 Open Access Article
Open Access ArticleEffect of calcium doping on the electrocatalytic activity of the Bi1−xCaxFeO3−δ oxygen electrode for solid oxide fuel cells†
Liang Wang,
Tian Xia*,
Liping Sun,
Qiang Li * and
Hui Zhao
* and
Hui Zhao
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, P. R. China. E-mail: xiatian@hlju.edu.cn; liqiang@hlju.edu.cn
First published on 16th January 2023
Abstract
For solid oxide fuel cell (SOFC) applications, there remains a growing interest in developing efficient cathode catalysts. Herein, iron-based Ca-doped Bi1−xCaxFeO3−δ (BCFx, x = 0.1, 0.2, and 0.3) oxides are evaluated as potential cathode materials for SOFCs. The phase structure, thermal expansion behavior, electrical conductivity, and electrocatalytic properties for the oxygen reduction reaction (ORR) of the BCFx cathodes are systematically characterized. Among all compositions, the Bi0.8Ca0.2FeO3−δ (BCF0.2) cathode exhibits the highest oxygen vacancy concentration and considerable electrocatalytic activity, demonstrating the lowest polarization resistance (0.11 Ω cm2) and largest exchange current density of 41.91 mA cm−2 at 700 °C. The BCF0.2 cathode-based single cell delivers excellent output performance, yielding a maximum power density of 760 mW cm−2 700 °C along with exceptional stability over a period of 60 h. This work highlights the Ca-doping strategy for enhancing electrocatalytic activity of the cathode electrocatalysts in SOFCs.
1. Introduction
Developing desirable cathode materials with outstanding electrocatalytic activity is a challenge for practical application in solid oxide fuel cells (SOFCs).1,2 Among these materials, the Fe-based cathodes have attracted much attention due to their low cost, outstanding thermo-chemical stability, and acceptable electrocatalytic activity.3–5In recent years, some efforts have been devoted to bismuth ferrite oxide (BiFeO3, BFO) with low thermal expansion coefficient and excellent chemical compatibility, giving rise to efficient utilizations for the ORR and oxygen evolution reaction (OER).6–8 Nevertheless, a secondary phase appears in BFO at 447–767 °C, which limits its practical applications.9 Thus, improving thermodynamic stability is considered to be crucial to the BFO catalyst. The cation doping seems to be an effective strategy for stabilizing the phase structure of BFO.10,11 Baek et al. reported that the Sr2+ doping significantly enhances oxygen surface exchange rate and thermal stability in Bi1−xSrxFeO3−δ (BSF).12 The BSF materials are proposed to be potential cathodes for SOFCs because of their excellent electrocatalytic activity.13 However, the electrical conductivity of BSF is not adequate (∼1–2 S cm−1 in the temperature range of 300 and 900 °C). Recently, it is found that partial substitution of Ca2+ for Bi3+ can promote the chemical compatibility and electrocatalytic performance.14 Moreover, the Ca doping also enhanced the electrical conductivity of BFO.15 Increased oxygen vacancy concentration in the bulk may be responsible for improved structural stability.16 In this scheme, we believe that Bi1−xCaxFeO3−δ should be promising cathode materials for SOFCs.
In this work, a series of Bi1−xCaxFeO3−δ (BCFx, x = 0.1, 0.2, and 0.3) oxides are synthesized and evaluated as potential Fe-based cathode electrocatalysts for SOFCs. The crystal structure, surface states, and thermal expansion behavior are systematically characterized. The electrochemical properties and electrode reaction kinetics for ORR are discussed in detail. Furthermore, the BCFx cathode-based fuel cell shows satisfactory SOFCs performance.
2. Experimental section
2.1 Materials preparation
Bi1−xCaxFeO3−δ (BCFx, x = 0.1, 0.2, and 0.3) oxides were synthesized by a sol–gel method. Stoichiometric amounts of Bi(NO3)3·3H2O, Ca(NO3)2·4H2O, and Fe(NO3)3·9H2O were mixed and dissolved into deionized water to form a transparent solution, with additions of citric acid and EDTA (citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDTA
EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) total metal ion = 2
total metal ion = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in molar ratio). The above solution was heated at 100 °C to produce the viscous gel. Afterwards, this gel was heated at 180 °C for 6 h, and then calcined at 850 °C for 12 h to obtain the final products.
1 in molar ratio). The above solution was heated at 100 °C to produce the viscous gel. Afterwards, this gel was heated at 180 °C for 6 h, and then calcined at 850 °C for 12 h to obtain the final products.
2.2 Material characterization
The phase composition of BCFx powders was analyzed using an X-ray diffractometer (XRD, Bruker AXS D8 Advance) with a Cu-Kα radiation (λ = 1.5418 Å) source in the 2θ range of 20–80°. The microstructure of the cells was observed using scanning electron microscopy (SEM) (Supra 55 Sapphire Carl ZEISS). The conductivity of BCFx was measured using standard four-probe method with a Keithley 2700 digital multimeter. The BCFx powders were pressed at a pressure of 230 MPa and then sintered at 900 °C for 12 h to obtain the rectangular bars (12 × 2 × 1.5 mm). Thermal expansion coefficients (TECs) of BCFx were recorded with a dilatometer (SETARAMM Setsys 18) within a temperature range of 50–800. X-ray photoelectron spectra (XPS) were conducted on a Kratos Axis Ultra DLD instrument with a radiation source of Al Kα (1486.6 eV). Electron paramagnetic resonance (EPR) spectra were performed with Bruker EPR100d X-band spectrometer. Thermogravimetric (TG) tests were performed using TG/DTA 6300 thermal analyzer (PerkinElmer, USA) in air atmosphere in temperature range of 50–800 °C. The oxygen non-stoichiometry value (δ) of samples at elevated temperatures was calculated by thermogravimetric analysis (TGA) and iodometric titration data.2.3 Electrochemical characterization
The dense Ce0.9Gd0.1O1.9 (GDC) electrolyte (thickness: ∼350 mm, diameter: ∼15 mm) was fabricated by pressing GDC powder (Ningbo SuoFuRen Energy Co. Ltd) and then sintered at 1400 °C for 24 h. To fabricate symmetric cells with the BCFx| GDC|BCFx configuration, the cathode ink was prepared by thoroughly mixing BCFx powder and organic binder. The cathode ink was symmetrically printed on both sides of the GDC substrate and then calcined at 900 °C for 4 h to obtain symmetrical cells. Electrochemical impedance spectroscopy (EIS) was acquired on symmetric cells with an electrochemical workstation (Autolab PGSTAT302N) in the frequency range of 10−2–106 Hz at 500–700 °C. For CO2 tolerance experiment, EIS of the cathode was tested at 700 °C in a mixture atmosphere with different CO2 concentrations (1, 3, 5 and 10 vol.% in air). For preparing the three-electrode cells, the cathode ink is deposited onto one side of the GDC electrolyte as the working electrode. Silver paste was used as the counter electrode and was painted on the opposite side of GDC substrate. The silver reference electrode was placed on the same side of the working electrode. The anode-supported fuel cells (NiO-YSZ|(YSZ, Zr0.84Y0.16O2)|GDC|BCFx) were fabricated for the fuel cell test. The half-cells of NiO-YSZ|YSZ|GDC were purchased from Ningbo SuoFuRen Energy Co. Ltd. The cathode ink was painted onto the GDC buffer layer and then calcined at 900 °C for 4 h. The single cell is sealed at the end of an alumina tube with high-temperature ceramic binder. The anode side is supplied with humidified H2 (80 mL min−1) and the cathode side is exposed to ambient air. Current–voltage (I–V) curves of the fuel cells were tested with an electrochemical workstation (IM6ex, ZAHNER) over a temperature range of 500–700 °C.3. Results and discussion
Fig. 1a shows the XRD patterns of the BCFx powders after calcining at 850 °C for 12 h. The BCFx materials have a cubic perovskite structure with space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, without any secondary phases. Furthermore, to understand detailed crystal structure of BCFx, the XRD patterns were refined using Rietveld refinement method (Fig. 1b–d). As can be seen clearly, the lattice parameters decrease gradually with increasing the Ca2+ concentration. This fact is mainly attributed to smaller ionic radius of Ca2+ (1 Å) than that of Bi3+ (1.04 Å), leading to the contraction of unit cell volume.17 The acceptable reliable factors mean a rationality for structural refinements. Aiming at probing chemical compatibility between BCFx and GDC, the BCFx–GDC mixtures were calcined at 850 °C for 12 h. The relevant XRD patterns are shown in Fig. S1.† It is found that all diffraction peaks are assigned to both BCFx and GDC components. Not any visible impurities can be detected in the mixtures, indicating that BCFx has a good chemical compatibility with GDC electrolyte.
m, without any secondary phases. Furthermore, to understand detailed crystal structure of BCFx, the XRD patterns were refined using Rietveld refinement method (Fig. 1b–d). As can be seen clearly, the lattice parameters decrease gradually with increasing the Ca2+ concentration. This fact is mainly attributed to smaller ionic radius of Ca2+ (1 Å) than that of Bi3+ (1.04 Å), leading to the contraction of unit cell volume.17 The acceptable reliable factors mean a rationality for structural refinements. Aiming at probing chemical compatibility between BCFx and GDC, the BCFx–GDC mixtures were calcined at 850 °C for 12 h. The relevant XRD patterns are shown in Fig. S1.† It is found that all diffraction peaks are assigned to both BCFx and GDC components. Not any visible impurities can be detected in the mixtures, indicating that BCFx has a good chemical compatibility with GDC electrolyte.
 | ||
| Fig. 1 (a) Room temperature XRD patterns of the BCFx powders. Rietveld refinement profiles of (b) BCF0.1, (c) BCF0.2, and (d) BCF0.3. | ||
Fig. 2a shows the electrical conductivities of the BCFx samples over the temperature range of 100–800 °C in air. The electrical conductivity of all perovskites increases at elevated temperature, suggesting a semi-conducting nature arising from small polaron-type hopping.18 The maximum conductivities are 6.43, 6.74, and 6.48 S cm−1 for BCF0.1, BCF0.2, and BCF0.3 at 800 °C, respectively. These values surpass that of undoped BiFeO3 (1.32 S cm−1)6 and are comparable to those of high-performance Fe-based cathode materials, such as Bi0.5Sr0.5Fe0.9Nb0.1O3−δ (3.3 S cm−1),19 BaFe0.95Sn0.05O3−δ (7.8 S cm−1),20 and Bi0.5Sr0.5Fe0.95Zr0.05O3−δ (5.97 S cm−1).21
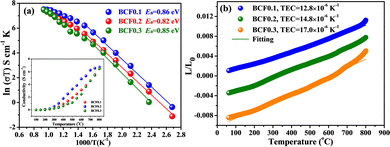 | ||
| Fig. 2 (a) Arrhenius plots of electrical conductivity of BCFx in air. (b) Thermal expansion curves of BCFx in the temperature range of 50–800 °C. | ||
Fig. 2b presents the thermal expansion curves of the BCFx samples in the temperature range of 50–800 °C. The average TEC value of BCFx increases with increasing the doping fraction. As usual, the TEC value is inversely proportional to the bonding energy of oxides.22 It is noteworthy that the bonding energy of Bi–O is larger than that of Ca–O, thereby giving rise to increased TEC. The average TEC values are 12.8, 14.8, and 17.0 × 10−6 K−1 for BCF, BCF0.2, and BCF0.3, respectively. These TEC values here are much lower than those of some Fe-based cathodes23–25 and are compatible with the GDC electrolyte (13.0 × 10−6 K−1),26 which benefits for the thermo-chemical stability between cathode and electrolyte.
Fig. 3a shows the O 1s XPS spectra of the BCFx materials. All O 1s spectra consist of two peaks. The peaks at 531.3–531.6 eV correspond to surface adsorbed oxygen (Oads), while the peaks at 528.4–528.9 eV are associated with the lattice oxygen (Olat).27 The percentages of Oads/Olat derived from the peak areas are summarized in Fig. 3b. Noticeably, the BCF0.2 sample has the largest Oads/Olat value (2.42), signifying that BCF0.2 possesses great surface oxygen adsorption properties.28 Fig. 3c displays the Fe 2p3/2 spectra of the BCFx samples. According to the reports, three subpeaks centered at 711.7–712.1 eV, 710–710.9 eV, and 709.0–709.4 eV are related to Fe4+, Fe3+, and Fe2+, respectively,29,30 and the fitting results of are listed in Table S1.† The average valence states of Fe are +3.058, +3.119, and +3.233 for BCF0.1, BCF0.2, and BCF0.3, respectively. Meanwhile, the calculated oxygen non-stoichiometry (δ) values of BCFx are 0.021, 0.041, and 0.034 for BCF0.1, BCF0.2, and BCF0.3, respectively. Furthermore, EPR spectroscopy was used to probe the presence of oxygen vacancies in the BCFx samples, as presented in Fig. 3d. The main resonance line is located at a magnetic field strength of 321.14 G, which is consistent with typical EPR signal with the g value of 2.228.31 This originates from the unpaired electrons trapped in oxygen vacancy species. Furthermore, the oxygen non-stoichiometry of BCFx materials at elevated temperatures was investigated by the TGA (Fig. S2a†). We can find that before 300 °C, the three materials show slow weight loss, mainly because the materials show adsorbed water vapor and hydrocarbons. With the further increase of temperature, the material begins to show obvious weight loss, mainly due to the loss of lattice oxygen in the material, which will also cause the change of Fe valence state and the formation of oxygen vacancies. The oxygen non-stoichiometry (δ) of the BCFx samples at elevated temperatures was explored by TGA in air, as presented in Fig. S2b.† The δ values were determined by TGA results and the initial oxygen non-stoichiometry (δ0) values at room temperature were obtained by the iodometric titration. The BCF0.2 possesses the largest oxygen vacancy concentration, indicating its excellent oxygen ions mobility and promoted ORR activity.
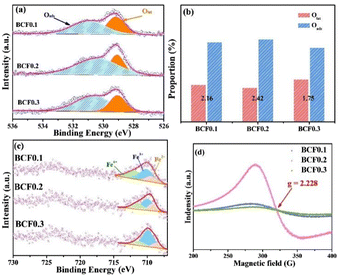 | ||
| Fig. 3 (a) O 1s XPS spectra, (b) the percentages of Oads and Olat, and (c) Fe 2p XPS spectra of the BCFx samples. (d) EPR spectra of the BCFx samples. | ||
The ORR activity of the BCFx electrodes with symmetric configuration (BCFx|GDC|BCFx) was evaluated by EIS. As shown in Fig. 4a, the RP values of BCF0.1, BCF0.2, and BCF0.3 electrodes are 0.27, 0.11, and 0.62 Ω cm2 at 700 °C, respectively. Among all cathodes, the BCF0.2 electrode gives the lowest RP values at 600–700 °C (Fig. 4b), reflecting the best electrochemical performance of BCF0.2. This performance of BCF0.2 outperforms many cobalt-free electrodes (Fig. 4c).32–36 Fig. 4d shows the Arrhenius plots of Rp for the BCFx cathodes. The activation energy (Ea) values of the BCF0.1, BCF0.2, and BCF0.3 cathodes are 124.4, 110.8, and 122.4 kJ mol−1, respectively. The lowest Ea facilitates the oxygen mobility and electrocatalytic activity of BCF0.2 and is smaller than those of reported Fe-based cathodes, e. g. La0.8Ca0.2Fe0.8Ni0.2O3−δ (178 kJ mol−1),32 LaFe0.8Cu0.2O3−δ (159.2 kJ mol−1),37 La0.5Sr0.5Fe0.9Mo0.1O3−δ (146.6 kJ mol−1),38 and PrBaFe2O5+δ (123.5 kJ mol−1).39
The sintering temperature plays a key role in the electrode performance. Fig. 5a shows the EIS spectra of the BCF0.2 electrode calcined at different temperatures (850 °C, 900 °C, and 950 °C). When sintering temperature is 900 °C, the RP value reaches the lowest level measured at 700 °C, manifesting that appropriate calcining temperature may make the best electrochemical performance. Furthermore, the effect of calcining temperature on the electrode microstructure is also explored (Fig. 5b). At the lower calcining temperature (850 °C), the electrode layer shows poor connection between cathode particles. The surface-section view reveals a moderate porous structure with well-connected network after calcining at 900 °C. Such microstructure provides convenient gas diffusion and charge transfer, further improving electrocatalytic ORR activity of the electrode.40 However, the cathode particles aggregate at higher treatment temperature (950 °C), resulting in decreased ORR kinetics and increased polarization resistance.41 Thus, the electrode should be calcined at 900 °C to realize the best electrochemical performance toward ORR.
 | ||
| Fig. 5 (a) EIS spectra of the BCF0.2 cathode at different sintering temperatures and then measured at 600–700 °C. (b) SEM images of the BCF0.2 cathode calcined at different temperatures. | ||
To go insight into the ORR mechanism, the EIS spectra of the BCF0.2 cathode were tested at different oxygen partial pressures (pO2) at 700 °C (Fig. 6a). All impedance spectra consist of high-frequency and low-frequency arcs, indicating that two reaction steps occur for ORR at least. The impedance data was fitted using the equivalent circuit R1 − (RHF/CPEHF) − (RLF/CPELF), where R1 is the ohmic resistance from the electrolyte and measuring device, RHF and RLF represent high-frequency and low-frequency resistance, and CPEHF and CPELF are the constant phase elements. As we know, the relationship between RP and pO2 can be expressed by the following equation: RP = k(pO2)−n (1), where k is the independent constant, and the n values represent different ORR steps on the electrode.42 The dependence of RHF and RLF for the BCF0.2 electrode on pO2 at 700 °C is plotted in Fig. 6 (b). The n value is 0.07 in the high-frequency region, which is indicative of oxide-ion transport from three-phase boundary (TPB) to electrolyte (n = 0).43 The characteristic n value related to the low-frequency region is 0.51, suggesting the oxygen adsorption/dissociation process on the catalyst surface (n = 1/2).44 Bode plots of impedance spectra are further discussed, as shown in Fig. 6c. Bode curves can be involved into two peaks: high-frequency peak (PHF) and low-frequency peak (PLF). The peak area for PHF presents a slight dependence on pO2, while PLF dramatically changes with pO2. The results further confirms that the oxygen adsorption-dissociation process is the rate-limiting step for ORR, as schematically illustrated in Fig. 6d. In addition, the ORR steps at the BCF0.2 electrode interface was also clarified by calculating the characteristic capacitance (C) and relaxation frequency (f).45 The CHF and fHF values in high-frequency range are 10−3–10−2 F cm−2 and 103–104 Hz (Fig. 6e and f, and Table S2†), respectively, which is related to the charge transfer reaction.46 For the low-frequency contribution, the CLF and fLF values are 0.2–0.3 F cm−2 and 10−1–102 Hz, respectively, corresponding to oxygen adsorption/dissociation process.47
To further develop the bifunctional application of BCF0.2 in the ORR and OER manners, the polarization curves were tested with a three-electrode cell using chronoamperometry method. The overpotentials for both ORR and OER models decrease with increasing temperature, suggesting enhanced electrocatalytic activity at elevated temperature. For the ORR mode, the current density reaches −268 mA cm−2 at an overpotential of −55 mV. This current density value is larger than those of some cobalt-based cathodes at the same overpotential.48,49 For the OER mode, the anodic overpotential is as low as 37.4 mV when the current density is 284 mA cm−2. Furthermore, the important parameter, exchange current density (i0), is used to evaluate the ORR activity. Tafel plots of the BCF0.2 cathode at 600–700 °C are shown in Fig. 7b. The i0 can be calculated by the Boult Volmer equation: η = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(i)/αZF − RT
ln(i)/αZF − RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(i0)/αZF (2).50 The corresponding i0 values of BCF0.2 are 11.67, 31.42, and 41.91 mA cm−2 at 600, 650, and 700 °C, respectively. At 700 °C, the i0 value is superior to those of some reported cobalt-free cathodes, such as Pr2CuO4 (9.8 mA cm−2),51 Ca2Fe1.3Mn0.7O5+δ (18.2 mA cm−2),52 and Pr0.8Sr0.2FeO3−δ (34.3 mA cm−2).53 Resultantly, the BCF0.2 catalyst is proposed to be utilized as promising bifunctional electrode.
ln(i0)/αZF (2).50 The corresponding i0 values of BCF0.2 are 11.67, 31.42, and 41.91 mA cm−2 at 600, 650, and 700 °C, respectively. At 700 °C, the i0 value is superior to those of some reported cobalt-free cathodes, such as Pr2CuO4 (9.8 mA cm−2),51 Ca2Fe1.3Mn0.7O5+δ (18.2 mA cm−2),52 and Pr0.8Sr0.2FeO3−δ (34.3 mA cm−2).53 Resultantly, the BCF0.2 catalyst is proposed to be utilized as promising bifunctional electrode.
 | ||
| Fig. 7 (a) Polarization curves of the BCF0.2 electrode in the ORR and OER models at 600–700 °C. (b) Tafel plots of the BCF0.2 cathode at 600–700 °C. | ||
To further investigate electrocatalytic activity of the cathode, an anode-supported single cell with the configuration of NiO-YSZ|YSZ|GDC|BCFx was fabricated. Fig. 8a–c show the I–V and I–P curves of single cells with the BCFx cathodes at 600–700 °C using humidified hydrogen (3% H2O) and ambient air as the fuel and oxidant, respectively. The maximal power densities (MPD) of single cells are 634, 760, and 594 mW cm−2 for BCF0.1, BCF0.2 and BCF0.3 at 700 °C, respectively. The BCF0.2 cathode-based single cell delivers the best output performance, and the MPD value is higher than those of Fe-based cathode-containing fuel cells, such as BaBi0.05Co0.8Nb0.15O3−δ (218 mW cm−2),54 Ba0.95La0.05Fe0.8Zn0.2O3−δ (266.5 mW cm−2),55 and SrFe0.8Sb0.2O3−δ (450 mW cm−2).56 Moreover, the long-term stability was further tested at a constant voltage of 0.5 V at 700 °C. The single cell shows stable MPD and current density over a period of 60 h operation without negligible degradation rate (Fig. 8d), indicating exceptional operating stability of the fuel cell.
 | ||
| Fig. 8 (a–c) I–V and I–P curves of the NiO-YSZ|YSZ|GDC|BCFx single cells at 600–700 °C. (d) Long-term stability test of single cell with the BCF0.2 cathode at 700 °C. | ||
CO2 tolerance is a prominent parameter to evaluate the possible application of the cathode. In general, alkaline earth-containing electrode oxide are always generated with CO2 form a carbonate on the cathode surface, which are averse to the adsorption and diffusion of oxygen.57 To assess the CO2 tolerance of BCF0.2, the EIS spectra are measured at 700 °C in air with different CO2 concentration. As shown the Fig. S3a,† it can be found that the polarization resistance values of BCF0.2 cathode increases with the CO2 concentration. Obviously, the polarization resistance values of the electrode increased from 0.11 to 0.156 Ω cm2 and 0.224 to 0.347 Ω cm2 for BCF0.2 and Bi0.5Sr0.5FeO3−δ (BSF) cathode (Fig. S3b†). Compared with BSF electrode, it can be found that the Ca doped electrode has better CO2 tolerance than the Sr doped electrode.
4. Conclusions
In summary, the Fe-based BCFx oxides have been synthesized and evaluated as efficient cathode catalysts for SOFCs. The Ca2+ substitution promotes the amount of absorbed oxygen and enhances the cathode performance of BCFx. Among all components, the BCF0.2 cathode exhibits excellent ORR activity, as evidenced by the lowest Rp (0.11 Ω cm2) and highest MPD (0.76 W cm−2) at 700 °C. Furthermore, EIS analysis indicates that the oxygen adsorption/dissociation process is the main rate-limiting step toward ORR. Additionally, the BCF0.2 electrode yields a higher current density in both ORR and OER manners, demonstrating bifunctional characteristic of the BCF0.2 electrode. This study endows an effective way for designing novel cathode electrocatalysts in SOFCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was supported by National Natural Science Foundation of China (51972100) and Natural Science Foundation of Heilongjiang Province (ZD2022E007).References
- Z. P. Shao and S. M. Haile, Nature, 2004, 431, 170–173 CrossRef CAS PubMed.
- X. M. Xu, Y. L. Pan, Y. J. Zhong, R. Ran and Z. P. Shao, Mater. Horiz., 2020, 7, 2519–2565 RSC.
- J. M. Porras-Vazquez, T. Pike, C. A. Hancock, J. F. Marco, F. J. Berry and P. R. Slater, J. Mater. Chem. A, 2013, 38, 11834–11841 RSC.
- J. T. Gao, Q. Li, W. W. Xia, L. P. Sun, L. H. Huo and H. Zhao, ACS Sustainable Chem. Eng., 2019, 7, 18647–18656 CrossRef CAS.
- T. Hong, M. Y. Zhao, K. Brinkman, F. L. Chen and C. G. Xia, ACS Appl. Mater. Interfaces, 2017, 9, 8659–8668 CrossRef CAS PubMed.
- R. A. Afzal, K. Y. Park, S. H. Cho, N. I. Kim, S. R. Choi, J. H. Kim, H. T. Lim and J. I. Park, RSC Adv., 2017, 7, 47643–47653 RSC.
- T. W. Chen, P. Kalimuthu, G. Anushya, S. M. Chen, R. Ramachandran, V. Mariyappan and D. C. Muthumala, Materials, 2021, 14, 2976 CrossRef CAS PubMed.
- I. Papadas, J. A. Christodoulides, G. Kioseoglou and G. S. Armatas, J. Mater. Chem. A, 2015, 3, 1587–1593 RSC.
- S. M. Selbach, M. A. Einarsrud and T. Grande, Chem. Mater., 2009, 21, 169–173 CrossRef CAS.
- L. Li, Z. Q. Kong, B. W. Yao, H. Yang, Z. H. Gao, L. J. Xu, F. F. Dong, M. Ni and Z. Lin, Chem. Eng. J., 2020, 396, 125237 CrossRef CAS.
- P. Anand, D. P. Jaihindh, W. K. Chang and Y. P. Fu, Appl. Surf. Sci., 2021, 540, 148387 CrossRef CAS.
- D. Baek, A. Kamegawa and H. Takamura, Solid State Ionics, 2014, 262, 691–695 CrossRef CAS.
- Y. J. Niu, J. Sunarso, W. Zhou, F. L. Liang, L. Ge, Z. H. Zhu and Z. P. Shao, Int. J. Hydrogen Energy, 2011, 36, 3179–3186 CrossRef CAS.
- M. Schrade, N. Masó, A. Perejón, L. A. Pérez-Maqueda and A. R. West, J. Mater. Chem. C, 2017, 5, 10077–10086 RSC.
- N. Masó and A. R. West, Chem. Mater., 2012, 24, 2127–2132 CrossRef.
- J. S. Lim, J. H. Lee, H. S. Park, R. Gao, T. Y. Koo, L. W. Martin, R. Ramesh and C. H. Yang, NPG Asia Mater., 2018, 10, 943–955 CrossRef CAS.
- F. Sánchez-De Jesús, A. M. Bolarín-Miró, C. A. Cortés-Escobedo, A. Barba-Pingarrón and F. Pedro-García, J. Alloys Compd., 2020, 824, 153944 CrossRef.
- J. W. Stevenson, T. R. Armstrong, L. R. Pederson and W. J. Weber, J. Electrochem. Soc., 1996, 143, 2722–2729 CrossRef CAS.
- L. Gao, Q. Li, L. P. Sun, L. H. Huo, H. Zhao and J. C. Grenier, J. Power Sources, 2017, 371, 86–95 CrossRef CAS.
- F. F. Dong, M. Ni, W. He, Y. B. Chen, G. M. Yang, D. J. Chen and Z. P. Shao, J. Power Sources, 2016, 326, 459–465 CrossRef CAS.
- J. T. Gao, D. Ma, H. Zhao, Q. Li, Z. Lü and B. Wei, Energy, 2022, 252, 124050 CrossRef CAS.
- A. R. Ruffa, J. Mater. Sci., 1980, 15, 2258–22567 CrossRef CAS.
- J. H. Zhang, F. Z. Han, C. X. Li and S. L. Zhang, J. Eur. Ceram. Soc., 2022, 42, 5801–5812 CrossRef CAS.
- Y. J. Wu, S. Wang, Y. Gao, X. Yu, H. T. Jiang, B. Wei and Z. Lü, J. Alloys Compd., 2022, 926, 166852 CrossRef CAS.
- X. Y. Lu, Y. Yang, Y. Z. Ding, Y. H. Chen, Q. W. Gu, D. Tian, W. L. Yu and B. Lin, Electrochim. Acta, 2017, 227, 33–40 CrossRef CAS.
- H. Hayashi, M. Kanoh, C. J. Quan, H. Inaba, S. R. Wang, M. Dokiya and H. Tagawa, Solid State Ionics, 2000, 13, 227–233 CrossRef.
- L. Gao, Q. Li, L. P. Sun, L. H. Huo, H. Zhao and J. C. Grenier, J. Mater. Chem. A, 2018, 6, 15221–15229 RSC.
- J. W. Yin, Y. M. Yin, J. Lu, C. M. Zhang, N. Ming and Z. F. Ma, J. Phys. Chem., 2014, 118, 13357–13368 CrossRef CAS PubMed.
- C. C. Wang, M. Gholizadeh, B. X. Hou and X. C. Fan, RSC Adv., 2021, 11, 7–14 RSC.
- T. Z. Ma, T. Xia, Q. Li, L. P. Sun, L. H. Huo and H. Zhao, J. Eur. Ceram. Soc., 2022, 42, 490–498 CrossRef CAS.
- C. Z. Sun, Y. Kong, L. Shao, K. N. Sun and N. Q. Zhang, J. Power Sources, 2020, 459, 228017 CrossRef CAS.
- N. O. Vitoriano, C. B. López, A. Hauch, I. R. D. Larramendi and T. Rojo, Int. J. Hydrogen Energy, 2014, 39, 6675–6679 CrossRef.
- B. Wei, Z. Lu, X. Q. Huang, M. L. Liu, N. Li and W. H. Su, J. Power Sources, 2008, 176, 1–8 CrossRef CAS.
- E. P. Murray, M. J. Sever and S. A. Barnett, Solid State Ionics, 2002, 148, 27–34 CrossRef.
- Y. J. Niu, J. Sunarso, F. L. Liang, W. Zhou, Z. H. Zhu and Z. P. Shao, J. Electrochem. Soc., 2011, 158, B132–B138 CrossRef CAS.
- J. M. Porras-Vazquez, T. Pike, C. A. Hancock, J. F. Marco, F. J. Berry and P. R. Slater, J. Mater. Chem. A, 2013, 1, 11834–11841 RSC.
- A. Idrees, X. N. Jiang, G. Liu, H. Luo, G. Q. Jia, Q. Y. Zhang, L. Jiang, X. N. Li and B. M. Xu, ChemistryOpen, 2018, 7, 688–695 CrossRef CAS PubMed.
- M. Wu, H. Cai, F. Jin, N. Sun, J. Xu, L. Zhang, X. Han, S. Wang, X. Su, W. Long, L. Wang and L. Zhang, J. Eur. Ceram. Soc., 2021, 41, 2682–2690 CrossRef CAS.
- G. D. Li, Y. J. Gou, X. J. Cheng, Z. Bai, R. Z. Ren, C. M. Xu, J. S. Qiao, W. Sun, Z. H. Wang and K. N. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 34282–34291 CrossRef CAS PubMed.
- W. W. Xia, Q. Li, L. P. Sun, L. H. Huo and H. Zhao, J. Alloys Compd., 2020, 835, 1545406 CrossRef.
- L. Gao, M. Z. Zhu, T. Xia, Q. Li, T. S. Li and H. Zhao, Electrochim. Acta, 2018, 289, 428–436 CrossRef CAS.
- J. R. Wilson, D. T. Schwartz and S. B. Adler, Electrochim. Acta, 2006, 51, 1389–1402 CrossRef CAS.
- Y. Takeda, R. Kanno, M. Noda, Y. Tomida and O. Yamamoto, J. Electrochem. Soc., 1987, 134, 2656–2661 CrossRef CAS.
- E. Siebert, A. Hammouche and M. Kleitz, Electrochim. Acta, 1995, 40, 1741–1753 CrossRef CAS.
- M. J. Escudero, A. Aguadero, J. A. Alonso and L. Daza, Electroanal. Chem., 2007, 611, 107–116 CrossRef CAS.
- A. B. Yu, T. Xia, L. P. Sun, Q. Li, L. H. Huo and H. Zhao, J. Alloys Compd., 2020, 837, 155563 CrossRef CAS.
- X. N. Li, X. N. Jiang, S. L. Pang, Q. Wang, Z. X. Su and Q. Y. Zhang, Int. J. Hydrogen Energy, 2011, 36, 13850–13857 CrossRef CAS.
- M. M. Guo, Q. Li, J. T. Gao, L. P. Sun, L. H. Huo and H. Zhao, J. Alloys Compd., 2021, 858, 158265 CrossRef CAS.
- F. C. Meng, T. Xia, J. P. Wang, Z. Shi and H. Zhao, J. Power Sources, 2015, 293, 741–750 CrossRef CAS.
- B. C. H. Steele, Solid State Ionics, 1995, 75, 157–165 CrossRef CAS.
- C. Sun, Q. Li, L. P. Sun, H. Zhao and L. H. Huo, Mater. Res. Bull., 2014, 53, 65–69 CrossRef CAS.
- Q. Li, L. P. Sun, X. Zeng, H. Zhao, L. H. Huo, J. C. Grenier, J. M. Bassat and F. Mauvy, J. Power Sources, 2013, 238, 11–16 CrossRef CAS.
- J. H. Piao, K. N. Sun, N. Q. Zhang, X. B. Chen, S. Xu and D. R. Zhou, J. Power Sources, 2007, 172, 633–640 CrossRef CAS.
- X. W. Meng, G. H. Long, S. X. Liu, Y. Ji, M. J. Pang, B. Wang, S. Q. Lü and J. H. Yang, Int. J. Hydrogen Energy, 2015, 40, 6935–6941 CrossRef CAS.
- Z. Wang, P. F. Lv, L. Yang, R. Guan, J. D. Jiang, F. J. Jin and T. M. He, Ceram. Int., 2020, 46, 18216–18223 CrossRef CAS.
- Y. Q. Meng, L. Sun, J. Gao, W. Z. Tan, C. S. Chen, J. X. Yi, H. J. M. Bouwmeester, Z. H. Sun and K. S. Brinkman, ACS Appl. Mater. Interfaces, 2019, 11, 11498–11506 CrossRef CAS PubMed.
- M. M. Guo, T. Xia, Q. Li, L. P. Sun and H. Zhao, J. Eur. Ceram. Soc., 2021, 41, 6531–6538 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06750a |
| This journal is © The Royal Society of Chemistry 2023 |


