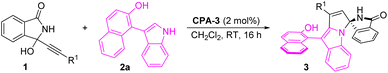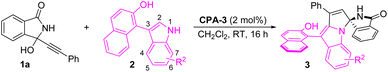Asymmetric organocatalytic (3 + 2) annulation of propargylic alcohols with indolylnaphthalenols: synergistic construction of axial and central chirality†
Yan
Xia‡
a,
Meiwen
Liu‡
b,
Chenxiao
Qian
b,
Pengfei
Li
 *b,
Mingxin
Dong
*b,
Mingxin
Dong
 *a and
Wenjun
Li
*a and
Wenjun
Li
 *a
*a
aDepartment of Medicinal Chemistry, School of Pharmacy, Qingdao University, Qingdao, Shandong 266021, China. E-mail: mxdong64@qdu.edu.cn; liwj@qdu.edu.cn
bShenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, College of Science, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China. E-mail: lipf@sustech.edu.cn; flyli1980@gmail.com
First published on 14th November 2022
Abstract
Organocatalytic enantioselective construction of chiral spiro N,N-acetal carbon stereocenters and axially chiral 3-arylindoles has been achieved via a chiral phosphoric acid (CPA)-catalyzed (3 + 2) annulation of α-(3-isoindolinonyl) propargylic alcohols with 1-(3-indolyl)naphthalen-2-ols, affording a broad scope of pyrrolo[1,2-a]indoles bearing both enantioenriched spiro isoindolinone-indoline and atropisomeric naphthalenol frameworks. Based on control experiments and our previous work, a possible mechanism was proposed accordingly.
Introduction
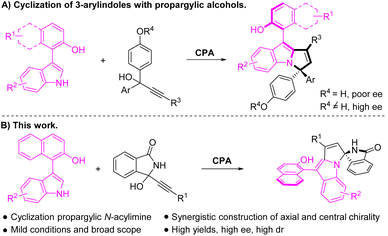
During the past few years, organocatalytic atroposelective construction of axially chiral biaryls has attracted much attention and achieved fast development.1 Notably, it has become an emerging research area of organocatalytic construction of axially chiral indole-based structures,2 including N-arylindoles,3 3-arylindoles,4 2-arylindoles,5 bisindoles,6 and other frameworks.7 In particular, the chiral phosphoric acid (CPA)-catalyzed reactions of naphthyl-indoles developed by Shi et al. provided an efficient synthetic strategy towards axially chiral 3-arylindoles.8 Despite these impressive achievements, most results were limited to the construction of exclusive axial chirality. The cooperatively controlling axial and central chirality still remains an important issue in this field.9 Therefore, it is highly desirable to develop an efficient protocol to construct 3-arylindole scaffolds with multiple chiral elements.Recently, the catalytic enantioselective construction of axially chiral allenes has been well established.10 In particular, the CPA-catalyzed reactions of functionalized propargylic alcohols provided robust access to axially chiral allenes via asymmetric conjugate addition of in situ formed propargylic p-quinone methides (p-QMs),11 propargylic aza-p-quinone methides (aza-p-QMs),12 and propargylic methyleneindoles.13 Independently, Shi and coworkers realized the asymmetric synthesis of aryl-pyrroloindoles with axial and central chirality via an organocatalytic asymmetric (2 + 3) cyclization of 3-arylindoles with propargylic alcohols (Scheme 1A).14 They also found that the auxiliary group of propargylic alcohols played a key role in controlling the enantioselectivity. Based on our previous work on the reactions of functionalized propargylic alcohols,11b,12a,13c–f we have successfully developed diverse organocatalytic reactions of α-(3-isoindolinonyl) propargylic alcohols,15a,b which in situ generated β,γ-alkynyl-α-imines under acidic conditions.16 As a continuation of our effort in organocatalytic reactions of propargylic alcohols, we have a high motivation to explore reactions of α-(3-isoindolinonyl) propargylic alcohols with challenges in terms of regio- and stereoselectivities. Here, we reported a CPA-catalyzed regio- and enantioselective reaction of 2-indolylnaphthalenols with α-(3-isoindolinonyl) propargylic alcohols (Scheme 1B). Notably, the protocol afforded a broad scope of pyrrolo[1,2-a]indoles containing both enantioenriched spiro isoindolinone-indoline and atropisomeric naphthalenol frameworks.
Results and discussion
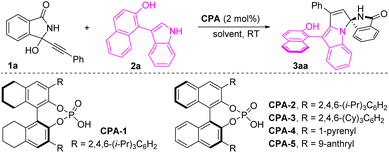
Initially, we optimized the reaction conditions with the model reaction between α-(3-isoindolinonyl) propargylic alcohol 1a and 2-(3-indolyl)naphthalenol 2a. Pleasingly, the desired product 3aa was obtained in 66% yield with 91% ee and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr from the CPA-1 catalyzed reaction (Table 1, entry 1). To improve the efficiency and selectivity, the screening of CPAs was carefully carried out (Table 1, entries 2–5). Encouragingly, using CPA-3 as the catalyst enabled the formation of product 3aa in 92% yield with 95% ee and 15
1 dr from the CPA-1 catalyzed reaction (Table 1, entry 1). To improve the efficiency and selectivity, the screening of CPAs was carefully carried out (Table 1, entries 2–5). Encouragingly, using CPA-3 as the catalyst enabled the formation of product 3aa in 92% yield with 95% ee and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 1, entry 3). With CPA-3 as the catalyst, the effect of reaction media was investigated (Table 1, entries 6–12) and dichloromethane (CH2Cl2) was identified as a suitable solvent. On decreasing the catalyst loading to 1 mol%, the desired product 3aa was obtained in 75% yield with 94% ee and 11
1 dr (Table 1, entry 3). With CPA-3 as the catalyst, the effect of reaction media was investigated (Table 1, entries 6–12) and dichloromethane (CH2Cl2) was identified as a suitable solvent. On decreasing the catalyst loading to 1 mol%, the desired product 3aa was obtained in 75% yield with 94% ee and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr after a prolonged reaction time (Table 1, entry 13).
1 dr after a prolonged reaction time (Table 1, entry 13).
| Entrya | CPA | Solvent | Yieldb [%] | eec [%] | drd |
|---|---|---|---|---|---|
| a Unless noted, a mixture of 1a (0.05 mmol), 2a (0.06 mmol) and CPA (2 mol%) in the solvent (0.3 mL) was stirred at room temperature (RT) for 16 h. b Isolated yield. c Determined by chiral HPLC analysis. d Determined by 1H NMR analysis. e CPA-3 (1 mol%), 36 h. | |||||
| 1 | CPA-1 | CH2Cl2 | 3aa, 66 | 91 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | CPA-2 | CH2Cl2 | 3aa, 74 | 91 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | CPA-3 | CH2Cl2 | 3aa, 92 | 95 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | CPA-4 | CH2Cl2 | 3aa, 80 | 34 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | CPA-5 | CH2Cl2 | 3aa, 60 | 80 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | CPA-3 | CHCl3 | 3aa, 67 | 84 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | CPA-3 | CCl4 | 3aa, 82 | 60 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | CPA-3 | ClCH2CH2Cl | 3aa, 88 | 89 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | CPA-3 | Toluene | 3aa, 66 | 80 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | CPA-3 | PhCl | 3aa, 89 | 80 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | CPA-3 | PhCF3 | 3aa, 75 | 70 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | CPA-3 | Xylenes | 3aa, 93 | 70 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13e | CPA-3 | CH2Cl2 | 3aa, 75 | 94 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
With the established optimal conditions, the substrate scope was then examined (Table 2). Generally, a series of propargylic alcohols with different residues (R1) 1a–m reacted smoothly with 2-(3-indolyl)naphthalenol 2a to furnish the corresponding products 3aa–ma in good to high yields (77–93%) with excellent diastereo- (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–>20
1–>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantioselectivities (94–97%). Importantly, various groups could be introduced into the aromatic ring of substituent R1 with a slight effect on the reaction. Neither a distinct electron effect nor a significant steric hindrance effect was observed (Table 2, entries 1–13). Heteroaromatic propargylic alcohol 1n (R1 = 2-thienyl) was also compatible to afford the desired product 3na in 85% yield with 95% ee and >20
1) and enantioselectivities (94–97%). Importantly, various groups could be introduced into the aromatic ring of substituent R1 with a slight effect on the reaction. Neither a distinct electron effect nor a significant steric hindrance effect was observed (Table 2, entries 1–13). Heteroaromatic propargylic alcohol 1n (R1 = 2-thienyl) was also compatible to afford the desired product 3na in 85% yield with 95% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 2, entry 14). Exceptionally, the aliphatic group (R1 = cyclopropyl) affected the stereoselectivity drastically, leading to the formation of racemic product 3oa in 83% yield (Table 2, entry 15). The absolute configuration of 3na was unambiguously confirmed by X-ray crystallography.17 The absolute configurations of other products 3 were assigned by analogy.
1 dr (Table 2, entry 14). Exceptionally, the aliphatic group (R1 = cyclopropyl) affected the stereoselectivity drastically, leading to the formation of racemic product 3oa in 83% yield (Table 2, entry 15). The absolute configuration of 3na was unambiguously confirmed by X-ray crystallography.17 The absolute configurations of other products 3 were assigned by analogy.
| Entrya | R1 | Yieldb [%] | eec [%] | drd |
|---|---|---|---|---|
| a Unless noted, 1 (0.05 mmol), 2a (0.06 mmol), and CPA-3 (2 mol%) in CH2Cl2 (0.3 mL) were stirred at RT for 16 h. b Isolated yields. c Determined by chiral HPLC analysis. d Determined by 1H NMR analysis. | ||||
| 1 | Ph | 3aa, 92 | 95 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 4-FC6H4 | 3ba, 92 | 95 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 4-ClC6H4 | 3ca, 88 | 96 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4d | 4-BrC6H4 | 3da, 80 | 97 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4-MeC6H4 | 3ea, 91 | 95 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 4-MeOC6H4 | 3fa, 83 | 96 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 4-t-BuC6H4 | 3ga, 93 | 95 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 4-PhC6H4 | 3ha, 83 | 97 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 3-ClC6H4 | 3ia, 85 | 97 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 3-BrC6H4 | 3ja, 85 | 94 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 3-MeC6H4 | 3ka, 81 | 96 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 2-ClC6H4 | 3la, 77 | 97 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 2-BrC6H4 | 3ma, 91 | 95 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 2-Thienyl | 3na, 85 | 95 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | Cyclopropyl | 3oa, 83 | 0 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The scope of 2-(3-indolyl)naphthalenols 2 was also examined by the CPA-3 catalyzed reaction of α-(3-isoindolinonyl) propargylic alcohol 1a. As shown in Table 3, all the probed 2-(3-indolyl)naphthalenols 2b–g reacted smoothly with propargylic alcohol 1a to furnish the corresponding pyrrolo[1,2-a]indoles 3ab–ag in 77–95% yields with 73–96% ee and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–>20
1–>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Notably, both electron-donating and electron-withdrawing substituents on the benzene ring (R2) had a slight effect on the excellent outcome. Taken together, organocatalytic (3 + 2)-annulation of propargylic alcohols with indolylnaphthalenols was developed for the asymmetric construction of 3-arylindole scaffolds with axial and central chirality.
1 dr. Notably, both electron-donating and electron-withdrawing substituents on the benzene ring (R2) had a slight effect on the excellent outcome. Taken together, organocatalytic (3 + 2)-annulation of propargylic alcohols with indolylnaphthalenols was developed for the asymmetric construction of 3-arylindole scaffolds with axial and central chirality.
| Entrya | R2 | Yieldb [%] | eec [%] | drd |
|---|---|---|---|---|
| a Unless noted, 1a (0.05 mmol), 2 (0.06 mmol), and CPA-3 (2 mol%) in CH2Cl2 (0.3 mL) were stirred at RT for 16 h. b Isolated yields. c Determined by chiral HPLC analysis. d Determined by 1H NMR analysis. | ||||
| 1 | 4-Cl | 3ab, 86 | 88 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 5-F | 3ac, 86 | 73 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 5-Me | 3ad, 93 | 96 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 6-F | 3ae, 77 | 96 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 6-Cl | 3af, 95 | 95 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 7-Me | 3ag, 80 | 80 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
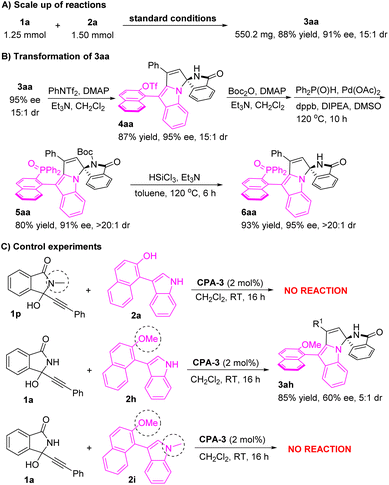
The scale-up of the reaction was surveyed to demonstrate the utility of the protocol. Under the standard conditions, the reaction on a scale of 1.25 mmol gave product 3aa in 88% yield with 91% ee and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 2A). The synthetic transformation of compound 3aa was also investigated (Scheme 2B). Esterification of the enantioenriched pyrrolo[1,2-a]indole 3aa with PhNTf2 afforded triflate 4aa in 87% yield without losing the stereoselectivity. The protection of amide with Boc2O was followed by a phosphorylation reaction to give phosphine oxide 5aa in 80% yield with 91% ee and >20
1 dr (Scheme 2A). The synthetic transformation of compound 3aa was also investigated (Scheme 2B). Esterification of the enantioenriched pyrrolo[1,2-a]indole 3aa with PhNTf2 afforded triflate 4aa in 87% yield without losing the stereoselectivity. The protection of amide with Boc2O was followed by a phosphorylation reaction to give phosphine oxide 5aa in 80% yield with 91% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. After treatment with HSiCl3, the Boc group was removed to afford product 6aa in 93% yield with 95% ee and >20
1 dr. After treatment with HSiCl3, the Boc group was removed to afford product 6aa in 93% yield with 95% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. To shed light on the reaction mechanism, several control experiments were carried out. As illustrated in Scheme 2C, propargylic alcohol 1p blocked the amide with the methyl group and failed to react with 2-(3-indolyl)naphthalenol 2a under the standard conditions, which indicated that the free amide residue played a key role in the catalytic process. When 3-(2-methoxynaphthalen-1-yl)-1H-indole 2h was employed to react with propargylic alcohol 1a, the desired product 3ah was obtained in 85% yield with 60% ee and 5
1 dr. To shed light on the reaction mechanism, several control experiments were carried out. As illustrated in Scheme 2C, propargylic alcohol 1p blocked the amide with the methyl group and failed to react with 2-(3-indolyl)naphthalenol 2a under the standard conditions, which indicated that the free amide residue played a key role in the catalytic process. When 3-(2-methoxynaphthalen-1-yl)-1H-indole 2h was employed to react with propargylic alcohol 1a, the desired product 3ah was obtained in 85% yield with 60% ee and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. These results disclosed that the hydroxyl group played an important role in controlling the stereoselectivity. Furthermore, no reaction occurred between propargylic alcohol 1a and 1-methyl-3-(2-methoxynaphthalen-1-yl)indole 2i, which indicated that the free amino of the indole ring also played a key role in controlling the efficiency.
1 dr. These results disclosed that the hydroxyl group played an important role in controlling the stereoselectivity. Furthermore, no reaction occurred between propargylic alcohol 1a and 1-methyl-3-(2-methoxynaphthalen-1-yl)indole 2i, which indicated that the free amino of the indole ring also played a key role in controlling the efficiency.
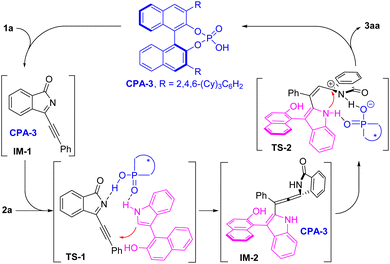
Based on these results, a proposed reaction mechanism is shown in Scheme 3. In the presence of CPA-3, propargylic alcohol 1a was dehydrated to give propargylic N-acylimine intermediate IM-1, followed by asymmetric 1,4-addition of 2-(3-indolyl)naphthalenol 2avia transient state TS-1 to form chiral allene intermediate IM-2. Protonation of allene IM-2 and subsequent enantioselective intramolecular annulation via transient state TS-2 furnished the desired product 3aa and re-generated catalyst CPA-3.
Conclusions
In conclusion, we have developed a CPA-catalyzed asymmetric reaction of α-(3-isoindolinonyl) propargylic alcohols with 1-(3-indolyl)naphthalen-2-ols. The protocol provided efficient access to a broad range of pyrrolo[1,2-a]indoles bearing both enantioenriched spiro isoindolinone-indoline and atropisomeric naphthalenol frameworks under mild conditions. Notably, this work further expanded the reaction scope of α-(3-isoindolinonyl) propargylic alcohols and enriched the chemistry of functionalized propargylic alcohols.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Natural Science Foundation of Shandong Province (ZR2021MB026), the Special Funds of the Taishan Scholar Program of Shandong Province (tsqn201812047), the Shenzhen Innovation of Science and Technology Commission (20200925151614002), and the Guangdong Provincial Key Laboratory of Catalysis (2020B121201002). The authors acknowledge the assistance of SUSTech Core Research Facilities, Xiaoyong Chang (X-ray) and Yang Yu (HRMS).References
- For selected reviews, see: (a) Y.-B. Wang and B. Tan, Construction of Axially Chiral Compounds via Asymmetric Organocatalysis, Acc. Chem. Res., 2018, 51, 534–547 CrossRef CAS PubMed; (b) B.-C. Da, S.-H. Xiang, S. Li and B. Tan, Chiral Phosphoric Acid Catalyzed Asymmetric Synthesis of Axially Chiral Compounds, Chin. J. Chem., 2021, 39, 1787–1796 CrossRef CAS; (c) J. K. Cheng, S.-H. Xiang, S. Li, L. Ye and B. Tan, Recent Advances in Catalytic Asymmetric Construction of Atropisomers, Chem. Rev., 2021, 121, 4805–4902 CrossRef CAS PubMed; (d) R. Song, Y. Xie, Z. Jin and Y. R. Chi, Carbene-Catalyzed Asymmetric Construction of Atropisomers, Angew. Chem., Int. Ed., 2021, 60, 26026–26037 CrossRef CAS; (e) J. K. Cheng, S.-H. Xiang and B. Tan, Organocatalytic Enantioselective Synthesis of Axially Chiral Molecules: Development of Strategies and Skeletons, Acc. Chem. Res., 2022, 55, 2920–2937 CrossRef CAS.
- (a) D. Bonne and J. Rodriguez, Enantioselective Synthesis of Atropisomers Featuring a Five-membered Ring, Chem. Commun., 2017, 53, 12385–12393 RSC; (b) T.-Z. Li, S.-J. Liu, W. Tan and F. Shi, Catalytic Asymmetric Construction of Axially Chiral Indole-Based Frameworks: An Emerging Area, Chem. – Eur. J., 2020, 26, 15779–15792 CrossRef CAS PubMed; (c) H.-H. Zhang and F. Shi, Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications, Acc. Chem. Res., 2022, 55, 2562–2580 CrossRef CAS PubMed.
- (a) W. Xia, Q.-J. An, S.-H. Xiang, S. Li, Y.-B. Wang and B. Tan, Chiral Phosphoric Acid Catalyzed Atroposelective C-H Amination of Arenes, Angew. Chem., Int. Ed., 2020, 59, 6775–6779 CrossRef CAS; (b) S. Huang, H. Wen, Y. Tian, P. Wang, W. Qin and H. Yan, Organocatalytic Enantioselective Construction of Chiral Azepine Skeleton Bearing Multiple-Stereogenic Elements, Angew. Chem., Int. Ed., 2021, 60, 21486–21493 CrossRef CAS.
- (a) H.-H. Zhang, C.-S. Wang, C. Li, G.-J. Mei, Y. Li and F. Shi, Design and Enantioselective Construction of Axially Chiral Naphthyl-Indole Skeletons, Angew. Chem., Int. Ed., 2017, 56, 116–121 CrossRef CAS PubMed; (b) L.-W. Qi, J.-H. Mao, J. Zhang and B. Tan, Organocatalytic Asymmetric Arylation of Indoles Enabled by Azo Groups, Nat. Chem., 2018, 10, 58–64 CrossRef CAS; (c) S. Lu, J.-Y. Ong, H. Yang, S. B. Poh, X. Liew, C. S. D. Seow, M. W. Wong and Y. Zhao, Diastereo- and Atroposelective Synthesis of Bridged Biaryls Bearing an Eight-Membered Lactone through an Organocatalytic Cascade, J. Am. Chem. Soc., 2019, 141, 17062–17067 CrossRef CAS PubMed; (d) S. Zhu, Y.-H. Chen, Y.-B. Wang, P. Yu, S.-Y. Li, S.-H. Xiang, J.-Q. Wang, J. Xiao and B. Tan, Organocatalytic Atroposelective Construction of Axially Chiral Arylquinones, Nat. Commun., 2019, 10, 4268 CrossRef; (e) G. D. Bisag, D. Pecorari, A. Mazzanti, L. Bernardi, M. Fochi, G. Bencivenni, G. Bertuzzi and V. Corti, Central-to-Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole-Quinoline Atropisomers, Chem. – Eur. J., 2019, 25, 15694–15701 CrossRef CAS; (f) Y.-H. Chen, H.-H. Li, X. Zhang, S.-H. Xiang, S. Li and B. Tan, Organocatalytic Enantioselective Synthesis of Atropisomeric Aryl-p-Quinones: Platform Molecules for Diversity-Oriented Synthesis of Biaryldiols, Angew. Chem., Int. Ed., 2020, 59, 11374–11378 CrossRef CAS; (g) W.-Y. Ding, P. Yu, Q.-J. An, K. L. Bay, S.-H. Xiang, S. Li, Y. Chen, K. N. Houk and B. Tan, DFT-Guided Phosphoric-Acid-Catalyzed Atroposelective Arene Functionalization of Nitrosonaphthalene, Chem, 2020, 6, 2046–2059 CrossRef CAS.
- (a) Y.-L. Hu, Z. Wang, H. Yang, J. Chen, Z.-B. Wu, Y. Lei and L. Zhou, Conversion of Two Stereocenters to One or Two Chiral Axes: Atroposelective Synthesis of 2,3-Diarylbenzoindoles, Chem. Sci., 2019, 10, 6777–6784 RSC; (b) L. Peng, K. Li, C. Xie, S. Li, D. Xu, W. Qin and H. Yan, Organocatalytic Asymmetric Annulation of ortho-Alkynylanilines: Synthesis of Axially Chiral Naphthyl-C2-indoles, Angew. Chem., Int. Ed., 2019, 58, 17199–17204 CrossRef CAS.
- (a) C. Ma, F. Jiang, F.-T. Sheng, Y. Jiao, G.-J. Mei and F. Shi, Design and Catalytic Asymmetric Construction of Axially Chiral 3,3′-Bisindole Skeletons, Angew. Chem., Int. Ed., 2019, 58, 3014–3020 CrossRef CAS PubMed; (b) F.-T. Sheng, Z.-M. Li, Y.-Z. Zhang, L.-X. Sun, Y.-C. Zhang, W. Tan and F. Shi, Atroposelective Synthesis of 3,3′-Bisindoles Bearing Axial and Central Chirality: Using Isatin-Derived Imines as Electrophiles, Chin. J. Chem., 2020, 38, 583–589 CrossRef CAS; (c) K.-W. Chen, Z.-S. Wang, P. Wu, X.-Y. Yan, S. Zhang, Y.-C. Zhang and F. Shi, Catalytic Asymmetric Synthesis of 3,3′-Bisindoles Bearing Single Axial Chirality, J. Org. Chem., 2020, 85, 10152–10166 CrossRef CAS; (d) F.-T. Sheng, S. Yang, S.-F. Wu, Y.-C. Zhang and F. Shi, Catalytic Asymmetric Synthesis of Axially Chiral 3,3′-Bisindoles by Direct Coupling of Indole Rings, Chin. J. Chem., 2022, 40, 2151–2160 CrossRef CAS.
- (a) C.-S. Wang, T.-Z. Li, S.-J. Liu, Y.-C. Zhang, S. Deng, Y. Jiao and F. Shi, Axially Chiral Aryl-Alkene-Indole Framework: A Nascent Member of the Atropisomeric Family and Its Catalytic Asymmetric Construction, Chin. J. Chem., 2020, 38, 543–552 CrossRef CAS; (b) J.-Y. Wang, M. Sun, X.-Y. Yu, Y.-C. Zhang, W. Tan and F. Shi, Atroposelective Construction of Axially Chiral Alkene-Indole Scaffolds via Catalytic Enantioselective Addition Reaction of 3-Alkynyl-2-indolylmethanols, Chin. J. Chem., 2021, 39, 2163–2171 CrossRef CAS; (c) Q.-Q. Hang, S.-F. Wu, S. Yang, X. Wang, Z. Zhong, Y.-C. Zhang and F. Shi, Design and Catalytic Atroposelective Synthesis of Axially Chiral Isochromenone-indoles, Sci. China: Chem., 2022, 65, 1929–1937 CrossRef CAS.
- F. Jiang, K.-W. Chen, P. Wu, Y.-C. Zhang, Y. Jiao and F. Shi, A Strategy for Synthesizing Axially Chiral Naphthyl-Indoles: Catalytic Asymmetric Addition Reactions of Racemic Substrates, Angew. Chem., Int. Ed., 2019, 58, 15104–15110 CrossRef CAS.
- X.-F. Bai, Y.-M. Cui, J. Cao and L.-W. Xu, Atropisomers with Axial and Point Chirality: Synthesis and Applications, Acc. Chem. Res., 2022, 55, 2545–2561 CrossRef CAS PubMed.
- (a) M. Ogasawara, Catalytic Enantioselective Synthesis of Axially Chiral Allenes, Tetrahedron: Asymmetry, 2009, 20, 259–271 CrossRef CAS; (b) J. Ye and S. Ma, Conquering Three-carbon Axial Chirality of Allenes, Org. Chem. Front., 2014, 1, 1210–1224 RSC; (c) R. K. Neff and D. E. Frantz, Recent Advances in the Catalytic Syntheses of Allenes: A Critical Assessment, ACS Catal., 2014, 4, 519–528 CrossRef CAS; (d) Q. H. Li, X. Jiang, K. Wu, R. Q. Luo, M. Liang, Z. H. Zhang and Z. Y. Huang, Research Progress on the Catalytic Enantioselective Synthesis of Axially Chiral Allenes by Chiral Organocatalys, Curr. Org. Chem., 2020, 24, 694–708 CrossRef CAS; (e) X. Wang, X. Chen, W. Lin, P. Li and W. Li, Recent Advances in Organocatalytic Enantioselective Synthesis of Axially Chiral Allenes, Adv. Synth. Catal., 2022, 364, 1212–1222 CrossRef CAS.
- (a) D. Qian, L. Wu, Z. Lin and J. Sun, Organocatalytic Synthesis of Chiral Tetrasubstituted Allenes from Racemic Propargylic Alcohols, Nat. Commun., 2017, 8, 567–575 CrossRef PubMed; (b) P. Zhang, Q. Huang, Y. Cheng, R. Li, P. Li and W. Li, Remote Stereocontrolled Construction of Vicinal Axially Chiral Tetrasubstituted Allenes and Heteroatom-Functionalized Quaternary Carbon Stereocenters, Org. Lett., 2019, 21, 503–507 CrossRef CAS PubMed.
- (a) L. Zhang, Y. Han, A. Huang, P. Zhang, P. Li and W. Li, Organocatalytic Remote Stereocontrolled 1,8-Additions of Thiazolones to Propargylic Aza-p-quinone Methides, Org. Lett., 2019, 21, 7415–7419 CrossRef CAS PubMed; (b) M. Chen, D. Qian and J. Sun, Organocatalytic Enantioconvergent Synthesis of Tetrasubstituted Allenes via Asymmetric 1,8-Addition to aza-para-Quinone Methides, Org. Lett., 2019, 21, 8127–8131 CrossRef CAS PubMed.
- (a) X. Li and J. Sun, Organocatalytic Enantioselective Synthesis of Chiral Allenes: Remote Asymmetric 1,8-Addition of Indole Imine Methides, Angew. Chem., Int. Ed., 2020, 59, 17049–17054 CrossRef CAS; (b) W.-R. Zhu, Q. Su, H.-J. Diao, E.-X. Wang, F. Wu, Y.-L. Zhao, J. Weng and G. Lu, Enantioselective Dehydrative γ-Arylation of α-Indolyl Propargylic Alcohols with Phenols: Access to Chiral Tetrasubstituted Allenes and Naphthopyrans, Org. Lett., 2020, 22, 6873–6878 CrossRef CAS PubMed; (c) Z. Wang, X. Lin, X. Chen, P. Li and W. Li, Organocatalytic Stereoselective 1,6-Addition of Thiolacetic Acids to Alkynyl Indole Imine Methides: Access to Axially Chiral Sulfur-containing Tetrasubstituted Allenes, Org. Chem. Front., 2021, 8, 3469–3474 RSC; (d) Y. Wu, Z. Yue, C. Qian, X. Chen, F. Li, P. Li and W. Li, Organocatalytic Enantioselective Construction of Axially Chiral Tetrasubstituted Allenes via 1,6-Addition of Alkynyl Indole Imine Methides with 2-Substituted Indoles, Asian J. Org. Chem., 2022, 11, e202100724 CAS; (e) X. Lin, B. Shen, Z. Wang, Y. Cheng, X. Chen, P. Li, P. Yu and W. Li, Organocatalytic Enantioselective 1,10-Addition of Alkynyl Indole Imine Methides with Thiazolones: An Access to Axially Chiral Tetrasubstituted Allenes, Org. Lett., 2022, 24, 4914–4918 CrossRef CAS PubMed; (f) Z. Wang, Y. Cheng, Z. Yue, X. Chen, P. Li and W. Li, Organocatalytic Asymmetric 3-Allenylation of Indoles via Remote Stereocontrolled 1,10-Additions of Alkynyl Indole Imine Methides, Asian J. Org. Chem., 2022, 11, e202200399 CAS.
- (a) X. Yuan, X. Wu, F. Peng, H. Yang, C. Zhu and H. Fu, Organocatalytic Asymmetric Synthesis of Arylindolyl Indolin-3-ones with Both Axial and Central Chirality, Chem. Commun., 2020, 56, 12648–12651 RSC; (b) P. Wu, L. Yu, C.-H. Gao, Q. Cheng, S. Deng, Y. Jiao, W. Tan and F. Shi, Design and Synthesis of Axially Chiral Aryl-pyrroloindoles via the Strategy of Organocatalytic Asymmetric (2 + 3) Cyclization, Fundam. Res., 2022 DOI:10.1016/j.fmre.2022.01.002.
- (a) C. Qian, M. Liu, J. Sun and P. Li, Chiral Phosphoric Acid-catalyzed Regio- and Enantioselective Reactions of Functionalized Propargylic Alcohols, Org. Chem. Front., 2022, 9, 1234–1240 RSC; (b) R. A. Unhale, M. M. Sadhu and V. K. Singh, Chiral Brønsted Acid Catalyzed Enantioselective Synthesis of Spiro-Isoindolinone-Indolines via Formal [3 + 2] Cycloaddition, Org. Lett., 2022, 24, 3319–3324 CrossRef CAS PubMed.
- (a) J. Yang, Z. Wang, Z. He, G. Li, L. Hong, W. Sun and R. Wang, Organocatalytic Enantioselective Synthesis of Tetrasubstituted α-Amino Allenoates by Dearomative γ-Addition of 2,3-Disubstituted Indoles to β, γ-Alkynyl-α-imino Esters, Angew. Chem., Int. Ed., 2020, 59, 642–647 CrossRef CAS; (b) F. Li, S. Liang, Y. Luan, X. Chen, H. Zhao, A. Huang, P. Li and W. Li, Organocatalytic Regio-, Diastereo- and Enantioselective γ-Additions of Isoxazol-5(4H)-ones to β,γ-Alkynyl-α-imino Esters for the Synthesis of Axially Chiral Tetrasubstituted α-Amino Allenoates, Org. Chem. Front., 2021, 8, 1243–1248 RSC; (c) M. Liu, C. Qian and P. Li, Organocatalytic Regio- and Enantioselective N-Alkylation of Isoxazol-5-ones, Eur. J. Org. Chem., 2021, e202101415 Search PubMed.
- CCDC 2194465 (3na).
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and spectroscopic data. CCDC 2194465. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qo01625g |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |