Open Access Article
Open Access ArticleThe impact of solution vs. slurry vs. mechanochemical syntheses upon the sorption performance of a 2D switching coordination network†
Shi-Qiang
Wang‡
 *ab,
Shaza
Darwish‡
*ab,
Shaza
Darwish‡
 a and
Michael J.
Zaworotko
a and
Michael J.
Zaworotko
 *a
*a
aBernal Institute, Department of Chemical Sciences, University of Limerick, Limerick V94 T9PX, Republic of Ireland. E-mail: xtal@ul.ie
bInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way 138634, Singapore. E-mail: wangsq@imre.a-star.edu.sg
First published on 19th April 2023
Abstract
The selection and optimization of synthesis routes for porous metal–organic materials are critical for their large-scale manufacture but remain largely underexplored. In this study, we compare mechanochemistry vs. slurry vs. solution methods for the synthesis of a 1D chain coordination polymer {[Co(bpy)(NCS)2(H2O)2]·bpy}n (chn-1-Co-NCS-H2O) that is an intermediate to the 2D switching coordination network [Co(bpy)2(NCS)2]n, sql-1-Co-NCS (1 = bpy = 4,4′-bipyridine). Although neat mechanosynthesis using Co(NCS)2 and bpy as the starting materials failed, both water slurry and water-assisted mechanochemical syntheses afforded the desired intermediate, chn-1-Co-NCS-H2O, in high yield. Nevertheless, the resulting sql-1-Co-NCS products were observed to exhibit different CO2 sorption profiles depending on the synthesis methods used to prepare chn-1-Co-NCS-H2O. This study reveals that water can play an important role in mechanosynthesis, not only by inducing and accelerating the reaction process, but also by enhancing product quality in a manner that is not readily detectable by PXRD.
Introduction
Switching coordination networks (CNs) are a subclass of the third generation coordination polymers that undergo guest-induced structural transformation(s) between “closed” nonporous and “open” porous phases.1–3 They represent a relatively small but potentially important and steadily growing type of flexible metal–organic frameworks (FMOFs) or soft porous crystals (SPCs).4–9 The characteristic features of switching CNs are their responsiveness to specific guest species and the resulting stepped or type F-IV sorption isotherms, which could enhance the working capacity and selectivity for gas storage and separation applications.10–13 It is widely recognized that CNs or MOFs have the potential to compete with conventional sorbents such as zeolites and activated carbons,14–18 however, the promise of large-scale applications is largely unrealized despite >118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MOF entries having been archived in the Cambridge Structural Database.19 A major issue that hinders the further development of CNs as industrial sorbents is their amenability to synthesis at large scale. This is perhaps the biggest hurdle between laboratory bench discovery, where solution synthesis has dominated, and commercial development, where the cost and efficiency of process methodology become paramount.20–22
000 MOF entries having been archived in the Cambridge Structural Database.19 A major issue that hinders the further development of CNs as industrial sorbents is their amenability to synthesis at large scale. This is perhaps the biggest hurdle between laboratory bench discovery, where solution synthesis has dominated, and commercial development, where the cost and efficiency of process methodology become paramount.20–22
With respect to the discovery of CNs, solvent mediated methods such as solvent diffusion (SD) and solvothermal (ST) methods are commonly used for mg and g scale synthesis. They offer an advantage of tending to result in single crystals, which enables determination of crystal structures via single crystal X-ray diffraction. Nevertheless, with respect to process development, more efficient approaches are desired for scale up of targeted CNs since SD and ST methods are generally low-yield, high-waste and time-consuming.22 Recently, mechanochemical synthesis methods such as ball milling (BM), a batch method, or twin-screw extrusion (TSE), a continuous method, have emerged as candidates for large scale synthesis of CNs, as well as alloys, oxides, halides, cocrystals, polymers, and composites.23,24 BM and TSE offer the potential of high space–time yield, low-waste processes and can thereby address the inefficiencies of solvent-based methods. Further, IUPAC recently highlighted mechanochemistry as one of “the top ten technologies to change the world”.25 However, the mantra that “the best solvent is no solvent” does not necessarily apply to water,26–28 especially water slurry (WS), which could be suitable for large-scale manufacture of water-stable, water-insoluble CNs.29–32
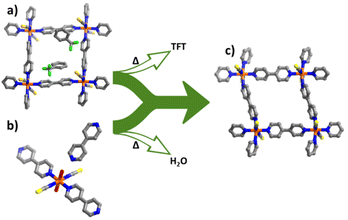
Recently, we reported that the previously known square lattice (sql) CN,33,34 [Co(bpy)2(NCS)2]n (bpy = 4,4′-bipyridine), sql-1-Co-NCS, exhibits recyclable switching between its “closed” nonporous and “open” porous phases induced by CO2 or C8 aromatics.35,36 We coined the term “switching adsorbent layered materials” (SALMAs) for such sorbents and they hold promise for gas storage and hydrocarbon separations,36–42 prompting us to explore the feasibility of their large-scale manufacture. In our previous work,35 we prepared sql-1-Co-NCS by applying heat under vacuum to its guest-loaded variant sql-1-Co-NCS·2TFT (TFT = α,α,α-trifluorotoluene), which was synthesized by self-assembly of Co(NCS)2 and bpy using the SD method in the presence of organic solvents (Fig. 1a and c and S1a†). This procedure generally takes 1–2 weeks to grow crystals and results in significant solvent waste (ethanol and TFT). On the other hand, it was found that the 1D chain coordination polymer {[Co(bpy)(NCS)2(H2O)2]·bpy}n (chn-1-Co-NCS-H2O) has the requisite stoichiometry to serve as an intermediate for preparing sql-1-Co-NCS simply by heating (Fig. 1b and S2†).33,34 A similar phenomenon was also reported for {[Cu(bpy)(BF4)2(H2O)2]·bpy}n (preELM-11) and [Cu(bpy)2(BF4)2(H2O)2]n (ELM-11).43 Whereas the structural transformation from sql-1-Co-NCS·2TFT to sql-1-Co-NCS involves noncovalent bonds only and chemical bonds are involved in activation of chn-1-Co-NCS-H2O, the conditions required by the two routes are, perhaps surprisingly, comparable (i.e. 50 °C under vacuum for several hours).
With the above synthesis routes in hand, we wondered if mechanochemistry would be feasible for direct preparation of sql-1-Co-NCS or its intermediate chn-1-Co-NCS-H2O, since we and others have been investigating the use of mechanochemical synthesis to prepare coordination polymers and related materials.44–50 In particular, we have previously reported that reaction of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of bpy and hydrated metal nitrates via BM and TSE afforded four 1D chain coordination polymers.44 Herein, we compare the effectiveness of mechanochemical synthesis for preparation of chn-1-Co-NCS-H2O with solution and slurry methods. We also assess if the sorption properties of sql-1-Co-NCS are impacted by the synthesis methods used to prepare its intermediate, chn-1-Co-NCS-H2O.
1 molar ratio of bpy and hydrated metal nitrates via BM and TSE afforded four 1D chain coordination polymers.44 Herein, we compare the effectiveness of mechanochemical synthesis for preparation of chn-1-Co-NCS-H2O with solution and slurry methods. We also assess if the sorption properties of sql-1-Co-NCS are impacted by the synthesis methods used to prepare its intermediate, chn-1-Co-NCS-H2O.
Results and discussion
With respect to the direct mechanosynthesis of sql-1-Co-NCS, we first investigated neat BM experiments with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of Co(NCS)2 and bpy, consistent with the chemical formula of sql-1-Co-NCS (Fig. S1b†). PXRD revealed that the reaction between Co(NCS)2 and bpy did not occur during 5–30 min of BM, and the starting materials became amorphous with color change after 1–2 h of BM (Fig. S3 and S4†). We anticipated that the presence of water in the reaction medium might be important, as exemplified by success in previous reports.51–55 We then conducted mechanochemical synthesis of chn-1-Co-NCS-H2O by adding aliquots of water to the BM experiments. It was observed that such water-assisted BM reactions afforded chn-1-Co-NCS-H2O and the amount of water used was found to be a key parameter (Fig. 2). When the M
2 molar ratio of Co(NCS)2 and bpy, consistent with the chemical formula of sql-1-Co-NCS (Fig. S1b†). PXRD revealed that the reaction between Co(NCS)2 and bpy did not occur during 5–30 min of BM, and the starting materials became amorphous with color change after 1–2 h of BM (Fig. S3 and S4†). We anticipated that the presence of water in the reaction medium might be important, as exemplified by success in previous reports.51–55 We then conducted mechanochemical synthesis of chn-1-Co-NCS-H2O by adding aliquots of water to the BM experiments. It was observed that such water-assisted BM reactions afforded chn-1-Co-NCS-H2O and the amount of water used was found to be a key parameter (Fig. 2). When the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O corresponded to its chemical formula (i.e. 1
H2O corresponded to its chemical formula (i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Fig. S1c†), the formation of chn-1-Co-NCS-H2O was not achieved after 5 min of BM. Increasing the M
2, Fig. S1c†), the formation of chn-1-Co-NCS-H2O was not achieved after 5 min of BM. Increasing the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio to 1
H2O ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was also unsuccessful. These results suggested to us that more water might be needed to accelerate the reaction. Indeed, pure samples of chn-1-Co-NCS-H2O were ultimately obtained when M
3 was also unsuccessful. These results suggested to us that more water might be needed to accelerate the reaction. Indeed, pure samples of chn-1-Co-NCS-H2O were ultimately obtained when M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O is 1
H2O is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 or higher, although ratios of 1
4 or higher, although ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and above resulted in solids that were caked.
8 and above resulted in solids that were caked.
We next investigated the preparation of chn-1-Co-NCS-H2O by TSE. Neat TSE resulted in a physical mixture of starting materials, consistent with the neat BM results. For water-assisted TSE (Fig. S5†), we added water to the starting materials by using an automatic syringe pump with variable water flow rates (Table S1†). The parameters of feed rate and screw speed were empirically optimized at 50 g h−1 and 50 rpm, respectively. PXRD studies revealed that chn-1-Co-NCS-H2O was successfully formed with 18–26 mL h−1 of water flow rate (Fig. S6†) while overwetting occurred when water flow reached 26 mL h−1 or above (Fig. S7†).
That water plays a critical role in preparing chn-1-Co-NCS-H2O using BM and TSE prompted us to consider the WS method and chn-1-Co-NCS-H2O was indeed obtained by stirring a slurry of Co(NCS)2 (1 mmol, 175 mg) and bpy (2 mmol, 312 mg) in water (10 mL) for 3 h (Fig. 3a and S8a†). This finding contradicts our previous report on chn-1-M-NO3 materials, in which WS resulted in different phases to those obtained through BM and TSE methods.44 In addition, 10× scale-up of WS afforded almost 5 g of chn-1-Co-NCS-H2O in ca. 95% yield, which is a more efficient outcome than slurry in ethanol conducted for 3 days.34 We also prepared single-crystals of chn-1-Co-NCS-H2O by SD method using water and ethanol as the solvents,33 but it took 2 weeks to obtain reasonable yield of product (Fig. S8b†). chn-1-Co-NCS-H2O is therefore accessible by four methods: BM, TSE, WS and SD. SEM images of the BM and WS products are presented in Fig. 3. In general, sample morphologies may vary due to the different synthesis methods and conditions. The particle sizes of BM products were observed to be in the range of 200–500 nm and the primary particles further agglomerate to form aggregates with a wide diameter range from 10 to 200 μm (Fig. S9a–e†). The particle shapes of BM products appeared to have no regular facets (ellipsoid or sub-rounded), while the WS sample was observed to be flatter (plate-like) and formed agglomerates in a narrower diameter range of 5 to 50 μm (Fig. S9f†).
 | ||
| Fig. 3 SEM images of chn-1-Co-NCS-H2O for (a) BM1206, (b) BM1208, (c) BM1210, (d) BM1212, (e) BM1215 and (f) WS product. | ||
The SD, WS, BM and TSE products were found to exhibit matching PXRD patterns with the calculated PXRD of chn-1-Co-NCS-H2O (Fig. 4a). The mechanochemically produced samples (e.g., BM128 and TSE3, M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O are 1
H2O are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, respectively) were observed to exhibit broader PXRD peaks than those produced by SD or WS. The TGA thermograms also exhibit differences between the samples prepared by BM/TSE and SD/WS (Fig. 4b). TGA of chn-1-Co-NCS-H2O produced by SD/WS revealed 6.9 wt% loss before 100 °C, corresponding to loss of two aqua ligands per formula unit (calc. 6.9 wt%) and the formation of sql-1-Co-NCS, while BM/TSE products did not fully transform until 120 °C. This could be attributable to the compacted nature and agglomeration of the BM and TSE particles.
12, respectively) were observed to exhibit broader PXRD peaks than those produced by SD or WS. The TGA thermograms also exhibit differences between the samples prepared by BM/TSE and SD/WS (Fig. 4b). TGA of chn-1-Co-NCS-H2O produced by SD/WS revealed 6.9 wt% loss before 100 °C, corresponding to loss of two aqua ligands per formula unit (calc. 6.9 wt%) and the formation of sql-1-Co-NCS, while BM/TSE products did not fully transform until 120 °C. This could be attributable to the compacted nature and agglomeration of the BM and TSE particles.
In order to determine if the intermediate chn-1-Co-NCS-H2O prepared from different methods and conditions could impact the gas sorption properties of sql-1-Co-NCS, 195 K CO2 sorption isotherms were collected on each sample after activation at 50 °C in vacuo for 10 h (Fig. 5). For the SD sample, it exhibited the expected switching isotherm with a steep adsorption step at 10 kPa as observed in our previous study.35 The WS sample exhibited almost the same CO2 uptake (138 cm3 g−1) but it showed a more gradual adsorption curve after the sharp gate opening. As a result, the WS sample did not reach full CO2 uptake until ca. 30 kPa. Notably, some mechanochemical samples (e.g., BM126, BM128 and TSE3) showed not only even more gradual sorption curves, but also lower CO2 uptakes (ca. 115–123 cm3 g−1). This could be attributable to differences in crystallinity and/or particle size.56–58 It can be addressed by adding larger water aliquots into the mechanosynthesis (e.g., BM1210). It is reasonable to assert that stress caused by mechanical forces may affect the crystal quality of BM and TSE samples and may also induce some defects and mechanical damage in the coordination networks. This may require in situ monitoring techniques for more in-depth insights.59–61
Conclusions
Whereas it is recognized that the profiles (e.g., shape and uptake) of gas sorption isotherms can vary to some extent for the same CNs prepared by different methods or the same methods under different conditions,61–63 it is reasonable to note that flexible/switching CNs such as sql-1-Co-NCS might be more sensitive than rigid CNs to the particle size and morphology.56–58 In this study, milder conditions and methods such as WS were found to retain more comparable sorption performance to their single-crystal counterparts. In addition, this work suggests that it is unsuitable to estimate product quality of sorbents just based on PXRD and/or TGA. Rather, gas sorption testing offered a more quantitative approach to estimate the overall quality of products obtained. Further, we found that neat BM and TSE methods were ineffective whereas water-assisted mechanosynthesis required an appropriate amount of water otherwise CO2 sorption performance was compromised. Considering elapsed time, ease of operation, yield and waste, WS is at least in this case a promising alternative, apart from mechanosynthesis, for the scale-up preparation of chn-1-Co-NCS-H2O and its analogue materials. Indeed, the Fe and Ni variants of chn-1-Co-NCS-H2O were also prepared even using three ingredients (FeSO4/NiSO4, NaSCN, and bpy) via the WS method.39 Further studies to explore WS and mechanochemical methods for other CN systems are in progress.Author contributions
Shi-Qiang Wang: methodology, investigation, formal analysis, writing – original draft; Shaza Darwish: methodology, investigation, writing – review & editing; Michael J. Zaworotko: supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the support of Science Foundation Ireland (16/IA/4624) and the Irish Research Council (IRCLA/2019/167).References
- S.-Q. Wang, S. Mukherjee and M. J. Zaworotko, Spiers Memorial Lecture: Coordination networks that switch between nonporous and porous structures: an emerging class of soft porous crystals, Faraday Discuss., 2021, 231, 9–50 RSC.
- S. Kitagawa and M. Kondo, Functional micropore chemistry of crystalline metal complex-assembled compounds, Bull. Chem. Soc. Jpn., 1998, 71, 1739–1753 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. I. Noro, Functional porous coordination polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- K. Uemura, R. Matsuda and S. Kitagawa, Flexible microporous coordination polymers, J. Solid State Chem., 2005, 178, 2420–2429 CrossRef CAS.
- S. Horike, S. Shimomura and S. Kitagawa, Soft porous crystals, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Flexible metal-organic frameworks, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- Z. Chang, D.-H. Yang, J. Xu, T.-L. Hu and X.-H. Bu, Flexible Metal-Organic Frameworks: Recent Advances and Potential Applications, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- J. H. Lee, S. Jeoung, Y. G. Chung and H. R. Moon, Elucidation of flexible metal-organic frameworks: Research progresses and recent developments, Coord. Chem. Rev., 2019, 389, 161–188 CrossRef CAS.
- S. B. Peh, A. Karmakar and D. Zhao, Multiscale Design of Flexible Metal–Organic Frameworks, Trends Chem., 2020, 2, 199–213 CrossRef CAS.
- F. Bigdeli, C. T. Lollar, A. Morsali and H.-C. Zhou, Switching in Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2020, 59, 4652–4669 CrossRef CAS PubMed.
- B. Qian, Z. Chang and X. H. Bu, Functionalized Dynamic Metal-Organic Frameworks as Smart Switches for Sensing and Adsorption Applications, Top. Curr. Chem., 2019, 378, 5 CrossRef PubMed.
- G. Férey and C. Serre, Large breathing effects in three-dimensional porous hybrid matter: facts, analyses, rules and consequences, Chem. Soc. Rev., 2009, 38, 1380–1399 RSC.
- S. Bureekaew, S. Shimomura and S. Kitagawa, Chemistry and application of flexible porous coordination polymers, Sci. Technol. Adv. Mater., 2008, 9, 014108 CrossRef PubMed.
- A. U. Czaja, N. Trukhan and U. Müller, Industrial applications of metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341, 974 CrossRef CAS PubMed.
- P. Silva, S. M. F. Vilela, J. P. C. Tomé and F. A. Almeida Paz, Multifunctional metal–organic frameworks: from academia to industrial applications, Chem. Soc. Rev., 2015, 44, 6774–6803 RSC.
- Z. Chen, M. C. Wasson, R. J. Drout, L. Robison, K. B. Idrees, J. G. Knapp, F. A. Son, X. Zhang, W. Hierse, C. Kühn, S. Marx, B. Hernandez and O. K. Farha, The state of the field: from inception to commercialization of metal–organic frameworks, Faraday Discuss., 2021, 225, 9–69 RSC.
- D. Liu, J.-P. Lang and B. F. Abrahams, Highly Efficient Separation of a Solid Mixture of Naphthalene and Anthracene by a Reusable Porous Metal–Organic Framework through a Single-Crystal-to-Single-Crystal Transformation, J. Am. Chem. Soc., 2011, 133, 11042–11045 CrossRef CAS PubMed.
- P. Z. Moghadam, A. Li, S. B. Wiggin, A. Tao, A. G. Maloney, P. A. Wood, S. C. Ward and D. Fairen-Jimenez, Development of a Cambridge Structural Database subset: a collection of metal–organic frameworks for past, present, and future, Chem. Mater., 2017, 29, 2618–2625 CrossRef CAS.
- M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, New synthetic routes towards MOF production at scale, Chem. Soc. Rev., 2017, 46, 3453–3480 RSC.
- J. Ren, X. Dyosiba, N. M. Musyoka, H. W. Langmi, M. Mathe and S. Liao, Review on the current practices and efforts towards pilot-scale production of metal-organic frameworks (MOFs), Coord. Chem. Rev., 2017, 352, 187–219 CrossRef CAS.
- N. Stock and S. Biswas, Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. Harris, G. Hyett and W. Jones, Mechanochemistry: opportunities for new and cleaner synthesis, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- D. E. Crawford and J. Casaban, Recent Developments in Mechanochemical Materials Synthesis by Extrusion, Adv. Mater., 2016, 28, 5747–5754 CrossRef CAS PubMed.
- F. Gomollón-Bel, Ten Chemical Innovations That Will Change Our World: IUPAC identifies emerging technologies in Chemistry with potential to make our planet more sustainable, Chem. Int., 2019, 41, 12–17 Search PubMed.
- R. A. Sheldon, Green solvents for sustainable organic synthesis: state of the art, Green Chem., 2005, 7, 267–278 RSC.
- M. Gaab, N. Trukhan, S. Maurer, R. Gummaraju and U. Müller, The progression of Al-based metal-organic frameworks–From academic research to industrial production and applications, Microporous Mesoporous Mater., 2012, 157, 131–136 CrossRef CAS.
- H. Reinsch, “Green” Synthesis of Metal-Organic Frameworks, Eur. J. Inorg. Chem., 2016, 2016, 4290–4299 CrossRef CAS.
- M. Sanchez-Sanchez, N. Getachew, K. Diaz, M. Diaz-Garcia, Y. Chebude and I. Diaz, Synthesis of metal-organic frameworks in water at room temperature: salts as linker sources, Green Chem., 2015, 17, 1500–1509 RSC.
- K. Guesh, C. A. D. Caiuby, Á. Mayoral, M. Díaz-García, I. Díaz and M. Sanchez-Sanchez, Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water, Cryst. Growth Des., 2017, 17, 1806–1813 CrossRef CAS.
- C. Avci-Camur, J. Perez-Carvajal, I. Imaz and D. Maspoch, Metal Acetylacetonates as a Source of Metals for Aqueous Synthesis of Metal–Organic Frameworks, ACS Sustainable Chem. Eng., 2018, 6, 14554–14560 CrossRef CAS.
- S. Dai, F. Nouar, S. Zhang, A. Tissot and C. Serre, One-Step Room-Temperature Synthesis of Metal(IV) Carboxylate Metal-Organic Frameworks, Angew. Chem., Int. Ed., 2021, 60, 4282–4288 CrossRef CAS PubMed.
- J. Lu, T. Paliwala, S. C. Lim, C. Yu, T. Y. Niu and A. J. Jacobson, Coordination polymers of Co(NCS)2 with pyrazine and 4,4′-bipyridine: Syntheses and structures, Inorg. Chem., 1997, 36, 923–929 CrossRef CAS.
- M. Wriedt and C. Näther, Preparation of New Ligand-Deficient Thiocyanato Compounds with Cooperative Magnetic Phenomena by Thermal Decomposition of Their Ligand-Rich Precursors, Eur. J. Inorg. Chem., 2010, 2010, 3201–3211 CrossRef.
- S.-Q. Wang, Q.-Y. Yang, S. Mukherjee, D. O'Nolan, E. Patyk-Kazmierczak, K.-J. Chen, M. Shivanna, C. Murray, C. C. Tang and M. J. Zaworotko, Recyclable switching between nonporous and porous phases of a square lattice (sql) topology coordination network, Chem. Commun., 2018, 54, 7042–7045 RSC.
- S.-Q. Wang, S. Mukherjee, E. Patyk-Kaźmierczak, S. Darwish, A. Bajpai, Q.-Y. Yang and M. J. Zaworotko, Highly Selective, High-Capacity Separation of o-Xylene from C8 Aromatics by a Switching Adsorbent Layered Material, Angew. Chem., Int. Ed., 2019, 58, 6630–6634 CrossRef CAS PubMed.
- N. Kumar, S.-Q. Wang, S. Mukherjee, A. A. Bezrukov, E. Patyk-Kaźmierczak, D. O'Nolan, A. Kumar, M.-H. Yu, Z. Chang, X.-H. Bu and M. J. Zaworotko, Crystal engineering of a rectangular sql coordination network to enable xylenes selectivity over ethylbenzene, Chem. Sci., 2020, 11, 6889–6895 RSC.
- S.-Q. Wang, X.-Q. Meng, M. Vandichel, S. Darwish, Z. Chang, X.-H. Bu and M. J. Zaworotko, High Working Capacity Acetylene Storage at Ambient Temperature Enabled by a Switching Adsorbent Layered Material, ACS Appl. Mater. Interfaces, 2021, 13, 23877–23883 CrossRef CAS PubMed.
- S.-Q. Wang, S. Darwish, D. Sensharma and M. J. Zaworotko, Tuning the switching pressure in square lattice coordination networks by metal cation substitution, Mater. Adv., 2022, 3, 1240–1247 RSC.
- S.-Q. Wang, S. Darwish, X.-Q. Meng, Z. Chang, X.-H. Bu and M. J. Zaworotko, Acetylene storage performance of [Ni(4,4′-bipyridine)2(NCS)2]n, a switching square lattice coordination network, Chem. Commun., 2022, 58, 1534–1537 RSC.
- E. Patyk-Kaźmierczak, M. Kaźmierczak, S.-Q. Wang and M. J. Zaworotko, Pressure-Induced Structural Effects in the Square Lattice (sql) Topology Coordination Network sql-1-Co-NCS·4OX, Cryst. Growth Des., 2023, 23, 2055–2064 CrossRef PubMed.
- S.-Q. Wang, S. Darwish and M. J. Zaworotko, Adsorbate-dependent phase switching in the square lattice topology coordination network [Ni(4,4′-bipyridine)2(NCS)2]n, Chem. Commun., 2023, 59, 559–562 RSC.
- A. Kondo, H. Noguchi, S. Ohnishi, H. Kajiro, A. Tohdoh, Y. Hattori, W.-C. Xu, H. Tanaka, H. Kanoh and K. Kaneko, Novel expansion/shrinkage modulation of 2D layered MOF triggered by clathrate formation with CO2 molecules, Nano Lett., 2006, 6, 2581–2584 CrossRef CAS PubMed.
- S. Darwish, S.-Q. Wang, D. M. Croker, G. M. Walker and M. J. Zaworotko, Comparison of Mechanochemistry vs Solution Methods for Synthesis of 4,4′-Bipyridine-Based Coordination Polymers, ACS Sustainable Chem. Eng., 2019, 7, 19505–19512 CrossRef CAS.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent, Chem. Sci., 2015, 6, 1645–1649 RSC.
- P. A. Julien, C. Mottillo and T. Friščić, Metal–organic frameworks meet scalable and sustainable synthesis, Green Chem., 2017, 19, 2729–2747 RSC.
- H. Yu, Q.-F. Xu, Z.-R. Sun, S.-J. Ji, J.-X. Chen, Q. Liu, J.-P. Lang and K. Tatsumi, Unique formation of two different W/Ag/S clusters from the same components via a low heating temperature solid state reaction and a solution reaction and their third-order NLO properties in solution, Chem. Commun., 2001, 2614–2615 RSC.
- J.-X. Chen, X.-Y. Tang, Y. Chen, W.-H. Zhang, L.-L. Li, R.-X. Yuan, Y. Zhang and J.-P. Lang, Formation of Four Different [MoOS3Cu3]-Based Coordination Polymers from the Same Components via Four Synthetic Routes, Cryst. Growth Des., 2009, 9, 1461–1469 CrossRef CAS.
- F. Wang, C.-Y. Ni, Q. Liu, F.-L. Li, J. Shi, H.-X. Li and J.-P. Lang, [Pb(Tab)2(4,4′-bipy)](PF6)2: two-step ambient temperature quantitative solid-state synthesis, structure and dielectric properties, Chem. Commun., 2013, 49, 9248–9250 RSC.
- W.-H. Zhang, Z.-G. Ren and J.-P. Lang, Rational construction of functional molybdenum (tungsten)–copper–sulfur coordination oligomers and polymers from preformed cluster precursors, Chem. Soc. Rev., 2016, 45, 4995–5019 RSC.
- T. Friščić and L. Fábián, Mechanochemical conversion of a metal oxide into coordination polymers and porous frameworks using liquid-assisted grinding (LAG), CrystEngComm, 2009, 11, 743–745 RSC.
- W. Yuan, T. Friščić, D. Apperley and S. L. James, High reactivity of metal–organic frameworks under grinding conditions: parallels with organic molecular materials, Angew. Chem., Int. Ed., 2010, 49, 3916–3919 CrossRef CAS PubMed.
- G. A. Bowmaker, Solvent-assisted mechanochemistry, Chem. Commun., 2013, 49, 334–348 RSC.
- Y.-H. Huang, W.-S. Lo, Y.-W. Kuo, W.-J. Chen, C.-H. Lin and F.-K. Shieh, Green and rapid synthesis of zirconium metal–organic frameworks via mechanochemistry: UiO-66 analog nanocrystals obtained in one hundred seconds, Chem. Commun., 2017, 53, 5818–5821 RSC.
- B. Karadeniz, A. J. Howarth, T. Stolar, T. Islamoglu, I. Dejanovic, M. Tireli, M. C. Wasson, S. Y. Moon, O. K. Farha, T. Friscic and K. Uzarevic, Benign by Design: Green and Scalable Synthesis of Zirconium UiO-Metal-Organic Frameworks by Water-Assisted Mechanochemistry, ACS Sustainable Chem. Eng., 2018, 6, 15841–15849 CrossRef CAS.
- Y. Sakata, S. Furukawa, M. Kondo, K. Hirai, N. Horike, Y. Takashima, H. Uehara, N. Louvain, M. Meilikhov and T. Tsuruoka, Shape-memory nanopores induced in coordination frameworks by crystal downsizing, Science, 2013, 339, 193–196 CrossRef CAS PubMed.
- H. Miura, V. Bon, I. Senkovska, S. Ehrling, S. Watanabe, M. Ohba and S. Kaskel, Tuning the gate-opening pressure and particle size distribution of the switchable metal–organic framework DUT-8(Ni) by controlled nucleation in a micromixer, Dalton Trans., 2017, 46, 14002–14011 RSC.
- V. Bon, N. Busov, I. Senkovska, N. Bönisch, L. Abylgazina, A. Khadiev, D. Novikov and S. Kaskel, The importance of crystal size for breathing kinetics in MIL-53(Al), Chem. Commun., 2022, 58, 10492–10495 RSC.
- A. D. Katsenis, A. Puskaric, V. Strukil, C. Mottillo, P. A. Julien, K. Uzarevic, M. H. Pham, T. O. Do, S. A. Kimber, P. Lazic, O. Magdysyuk, R. E. Dinnebier, I. Halasz and T. Friscic, In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework, Nat. Commun., 2015, 6, 6662 CrossRef CAS PubMed.
- T. Stolar, L. Batzdorf, S. Lukin, D. Žilić, C. Motillo, T. Friščić, F. Emmerling, I. Halasz and K. Užarević, In Situ Monitoring of the Mechanosynthesis of the Archetypal Metal–Organic Framework HKUST-1: Effect of Liquid Additives on the Milling Reactivity, Inorg. Chem., 2017, 56, 6599–6608 CrossRef CAS PubMed.
- P. A. Julien, K. Uzarevic, A. D. Katsenis, S. A. Kimber, T. Wang, O. K. Farha, Y. Zhang, J. Casaban, L. S. Germann, M. Etter, R. E. Dinnebier, S. L. James, I. Halasz and T. Friscic, In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework, J. Am. Chem. Soc., 2016, 138, 2929–2932 CrossRef CAS PubMed.
- D. Prochowicz, K. Sokołowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friščić and J. Lewiński, A mechanochemical strategy for IRMOF assembly based on pre-designed oxo-zinc precursors, Chem. Commun., 2015, 51, 4032–4035 RSC.
- K. Užarević, T. C. Wang, S.-Y. Moon, A. M. Fidelli, J. T. Hupp, O. K. Farha and T. Friščić, Mechanochemical and solvent-free assembly of zirconium-based metal–organic frameworks, Chem. Commun., 2016, 52, 2133–2136 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystallographic data, PXRD patterns, SEM images, etc. CCDC 2014135. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00391d |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |