 Open Access Article
Open Access ArticleRecovery of vanadium and nickel from heavy oil fly ash (HOFA): a critical review
Ashraf Bakkar *a,
Mohamed M. El-Sayed Selemanb,
Mohamed M. Zaky Ahmedc,
Saeed Harba,
Sami Goren
*a,
Mohamed M. El-Sayed Selemanb,
Mohamed M. Zaky Ahmedc,
Saeed Harba,
Sami Goren a and
Eskander Howsawia
a and
Eskander Howsawia
aDepartment of Environmental Engineering, College of Engineering at Al-Leith, Um Al-Qura University, Al-Lith 28434, Saudi Arabia. E-mail: atbakkar@uqu.edu.sa
bDepartment of Metallurgical and Materials Engineering, Faculty of Petroleum and Mining Engineering, Suez University, Suez 43512, Egypt
cMechanical Engineering Department, College of Engineering at Al Kharj, Prince Sattam Bin Abdulaziz University, Al Kharj 11942, Saudi Arabia
First published on 21st February 2023
Abstract
Heavy oil fly ash “HOFA” is the fly ash generated in power stations using heavy oil as fuel. HOFA is considered a hazardous waste because it contains considerable amounts of heavy metals. However, it contains significant amounts of vanadium “V” and nickel “Ni”, which are precious metals for manufacturing processes. This paper presents a critical review of various approaches described in the literature for the recovery of V and Ni from HOFA, including processes of leaching, chemical precipitation, solvent extraction, and ion exchange. The optimum operational parameters and their effects on recovery efficiency are discussed. The digestion mixtures of strong mineral acids used for dissolving all metals present in HOFA are also highlighted. The leaching processes of V and Ni use mainly acidic and alkaline solutions. Bioleaching is a promising environmentally friendly approach for the recovery of V and Ni through using appropriate bacteria and fungi. After leaching, V and Ni compounds are recovered and purified using various techniques, including chemical precipitation, solvent extraction, and ion exchange. In most cases, V and Ni are recovered as thermally decomposable compounds that undergo calcination to produce V2O5 and NiO. Eventually, V and Ni are recovered as pure oxides in most approaches, but pure metals are obtained in exceptional procedures.
1. Introduction
Heavy oil fly ash (HOFA) is a waste product resulting from the combustion of heavy fuel oil. Heavy oil is the most used fuel for heating boilers at power stations and water desalination plants in Saudi Arabia and many countries worldwide. For instance, the annual consumption of heavy oil in power stations and desalination plants is more than 40 million tons in Saudi Arabia, and more than 4 thousand tons in thermal power stations in Egypt.1 The combustion of heavy fuel in power stations generates great amounts of fly ash, which is expected to increase in the next few years due to the growing rate of energy demand. Burning 1000 liters of heavy oil generates about 3 kg of fly ash.2 The HOFA produced is mainly composed of unburned carbon, with a great percentage of 64–97,3 and inorganic compounds, including oxides of vanadium, nickel, magnesium, aluminum, zinc, iron, and others. Sulfates of those metals might be included too.4Vanadium(V) and nickel (Ni) are considered the most toxic metallic species present in HOFA.5,6 They are easily leachable by rainwater in disposal landfills. On the other hand, V and Ni have wide applications in the industry; they are used as alloying elements in steel and other special alloys.7,8 Therefore, the recovery of V and Ni from industrial wastes is of great interest in terms of environmental and economic aspects. In general, V and Ni are present in HOFA with contents of 2–5 wt% according to many studies characterized the fly ash.9–11 The chemical composition of HOFA varies depending on the composition of the heavy oil fuel, the operating conditions, and the reagents added. However, higher levels of V and Ni were detected in heavy oil fly ashes (HOFAs) produced worldwide in various power stations.12
Both V and Ni are considered precious metals and their rapid and continuously increasing demand is countered by their low concentrations in raw minerals. V is usually recovered from the raw materials produced as a by-product during the extraction of other metals. V is used in a wide range as an alloying element for a diverse range of super alloys of steel, stainless steel, nickel, and titanium.7,13,14 Many applications are contributing to the increased demand for V, for example, V redox battery (V flow battery) is a promising solution for the global problem of seeking large scale energy storage.15–17
Heavy oil fuel is the major fuel used in Saudi Arabia, it contributes to more than 70% of national energy production.3,18 The bulk density of HOFA ranges from 0.26 g cm−3 (ref. 19) to 0.52 g cm−3.20 However, most studies reported that HOFA has a density of around 0.33 g cm−3. This means that the specific volume of HOFA is about 3 cm3 g−1. Consequently, the dumping of HOFA needs spacious landfill sites that increase the environmental consequences. The large quantity of HOFA generated along wide Saudi Arabia has become a pressing concern from a safe disposal point of view.
Although the HOFA contains V and Ni in economical concentrations comparable to that found naturally in mineral recourses, its disposal in landfills leads to the leaching of V and Ni that poisons the soil. Consequently, real efforts have to be directed to allow the recovery of these valuable metals and the safe re-utilization of fly ash residue.
2. Digestion and chemical composition of HOFA
Digestion aims at the dissolution of various metals (major and trace) present in HOFA in an aqueous solution that can be undergone subsequent analysis using atomic spectrometric techniques. Mixtures of strong inorganic acids such as HCl, HNO3, HF, and H2SO4 are the most commonly used dissolvents for the direct digestion of fly ash. It is reported that HCl digestion can completely dissolve all cations, except for Si and partly Ti. However, HF acid can dissolve silicates and completely extract Si and Ti.21,22 The mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 concentrated HCl–HNO3 with water, dissolved 49 elements.23 The most common digestion methods of fly ash for extraction of heavy metals involve the use of aqua regia solution (3
1 concentrated HCl–HNO3 with water, dissolved 49 elements.23 The most common digestion methods of fly ash for extraction of heavy metals involve the use of aqua regia solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HCl–HNO3) and reverse aqua regia solution (1
1 HCl–HNO3) and reverse aqua regia solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 HCl–HNO3). Digestion procedures include using a hot plate, a microwave oven and ultrasonic baths. With the aid of microwave and ultrasonic techniques, the digestion time can be reduced with higher dissolution efficiency.24 Digestion is commonly preceded by the preparation of ash samples through drying at elevated temperatures. Table 1 summarizes some digestion procedures used for the chemical analysis of HOFA elements.
3 HCl–HNO3). Digestion procedures include using a hot plate, a microwave oven and ultrasonic baths. With the aid of microwave and ultrasonic techniques, the digestion time can be reduced with higher dissolution efficiency.24 Digestion is commonly preceded by the preparation of ash samples through drying at elevated temperatures. Table 1 summarizes some digestion procedures used for the chemical analysis of HOFA elements.
| Digestion solution | Preparation of the ash | Temp., °C | Time | S/L ratio | Assisting technique | Analysis technique | Ref. |
|---|---|---|---|---|---|---|---|
| Conc. HCl (2.0 mL) + HClO4 (5.0 mL) + HNO3 (5.0 mL) | Drying at 110 °C for 2 h, ground + −200 mesh seiving | Room temperature | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 g ml−1 20 g ml−1 |
Heating slowly at 100 °C for 1 h on a hot plate with constant stirring till drying, then conc. HNO3 + drying (3 times). Finally the solid residue was redissolved in dilute HNO3 | ICP-OES | 26 | |
| Aqua regia | Drying at 110 °C for 2 h, ground + −200 mesh seiving | The cooled solution was filtered in a 100 mL volumetric flask and diluted with distilled water before analysis | 26 | ||||
Reverse aqua regia solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 HCl–HNO3) 3 HCl–HNO3) |
Crushing + sieving (−100 μm) | 250 °C | 300 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 g ml−1 5 g ml−1 |
ICP-AES | 51 | |
| A mixture of acids composed of HClO4, H2SO4 and HNO3 | 35 | ||||||
| Aqua regia | Drying t 110 °C + sieving (−500 μm) | 0.005–0.015 g ml−1 | Microwave furnace | ICP-AES | 27 | ||
| Hydrofluoric acid + aqua regia | Burned at 550 °C for 24 h | Hydrofluoric acid and aqua regia | AAS | 28 | |||
| H2SO4 | ICP-OES & titration | 37 | |||||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HNO3–Hf solution 1 HNO3–Hf solution |
Drying at 105 °C | 120 °C | 120 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 g ml−1 40 g ml−1 |
Autoclave | ICP-AES & titration | 38 |
| HNO3–Hf solution | Drying at 105 °C | 120 °C | 120 min | Autoclave | ICP-AES | 40 | |
| Hydrofluoric acid (2.5 mL) and nitric acid (7.5 ml) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g ml−1 10 g ml−1 |
ICP-AES | 69 | ||||
| 25% nitric acid solution | 0.1 g of fly ash was mixed with 1 g lithium borate mixture + heating at 1000 °C for 1 h | 60 °C | ICP-AES | 52 | |||
| Hot mixed acid composed of concentrated HClO4, H2SO4, and HNO3 | ICP-AES | 31 | |||||
| Aqua regia | Crushing + sieving (−75 μm) + washing with hexane and acetone + drying for 30 min at 70 °C | 50 °C | 24 h | ICP-OES | 46 | ||
| Aqua regia | Drying 120 °C, 24 h | 100 ± 5 °C | 2 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g ml−1 10 g ml−1 |
Digestion was repeated thrice using a hot plate with magnetic stirrer, a flask, and a condenser | ICP-OES | 47 |
The mostly used spectrometric techniques are atomic absorption spectrometry “AAS”, inductively coupled plasma-optical emission spectrometry “ICP-OES”, and inductively coupled plasma-mass spectrometry “ICP-MS”.24,25 ICP-OES has widely been used to analyze an enormous range of coal and fly ashes. The main superiority of ICP over AAS techniques include its capability of conducting simultaneous multi-element analysis with higher sensitivity and reliability.24 The carbon content in the fly ash (found as carbon, carbonate, and residual oily substance) is evaluated by thermal decomposition for 6 h at 1000 °C. The weight loss is considered to be the carbon content that is found to be 85% in a HOFA sample.26,27 However, Tsai and Tsai28 measured the contents of carbon, hydrogen, and nitrogen in the fly ash using the elemental analyzer (Heraeus CHNRapid Elemental analyzer). The sulfur content in the fly ash is determined using X-ray fluorescence spectrometer according to old and recent researches,29,30 but Tsai and Tsai28 measured the sulfur in the fly ash by a Tacussel Coulomax 78 Elemental analyzer.
The chemical analysis of HOFA shows that it is composed mainly of carbon, in addition to some metallic elements. Table 2 reveals the chemical composition of fly ash samples produced by burning heavy oil in different power plants and desalination plants. The ash contains high levels of V and Ni, and highly toxic elements such as arsenic, cadmium, cobalt, chromium, lead, and selenium. V content reaches up to 5 wt%,11 but the maximum content of Ni is 1.8 wt%.31
| Element | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref. 20, PP | Ref. 20, DP | Ref. 19 | Ref. 9 | Ref. 11 | Ref. 40 | Ref. 10 | Ref. 31 | Ref. 47 | |
| a PP = power plant; DP = desalination plant. | |||||||||
| Carbon (%) | 94.40 | 56.70 | 85.56 | 51.86 | 67.4 | 77.40 | |||
| Sulfur (%) | 7.77 | 27.18 | ND | 8.6 | 7.10 | ||||
| Vanadium (ppm) | 9072 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 044 044 |
2958 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 487 487 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000 |
7670 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 900 900 |
3540 |
| Nickel (ppm) | 2382 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 633 633 |
1762 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 852 852 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 400 400 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000 |
6800 | 1055 |
| Cadmium (ppm) | 1.65 | 3.7 | 3.28 | 1.59 | 60.4 | ||||
| Arsenic (ppm) | 2.54 | 1846 | 2.24 | 68.29 | 128.5 | ||||
| Cobalt (ppm) | 2.88 | 12.33 | 3.28 | 247.79 | 2200 | 60.7 | |||
| Chromium (ppm) | 36.79 | 113.09 | 4.06 | 107.60 | 8000 | 70.4 | |||
| Selenium (ppm) | 1.00 | 6.81 | 11.60 | 13.20 | 7.25 | ||||
| Lead (ppm) | 17.09 | 13.94 | 11.00 | 116.10 | 27.85 | ||||
| Zinc (ppm) | 21.92 | 118 | 130 | 592.10 | 1110 | 4000 | 65.5 | ||
| Copper (ppm) | 10.44 | 50.40 | 170.4 | 120.30 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
57.12 | |||
| Iron (ppm) | 7210 | 8771 | 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
8000 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1400 | 176.5 | ||
| Magnesium (ppm) | 6971 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 608 |
2000 | 3430 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
3615 | |||
| Manganese (ppm) | 23.9 | 149.26 | 12.8 | ||||||
| Calcium (ppm) | 582.3 | 4121.2 | 2300 | 3380 | |||||
| Sodium (ppm) | 1395 | 7555 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1310 | |||||
| Alumnium (ppm) | 3541 | 1041.8 | 1040 | 2870 | 2500 | 642.4 | |||
| Barium (ppm) | 7.42 | 49.68 | 69.0 | ||||||
| Others (ppm) | Hg, 0.25 | Mo, 3500 | Si, 8000 | Si, 800; O, 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
|||||
3. Leaching of V and Ni from HOFA
Leaching of HOFA is the first main step for the recovery of V and Ni. The recovery procedure varies with the type of ash depending on its own physicochemical properties, particularly the chemical composition.32 Research is directed to reach optimal leaching conditions, which include the leaching agents, temperature, residence time (contact time), agitation speed, particle size, and solid to liquid ratio (S/L).22Aburizaiza26 studied the leachability of V, Ni, and Fe from HOFA generated in three different power and desalination plants using various leaching agents, including water, H2SO4, HNO3, and HCl. It was found that the most leachable metal is V with exothermic and spontaneous dissolution reactions. For a comparison investigation of the used agents' leachability, acidic solutions were tested with a concentration of 1 M and S/L ratio of 1/50 g ml−1 at room temperature. Considerable metal recovery is obtained at 20 min. Within this time, the leachability for Ni follows the order; HCl > H2SO4 > HNO3 > H2O. For V and Fe, the leaching agents are ranked as H2SO4 > HCl > HNO3 > H2O. However, a longer time for 1 h ranks HCl and HNO3 in a higher rank former to H2SO4.26 On the other hand, alkaline leaching using sodium hydroxide “NaOH” and ammonium hydroxide “NH4OH” have the advantage to leach V leaving Ni and Fe undissolved in the ash.26,28
An overall review of the literature shows that the approaches proposed for the recovery of V and Ni are mainly performed through acidic or alkaline leaching processes. Therefore, the recovery methods can be classified, according to the leaching solution used, to be acidic and alkaline processes. Additionally, water leaching and bioleaching are recently researched in systematic approaches to recover V and Ni from fly ash.
3.1. Acidic leaching
H2SO4 is widely used in leaching processes by the virtue of its high economically advantageous due to its distinct lower cost.22,33 H2SO4 acid dissolves most of the metallic cations, such as V, Ni, Fe, Mg, and others, forming metal sulfates. V compounds can be then precipitated by adding various agents. Ni can also be precipitated as Ni compounds, or pure Ni can be electrochemically deposited from a Ni sulfate solution.Amer34 studied the selective recovery of V and Ni by direct H2SO4 leaching of Egyptian boiler ash under oxygen pressure to produce V2(SO4)3 and NiSO4. The dissolution of V and Ni is explained in the next two equations:
| V2O3 + 3H2SO4 = V2(SO4)3 + 3H2O | (1) |
| NiO + H2SO4 = NiSO4 + H2O | (2) |
The optimum parameters for leaching are established to be: temperature of 200 °C, leaching period of 15 min, O2 pressure of 15 bar, H2SO4 concentration of 60 g l−1, and S/L ratio of 1. V is precipitated from the acidic sulfate solution as vanadium hydroxide “V(OH)3” by adding NH4OH and neutralization. On the other hand, Ni metal can be electrodeposited from NiSO4 electrolyte. Leaching under O2 pressure is applied to oxidize ferrous sulfate to ferric sulfate (eqn (3)). The latter hydrolyzes thereafter and leads to Fe precipitation as ferric oxide (eqn (4)), leaving V and Ni in the leaching solution.
| 2FeSO4 + H2SO4 + 1/2 O2 = Fe2(SO4)3 + H2O | (3) |
| Fe2(SO4)3 + 3H2O = Fe2O3 + 3H2SO4 | (4) |
Barik et al.35 developed a process for recovering V and Ni from an industrial Ni and V-rich solid waste. The process includes H2SO4 leaching, solvent extraction, precipitation, and crystallization. Both V and Ni were leached out with 98% recovery by 1.35 M H2SO4 at 40 °C for 90 min. V is selectively extracted from the liquor with 40% LIX 84-I at pH 0.5 and precipitated as ammonium metavanadate “NH4VO3” by adding NH4OH. The Ni dissolved in the raffinate is selectively separated as Ni oxalate “NiC2O4” by using ammonium oxalate “(NH4)2C2O4”. Finally, pure Ni oxide “NiO” is obtained by calcination of Nickel oxalate “NiC2O4” at 450 °C for 2 h. On the other hand, the NH4VO3 can be calcined to give V pentoxide “V2O5”.36
Nazari et al.37 optimized the parameters of the H2SO4 leaching process to maximize the recovery of V and Ni. The optimum conditions for the highest recovery obtained, for V of 94% and for Ni of 81% are: H2SO4 concentration of 19.5%, temperature of 80 °C, S/L ratio of 9.15 wt%, and leaching time of 2 h.
Vitolo et al.38 recovered V as V2O5 from fly ash, through a three-step process consisting of H2SO4 leaching, oxidative precipitation, and washing. The operating parameters of leaching are reported to be boiling H2SO4 of 1 M concentration for 30 min with L/S ratio of 3 ml g−1. The recovery of V is around 90%. The oxidative precipitation process is performed by adding sodium chlorate “NaClO3” as an oxidative agent of V(IV) to V(V).39 This oxidation of V is accompanied with the evolution of H and consequently leads to a decrease in pH. Na2CO3 is added to keep the ideal pH for V2O5 precipitation. Finally, the V2O5 precipitated is washed with an acidic solution to remove Na and other impurities.
Vitolo et al.40 developed their three-step process38 by preceding it with a controlled burning of fly ash in order to reduce its carbon content. The reduction of carbonaceous fraction in the fly ash increases the V recovery in the following leaching step to reach 97%. The optimum temperature of the burning step is found to be 850 °C and leads to greater overall V recovery (83%) in the form of V2O5 with lower content of impurities. Higher burning temperature (above 950 °C) leads to lower V recovery due to the fusion and volatilization of V and the formation of complicated V–Ni refractory compounds.
Navarro et al.27 conducted leaching experiments of HOFA in 0.5 M H2SO4 solution with L/S ratio of 4 ml g−1 for 24 h at room temperature and an agitation speed of 200 rpm. The leaching step was followed by rinsing for 1 h in water with L/S ratio of 4 ml g−1. V recovery is recorded to be 98%, while Ni recovery is close to 12%.
Aburizaiza26 illustrated a multi-step procedure for leaching and extraction of Ni, V, and Fe from HOFA, following the next steps:
(1) Acidic leaching of fly ash in digestion solution composed of mineral acids (perchloric acid “HCLO4” and/or HCl), and hydrogen peroxide “H2O2”.
(2) Organic extraction of metals; in this step, the leachate is mixed and stirred with ammonium pyrollidine dithiocarbomate “C5H9NS2·NH3” in chloroform “CHCl3”. After equilibrium, a layer of an organic phase – containing the metallic cations – is formed. The results reveal compete extraction of aimed metal ions (V, Ni, and Fe) in the organic phase.
(3) Stripping of V, Ni, and Fe from the organic phase by mixing it with 1 M HNO3 containing Hg2+ ions. Thus, the resultant aqueous nitric acid solution contains metallic cations (V3+, Ni2+, Fe2+).
(4) Extraction of total Ni in the stripped nitric acid solution by adding dimethylglyoxime “C4H8N2O2” in chloroform, that forms again an organic phase containing Ni2+ ions and leave V3+ and Fe2+ ions dissolved in the aqueous nitric acid solution phase. The Ni-contained organic phase is separated and evaporated till dryness to recover nickel in the form of NiO.
(5) Extraction of total Fe in the aqueous nitric acid solution phase (remained in step 4) by treating with 4,4-pypyridyl. The Fe-contained organic phase is separated and undergoes evaporation and ignition to obtain Fe in the form of FeO.
(6) The remained aqueous solution contains vanadium as V3+ and other trace elements.
Analysis and materials balance reveal higher recovery values of Ni and Fe when their extracted concentrations are compared with that in the digestion solution. However, the study does not suggest an approach to extract V from the final aqueous solution. Moreover, the approach consumed a lot of chemicals to treat a small quantity of fly ash.26
Aiming at recovering of V and Ni and making the fly ash harmless, Tokuyama et al.31 leached HOFA in two steps. The first step uses water to dissolve Ni, Zn, Mg, Al, and Fe; and the second utilizes H2SO4 to dissolve V. The authors also found that the leaching efficiency of H2SO4 is comparable to that of HCl. On the other hand, the authors reported that selective leaching of V can be achieved by leaching with concentrated NaOH.
Tsygankova et al.12 reported that the leaching process of HOFA depends not only on its chemical composition but also on its phase composition. They presented the results of acidic leaching for three fly ash types with different chemical and phase compositions. Each fly ash has its own optimum leaching parameters in acidic solutions, that range from 5 to 9% H2SO4 concentration at temperatures between 20 and 80 °C for time periods extending to 30 and 60 min. V is precipitated as V2O5 through oxidation of the leaching solution with H2O2 at 20 °C for 30 min. Precipitation is completed by subsequent heating at 95 °C and pH of about 1.8 to 2.
Khalafalla et al.41 leached the concentrate of petroleum fly ash in H2SO4 solution with the aim at recovering V, Ni, and Zn. The fly ash is physically concentrated using high tension left magnet to raise the contents of V2O5, NiO, and ZnO to 18.1%, 11.18%, and 8.11%, respectively. Thereafter, the leaching process, with 180 g per l H2SO4 in the presence of 4% MnO2 as an oxidant at 80 °C for 10 h, is conducted to leach 96.5 of V, 94.8% of Ni, and 99.1% of Zn. Eventually, V is recovered from the leach liquor by solvent extraction with 3% Alamine 336 in kerosene, leaving Ni and Zn dissolved in the raffinate solution. V is then stripped from the organic phase by adding H2SO4 and precipitated as V2O5·H2O compound. On the other hand, Ni and Zn are co-precipitated, by adding Na2S to the raffinate solution, forming (Zn–Ni) sulfide cake, which can be suggested to undergo hydrometallurgical and solvometallurgical42 approaches for recovery and separation of Zn and Ni.43–45
Rahimi et al.46 developed a novel approach for extracting V from HOFA using lemon juice by the leaching effect of organic acids contained therein. Lemon juice contains 90 mg g−1 citric acid, 0.86 mg g−1 malic acid, and 1.24 mg g−1 ascorbic acid. The concept was to use an environmentally friendly approach to get organic acids alternative to the creation of such acids by the very slow microbial growth in bioleaching. The authors46 dissolved V with the highest recovery of 88.7% using optimal conditions of 2 h ultrasound leaching of HOFA in 27.9% lemon juice solution containing 10% H2O2 at 35 °C and S/L ratio of 0.01%. After the leaching step, sodium carbonate “Na2CO3” is added to raise pH to 9–10, and CaCl2 is put in the liquor to precipitate Al and Fe. Thereafter, V is precipitated as highly pure NH4VO3 by adding ammonium chloride “NH4Cl”. Eventually, NH4VO3 is calcined at 500 °C to produce vanadium pentoxide “V2O5”. The assisting leaching agents, both H2O2 and ultrasound, are found to have an essential effect in accelerating the V dissolution kinetics.
The approach to leach HOFA by the organic acids in lemon juice is novel and environmentally friendly. Nevertheless, the S/L ratio used of only 0.01% necessitates using great amounts of lemon juice. Moreover, the results lack more research to be more reliable.
3.2. Alkaline leaching
Many studies on alkaline leaching of HOFA reported that Ni is insoluble in alkali solutions, which have the superiority for selective leaching of V with higher recovery efficiency. (e.g. Ref. 26, 28, 31 and 47). Tsai and Tsai28 suggested a two-stage leaching method for HOFA. In the first stage, Ni is recovered by leaching the fly ash in ammonia water containing ammonium sulfate “(NH4)2SO4”. In the second stage, the ash residue is leached in an alkaline (NaOH) solution to recover V. However, the approach was not adopted in recent studies because of the lower recovery of Ni (60%) in the ammonia/(NH4)2SO4 solution, which dissolves also 8% of V in the first stage. Moreover, the study didn't conduct or suggest an approach for the final recovery of V and Ni from the leaching solution.Al-Zuhairi48 extracted V with a high recovery yield (98%) from the residual of fired crude oil in a power station by alkaline leaching method using sodium hydroxide “NaOH” solution. The conditions for maximum extraction of V are reported to be 2 M NaOH concentration at 100 °C for 2 h. The leachate is then acidified, to lower its pH, and treated with ammonium hydroxide “NH4OH” in order to precipitate V as NH4VO3. The latter is calcined at a temperature of less than 690 to produce V2O5.
Navarro et al.27 reported that alkaline leaching of HOFA by NaOH is preferred to acidic leaching because it is more selective for V, although alkaline leaching has lower leaching efficiency compared to acidic leaching by H2SO4. Alkaline leaching by NaOH is performed using 2 M NaOH solution with L/S ratio of 4 ml g−1 for 24 h at room temperature and agitation of 200 rpm. Leaching is followed by 1 h water rinsing 3 times. Vanadium recovery reaches 90%, but successive leaching and rinsing for 6 times, 1 h for each time, increases V recovery to 98%. The temperature is found to have a very limited influence on the efficiency and kinetics of V leaching when the contact time reaches 12 h. Sodium carbonate “Na2CO3” is also tested for leaching HOFA with the aim of increasing the selectivity V recovery with avoiding Si leaching. However, 80% of V is recovered by leaching treatment using 0.66 M Na2CO3 solution at the same condition applied for NaOH leaching. Compared to NaOH leaching, Na2CO3 leaching shows higher vanadium selectivity with lower leaching efficiency.
Hakimi et al.49 established a flowchart for the extraction of V with a high recovery yield through alkaline leaching of V-rich HOFA using NaOH solution at 95 °C for 4 h. After the leaching, the alkaline leachate is neutralized by adding 4.5 M H2SO4 solution. When the pH is adjusted to 8, dissolved Al and Si are deposited leaving V dissolved as sodium vanadate in the filtrate. The latter is treated with an ammonium compound to precipitate V as NH4VO3, which is calcined at 450 °C for 1 h to produce V2O5.
Akita et al.50 studied the recovery of nickel and vanadium from oil fly ash in a two-step leaching process. Ni is leached with NH4Cl solution in the first step, followed by Na2CO3 leaching of the residual ash to dissolve vanadium in the second step. Vanadium is then recovered by solvent extraction with TOA/toluene solution followed by precipitation as NH4VO3 with NH4Cl. On the other hand, Nickel is recovered as NiS from its leach liquor by precipitation with Na2S.
Al-Ghouti et al.51 conducted leaching of HOFA in a two main steps process for recovery of nickel and vanadium. In the first step, Ni is selectively extracted using NH4Cl/NH3 solution composed of 2 M NH4CL and 2 M NH3, at 50 °C with L/S ratio of about 19 ml g−1, under agitation for 6 h. The leachate is then treated with sodium sulfide “Na2S” to precipitate Ni as NiS with a recovery yield of 56%. In the second step, V is extracted from the Ni-free residual ash by leaching in an aqueous solution of sodium carbonate with a concentration of 2 M Na2CO3 at 70 °C and pH 5.5 under agitation for 6 h. However, Na2CO3 solution strips other cations like Fe, Mg, and Ca along with V. Selective extraction of V is conducted by adding tri-ethylamine/toluene solution “0.1 M of (CH3CH2)3N in toluene” to the Na2CO3 solution. The organic layer is separated and mixed again with a new 2 M Na2CO3 solution for 3 h to extract V in an aqueous layer. The extracted V is precipitated with a maximum recovery of 45% by mixing the resulting aqueous layer with 1.3 M NH4Cl for 20 h.
Stas et al.11 established a two-stage leaching process for the recovery of vanadium, nickel, and molybdenum from HOFA. In the first stage, namely alkaline leaching, V and Mo are recovered using NaOH solution. The residual fly ash is then leached within the following second leaching stage (acidic leaching) by H2SO4 to recover Ni. The highest V recovery (∼90%) is achieved in 8 M NaOH solution with L/S ratio of 5 ml g−1 at 100 °C for 3 h. The resultant alkaline leaching solution containing V and Mo is cooled with mild agitation to 5 °C for 1 h to precipitate V as sodium vanadate, which is separated by filtration leaving the filtrate containing sodium molybdate. The latter filtrate is acidified with HNO3 and heated to 90 °C to precipitate Mo as H2MoO4. On the other hand, the sodium vanadate is re-dissolved in 5% HNO3 solution (pH = 8), and (NH4)2SO4 is added to the solution to precipitate V as NH<sub4></sub>VO<sub3></sub>. The NH<sub4></sub>VO<sub3></sub> transforms to V2O5 by calcination at 500 for 24 h. In the second stage (acidic leaching), the residual ash is leached by 5 M H2SO4 solution for 3 h at 100 °C and L/S ratio of 4 ml g−1 to extract Ni with a recovery yield of 80%. The acidic leachate is then treated with NaOH solution in two steps to raise the pH and precipitate Fe and the remaining V. Eventually, sodium carbonate “Na2CO3” is added to precipitate Ni as nickel carbonate “NiCO3”.
Howsawi et al.47 conducted alkaline leaching experiments for recovery of V from HOFA sample delivered from a power station plant in Saudi Arabia. 2 M NaOH solution dissolves 94.4% of V found in HOFA in a leaching experiment for 2 h at 110 °C and L/S ratio of 8 ml g−1. A Graham condenser is used to cool and condense vapor back to the leaching solution. The filtrate is acidified to pH 2 by adding HNO<sub3></sub>, and then NH4OH is gradually added until pH 7 to precipitate V as NH4VO3.
3.3. Water leaching
A three-step process, beginning with carbon burning and followed by salt roasting and water leaching, was conducted on HOFA containing 85 wt% unburned C and 2.2 wt% V.52 The burning step for 4 h at 650 °C removes the most of carbon and consequently raises the V content up to 19 wt%. Then, the burned ash (enriched with V) is roasted for 4 h at 650 °C with sodium carbonate “Na2CO3” in order to convert V oxides to sodium metavanadate “NaVO3” (eqn (5) and (6)), which is a water-soluble vanadium compound.52,53| V2O5 + Na2CO3 = 2NaVO3 + CO2 (g) | (5) |
| V2O3 + Na2CO3 + O2 (g) = 2NaVO3 + CO2 (g) | (6) |
The roasted ash, containing V as water-soluble NaVO3, is leached with water for 4 h at 60 °C with L/S ratio of 50 ml g−1. Water leaching selectively dissolves V, leaving Fe and Ni compounds undissolved in the ash residue, and 92 wt% of V is recovered. After the leaching process, V is precipitated as NH4VO3 from the leaching solution by adding NH4)2SO4.49 The precipitated NH<sub4></sub>VO<sub3></sub> is separated by filtration, dried, and calcined to produce vanadium oxide “V2O5” according to the next precipitation and calcination reaction equations:
Precipitation:
| 2NaVO3 + (NH4)2SO4 = 2NH4VO3 + Na2SO4 | (7) |
Calcination:
| 2NH4VO3 = V2O5 + 2 NH3 + H2O | (8) |
A more recent approach for converting V compounds into water-soluble NaVO3 through roasting with NaCl is reported by Ibrahim et al.54 The authors leached vanadium in distilled water, at L/S ratio of 10 ml g−1, with a high recovery (95.3%) from HOFA that was previously roasted with 20 wt% NaCl for 2.5 h. The roasting temperature is optimized, and 850 °C is found to be the appropriate temperature for the reaction of sodium chloride with vanadium-bearing minerals in the presence of excess oxygen to form NaVO3 according to the equations (eqn (9) and (10)):
| 2NaCl + 2VO2 + O2 → 2NaVO3 + Cl2 ↑ | (9) |
| 2NaCl + V2O5 + 1/2 O2 → 2NaVO3 + Cl2 ↑ | (10) |
| 2NaCl + 2Fe2VO4 + 2O2 → 2NaVO3 + 2Fe2O3 + Cl2 ↑ | (11) |
However, higher temperature up to 1000 °C leads to the formation of refractory compounds due to the sintering of V, Ni, Al, and Fe compounds and the melting of NaCl.55 Eventually, the study showed that V can be easily extracted from NaVO3 through water leaching. The solid residue can undergo a further acidic leaching process to extract Ni.
3.4. Bioleaching
Bioleaching is a developed approach for extraction of valuable metals from fly ashes with the advantage of avoiding high amounts and concentrations of chemical reagents used in acidic and alkaline leaching processes.56–59 In bioleaching, several kinds of microorganisms including various species of bacteria and fungi are utilized to leach and recover a variety of metals from solid materials. The most common bacteria used in bioleaching belong to the thiobacilli genus, which are considered as acidophilic species, such as Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans.60Rastegar et al.60 optimized the recovery of V, Ni, and Cu from HOFA using Acidithiobacillus ferrooxidans bacteria “A. ferrooxidans”. The bacteria were allowed to grow in a medium containing 3 g per l (NH4)2SO4, 0.5 g per l MgSO4·7H2O, 0.5 g per l K2HPO4·3H2O, 0.1 g per l KCl, 0.01 Ca(NO3)2, and 44.22 g per l FeSO4·7H2O. The fly ash is added to this medium with S/L ratio ranging between 1% and 4%. Vanadium, nickel, and copper are simultaneously leached with maximum recoveries of 74%, 95%, and 88%, respectively, using optimum parameters; including Fe2+ concentration of 2.6 g l−1, pH of 1.3, S/L ratio of 0.01 (w/v). After bioleaching step, Ni and Cu are separated from the leachate liquid by raising its pH up to 9–10 using NaOH. V is then precipitated as NH4VO3 by adding NH4Cl. Pure V2O5 is obtained by calcination of NH4VO3 for 4 h at 550 °C.
Additionally, the authors60 investigated a hydrothermal method for producing NaV6O15 nanorods, which can be used in the synthesis of rechargeable lithium batteries. A suspension of V2O5 powder, in water with additions of H2O2 and NaCl, is undergone vigorous stirring for 2 h until obtaining an orange suspension of NaV6O15 nanorods that are washed, dried, and annealed at 500 °C.
Wei et al.61 reported that A. ferrooxidans bacteria utilize sulfur and ferrous sulfate for growth and produce H2SO4. The authors investigated A. ferrooxidans for bioleaching of V from a V-containing shale. Maximum recovery of 62% V is achieved with S/L ratio of 0.02, 5 g l−1 initial Fe2+ concentration, pH 2, 10% inoculum percentage, and 1.2 days bioleaching time.
Rasoulnia et al.62 studied the bioleaching of HOFA using the fungus Penicillium simplicissimum, which produces high amounts of organic acids (citrate, gluconate, oxalate). The fungus is activated and cultivated on PDA (potato dextrose agar) Petri dishes for 7 days as incubation time at 30 °C. The fungus is inoculated to bioleaching medium with the following composition: 100 g per l sucrose, 1.5 g per l NaNO3, 0.5 g per l KH2PO4, 0.025 g per l MgSO4·7H2O, 0.025 g per l KCl, and 1.6 g per l yeast extract. Bioleaching experiments of the ash are conducted in 100 ml of Penicillium simplicissimum medium with S/L ratio of 0.01 g ml−1 at 30 °C under stirring with 130 rpm. Among three leaching methods studied by the authors, the method, namely spent-medium leaching, is found to achieve the highest recovery of V and Ni. In this method, the fungus is endowed to grow for 15 days and create organic acids. The ash sample is then added to the bioleaching medium and undergoes leaching by the organic acids produced by fungus. The subsequent analysis of the filtrate showed that the recovery of V is 96.3% and of Ni is 40.1%. Fe is also extracted but with a lower recovery yield, which can be increased to be 48.3% if the original ash was preheated at 400 °C. However, the thermal pretreatment of the ash reduces the recovery of V and Ni due to the formation of less soluble compounds.
In a comparison study between the bioleaching efficiency of the acid producing fungi, Aspergillus niger and Penicillium simplicissimum, Rasoulnia and Mousavi63 found that the maximal recovery of V (97%) and Ni (50%) is obtained using Aspergillus niger. The higher bioleaching efficiency of Aspergillus enables using higher pulp density of added ash with S/L ratio of 3.2% (w/v) under optimal conditions of 7 days leaching duration and 60 °C leaching temperature.
Another fungal bioleaching approach was recently investigated by Seddiek et al.64 The fungal isolate Cladosporium cladosporioides is grown on Czapek's Dox agar medium with a composition of 30 g sucrose, 15 g agar, 1 g KH2PO4, 2 g NaNO3, 0.5 g KCl, 0.5 g MgSO4·7H2O, traces FeSO4·5H2O, and 5 g yeast extract in 1 liter. The main organic acid produced by this fungus is malic acid. The optimum parameters for bioleaching of V and Ni from HOFA are; the fly ash concentration is 1% (w/v), pH is 6 for V and pH is 8 for Ni, and the bioleaching time is 10 days. The maximum recovery obtained for V and Ni is 65.4% and 74.6%, respectively. The thermal roasting pretreatment preceding the bioleaching process reduce the recovery efficiency of both V and Ni. This is attributed to the fact that the increase in the concentration of metallic elements in the ash due to the thermal pretreatment has an inhibitory effect on the growth of fungus.62,64
4. Recovery of V and Ni from the leaching solutions
Leaching processes dissolve V and Ni with other metals in aqueous solutions. V and Ni can be extracted through main methods, including chemical precipitation, solvent extraction, and ion exchange.4.1. Chemical precipitation
Chemical precipitation is conducted by adding an ammonium compound (e.g. NH4OH, NH4Cl, (NH4)2SO4, (NH4)2S2O8, or NH4NO3) to the leaching solution to precipitate V as NH4VO3 at pH 7 to 9, see Table 3. NH4Cl is preferred to use because of its low cost. Also, an increasing the amount of NH4Cl induces the precipitation of NH4VO3 and decreases its solubility.65 NH4VO3 can be directly processed to fabricate NaV6O15, which is investigated as a high-performance cathode in lithium ion batteries, sodium ion batteries, and zinc ion batteries.66–68| Approach | Preparation of the ash | Leaching agent | Temp., °C | Time | S/L ratio | Leaching recovery efficiency | Extraction approach | Final product | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Acidic leaching | The ash was crushed, ground and sieved (−250 μm) | H2SO4 (60 g l−1), under O2 pressure of 15 bar | 200 °C | 15 min | 1 | 95% | Precipitation of V as V(OH)3 by adding NH4OH electrodeposition of Ni | V2O5 & Ni | 34 |
| Acidic leaching | H2SO4 (1.35 M), with stirring 300 rpm | 40 °C | 90 min | 1/5 g ml−1 | 98% V, 98% Ni | Solvent extraction of V by LIX 84-I at pH 0.5; followed by precipitation as NH4VO3 by adding NH4OH | V2O5 & NiO | 35 | |
| Precipitation of Ni as NiC2O4 by adding (NH₄)₂C₂O₄ to the raffinate | |||||||||
| Acidic leaching | Crushing + sieving (−75 μm) | H2SO4 (conc. 19.5%) | 80 °C | 120 min | 9.15 wt% | 94% V, 81% Ni | 37 | ||
| Acidic leaching | Boiling H2SO4 (conc. 1 M) | Boiling (∼100 °C) | 30 min | 1/3 g ml−1 | ∼90% | Precipitation of V as V2O5 by adding NaClO3 + Na2CO3 | V2O5 | 38 | |
| Acidic leaching | Burning of the ash at 850 °C | Boiling H2SO4 (conc. 2 M) | Boiling (∼100 °C) | 60 min | 1/7 g ml−1 | 97% | Precipitation of V as V2O5 by adding NaClO3 + Na2CO3 | V2O5 with higher purity | 40 |
| Acidic leaching | Drying t 110 °C + sieving (−500 μm) | H2SO4 (conc. 0.5 M) | RT, agitation speed 200 rpm | 24 h | 1/4 g ml−1 | 98% V; 68% al; 42 Fe; 12% Ni; 4% Si | Precipitation of V as NH4VO3 by adding NH4Cl (1 M) pH 5 + agitation for 7 days | NH4VO3 | 27 |
| Acidic leaching | (HCLO4 + HCl + H2O2) | High recovery of V, Ni, and Fe | Solvent extraction using organic compounds and HNO3 in sequential procedure | NiO and FeO in two separation steps, leaving V dissolved in aqueous solution | 26 | ||||
| Water leaching + acidic leaching | Water to dissolve Ni, Zn, mg, al, and Fe; followed by H2SO4 (1 M) to dissolve V | 25 °C | 360 min | 1/25 g ml−1 | Precipitation of Al and Fe after water leaching by adding NaOH + ion exchange to recover Ni, Zn, and V | 31 | |||
| Acidic leaching | H2SO4 (conc. 5–9%) | 20–80 °C | 30–60 min | 1/4 g ml−1 | Precipitation of V as V2O5, after oxidation by H2O2, by static heating for 2 h at 95 °C and pH 1.8–2 | V2O5 | 12 | ||
| Acidic leaching | Physical concentration using high tension magnetic separation | H2SO4 (180 g l−1) + 4% MnO2 | 80 °C | 600 min | 1/10 g ml−1 | 96.5% V, 94.8% Ni | Solvent extraction of V using Alamine 336/kerosine and H2SO4 stripping; precipitation of Ni & Zn by adding Na2S | V2O5·H2O; Zn–Ni sulfide cake | 41 |
| Ultrasound-assisted acidic leaching | Crushing + sieving (−75 μm) + washing with hexane and acetone + drying for 30 min at 70 °C | 27.9% lemon juice solution containing 10% H2O2 | Initial = 35 °C | 120 min | 0.01% | 88.7% | Precipitation of V as NH4VO3 by adding NH4Cl | V2O5 | 46 |
| Acidic leaching | Drying at 105 °C | H2SO4 (0.5 N), pH 1 | 30 °C | 120 min | 1/5 g ml−1 | 65% for V 60% for Ni, 42% for Fe | 28 | ||
| Alkaline leaching | Drying at 105 °C | NaOH (2 N), pH 14 | 30 °C | 120 min | 1/5 g ml−1 | 80% for V | 28 | ||
| Alkaline leaching | Drying at 105 °C | NH4OH (4 N), pH 10 | 30 °C | 120 min | 1/5 g ml−1 | 50% for V 60% for Ni | 28 | ||
| Alkaline leaching | Drying at 105 °C | (NH4OH (0.25 N) + (NH4)2SO4 (4 N); pH 8.5 | 30 °C | 120 min | 1/5 g ml−1 | 80% for V 60% for Ni | 28 | ||
| Alkaline leaching | NaOH, 2 M | 100 °C | 120 min | V (98%) | Precipitation of V as NH4VO3 by adding NH4OH′′ after acidification by HNO3 | NH4VO3 is calcined at <690 °C to produce V2O5 | 48 | ||
| Alkaline leaching | Drying at 110 °C + sieving (−500 μm) | NaOH, 2 M + water rinsing of residual ash (for 1 h, 3 times) | RT, agitation speed 200 rpm | 24 h + 6 times repeating washing/rinsing step | 1/4 g ml−1 | V (98%) | Controlling pH to be 8 for precipitation of Al. Then, adding NH4CL (1 M, pH 5) for precipitation of V (99 %V) | NH4VO3 | 27 |
| Alkaline leaching | Drying at 110 °C + sieving (−500 μm) | Na2CO3 0.66 M + water rinsing | RT, agitation speed 200 rpm | 24 h + 6 times repeating washing/rinsing step | 1/4 g ml−1 | V (80%) | Precipitation of V by adding NH4CL (1 M, pH 5) | NH4VO3 | 27 |
| Alkaline leaching | NaOH, 2.5 M | 100 °C | 240 min | 1/2.7 g ml−1 | V (99.6%) | Precipitation of V as NH4VO3 by adding of NH4NO3 (pH = 6–7, 100 °C, 4 h) | NH4VO3 is calcined at 450 °C to produce V2O5 | 49 | |
| Alkaline leaching | 2 M NH4Cl + NH4OH | 50 °C | 300 min | Ni (59.1%) | Precipitation of Ni by adding Na2S | NiS | 50 | ||
| Alkaline leaching | Leaching in NH4Cl/NH4OH solution for recovering Ni | 2 M Na2CO3 solution | 70 °C | 420 min | V (63%) | Solvent extraction of V is conducted using TOA/toluene + precipitation of V by adding NH4CL | NH4VO3 | 50 | |
| Alkaline leaching NH4Cl/NH3 solution | Crushing + sieving (−100 μm) | 2 M NH4Cl + 2 M NH3 | 50 °C | 300 min | 1/19 g ml−1 | Ni (56%) | Precipitation of Ni by adding Na2S | NiS | 51 |
| Alkaline leaching Na2CO3 solution | Leaching in NH4Cl/NH3 solution for recovering Ni | 2 M Na2CO3 solution | 70 °C | 300 min | 1/20 g ml−1 | V (45%) | Solvent extraction of V is conducted using tri-ethylamine/toluene + precipitation of V by adding NH4CL | NH4VO3 | 51 |
| Alkaline leaching | NaOH, 8 M | 100 °C | 180 min | 1/5 g ml−1 | V (90%) | Cooling to 5 °C + gentle agitation to precipitate NaVO3, that is dissolved in HNO3 + adding (NH4)2SO4 to precipitate NH4VO3 | NH4VO3 is calcined to produce V2O5 | 11 | |
| Acidic leaching | Leaching in NaOH for recovering V & Mo | H2SO4, 5 M | 100 °C | 180 min | 1/4 g ml−1 | Ni (80%) | Precipitation of Ni as NiCO3 by adding Na2CO3 | NiCO3 | 11 |
| Alkaline leaching | NaOH, 2 M | 110 °C | 120 min | 1/8 g ml−1 | V (94.4%) | Precipitation of V as NH4VO3 by adding of NH4OH (pH = 6–7, 100 °C, 4 h) | NH4VO3 | 47 | |
| Water leaching | Burning for 4 h at 650 °C + roasting for 4 h with Na2CO3 at 650 °C | H2O | 60 °C | 240 min | 1/50 g ml−1 | 92% | Precipitation of V as NH4VO3 by adding (NH4)2SO4 | V2O5 | 52 |
| Water leaching | Roasting for 2.5 h with 20 wt% NaCl at 850 °C | H2O | RT | 90 min | 1/10 g ml−1 | 95.3% | Precipitation of V as NH4VO3 by adding NH4Cl | NH4VO3 | 54 |
Hakimi et al.49 optimized the precipitation of V as NH4VO3 compound by adding an ammonium compound to the filtrate after the leaching process. They compared individual use of three ammonium compounds, namely (NH4)2SO4 ammonium persulfate “(NH4)2S2O8”, and ammonium nitrate “NH4NO3”. The highest yield of V extraction (99.6%) is obtained by using NH4NO3, because the nitrate anion is a strong oxidizing agent that completely oxidizes the V cations to the highest oxidation state of V5+. Otherwise, H2O2 can be added to complete the oxidation state of V compounds to the valence state of +5 as recommended by many studies.12,34,37 Oxidation increases the recovery efficiency of V and facilitates the precipitation of V compounds because V5+ is less soluble than V4+.
V, in the form V5+ cations, is precipitated as NH4VO3, which is subsequently calcined to produce the prevalent final product “V2O5”. However, Vitolo et al.38,40 precipitated V2O5 directly from H2SO4 leachate by adding NaClO3, as an oxidant, with adjusting pH at about 2 or 3. However, adjusting pH with Na2CO3 pollutes the V2O5 precipitated with some Na. This necessitates repeat washing with an acidic solution to remove Na. Consequently, the oxidant H2O2 is preferred to eliminate contamination with alkali elements.12
Nickel is dissolved as NiSO4 in H2SO4 leaching solutions. Some studies34,69–71 suggested the application of electrowinning technique to obtain pure Ni. Nevertheless, these studies didn't conduct electrodeposition experiments and were confined to suggestions. However, the electrochemical deposition of Ni from NiSO4 electrolyte is well known process.72–76
Ni is precipitated as NiCO3 from pH-modified H2SO4 leaching solution by adding Na2CO3.11 Also, Ni is precipitated from alkaline NH4Cl/NH3 leaching solution as NiS by adding Na2S.51 Nickel oxalate “NiC2O4”, which can be easily transformed to NiO by calcination, is precipitated after adding ammonium oxalate “(NH4)2C2O4” to H2SO4 leaching solution.35
4.2. Solvent extraction
Solvent extraction is the process in which the element or compound aimed to be extracted transfers from one solvent to another due to the difference in solubility or distribution coefficient between these two immiscible (or slightly soluble) solvents.77–79 It has a greater separation efficiency than chemical precipitation and a higher selectivity degree than the ion exchange method with faster mass transfer. Therefore, solvent extraction has long been utilized for vanadium extraction (since 1950s).80The extractants used are decided according to the leaching solution, its pH, and the dissolved metal valency. Vanadium is generally found as V4+ or V5+ cations in the leaching solutions of HOFA. For extracting V4+, organophosphorus acids, such as D2EHPA “di(2-ethylhexyl)phosphoric acid”, Cyanex 272 “bis(2,4,4-trimethylpentyl)phosphinic acid”, and EHEHPA “2-ethylhexylphosphonic acid mono-2-ethyl hexyl ester” are used. On the other hand, TOA “trioctylamine”, Alamine 336 “trialkylamine”, LIX 860-I “trialkylamine”, and NBEA “N-(2-hydroxy-5-nonylbenzyl)-β,β-dihydroxyethylamine” are used for extracting V5+.80,81 Ionic liquids “ILs” are recently used as extractants for vanadium from aqueous solutions; Examples of such novel ILs are trihexyl(tetradecyl) phosphonium bis(2,4,4-trimethylpentyl) phosphinate “Cyphos IL 104”82 and 1-octyl-3-methylimidazoliumchloride “[Omim]Cl”.83 Table 4 summarizes some procedures and solvent extraction conditions for V and Ni reported in the literature.
| Solvent | Target metal valence | Solvent composition | O/A ratio | Proper pH | Extracting efficiency | Stripping agent | Stripping O/A ratio | Stripping efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| LIX 84-1 | V(V) | 40% LIX 84-1 in kerosene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.35–1.0 | >99.9% | 15% NH4OH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
99.9% | 35 |
| Aliquat 336 | V(V) | 0.06 M aliquat 336 in kerosene +5% isodecanol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.0 | 99.0% | NH4OH/NH4Cl | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
48.0% | 27 |
| NBEA | V(V) | 0.2 M NBEA in octan | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.5 | 98.0% | 81 | |||
| D2EHPA | V(IV) | 10% D2EHPA + 5% TBP,+ 85% sulfonated kerosene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2.5–2.8 | 99.0% | 1.5 M H2SO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
99.0% | 85 |
| D2EHPA | V(IV) | 10% D2EHPA + 5% TBP,+ 85% sulfonated kerosene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
≤2.5 | 96.12% | 87 | |||
| D2EHPA + Cyanex 272 | V(IV) | 20% V (0.25 M D2EHPA and 0.35 M Cyanex 272) + 80% V kerosene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.5 | 80% | n/a | 86 | ||
| D2EHPA + Cyanex 272 | Ni(II) | 20% V (0.25 M D2EHPA and 0.35 M Cyanex 272) + 80% V kerosene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.5 | 90% | n/a | 86 | ||
| Cyphos IL 104 | V(V) | Cyphos IL 104 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.5–2 | 99.07% | 1.5 M HNO3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99.99% | 82 |
| [Omim]Cl IL | V(V) | 0.2 M of [Omim]Cl IL in 1 L of n-pentanol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8.05 | 97.93% | 83 | |||
| HBL110 | Ni(II) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98% | Dilute H2SO4 solution + electrowinning | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95% | 71 |
The solvent extraction efficiency is influenced by the vanadium complex, which is determined by the solution pH and the concentration of V. Zeng and Cheng84 reported that the pH in the range between 1.5 and 2.5 is optimum to extract V(IV) by D2EHPA with a concentration of 5–20% in kerosene. However, Liu et al.85 found that the solution pH must be in the range from 2.5 to 2.8 to extract V(IV) by D2EHPA, and that further increase in the pH leads to co-extraction of Fe and Al. Noori et al.86 used a mixture of 0.25 M D2EHPA and 0.35 M Cyanex 272 to extract V(IV) at pH 2.5 and extract Ni(II) at pH 5.5. Tang et al.87 illustrated the chemical behavior of V extraction from the aqueous leachate of stone coal. The authors reported that the extraction efficiency increases as the pH increases up to 2.5. The leachate should be treated with SO2 before extraction to reduce V(V) to V(IV) which is extracted more efficiently by D2EHPA.
Barik et al.35 used the commercial solvent extractant LIX 84-I “2-hydroxy-5-nonylacetophenone oxime” diluted with kerosene (40% v/v) to selectively recover V from an acidic aqueous solution. V is extracted with a recovery efficiency of 99.9% when the ratio of organic phase to aqueous phase “O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio” is 1
A ratio” is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and pH is 0.5 at 25 °C. Then, V is stripped from V-loaded LIX84-I as NH3VO3 by equilibration with 15% NH4OH aqueous solution with O
1, and pH is 0.5 at 25 °C. Then, V is stripped from V-loaded LIX84-I as NH3VO3 by equilibration with 15% NH4OH aqueous solution with O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio of 1
A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Eventually, NH3VO3 is crystallized by evaporation of the stripped solution.
3. Eventually, NH3VO3 is crystallized by evaporation of the stripped solution.
With the aim of extracting vanadium from the NaOH alkaline leachate, Navarro et al.27 applied two different procedures; one is named as selective precipitation, and the other is termed as solvent extraction. In the selective precipitation procedure, the alkaline leachate is neutralized by adding H2SO4 to control the pH to 8 in order to precipitate Si and Al, leaving V in the solution. Further pH lowering of the alkaline leachate to be 5 by extra H2SO4, with the addition of 1 M NH4Cl solution, leads to the precipitation of vanadium as ammonium vanadate. On the other hand, solvent extraction is conducted by using Aliquat 336 which extracts metals by acting as a liquid anion exchanger. Aliquat 336 is added to kerosene with a concentration of 0.06 M and 5% isodecanol, forming an organic phase that is mixed with the same volume of H2O2-pretreated alkaline leachate for 30 min at pH controlled to be 3. The solvent extraction procedure results in significant extraction of V but is contaminated with Al. Also, it is difficult to strip V from the organic phase. Therefore, a selective precipitation procedure using NH4Cl solution is endorsed as the final step after alkaline leaching.
Mahandra et al.82 extracted V(V) from the leaching solution of spent V2O5 catalyst using Cyphos IL 104. HNO3 is used as a stripping agent to strip V from the organic phase. The A/O ratio for extraction is 3/1 but for stripping is 1/3.
Zeng et al.71 proposed a new approach for the direct extraction of Ni from H2SO4 leach solutions of low grade Ni resources using a novel solvent extractant HBL110. This extractant can selectively recover Ni with an efficiency of 98% leaving Fe, Al, Mn, Cr, and Ca in the leaching solution. The Ni-loaded organic solution is stripped by dilute H2SO4 solution. The O/A ratio of extraction and of stripping is 1. Ni can be eventually extracted by electrowinning with an overall recovery of 95%. The process is advantageous because it consumes low chemicals with a short flowchart procedure and achieves high recovery of Ni with efficient removal of impunities. Therefore, the authors reported that the process shows proper economical and environmental advantages.
4.3. Ion exchange
The ion exchange approach is a process to separate and extract selected metals from their solutions using ion exchange resins. The ion exchange processes have the advantage of the separation of target ions without using an excess of chemicals, with enabling the application of closed water recycling systems that eliminate additional equipment for wastewater treatment. The ion exchange resins used to adsorb vanadium are generally classified as anionic exchange resins and chelating resins. Anionic resins react with and separate pentavalent vanadate ions “V(V)” from other impurities in aqueous solutions, but chelating resins are used to extract V(IV) from acidic solutions.80,88 Although there are several papers on using ion exchange resins for the separation of vanadium ions from other impurities in various media, the literature on the extraction of vanadium from fly ash leachate is limited. However, ion exchange technology is primarily applied on the experimental scale and is rarely applied in the industry.88Tokuyama et al.31 developed a process to recover V and Ni and to make the fly ash less harmful. The process includes two-step leaching and ion exchange. The first leaching step uses water to dissolve Ni, Zn, Mg, Al, and Fe. The second leaching step is applied to dissolve V using H2SO4. The solution after water leaching is treated with NaOH to reach pH 5 to precipitate Al and about 80% of Fe. The remaining Fe dissolved as Fe(II) is oxidized to Fe(III) by adding H2O2. Recovery of Ni and Zn is then conducted by ion exchange with CR20 resin. Nickel can be selectively recovered from the resin because of a distinct inequality in the ion exchange isotherm between Ni and Zn. On the other hand, vanadium is selectively recovered from the second acidic leachate by using the resin C467, because this resin has the highest selectivity for V against Fe.
Yu et al.89 utilized trioctyl methyl ammonium chloride-impregnated resins “TOMAC-IRs” to extract vanadium from a V-containing acidic solution. TOMAC-IRs adsorbed V(V) with a higher efficiency from V-containing solution with initial pH of 1.8 and S/L ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g ml−1 in a contact time of 4 h. The adsorbed vanadium in TOMAC-IRs is then desorbed with 6 mol l−1 NaOH solution.
200 g ml−1 in a contact time of 4 h. The adsorbed vanadium in TOMAC-IRs is then desorbed with 6 mol l−1 NaOH solution.
Seggiani et al.69 investigated the extraction of nickel from sulfate solution leachate of Orimulsion fly ash after vanadium recovery using ion exchange chelating resins. Three commercial chelating resins, namely Lewatit TP-207, Purolite S-930 and Amberlite IRC-748, are examined for uptake of Ni from the fly ash leachate at pH 4. The results showed that TP207 resin displays higher extraction of Ni(II) with the recovery of 92–95%. Nickel is then stripped from the resin by 10% H2SO4 solution. Nickel can be eventually recovered from the resultant solution containing NiSO4, as metallic nickel by electrowinning.70,90
5. Calcination
In consequence of the recovery of V and Ni in the form of their chemical compounds (NH4VO3 and NiC2O4), as mentioned above, calcination is conducted to produce V2O5 and NiO. Vanadium pentoxide “V2O5” is widely used in the synthesis of modern high-performance batteries, electrochromic devices, sensors, and photocatalysts.91–93 Nickel oxide “NiO” has a variety of specialized applications in thin film solar cells, superior rechargeable batteries, and electrochromic devices.93,94 Both V2O5 and NiO, even with low purity grades, are widely used in the production of steel alloys.93,95The precipitated NH4VO3 is separated from the leaching solution by filtration, dried, and calcined to produce V2O5 according to eqn (8) “2NH4VO3 → V2O5 + 2NH3 + H2O”. Liu et al.96 reported that the optimum conditions for calcination of NH4VO3 to obtain V2O5 are to be 400 °C for 36 min, based on experimentation of a sample weighing 4.25 g. Nevertheless, most of the studies conducted the calcination process of NH4VO3 at higher temperatures and for longer times. Stas et al.11 calcined NH4VO3 at 500 °C for 24 h. However, most of the old and recent studies conducted the calcination process in shorter times at the temperature range of 500–690 °C, e.g. 1 h at 500 °C,97 30 min at 690 °C,48 4 h at 550 °C 46, 1 h at 450 °C.49
Nickel is recovered from the acidic leachate of HOFA as NiC2O4 by adding NH4)2C2O4.35 Nickel oxide “NiO” is obtained by calcination of NiC2O4 at 450 °C for 2 h. The thermal decomposition of (NH4)2C2O4 has been demonstrated to occur following the next reactions:98
| NiC2O4 → NiO + CO + CO2 | (12) |
| NiO + CO → Ni + CO2 | (13) |
Du et al.98 fabricated Ni/NiO nanocomposite by direct calcination of NiC2O4, and controlled the Ni metal content in the NiO by controlling the temperature and atmosphere of the calcination. The produced Ni/NiO composite demonstrated high electrochemical performance as an anode in lithium-ion battery. However, Rakshit et al.99 and Gomaa et al.100 reported that the calcination in air, where excess of O2, converts NiC2O4 into NiO.
Also, NiCO3, precipitated by the addition of Na2CO3 to the acidic leachate of HOFA 11, can also undergo thermal decomposition at 450 °C to produce NiO, according to the equation “NiCO3 → NiO + CO2”.101,102
6. Concluding summary and recommendations
In summary, Fig. 1 displays an overall flowchart of the recovery of V and Ni from HOFA with showing the most common steps following the main three optional leaching approaches (acidic leaching, alkaline leaching, and water leaching). Also, Table 5 summarizes the advantages and limitations of all leaching and recovery processes reviewed in this paper.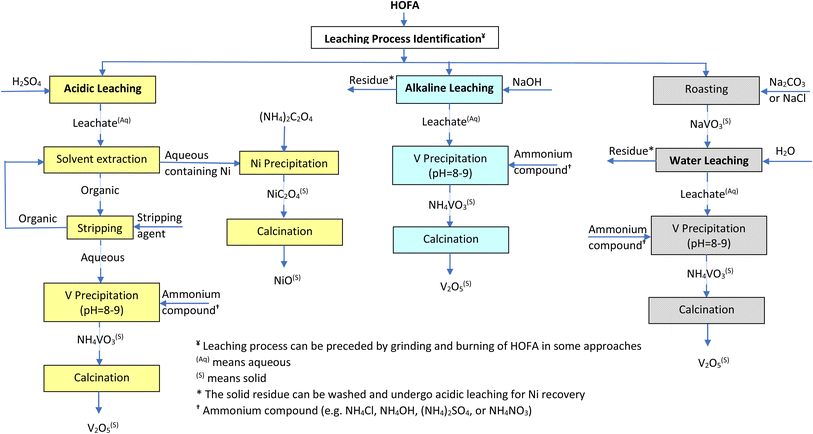 | ||
| Fig. 1 Overall flowchart of the recovery of V and Ni from HOFA showing the most common steps following the main three optional leaching approaches. | ||
| Process | Type | Advantages | Limitations |
|---|---|---|---|
| Pre-treatment of HOFA | Burning (400–850 °C) | Reduces the C content of HOFA | Gaseous emissions |
| Reduces the volume of HOFA | Possible volatilization of V compounds | ||
| Increases the V & Ni contents in the ash | Possible fusion and formation of V–Ni refractory compounds at higher temperatures (>900 °C) | ||
| Increases the V & Ni recovery, and decreases the consumption of chemical agents in the leaching process | Changes the ash pH that may adverse the V & Ni recovery in bioleaching process | ||
| Possibility of recovering the heat of combustion of carbonaceous constituents of HOFA | |||
| Roasting (mostly with Na2CO3 or NaCl, at 650–850 °C) | Is necessary prior to water leaching to produce water soluble NaVO3 compound | Gaseous emissions | |
| Leads to selective recovery of V | Possible sintering of V & Ni at high temperatures | ||
| Reduces the volume of HOFA | Possible fusion of NaCl at high temperatures | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Leaching | Acidic leaching (H2SO4 is widely used) | Higher extraction rate | No selectivity |
| Lower cost | More waste solutions | ||
| Dissolves all metals, so residual HOFA can be reused in some applications | More complicated steps for selective recovery of V and Ni | ||
| Alkaline leaching (NaOH is widely used) | Selective recovery of V | Ni is nonrecoverable | |
| Good extraction rate | For Ni recovery, the ash should undergo a next acidic leaching process | ||
| Simple flowchart process | The solid ash residue keeps its polluting metals | ||
| Water leaching | Lower cost and wide availability | Should be preceded by a roasting step | |
| Avoids high amounts of chemical reagents | Lower recovery efficiency | ||
| Simple flowchart process | No attempts to recover Ni | ||
| Bioleaching | Environmentally friendly | Very slow | |
| Avoids high amounts of chemical reagents | The S/L ratio is very low | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Recovery | Chemical precipitation (using ammonium compounds) | Lower cost | Weak selectivity |
| Straightforward process | Lower metal purity | ||
| Ion exchange | Avoids using excess of chemicals | Lower selectivity degree | |
| Good recovery rate | Resin needs refreshening | ||
| Long flowchart process | |||
| Solvent extraction | Greater separation efficiency | Narrow pH range | |
| Higher selectivity degree | Is influenced by the metal valency | ||
| Faster extraction rate | Long flowchart process | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Calcination | Simple process | Gaseous emissions | |
| Extraction of vanadium pentoxide and nickel oxide that have great and modern applications | Extraction of metal oxide, not pure metal | ||
The recovery of V and Ni from HOFA commences with the leaching process, which is considered as the first main step in recovery. The leaching processes mostly applied are classified as acidic processes and alkaline processes, in addition to the water leaching process.
Although acidic leaching can be performed by one of the strong mineral acids such as HCl, H2SO4, or HNO3, and studies reported that HCl and H2SO4 have comparable leaching efficiencies, H2SO4 is preferred due to its significantly lower cost and its wide application in leaching of many metals. In addition to V and Ni, H2SO4 dissolves almost all metals present in HOFA, such as Mo, Mg, Mn, and others.
However, alkaline leaching using NaOH has the advantage of selective leaching of V, leaving Ni undissolved in the ash because it is insoluble in alkaline solutions. Although NH4OH and Na2CO3 solutions were investigated in some studies as alkaline leaching agents, NaOH has the highest selectivity with the highest leaching efficiency for V.
Based on the advantage of selective solubility of V by NaOH solution, a two-stage leaching process has been developed and can be adopted to recover V and Ni; In the first stage (alkaline leaching), V is recovered by NaOH solution. The residual solid fly ash is then leached by H2SO4 solution (acidic leaching) to recover Ni.
Water leaching is significantly weak if compared with acidic and alkaline leaching. However, the approaches reported on water leaching of HOFA preceded the leaching process with a roasting step. The roasting of ash with Na2CO3 salt or NaCl salt converts vanadium compounds into NaVO3 which is a water-soluble compound. The roasting temperature should not be too high to bring about sintering of V and Ni compounds.
Bioleaching is an environmentally friendly approach that utilizes specific species of bacteria and fungi for yielding acidic media that leach V and Ni from HOFA. “Acidithiobacillus ferrooxidans” bacteria utilize sulfur and ferrous sulfate for the production of H2SO4, which leaches V and Ni from HOFA, but with much lower recovery efficiency than traditional acidic leaching.
The fungi “Aspergillus niger” and “Penicillium simplicissimum” are endowed to grow for days and create organic acids (citrate, gluconate, oxalate), that leach V and Ni when mixed with the ash. The fungal isolate Cladosporium cladosporioides yields malic acid that extracts V and Ni from HOFA.
Alternatively to the bioleaching approach which is very slow, another environmentally friendly trial used lemon juice – which contains few contents of citric acid, malic acid, and ascorbic acid – to recover V from HOFA with a considerable recovery efficiency. However, the leaching experiment has a very low pulp density of added ash, and further research is recommended to conduct to have more reliable results.
Recovery of V and Ni from the leaching solution can be conducted by chemical precipitation, solvent extraction, or ion exchange. Direct chemical precipitation of V as NH3VO3 is achieved by adding an ammonium compound (e.g. NH4OH, NH4Cl, (NH4)2SO4, (NH4)2S2O8, or NH4NO3) to the leaching solution with pH of 8 to 9. NH4Cl is preferred more than other compounds because of its lower cost. However, NH4NO3 yields the highest V recovery because of its oxidizing effect on V cations to V5+. On the other hand, Ni is precipitated as NiCO3 from pH modified H2SO4 leaching solution by adding Na2CO3, and as nickel oxalate “NiC2O4” by adding (NH4)2C2O4. Solvent extraction has a higher recovery efficiency than chemical precipitation and a higher degree of selectivity than the ion exchange method with faster mass transfer.
The recovered NH4VO3 is straightforwardly calcined at temperatures ranging from 400 °C to 550 °C to produce vanadium pentoxide “V2O5”. On the other hand, NiC2O4 is easily transformed into NiO by calcination at 450 °C. Also, Ni leached as NiSO4 in an H2SO4 leaching solution can be electrodeposited as a pure metal. Both V2O5 and NiO are used widely in the synthesis of modern high-performance batteries and electrochromic devices, and in other superior applications, as well as in the production of special alloy steel.
HOFAs generated from several power stations and desalination plants have diverse chemical compositions, as well as having low to tiny contents of V and Ni. Therefore, there is no recovery method has been yet adopted to be the best approach. In this regard, further research is recommended to optimize the recovery approach individually adopted for a particular HOFA.
It is also recommended to direct further research to increase the recovery efficiency with lowering pollution through reducing the chemical agents used, reutilizing the waste solutions, and minimizing gaseous emissions. For example, the utilization of ultrasound can reduce the time, temperature, and chemical agents of leaching processes. Also, using seawater instead of fresh water in water leaching can be experimented to raise the recovery efficiency. Moreover, pre-water-washing of HOFA preceding the roasting can be suggested to dissolve metal sulfates that will reduce emission of sulfur oxides.
Author contributions
Conceptualization: A. Bakkar, and E. Howsawi; Data curation: A. Bakkar, S. Harb, and E. Howsawi; Formal analysis: A. Bakkar, M. M. E. S. Seleman, and S. Goren; Funding acquisition: A. Bakkar, and E. Howsawi; Methodology: A. Bakkar, M. M. Z. Ahmed; S. Goren; Project administration: A. Bakkar, and E. Howsawi; Resources: A. Bakkar, S. Goren, and S. Harb; Software: S. Goren, and M. M. Z. Ahmed; Supervision: A. Bakkar, and E. Howsawi; Visualization: A. Bakkar, S. Goren, and M. M. E. S. Seleman; Writing – original draft: A. Bakkar, S. Harb, S. Goren, M. M. E. S. Seleman, and E. Howsawi; Writing – review & editing: E. Howsawi and M. M. Z. Ahmed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (23UQU4331139DSR001).References
- M. Alshaaer, M. Shqair, H. G. Abdelwahed, K. Abuhasel and M. Z. Toro, Stabilization of heavy oil fly ash (HFO) for construction and environmental purposes, Int. J. Appl. Eng. Res., 2017, 12(4), 488–497 Search PubMed.
- Y. M. Hsieh, and M. S. Tsai, An investigation of the characteristics of unburned carbon in oil fly ash, in Environmental Challenges and Greenhouse Gas Control for Fossil Fuel Utilization in the 21st Century, ed. M. M. Maroto-Valer, C.Song and Y. Soong, Springer, Boston, MA, 2002, pp. 387–401, DOI:10.1007/978-1-4615-0773-4_27.
- S. Salehin, A. S. Aburizaiza and M. A. Barakat, Recycling of residual oil fly ash: Synthesis and characterization of activated carbon by physical activation methods for heavy metals adsorption, Int. J. Environ. Res., 2015, 9(4), 1201–1210, DOI:10.22059/IJER.2015.1010.
- J. Liu, Recovery of vanadium and nickel from oil fly ash, Master thesis, Memorial University of Newfoundland, 2017.
- E. Tatar, G. M. Csiky, V. G. Mihucz and G. Zaray, Investigation of adverse health effects of residual oil fly ash emitted from a heavy-oil-fuelled Hungarian power plant, Microchem. J., 2005, 79, 263–269, DOI:10.1016/j.microc.2004.10.021.
- A. M. Hameed, An eco-friendly ultrasound-assisted deep eutectic solvent-based liquid–phase microextraction method for enrichment and quantification of nickel in environmental samples, J. Umm Al-Qura Univ. Appl. Sci., 2022, 8, 57–68, DOI:10.1007/s43994-022-00009-2.
- J.-C. Lee, E.-Y. K. Kurniawan, K. W. Chung, R. Kim and H.-S. Jeon, A review on the metallurgical recycling of vanadium from slags: towards a sustainable vanadium production, J. Mater. Res. Technol., 2021, 12, 343–364, DOI:10.1016/j.jmrt.2021.02.065.
- M. Z. Mubarok and L. I. Hanif, Cobalt and nickel separation in nitric acid solution by solvent extraction using Cyanex 272 and Versatic 10, Proc. Chem., 2016, 19, 743–750, DOI:10.1016/j.proche.2016.03.079.
- A. Mofarrah and T. Husain, Use of heavy oil fly ash as a color ingredient in cement mortar, Int. J. Concr. Struct. Mater., 2013, 7, 11–117, DOI:10.1007/s40069-013-0042-3.
- Z. Aslam, I. A. Hussein, R. A. Shawabkeh, M. A. Parves, W. Ahmad and Ihsanullah, Adsorption kinetics and modeling of H2S by treated waste oil fly ash, J. Air Waste Manag. Assoc., 2019, 69(2), 246–257, DOI:10.1080/10962247.2018.1536004.
- J. Stas, A. Dahdouh and O. Al-chayah, Recovery of vanadium, nickel and molybdenum from fly ash of heavy oil-fired electrical power station, Chem. Eng., 2007, 51(2), 67–70, DOI:10.3311/pp.ch.2007-2.11.
- M. V. Tsygankova, V. I. Bukin, E. I. Lysakova, A. G. Smirnova and A. M. Reznik, The recovery of vanadium from ash obtained during the combustion of fuel oil at thermal power stations, Russ. J. Non-Ferrous Metals, 2011, 52(1), 19–23, DOI:10.3103/S1067821211010251.
- R. R. Moskalyk and A. M. Alfantazi, Processing of vanadium: a review, Miner. Eng., 2003, 6(9), 793–805, DOI:10.1016/S0892-6875(03)00213-9.
- A. Nasimifar and J. Mehrabani, A review on the extraction of vanadium pentoxide from primary, secondary, and co-product sources, Int. J. Min. Geol. Eng., 2022, 56(4), 361–382, DOI:10.22059/ijmge.2022.319012.594893.
- M. Petranikova, A. H. Tkaczyk, A. Bart, A. Amato, V. Lapkovskis and C. Tunsu, Vanadium sustainability in the context of innovative recycling and sourcing development, Waste Manag., 2020, 113, 521–544, DOI:10.1016/j.wasman.2020.04.007.
- L. da Silva Lima, M. Quartier, A. Buchmayr, D. Sanjuan-Delmás, H. Laget, D. Corbisier and J. Dewulf, Life cycle assessment of lithium-ion batteries and vanadium redox flow batteries-based renewable energy storage systems, Sustain. Energy Technol. Assessments, 2021, 46, 101286, DOI:10.1016/j.seta.2021.101286.
- G. J. Simandl and S. Paradis, Vanadium as a critical material: economic geology with emphasis on market and the main deposit types, Appl. Earth Sci., 2022, 131(4), 218–236, DOI:10.1080/25726838.2022.2102883.
- T. Alamro, Assessment of Compression Ignition Engine's Performance and Emissive Attributes Powered with Hybrid Biofuels, J. Umm Al-Qura Univ. Eng. Archit., 2021, 12(2), 1–6 Search PubMed.
- A. Mofarrah and T. Husain, Evaluation of environmental pollution and possible management options of heavy oil fly ash, J. Mater. Cycles Waste Manag., 2013, 15, 73–81, DOI:10.1007/s10163-012-0090-9.
- M. H. Al-Malack, A. A. Bukhari, O. S. Al-Amoudi, H. H. Al-Muhanna and T. H Zaidi, Characteristics of fly ash produced at power and water desalination plants firing fuel oil, Int. J. Environ. Res., 2013, 7(2), 455–466, DOI:10.22059/ijer.2013.624.
- E. P. Shevko, S. B. Bortnikova, N. A. Abrosimova, V. S. Kamenetsky, S. P. Bortnikova, G. L. Panin and M. Zelenski, Trace elements and minerals in fumarolic sulfur: the case of Ebeko Volcano, Kuriles, Geofluids, 2018, p. 4586363, DOI:10.1155/2018/4586363.
- J.-L. Mukaba, C. P. Eze, O. Pereao and L. F. Petrik, Rare Earths' Recovery from phosphogypsum: An overview on direct and indirect leaching techniques, Minerals, 2021, 11(10), 1051, DOI:10.3390/min11101051.
- E. Sahlin and B. Magnusson, Survey analysis and chemical characterization of solid inhomogeneous samples using a general homogenization procedure including acid digestion, drying, grinding and briquetting together with X-ray fluorescence, Talanta, 2012, 97, 63–72, DOI:10.1016/j.talanta.2012.03.063.
- A. Saydut, Microwave acid digestion for the determination of metals in subbituminous coal bottom ash by ICP-OES, Energy Explor. Exploit., 2010, 28(2), 105–115, DOI:10.1260/0144-5987.28.2.105.
- S. Goren, Heavy metal analysis and stabilization of medical waste incineration ash, Asian J. Chem., 2011, 23(8), 3569–3575 CAS.
- A. Aburizaiza, Sequential leaching of vanadium from heavy fuel oil fly ash generated from Saudi Arabia thermal power plants, Curr. J. Appl. Sci. Tech., 2019, 32(4), 1–17, DOI:10.9734/CJAST/2019/46032.
- R. Navarro, J. Guzman, I. Saucedo, J. Revilla and E. Guibal, Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes, Waste Manag., 2007, 27(3), 425–438, DOI:10.1016/j.wasman.2006.02.002.
- S. L. Tsai and M. S. Tsai, A study of the extraction of vanadium and nickel in oil-fired fly ash, Resour. Conserv. Recycl., 1998, 22(3–4), 63–176, DOI:10.1016/S0921-3449(98)00007-X.
- K. Furuya, Y. Kato, T. Kikuchi and Y. Gohshi, State analysis of sulfur in coal and coal fly ash by double-crystal X-ray fluorescence spectrometry, Mikrochim. Acta, 1983, 80, 263–270, DOI:10.1007/BF01213120.
- J. Wilcox, B. Wang, E. Rupp, R. Taggart, H. Hsu-Kim, M. L. S. Oliveira, C. M. N. L. Cutruneo, S. Taffarel, L. F. O. Silva, S. D. Hopps, G. A. Thomas and J. C. Hower, Observations and assessment of fly ashes from high-sulfur bituminous coals and blends of high-sulfur bituminous and subbituminous coals: environmental processes recorded at the macro- and nanometer scale, Energy Fuels, 2015, 29(11), 7168–7177, DOI:10.1021/acs.energyfuels.5b02033.
- H. Tokuyama, S. Nii, F. Kawaizumi and K. Takahashi, Process development for recovery of vanadium and nickel from heavy oil fly ash by leaching and ion exchange, Separ. Sci. Technol., 2003, 38(6), 1329–1344, DOI:10.1081/SS-120018812.
- A. S. Meawad, D. Y. Bojinova and Y. G. Pelovski, An overview of metals recovery from thermal power plant solid wastes, Waste Manag., 2010, 30, 2548–2559, DOI:10.1016/j.wasman.2010.07.010.
- K. Binnemans and T. Jones, Methanesulfonic acid (MSA) in hydrometallurgy, J. Sustain. Metall., 2022 DOI:10.1007/s40831-022-00641-6.
- A. M. Amer, Processing of Egyptian boiler-ash for extraction of vanadium and nickel, Waste Manag., 2002, 22, 515–520, DOI:10.1016/S0956-053X(02)00008-9.
- S. P. Barik, K. H. Park and C. W. Nam, Process development for recovery of vanadium and nickel from an industrial solid waste by a leaching solvent extraction technique, J. Environ. Manag., 2014, 146, 22e28, DOI:10.1016/j.jenvman.2014.06.032.
- A. Akcil, F. Vegliò, F. Ferella, M. D. Okudan and A. Tuncuk, A review of metal recovery from spent petroleum catalysts and ash, Waste Manag., 2015, 45, 420–430, DOI:10.1016/j.wasman.2015.07.007.
- E. Nazari, F. Rashchi, M. Saba and S. M. J. Mirazimi, Simultaneous recovery of vanadium and nickel from power plant fly ash: optimization of parameters using response surface methodology, Waste Manag., 2014, 34, 2687–2696, DOI:10.1016/j.wasman.2014.08.021.
- S. Vitolo, M. Seggiani, S. Filippi and C. Brocchini, Recovery of vanadium from heavy oil and orimulsion fly ashes, Hydrometallurgy, 2000, 57(2), 141–149, DOI:10.1016/S0304-386X(00)00099-2.
- S. Liu, W. Xue and L. Wang, Extraction of the rare element vanadium from vanadium-containing materials by chlorination method: a critical rReview, Metals, 2021, 11(8), 1301, DOI:10.3390/met11081301.
- S. Vitolo, M. Seggiani and F. Falaschi, Recovery of vanadium from a previously burned heavy oil fly ash, Hydrometallurgy, 2001, 62(3), 145–150, DOI:10.1016/S0304-386X(01)00193-1.
- M. S. Khalafalla, W. M. Abdellah, H. A. A. Khoziem and A. A. M. Abd El-Hamid, Ecological treatment of El Kriymat boiler ash for recovering vanadium, nickel and zinc from sulfate leach liquor, J. Mater. Cycles Waste Manag., 2023, 25, 441–455, DOI:10.1007/s10163-022-01550-2.
- K. Binnemans and P. T. Jones, Solvometallurgy: an emerging branch of extractive metallurgy, J. Sustain. Metall., 2017, 3, 570–600, DOI:10.1007/s40831-017-0128-2.
- A. R. Weshahy, A. A. Gouda, B. M. Atia, A. K. Sakr, J. S. Al-Otaibi, A. Almuqrin, M. Y. Hanfi, M. I. Sayyed, R. El Sheikh, H. A. Radwan, F. S. Hassen and M. A. Gado, Efficient recovery of rare earth elements and zinc from spent Ni-metal hydride batteries: statistical studies, Nanomaterials, 2022, 12(13), 2305, DOI:10.3390/nano12132305.
- A. Bakkar and V. Neubert, Recycling of cupola furnace dust: extraction and electrodeposition of zinc in deep eutectic solvents, J. Alloys Compd., 2019, 771, 424–432, DOI:10.1016/j.jallcom.2018.08.246.
- A. Bakkar, Electrochemical synthesis of silicon and gallium arsenide photovoltaic thin films: a critical review and a novel approach, Mat. Sci. Forum, 2020, 1008, 84–96, DOI:10.4028/www.scientific.net/msf.1008.84.
- G. Rahimi, S. O. Rastegar, F. R. Chianeha and T. Gu, Ultrasound-assisted leaching of vanadium from fly ash using lemon juice organic acids, RSC Adv., 2020, 10, 1685–1696, 10.1039/C9RA09325G.
- E. Howsawi, S. Harb and A. Bakkar, Opportunities for recycling of heavy oil fly ash (HOFA) generated in power stations, IEEES 13, 2022, pp. 271–275, ISBN: 978-603-8183-80-9 Search PubMed.
- M. A. H. Al-Zuhairi, Vanadium extraction from residual of fired crude oil in power plants, Iraqi J. Mech. Mater. Eng., 2014, 14(4), 423–431 Search PubMed . https://www.iasj.net/iasj/article/98371.
- M. Hakimi, P. T. Kiani, M. Alikhani, N. Feizi, A. M. Bajestani and P. Alimard, Reducing environmental pollution of fuel fly ash by extraction and removal vanadium pentoxide, Solid Fuel Chem., 2020, 54(5), 337–342, DOI:10.3103/S0361521920050055.
- S. Akita, T. Maeda and H. Takeuchi, Recovery of vanadium and nickel in fly ash from heavy oil, J. Chem. Technol. Biotechnol., 1995, 62(4), 345–350, DOI:10.1002/jctb.280620406.
- M. A. Al-Ghouti, Y. S. Al-Degs, A. Gharir, H. Koury and M. Ziedan, Extraction and separation of vanadium and nickel from fly ash produced in heavy fuel power plants, Chem. Eng. J., 2011, 173, 191–197, DOI:10.1016/j.cej.2011.07.080.
- M. Jung and B. Mishra, Vanadium recovery from oil fly ash by carbon removal and roast-leach process, JOM, 2018, 70, 168–172, DOI:10.1007/s11837-017-2653-7.
- M. Jung and B. Mishra, Vanadium recovery method, US10486983B2, 2019.
- A. H. Ibrahim, X. Lyu, B. M. Atia, M. A. Gado and A. B. ElDeep, Phase transformation mechanism of boiler ash roasted with sodium salt for vanadium extraction, J. Mater. Cycles Waste Manag., 2023, 25, 86–102, DOI:10.1007/s10163-022-01512-8.
- H. Jammulamadaka and S. V. Pisupati, A critical review of extraction methods for vanadium from petcoke ash, Fuels, 2023, 4(1), 58–74, DOI:10.3390/fuels4010005.
- M. S. Farahani, S. Yaghmaei, S. M. Mousavi and F. Amiri, Bioleaching of heavy metals from a petroleum spent catalyst using Acidithiobacillus thiooxidans in a slurry bubble column bioreactor, Sep. Purif. Technol., 2014, 132, 41–49, DOI:10.1016/j.seppur.2014.04.039.
- V. A. Snegirev and T. M. Sabirova, State-of-the-art and problems of bioleaching of metals from ash-and-slag wastes, Metallurgist, 2021, 65, 794–807, DOI:10.1007/s11015-021-01217-7.
- M. Gavrilescu, Microbial recovery of critical metals from secondary sources, Bioresour. Technol., 2022, 344(Part A), 126208, DOI:10.1016/j.biortech.2021.126208.
- M. M. Koutb, E. A. Hassan, F. M. Morsy and M. M. K. Bagy, Optimization of keratinase production by keratinolytic fungus Chrysosporium tropicum and its potentiality in biodegradation of chicken feathers, J. Umm Al-Qura Univ. Appl. Sci., 2022 DOI:10.1007/s43994-022-00020-7.
- S. O. Rastegar, S. M. Mousavi and S. A. Shojaosadati, Bioleaching of an oil-fired residual: process optimization and nanostructure NaV6O15 synthesis from the bioleachate, RSC Adv., 2015, 5(51), 41088–41097, 10.1039/C5RA00128E.
- D. Wei, T. Liu, Y. Zhang, Z. Cai, J. He and C. Xu, Vanadium Bioleaching Behavior by Acidithiobacillus ferrooxidans from a Vanadium-Bearing Shale, Minerals, 2018, 8(1), 24, DOI:10.3390/min8010024.
- P. Rasoulnia, S. M. Mousavi, S. O. Rastegar and H. Azargoshasb, Fungal leaching of valuable metals from a power plant residual ash using Penicillium simplicissimum : Evaluation of thermal pretreatment and different bioleaching methods, Waste Manag., 2016, 52, 309–317, DOI:10.1016/j.wasman.2016.04.004.
- P. Rasoulnia and S. M. Mousavi, V and Ni recovery from a vanadium-rich power plant residual ash using acid producing fungi: Aspergillus niger and Penicillium simplicissimum, RSC Adv., 2016, 6(11), 9139–9151, 10.1039/C5RA24870A.
- H. A. Seddiek, Y. M. Shetaia, K. F. Mahmound, I. E. El-Aassy and S. S. Hussien, Bioleaching of Egyptian fly ash using cladosporium cladosporioides, Ann. Biol., 2021, 37(1), 18–22 Search PubMed.
- F. Liu, P. Ning, H. Cao, Z. Li and Y. Zhang, Solubilities of NH4VO3 in the NH3–NH4+–SO42–Cl–H2O system and modeling by the bromley–zemaitis equation, J. Chemical Eng. Data, 58(5), 1321–1328, DOI:10.1021/je4000873.
- F. Hu, W. Jiang, Y. Dong, X. Lai, L. Xiao and X. Wu, Synthesis and electrochemical performance of NaV6O15 microflowers for lithium and sodium ion batteries, RSC Adv., 2017, 7, 29481–29488, 10.1039/C7RA04388K.
- R. Li, C. Guan, X. Bian, X. Yu and F. Hu, NaV6O15 microflowers as a stable cathode material for high-performance aqueous zinc-ion batteries, RSC Adv., 2020, 10(12), 6807–6813, 10.1039/D0RA00365D.
- Y. Ran, P. Hong, J. Ren, B. Wang, M. Xiao, Y. Chen, X. Xiao and Y. Wang, V2O5/NaV6O15 nanocomposites synthesized by molten salt method as a high-performances cathode material for aqueous zinc-ion batteries, Nanotechnology, 2022, 33(11), 115402, DOI:10.1088/1361-6528/ac3fe1.
- M. Seggiani, S. Vitolo and S. D'Antone, Recovery of nickel from Orimulsion fly ash by iminodiacetic acid chelating resin, Hydrometallurgy, 2006, 81(1), 9–14, DOI:10.1016/j.hydromet.2005.09.005.
- B. Robotin, A. Ispas, V. Coman, A. Bund and P. Ilea, Nickel recovery from electronic waste II Electrodeposition of Ni and Ni–Fe alloys from diluted sulfate solutions, Waste Manag., 2013, 33(11), 2381–2389, DOI:10.1016/j.wasman.2013.06.001.
- L. Zeng, G. Zhang,L. Xiao, and Z. Cao, Direct solvent extraction of nickel from sulfuric acid leach solutions of low grade and complicated nickel resources using a novel extractant of HBL110, in Rare Metal Technology, ed. S. Alam, H. Kim, N. R. Neelameggham, T. Ouchi and H. Oosterhof, Springer, 2016, pp. 47–53, DOI:10.1007/978-3-319-48135-7_5.
- G. A. DiBari, Nickel plating, Met. Finish., 1999, 97(1), 289–290, DOI:10.1016/S0026-0576(00)83085-8.
- E. Kuzeci, R. Kammel and S. K. Gogia, Electrowinning of nickel from aqueous sulphate bath in the presence of metallic and LIX64N impurities, Mineral Process. Extract. Metall. Rev., 1992, 10(1), 57–69, DOI:10.1080/08827509208914075.
- F. K. Crundwell, M. S. Moats, V. Ramachandran, T. G. Robinson, and W. G. Davenport, Electrowinning of nickel from purified nickel solutions, in Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, ed. K. F. Crundwell, M. S. Moats, V. Ramachandran, T. G. Robinson and W. G. Davenport, Elsevier, 2011, pp. 327–345, ISBN 9780080968094, DOI:10.1016/B978-0-08-096809-4.10026-7.
- Z. T. Althagafi, J. T. Althakafy, B. A. Al Jahdaly and M. I. Awad, Differential electroanalysis of dopamine in the presence of a large excess of ascorbic acid at a nickel oxide nanoparticle-modified glassy carbon electrode, J. Sensors, 2020, 8873930, DOI:10.1155/2020/8873930.
- M. Próchniak and M. Grdeń, Electrochemical deposition of nickel from aqueous electrolytic baths prepared by dissolution of metallic powder, J. Solid State Electrochem., 2022, 26, 431–447, DOI:10.1007/s10008-021-05084-9.
- H. Chen and L. Wang, Posttreatment Strategies for Biomass Conversion, in Technologies for Biochemical Conversion of Biomass, ed. H. Chen and L. Wang, Academic Prss, 2017, pp. 97–217, DOI:10.1016/B978-0-12-802417-1.00008-9.
- I. R. Rodrigues, C. Deferm, K. Binnemans and S. Riaño, Separation of cobalt and nickel via solvent extraction with Cyanex-272: batch experiments and comparison of mixer-settlers and an agitated column as contactors for continuous counter-current extraction, Sep. Purif. Technol., 2022, 296, 121326, DOI:10.1016/j.seppur.2022.121326.
- S. M. S. Iqubal, Review on kinetic studies of α-hydroxy acids (glycolic, mandelic, citric, tartaric and malic) and some other organic compounds with water soluble nano particles of colloidal MnO2 in absence and presence of non-ionic surfactant (TX-100), J. Umm Al-Qura Univ. Appl. Sci., 2022, 8, 79–84, DOI:10.1007/s43994-022-00015-4.
- J. H. Zhang, W. Zhang, L. Zhang and S. G. Gu, A Critical review of technology for Selective recovery of Vanadium from leaching solution in V2O5 production, Solvent Extr. Ion Exch., 2014, 32, 221–248, DOI:10.1080/07366299.2013.877753.
- L. D. Kurbatova, O. V. Koryakova, M. S. Valova and I. N. Ganebnykh, The Peculiarities of extraction of vanadium(V) by aminophenols from sulfuric acid solutions, J. Solution Chem., 2021, 50, 823–832, DOI:10.1007/s10953-021-01089-0.
- H. Mahandra, R. Singh and B. Gupta, Recovery of vanadium (V) from synthetic and real leach solutions of spent catalyst by solvent extraction using Cyphos IL 104, Hydrometallurgy, 2020, 196, 105405, DOI:10.1016/j.hydromet.2020.105405.
- J. He, W. Tao and G. Dong, Study on extraction performance of vanadium (V) from aqueous solution by octyl-imidazole ionic liquids extractants, Metals, 2022, 12(5), 854, DOI:10.3390/met12050854.
- L. Zeng and C. Y. Cheng, A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part I: Metallurgical processes, Hydrometallurgy, 2009, 98(1–2), 1–9, DOI:10.1016/j.hydromet.2009.03.010.
- Y.-h. Liu, C. Yang, P.-y. Li and S.-q. Li, A new process of extracting vanadium from stone coal, Int. J. Miner., Metall. Mater., 2010, 17(4), 381–388, DOI:10.1007/s12613-010-0330-8.
- M. Noori, F. Rashchi, A. Babakhani and E. Vahidi, Selective recovery and separation of nickel and vanadium in sulfate media using mixtures of D2EHPA and Cyanex 272, Sep. Purif. Technol., 2014, 136, 265–273, DOI:10.1016/j.seppur.2014.08.038.
- Y. Tang, G. Ye, H. Zhang, X. Kang, S. Zhu and X. Liang, Solvent extraction of vanadium with D2EHPA from aqueous leachate of stone coal after low–temperature sulfation roasting, Colloids Surf. A Physicochem. Eng. Asp., 2022, 650, 129584, DOI:10.1016/j.colsurfa.2022.129584.
- H. Peng, J. Guo, B. Li and H. Huang, Vanadium properties, toxicity, mineral sources and extraction methods: a review, Environ. Chem. Lett., 2022, 20, 1249–1263, DOI:10.1007/s10311-021-01380-y.
- C. Yu, S. Bao, Y. Zhang and B. Chen, Separation and adsorption of V(V) from canadium-containing solution by TOMAC-impregnated resins, Chem. Eng. Res. Des., 2021, 174, 405–413, DOI:10.1016/j.cherd.2021.08.019.
- F. Veglio, R. Quaresima, P. Fornari and S. Ubaldini, Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning, Waste Manag., 2003, 23, 245–252, DOI:10.1016/S0956-053X(02)00157-5.
- X. Liu, J. Zeng, H. Yang, K. Zhou and D. Pan, V2O5-Based nanomaterials: synthesis and their applications, RSC Adv., 2018, 8(8), 4014–4031, 10.1039/C7RA12523B.
- S. Siriroj, J. Padchasri, A. Montreeuppathum, J. Lomon, N. Chanlek, Y. Poo-arporn, P. Songsiriritthigul, S. Rujirawat and P. Kidkhunthod, Enhancement of V2O5 Li-ion cathode stability by Ni/Co doped Li-borate-based glass, RSC Adv., 2022, 12(40), 26111–26115, 10.1039/D2RA04353J.
- S. I. Basha, A. Aziz, M. Maslehuddin, S. Ahmad, A. S. Hakeem and M. Mizanur Rahman, Characterization, processing, and application of heavy fuel oil ash, an industrial waste material – A Review, Chem. Rec., 2020, 20, 1568–1595, DOI:10.1002/tcr.202000100.
- Y. Li, F. Duan, S. Yang, Q. Deng, S. Liu and C. Peng, Design and synthesis of hierarchical NiO/Ni3V2O8 nanoplatelet arrays with enhanced lithium storage properties, RSC Adv., 2019, 9(67), 39536–39544, 10.1039/C9RA08252B.
- M. A. M. Ahssi, M. A. Erden, M. Acarer and H. Çuğ, The effect of nickel on the microstructure, mechanical properties and corrosion properties of niobium–vanadium microalloyed powder metallurgy steels, Materials, 2020, 13, 4021, DOI:10.3390/ma13184021.
- B.-g. Liu, J.-h. Peng, R.-d. Wan, L.-b. Zhang, S. h. Guo and S.-m. Zhang, Optimization of preparing V2O5 by calcination from ammonium metavanadate using response surface methodology, Trans. Nonferrous Metals Soc. China, 2011, 21(3), 673–678, DOI:10.1016/S1003-6326(11)60764-4.
- Y. Chen, Q. Feng, Y. Shao, G. Zhang, L. Ou and Y. Lu, Investigations on the extraction of molybdenum and vanadium from ammonia leaching residue of spent catalyst, Int. J. Mineral Proc., 2006, 79(1), 42–48, DOI:10.1016/j.minpro.2005.11.009.
- M. Du, Q. Li and H. Pang, Oxalate-derived porous prismatic nickel/nickel oxide nanocomposites toward lithium-ion battery, J. Colloid Interface Sci., 2020, 580, 614–622, DOI:10.1016/j.jcis.2020.07.009.
- S. Rakshit, S. Chall, S. S. Mati, A. Roychowdhury, S. P. Moulik and S. C. Bhattacharya, Morphology control of nickel oxalate by soft chemistry and conversion to nickel oxide for application in photocatalysis, RSC Adv., 2013, 3(17), 6106–6116, 10.1039/C3RA21978J.
- A. A. Gomaa, A. Abdelkader, S. A. Halawy and M. A. Mohamed, Preparation and characterization of nanocrystalline NiO by the thermal decomposition of oxalate salt for the dehydrogenation of 2-butanol to methyl ethyl ketone, Aswan Univ. J. Env. Studies, 2(3), 178–189, DOI:10.21608/aujes.2021.77260.1025.
- D. G. Arsher, Thermodynamic properties of import to environmental processes and remediation. II. Previous thermodynamic property values for nickel and some of its compounds, J. Phys. Chem. Ref. Data, 1999, 28(5), 1485–1507, DOI:10.1063/1.556044.
- M. Javanmardi, R. Emadi and H. Ashrafi, Synthesis of nickel aluminate nanoceramic compound from aluminum and nickel carbonate by mechanical alloying with subsequent annealing, Trans. Nonferrous Metals Soc. China, 2016, 26(11), 2910–2915, DOI:10.1016/S1003-6326(16)64420-5.
| This journal is © The Royal Society of Chemistry 2023 |
