CrossRef.
- J. Caeiro, D. Pena, A. Cobas, D. Perez and E. Guitián, Adv. Synth. Catal., 2006, 348, 2466–2474 CrossRef CAS.
- J. B. Feltenberger, R. Hayashi, Y. Tang, E. S. Babiash and R. P. Hsung, Org. Lett., 2009, 11, 3666–3669 CrossRef CAS PubMed.
- Z.-X. Ma, J. B. Feltenberger and R. P. Hsung, Org. Lett., 2012, 14, 2742–2745 CrossRef CAS PubMed.
- Z. Xiong, J. Duan, X. Li, X. Wang, Y. Addepalli, M. Lu, W. Yao, L. He and Z. Wang, J. Org. Chem., 2021, 86, 12403–12411 CrossRef CAS PubMed.
- J. Shi, H. Xu, D. Qiu, J. He and Y. Li, J. Am. Chem. Soc., 2017, 139, 623–626 CrossRef CAS PubMed.
- C. Lu, A. V. Dubrovskiy and R. C. Larock, J. Org. Chem., 2012, 77, 2279–2284 CrossRef CAS PubMed.
- D. Chang, D. Zhu and L. Shi, J. Org. Chem., 2015, 80, 5928–5933 CrossRef CAS PubMed.
- I. Briguglio, S. Piras, P. Corona, E. Gavini, M. Nieddu, G. Boatto and A. Carta, Eur. J. Med. Chem., 2015, 97, 612–648 CrossRef CAS PubMed.
- Y. Ren, L. Zhang, C.-H. Zhou and R.-X. Geng, Med. Chem., 2014, 4, 640–662 Search PubMed.
- X.-M. Peng, G.-X. Cai and C.-H. Zhou, Curr. Top. Med. Chem., 2013, 13, 1963–2010 CrossRef CAS PubMed.
- R. R. Kale, V. Prasad, P. P. Mohapatra and V. K. Tiwari, Monatsh. Chem., 2010, 141, 1159–1182 CrossRef CAS.
- E. Loukopoulos and G. E. Kostakis, Coord. Chem. Rev., 2019, 395, 193–229 CrossRef CAS.
- T. Jin and Y. Yamamoto, Angew. Chem., Int. Ed., 2007, 46, 3323–3325 CrossRef CAS PubMed.
- C. Spiteri, S. Keeling and J. E. Moses, Org. Lett., 2010, 12, 3368–3371 CrossRef CAS PubMed.
- Z. Liu, F. Shi, P. D. Martinez, C. Raminelli and R. C. Larock, J. Org. Chem., 2008, 73, 219–226 CrossRef CAS PubMed.
- P. Li, J. Zhao, C. Wu, R. C. Larock and F. Shi, Org. Lett., 2011, 13, 3340–3343 CrossRef CAS PubMed.
- G. Chen, M. Hu and Y. Peng, J. Org. Chem., 2018, 83, 1591–1597 CrossRef CAS PubMed.
- P. Sureshbabu, V. Bhajammanavar, V. S. K. Choutipalli, V. Subramanian and M. Baidya, Chem. Commun., 2022, 58, 1187–1190 RSC.
- L. Campbell-Verduyn, P. H. Elsinga, L. Mirfeizi, R. A. Dierckx and B. L. Feringa, Org. Biomol. Chem., 2008, 6, 3461–3463 RSC.
- G. Singh, R. Kumar, J. Swett and B. Zajc, Org. Lett., 2013, 15, 4086–4089 CrossRef CAS PubMed.
- F. Shi, J. P. Waldo, Y. Chen and R. C. Larock, Org. Lett., 2008, 10, 2409–2412 CrossRef CAS PubMed.
- H. Ankati and E. Biehl, Tetrahedron Lett., 2009, 50, 4677–4682 CrossRef CAS.
- S. Chandrasekhar, M. Seenaiah, C. L. Rao and C. R. Reddy, Tetrahedron, 2008, 64, 11325–11327 CrossRef CAS.
- F. Zhang and J. E. Moses, Org. Lett., 2009, 11, 1587–1590 CrossRef CAS PubMed.
- M. Kleoff, L. Boeser, L. Baranyi and P. Heretsch, Eur. J. Org. Chem., 2021, 2021, 979–982 CrossRef CAS.
- S. Yoshida, T. Morita and T. Hosoya, Chem. Lett., 2016, 45, 726–728 CrossRef CAS.
- J. Guo, I. C. Kiran, R. S. Reddy, J. Gao, M. Tang, Y. Liu and Y. He, Org. Lett., 2016, 18, 2499–2502 CrossRef CAS PubMed.
- J. Yuan, X. Y. Wu and F. Sha, Eur. J. Org. Chem., 2016, 2016, 2929–2932 CrossRef CAS.
- H. Xu, J. He, J. Shi, L. Tan, D. Qiu, X. Luo and Y. Li, J. Am. Chem. Soc., 2018, 140, 3555–3559 CrossRef CAS PubMed.
- H. Ryu, J. Seo and H. M. Ko, J. Org. Chem., 2018, 83, 14102–14109 CrossRef CAS PubMed.
- Y.-K. Li, M.-X. Cui, F. Sha, Q. Li and X.-Y. Wu, Org. Biomol. Chem., 2019, 17, 8963–8968 RSC.
- A. S. Alshreimi, G. Zhang, T. W. Reidl, R. L. Peña, N. G. Koto, S. M. Islam, D. J. Wink and L. L. Anderson, Angew. Chem., Int. Ed., 2020, 59, 15244–15248 CrossRef CAS PubMed.
- H. Yuan, D. L. Lu, C. Liang and D. L. Mo, Adv. Synth. Catal., 2022, 364, 1409–1414 CrossRef CAS.
- Q. Jin, D. Zhang and J. Zhang, RSC Adv., 2020, 10, 30620–30623 RSC.
- D. K. Kim, J. Y. Son, I. Jung, N. Heo, S. H. Han, D. Kim and P. H. Lee, Adv. Synth. Catal., 2021, 363, 1358–1367 CrossRef CAS.
- W. Zhang, J. Bu, L. Wang, P. Li and H. Li, Org. Chem. Front., 2021, 8, 5045–5051 RSC.
- H. Takikawa, A. Nishii, H. Takiguchi, H. Yagishita, M. Tanaka, K. Hirano, M. Uchiyama, K. Ohmori and K. Suzuki, Angew. Chem., Int. Ed., 2020, 59, 12440–12444 CrossRef CAS PubMed.
- Y. Minami, Y. Furuya and T. Hiyama, Chem. - Eur. J., 2020, 26, 9471–9474 CrossRef CAS PubMed.
- T. Kubo, T. Fujita and J. Ichikawa, Chem. Lett., 2020, 49, 264–266 CrossRef CAS.
- J. He, Z. Jia, H. Tan, X. Luo, D. Qiu, J. Shi, H. Xu and Y. Li, Angew. Chem., Int. Ed., 2019, 58, 18513–18518 CrossRef CAS PubMed.
- X. Xiao and T. R. Hoye, J. Am. Chem. Soc., 2019, 141, 9813–9818 CrossRef CAS PubMed.
- J.-C. Castillo, J. Quiroga, R. Abonia, J. Rodriguez and Y. Coquerel, Org. Lett., 2015, 17, 3374–3377 CrossRef CAS PubMed.
- J.-C. Castillo, A. Tigreros, Y. Coquerel, J. Rodríguez, M. A. Macías and J. Portilla, ACS Omega, 2019, 4, 17326–17339 CrossRef CAS PubMed.
- J. Torres-Alacan, J. Org. Chem., 2016, 81, 1151–1156 CrossRef CAS PubMed.
- S. Stokes, M. Bekkam, M. Rupp and K. T. Mead, Synlett, 2012, 2012, 389–392 Search PubMed.
- J.-A. García-López and M. F. Greaney, Chem. Soc. Rev., 2016, 45, 6766–6798 RSC.
- H. Takikawa, A. Nishii, T. Sakai and K. Suzuki, Chem. Soc. Rev., 2018, 47, 8030–8056 RSC.
- I. Pozo, E. Guitián, D. Pérez and D. Peña, Acc. Chem. Res., 2019, 52, 2472–2481 CrossRef CAS PubMed.
- Y.-L. Wei, G. Dauvergne, J. Rodriguez and Y. Coquerel, J. Am. Chem. Soc., 2020, 142, 16921–16925 CrossRef CAS PubMed.
- T. R. Silva, V. V. Souza and C. Raminelli, ChemistrySelect, 2022, 7, e202203039 CAS.
- S. Liu, P. Xie, L. Wu, J. Zhao, Z. Cai and L. He, Org. Chem. Front., 2022, 9, 1550–1555 RSC.
- A. Guin, R. N. Gaykar, S. Deswal and A. T. Biju, Org. Lett., 2021, 23, 7456–7461 CrossRef CAS PubMed.
- Z. Wang, Y. Addepalli and Y. He, Org. Lett., 2018, 20, 644–647 CrossRef CAS PubMed.
- T. Aoki, S. Koya, R. Yamasaki and S. Saito, Org. Lett., 2012, 14, 4506–4509 CrossRef CAS PubMed.
- D. A. Candito, J. Panteleev and M. Lautens, J. Am. Chem. Soc., 2011, 133, 14200–14203 CrossRef CAS PubMed.
- A. Z. Bradley and R. P. Johnson, J. Am. Chem. Soc., 1997, 119, 9917–9918 CrossRef CAS.
- K. Miyawaki, R. Suzuki, T. Kawano and I. Ueda, Tetrahedron Lett., 1997, 38, 3943–3946 CrossRef CAS.
- B. Baire, D. Niu, P. H. Willoughby, B. P. Woods and T. R. Hoye, Nat. Protoc., 2013, 8, 501–508 CrossRef CAS PubMed.
- D. Niu and T. R. Hoye, Nat. Chem., 2014, 6, 34–40 CrossRef CAS PubMed.
- D. Niu, P. H. Willoughby, B. P. Woods, B. Baire and T. R. Hoye, Nature, 2013, 501, 531–534 CrossRef CAS PubMed.
- L. L. Fluegel and T. R. Hoye, Chem. Rev., 2021, 121, 2413–2444 CrossRef CAS PubMed.
- H. Shen, X. Xiao and T. R. Hoye, Org. Lett., 2019, 21, 1672–1675 CrossRef CAS PubMed.
- Y. Wang, L. Zheng and T. R. Hoye, Org. Lett., 2018, 20, 7145–7148 CrossRef CAS PubMed.
- R. Karmakar, P. Mamidipalli, S. Y. Yun and D. Lee, Org. Lett., 2013, 15, 1938–1941 CrossRef CAS PubMed.
- D. Lee and S. Ghorai, Silver Catalysis in Organic Synthesis, 2019, 33–83 Search PubMed.
- K.-P. Wang, S. Y. Yun, P. Mamidipalli and D. Lee, Chem. Sci., 2013, 4, 3205–3211 RSC.
- N.-K. Lee, S. Y. Yun, P. Mamidipalli, R. M. Salzman, D. Lee, T. Zhou and Y. Xia, J. Am. Chem. Soc., 2014, 136, 4363–4368 CrossRef CAS PubMed.
- J. Chen, B. Baire and T. R. Hoye, Heterocycles, 2014, 88, 1191 CrossRef CAS PubMed.
- S. Arora, J. Zhang, V. Pogula and T. R. Hoye, Chem. Sci., 2019, 10, 9069–9076 RSC.
- J. K. Vandavasi, W.-P. Hu, C.-T. Hsiao, G. C. Senadi and J.-J. Wang, RSC Adv., 2014, 4, 57547–57552 RSC.
- T. Wang and T. R. Hoye, J. Am. Chem. Soc., 2016, 138, 13870 CrossRef CAS PubMed.
- J. Zhang, A. C. Page, V. Palani, J. Chen and T. R. Hoye, Org. Lett., 2018, 20, 5550–5553 CrossRef CAS PubMed.
- X. Xiao and T. R. Hoye, Nat. Chem., 2018, 10, 838–844 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |