Open Access Article
Open Access ArticleHigh strontium adsorption performance of layered zirconium phosphate intercalated with a crown ether
Lina Wu abc,
Huiping Wang*bc,
Xiangqian Kongbc,
Haibo Wei
abc,
Huiping Wang*bc,
Xiangqian Kongbc,
Haibo Wei bc,
Sheng Chenbc and
Lisheng Chi
bc,
Sheng Chenbc and
Lisheng Chi *bc
*bc
aCollege of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, China
bFujian Science and Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350116, China
cFujian Key Laboratory of Fuel and Materials in Clean Nuclear Energy System, Fujian Institute of Research on the Structure of Matter, CAS, Fuzhou, Fujian 350002, China. E-mail: lchi@fjirsm.ac.cn
First published on 21st February 2023
Abstract
Effective removal of strontium isotopes in radioactive waste streams has important implications for the environment and the sustainable development of nuclear energy. In this work, a zirconium phosphate/18-crown-ether-6 (ZrP/18C6) composite was prepared using the intercalation method by loading crown ether into zirconium phosphate. The composite was structurally and morphologically characterized by XRD, FT-IR, XPS, and SEM. The adsorption experiments of Sr2+ onto the ZrP/18C6 composite were conducted as a function of temperature, pH, Sr2+ concentration and competing ions. The results indicate ZrP/18C6 can adsorb 98.6% of Sr2+ within 30 minutes at an Sr2+ concentration of 100 mg L−1 and maintain a high removal rate with a distribution coefficient of 7 × 105 mL g−1 when Sr2+ is at a low level of 4.28 mg L−1. The ZrP/18C6 composite reached a maximum adsorption capacity of 195.74 mg g−1 at an Sr2+ concentration of 380 mg L−1, which is significantly higher than the 43.03 mg g−1 of α-ZrP. The adsorption performance of Sr2+ onto ZrP/18C6 is not significantly affected by temperature, pH and competing ions. Furthermore, the adsorption kinetics and thermodynamics were analyzed based on the adsorption data obtained in the present work. It is shown that the adsorption of Sr2+ onto ZrP/18C6 follows the pseudo-second-order model and the Langmuir monolayer model, respectively. Additionally, the adsorption mechanism of Sr2+ by ZrP/18C6 is discussed.
1 Introduction
Sr-90 is one of the fission products decayed from U-235 when a nuclear power plant is in operation.1 It has a long half-life of 29 years and is one of the most radioactive nuclides in nuclear waste streams.2 Moreover, due to the high similarity between strontium and calcium in chemical properties, on entering human body, strontium can be easily adsorbed by bone, which could cause bone cancer and leukemia.3,4 Therefore, efficient removal of strontium isotopes from nuclear wastes is of great importance.Many studies have been conducted on removal of strontium isotopes from nuclear waste streams. The main methods studied include chemical precipitation,5 solvent extraction,6,7 membrane treatment,8,9 biological methods,10,11 and adsorption methods.12–14 Among these methods, the adsorption method has advantages over other methods in treatment efficiency, chemical reagent dosage, and separation cost. Therefore, in the last decades, a lot of adsorbents have been developed.15–17 However, the adsorbent materials for the removal of Sr isotopes with a fast adsorption rate, good stability, and excellent adsorption performance have been rarely reported.18,19
α-ZrP (Zr(HPO4)2·H2O) is a two-dimensional layered phosphate with a layer spacing of 0.76 nm and a large number of P–OH groups between the layers.20,21 The hydrogen protons in P–OH groups can be exchanged with other metal ions,22–24 demonstrating their good ion-exchange properties. Furthermore, the zirconium phosphate material has a good adsorption capacity for alkali metals and alkaline earth metals, as well as for Sr-90 in radioactive waste streams.12,25,26 In addition, as an inorganic adsorbent, it has good thermal stability, acid resistance, good mechanical strength, and ease of synthesis.22,25 As the weak interlayer force makes the α-ZrP layer spacing adjustable, the guest molecules can be inserted under certain conditions to change the properties of the host or the guest.23,27,28 The pre-intercalation method is generally used to prepare composite of host and guest materials, and the pre-intercalation materials used to adjust interlayer space of the zirconium phosphate are mainly amine molecules.28 After insertion of an amine molecule, the carbon skeleton of the amine molecule and the layer of the zirconium phosphate are maintained as a single parallel structure or a double-layer oblique insertion structure, which plays a vital role in expanding the interlayer space.29,30 As a result, a number of adsorbents based on zirconium phosphate material with excellent adsorption properties have been developed.27,31
The crown ether is composed of multiple ethoxy (C–C–O) units, and the multiple powered oxygen atoms in the structure allow it to form host–guest complexes with alkali and alkaline earth metal ions.32,33 As the diameter of Sr2+ (0.23 nm) is close to the size of the cavity in 18-crown-ether-6 (18C6) and its derivatives, which is in the range of 0.26–0.32 nm, Sr2+ can enter the cavity to interact with O atoms in 18C6 to form stable complexes.19,34 As a result, the 18C6 has become the most studied extractant in the removal of Sr-90 from highly radioactive waste streams.7,35–38 However, as an organic material, post-treatment of the crown ether after nuclear waste adsorption poses a challenge.38
In this experiment, the target products were synthesized by a pre-intercalation method. The pre-supporter material used in zirconium phosphate is n-butylamine molecule.22 The interlayer space in zirconium phosphate is about 1.8 nm after intercalating with the n-butylamine molecule, which is close to the molecular width of 18-crown-ether-6.28,39 Therefore, n-butylamine was finally selected as the intercalator to prepare the pre-propellant material in this study. In the present work, based on the structure character of α-ZrP, 18-crown-ether-6 was successfully inserted into the interlayers to form the ZrP/18C6 composite, which is evaluated for its adsorption properties and stability in the removal of strontium ions from simulated nuclear wastes. The study reveals that ZrP/18C6 is a promising adsorbent material for the removal of strontium ions from nuclear waste streams.
2 Experimental
2.1 Chemicals
Zirconium oxychloride octahydrate (ZrOCl2·8H2O, 99%), orthophosphoric acid (H3PO4, 85 wt%), hydrofluoric acid (HF, 99%), n-butylamine (CH3(CH2)3NH2, 99%), anhydrous ethanol (CH3CH2OH, 99.7%), strontium nitrate (Sr (NO3)2, 99%), sodium nitrate (NaNO3, 99%), and potassium nitrate (KNO3, 99%) were purchased from Shanghai Sinopharm Chemical Reagent Company. 18-crown-ether-6 (C12H24O6, 98%) was obtained from Shanghai Aladdin Biochemical Technology Company. All chemicals above were used as received without further purification. Ultra-pure water was provided by ultra-pure water purification equipment (Direct-Q5UV Millipore, 18.2 MΩ cm @ 25 °C).2.2 Characterization
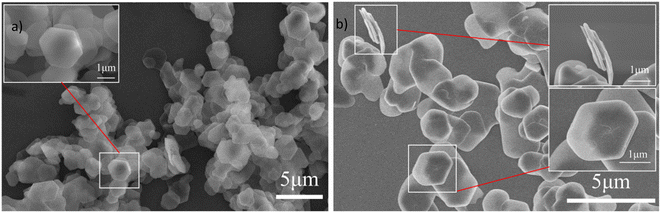
X-ray diffraction (XRD) was performed on a Rigaku Miniflex 600 in the 2θ range from 3° to 80° with a scan rate of 5° min−1 to characterize the crystal structure of the samples before and after adsorption of Sr2+ ions. Fourier transform infrared spectroscopy (FT-IR) was used to characterize the functional groups of the composites. The IR spectra were collected on a Bruker Optics Vertex70 infrared spectrometer in a scan range from 400 cm−1 to 4000 cm−1. The elemental composition and elemental electron binding energies of the composites were characterized by X-ray photoelectron spectroscopy (XPS) and obtained on a ThermoFisher Escalab 250Xi X-ray photoelectron spectrometer. The morphology and microstructure of the adsorbents were observed by Hitachi SU8010 field emission scanning electron microscope (SEM). Thermogravimetric analysis (TGA) was conducted using a Netzsch STA 499F3 type thermal analyzer to determine the thermal stability of the samples in the temperature range from 30 °C to 950 °C under a nitrogen gas flow. The ion concentration in the solution was measured using a PerkinElmer Avio 200 inductively coupled plasma emission spectrometry (ICP-OES).2.3 Preparation of ZrP/18C6 composites
The α-ZrP was synthesized using the fluorine reflux method as follows.21,35 100 mL of 3 mol L−1 phosphoric acid (H3PO4) was mixed well with 9.72 g of zirconium oxychloride octahydrate (ZrOCl2·8H2O), followed by adding 6 mL of hydrofluoric acid (HF) and refluxing at a temperature of 100 °C for 24 hours. After cooling, the reaction product was washed with ultrapure water several times and dried in a vacuum oven at 70 °C overnight. The product was identified by XRD to be α-zirconium phosphate (α-ZrP).Then, 1 g of α-zirconium phosphate (α-ZrP) was dispersed into 100 mL of 1 mol L−1 n-butylamine solution, followed by stirring in an oscillator for 5 days. The reaction product was separated by centrifugation and washed several times to remove the excess n-butylamine. The product was dried at 70 °C and named β-zirconium phosphate (β-ZrP).
Finally, β-ZrP and 18-crown-ether-6 were uniformly dispersed into anhydrous ethanol solution. The mixed solution was shaken in an oscillator for 5 days. The final product was separated by centrifugation, washed several times with ultrapure water, and dried in a drying oven at 70 °C. The obtained zirconium phosphate/crown-ether composite (namely, ZrP/18C6) was white powder and used for the following studies.
2.4 Batch adsorption experiments
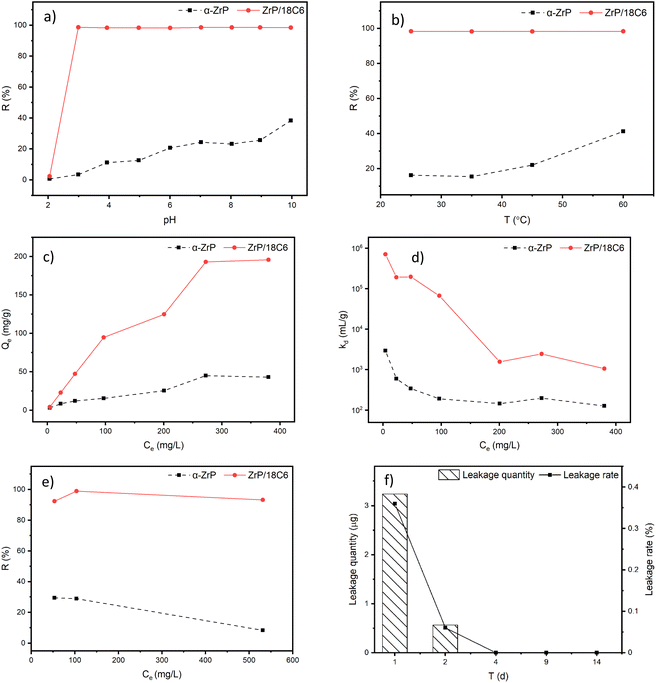
Batch adsorption experiments of Sr2+ on the α-ZrP and ZrP/18C6 materials were performed in polyethylene centrifuge tubes. 20 mg adsorbent was added to 20 mL of the Sr2+ solution and the solution was shaken in an oscillator for 6 h until adsorption equilibrium was reached. The adsorbent was removed with an injection filter and the concentration of Sr2+ ions in the solution was measured using ICP-OES before and after adsorption. Due to large variation in nuclear waste streams, the adsorption performance is investigated in the aqueous solution, which is partly related to the actual nuclear waste environments.In this study, the effects of pH, temperature, competing ions, and initial concentration of Sr2+ on the adsorption performance of ZrP/18C6 and α-ZrP were carried out. The solution pH was adjusted to 2, 3, 4, 5, 6, 7, 8, 9 and 10 using 0.1 mol L−1 nitric acid and 0.1 mol L−1 sodium hydroxide. Na+ and K+ were selected as competing ions for testing adsorption of Sr2+ on ZrP/18C6. In addition, the desorption of Sr2+ adsorbed on ZrP/18C6 was studied.
The equilibrium adsorption capacity (Qe, mg g−1), adsorption efficiency (R, %), and distribution coefficient (K, mL g−1) of the adsorbents on strontium ions were determined using the following eqn (1)–(3):
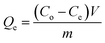 | (1) |
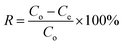 | (2) |
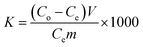 | (3) |
3 Results and discussion
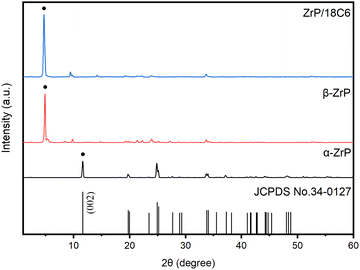
3.1 Structural characterizations
The peak at 11.64° can be used to characterize the interlayer separation. Based on the Bragg equation expressed with the following eqn (4):
2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ θ = nλ
| (4) |
The peak at 11.64° in α-ZrP corresponds to the interlayer separation of 0.760 nm. Intercalation of n-butylamine into α-ZrP forms β-ZrP, leading to expanding of the interlayer separation.31 Therefore, the diffraction peak shifts to the lower angle at 4.91° in β-ZrP, which corresponds to the interlayer separation of 1.798 nm. This implies that n-butylamine was successfully inserted into zirconium phosphate. The XRD pattern of the ZrP/18C6 composite is similar to that of β-ZrP. However, the peak further shifts to a lower angle at 4.72°, which is ascribed to substitution of 18-crown-ether-6 for n-butylamine. Intercalation of 18-crown-ether-6 forces the interlayer space to open wider to 1.871 nm as the size of 18-crown-ether-6 is larger than n-butylamine. As a result, the ZrP/18C6 composite provides more room between the layers for adsorbate. As the 18-crown-ether-6 has been known as a highly selective adsorbent for Sr2+,34,40 the ZrP/18C6 composite could be prospective for an Sr2+ adsorbent with high adsorption capacity.
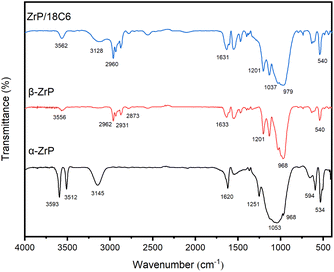
Several new peaks appeared after n-butylamine was inserted into α-ZrP to replace H2O. The 3556 cm−1 peak is due to the N–H stretching vibration. The 2962 cm−1 and 2873 cm−1 peaks are due to stretching vibrations of the –CH3 group in the inserted n-butylamine.35
When 18-crown-ether-6 was inserted into β-ZrP, the water molecule was also entered. The 3128 cm−1 broad peak is due to stretching vibration of the –OH group, the 1037 cm−1 peak is due to stretching vibration of the C–O–C group, and the 2960 cm−1 peak is due to stretching vibration of the –CH2 group in crown ether. The results indicate that 18-crown-ether-6 has been successfully compounded with zirconium phosphate.
3.2 Stability
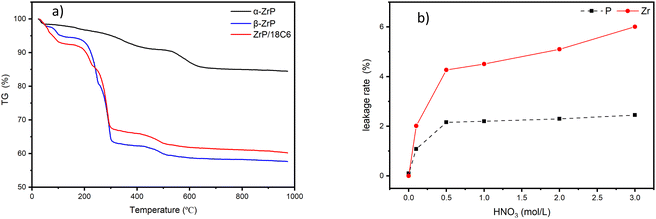
In practice, an adsorbent must demonstrate fair stability for its application.31 The thermal stability of the composites was tested by thermogravimetry under the nitrogen atmosphere (Fig. 5(a)). In the temperature from 30 °C to 950 °C, the weight loss is mainly composed of three parts. The first weight loss occurs at the temperature ≦120 °C due to the evaporation of water from the surface of the material. The second weight loss occurs at the temperature between 120 °C and 300 °C due to loss of crystallized water within the structure, which is the main weight loss. The final weight loss is mainly due to escape of inserted n-butylamine and 18-crown-ether-6 in the interlayer at the temperature from 300 °C to 600 °C.The acid resistance of the adsorbents was tested for possible application in treatment of nuclear waste streams. The acid resistance was investigated by measuring leakage rates of P and Zr after soaking the 10 mg materials in 20 mL nitric acid with different concentrations for 24 hours at room temperature (Fig. 5(b)). The leakage rate of ion is calculated using eqn (5).
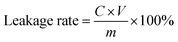 | (5) |
With the increase in nitric acid concentration, the leakage rates of P and Zr are up to 2% and 6%, respectively. The low leakage rates of P and Zr indicate that the composites have good stability under the acid conditions tested.
3.3 Adsorption performance of adsorbents
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.75
1, 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
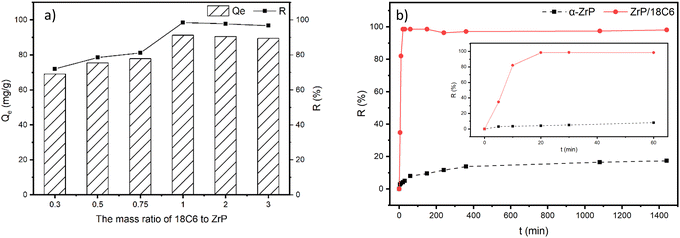
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used, and all the adsorption experiments were performed under the same conditions (Fig. 6(a)). The results show that the adsorption capacity and removal rate of the composites for Sr2+ increased significantly with increasing the inserted amount of 18-crown-ether-6. The adsorption capacity reached the maximum at a mass ratio of 1
1 was used, and all the adsorption experiments were performed under the same conditions (Fig. 6(a)). The results show that the adsorption capacity and removal rate of the composites for Sr2+ increased significantly with increasing the inserted amount of 18-crown-ether-6. The adsorption capacity reached the maximum at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by slightly decreasing when the mass of crown ether was further increased. This can be ascribed to substitution of 18C6 for n-butylamine located in the interlayer of zirconium phosphate, which increases the adsorption sites due to the larger size of 18C6. However, excessive insertion leads to a reduction in the free space between layers.35 This increases the difficulty of Sr2+ entering the interlayer, which in turn reduces the adsorption capacity. Based on the results obtained in this test, the 18-crown-ether-6 and zirconium phosphate composite (ZrP/18C6) with a mass ratio of 1
1, followed by slightly decreasing when the mass of crown ether was further increased. This can be ascribed to substitution of 18C6 for n-butylamine located in the interlayer of zirconium phosphate, which increases the adsorption sites due to the larger size of 18C6. However, excessive insertion leads to a reduction in the free space between layers.35 This increases the difficulty of Sr2+ entering the interlayer, which in turn reduces the adsorption capacity. Based on the results obtained in this test, the 18-crown-ether-6 and zirconium phosphate composite (ZrP/18C6) with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used for further study of Sr2+ ions removal in the following studies.
1 was used for further study of Sr2+ ions removal in the following studies.
3.4 Adsorption mechanism of Sr2+ onto ZrP/18C6
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (6) |
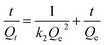 | (7) |
The adsorption data of the ZrP/18C6 adsorbent were fitted to the kinetic models (Fig. 8), and the corresponding model fitting parameters are obtained (Table 1). The adsorption data have a better fit to the pseudo-second order kinetic model with R2 = 0.99996 than the pseudo-first order model. Meanwhile, the equilibrium adsorption amount of 89.605 mg g−1 obtained from the fitting is in good agreement with 89.51 mg g−1 obtained from the experiment. It is concluded that the adsorption of Sr2+ onto ZrP/18C6 proceeds with the pseudo-second order model.46 Therefore, the adsorption process is dominated by chemisorption.47
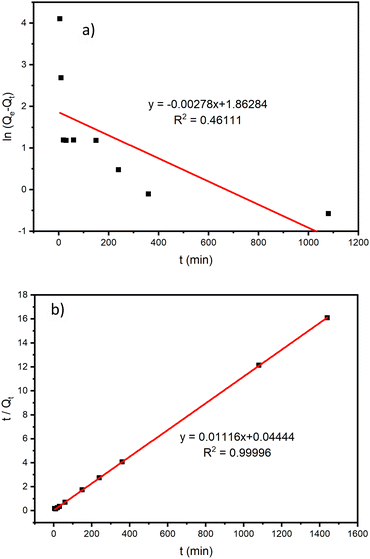 | ||
| Fig. 8 Kinetic model fitting curves on Sr2+ adsorption by ZrP/18C6. (a) Pseudo-first order kinetics model. (b) Pseudo-second order kinetics model. | ||
| Model | Pseudo-first order | Pseudo-second order | ||||
|---|---|---|---|---|---|---|
| Modeling parameters | Qe (mg g−1) | k1 (min−1) | R2 | Qe (mg g−1) | k2 (g min−1 mg−1) | R2 |
| Value | 6.442 | 0.0028 | 0.46111 | 89.605 | 0.0028 | 0.99996 |
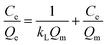 | (8) |
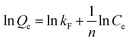 | (9) |
To further understand the adsorption process of Sr2+ on ZrP/18C6, the adsorption data obtained at 25 °C were fitted to the models (Fig. 9), and the corresponding model fitting parameters were obtained (Table 2). It can be found that the isotherm Langmuir adsorption model is better fitted to the data with R2 = 0.95860 than the Freundlich adsorption model. Moreover, the equilibrium adsorption amount of 187.617 mg g−1 obtained from the isotherm Langmuir adsorption model was closer to the experimental data of 195.74 mg g−1. The theoretical model analysis indicates that the Sr2+ ions are adsorbed onto the ZrP/18C6 composite mainly via the monolayer process.50
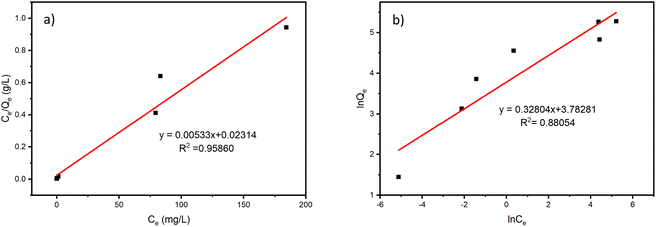 | ||
| Fig. 9 Thermodynamics isotherm model fitting curves of Sr2+ adsorption by ZrP/18C6. (a) Langmuir adsorption isotherm model. (b) Freundlich adsorption isotherm model. | ||
| Model | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Modeling parameters | Qm (mg g−1) | kL (mg−1) | R2 | 1/n | kF (Ln mgn−1 g−1) | R2 |
| Value | 187.617 | 0.230 | 0.95860 | 0.32804 | 43.939 | 0.88054 |
The adsorption experiments conducted in this study confirm that the ZrP/18C6 composite has a high adsorption capacity at 195.74 mg g−1 when the equilibrium concentration of Sr2+ was at 380 mg L−1, which is 4.5 times higher than α-ZrP. The XRD patterns of ZrP/18C6 before and after adsorption were compared (Fig. 10(a)). It is shown that after adsorption the diffraction peak at 4.72° was shifted to a higher angle of 4.93° peak. This indicates that the interlayer separation in the ZrP/18C6 structure became narrower after adsorption owing to the strong complexation of Sr2+ with oxygen atoms in 18-crown-ether-6 and –OH groups in zirconium phosphate. As the smaller free interlayer space limit occurrence of multilayer adsorption, Sr2+ ions are mainly adsorbed onto the ZrP/18C6 via the monolayer process, which is consistent with the isotherm Langmuir model.
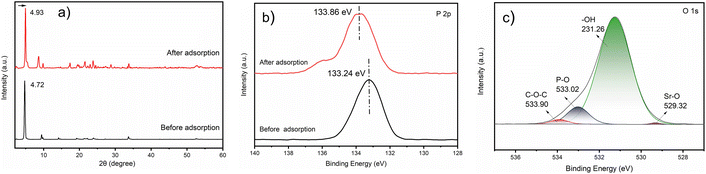 | ||
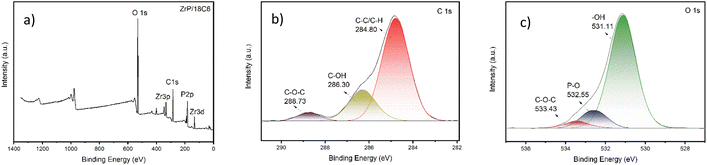
| Fig. 10 The change of ZrP/18C6 before and after Sr2+ adsorption (a) XRD patterns. (b) XPS spectra of P 2p. (c) XPS spectra of O 1s after Sr2+ adsorption. | ||
The P 2p and O 1s XPS of ZrP/18C6 before and after Sr2+ adsorption was collected (Fig. 10(b) and (c)). A shift of 0.62 eV in the P 2p peak and 0.47 eV in the fitted P–O bond of the O 1s peak were observed after Sr2+ adsorption. These data confirm that strong binding of Sr2+ to the P–O bond in zirconium phosphate is formed during the adsorption.51,52 Meanwhile, a new peak at 529.32 eV appeared in the O 1s peak, which is ascribed to the Sr–O bond,53 and the fitted C–O–C bond in the O 1s peak also changed by 0.47 eV after adsorption. These data suggest that 18-crown-ether-6 is involved in the adsorption process by reacting Sr2+ with oxygen atoms in the crown ether.40 Therefore, Sr2+ are adsorbed onto ZrP/18C6 via the chemisorption process at a relatively high speed.
4 Conclusions
In the present study, zirconium phosphate/18-crown-ether-6 composite (ZrP/18C6) was successfully synthesized by expanding the interlayer space of α-ZrP with n-butylamine. The effects of the environmental factors and Sr2+ concentration on the adsorption behavior of Sr2+ onto ZrP/18C6 were systematically investigated to examine its adsorption performance and adsorption mechanism. The ZrP/18C6 composite demonstrates an excellent adsorption capacity of 195.74 mg g−1 for Sr2+ at the equilibrium concentration of 380 mg L−1, which was little affected by the solution temperature, pH and competing ions. The material shows a quick adsorption rate of 98.6% within 30 min and a high affinity for Sr2+ with a distribution coefficient of 7 × 105 mL g−1 at a low Sr2+ concentration of 4.28 mg L−1. In addition, the composite adsorbent exhibits good thermal stability and acid resistance.Analyses of the adsorption data, in combination with XRD and XPS data, reveal that Sr2+ ions are adsorbed onto ZrP/18C6 via the Langmuir monolayer model that is mainly controlled by the chemisorption process. The desorption data show that Sr2+ ions adsorbed onto the ZrP/18C6 material are well preserved with a low leakage rate of 0.5% in a 14 day experiment. Therefore, the layered zirconium phosphate/18-crown-ether-6 (ZrP/18C6) composite is a promising candidate adsorbent for application in the removal of Sr2+ from nuclear waste streams. However, further experiments should be conducted on its stability under radiation conditions and its adsorption performance in actual nuclear waste streams.
Author contributions
Lina Wu performed the experiments, analyzed the data and wrote the manuscript. Huiping Wang provided helpful discussions and performed the characterizations. Xiangqian Kong and Haibo Wei collected and analyzed the data. Sheng Chen reviewed the manuscript. Lisheng Chi revised the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (#2021ZR108).Notes and references
- R. Natarajan, Prog. Nucl. Energy, 2017, 101, 118–132 CrossRef CAS.
- S. K. Sahoo, N. Kavasi, A. Sorimachi, H. Arae, S. Tokonami, J. W. Mietelski, E. Lokas and S. Yoshida, Sci. Rep., 2016, 6, 23925 CrossRef CAS PubMed.
- A. Burger and I. Lichtscheidl, Sci. Total Environ., 2019, 653, 1458–1512 CrossRef CAS PubMed.
- S. Pors Nielsen, Bone, 2004, 35, 583–588 CrossRef CAS PubMed.
- Y. Guo, N. T. Hong Nhung, X. Dai, C. He, Y. Wang, Y. Wei and T. Fujita, Front. Bioeng. Biotechnol., 2022, 10, 819407 CrossRef PubMed.
- P. N. Khan, A. Bhattacharyya, J. N. Sharma and S. Manohar, J. Hazard. Mater., 2020, 397, 122476 CrossRef CAS PubMed.
- M. A. Momen and M. L. Dietz, React. Funct. Polym., 2021, 160, 104829 CrossRef CAS.
- R. K. Vishwakarma, P. K. Narayanam, R. Umamaheswari and K. Sundararajan, J. Environ. Manage., 2021, 298, 113443 CrossRef CAS PubMed.
- Y. K. Kim, S. Kim, Y. Kim, K. Bae, D. Harbottle and J. W. Lee, Appl. Surf. Sci., 2019, 493, 165–176 CrossRef CAS.
- D. Sofronov, M. Rucki, V. Varchenko, E. Bryleva, P. Mateychenko and A. Lebedynskiy, J. Environ. Chem. Eng., 2022, 10, 106944 CrossRef CAS.
- S. Eun, J. Ryu, H. Kim, H. J. Hong and S. Kim, J. Environ. Manage., 2021, 297, 113389 CrossRef CAS PubMed.
- J. Zhang, L. Chen, X. Dai, L. Chen, F. Zhai, W. Yu, S. Guo, L. Yang, L. Chen, Y. Zhang, L. He, C. Chen, Z. Chai and S. Wang, Chem. Commun., 2021, 57, 8452–8455 RSC.
- G. Kim, D. S. Lee, H. Eccles, S. M. Kim, H. U. Cho and J. M. Park, RSC Adv., 2022, 12, 18936–18944 RSC.
- L. Yin, X. Kong, X. Shao and Y. Ji, J. Environ. Chem. Eng., 2019, 7, 103073 CrossRef CAS.
- S. İnan, J. Radioanal. Nucl. Chem., 2022, 331, 1137–1154 CrossRef.
- D. Alby, C. Charnay, M. Heran, B. Prelot and J. Zajac, J. Hazard. Mater., 2018, 344, 511–530 CrossRef CAS PubMed.
- N. A. Bezhin, I. I. Dovhyi, S. V. Kapranov, N. I. Bobko, V. V. y. Milyutin, V. O. Kaptakov, E. A. Kozlitin and I. G. Tananaev, J. Radioanal. Nucl. Chem., 2021, 328, 1199–1209 CrossRef CAS.
- B. Aguila, D. Banerjee, Z. Nie, Y. Shin, S. Ma and P. K. Thallapally, Chem. Commun., 2016, 52, 5940–5942 RSC.
- E. P. Horwitz, M. L. Dietz and D. E. Fisher, Solvent Extr. Ion Exch., 1990, 8, 199–208 CrossRef CAS.
- M. Pica, A. Donnadio and M. Casciola, Coord. Chem. Rev., 2018, 374, 218–235 CrossRef CAS.
- A. Contreras-Ramirez, S. Tao, G. S. Day, V. I. Bakhmutov, S. J. L. Billinge and H. C. Zhou, Inorg. Chem., 2019, 58, 14260–14274 CrossRef CAS PubMed.
- A. Bashir, S. Ahad, L. A. Malik, A. Qureashi, T. Manzoor, G. N. Dar and A. H. Pandith, Ind. Eng. Chem. Res., 2020, 59, 22353–22397 CrossRef CAS.
- M. V. Ramos-Garcés, J. González-Villegas, A. López-Cubero and J. L. Colón, Acc. Mater. Res., 2021, 2, 793–803 CrossRef.
- A. Clearfield and J. A. Stynes, J. Inorg. Nucl. Chem., 1964, 26, 117–129 CrossRef CAS.
- M. Pica, Molecules, 2021, 26, 2392 CrossRef CAS PubMed.
- Z. Li, E. L. Vivas, Y. J. Suh and K. Cho, J. Environ. Chem. Eng., 2022, 10, 107333 CrossRef CAS.
- J. Zhang, L. Feng, Y. Jian, G. Luo, M. Wang, B. Hu, T. Liu, J. Li, Y. Yuan and N. Wang, Chem. Eng. J., 2022, 429, 132265 CrossRef CAS.
- W. Mu, Q. Yu, B. Chen, X. Li, H. Wei, Y. Yang and S. Peng, J. Mol. Liq., 2021, 323, 114585 CrossRef CAS.
- A. Clearfield and G. D. Smith, J. Inorg. Nucl. Chem., 1968, 30, 327–329 CrossRef CAS.
- A. Clearfield and R. M. Tindwa, J. Inorg. Nucl. Chem., 1979, 41, 871–878 CrossRef CAS.
- W. Mu, Y. Yang, B. Chen, X. Li, H. Wei, Y. Yang and s. Peng, Sep. Purif. Technol., 2022, 291, 120605 CrossRef CAS.
- H. X. Zhang, Z. X. Huang, P. Y. Zhao, Y. Hou, J. J. Guo and Y. C. Wu, Mater. Res. Express, 2019, 6, 125095 CrossRef CAS.
- S. Avramescu, S. Petrescu, D. C. Culita, M. Tudose, A. Hanganu, I. Zarafu and P. Ionita, J. Nanopart. Res., 2020, 22, 194 CrossRef CAS.
- N. A. Bezhin and I. I. Dovhyi, Russ. Chem. Rev., 2015, 84, 1279–1293 CrossRef CAS.
- W. Mu, Q. Yu, J. Gu, X. Li, Y. Yang, H. Wei and S. Peng, Sep. Purif. Technol., 2020, 240, 116658 CrossRef CAS.
- W. Mu, B. Chen, Y. Yang, X. Li, H. Wei, Y. Yang and S. Peng, J. Phys. Chem. Solids, 2022, 163, 110604 CrossRef CAS.
- J. D. Law, D. J. Wood and R. S. Herbst, Sep. Sci. Technol., 1997, 32, 223–240 CrossRef CAS.
- Z. Wei, Y. Gao, Y. Zhou, C. Jiao, M. Zhang, H. Hou and W. Liu, J. Serb. Chem. Soc., 2020, 85, 909–922 CrossRef CAS.
- W. Mu, B. Chen, Q. Yu, X. Li, H. Wei, Y. Yang and S. Peng, J. Mol. Liq., 2021, 326, 115307 CrossRef CAS.
- C. Guo, M. Yuan, L. He, L. Cheng, X. Wang, N. Shen, F. Ma, G. Huang and S. Wang, CrystEngComm, 2021, 23, 3349–3355 RSC.
- J. R. Ferraro, J. Chem. Educ., 1961, 38, 201 CrossRef CAS.
- S. Pourbeyram, Ind. Eng. Chem. Res., 2016, 55, 5608–5617 CrossRef CAS.
- S. M. Fernández-Valverde, A. Contreras-Ramírez, E. Ordóñez-Regil, M. E. Fernández-García and M. Pérez-Álvarez, J. Solid State Chem., 2013, 198, 238–245 CrossRef.
- Y. S. Ho, Scientometrics, 2004, 59, 171–177 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- Y. S. Ho, J. Hazard. Mater., 2006, 136, 681–689 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Process Saf. Environ. Prot., 1998, 76, 332–340 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- H. Freundlich, Z. Phys. Chem., Stoechiom. Verwandtschaftsl., 1906, 57, 385–470 CAS.
- H. Swenson and N. P. Stadie, Langmuir, 2019, 35, 5409–5426 CrossRef CAS PubMed.
- Z. Jiao, Y. Meng, C. He, X. Yin, X. Wang and Y. Wei, Microporous Mesoporous Mater., 2021, 318, 111016 CrossRef CAS.
- Q. Gao, J.-F. Xie, Y.-T. Shao, C. Chen, B. Han, K.-S. Xia and C.-G. Zhou, Chem. Eng. J., 2017, 313, 197–206 CrossRef CAS.
- J.-C. Dupin, D. Gonbeau, P. Vinatier and A. Levasseur, Phys. Chem. Chem. Phys., 2000, 2, 1319–1324 RSC.
| This journal is © The Royal Society of Chemistry 2023 |