Open Access Article
Open Access ArticleFirst principles calculation to investigate the effect of Mn substitution on Cu site in CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) system
F. F. Alharbia,
Shahid Mehmoodb,
Zahid Alib,
Salma Amanc,
Rabia Yasmin Khosad,
Vladimir G. Kostishyne,
Sergei V. Trukhanov ef,
M. I. Sayyedg,
Daria I. Tishkevich
ef,
M. I. Sayyedg,
Daria I. Tishkevich *f and
Alex V. Trukhanov
*f and
Alex V. Trukhanov ef
ef
aDepartment of Physics, College of Science, Princess Nourah Bint Abdulrahman University, P. O. Box 84428, Riyadh 11671, Saudi Arabia
bCenter for Computational Materials Science, Department of Physics, University of Malakand, Chakdara, Dir (Lower), 18800 Pakistan
cInstitute of Physics, Khwaja Fareed University of Engineering and Information Technology, Abu Dhabi Road, Rahim Yar Khan – 64200, Pakistan. E-mail: salma.physics.kfu@gmail.com
dUniversity of Education, Lahore, Dera Ghazi Khan Campus, D. G. Khan, 32200, Pakistan
eSmart Sensors Laboratory, Department of Electronic Materials Technology, National University of Science and Technology MISiS, 119049 Moscow, Russia
fLaboratory of Magnetic Films Physics, Scientific-Practical Materials Research Centre of National Academy of Sciences of Belarus, 220072 Minsk, Belarus
gDepartment of Physics, Faculty of Science, Isra University, 1162 Amman, Jordan
First published on 26th April 2023
Abstract
Structural, electronic, elastic and magnetic properties of CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) system have been carried out through DFT using GGA, GGA+U and HF potential. The investigation of structural optimization reveals that lattice parameters of the understudy system is reliable with the reported results and are increasing with the Mn substitution due to their greater atomic radii as compare to Cu atom. Both the cohesive energy and the enthalpy show that CeCu3V4O12 is the most thermodynamically stable among these compounds. When Mn is replaced by Cu in these compounds, not only it become semi-metals, but the host compound also changes from non-magnetic to anti-ferromagnetic and their electrical resistance provides further credence to their electronic behavior. Mechanical stability, anisotropy, and ductility are all demonstrated through the elastic characteristics of these compounds. Due to anti-ferromagnetic ductile nature of the Mn base compounds, it is expected that the compounds in the system may use for spintronic application and in magnetic cloaking devices.
Introduction
Pulsed laser deposition (PLD) enables the hetero-epitaxial development of functional oxides, providing advantages for controlling their structural and physical properties. For instance, the critical temperature of superconducting cuprates is elevated by lateral compressive strain to a greater extent than by equivalent hydrostatic pressure.1 Bismuth ferrites on LaAlO3 and YAlO3 (ref. 2–5) are two examples of films where epitaxial stress caused by lattice mismatches influences the ferroelectric characteristics or even changes the morpho-tropic phase. Similarly, doping can produce electronic or magnetic changes in certain perovskites manganates6 or cobaltaties.7 Many magnetic thin films show strain-induced magnetic anisotropy.8–10 The occurrences above are primarily due to the interaction between the lattice changing and physical characteristics that determine the functionality of the oxides. In this investigation, we focus on a specific subset of perovskite oxides known as “quadruple perovskites,” which have the general formula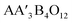 . A′–B inter-metallic charge transfer massive dielectric permittivity, oxygen reaction catalysis, negative thermal expansion and half-metallic11–18 characteristics are just some of the many unusual qualities that have garnered them a lot of attention. The A site is often filled by alkali to rare-earth metals. In contrast, the A′ site is normally taken by Cu2+ or Mn3+ elements.
. A′–B inter-metallic charge transfer massive dielectric permittivity, oxygen reaction catalysis, negative thermal expansion and half-metallic11–18 characteristics are just some of the many unusual qualities that have garnered them a lot of attention. The A site is often filled by alkali to rare-earth metals. In contrast, the A′ site is normally taken by Cu2+ or Mn3+ elements.
The cation ratio between the 12-coordinate A cation and the 3-coordinate A′ cation is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The sizeable octahedral tilting of BO6 stabilizes this A-site order. The quadruple perovskite oxides have a lattice parameter of 3.6–3.7 Å, making them quite dense. This could make sample preparation more challenging; conventional methods often include high-pressure synthesis at several giga pascal's. CaCu3Mn4O12 (ref. 19–22) which is semiconducting, may be transformed into metal by replacing the Ca atoms with La or Bi atoms,10,11 demonstrating the intriguing consequences seen for A-site replacements in these perovskites.
3. The sizeable octahedral tilting of BO6 stabilizes this A-site order. The quadruple perovskite oxides have a lattice parameter of 3.6–3.7 Å, making them quite dense. This could make sample preparation more challenging; conventional methods often include high-pressure synthesis at several giga pascal's. CaCu3Mn4O12 (ref. 19–22) which is semiconducting, may be transformed into metal by replacing the Ca atoms with La or Bi atoms,10,11 demonstrating the intriguing consequences seen for A-site replacements in these perovskites.
Charge disproportionation is also present in the isotropic combination CaCu3Fe4O12. At the same time, the introduction of La or Bi into the A-site results in a markedly different pattern of inter-site charge transfer. The concentrated electrons in the A-site cations are accountable for their magnetic properties. In contrast, the oxygen atoms in the B-site cations play a significant function in the material's electronic nature.9,12 Perovskites arranged along the A-site with van der Waals valence at the B-sites exhibit intriguing behaviors. Paramagnetic and metallic properties are exhibited by Cu at A′ and V at B sites, respectively. When V is substituted into the A′-site, the resulting change in the energy level of the Fermi level is distinct from combinations with Mn in the B-site or Fe.13 Compounds in which A′-site is composed of both Mn and Cu and B-site is occupied by V are rare and less studied such as CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) system. CeCu3V4O12 is studied by Kadyrova et al.23 and reported their metallic paramagnetic nature. CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) is only synthesized by the same group and only their structure properties were studied.24 CeMn3V4O12 were studied by Kadyrova et al. experimentally25 and theoretical26 study demonstrates that the anti-ferromagnetism in this compound is due to Mn–O–Mn super exchange interaction.
The study of doping of elements on B-site in 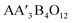 compounds are available extensively27–29 while very little study on the physical properties of doping at A′-site is reported such that transition from ferrimagnetic to anti-ferromagnetic in LaCu3Mn4O12 (ref. 30) and from ferromagnetic to anti-ferromagnetic in RCu3Mn4O12 (R = Nd and Pr)31 and recently spin glass state is reported in NdCu3Mn4O12 by Mn substitutions at Cu site.32 Keeping in view the effect of substitution at Cu site, the present study is performed to investigate the electronic structure in addition to elastic, thermoelectric and magnetic properties. The exchange correlation GGA, GGA with Hubbard U and HF are used within the WEIN2K package following the density functional theory (DFT) techniques for cutting-edge magnetic memory devises and spintronic technology.
compounds are available extensively27–29 while very little study on the physical properties of doping at A′-site is reported such that transition from ferrimagnetic to anti-ferromagnetic in LaCu3Mn4O12 (ref. 30) and from ferromagnetic to anti-ferromagnetic in RCu3Mn4O12 (R = Nd and Pr)31 and recently spin glass state is reported in NdCu3Mn4O12 by Mn substitutions at Cu site.32 Keeping in view the effect of substitution at Cu site, the present study is performed to investigate the electronic structure in addition to elastic, thermoelectric and magnetic properties. The exchange correlation GGA, GGA with Hubbard U and HF are used within the WEIN2K package following the density functional theory (DFT) techniques for cutting-edge magnetic memory devises and spintronic technology.
Results and discussion
Structural analysis
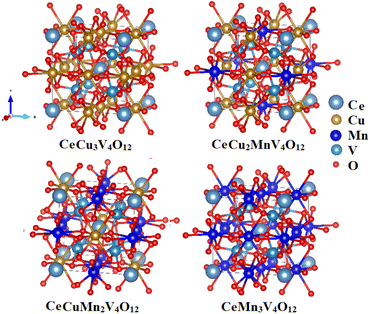
The structural variables of the CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) system were optimized in the realm of density functional theory (DFT) using GGA, GGA+U, and HF potentials with the assistance of experimental structure parameters.23–25 The ground state energy of each unit cell shown in Fig. 1 against the ground state volume is estimated by appropriate the Birch–Murnaghan equation of state33 and presented in Fig. 2. Table 1 lists the evaluated including the energy of the ground state (Eo), the lattice constants (ao), the Cohesive energy (Ecoh) and the formation enthalpy (ΔH). The calculated ao from the Table 1 for CeCu3V4O12 is 7.327, for CeCu2MnV4O12 is 7.358, CeCuMn2V4O12 7.431 and for CeCu3V4O12 is 7.462 Å respectively. The results are consistent with experiments and theoretical results,23–26 as shown by comparing the evaluated and reported ao depicted in Fig. 3 and Table 1. The replacement of Mn on Cu site does not change the symmetry similarly in LaCu3−xMnxMn4O12 (x = 1 and 2) and RCu3−xMnxMn4O12 R = Pr, Nd and (x = 1 and 2)30,31 and only leading to increasing the ao of these compounds from CeCu3V4O12 to CeMn3V4O12. The ao of these compounds are growing due to greater ionic radius of Mn than Cu (Mn = 0.097 Å and Cu = 0.087 Å) as seen from the Fig. 2. The estimated ground state energies for CeCu3V4O12 is −37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 034.439 Ry, CeCu2MnV4O12 is −36
034.439 Ry, CeCu2MnV4O12 is −36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039.447 Ry, CeCuMn2V4O12 is −35
039.447 Ry, CeCuMn2V4O12 is −35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058.458 and CeCu3V4O12 is −34
058.458 and CeCu3V4O12 is −34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 057.875 respectively.
057.875 respectively.
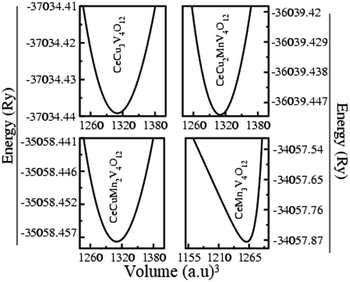 | ||
| Fig. 2 Optimized ground state energies vs. volume of the perovskites CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3). | ||
| Parameters | GGA | GGA+U | HF | EXP. | Others |
|---|---|---|---|---|---|
| CeCu3V4O12 | |||||
| a0 (Å) | 7.293 | 7.303 | 7.327 | 7.329 (ref. 23), 7.326 (ref. 24) | |
| E0 (Ry) | −37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 034.419 034.419 |
−37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 034.071 034.071 |
−37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 034.439 034.439 |
||
| Ecoh (Ry) | −41.960 | −41.612 | −41.980 | ||
| ΔH (Ry) | −2.098 | −2.080 | −2.099 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| CeCu2MnV4O12 | |||||
| a0 (Å) | 7.321 | 7.341 | 7.358 | 7.360 (ref. 24) | |
| E0 (Ry) | −36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039.443 039.443 |
−36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039.431 039.431 |
−36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039.447 039.447 |
||
| Ecoh (Ry) | −39.092 | −39.080 | −39.096 | ||
| ΔH (Ry) | −1.954 | −1.950 | −1.954 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| CeCuMn2V4O12 | |||||
| a0 (Å) | 7.393 | 7.422 | 7.431 | 7.432 (ref. 24) | |
| E0 (Ry) | −35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058.441 058.441 |
−35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058.402 058.402 |
−35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058.458 058.458 |
||
| Ecoh (Ry) | −37.521 | −37.501 | −37.546 | ||
| ΔH (Ry) | −1.875 | −1.873 | −1.876 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| CeMn3V4O12 | |||||
| a0 (Å) | 7.411 | 7.436 | 7.462 | 7.480 (ref. 24), 7.469 (ref. 25) | 7.46 (ref. 26) |
| E0 (Ry) | −34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 057.790 057.790 |
−34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056.515 056.515 |
−34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 057.875 057.875 |
||
| Ecoh (Ry) | −35.291 | −34.201 | −35.931 | ||
| ΔH (Ry) | −1.760 | −1.746 | −1.764 | ||
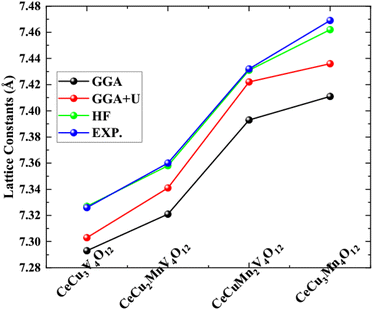 | ||
| Fig. 3 Correlation between the computed and observed lattice constant of perovskite structures CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3). | ||
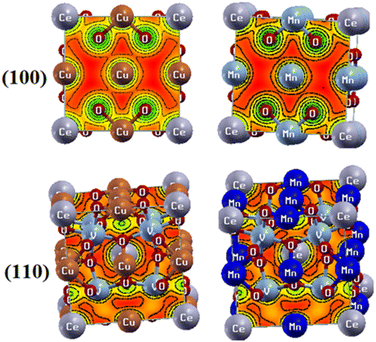
As shown in Table 1, Ecoh and ΔH are crucial stability factors that have been determined.26,34 In a compound, the Ecoh value is independent of the particle size and number of constituents. A larger value indicates a more firmly bound system because of electron nuclei interaction. Ecoh and ΔH for the system CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) lie in the range of −41.612 to −41.980, −2.080 to −2.099 Ry, −39.80 to −39.096, −1.950 to −1.954 Ry, −37.501 to −37.521, −1.873 to −1.876 and −35.201 to −35.931 Ry and −1.746 to −1.764 Ry consequently. Ecoh and ΔH shows that the system CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) is thermodynamic stable and the compound CaCu3V4O12 is show greater stability compare to all other compounds. Comparing the Ecoh and ΔH with the B0 for these compounds CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) through Tables 1 and 2 show in Fig. 4. The figure discloses that all stability factors are declining from Cu base compound to Mn containing compounds shows that the reduction in stability occurs from Cu to Mn and reveal the more stable nature of CaCu3V4O12 compound. Electronic charge density (ECD) in the (100) and (110) planes of CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) are shown in Fig. 5 to shed light on the character of the chemical bonds between the atoms. By comparing the ECD via (100) and (110) planes, we can see that the Ce–O bond is ionic while the Mn/V–O bond is covalent. Fig. 5 shows that when one moves from Cu-based to Mn-containing compounds, the covalency between Mn/V–O raises and the ionic character of the Ce–O bond decreases. However from the Pauling's method35,36 using the formula % ionic character (P) = (1 − e−1/4(ECe/Mn/V−EO)2(100%)), where ECe/Mn/V and EO is the electro-negativity of Ce/Mn/V and O atoms which suggest that Ce–O bonds are 75.24% and 73.96% ionic in nature and Mn–O and V–O bonds are respectively 40.95% and 44.09% covalent. Which further verifies the trend toward greater covalency and decreasing iconicity.
| Mechanical parameters | CeCu3V4O12 | CeCu2MnV4O12 | CeCuMn2V4O12 | CeMn3V4O12 |
|---|---|---|---|---|
| C11 (GPa) | 408.763 | 397.489 | 389.314 | 385.781 |
| C12 (GPa) | 168.540 | 158.764 | 150.198 | 145.567 |
| C44 (GPa) | 131.506 | 125.696 | 121.344 | 117.632 |
| Gv (GPa) | 126.948 | 123.163 | 120.560 | 118.609 |
| GR (GPa) | 126.698 | 123.084 | 120.553 | 118.609 |
| GH (GPa) | 126.823 | 123.123 | 120.556 | 118.615 |
| B0 (GPa) | 248.614 | 238.336 | 230.132 | 225.633 |
| B0/GH | 1.960 | 1.935 | 1.908 | 1.902 |
| Y (GPa) | 325.451 | 315.196 | 307.913 | 302.802 |
| HV (Chen) (GPa) | 12.46 | 12.42 | 12.48 | 12.40 |
| HV (Tian) (GPa) | 13.19 | 13.11 | 13.12 | 13.02 |
| HV (Teter) (GPa) | 19.15 | 18.59 | 18.20 | 17.91 |
| HV (Miao) (GPa) | 18.46 | 18.69 | 18.94 | 19.01 |
| HV (MazhniK) (GPa) | 16.3 | 15.7 | 15.2 | 14.9 |
| C′′ | 37.033 | 33.064 | 29.197 | 27.930 |
| A | 1.094 | 1.053 | 1.016 | 0.979 |
| Y | 0.281 | 0.279 | 0.277 | 0.276 |
| ζ | 0.696 | 0.676 | 0.657 | 0.643 |
| λ | 163.981 | 156.227 | 149.758 | 146.552 |
| μ | 126.948 | 123.163 | 120.560 | 118.622 |
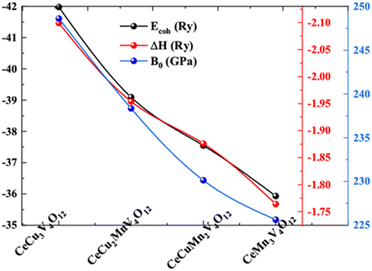 | ||
| Fig. 4 Correlation between cohesive energy, enthalpy, and bulk modulus of perovskite phases CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3). | ||
Elastic properties
Specific boundary factors are connected to the materials functional applications and a material's elastic constants (Cij) show how it resists external stress. We are conscious of how structural materials react to applied pressure within their elastic range. In addition, a comprehensive knowledge of the elastic constants of the materials is essential for real-world applications. A solid's elastic and mechanical property are strongly linked to crystal symmetry orientations, mechanical stability, and anisotropic indices,37–39 the WIEN2k software's integrated IRElast package is used to calculate the elastic parameters of the CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) system described in Table 2. Mechanical factors; as shear modulus (GH), bulk modulus (B0), Reuss shear modulus (GR), Young's modulus (Y), Poisson's ratio (υ), Paugh ratio (B0/GH), shear constant (C′), hardness parameters (HV), Cauchy pressure (C′′), Kleinman's parameter (ζ), anisotropic factor (A), and Lame's constants (λ and μ) are examine using Cij, listed in Table 2. All CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) compounds exhibit mechanical stability as they meet the physical standards for the cubic phase compounds (C11 + 2C12 > 0, C11 − C12 > 0 and C44 > 0) as specified in (ref. 40).The value of GR, Gv and GH from the Table 2 for CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) compounds are in the range of 118.609 to 126.948, 118.609 to 126.698 and 118.615 to 126.832 GPa, respectively. This shows that CeCu3V4O12 has more opposition to plastic deformation than the CeCu3−xMnxV4O12 (x = 1, 2 and 3). In response to an applied field, B0 quantifies the amount of material resistance.41,42 B0 describes the increase in pressure that occurs with a negligible reduction in volume. In all cases, the calculated B0 value lies between 225.633 and 248.614 GPa. As shown in Fig. 4 from the EC and H, these results indicate that CeCu3V4O12 is the most resistant of the studied compounds to applying an external field.
The Pugh ratio explains why some materials are ductile while others are brittle. The material is ductile if the B0/GH ratio exceeds the critical threshold and brittle if it is less than 1.75.43 These compounds are ductile, as their Pugh ratios are more than the threshold value, coming in at 1.906, 1.935, 1.908, and 1.902, respectively.
The stiffness of the material is characterized by a higher value for the Young modulus (Y). The elevated value of Y represents stiffness of the material and vice versa. The evaluated values of Y for these compounds vary from 302.802 to 325.451 GPa, demonstrating that all compounds show stiffness while CeCu3V4O12 is more rigid than others.
The hardness by different methods proposed by X. Chen et al., N. Miao et al., D. M. Teter, Y. Tian et al., and E. Mazhnik et al.44–49 and presented in Table 2. It can be observed from Table 2, the CeCuMn2V4O12 compound have maximum hardness value according to the Chen formalism and Tian, Miao and Mazhnik equations show that CeCu3V4O12 is hardest among the series. These compounds' proper hardness is exceptionally challenging in light of these findings. Mazhnik et al. have produced the most well-liked and widely accepted approach. The method uses the Y and υ decides the hardness. According to Mazhnik approach the CeCu3V4O12 compound is the harder than the rest which is also indicated by the young modulus.
While investigating materials' ductile and brittle properties, the Cauchy pressure is also considered.50 Materials with a positive value of Cauchy pressure are ductile, and those with a negative value are brittle. Pugh ratio estimates that all these compounds are in the ductile range, with values between 27.390 and 37.033 GPa.
The isotropic behavior of the material can be explained by its isotropic factor (A).
Microcracks form as a result,51 where A is equal to one for isotropic materials and 0 for anisotropic ones. All the compounds had A values between 0.979 and 1.094, indicating they are anisotropic.
The compressibility of a substance can be represented by Poisson's ratio (υ). These substances are calculated to be between 0.276 and 0.281 by measuring the shear and bulk moduli. Lower values in the range indicate unchanged in the symmetry, while higher values indicate whether volumetric changes have occurred. The calculated Poisson's ratios for the compounds under consideration, shown in Table 2, demonstrate volume fluctuation.
The Kleinman parameter (ζ) specifies the elasticity of the material's bonding. Using elastic coefficients, Harrison52 developed a formula to determine the ζ; Kleinman's work demonstrates that ζ = 0 denotes bond bending, whereas = 1 denotes bond stretching. Considering that the evaluated value for the complete set of compounds is between 0.643 and 0.696, we may infer that bond bending and stretching are possible in all combinations; this property is referred to ductility of these compounds. Bond bending overtakes bond stretching as the value approaches 1.
The values of Y and υ determine Lame's constants (μ and λ). Changing the value of Y has a significant impact on both μ and λ.53 Since Y is low, lower are the values of μ and λ. All the compounds considered are tough and stable, with Lame's constants falling in the range of 146.552–163.981 and 118.622–126.948. Overall, their elastic characteristics corroborate the compounds' ductile and anisotropic mechanical stability.
Electronic properties
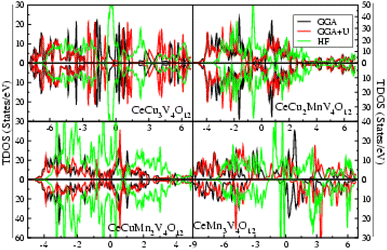
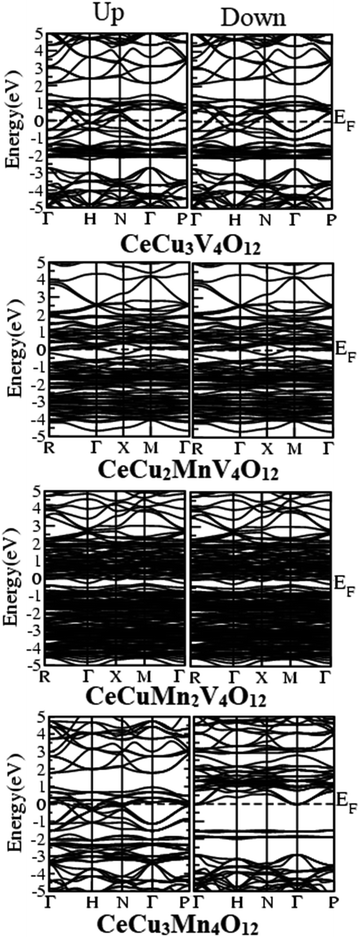
According to literature, the A′-site atom is the cause for the magnetic character; the B-site atom is the cause for the electronic nature in several quadruple perovskites that consist of binary magnetic elements. Adding a third magnetic element to the perovskite formula makes it very challenging to demonstrate the electrical and magnetic nature of the quadruple perovskite family. The total density of states (TDOS) plotted in Fig. 6 against the GGA, GGA+U, and HF potentials for the three magnetic perovskite sub-lattice systems helps clarify the ambiguity surrounding their relative metallicity. The figure discloses that all the compounds are metallic through GGA potential while GGA+U and HF gives metallic nature of CeCu3−xMnxV4O12 (x = 0, 1, 2) except CeCu3Mn4O12 which is semi-metallic in nature. It has been reported, both theoretically and empirically, that CeCu3Mn4O12 (ref. 25 and 26) is semi-metallic wears experimentally it is reported that all others compounds are23,24 metals, which reveal that the electrical properties of compounds change when Mn is completely replaced by Cu. The results obtained by GGA+U and HF are similar with slight difference such that all gives the same electronic character of the understudy compounds.The electronic behavior of the CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) compounds are also confirmed by calculating their band structure using HF potential and provided in Fig. 7. The figure disclose that the compounds CeCu3−xMnxV4O12 (x = 0, 1, 2) are metallic such that energy bands are overlapping at the Fermi level while CeCu3Mn4O12 is semi-metallic due to overlapping of band in the up states and in spin down state no crossing over at the Fermi level occurs. Correspondingly the isotropic compounds show the same behavior such that the transition from metal to semiconductor occurs with the substitution of Ti and Fe at Mn site in CaCu3Mn4O12, BiCu3Mn4O12 and LaCu3Mn4O12.54–56
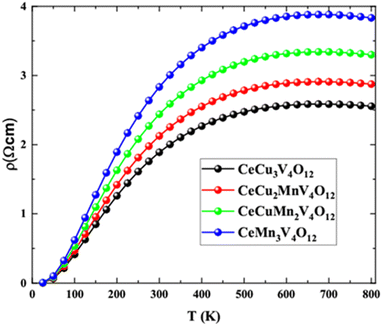
The electrical resistivity for CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) system is determined by BoltzTraP code57 from 0 to 800 K temperature range and displayed in Fig. 8. The thermal velocity of electrons raise with rising temperature, leading to more collisions among free electrons and causing an increase in resistance, as shown in the graph for electrical resistivity. Electrical resistivity for CeCu3V4O12 is 1.88 Ω cm, for CeCu2MnV2O12 is 2.12 Ω cm, CeCuMn2V2O12 is 2.44 Ω cm and for CeCu3Mn4O12 is 2.83 Ω cm at room temperature. The resistivity of CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) are consistent with the experiment and with previous results.23–26 Although the resistivity of CeCu3V4O12 is smaller and increases with Mn concentration. Similarly the resistivity of the CaCu3Mn4O12, BiCu3Mn4O12 and LaCu3Mn4O12 is also increases with Ti,54 and with Fe doping.55,56
Magnetic properties
Ground state magnetic energies are estimated for various magnetic configurations, including non-magnetic (NM), ferromagnetic (FM), and different types of anti-ferromagnetic (AFM).58,59 This allows one to determine the stability of magnetic phase of CeCu3−xMnxV4O12 (x = 0, 1, 2, and 3) compounds. Fig. 9 depicts the energy of the magnetic ground state as a function of volume for these configurations. Fig. 9 shows CeCu3V4O12 and CeCu3−xMnxV4O12 (x = 1, 2, and 3) have reduced ground state energies due to their respective NM ordering and AFM nature, respectively. The findings regarding their magnetic properties are consistent with previous experimental and published data. Nevertheless, Mn doping causes a shift from the NM to the AFM phase24–26 in the host CeCu3V4O12 compound.23 Table 3 displays the estimated magnetic moments of the elements constituting these perovskites. Table 3 clearly shows that the magnetic moments of a copper atom are 0.285 and 0.180 and 0.167μB; magnetic moments of Mn atom are 4.073, 4.175 and 4.274μB; for V atom are 0.052, 1.434, 1.347 and 1.254μB respectively in CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) compounds. The magnetic moment of Mn and V are reliable with the other (4.27 and 1.25μB)26 observed results for CeCu3Mn4O12 which make the existing outcomes coherent and reasonable compare to others compounds. The magnetic moments of the Mn atom increases due to increase in its concentration and increases in the lattice constants of the system60 and with the presence of the high Mn ion, same with the case in RCu3−xMnxMn4O12 (x = 0,1,2; R = Pr and Nd).31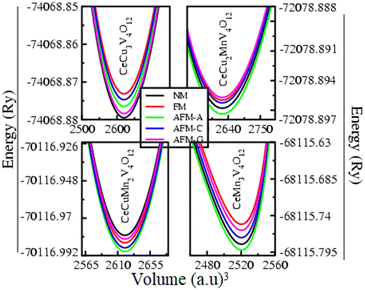 | ||
| Fig. 9 Optimized ground state magnetic energy versus volume of the perovskites CeCu3−xMnxV44O12 (x = 0, 1, 2 and 3). | ||
| Parameters | CeCu3V4O12 | CeCu2MnV4O12 | CeCuMn2V4O12 | CeMn3V4O12 |
|---|---|---|---|---|
| Cu (μB) | 0.285 | 0.180 | 0.167 | — |
| Mn (μB) | — | 4.073 | 4.175 | 4.274 |
| V (μB) | 0.052 | 1.432 | 1.347 | 1.254 |
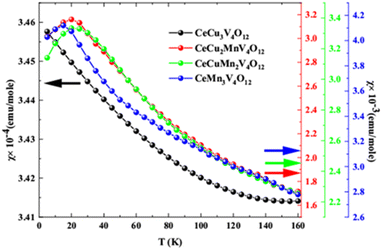
BoltzTraP code57 is employed to find out the magnetic susceptibility and derivative of the magnetic susceptibility of CeCu3−xMnxV4O12 (x = 0, 1, 2, and 3) compounds. Magnetic susceptibilities calculated using the Curie–Weiss law61 are depicted in Fig. 10.
The decreasing magnetic susceptibility with increasing temperature of CeCu3V4O12 indicates its NM nature. In contrast, the growing and subsequently decreasing magnetic susceptibility of CeCu2MnV4O12, CeCuMn2V4O12, CeCu3Mn4O12 indicates their AFM nature (as seen in the image). These compounds' magnetic susceptibility curves are incredibly comparable to the experimentally reported results for CeCu3Mn4O12.25,26 To determine the critical temperature (TN) the (dχ/dT) is considered for the CaCu3−xMnxV4O12 (x = 1, 2 and 3) system shown in Fig. 11. The anomalies for CaCu3−xMnxV4O12 (x = 1, 2 and 3) system can be seen from Fig. 11 and Table 3. It is obvious that the abnormality happens at 13, 18 and 10 K correspondingly for CaCu3−xMnxV4O12 (x = 1, 2 and 3). This work is reasonable and logical because the calculated TN of CeCu3Mn4O12 agrees well with the previously reported values (11.7 and 8 K).24–26 These compounds are essential for memory and spintronics because of their anti-ferromagnetic character, as demonstrated by the magnetic energy optimization and magnetic susceptibilities.62 Aside from their practical uses, the absence of magnetic moments in these materials renders them unnoticeable to magnetic probes and insensible to instability in the magnetic field.63 These materials' anti-ferromagnetic moments would affect their neighbors in spite of how closely the elements are arranged in the device. Still, they would also produce zero stray fields and eradicate the unintended magnetic crosstalk between neighboring devices.64 Furthermore, the exchange between the dissimilar states of anti-ferromagnetic tends to be numerous times faster than ferromagnetic, making them very promising in increasing ultrafast and ultrahigh-density spy circuits.65
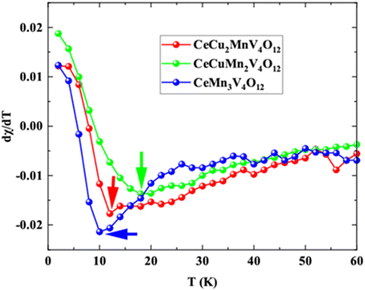 | ||
| Fig. 11 The differential of magnetic susceptibility with respect to temperature in perovskite structures CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3). | ||
Thus, these materials are great for memory and spintronics since their AFM nature allows them for highly concentrated devices.
Computational detail
The FP-LAPW approach in the DFT utilized in the WEIN2k package, was used to carry out the current investigation.66 The GGA,67–69 GGA+U and HF is employed to deal with the exchange and correlation.70 The HF potential treat the correlated system (3d and 4f electron) very well especially in structure and electronic calculation,71,72 due to the presence of 3d and 4f elements in the studied compounds hybrid functional are also used. The technique illustrated by Dudarev et al.73 deal with the electronic correlation effect on Ce-f, Mn, Cu and V-d orbital's. According to the literature,26,74,75 the effective U value chosen to be 4 eV per Ce, 4 eV per Mn, 5 eV per Cu and 2 eV per V respectively and the exact exchange α for Ce is 0.35 eV is used throughout the calculation.The interstitial region is extended on a plane wave basis with Kmax = 6/RMT as the cut-off value, the wave function is ubiquitous in the spherical harmonics contained within the muffin-tin spheres, which together make up the unit cell of the FPLAPW technique. For convergence to be guaranteed for less than one mRy a.u−1, the Brillouin zone path employs a thousand K-point grids. Self-consistent field calculations76 are used to compute the magnetic moments of the component elements in these compounds. Magnetic susceptibilities are evaluated using the BoltzTraP tool57 and elastic properties are anticipated by IRElast package.77
Conclusion
Structural, electronic and magneto-elastic properties of CeCu3−xMnxV4O12 (x = 0, 1, 2 and 3) compounds have been studied in the domain of DFT. The examination of structural optimization reveals that the structural parameters of the CaCu3−xMnxV4O12 (x = 0, 1 and 2) system are well consistent with experiments and are increases with Mn concentration. Electronic properties disclose the conductor nature of CeCu3−xMnxV4O12 (x = 0, 1 and 2) while semi-metallic nature of CeMn3V4O12. Beside this electrical resistivity also acknowledged their electronic nature. These elastic characteristics prove that these compounds are anisotropic, ductile as well as mechanically stable. CeCu3V4O12 is paramagnetic and CeCu3−xMnxV4O12 (x = 1, 2 and 3) is anti-ferromagnetic determined from the spin dependent energy optimization. Their paramagnetic and anti-ferromagnetic nature is also confirmed from their magnetic susceptibility and the Néel temperature for CeCu3−xMnxV4O12 (x = 1, 2 and 3) compounds are 13, 18 and 10 K respectively estimated from the dχ/dT. The anti-ferrimagnetic ductile nature of CeCu3−xMnxV4O12 (x = 1, 2 and 3) compound identifies them promising materials for use in spintronics and magnetic cloaking.Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to legal or ethical reasons.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R55), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.References
- J. P. Locquet, J. Perret and J. Fompeyrine, et al., Nature, 1998, 394, 453–456 CrossRef CAS.
- K. J. Choi, M. Biegalski, Y. L. Li and A. Sharan, et al., Science, 2004, 306, 1005–1009 CrossRef CAS PubMed.
- D. I. Tishkevich, A. I. Vorobjova and A. V. Trukhanov, et al., Solid State Phenom., 2020, 299, 281–286 Search PubMed.
- D. Sando, A. Barthelémy and M. Bibes, J. Phys.: Condens. Matter, 2014, 26, 473201 CrossRef CAS PubMed.
- A. V. Trukhanov, D. I. Tishkevich and S. V. Podgornaya, et al., Nanomaterials, 2022, 12, 868 CrossRef CAS PubMed.
- Y. Konishi, Z. Fang and M. Izumi, et al., J. Phys. Soc. Jpn., 1999, 68, 3790–3793 CrossRef CAS.
- W. S. Choi, J.-H. Kwon and H. Jeen, et al., Nano Lett., 2012, 12, 4966–4970 CrossRef CAS PubMed.
- A. Vorobjov, D. Tishkevich and D. Shimanovich, et al., RSC Adv., 2021, 11, 3952 RSC.
- H. Boschker, M. Mathews and E. P. Houwman, et al., Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 214425 CrossRef.
- A. Fedotov, V. Shendyukov and L. Tsybulskaya, et al., J. Alloys Compd., 2021, 887, 161451 CrossRef CAS.
- A. A. Belik, J. Chem. Soc., Dalton Trans., 2018, 47, 3209–3217 RSC.
- T. B. Adams, D. C. Sinclair and A. R. West, Adv. Mater., 2002, 14, 1321–1323 CrossRef CAS.
- Y. Long, N. Hayashi and T. Saito, et al., Nature, 2009, 458, 60–63 CrossRef CAS PubMed.
- Y. Long, T. Kawakami and W. Chen, et al., Chem. Mater., 2012, 24, 2235–2239 CrossRef CAS.
- I. Yamada, S. Marukawa and M. Murakami, et al., Appl. Phys. Lett., 2014, 105, 231906 CrossRef.
- S. Yagi, I. Yamada and H. Tsukasaki, et al., Nat. Commun., 2015, 6, 8249 CrossRef PubMed.
- I. Yamada, H. Fujii and A. Takamatsu, et al., Adv. Mater., 2017, 29, 1603004 CrossRef PubMed.
- W. Chen, M. Mizumaki and H. Seki, et al., Nat. Commun., 2014, 5, 3909 CrossRef CAS PubMed.
- D. I. Tishkevich, T. I. Zubar and A. L. Zhaludkevich, et al., Nanomaterials, 2022, 12, 1642 CrossRef CAS PubMed.
- Y. Lin, Y. B. Chen and T. Garret, et al., Appl. Phys. Lett., 2002, 81, 631–633 CrossRef CAS.
- D. Tishkevich, A. Vorobjova and D. Shimanovich, et al., Nanomaterials, 2021, 11, 1775 CrossRef CAS PubMed.
- A. I. Vorobjova, D. I. Tishkevich and E. A. Outkina, et al., Nanomaterials, 2022, 12, 1344 CrossRef CAS PubMed.
- N. I. Kadyrova, Y. G. Zainulin and V. L. Volkov, et al., J. Inorg. Chem., 2008, 53, 1542–1545 Search PubMed.
- N. I. Kadyrova, Y. G. Zainulin and N. V. Melnikova, et al., J. Inorg. Chem., 2018, 82, 804–806 CAS.
- N. I. Kadyrova, Y. G. Zainulin and A. P. Tyutyunnik, et al., Russ. J. Inorg. Chem., 2017, 62, 103–110 CrossRef CAS.
- S. Mehmood, Z. Ali and I. Khan, et al., Mater. Chem. Phys., 2020, 254, 123229 CrossRef CAS.
- S. Mehmood, Z. Ali and N. Alwadai, et al., J. Phys. Chem. Solids, 2023, 174, 111162 CrossRef CAS.
- S. Mehmood and Z. Ali, J. Appl. Physiol., 2023, 129, 76 CAS.
- S. Mehmood, Z. Ali and I. Ahmad, Mater. Chem. Phys., 2023, 295, 127164 CrossRef CAS.
- A. Munoz, M. J. M. Lope and M. Retuerto, et al., J. Appl. Phys., 2008, 104, 083911 CrossRef.
- M. Retuerto, M. J. M. Lope and J. S. Benitez, et al., J. Appl. Phys., 2010, 108, 083905 CrossRef.
- Q. Zhang, Y. Breard and V. Hardy, Inorg. Chem., 2022, 61, 5792–5799 CrossRef CAS PubMed.
- F. Birch, Phys. Rev., 1947, 71, 809 CrossRef CAS.
- S. Mehmood, Z. Ali and I. Khan, et al., J. Electron. Mater., 2020, 49, 3780–3790 CrossRef CAS.
- M. Lenglet, Act. Passive Electron. Compon., 2004, 27, 1–60 CrossRef.
- Y. Slimani, N. A. Algarou and M. A. Almessiere, et al., Arabian J. Chem., 2021, 14, 102992 CrossRef CAS.
- A. Bouhemadou, O. Boudrifa and N. Guechi, Comput. Mater. Sci., 2014, 81, 561–574 CrossRef CAS.
- A. Tasnim, Md. Mahamudujjaman and Md. A. Afzal, et al., Results Phys., 2023, 45, 106236 CrossRef.
- M. S. Hossain, N. Jahan and M. M. Hossain, et al., Mater. Today Commun., 2023, 34, 105147 CrossRef CAS.
- L. Salik, A. Bouhemadou and K. Boudiaf, et al., J. Supercond. Novel Magn., 2020, 33, 1091–1102 CrossRef CAS.
- M. H. K. Rubel, M. A. Hossain and M. K. Hossain, et al., Results Phys., 2022, 42, 105977 CrossRef.
- F. Khelfaoui, M. Ameri and D. Bensaid, J. Supercond. Novel Magn., 2018, 31, 3183–3192 CrossRef CAS.
- S. F. Pugh, Philos. Mag., 1954, 45, 823 CAS.
- X. Q. Chen, H. Niu, D. Li and Y. Li, Intermetallics, 2011, 19, 1275–1281 CrossRef CAS.
- Y. Tian, B. Xu and Z. Zhao, Int. J. Refract. Hard Met., 2012, 33, 93–106 CrossRef CAS.
- D. M. Teter, MRS Bull., 1998, 23, 22–27 CrossRef CAS.
- N. Miao, B. Sa, J. Zhou and Z. Sun, Comput. Mater. Sci., 2011, 50, 1559–1566 CrossRef CAS.
- E. Mazhnik and A. R. Oganov, J. Appl. Phys., 2019, 126, 125109 CrossRef.
- R. Islam, M. M. Hossain and M. A. Ali, et al., RSC Adv., 2022, 12, 32994 RSC.
- D. G. Pettifor, Mater. Sci. Technol., 1992, 8, 345 CrossRef CAS.
- H. Fu, D. Li and F. Peng, et al., Comput. Mater. Sci., 2008, 44, 774 CrossRef CAS.
- A. I. Vorobjova, D. L. Shimanovich and O. A. Sycheva, et al., Russ. Microelectron., 2019, 48(2), 107–118 CrossRef CAS.
- L. Kleinman, Phys. Rev., 1962, 128, 2614 CrossRef CAS.
- H. Falcon, J. A. Alonso and J. S. Bentez, J. Phys.: Condens. Matter, 2006, 18, 6841–6852 CrossRef CAS.
- P. Kayser, M. J. M. Lope and J. A. Alonso, et al., Solid State Chem., 2013, 204, 78–85 CrossRef CAS.
- J. S. Bentez, M. RetuertoI and M. Jesus, et al., Z. Kristallogr., 2010, 225, 201–208 Search PubMed.
- G. K. H. Madsen and D. J. Singh, Comput. Phys. Commun., 2006, 175, 67 CrossRef CAS.
- Q. Zhao, Y. Y. Yin and J. H. Dai, et al., Chin. Phys. B, 2016, 25, 020701 CrossRef.
- A. L. Kozlovskiy, I. E. Kenzhina and M. V. Zdorovets, et al., Ceram. Int., 2019, 45, 17236–17242 CrossRef CAS.
- J. Yang, Z. Xiao and Z. Li, et al., Computational Condensed Matter, 2014, 1, 51–57 CrossRef.
- S. Mehmood, Z. Ali and I. Khan, et al., Mater. Chem. Phys., 2017, 196, 222 CrossRef CAS.
- T. Jungwirth, X. Marti and P. Wadley, et al., Nat. Nanotechnol., 2016, 11, 231–241 CrossRef CAS PubMed.
- J. Zelezny, P. Wadley and K. Olejnik, et al., Nat. Phys., 2018, 14, 220–228 Search PubMed.
- S. Mehmood and Z. Ali, Mater. Chem. Phys., 2022, 282, 125915 CrossRef CAS.
- X. Li and J. Yang, Natl. Sci. Rev., 2016, 3, 365–381 CrossRef CAS.
- P. Blaha, K. Schwarz and F. Tran, et al., J. Chem. Phys., 2020, 152, 07410 CrossRef PubMed.
- A. M. Asiri, M. K. Shahzad and S. Hussain, et al., Heliyon, 2023, 9, e14112 CrossRef CAS PubMed.
- M. K. Shahzad, S. T. Mujtaba and S. Hussain, et al., RSC Adv., 2022, 12, 27517 RSC.
- A. M. Asiri, M. K. Shahzad and S. Hussain, et al., Heliyon, 2023, 9, e13687 CrossRef PubMed.
- L. Wang, T. Maxisch and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 195107 CrossRef.
- F. Tran, D. Koller and P. Blahaaq, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 134406 CrossRef.
- A. S. Botana, F. Tran and V. Pardo, et al., Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 235118 CrossRef.
- S. Dudarev, G. Botton and S. Savrasov, et al., Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505 CrossRef CAS.
- D. I. Tishkevich, A. I. Vorobjova and A. A. Bondaruk, et al., Nanomaterials, 2022, 12, 2382 CrossRef CAS PubMed.
- A. Shah, Z. Ali and S. Mehmood, et al., J. Electron. Mater., 2019, 49, 1230 CrossRef.
- J. C. Slater, Phys. Rev., 1953, 91, 528 CrossRef CAS.
- M. Jamal, M. Bilal and I. Ahmad, et al., J. Alloys Compd., 2018, 735, 469 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |