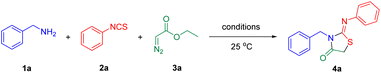Open Access Article
Open Access ArticleSynthesis of 2-iminothiazolidin-4-ones via copper-catalyzed [2 + 1 + 2] tandem annulation†
Mingming Zhaoab,
Yiming Guoab,
Qi Wangab,
Lanqi Liuab,
Shujie Zhangab,
Wei Guo *c,
Lin-Ping Wu
*c,
Lin-Ping Wu *ab and
Fayang G. Qiu
*ab and
Fayang G. Qiu *ab
*ab
aGuangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou 510530, P. R. China. E-mail: wu_linping@gibh.ac.cn; qiu_fayang@gibh.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cKey Laboratory of Organo-Pharmaceutical Chemistry of Jiangxi Province, Gannan Normal University, Ganzhou 341000, P. R. China. E-mail: guoweigw@126.com
First published on 12th January 2023
Abstract
In this paper, an efficient synthesis of 2-iminothiazolidin-4-ones through a copper-catalyzed tandem annulation reaction of alkyl amines, isothiocyanates and diazo acetates is presented. Notable advantages of this [2 + 1 + 2] cyclization methodology include readily accessible starting materials, simple operation, mild reaction conditions, high yields, step-economy and diverse functional group tolerance. In addition, the reaction is applicable to the gram scale synthesis and the preparation of bioactive molecules.
Introduction
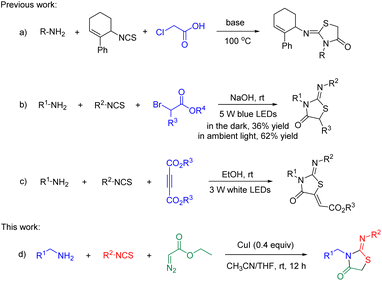
The thiazolidine skeleton, a privileged heterocyclic motif, is ubiquitous in various natural compounds, drug candidates, and pharmacologically active molecules. Specifically, 2-iminothiazolidin-4-one derivatives have attracted much attention due to their broad biological activities1 and have thus been developed as sphingosine-1-phosphate receptor agonists,2 carbonic anhydrase IX inhibitors,3 selective glycogen synthase kinas-3β inhibitors,4 cell division cycle dual phosphatases inhibitors,5 entamoeba histolytica inhibitors,6 and human carbonic anhydrase IX inhibitors.7 Owing to their pharmaceutical importance, some powerful synthetic methodologies have been developed to access 2-iminothiazolidin-4-ones.8 In addition, the one-pot three-component cyclization has been successfully explored for the acquisition of 2-iminothiazolidin-4-ones via amines, isothiocycanates, with chloroacetic acid9 (Scheme 1a)/α-bromoesters10 (Scheme 1b)/alkyl acetylenedicarboxylates11 (Scheme 1c).Despite the great progress, the reported methods suffer from several drawbacks such as high temperature, microwave-irradiation, or difficult to scale-up (visible-light-promoted, Scheme 1b and c). Therefore, there is an urgent need to develop concise and scalable methods for the formation of 2-iminothiazolidin-4-ones under mild reaction conditions.
Diazocarbonyl compounds are a class of versatile and easily accessible building blocks containing two functional groups, thereby affording rich chemistry under different reaction conditions.12 For instance, various cyclopropanations, C–H alkylation, C–X insertion, the Wolff rearrangement, and dipolar cycloaddition reactions have been established.13 In the past few years, the transition-metal-catalyzed diazocarbonyl compounds have been applied to the assembly of N-heterocycles via carbene transfer reactions and such reactions have emerged as a powerful tool in synthetic organic chemistry.14 Here we designed a novel three-component tandem strategy to prepare the 2-iminothiazolidin-4-ones via the copper-catalyzed [2 + 1 + 2] cyclization of alkyl amines, isothiocyanates, and diazo esters (Scheme 1d).
Results and discussion
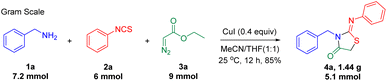
Our investigations were started with the reaction of phenylmethanamine 1a, phenyl isothiocyanate 2a, and ethyl 2-diazoacetate 3a in CH3CN at room temperature (25 °C) (Table 1). As shown in Table 1, a brief screening of the copper catalysts and iodine reagents suggested that CuI was the best choice (entries 1–12). In this case, 2-iminothiazolidin-4-one (4a) was obtained in 78% yield (entry 5). As expected, no target product was found in the absence of copper catalyst (entry 13). Subsequently, the amount of CuI (Tables S1†), the molar ratio of reactants (Tables S2†), reaction time (Tables S3†) and reaction temperature (Tables S4†) were optimized. However, the yield of 4a was not improved (see the ESI Tables S1–S4† and Table 1, entry 6). For example, the yield was decreased to 51% when the reaction was carried out in CH3CN for 6 h (entry 6). Then, the effect of solvents was also investigated (entries 14–19). It was found that the mixture of CH3CN with THF (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was a more efficient than other solvent systems, affording 4a in 85% yield (entry 19). In addition, when the reaction was performed under N2 or O2 atmosphere, the transformations proceeded without much difference (entries 20–21), illustrating that the effect of oxygen was insignificant.
1) was a more efficient than other solvent systems, affording 4a in 85% yield (entry 19). In addition, when the reaction was performed under N2 or O2 atmosphere, the transformations proceeded without much difference (entries 20–21), illustrating that the effect of oxygen was insignificant.
| Entry | Catalyst | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.222 mmol), 2a (0.185 mmol), 3a (0.278 mmol) and catalyst (0.4 equiv.) in solvent (2 mL), open to air at room temperature (25 °C).b Isolated yield.c Reaction under N2 atmosphere.d Reaction under O2 atmosphere. | ||||
| 1 | CuCl2 | CH3CN | 12 | 38 |
| 2 | CuBr2 | CH3CN | 12 | 48 |
| 3 | CuCl | CH3CN | 12 | 41 |
| 4 | CuBr | CH3CN | 12 | 51 |
| 5 | CuI | CH3CN | 12 | 78 |
| 6 | CuI | CH3CN | 6 | 51 |
| 7 | Cu(OAc)2 | CH3CN | 12 | 0 |
| 8 | Cu(OTf)2 | CH3CN | 12 | 31 |
| 9 | Cu(TFA)2 | CH3CN | 12 | 65 |
| 10 | NBS | CH3CN | 12 | 22 |
| 11 | NIS | CH3CN | 12 | 42 |
| 12 | I2 | CH3CN | 12 | 49 |
| 13 | — | CH3CN | 12 | 0 |
| 14 | CuI | DMF | 12 | 53 |
| 15 | CuI | Acetone | 12 | 68 |
| 16 | CuI | EtOH | 12 | 48 |
| 17 | CuI | MeNO2 | 12 | 56 |
| 18 | CuI | THF | 12 | 82 |
| 19 | CuI | CH3CN/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
12 | 85 |
| 20c | CuI | CH3CN/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
12 | 81 |
| 21d | CuI | CH3CN/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
12 | 78 |
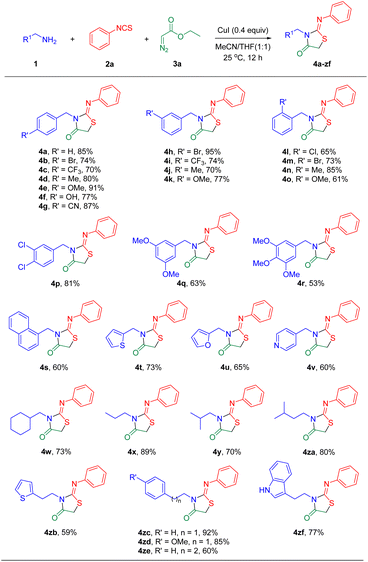
With the optimal reaction conditions in hand, the substrate scope of this transformation was examined. As shown in Scheme 2, the scope of alkyl amines 1 was illustrated and the reaction was performed on the open air conditions at room temperature (25 °C). In general, the results revealed that benzylamines 1b–1o bearing either an electron-donating group (CH3, OCH3, and OH) or an electron-withdrawing group (Cl, Br, CF3, and CN) on the para-/meta-/ortho-position of the aromatic ring reacted with phenyl isothiocyanate 2a and ethyl 2-diazoacetate 3a smoothly to furnish products 4b–4o in good to excellent yields. Moreover, disubstituted and trisubstituted benzylamines were able to undergo this transformation to afford the desired products 4p–4r in moderate to good isolated yields. Further, naphthalen-1-ylmethanamine, thiophen-2-ylmethanamine, furan-2-ylmethanamine, and pyridin-4-ylmethanamine were applicable to this reaction system providing 4s–4v in moderate yields. When cyclohexylmethanamine and aliphatic amines were employed as competent partners with 2a and 3a, the corresponding products 4w–4za were obtained in good yields. To our delight, the use of 2-(thiophen-2-yl)ethan-1-amine, 2-phenylethan-1-amine, and 3-phenylpropan-1-amine also generated the desired 2-iminothiazolidin-4-ones 4zb–4ze in 59–92% yields. Notably, tryptamine afforded product 4zf in 77% yield. However, aniline failed to give the desired product, which might be due to the conjugation effect between the aromatic ring and the nitrogen atom.
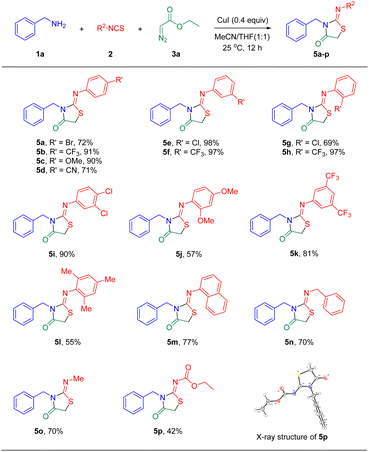
The scope of isothiocyanates under similar conditions was then investigated, and the results are summarized in Scheme 3. As expected, isothiocyanatobenzenes containing –Cl, –Br, –CF3, –OCH3, –CN groups at the para-, meta-, or ortho-position reacted well with phenylmethanamine 1a and ethyl 2-diazoacetate 3a, producing the desired cyclization products 5a–5h in moderate to excellent yields. Furthermore, disubstituted and trisubstituted isothiocyanatobenzenes provided the desired products 5i–5l as well. For 1-napthyl isothiocyanate, the target product 5m was formed in 77% yield, whereas (isothiocyanatomethyl)benzene gave the corresponding product 5n in 70% yield. In addition, aliphatic isothiocyanates were tried. To our delight, isothiocyanatomethane demonstrated comparable reactivity and provided the desired product 5o in 70% yield. Notably, the CuI catalyzed [2 + 1 + 2] tandem annulation of 1a, o-ethyl carbonisothiocyanatidate 2p, and ethyl 2-diazoacetate 3a at room temperature (25 °C) afforded the 2-iminothiazolidin-4-one 5p in 42% yield. The geometry of the imine double bond at 5p was determined via X-ray crystallographic analysis (see the ESI† for details). Further, other substituted diazo compounds were screened under the standardized protocol. However, ethyl 2-diazo-3-oxo-3-phenylpropanoate, ethyl 2-diazo-3-oxobutanoate, or ethyl 2-diazo-2-phenylacetate couldn't be transformed into the target product because of a steric hindrance effect.
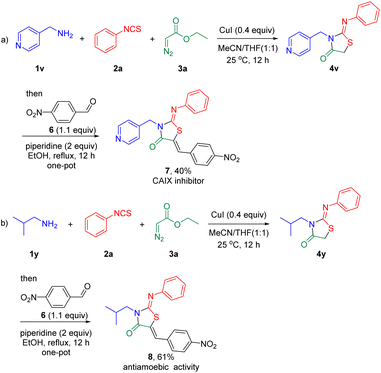
As shown in Scheme 4, the present method can be performed in gram scale. In addition, this tandem annulation was used to furnish bioactive molecules (Scheme 5). For instance, the reaction of 1v, phenyl isothiocyanate 2a, ethyl 2-diazoacetate 3a, and 4-nitrobenzalde 6 led to 7, a human carbonic anhydrase IX inhibitor,7 in 40% yield via a one-pot annulation (Scheme 5a). Similarly, an entamoeba histolytica inhibitor6 8 was obtained in 61% yield (Scheme 5b).
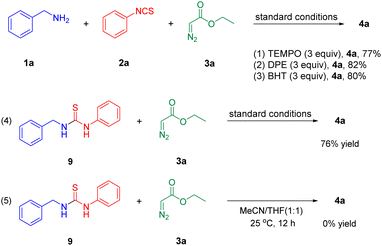
To gain insight into the mechanism of this reaction, we carried out some control experiments (Scheme 6). Addition of 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO), 2,6-di-tert-butyl-4-methylphenol (BHT), or 1,1-diphenylethylene (DPE), showed no significant effects on the yield of the desired product 4a (eqn (1)–(3)), indicating that a radical pathway is unlikely in this reaction. Furthermore, the reaction of 1-benzyl-3-phenylthiourea 9 (ref. 10, 11 and 15) with ethyl 2-diazoacetate 3a carried out under the standard conditions provided 4a in 76% isolated yield, implying that 9 might be a reasonable intermediate in this transformation (eqn (4)). However, when 1-benzyl-3-phenylthiourea 9 was used as the substrate in the absence of CuI, no desired product 4a was detected, suggesting that CuI is essential to the reaction (eqn (5)).
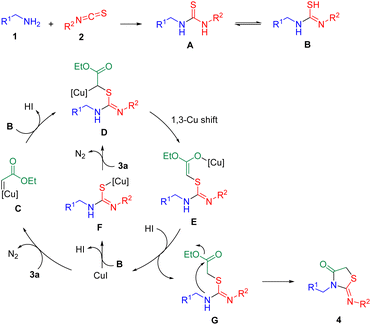
According to these experimental results, a plausible reaction mechanism for the copper-catalyzed tandem annulation reaction is suggested in Scheme 7. First, the reaction of alkyl amine (1) and isothiocyanate (2) gives intermediate A.10,11,15 Next, the intermediate B is obtained from A via isomerization.10,16 Then the coordination of 3a to the catalyst (CuI) affords the active Cu(I) carbene intermediate C through the extrusion of one N2 molecule.17 Subsequently, nucleophilic attack of intermediate B on Cu(I) carbene intermediate C forms intermediate D, which further generates intermediate E through 1,3-Cu shift. Intermediate E is protonated to produce intermediate G with concomitant regeneration of CuI catalyst. Finally, G undergoes an intramolecular ammonolysis/cyclization to yield the desired product 4. In the meantime, it is also possible that CuI first reacts with intermediate B to provide intermediate F, followed by metal carbene generation via its reaction with 3a to give D.
Conclusions
In conclusion, we have explored a novel copper-catalyzed tandem annulation reaction of alkyl amines, isothiocyanates, and diazo esters for the construction of 2-iminothiazolidin-4-ones under mild reaction conditions. This transformation represents a copper-catalyzed C–S/C–N bond formation strategy in a one-pot protocol without the requirement of any external bases, ligands, or oxidants. It shows wide functional group tolerance, high yields, and high step economy. In addition, this protocol is applicable to the gram scale synthesis and the preparation of bioactive molecules with such structural motif, which may facilitate the research of such bioactive molecules in medicinal chemistry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (NNSFC) (Grant No. 21971243) for financial support.References
- P. Vicini, A. Geronikaki, K. Anastasia, M. Incerti and F. Zani, Bioorg. Med. Chem., 2006, 14, 3859–3864 CrossRef CAS PubMed.
- M. H. Bolli, S. Abele, C. Binkert, R. Bravo, S. Buchmann, D. Bur, J. Gatfield, P. Hess, C. Kohl, C. Mangold, B. Mathys, K. Menyhart, C. Müller, O. Nayler, M. Scherz, G. Schmidt, V. Sippel, B. Steiner, D. Strasser, A. Treiber and T. Weller, J. Med. Chem., 2010, 53, 4198–4211 CrossRef CAS PubMed.
- G. Bianco, R. Meleddu, S. Distinto, F. Cottiglia, M. Gaspari, C. Melis, A. Corona, R. Angius, A. Angeli, D. Taverna, S. Alcaro, J. Leitans, A. Kazaks, K. Tars, C. T. Supuran and E. Maccioni, ACS Med. Chem. Lett., 2017, 8, 792–796 CrossRef CAS PubMed.
- M. Arfeen, S. Bhagat, R. Patel, S. Prasad, I. Roy, A. K. Chakraborti and P. V. Bharatam, Eur. J. Med. Chem., 2016, 121, 727–736 CrossRef CAS PubMed.
- S. Huber-Villaume, G. Revelant, E. Sibille, S. Philippot, A. Morabito, S. Dunand, P. Chaimbault, D. Bagrel, G. Kirsch, S. Hesse and H. Schohn, Bioorg. Med. Chem., 2016, 24, 2920–2928 CrossRef CAS PubMed.
- M. Mushtaque, F. Avecilla and A. Azam, Eur. J. Med. Chem., 2012, 55, 439–448 CrossRef CAS PubMed.
- M. F. Ansari, D. Idrees, M. I. Hassan, K. Ahmad, F. Avecilla and A. Azam, Eur. J. Med. Chem., 2018, 144, 544–556 CrossRef CAS PubMed.
- (a) D. Kumar, M. Sonawane, B. Pujala, V. K. Jain, S. Bhagat and A. K. Chakraborti, Green Chem., 2013, 15, 2872–2884 RSC; (b) S. Kasmi-Mir, A. Djafri, L. Paquin, J. Hamelin and M. Rahmouni, Molecules, 2006, 11, 597–602 CrossRef CAS PubMed; (c) A. Saeed, N. Abbas and U. Flörke, J. Braz. Chem. Soc., 2007, 18, 559–565 CrossRef CAS; (d) D. R. St. Laurent, Q. Gao, D. Wu and M. H. Serrano-Wu, Tetrahedron Lett., 2004, 45, 1907–1910 CrossRef.
- M. Sathishkumar, S. Nagarajan, P. Shanmugavelan, M. Dinesh and A. Ponnuswamy, Beilstein J. Org. Chem., 2013, 9, 689–697 CrossRef PubMed.
- W. Guo, M. Zhao, W. Tan, L. Zheng, K. Tao, L. Liu, X. Wang, D. Chen and X. Fan, J. Org. Chem., 2018, 83, 1402–1413 CrossRef CAS PubMed.
- W. Guo, K. Tao, L. Zheng, M. Zhao, W. Tan, L. Cai, Z. Xie, D. Chen and X. Fan, J. Org. Chem., 2019, 84, 6448–6458 CrossRef CAS PubMed.
- (a) M. Regitz and G. Maas, Diazo compounds: properties and synthesis, Academic Press, Orlando, FL, USA, 1986, p. 608 Search PubMed; (b) M. P. Doyle, M. A. McKervey and T. Ye, Modern catalytic methods for organic synthesis with diazo compounds: from cyclopropanes to ylides, J Wiley & Sons, New York, USA, 1998, p. 652 Search PubMed; (c) A. C. B. Burtoloso, P. B. Momo and G. L. Novais, An. Acad. Bras. Cienc., 2018, 90, 859–893 CrossRef CAS PubMed.
- (a) H. Lebel, J.-F. Marcoux, C. Molinaro and A. B. Charette, Chem. Rev., 2003, 103, 977–1050 CrossRef CAS PubMed; (b) F. Brackmann and A. de Meijere, Chem. Rev., 2007, 107, 4493–4497 CrossRef CAS PubMed; (c) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981–10080 CrossRef CAS PubMed; (d) D. Gillingham and N. Fei, Chem. Soc. Rev., 2013, 42, 4918–4931 RSC; (e) Z. Zhang and J. Wang, Tetrahedron, 2008, 64, 6577–6605 CrossRef CAS; (f) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704–724 CrossRef CAS PubMed.
- (a) Y.-P. Li, Z.-Q. Li and S.-F. Zhu, Tetrahedron Lett., 2018, 59, 2307–2316 CrossRef CAS; (b) A. C. B. Burtoloso, R. M. P. Dias and B. Bernardim, Acc. Chem. Res., 2015, 48, 921–934 CrossRef CAS.
- (a) L. C. de Sequeira Aguiar, G. M. Viana, M. V. dos Santos Romualdo, M. V. Costa and B. S. Bonato, Lett. Org. Chem., 2011, 8, 540–544 CrossRef CAS; (b) T. Bade and R. R. Vedula, J. Heterocycl. Chem., 2015, 52, 1883–1886 CrossRef CAS; (c) N. Tumula, N. Jatangi, R. K. Palakodety, S. Balasubramanian and M. Nakka, J. Org. Chem., 2017, 82, 5310–5316 CrossRef CAS PubMed; (d) J.-J. Chu, B.-L. Hu, Z.-Y. Liao and X.-G. Zhang, J. Org. Chem., 2016, 81, 8647–8652 CrossRef CAS PubMed; (e) S. Wangngae, M. Pattarawarapan and W. Phakhodee, J. Org. Chem., 2017, 82, 10331–10340 CrossRef CAS PubMed.
- W. Guo, M. Zhao, W. Tan, L. Zheng, K. Tao, L. Chen, M. Wang, D. Chen and X. Fan, Asian J. Org. Chem., 2018, 7, 1893–1897 CrossRef CAS.
- (a) I. Rivilla, W. M. C. Sameera, E. Alvarez, M. M. Díaz-Requejo, F. Maseras and P. J. Pérez, Dalton Trans., 2013, 42, 4132–4138 RSC; (b) J. Li, M. Tang, L. Zang, X. Zhang, Z. Zhang and L. Ackermann, Org. Lett., 2016, 18, 2742–2745 CrossRef CAS PubMed; (c) Z. Chen, X. Hu, J. Huang and W. Zeng, Org. Lett., 2018, 20, 3980–3983 CrossRef CAS PubMed; (d) M. Ioannou, M. J. Porter and F. Saez, Chem. Commun., 2002, 346–347 RSC; (e) J. Su, Q. Li, Y. Shao and J. Sun, Org. Lett., 2022, 24, 1637–1641 CrossRef CAS PubMed; (f) C. Yu, Y. Xu, X. Zhang and X. Fan, J. Org. Chem., 2022, 87, 7392–7404 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2190019. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra07872d |
| This journal is © The Royal Society of Chemistry 2023 |