Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tailoring the Fe → Pd interaction in cationic Pd(II) complexes via structural variation of the ligand scaffold of sterically demanding dppf-analogs and their P,N-counterparts†‡
Subhayan
Dey
 a,
Fabian
Roesler
a,
Clemens
Bruhn
a,
Zsolt
Kelemen
a,
Fabian
Roesler
a,
Clemens
Bruhn
a,
Zsolt
Kelemen
 *b and
Rudolf
Pietschnig
*b and
Rudolf
Pietschnig
 *a
*a
aInstitut für Chemie und CINSaT, University of Kassel, Heinrich Plett-Straße 40, 34132 Kassel, Germany. E-mail: pietschnig@uni-kassel.de
bDepartment of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Műegyetem Rkp. 3, 1111 Budapest, Hungary
First published on 8th May 2023
Abstract
Two 1,1′-azaphospha substituted dppf-analogues Fc′(NMe2)(PPh2) (Ph = C6H5, Fc′ = 1,1′-ferrocenediyl, 3a) and Fc′(NMe2)(PMes2) (Mes = 2,4,6-Me3C6H2, 3b) have been prepared, via reductive amination, followed by salt-metathesis (of 2), starting from 1,1′-azabromoferrocene 1. Their donor properties have been explored using heteronuclear NMR spectroscopy based on their 1JP–Se coupling, and the formation of PdCl2-complexes in comparison to a set of related dppf analogs with gradual steric variation, such as Fc′(PMes2)(PPh2) (5) and Fc′(PMes2)(PtBu2) (6). Chloride abstraction from these complexes, namely Fc′(PMes2)(PPh2)·PdCl2 (7), Fc′(PMes2)(PtBu2)·PdCl2 (8), and [Fc′(NMe2)(PPh2)]2·PdCl2 (9) using AgSbF6 produced the corresponding cationic Pd(II) complexes [Fc′(PMes2)(PtBu2)·PdCl][SbF6] (10), [Fc′(PPh2)(NMe2)·PdCl][SbF6]2 (11) and [Fc′(PPh2)(NMe2)·Pd(PPh2C5H5)][SbF6]2 (12) featuring Fe → Pd interactions. Variation of the counter anion by coordination of 3a to a chloride-free Pd(II) source furnished [Fc′(PPh2)(NMe2)·Pd(PPh3)][BF4]2 (13), [Fc′(PPh2)(NMe2)·Pd(PPh2)Fc′(NMe2)][BF4]2 (14), and [Fc′(PPh2)(NMe2)·PdP(p-OMe-C6H4)3][BF4]2 (15) with similar Fe → Pd interactions. Comparison with previously reported diphospha- and azaphospha- counterparts, revealed that 10 and 11 display the shortest and 15 the longest Fe–Pd bond, within their ligand scaffold congeners. DFT calculations performed on compounds 10–15 were further able to verify their intrinsic structural features and trends and shed light on the nature of the Fe → Pd bonding interactions which are furthermore consistent with CV measurements.
Introduction
Ferrocene, an extremely useful and unique building block, has remained at the centre of attraction for several decades now.1,2 Being an essential component in organometallic polymers,3–10 redox-tunable substances,6,11–13 organometallic drugs,14,15 and functional materials,9,16–20 ferrocene has played a vital role in homogenous catalysis.21–25 The principle reasons for its popularity in homogenous catalysis lie in the easy syntheses of its P-functionalized derivatives (such as dppf) and a flexible backbone,26,27 which provides an opportunity to stabilize various metal centers by attaining facile geometric changes.21,28 When such ferrocene-based ligands were further explored in respect to catalysis, it was found that bite angles (βn) of such ligands, which have a positive effect on the efficiency of catalysts,29,30 are significantly higher than those found for usual bisphosphanyl ligands (such as dppe),29–31 and can further be manipulated by changing the substituents on phosphorus.27 These aforementioned qualities made ferrocene-based bisphosphane ligands remarkably successful,21,22 and a search for new ligands, with optimized steric demand and donating ability, is still relevant to date.32–37Analogous heteroditopic P,N-ligands with a 1,1′-backbone are far less explored (type E, Fig. 1), unlike other heteroditopic amino ferrocene ligands (B–D, Fig. 1).33,54,66–69,71 The simultaneous presence of P and N donor atoms in a single molecule, are attractive to differentiate between their softness according to the HSAB concept and to foster hemilabile coordination. Recently the chemistry of κ3-bis(donor)ferrocenyl – transition-metal interactions has been reviewed as well.72
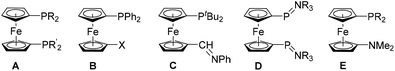 | ||
Fig. 1 Previously reported symmetrically and unsymmetrically substituted dppf-analogs (A–D), and herein reported 1,1′-azaphospha substituted ferrocenyl ligands (E, R = Ph, Mes). Known 1,1′-diphospha substituted dppf-analogues A: R = R′ = Ph, Mes, tBu etc.;23,27,32,38–48 1,1′-unsymmetrically substituted diphenylphosphano ferrocenes B: X = CHO,49,50 COOH,51 COOMe,51 CONEt2,52 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CBr2,49 C CBr2,49 C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH,49 HC CH,49 HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2,53 C CH2,53 C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N,54 CH2OH,34 SH,55 oxazoline,56 PO(OEt)2,57 COOH,58,59 OMe,60 C5H4N,61 bipyridine,62 HC N,54 CH2OH,34 SH,55 oxazoline,56 PO(OEt)2,57 COOH,58,59 OMe,60 C5H4N,61 bipyridine,62 HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6F5),63,64 CH2NH(CHMePh),50 CHN(CHMePh),50 3,5-dihydroazepine,65 N N(C6F5),63,64 CH2NH(CHMePh),50 CHN(CHMePh),50 3,5-dihydroazepine,65 N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C,54 N C,54 N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NHiPr)2,66 N C(NHiPr)2,66 N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NHC6H11)2,66 N C(NHC6H11)2,66 N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NHXyl)2,66 NH(CO)CH2PPh2,67 and NH(CH2)2PPh2;67 1,1′-unsymmetrically substituted di-tert-butylphosphano ferrocenes C;68,69 and bis(phosphinimine) ligands D: R = Et and Ph.70 Analogous heteroditopic P,N-ligands, presented in this report, E: R = Ph and Mes. C(NHXyl)2,66 NH(CO)CH2PPh2,67 and NH(CH2)2PPh2;67 1,1′-unsymmetrically substituted di-tert-butylphosphano ferrocenes C;68,69 and bis(phosphinimine) ligands D: R = Et and Ph.70 Analogous heteroditopic P,N-ligands, presented in this report, E: R = Ph and Mes. | ||
Here we report a general approach to ferrocene bridged 1,1′-disubstituted P,N-ligands of the general type E and explore their donor properties. Furthermore, we apply these dppf analogous P,N and closely related P,P ligands in the coordination of Pd(II) ions where depending on the counter-anions a gradual tuning of Fe → Pd interactions is achieved which besides structural investigation is explored with density functional theory (DFT) and electrochemical methods (CV).
Results and discussion
Synthesis and properties of ferrocene bridged P,N-ligands
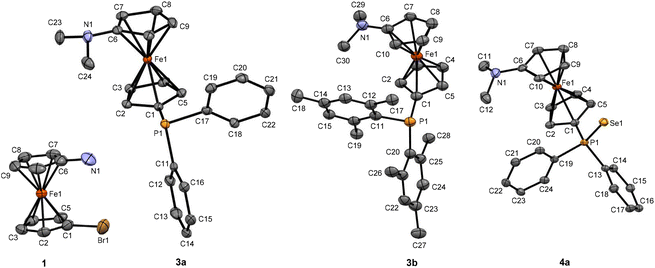
Whereas all previously reported azaphospha-substituted ferrocene ligands have been synthesized starting from BH3-protected ferrocenyl phosphanes,33,54,67,71 the synthesis of azaphosphanes 3 (with generic structure of E, depicted in Fig. 1) hinges upon successful and large-scale preparation of an unsymmetrically substituted 1,1′-azabromoferrocene 1. Although there are several synthetic strategies reported for compound 1,73–77 due to low obtainable yields,73,75–77 and apparently impure final product,73,74,76,77 a newly reported pathway, which involves Staudinger-type reaction on 1,1′-azidobromoferrocene (Scheme 1A),78 was used for this compound. Compound 1 was initially purified by column chromatography, followed by a flask-to-flask sublimation, which resulted in bright colored golden yellow crystals, which were found suitable for characterization by single crystal X-ray diffraction (Fig. 2 and S2, ESI‡).Adapting a known procedure for reductive amination on aminoferrocenes,77,79,80 compound 1 was further reacted with paraformaldehyde and NaCNBH3, in presence of glacial acetic acid to obtain N,N-dimethyl substituted 1,1′-azabromoferrocene 2 (Scheme 1B). When compound 2 was lithiated and in situ reacted with one equivalent of Ph2PCl or Mes2PCl, compound 3a and 3b were obtained in yields of 69% and 21%, respectively, calculated based on starting material 1 (Scheme 1B). Although the crystal structure of compound 3a was deposited in 2003 by Butler et al. (CCDC Deposition Number 217930‡, deposited on 07/10/2003),81 no further report, describing its synthesis and characterization, could be found despite our best effort. Therefore, synthetic details and complete characterization of compound 3a are presented in this report, whereas an independently developed crystallographic data set, obtained by X-ray analysis from a single crystal of 3a, was used for further discussion.
Although the solid-state molecular structures of 3a and 3b are very similar, their geometries at the phosphanyl unit differs owing to the different steric demand of the P-bonded substituents, with the sum of angles at phosphorus being larger by ca. 13° in mesityl substituted 3b than in phenyl substituted 3a (Fig. 2). This observation is consistent with related sterically demanding dppf analogs, Fc′(PMes2)(PPh2) and Fc′(PMes2)2,82,83 for which better donor ability has been found for Mes2P- than for Ph2P- groups, based on variation of the s-character of the lone pair at phosphorus with the geometry at this atom.23,82,83
To examine how the diarylphosphanyl groups in 3a and 3b are influenced by the amino unit in the molecule, we set out to determine the 1JP–Se values as a measure for their donating properties. To this end, 3a and 3b were reacted with red selenium forming selenophosphoranes 4a and 4b (Scheme 1B) featuring 1JP–Se coupling values of 757 Hz and 719 Hz (in Toluene-D8), respectively, which are ca. 4–6 Hz lower than those reported for Fc′(PSePh2)2 (761 Hz), Fc′(PSeMes2)(PSePh2) (763PSePh2 and 723PSeMes2 Hz), and Fc′(PSeMes2)2 (723 Hz) in the same solvent.23,82 The lower values for 1JP–Se in 4a and 4b indicate a better donor ability of the phosphanyl units as compared to their counterparts without the NMe2 unit. Moreover, by careful evaluation of the structure of 4a (Fig. 2), its molecular parameters were found in compliance with the comparable values of Fc′(PSeMes2)(PSePh2) and Fc′(PSePh2)2.83,84 To explore the overall electronic effect of simultaneous introduction of NMe2 and PR2 (R = Ph and Mes) units in this molecular scaffold, the redox properties of the metallocene units 3a and 3b have further been investigated using cyclic voltammetry (CV, Fig. S1, ESI‡), where the introduction of NMe2 unit shows a significant shift of Fc/Fc+ oxidation maxima (133 and 26 mV for 3a and 3b, respectively, where Fc denotes ferrocene) towards lower potentials, as compared to FcPPh2 and FcPMes2, respectively (Fig. S1, ESI‡).
Pd(II) complexes of Dppf-analogs
For mutual comparison, PdCl2 complexes of P,N ligand 3a and P,P ligands 5 and 6 have been prepared in dichloromethane by adapting a published procedure (Scheme 2).28,41,85 The 31P NMR resonances of the donor atoms in bisphosphane ligands 5 and 6 experience significant deshielding upon conversion into their respective PdCl2 complexes 7 and 8 with coordination shifts of Δδ ≈50 ppm. By contrast, the corresponding P,N ligand 3a shows no significant shift of its 31P resonance upon coordination in 9. The closely related mesityl substituted 3b did not undergo complex formation with PdCl2 in CH2Cl2 solution at room temperature, whereas at higher temperature in toluene solution unspecific reaction led to inseparable mixtures (Fig. S59 and S60, ESI‡).Single crystals, suitable for X-ray crystallography, could be obtained by slow diffusion of hexanes or pentane into solutions of complexes 7–9 in dichloromethane revealing bidentate coordination of the P,P ligands in 7 and 8 in contrast to monodentate coordination of the P,N ligand in 9 (Fig. 3).
To increase the metal ligand interaction, complexes 7–9 were further reacted with AgSbF6 as chloride abstracting reagent (Scheme 3). In case of complex 7, the all aryl substituted P,P ligand experiences irreversible oxidation leading to insoluble paramagnetic products. By contrast, chloride abstraction from complex 8 leads to formally cationic Pd-complex 10 with the P,P ligand remaining intact (Scheme 3A). For Pd complex 9, containing P,N ligand 3a in monodentate fashion, the reaction depends strongly on the stoichiometry of the reagents. For sub-stoichiometric and stoichiometric amounts of AgSbF6, chloride abstraction is successful and the twofold monodentate binding mode changes to bidentate with elimination of the second equivalent of ligand leading to 11 (Scheme 3B). With excess (2 eq.) of AgSbF6 a similar hapticity change of the P,N ligand occurs, however, both chloride ions are removed to form dicationic complex 12 (Scheme 3B). Within the latter, the coordination sphere around palladium is completed by cyclopentadienyl diphenylphosphane, which we anticipate stemming from fragmentation of the second ligand unit in 9 (Scheme 3B). A similar bidentate and dicationic complex 13 can be obtained directly starting from P,N ligand 3a by reaction with Pd(NCCH3)4(BF4)2 as palladium source in the presence of PPh3 (Scheme 3C). In the absence of an additional phosphane ligand, or in the presence of sterically very demanding phosphanes (e.g., P(o-tol)3 or PMes3), complex 14 is formed, where the second equivalent of ligand in the precursor acts as additional phosphane (Scheme 3C). Moreover, one might speculate about a potential role of 14 as precursor in the formation of 12. Interestingly, the additional phosphane ligand can be displaced within the ligand sphere of Pd as has been demonstrated by displacement of PPh3 by P(p-anisyl)3 yielding 15 (Scheme 3D). Similar phosphane displacement has been previously reported, which underwent much faster (reaction time 5 min),86 whereas displacement of PPh3 by P(p-OMe-C6H4)3 to 15 took longer time and higher reaction temperature (40 h, 50 °C) as followed by 31P NMR spectroscopy (Fig. S64–S67, ESI‡).
In their 31P NMR spectra the cationic Pd complexes 11–15 related to the P,N ligand show a coordination shift to lower field of roughly Δδ ≈12 ppm versus their neutral counterpart 9. By contrast, with the P,P ligand a significantly larger high-field shift is observed for cationic complex 10, compared with its neutral precursor 8, which is more pronounced for the mesityl substituted phosphanyl unit (Δδ = −63.6 ppm) than for the tert-Butyl substituted one (Δδ = −51.3 ppm). For completeness, it needs to be mentioned that the sterically more demanding P,N ligand 3b does not form analogous Pd(II) complexes even with Pd(NCCH3)4(BF4)2 as palladium source (Fig. S58, ESI‡).
We have been able to grow crystals suitable for single crystal X-ray diffraction for 10–15. For all cationic complexes (10–15), a bidentate coordination of one ferrocenylene bridged ligand is observed and a short contact between iron and palladium, which was absent in their neutral precursors 7–9 (Fig. 3, 4 and 5). In general, no direct interaction of the complex counter-anions (BF4−, SbF6−) with the Pd atom is present which also show no significant effect on the overall structure of their corresponding cations.
When analyzing and discussing the lengths of Fe–Pd distances for these complexes, one has to keep in mind that the difference in Cipso,Cp–P or Cipso,Cp–N bond lengths also affects the respective Fe–Pd distances and interactions for related diphospha- and diaza-substituted dppf analogs. Therefore, shorter Pd–Fe distances are to be expected for ferrocene based N,N ligand scaffolds compared with their P,P counterparts, whereas for related mixed P,N scaffolds, intermediate Pd–Fe distances can be anticipated. In turn that means only Pd–Fe distances for the same ligand scaffold are suitable for meaningful comparison. Remarkably, the Fe–Pd distances observed in complex 10 are the shortest for such ferrocenylene bridged bisphosphane ligands, observed so far. Considering the two independent molecules in the unit cell the Fe–Pd distances in 10 are 2.8369(10) Å and 2.7974(10) Å averaging to 2.817(1) Å, which all are substantially shorter than previously reported values for such complexes, ranging between 2.877(2)–3.0168(4) Å (entries 1–5, Table 1).85–89 Moreover, the short Fe–Pd contact in 10 comes with Cipso,Cp,Fc–P distances (1.790(5) and 1.797(5) Å for Molecule 10B, Table S2, ESI‡) complying to the average values of previously reported complexes (1.783–1.829 Å, entries 1–5, Table 1).85–89 Similarly complex 11 derived from P,N ligand 3a, features the shortest Fe–Pd contact for ferrocenylene bridged P,N ligands, observed so far. The Fe–Pd distance in 11 is 2.7384(18), which is shorter than previously reported values for such complexes ranging between 2.7590(5) to 2.7956(5) Å (entries 7 and 8, Table 1).66,67 By contrast, for the dicationic Pd complexes 12–15 derived from P,N ligand 3a, the longest Fe–Pd distances are also observed (2.811(3)–2.8349(11) Å, entry 9, Table 1), as compared to the previously reported complexes with ferrocene based P,N ligands (2.7590(5)–2.7956(5) Å, entries 7 and 8, Table 1).66,67
| Square-planar Pd(II) complexes | Avg. Cipso,Cp–E bond lengthb (Å) | Pd–Fe distance (Å) | Tilt angle αc (°) | Bite angle βn (°) | Torsion angle τc (°) | Ref. | |
|---|---|---|---|---|---|---|---|
| a Entire table is available in ESI, Table S1.‡ b Averages of two identical bonds from a single molecule (standard deviations are excluded). c Angles were calculated using Mercury as crystallographic software.90,91 d TFAB = tetrakis(pentafluorophenyl)borate,88 [B(C6F5)4]−. e Averages from all crystallographically independent entities in the unit cell (standard deviations are excluded). f Cy = C6H11. g Xyl = 2,4,6-Me3-C6H2. h Presented in current report. | |||||||
| 1 | [dppf·Pd(PPh3)][BF4]2 | 1.787e (E = P) | 2.8934e | 19.7e | 156.79e | 41.1 | 85 and 87 |
| [dppf·PdP(C5H4-p-F)3][BF4]2 | 1.784 (E = P) | 3.0014(4) | 22.1 | 157.54(3) | 26.9 | 86 | |
| 2 | [Fc′(PtBu2)(PPh2)·Pd(PPh3)][BF4]2 | 1.785(3) (E = PtBu), 1.777(3) (E = PPh) | 2.9310(5) | 19.7 | 156.11(3) | 42.6 | 85 |
| 3 | [Fc′(PtBu2)2·PdCl][SbCl6] | 1.797 (E = P) | 2.9389(4) | 19.8 | 162.34(2) | 30.5 | 85 |
| [Fc′(PtBu2)2·PdI][I] | 1.823e (E = P) | 2.9390e | 18.0e | 161.88e | 34.6 | 85 and 88 | |
| [Fc′(PtBu2)2·PdBr][TFAB]d | 1.829 (E = P) | 2.9395(18) | 19.2 | 163.04(5) | 31.0 | 88 | |
| [Fc′(PtBu2)2·Pd(C6H4-p-CN)][BF4]2 | 1.808 (E = P) | 2.9988(8) | 16.3 | 159.75(4) | 35.9 | 89 | |
| 4 | [Fc′[P(C6H11)2]2·Pd(PPh3)][BF4]2 | 1.782 (E = P) | 2.9339(5) | 18.9 | 156.63(3) | 38.2 | 85 |
| [Fc′[P(C6H11)2]2·Pd(PMe3)][BF4]2 | 1.783 (E = P) | 2.9567(10) | 20.4 | 157.20(6) | 41.7 | 85 | |
| 5 | [Fc′(CpPiPr2)2·Pd(PMe3)][BF4]2 | 1.786 (E = P) | 3.0168(4) | 19.1 | 158.09(2) | 32.6 | 85 |
| 6 | [Fc′(PMes2)(PtBu2)·PdCl][SbF6] (10)e | 1.795 (E = PtBu)e and 1.788 (E = PMes)e | 2.8369(10) and 2.7974(10) | 21.5e | 161.60(5)° and 161.46(5)° | 40.5 and 46.2 | |
| 7 | [Fc′[NC(NHiPr)2](PPh2)·PdCl][SbF6] | 1.775(2) (E = P) and 1.379(3) (E = N) | 2.7590(5) | 24.6 | 163.01(5) | 1.9 | 66 |
| [Fc′[NC(NHCy)2](PPh2)·PdCl][SbF6]f | 1.773(2) (E = P) and 1.388(2) (E = N) | 2.7956(5) | 22.8 | 162.46(5) | 5.7 | 66 | |
| [Fc′[NC(NHXyl)2](PPh2)·PdCl][SbF6]g | 1.770(2) (E = P) and 1.384(2) (E = N) | 2.7821(5) | 23.0 | 163.15(5) | 5.9 | 66 | |
| 8 | [Fc′[NH(CH2)2PPh2](PPh2)·Pd][SbF6]2 | 1.790(7) (E = P) and 1.419(9) (E = N) | 2.7889(9) | 21.3 | 164.4(2) | 9.8 | 67 |
| 9 | [Fc′(PPh2)(NMe2)·PdCl][SbF6]2 (11) | 1.783(11) (E = P) and 1.394(15) (E = N) | 2.7384(18) | 23.3 | 163.9(2) | 1.7 | |
| [Fc′(PPh2)(NMe2)·Pd(PPh2C5H5)][SbF6]2 (12) | 1.788(8) (E = P) and 1.420(9) (E = N) | 2.811(3) | 21.9 | 159.11(19) | 22.3 | ||
| [Fc′(PPh2)(NMe2)·Pd(PPh3)][BF4]2 (13) | 1.758(10) (E = P) and 1.415(12) (E = N) | 2.8289(19) | 20.9 | 158.9(2) | 20.4 | ||
| [Fc′(PPh2)(NMe2)·Pd(PPh2)Fc′(NMe2)][BF4]2 (14) | 1.766(6) (E = P) and 1.427(7) (E = N) | 2.8184(9) | 19.5 | 158.79(13) | 30.7 | ||
| [Fc′(PPh2)(NMe2)·PdP(p-OMe-C6H4)3][BF4]2 (15) | 1.770(7) (E = P) and 1.408(9) (E = N) | 2.8349(11) | 22.5 | 160.45(16) | 11.1 | ||
The short Fe–Pd distance in complex 10 may be interpreted as an attractive bonding interaction between these two metal centers. However, such a short contact may also be considered as a consequence of a large torsion (τ) or bite angle (βn) at the structurally flexible ferrocene scaffold owing to steric repulson between substituents R's around Pd (Fig. 6). Comparing the geometric parameters of the complex with the shortest Fe–Pd distance, 10, with related compounds, all three angular parameters show intermediate values. The tilt angle α (21.5°) in 10 is slightly smaller than that observed with a corresponding dppf complex (22.1°),86 yet larger than for its Fc′(PtBu2)2 analog (16.3°) (entries 1, 3 and 6, Table 1).89 Bite angle βn (161.60(5)° and 161.46(5)°) in 10 is slightly smaller than that observed with its Fc′(PtBu2)2 analog (162.34(2)°),85,88 yet larger than for its Fc′(PtBu2)(PPh2)·analog (156.11(3)°) (entries 1, 3 and 6, Table 1).85
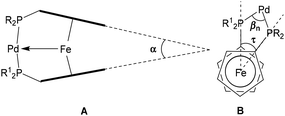 | ||
| Fig. 6 Molecular parameters of Pd(II) complexes of dppf analogs with Fe → Pd interactions with bite (βn), torsion (τ), and tilt (α) angles illustrated. | ||
While the torsion angle for 10 is 40.5°, the largest and smallest τ-values in related complexes are found for its Fc′(PtBu2)(PPh2)·analog (42.6°)85 and its dppf analog (26.9°),86 respectively (entries 1, 2 and 6, Table 1). In summary, these values indicate that the Fe–Pd distance is not a consequence of either steric repulsion or geometric distortion alone in the related molecule. In turn, the unusual shortening of Fe–Pd bond in compound 10 seems to be a compromise between minimized steric repulsion, rotational distortion, and secondary interaction of the ligand system in the solid state. Similarly, 11, featuring the shortest Fe–Pd bond reported for this complex type involving a P,N ligand shows no unusual bite (βn = 163.9(2)°) torsion (τ = 1.7°) and tilt (α = 23.3°) angles compared with previously reported analogs (entries 7–9, Table 1).66,67
For complexes 11–15 derived from P,N ligand 3a, the longest Fe–Pd distances had been observed. Interestingly, the torsion angles in 12–14 are 22.3° (12), 20.4° (13) and 30.7° (14) the largest found so far, where for related complexes values between 1.9–9.8° had been reported (entries 7–9, Table 1).66,67 Compared to its Fc′(NMe2)(PPh2) congeners 12–14 the torsion angle of 15 is slightly smaller (11.1°). Regarding their bite angles βn, complexes 11–15 feature lower values (158.79(13)°–163.9(2)°) as compared to previously reported related structures (162.46(5)–164.4(2)°) (entries 7–9, Table 1).66,67 Similarly, the tilt angles in complexes 11–15 are between 19.5° and 23.3°, slightly smaller compared to those for related complexes Fc′[NC(NHR)2](PPh2)·[PdCl][SbF6] (R = iPr, Cy and Xyl; entry 7, Table 1) and Fc′[NH(CH2)2PPh2][PPh2]·Pd[SbF6]2 (entry 8, Table 1) reported so far (21.3°–24.6°).66,67
To get deeper insight into the proposed interaction between the iron and palladium centers, DFT calculations were carried out (more details in the ESI‡). First, different DFT methods were tested on systems 10–15 (see Table S19, ESI‡) and the optimized geometries were compared with the obtained X-ray structures. Although the computed and X-ray structures are in good agreement, as a common motif the differences between the DFT calculated and the experimentally obtained Fe–Pd distances vary between 0.059–0.212 Å (Table S19, ESI‡). Without any doubt, it can be considered as a noticeably high error, especially if the description of the interaction between these two atoms is the main goal. One possible explanation of such discrepancy could be a flat potential energy surface (PES). Therefore, to test the plausibility of our inference, we performed PES scans with incremental increase or decrease of the Fe–Pd distances.92 First, three model systems were computed, where the bulky groups were repleaced by methyl substituents (16–18, Fig. 7) in order to keep the steric factors less dominant. Similar to our previuos study,93 the best match between computed and experimental structures was obtained using the ωB97X-D funcional, which was then employed for optimizing the molecular structures in this study. It can further be established that all the three structures are very flexible, where 0.200 Å elongation or contraction require only 1.0–4.8 kcal mol−1 energy investment and the flexibility increases in the order of 18 > 17 > 16. In case of 18, 0.3 Å stretching requires energy as low as 2.5–3.6 kcal mol−1. These data suggest a rather weak interaction between the two metals, especially in case of 18. In full agreement with our present study, only 2.3 kcal mol−1 stabilization energy was reported for [Pd(dtbpf)Cl]+.85 Such small energy values can easily be compensated by second order interactions in the crystal lattice.
 | ||
| Fig. 7 Potential energy surface (PES) scan for the elongation of the Fe–Pd distance in the model compounds 16–18. | ||
This hypothesis was fully supported by the X-ray structure of 10 where two independent molecules are present in the crystallographic unit cell differing in their geometric parameters, but otherwise feature identical steric and electronic parameters based on their identical constitution. However, careful comparison pointed at two separate, yet apparently interconnected, differences between them. When one molecule has a torsion angle substantially higher (46.2°) than the other (40.5°), the latter shows comparatively higher Pd–Fe distance (2.8369(10) Å) than the earlier (2.7974(10) Å) (Table S2, ESI‡). However, such correlation between molecular torsion and Pd–Fe distance is not completely uncommon in the literature and can further be observed for similar dppf-complexes depicted in Table 1 (entry 1), where [dppf·PdP(C5H4-p-F)3][BF4]2 shows higher Pd–Fe distance but lower torsion angle than [dppf·Pd(PPh3)][BF4]2. The remaining molecular parameters for aforementioned independent molecular entities of 10 are quite comparable, and sometimes equal in magnitude once the standard deviations are carefully considered (Table S1, ESI‡).
In case of the related compound [Fc′(NIm)2·Pd(NCMe)]2+, Tamm and co-workers demonstrated that the possible existence of a second (local) minimum on the PES, where the Fe–Pd distance is significantly longer.92 Therefore, we have further increased the distance between Pd and Fe centers in case of the model systems (16–18) and second minima were found around ∼3.78 Å (16′), ∼4.01 Å (17′) and ∼4.29 Å (18′), and the Pd atom adopts a slightly distorted T-shaped geometry, which is in full agreement with the earlier knowledge.92 In contrast to 16 and 17, in case of 18 the T-shaped, second minima (16′, 17′, 18′) is more stable by 8.1 kcal mol−1 (ΔEisomer-scan, Fig. 7).
Starting a new optimization without any restriction from the T-shaped structure (18′) verified the stability of this second isomer at higher level of theory as well (ΔEisomer = −8.8 kcal mol−1 at ωB97X-D/6-311+G**), which further suggests a rather weak Fe–Pd interaction for 18. A similar conclusion can be obtained performing the same calculations with the dppf ligand (ΔEisomer = −9.4 kcal mol−1 at ωB97X-D/6-311+G**). As expected, on further increasing steric hindrance the energy difference between the two isomers decreases and the stability order changes at a certain point.
Indeed, performing PES scans on compounds 10 and 18 revealed that the T-shaped isomers become more stable by 12.5 kcal mol−1 in case of 10. In contrast to 10, compound 11 (with rather less bulky substituents at the donor atoms) did not significantly differ from the model compound 17, i.e. the T-shaped isomer is less stable for 11 (ΔEisomer-scan = 11.9 kcal mol−1 for 11 and ΔEisomer-scan = 8.8 kcal mol−1 for 17, Fig. 7 and 8). Nataro and co-workers came to a similar conclusion, where introduction of two bulky tert-butyl groups at both phosphorus atoms (i.e. for dtbpf ligand) was able to prevent the formation of the T-shaped isomer, which in turn further prevented dimerization via formation of intermolecular Pd2Cl2 bridging unit (Fig. S62, ESI‡).85 Similarly, once the chlorine substituent was replaced by the more bulky phosphane ligands (12–15), the stability of the molecule with T-shaped geometry at Pd centres substantially decreased due to steric factors (compare PES scan for 17 and 12–15 in Fig. 7 and 8).
 | ||
| Fig. 8 Potential energy surface (PES) scan for the elongation of the Fe–Pd distance for the compounds 10–15. | ||
Apart from investigation of the energetic aspects, Kohn–Sham molecular orbitals of 10–15 (Fig S63 in the ESI‡) and Wiberg-indices of the Fe–Pd bonds, and Bader's Atoms-in-molecules (AIM) topology analyses (Tables S20, S21–S23 in the ESI‡) were performed in case of all investigated points of the PES scans of 16–18. Unsurprisingly, both descriptors suggest a stronger bond between the metal centers if the actual Fe–Pd distance is shorter. Interestingly, both the bond critical points and the Wiberg-indices exhibit almost perfect linear trends with the Fe–Pd bond lengths. Since the different DFT functionals deliver various Pd–Fe distances, the use of these descriptors requires careful application and serious consideration.
In line with the presence of a Fe → Pd bond, variation of a competing donor affects the Fe–Pd bond length. As compared to compounds 11–14, compound 15 contains a higher electron donating phosphine P(p-OMe-C6H4)3, and consequently an electronically more saturated Pd(II) atom.86 As a result, compound 15 shows a weaker and consequently longer Pd–Fe bond [2.8349(11) Å], as compared to those of 11 (2.7384(18) Å), 12 (2.811(3) Å), 13 (2.815(2) Å) and 14 (2.8184(9) Å) (entry 9, Table 1). The calculated bond lengths exhibit similar trends.
CV measurements have been made to explore how the redox properties of the ferrocene unit change through the interaction with the Pd(II) atom. While PdCl2 complex 8 shows a quasi-reversible oxidation with a half wave potential of 0.15 V (Fig. S1, ESI‡), upon chloride abstraction no comparable oxidation event was detectable for its cationic analog 10. For the related P,N complexes 11, 13 and 15 also no structured oxidation events were found. Only 14 shows a broad irreversible oxidation with a maximum at 1.10 V (Fig. S1, ESI‡), which we attribute to the P-coordinated freely rotating ferrocene moiety with an uncoordinated NMe2 unit only present in this compound. For the reduction of the complexes 13–15 on the other hand irreversible maxima were detected at −0.85 V (13), −1.20 V (14) and −0.89 V (15) (Fig. S1, ESI‡). These results suggest that Fe → Pd interactions are fairly strong in these complexes and consequently the Fe atom is no longer available for reversible oxidation within the potential window of the solvent.
General experimental section
All manipulations were performed under argon atmosphere unless mentioned otherwise. Prior to use, the glassware was dried in a drying oven at 120 °C. Solvents were distilled over drying agents, prescribed in the CRC Handbook of chemistry and subsequently stored under argon atmosphere over 4 Å molecular sieves. Solvents for column chromatography and aqueous workups were used (analytical grade supplied by VWR and Alfa-Aesar) without further purification. NMR solvents (purchased from Deutero) were degassed via a few cycles of freeze, pump and thaw, and finally stored over 3 Å molecular sieves under Argon atmosphere. Reagents and chemicals were purchased from commercial suppliers (Sigma-Aldrich, ABCR, Alfa-Aesar) and used as received. Ligands Fc′(PMes2)(PPh2)83 and Fc′(PMes2)(PtBu2)82 were synthesized following procedures, reported by Pietschnig et al.94 Although Fc′(NH2)Br has previously been synthesized following various procedures in yields of 71%,74 and 50% (calculated on the basis of starting Fc′Br2),73,77 in the current report, a recently published procedure was employed, due to its simplicity and higher yield (ca. 70–80%, calculated on the basis of Fc′Br2).78All solution-phase NMR spectra were measured with Varian 500VNMRS and Varian MR-400 spectrometers at 22 °C. Chemical shifts (δ in ppm) were expressed with respect to the following standards, set as 0 ppm: SiMe4 (for 1H and 13C), aqueous H3PO4 (for 31P), BF3·OEt2 (in CDCl3 for 11B) and CFCl3 (for 19F). The signals, resulting from the residual nondeuterated NMR solvents, were referenced as indicated in the literature.95 In addition to the standard notation of the signal multiplicity, pst, brs, brd and brm were used to abbreviate pseudo triplet, broad singlet, broad doublet and broad multiplet, respectively in order. The amount of residual solvents was verified by NMR analysis and the expected values for elemental analyses were calculated accordingly. When Electrospray ionization (ESI) and Atmospheric pressure chemical ionization (APCI) mass spectra were measured with a Finnigan LCQ Deca (ThermoQuest, San Jose, USA) instrument using samples dissolved in HPLC-quality thf, MALDI was measured with an UltraFlex ToF/ToF (Bruker Daltonics, Bremen, D) instrument, where an N2 laser with 337 nm wavelength and 3 ns pulse duration was used. Elemental analyses were performed without the presence of any external oxidizer in an EA 3000 Elemental Analyzer (EuroVector). The values of elemental analyses, reported in this article is actually the average of three consecutive readings taken from the purest specimens of each compound. X-ray diffraction experiments were performed using a STOE StadiVari [using either Mo-GENIX source (λ = 0.71073 Å), or Cu-GENIX source (λ = 1.54186 Å)] diffractometer. Structures were solved using dual space method (SHELXT) and were refined with SHELXL-2018.96 All non-hydrogen atoms were refined anisotropically, whereas hydrogen atoms were placed on adjacent atoms using a riding model. Further programs used in the structure analyses were Mercury and Platon.97–99
CV measurement was done with GB2202-C-VAC under Argon atmosphere, when samples were measured as a solution (1 mM) in dry and deoxygenated CH2Cl2, where anhydrous [Bu4N][PF6] was used as a conducting salt at a concentration of 0.1 M. The three-electrode cell consisted of a platinum working electrode, a silver counter electrode, and a silver pseudo reference electrode. The potential was driven on a WaveDriver 20 Bipotentiostat from Pine Research Instrument, and the electrochemical data was recorded via AfterMath (Ver. 1.5.9807, Pine Instrument). All redox processes were referenced using half-wave potentials of Fe(C5Me5)2 as a standard, which was added to the analysed solution. Its corresponding value was then subtracted from the recorded potentials to convert them to the Fc/Fc+ scale following established procedures,100 and finally evaluated with AfterMath and OriginPro.
Fc′(NMe2)Br (2)
On the basis of a known procedure to synthesize Fc′(NHCH2R)Br (when R = Ph, tBu),77 A solution of Fc′(NH2)Br (0.660 g, 2.36 mmol) in glacial acetic acid (18 mL) under argon was treated with paraformaldehyde (0.707 g, 23.54 mmol) and NaBH3CN (0.740 g, 11.78 mmol) and stirred at room temperature overnight. The reaction mixture was brought to pH 12 by addition of 60 mL of 10 M aqueous solution of NaOH and extracted with hexanes (4 × 20 mL). The combined organic extract was washed with water and brine, dried over anhydrous Na2SO4 and filtered. Column chromatography was performed on SiO2, using a solution of hexanes and NEt3 (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After removal of volatiles in high vacuum, compound Fc′(NMe2)Br was recovered as dark red oil (0.659 g, 2.14 mmol, 91%), which is slightly sensitive towards aerial oxygen and solidifies over standing for long time at rt. 1H NMR (C6D6): δ 2.27 (s, 6H, –NCH3), 3.54 (pst, 2H, α-H of C5H4NMe2), 3.86 (pst, 2H, α-H of C5H4Br), 3.89 (pst, 2H, β-H of C5H4NMe2), 4.42 (pst, 2H, β-H of C5H4Br). 13C NMR (C6D6): δ 41.95 (s, –NCH3), 57.66 (s, C of C5H4NMe2), 66.17 (s, C of C5H4NMe2), 66.42 (s, C of C5H4Br), 69.09 (s, C of C5H4Br), 78.54 (s, ipso-C of C5H4Br). MS (APCI) 308 [M], 309 [M + 1]. Anal. Calcd for C12H14BrFeN: C, 46.80; H, 4.58; N, 4.55. Found C, 46.69; H, 4.66; N, 4.63.
2). After removal of volatiles in high vacuum, compound Fc′(NMe2)Br was recovered as dark red oil (0.659 g, 2.14 mmol, 91%), which is slightly sensitive towards aerial oxygen and solidifies over standing for long time at rt. 1H NMR (C6D6): δ 2.27 (s, 6H, –NCH3), 3.54 (pst, 2H, α-H of C5H4NMe2), 3.86 (pst, 2H, α-H of C5H4Br), 3.89 (pst, 2H, β-H of C5H4NMe2), 4.42 (pst, 2H, β-H of C5H4Br). 13C NMR (C6D6): δ 41.95 (s, –NCH3), 57.66 (s, C of C5H4NMe2), 66.17 (s, C of C5H4NMe2), 66.42 (s, C of C5H4Br), 69.09 (s, C of C5H4Br), 78.54 (s, ipso-C of C5H4Br). MS (APCI) 308 [M], 309 [M + 1]. Anal. Calcd for C12H14BrFeN: C, 46.80; H, 4.58; N, 4.55. Found C, 46.69; H, 4.66; N, 4.63.
Fc′(NMe2)(PPh2) (3a)
n BuLi (0.9 mL, 2.5 M in hexanes, 2.25 mmol) was added dropwise to a 0 °C cooled solution of Fc′(NMe2)Br (0.659 g, 2.14 mmol) in a mixture of hexanes and thf (30 mL; hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thf; 9
thf; 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), resulting in a color change from pale yellow to bright orange. After the reaction mixture was stirred for 40 min at 0 °C, a solution of Ph2PCl (2.40 mmol) in a mixture of hexanes and thf (30 mL; hexanes
1), resulting in a color change from pale yellow to bright orange. After the reaction mixture was stirred for 40 min at 0 °C, a solution of Ph2PCl (2.40 mmol) in a mixture of hexanes and thf (30 mL; hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thf; 9
thf; 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added dropwise over ca. 5 minutes. The reaction mixture was warmed to ambient temperature and stirred overnight. After all the volatiles were removed under high vacuum, the product was extracted in a mixture of hexanes and toluene (50 mL, hexanes
1) was added dropwise over ca. 5 minutes. The reaction mixture was warmed to ambient temperature and stirred overnight. After all the volatiles were removed under high vacuum, the product was extracted in a mixture of hexanes and toluene (50 mL, hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 2
toluene, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and LiCl was removed by filtration. From this orange solution, solvents were removed under high vacuum, resulting in a yellow, sticky solid, which was further purified by crystallization in a mixture of hexanes and toluene (10 mL, hexanes
1), and LiCl was removed by filtration. From this orange solution, solvents were removed under high vacuum, resulting in a yellow, sticky solid, which was further purified by crystallization in a mixture of hexanes and toluene (10 mL, hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 4
toluene, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at −20 °C (crystallized yield 76%). 1H NMR (Toluene D8): δ 2.21 (s, 6H, –NCH3), 3.54 (pst, 2H, α-H of C5H4NMe2), 3.74 (pst, 2H, α-H of C5H4PPh2), 4.20 (pst, 2H, β-H of C5H4NMe2), 4.33 (pst, 2H, β-H of C5H4PPh2), 7.04–7.09 (m, 6H, m-, p-HPh), 7.44–7.48 (m, 4H, o-HPh). 13C NMR (Toluene D8): δ 41.87 (s, –NCH3), 56.16 (s, C of C5H4NMe2), 65.10 (s, C of C5H4NMe2), 69.77 (d, β-C of C5H4PPh2, J = 4 Hz), 72.21 (d, α-C of C5H4PPh2, J = 15 Hz), 75.41 (d, ipso-C of C5H4PPh2, J = 7 Hz), 116.64 (s, ipso-C of C5H4NMe2), 128.38 (d, m-CPh, J = 9 Hz), 134.00 (d, o-CPh, J = 20 Hz), 140.69 (d, p-CPh, J = 12 Hz). 31P{1H} NMR (Toluene D8): δ −16.1 (s, PPh2). MS (APCI) 414 [M + 1]. Anal. Calcd for C24H24FeNP: C, 69.75; H, 5.85; N, 3.39. Found: C, 69.88; H, 5.97; N, 3.31.
1) at −20 °C (crystallized yield 76%). 1H NMR (Toluene D8): δ 2.21 (s, 6H, –NCH3), 3.54 (pst, 2H, α-H of C5H4NMe2), 3.74 (pst, 2H, α-H of C5H4PPh2), 4.20 (pst, 2H, β-H of C5H4NMe2), 4.33 (pst, 2H, β-H of C5H4PPh2), 7.04–7.09 (m, 6H, m-, p-HPh), 7.44–7.48 (m, 4H, o-HPh). 13C NMR (Toluene D8): δ 41.87 (s, –NCH3), 56.16 (s, C of C5H4NMe2), 65.10 (s, C of C5H4NMe2), 69.77 (d, β-C of C5H4PPh2, J = 4 Hz), 72.21 (d, α-C of C5H4PPh2, J = 15 Hz), 75.41 (d, ipso-C of C5H4PPh2, J = 7 Hz), 116.64 (s, ipso-C of C5H4NMe2), 128.38 (d, m-CPh, J = 9 Hz), 134.00 (d, o-CPh, J = 20 Hz), 140.69 (d, p-CPh, J = 12 Hz). 31P{1H} NMR (Toluene D8): δ −16.1 (s, PPh2). MS (APCI) 414 [M + 1]. Anal. Calcd for C24H24FeNP: C, 69.75; H, 5.85; N, 3.39. Found: C, 69.88; H, 5.97; N, 3.31.
Fc′(NMe2)(PMes2) (3b)
n BuLi (0.9 mL, 2.25 mmol) was added dropwise to a 0 °C cooled solution of Fc′(NMe2)Br (0.659 g, 2.14 mmol) in a mixture of hexanes and thf (30 mL; hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thf; 9
thf; 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), resulting in a color change from pale yellow to bright orange. After the reaction mixture was stirred for 40 min at 0 °C, a solution of Mes2PX (X = Cl/Br, 48%/52%, FW = 327.914 g mol−1, 2.50 mmol) in a mixture of hexanes and thf (30 mL; hexanes
1), resulting in a color change from pale yellow to bright orange. After the reaction mixture was stirred for 40 min at 0 °C, a solution of Mes2PX (X = Cl/Br, 48%/52%, FW = 327.914 g mol−1, 2.50 mmol) in a mixture of hexanes and thf (30 mL; hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thf; 9
thf; 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added dropwise over ca. 5 minutes. The reaction mixture was warmed to ambient temperature and stirred overnight. After all the volatiles were removed under high vacuum, the product was extracted in a mixture of hexanes and toluene (50 mL, hexanes
1) was added dropwise over ca. 5 minutes. The reaction mixture was warmed to ambient temperature and stirred overnight. After all the volatiles were removed under high vacuum, the product was extracted in a mixture of hexanes and toluene (50 mL, hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 2
toluene, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and LiCl was removed by filtration. From this orange solution, solvents were removed under high vacuum, resulting in a yellow, sticky solid, which was further purified by crystallization in a mixture of hexanes and toluene (10 mL, hexanes
1), and LiCl was removed by filtration. From this orange solution, solvents were removed under high vacuum, resulting in a yellow, sticky solid, which was further purified by crystallization in a mixture of hexanes and toluene (10 mL, hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene, 4
toluene, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at −20 °C. Crystallized yield 23%. 1H NMR (Toluene D8): δ 2.09 (m, 6H, p-CH3 of Mes), 2.11 (m, 12H, o-CH3 of Mes), 2.38 (bs, 6H, –NCH3), 3.52 (m, 2H, α-H of C5H4NMe2), 3.84 (m, 2H, α-H of C5H4PMes2), 4.34 (m, 2H, β-H of C5H4NMe2), 4.45 (m, 2H, β-H of C5H4PMes2), 6.69 (brs, 4H, m-HMes). 13C NMR (Toluene D8): δ 20.84 (s, p-CH3Mes), 23.52 (d, o-CH3Mes, J = 15 Hz), 41.53 (s, –NCH3), 56.14 (s, C of C5H4NMe2), 65.00 (s, C of C5H4NMe2), 69.46 (d, β-C of C5H4PPh2, J = 4 Hz), 74.27 (d, α-C of C5H4PPh2, J = 19 Hz), 77.49 (d, ipso-C of C5H4PPh2, J = 11 Hz), 116.56 (s, ipso-C of C5H4NMe2), 130.41 (d, m-CPh, J = 3 Hz), 133.41 (d, o-HPh, J = 21 Hz), 142.50 (d, p-HPh, J = 15 Hz). 31P{1H} NMR (Toluene D8): δ −33.9 (s, PMes2). MS (APCI): m/z (%) 498 [M + 1]. HRMS (APCI) 498.2008 [M + 1]. Anal. Calcd for C30H36FeNP: C, 72.44; H, 7.29; N, 2.82. Found: C, 72.56; H, 7.36; N, 2.88.
1) at −20 °C. Crystallized yield 23%. 1H NMR (Toluene D8): δ 2.09 (m, 6H, p-CH3 of Mes), 2.11 (m, 12H, o-CH3 of Mes), 2.38 (bs, 6H, –NCH3), 3.52 (m, 2H, α-H of C5H4NMe2), 3.84 (m, 2H, α-H of C5H4PMes2), 4.34 (m, 2H, β-H of C5H4NMe2), 4.45 (m, 2H, β-H of C5H4PMes2), 6.69 (brs, 4H, m-HMes). 13C NMR (Toluene D8): δ 20.84 (s, p-CH3Mes), 23.52 (d, o-CH3Mes, J = 15 Hz), 41.53 (s, –NCH3), 56.14 (s, C of C5H4NMe2), 65.00 (s, C of C5H4NMe2), 69.46 (d, β-C of C5H4PPh2, J = 4 Hz), 74.27 (d, α-C of C5H4PPh2, J = 19 Hz), 77.49 (d, ipso-C of C5H4PPh2, J = 11 Hz), 116.56 (s, ipso-C of C5H4NMe2), 130.41 (d, m-CPh, J = 3 Hz), 133.41 (d, o-HPh, J = 21 Hz), 142.50 (d, p-HPh, J = 15 Hz). 31P{1H} NMR (Toluene D8): δ −33.9 (s, PMes2). MS (APCI): m/z (%) 498 [M + 1]. HRMS (APCI) 498.2008 [M + 1]. Anal. Calcd for C30H36FeNP: C, 72.44; H, 7.29; N, 2.82. Found: C, 72.56; H, 7.36; N, 2.88.
Selenophosphoranes of Fc′(NMe2)(PPh2) (3a) and Fc′(NMe2)(PMes2) (3b)
On the basis of a known procedure,82,83 a suspension of red Se (0.5 mmol) and Fc′(NMe2)(PPh2) or Fc′(NMe2)(PMes2) (0.2 mmol) in thf (20 mL) was stirred for 1 h at r.t. All the volatiles were removed under high vacuum (10−3 mbar) and the product was extracted with hot toluene. Analytically pure compound was precipitated from hot toluene by slow cooling. Note: If all the residual Se is not removed by single filtration attempt, the procedure of filtration must be carried out for multiple times before characterization.Fc′(NMe2)(PSePh2) (4a)
Yield 86%. 1H NMR (Toluene D8): δ 2.09 (s, 6H, –NCH3), 3.63 (pst, 2H, α-H of C5H4NMe2), 3.90 (pst, 2H, β-H of C5H4NMe2), 4.38 (m, 2H, α-H of C5H4PSePh2), 4.56 (m, 2H, β-H of C5H4PSePh2), 6.95–7.00 (m, 6H, m-, p-HPh), 7.80–7.85 (m, 6H, o-HPh). 13C NMR (Toluene D8): δ 41.67 (s, –NCH3), 56.56 (s, C of C5H4NMe2), 66.56 (s, C of C5H4NMe2), 70.61 (d, β-C of C5H4PSePh2, J = 10 Hz), 72.84 (d, α-C of C5H4PSePh2, J = 15 Hz), 117.63 (s, ipso-C of C5H4NMe2), 128.08 (d, o-CPh, J = 12 Hz), 130.81 (d, m-CPh, J = 3 Hz), 132.55 (d, p-CPh, J = 11 Hz), 135.30 (d, ipso-C of Ph). 31P{1H} NMR (Toluene D8): δ 36.7 (s, PSePh2, 1JP–Se = 757 Hz). 77Se NMR (toluene-d8): δ −296.3 (d, PSePh2, 1JP–Se = 757 Hz). MS (MALDI) 493 [M]. Anal. Calcd for C24H24SeFeNP: C, 58.56; H, 4.91; N, 2.85. Found: C, 58.83; H, 4.96; N, 2.90.Fc′(NMe2)(PSeMes2) (4b)
Yield 93%. 1H NMR (Toluene D8): δ 1.99 (s, 6H, p-CH3 of Mes), 2.17 (s, 6H, –NCH3), 2.47 (bs, 12H, o-CH3 of Mes), 3.66 (bs, 2H, β-H of C5H4PSeMes2), 4.03 (m, 2H, α-H of C5H4NMe2), 4.42 (m, 2H, β-H of C5H4NMe2), 5.01 (bs, 2H, α-H of C5H4PSeMes2), 6.54 (bd, 4H, m-H of Mes, J = 4 Hz). 13C NMR (Toluene D8): δ 24.47 (d, CH3 of Mes, J = 6 Hz), 41.55 (s, –NCH3), 56.89 (s, β-C of C5H4NMe2), 66.81 (s, α-C of C5H4NMe2), 70.58 (d, β-C of C5H4PSeMes2, J = 10 Hz), 76.08 (d, ipso-C of C5H4PSeMes2, J = 10 Hz), 79.14 (d, α-C of C5H4PSeMes2, J = 81.0 Hz), 117.54 (s, ipso-C of C5H4NMe2), 131.90 (d, o-CPh, J = 11 Hz), 139.28 (d, m-CPh, J = 3 Hz), 140.51 (bs, ipso-CPh). 31P{1H} NMR (Toluene D8): δ 15.8 (s, PSeMes2, 1JP–Se = 719 Hz). 77Se NMR (Toluene D8): δ −79.5 (d, PSeMes2, 1JP–Se = 719 Hz). MS (MALDI) 578 [M + 1]. Anal. Calcd for C30H36SeFeNP: C, 62.51; H, 6.30; N, 2.43. Found: C, 62.69; H, 6.55; N, 2.48.PdCl2 Complexes of Fc′(PMes2)(PPh2) (5), Fc′(PMes2)(PtBu2) (6) and Fc′(NMe2)(PPh2) (3a)
On the basis of a known procedure,33,54,101 a suspension of PdCl2 (0.1 mmol) and Fc′(PMes2)(PPh2) (5, 0.1 mmol) or Fc′(PMes2)(PtBu2) (6, 0.1 mmol) or Fc′(NMe2)(PPh2) (3a, 0.2 mmol) in DCM (20 mL) was stirred for 1 h at r.t. Analytically pure crystalline materials were obtained from DCM solution by slow diffusion of dry pentane at room temperature.Fc′(PMes2)(PPh2)·PdCl2 (7)
Yield 81%. 1H NMR (CD2Cl2): δ 1.64 (s, 3H, CH3 of Mes), 1.86 (s, 3H, CH3 of Mes), 2.23 (s, 3H, CH3 of Mes), 2.29 (s, 3H, CH3 of Mes), 2.61 (s, 3H, CH3 of Mes), 3.32 (s, 3H, CH3 of Mes), 3.58 (bs, 1H, H of C5H4), 4.14 (bs, 1H, H of C5H4), 4.15 (bs, 1H, H of C5H4), 4.20 (bs, 1H, H of C5H4), 4.37 (bs, 2H, H of C5H4), 4.56 (bs, 1H, H of C5H4), 5.59 (bs, 1H, H of C5H4), 6.67 (m, 1H, m-H of Mes), 6.79 (m, 1H, m-H of Mes), 6.84 (m, 1H, m-H of Mes), 6.90 (m, 1H, m-H of Mes), 7.44–7.57 (bm, 6H, m and p-H of Ph), 7.89–7.93 (bm, 2H, o-H of Ph), 8.09–8.13 (bm, 2H, o-H of Ph). 13C NMR (CD2Cl2): δ (126 MHz, CD2Cl2) δ 20.94 (d, CH3Mes, J = 9 Hz), 25.38 (d, CH3Mes, J = 7 Hz), 25.85 (d, CH3Mes, J = 2.4 Hz), 27.63 (d, CH3Mes, J = 7 Hz), 28.50 (dd, CH3Mes, J = 15, 5 Hz), 71.74 (d, H of C5H4, J = 6 Hz), 72.40 (d, H of C5H4, J = 5 Hz), 74.52 (d, H of C5H4, J = 10 Hz), 74.73 (d, H of C5H4, J = 7 Hz), 74.94 (d, H of C5H4, J = 12 Hz), 76.28 (dd, ipso-H of C5H4, J = 52, 6 Hz), 78.29 (d, H of C5H4, J = 6 Hz), 78.40 (d, H of C5H4, J = 4 Hz), 80.34 (dd, ipso-H of C5H4, J = 50, 7 Hz), 84.80 (d, H of C5H4, J = 22 Hz), 128.45 (dd, aryl-H, J = 76, 11 Hz), 130.55–131.03 (m, aryl-H), 131.53 (d, aryl-H, J = 3 Hz), 132.00 (d, aryl-H, J = 7 Hz), 133.25 (dd, aryl-H, J = 57, 5 Hz), 135.25 (dd, aryl-H, J = 32, 10 Hz), 139.56 (d, aryl-H, J = 3 Hz), 139.89 (d, aryl-H, J = 8 Hz), 141.53 (d, aryl-H, J = 2 Hz), 142.99 (d, aryl-H, J = 9 Hz). 31P{1H} NMR (CD2Cl2): δ 15.0 (d, PMes, J = 30 Hz), 31.4 (d, PPh, J = 30 Hz). MS (ESI) 780 [M] for [C40H40FeP2PdCl]+. Anal. calcd for C40H40Cl2FeP2Pd: C, 58.89; H, 4.94. Found: C, 59.23; H, 5.16.Fc′(PMes2)(PtBu2)·PdCl2 (8)
Yield 63%. 1H NMR (CD2Cl2): δ 1.25 (s, 3H, CH3 of Mes), 1.77 (s, 3H, CH3 of Mes), 1.60–2.10 (bs, 18H, PC(CH3)3), 2.27 (s, 3H, CH3 of Mes), 2.27 (s, 3H, CH3 of Mes), 3.86 (s, 3H, CH3 of Mes), 3.93 (pst, 1H, H of C5H4), 3.96 (s, 3H, CH3 of Mes), 4.14 (s, 1H, H of C5H4), 4.21 (pst, 1H, H of C5H4), 4.27 (s, 1H, H of C5H4), 4.56 (m, 1H, H of C5H4), 4.64 (d, 1H, H of C5H4), 4.71 (pst, 1H, H of C5H4), 4.94 (m, 1H, H of C5H4), 6.66 (m, 1H, m-H of Mes), 6.69 (s, 1H, m-H of Mes), 6.99 (m, 1H, m-H of Mes), 7.05 (m, 1H, m-H of Mes). 31P {1H} NMR (CD2Cl2): δ 15.8 (d, PMes, J = 34 Hz), 77.3 (d, PtBu, J = 34 Hz). MS (ESI) 740 [M] for cation [C36H48ClFeP2Pd]+. HRMS (MALDI) 739.1327 [C36H48FeP2PdCl]+. Anal. calcd for C36H48Cl2FeP2Pd: C, 55.73; H, 6.24. Found: C, 55.71; H, 5.88. Note: Although this compound was synthesized and crystallized from a solution of DCM, the crystallized species is difficult to dissolve in CD2Cl2, resulting in precipitation of crystallized compound in the NMR tube during measurement for prolonged time. As a result, the quality of 13C NMR obtained was very poor in terms of signal shape and ratio with base line (Fig. S61‡).[Fc′(NMe2)(PPh2)]2·PdCl2 (9)
Yield 48%. 1H NMR (Toluene-d8): δ 2.20 (brm, 12H, NCH3), 3.48 (brs, 4H, H of C5H4), 3.68 (brs, 4H, H of C5H4), 4.17 (brs, 4H, H of C5H4), 4.39 (brs, 4H, H of C5H4), 7.10 (brm, 12H, aryl-H), 7.42 (brm, 8H, aryl-H). 13C NMR (Toluene-d8) δ 41.81 (brs, NCH3), 56.16 (brs, C of C5H4), 65.04 (brs, C of C5H4), 67.07 (brm, ipso-C of C5H4PPh2), 70.07 (brs, C of C5H4), 116.62 (s, ipso-C of C5H4NMe2). 31P{1H} NMR (Toluene-d8): δ −16.2 (brm, PPh). MS (ESI) 968 [M + 1] for [C48H48ClFe2N2P2Pd]+. Anal. Calcd for C48H48Cl2Fe2N2P2Pd: C, 57.43; H, 4.82; N, 2.79. Found: C, 57.59; H, 5.03; N, 2.89.Reactions of AgSbF6 with Fc′(PMes2)(PtBu2)·PdCl2 (8), and [Fc′(NMe2)(PPh2)]2·PdCl2 (9)
Solid AgSbF6 (0.1 mmol, 0.034 g) was added into a DCM solution (20 mL) of Fc′(PMes2)(PtBu2)·PdCl2 (0.1 mmol) or [Fc′(NMe2)(PPh2)]2·PdCl2 (individually 0.1 and 0.05 mmol for selectively synthesizing 11 and 12, respectively). After stirring for overnight at rt., the solution was filtered through a PTFE syringe filter and the product was crystallized by diffusion with hexanes and washed with multiple times of pentanes (3 × 20 mL).[Fc′(PMes2)(PtBu2)·PdCl][SbF6] (10)
Yield 93%. 1H NMR (CD2Cl2): δ 1.58 (d, 18H, P–C-(CH3)3, J = 16 Hz), 2.28 (s, 6H, p-CH3 of Mes), 2.59 (s, 6H, o-CH3 of Mes), 4.00 (m, 2H, H of C5H4PMes2), 4.46 (m, 2H, H of C5H4PMes2), 5.40 (m, 2H, H of C5H4PPh2), 5.57 (m, 2H, H of C5H4PPh2), 6.95 (d, 4H, m-H of Mes, J = 4 Hz). 13C NMR (CD2Cl2): δ 21.12 (s, p-CH3 of Mes), 24.66 (d, o-CH3 of Mes, J = 8 Hz), 31.55 (s, P–C-(CH3)3), 38.17 (dd, P–C-(CH3)3, J = 12 and 5 Hz), 72.07 (d, C of C5H4, J = 7 Hz), 72.23 (d, C of C5H4, J = 11 Hz), 82.71 (d, C of C5H4, J = 6 Hz), 83.31 (d, C of C5H4, J = 6 Hz), 131.63 (d, m-C of Mes, J = 9 Hz), 142.95 (d, C of Ph, J = 3 Hz), 143.49 (dd, C of Ph, J = 11 and 2 Hz). 31P {1H} NMR (CD2Cl2): δ 26.0 (d, PtBu, J = 425 Hz), −47.8 (d, PMes, J = 425 Hz). 19F NMR (CD2Cl2): −134.2 to −111.3 (brm, SbF6). MS (ESI) 740 [M] for [C36H48ClFeP2Pd]+. Anal. Calcd for C36H48ClF6FeP2PdSb: C, 44.29; H, 4.96. Found: C, 44.53; H, 4.63.[Fc′(NMe2)(PPh2)·PdCl][SbF6] (11)
Yield 51%. 1H NMR (CD2Cl2): δ 3.06 (d, 6H, NCH3, J = 4 Hz), 3.38 (s, 2H, H of C5H4PPh2), 3.45 (s, 2H, H of C5H4PPh2), 5.83 (s, 2H, H of C5H4PPh2), 5.99 (s, 2H, H of C5H4PPh2), 7.54–7.56 (brm, 4H, aryl-HPh), 7.63 (d, 2H, aryl-HPh, J = 7 Hz), 8.15 (dd, 4H, aryl-HPh, J = 13, 8 Hz). 13C NMR (CD2Cl2): δ 45.72 (d, NCH3, J = 3 Hz), 60.41 (s, C of C5H4NMe2), 73.98 (d, C of C5H4PPh2, J = 11 Hz), 80.40 (s, C of C5H4NMe2), 87.75 (d, C of C5H4PPh2, J = 8 Hz), 123.29 (d, ipso-C of Ph, J = 63 Hz), 130.00 (d, aryl-C of Ph, J = 13 Hz), 133.79 (d, aryl-C of Ph, J = 3 Hz), 135.49 (d, aryl-C of Ph, J = 13 Hz). 19F NMR (CD2Cl2): −111.3 (brs, SbF6). 31P{1H} NMR (CD2Cl2): δ 1.2 (brs). Note: As compound 11 contains substantial amount of impurities, which could not be removed by multiple crystallization attempts, CHN measurements produced non-reproducible results. High resolution mass measurement (ESI) from this complex showed peak for cation [C24H24ClFeNPPd]+ at 553.9739 (calculated exact mass is 553.9719).[Fc′(NMe2)(PPh2)·PdPPh2(C5H5)][SbF6]2 (12)
Yield ca. 32% on the basis of starting [Fc′(NMe2)(PPh2)]2·PdCl2. 1H NMR (CD2Cl2): δ 2.51 (brm, 6H, NCH3), 3.38 (m, 2H, H of C5H4PPh2), 3.44 (pst, 2H, H of C5H4PPh2), 5.76 (pst, 2H, H of C5H4NMe2), 5.93 (d, 2H, H of C5H4NMe2, J = 2 Hz), 7.48 (brs, 5H, aryl-HPh), 7.56 (td, 4H, aryl-HPh, J = 8 and 3 Hz), 7.63 (dd, 4H, aryl-HPh, J = 9 and 7 Hz), 8.15 (dd, 4H, aryl-HPh, J = 13 and 8 Hz). 13C NMR (CD2Cl2): δ 45.75 (d, NCH3, J = 3 Hz), 60.41 (s, C of C5H4NMe2), 74.00 (d, C of C5H4PPh2, J = 11 Hz), 80.36 (s, C of C5H4NMe2), 87.71 (d, C of C5H4PPh2, J = 8 Hz), 130.03 (d, aryl-C of PPhC5H4, J = 13 Hz), 133.83 (d, aryl-C of PPhC5H4, J = 3 Hz), 135.52 (d, aryl-C of PPhC5H4, J = 13 Hz). 19F NMR (CD2Cl2): −120.6 (brs, SbF6). 31P{1H} NMR (CD2Cl2): δ −5.6 (d, PPh2Fc, J = 37 Hz), 20.6 (d, PPh2C5H4, J = 37 Hz). Note: As compound 12 contains substantial amount of impurities, which could not be removed by multiple crystallization attempts, CHN measurements produced non-reproducible results. High resolution mass measurement (MALDI) from this complex showed [M + 2] peak for cation [C41H39F6FeNP2PdSb]+ (or [Fc′(PPh2)(NMe2)·Pd(PC5H4Ph2)]+(SbF6)−, calculated exact mass for M + 2 is 1005.9884) at 1006.0550 and [C48H48F6Fe2N2P2PdSb]+ (or [Fc′(PPh2)(NMe2)·Pd(PPh2)Fc′(NMe2)]+(SbF6)−, calculated exact mass is 1166.9969) at 1167.1100. This result supports our speculation of [Fc′(PPh2)(NMe2)·Pd(PPh2)Fc′(NMe2)]+(SbF6)− being an intermediate in the formation of compound 12.[Fc′(NMe2)(PPh2)·PdPPh3][BF4]2 (13)
A DCM solution (15 mL) of PPh3 (0.039 g, 0.15 mmol) was added dropwise to another DCM solution (15 mL) of Fc′(NMe2)(PPh2) (0.062 g, 0.15 mmol) and [Pd(MeCN)4][BF4]2 (0.067 g, 0.15 mmol). After stirring at room temperature for 2 h the color of the reaction mixture changes from bluish green to dark red. All the volatiles were removed in high vacuum (10−3 mbar) and further extracted with DCM (15 mL) and crystalized via slow diffusion of hexanes at room temperature. Crystallized yield ca. 97%. 1H NMR (CD2Cl2): δ 2.46 (brs, 6H, NCH3), 3.74 (brs, 2H, H of C5H4PPh2), 4.28 (brs, 2H, H of C5H4PPh2), 5.59 (brs, 2H, H of C5H4NMe2), 5.74 (brs, 2H, H of C5H4NMe2), 7.45 (m, 10H, aryl-H of Ph), 7.53–7.65 (m, 15H, aryl-H of Ph). 13C NMR (CD2Cl2): δ 48.49 (s, NCH3), 61.87 (d, C of C5H4NMe2, J = 2 Hz), 73.67 (d, C of C5H4PPh2, J = 11 Hz), 80.98 (s, C of C5H4NMe2), 87.66 (d, C of C5H4PPh2, J = 9 Hz), 102.35 (d, ipso-C of C5H4NMe2, J = 4 Hz), 119.43 (d, ipso-C of C5H4PPh2, J = 65 Hz), 128.49 (d, ipso-C of Ph, J = 48 Hz), 130.15 (d, aryl-C of Ph, J = 13 Hz), 130.20 (d, aryl-C of Ph, J = 11 Hz), 133.01 (d, aryl-C of Ph, J = 3 Hz), 133.89 (d, aryl-C of PPh, J = 3 Hz), 134.89 (d, aryl-C of Ph, J = 12 Hz), 135.54 (d, aryl-C of PPhFc, J = 12 Hz). 11B NMR (CD2Cl2): δ −1.2 (s, BF4). 19F NMR (CD2Cl2): δ −151.0 (s, BF4). 31P {1H} NMR (CD2Cl2): δ −4.7 (d, PPh2Fc, J = 36 Hz), 21.1 (d, PPh3, J = 36 Hz). MS (ESI) 869 [M] for [C42H39BF4FeNP2Pd]+. Anal. Calcd for C43H41B2Cl2F8FeNP2Pd (13·CH2Cl2): C, 49.64; H, 3.97; N, 1.35. Found: C, 49.61; H, 4.14; N, 1.48.[Fc′(PPh2)(NMe2)·Pd(PPh2)Fc′(NMe2)][BF4]2 (14)
A DCM solution (15 mL) of PR3 (R = o-tollyl or Mes, 0.15 mmol) was added dropwise to another DCM solution (15 mL) of Fc′(NMe2)(PPh2) (0.15 mmol) and [Pd(MeCN)4] [BF4]2 (0.066 g, 0.15 mmol). After stirring at room temperature for 16 h, all the volatiles were removed in high vacuum (10−3 mbar) and further extracted with DCM (15 mL) and crystalized via slow diffusion of hexanes at room temperature. Crystals were thoroughly washed with toluene (3 × 10 mL), ether (3 × 10 mL) and pentane (2 × 10 mL) before characterization. Crystallized yield: 56%. 1H NMR (CD2Cl2): δ 2.48 (s, 6H, NCH3Int), 3.14 (brs, 6H, NCH3Ext), 3.58 (brs, 2H, H of C5H4Ext), 3.71 (brs, 2H, H of C5H4Int), 4.11 (brs, 2H, H of C5H4Int), 4.54 (brs, 2H, H of C5H4Ext), 4.72 (brs, 2H, H of C5H4Int), 4.75 (brs, 2H, H of C5H4Ext), 5.53 (brs, 2H, H of C5H4Int), 5.70 (brs, 2H, H of C5H4Int), 7.46–7.54 (brm, 8H, H of PhInt), 7.60–7.65 (brm, 8H, H of PhExt), 7.85–7.89 (brdd, 4H, H of PhInt & PhExt). 31P{1H} NMR (CD2Cl2): δ −3.4 (d, PPh2Int, J = 36 Hz), 20.3 (d, PPh2Ext, J = 36 Hz) (Fig. S43‡); −2.4 (d, PPh2Int, J = 36 Hz), 21.6 (d, PPh2Ext, J = 36 Hz) (Fig. S45‡). 31P NMR (CD2Cl2): δ −5.5 (brs, PPh2Int), −18.3 (brs, PPh2Ext) (Fig. S44‡). 11B NMR (CD2Cl2): δ −1.12 (s, BF4) (Fig. S44‡). 19F NMR (CD2Cl2): δ −151.11 (brs, BF4). HRMS (MALDI) 932.0970 and 769.0980 [M] for [C41H39FeNP2Pd]2+ and [C24H24FeNP2Pd]2+ (for sample showed in Fig. S43 and S44 of ESI‡). Anal. Calcd for C48H48B2F8Fe2N2P2Pd: C, 52.10; H, 4.37; N, 2.53. Found: C, 51.65; H, 4.44; N, 2.45 (for sample showed in Fig. S44 of ESI‡) and C, 50.09; H, 4.04; N, 2.50 (for sample showed in Fig. S43 of ESI‡). Note: The azaphosphaferrocene unit, which contains both the N-Pd and P-Pd bonds, has been termed as “Internal” (in short “Int”), whereas similar unit, which contains only P-Pd bond, has been termed as “External” (in short “Ext”). Compound 14 can also be synthesized (yield 73%) by reacting 3a with 0.5 eq. of [Pd(MeCN)4][BF4]2 (Fig. S45 of ESI‡; Anal. found: C, 51.89; H, 4.62; N, 2.45, Anal. Calcd for C48H48B2F8Fe2N2P2Pd: C, 52.10; H, 4.37; N, 2.53).[Fc′(PPh2)(NMe2)·PdP(p-OMe-C6H4)3][BF4]2 (15)
An acetone solution (15 mL) of [Fc′(NMe2)(PPh2)] [PdPPh3][BF4]2 (13, 0.143 g, 0.15 mmol) was added to another suspension of P(p-OMe-C6H4)3 (0.053 g, 0.015 mmol) in acetone (15 mL). The reaction mixture was stirred for 10 min at room temperature and subsequently heated at 50 °C for 40 h. The progress of the reaction was monitored by 31P NMR and all the volatiles were removed when no starting material peaks were observed (Fig. S50–S52, ESI‡). Crystallized yield (57%) was obtained by slow diffusion of hexanes in the solution of DCM. To remove all the residual phosphines, the crystals were thoroughly washed with Et2O (4 × 20 mL) before characterization. 1H NMR (CD2Cl2): δ 2.49 (d, 6H, NCH3, J = 3.7 Hz), 3.74 (s, 2H, H of C5H4), 3.82 (s, 9H, OCH3), 4.27 (pst, 2H, H of C5H4), 5.53 (pst, 2H, H of C5H4), 5.70 (s, 2H, H of C5H4), 6.93 (m, 6H, m-H of PhOMe), 7.46 (td, 6H, o-H of PhOMe, J = 7.8, 3.1 Hz,), 7.51–7.59 (m, 10H, H of Ph). 13C NMR (CD2Cl2): δ 48.57 (s, NCH3), 56.04 (s, OCH3), 61.66 (d, C of C5H4NMe2, J = 2 Hz), 73.35 (d, C of C5H4PPh2, J = 11 Hz), 80.31 (s, C of C5H4NMe2), 86.99 (d, C of C5H4PPh2, J = 9 Hz), 102.04 (d, ipso-C of C5H4NMe2, J = 4 Hz), 115.64 (d, aryl-C of PhOMe, J = 12.5 Hz), 119.67 (dm, ipso-C of PPhFc, J = 55 Hz), 119.87 (dd, ipso-C of PhOMe, J = 64, 4 Hz), 130.09 (d, aryl-C of PhOMe, J = 13 Hz), 133.77 (d, aryl-C of PPhFc, J = 3 Hz), 135.49 (d, aryl-C of PPhFc, J = 12 Hz), 136.42 (d, aryl-C of PPhFc, J = 13.1 Hz), 163.32 (d, p-C of PhOMe, J = 3 Hz). 31P {1H} NMR (CD2Cl2): δ −4.9 (d, PPh2, J = 32 Hz), 19.9 (d, P(p-OMe-C6H4)3, J = 32 Hz). 11B NMR (CD2Cl2): δ −1.2 (s, BF4). 19F NMR (CD2Cl2): δ −151.3 (s, BF4). MS (ESI) 959 [M] for [C45H45BF4FeNO3P2Pd]+. Anal. Calcd for C45H45B2F8FeNO3P2Pd: C, 51.69; H, 4.34; N, 1.34. Found: C, 51.67; H, 4.64; N, 1.30.Conclusions
Coordination of PdCl2 with a set of P,N and P,P ligands with 1,1′-ferrocenylene backbone has been explored. While for the P,N ligand monodentate binding was observed, the P,P ligands coordinated in bidentate fashion to the palladium atom. Chloride abstraction led to cationic Pd complexes with bidentate ligand coordination involving Fe → Pd interaction. With P,P ligand 8 this Fe–Pd distance in complex 10 was found shortest as compared to analogous previously published bisphosphane complexes. In the same vein, with P,N ligand 3a, the Fe–Pd distance in complex 11 was also shortest compared with analogous previously published complexes containing ferrocene bridged P,N ligands. By contrast, the same ligand 3a is also involved in the longest Fe–Pd distances observed for the here reported dicationic Pd complexes 12–15, as compared to those reported for complexes of analogous P,N ligands. The Fe–Pd distances were found to be determined primarily by the distances between the ferrocene moieties and the P–Pd–P/N scaffolds, and secondarily by a complex counterbalance of steric and molecular distortions. These structural trends were corroborated by DFT calculations which furthermore indicated a very shallow minimum on the potential energy surface. The stretching of the Fe–Pd bond can be easily counterbalanced by steric effects and secondary interactions. Consistent with these findings, CV measurements demonstrated that reversible iron centered oxidation, which was possible for the free ligand, is suspended by the Fe → Pd interaction.Author contributions
Supervision, writing and resources, R.P. and Z.K.; conceptualization, syntheses and characterization of all compounds, writing, and visualization, S.D.; electrochemical characterization, F.R., crystallographic structure optimization, C.B.; theoretical calculations, Z.K. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
Authors declare no conflicts to interest for this report.Acknowledgements
The authors would like to thank the ZFF Program for financial support. Z. K. is grateful for the general support of János Bolyai Research Scholarship, Project UNKP-22-5-BME-298 and TKP2021-EGA-02 provided by the Ministry of Innovation and Technology of Hungary.References
- P. Štěpnička, Ferrocene Chemistry, Eur. J. Inorg. Chem., 2022, 2022, e202200388 Search PubMed.
- P. Stepnicka, Forever young: the first seventy years of ferrocene, Dalton Trans., 2022, 51, 8085–8102 RSC.
- D. E. Herbert, U. F. Mayer and I. Manners, Strained metallocenophanes and related organometallic rings containing pi-hydrocarbon ligands and transition-metal centers, Angew. Chem., Int. Ed., 2007, 46, 5060–5081 CrossRef CAS PubMed.
- V. Bellas and M. Rehahn, Polyferrocenylsilane-based polymer systems, Angew. Chem., Int. Ed., 2007, 46, 5082–5104 CrossRef CAS PubMed.
- A. Alkan, L. Thomi, T. Gleede and F. R. Wurm, Vinyl ferrocenyl glycidyl ether: an unprotected orthogonal ferrocene monomer for anionic and radical polymerization, Polym. Chem., 2015, 6, 3617–3624 RSC.
- R. Pietschnig, Polymers with pendant ferrocenes, Chem. Soc. Rev., 2016, 45, 5216–5231 RSC.
- L. Cao, I. Manners and M. A. Winnik, Influence of the interplay of crystallization and chain stretching on micellar morphologies: Solution self-assembly of coil-crystalline poly(isoprene-block-ferrocenylsilane), Macromolecules, 2002, 35, 8258–8260 CrossRef CAS.
- F. Jäkle, M. Gallei and H. Qiu, 30 Year anniversary of polyferrocenylsilanes: An inspiration for new advances in main group and transition metal-containing polymers, Polymer, 2022, 254, 125062 CrossRef.
- S. Dey, D. Kargin, M. V. Höfler, B. Szathmári, C. Bruhn, T. Gutmann, Z. Kelemen and R. Pietschnig, Oligo- and polymerization of phospha [2]ferrocenophanes to one dimensional phosphorus chains with ferrocenylene handles, Polymer, 2022, 242, 124589 CrossRef CAS.
- B. Bagh, N. C. Breit, S. Dey, J. B. Gilroy, G. Schatte, K. Harms and J. Muller, Cyclic and linear polyferrocenes with silicon and tin as alternating bridges, Chemistry, 2012, 18, 9722–9733 CrossRef CAS PubMed.
- M. Saleem, H. Yu, L. Wang, Z. ul Abdin, H. Khalid, M. Akram, N. M. Abbasi and J. Huang, Review on synthesis of ferrocene-based redox polymers and derivatives and their application in glucose sensing, Anal. Chim. Acta, 2015, 876, 9–25 CrossRef CAS PubMed.
- X. Sui, X. Feng, M. A. Hempenius and G. J. Vancso, Redox active gels: synthesis, structures and applications, J. Mater. Chem. B, 2013, 1, 1658 RSC.
- J. Elbert, J. Mersini, N. Vilbrandt, C. Lederle, M. Kraska, M. Gallei, B. Stühn, H. Plenio and M. Rehahn, Reversible Activity Modulation of Surface-Attached Grubbs Second Generation Type Catalysts Using Redox-Responsive Polymers, Macromolecules, 2013, 46, 4255–4267 CrossRef CAS.
- C. Ornelas, Application of ferrocene and its derivatives in cancer research, New J. Chem., 2011, 35, 1973 RSC.
- G. Jaouen, A. Vessieres and S. Top, Ferrocifen type anti cancer drugs, Chem. Soc. Rev., 2015, 44, 8802–8817 RSC.
- R. L. Hailes, A. M. Oliver, J. Gwyther, G. R. Whittell and I. Manners, Polyferrocenylsilanes: synthesis, properties, and applications, Chem. Soc. Rev., 2016, 45, 5358–5407 RSC.
- S. Takahashi and J. I. Anzai, Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors, Materials, 2013, 6, 5742–5762 CrossRef CAS PubMed.
- P. Debroy and S. Roy, Recent advances in the synthesis and properties of ferrocenes having an unsaturated backbone, Coord. Chem. Rev., 2007, 251, 203–221 CrossRef CAS.
- D. P. Puzzo, A. C. Arsenault, I. Manners and G. A. Ozin, Electroactive inverse opal: a single material for all colors, Angew. Chem., Int. Ed., 2009, 48, 943–947 CrossRef CAS PubMed.
- D. Löber, S. Dey, B. Kaban, F. Roesler, M. Maurer, H. Hillmer and R. Pietschnig, 3D Micro/Nanopatterning of a Vinylferrocene Copolymer, Molecules, 2020, 25, 2438 CrossRef PubMed.
- K.-S. Gan and T. S. A. Hor, in Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Materials Science, ed. A. Togni and T. Hayashi, John Wiley & Sons, 2007, ch. 1, pp. 3–104 Search PubMed.
- S. W. Chien and T. S. A. Hor, in Ferrocenes: Ligands, Materials and Biomolecules, ed. A. Togni and T. Hayashi, John Wiley & Sons, 2008, ch. 2, pp. 33–116 Search PubMed.
- S. Dey and R. Pietschnig, Chemistry of sterically demanding dppf-analogs, Coord. Chem. Rev., 2021, 437, 213850 CrossRef CAS.
- J. Schulz, J. Antala, I. Cisarova and P. Stepnicka, Beyond phosphorus: synthesis, reactivity, coordination behaviour and catalytic properties of 1,1′-bis(diphenylstibino)ferrocene, Dalton Trans., 2023, 52, 1198–1211 RSC.
- P. Vosáhlo and P. Štěpnička, Assessing the role of substituents in ferrocene acylphosphines and their impact on gold-catalysed reactions, New J. Chem., 2023, 47, 4510–4520 RSC.
- A. Fihri, P. Meunier and J.-C. Hierso, Performances of symmetrical achiral ferrocenylphosphine ligands in palladium-catalyzed cross-coupling reactions: A review of syntheses, catalytic applications and structural properties, Coord. Chem. Rev., 2007, 251, 2017–2055 CrossRef CAS.
- T. J. Colacot and S. Parisel, in Ferrocenes: Ligands, Materials and Biomolecules, ed. P. Štěpnička, John Wiley & Sons, 2008, ch. 3, pp. 117–140 Search PubMed.
- G. Bandoli and A. Dolmella, Ligating ability of 1,1′-bis(diphenylphosphino)ferrocene: a structural survey (1994–1998), Coord. Chem. Rev., 2000, 209, 161–196 CrossRef CAS.
- M. N. Birkholz, Z. Freixa and P. W. van Leeuwen, Bite angle effects of diphosphines in C-C and C-X bond forming cross coupling reactions, Chem. Soc. Rev., 2009, 38, 1099–1118 RSC.
- P. Dierkes and P. W. N. M. van Leeuwen, The bite angle makes the difference: a practical ligand parameter for diphosphine ligands, J. Chem. Soc., Dalton Trans., 1999, 1519–1529 RSC.
- P. W. N. M. van Leeuwen, P. C. J. Kamer and J. N. H. Reek, The bite angle makes the catalyst, Pure Appl. Chem., 1999, 71, 1443–1452 CAS.
- P. Vosáhlo, I. Císařová and P. Štěpnička, Comparing the asymmetric dppf-type ligands with their semi-homologous counterparts, J. Organomet. Chem., 2018, 860, 14–29 CrossRef.
- K. Skoch, I. Cisarova, J. Schulz, U. Siemeling and P. Stepnicka, Synthesis and characterization of 1′-(diphenylphosphino)-1-isocyanoferrocene, an organometallic ligand combining two different soft donor moieties, and its Group 11 metal complexes, Dalton Trans., 2017, 46, 10339–10354 RSC.
- P. Štěpnička, in Ferrocenes: Ligands, Materials and Biomolecules, ed. P. Štěpnička, John Wiley & Sons, 2008, ch. 5, pp. 177–204 Search PubMed.
- H.-U. Blaser, W. Chen, F. Camponovo and A. Togni, in Ferrocenes: Ligands, Materials and Biomolecules, ed. P. Štěpnička, John Wiley & Sons, 2008, ch. 6, pp. 205–235 Search PubMed.
- P. Štěpnička and M. Lamač, in Ferrocenes: Ligands, Materials and Biomolecules, ed. P. Štěpnička, John Wiley & Sons, 2008, ch. 7, pp. 237–277 Search PubMed.
- U. Siemeling, in Ferrocenes: Ligands, Materials and Biomolecules, ed. P. Štěpnička, John Wiley & Sons, 2008, ch. 4, pp. 141–176 Search PubMed.
- A. L. Boyes, I. R. Butler and S. C. Quayle, Palladium(II) complexes of (diisopropylphosphino)-ferrocenes: improved catalysts for the Heck reaction, Tetrahedron Lett., 1998, 39, 7763–7766 CrossRef CAS.
- W. R. Cullen, T. J. Kim, F. W. B. Einstein and T. Jones, Structure of the hydrogenation catalyst [(PP)Rh(NBD)]ClO4, PP = (η5-(Me3C)2PC5H4)2Fe, and some comparative rate studies, Organometallics, 1983, 2, 714–719 CrossRef CAS.
- B. C. Hamann and J. F. Hartwig, Systematic Variation of Bidentate Ligands Used in Aryl Halide Amination. Unexpected Effects of Steric, Electronic, and Geometric Perturbations, J. Am. Chem. Soc., 1998, 120, 3694–3703 CrossRef CAS.
- O. V. Gusev, T. y. A. Peganova, A. M. Kalsin, N. V. Vologdin, P. V. Petrovskii, K. A. Lyssenko, A. V. Tsvetkov and I. P. Beletskaya, Palladium Complexes with Metallocene-Bridged Bidentate Diphosphine Ligands: Synthesis, Structure, and Catalytic Activity in Amination and Cross-Coupling Reactions, Organometallics, 2006, 25, 2750–2760 CrossRef CAS.
- I. R. Butler, W. R. Cullen, T. J. Kim, S. J. Rettig and J. Trotter, 1,1′-Bis(alkylarylphosphino)ferrocenes: synthesis, metal complex formation, and crystal structure of three metal complexes of Fe(η5-C5H4PPh2)2, Organometallics, 1985, 4, 972–980 CrossRef CAS.
- T.-Y. Dong and C.-K. Chang, A Novel Convenient Method to Synthesize Unsymmetrical 1,1′-Disubstituted Ferrocenes Consisting of Various Phosphino, Thiolato and Pyridyl Substituents, J. Chin. Chem. Soc., 1998, 45, 577–579 CrossRef CAS.
- L. E. Hagopian, A. N. Campbell, J. A. Golen, A. L. Rheingold and C. Nataro, Synthesis and electrochemistry of late transition metal complexes containing 1,1′-bis(dicyclohexylphosphino)ferrocene (dcpf). The X-ray structure of [PdCl2(dcpf)] and Buchwald–Hartwig catalysis using [PdCl2(bisphosphinometallocene)] precursors, J. Organomet. Chem., 2006, 691, 4890–4900 CrossRef CAS.
- A. Fihri, J.-C. Hierso, A. Vion, D. H. Nguyen, M. Urrutigoïty, P. Kalck, R. Amardeil and P. Meunier, Diphosphines of dppf-Type Incorporating Electron-Withdrawing Furyl Moieties Substantially Improve the Palladium-Catalysed Amination of Allyl Acetates, Adv. Synth. Catal., 2005, 347, 1198–1202 CrossRef CAS.
- M. Yamashita, J. V. C. Vicario and J. F. Hartwig, Trans Influence on the Rate of Reductive Elimination. Reductive Elimination of Amines from Isomeric Arylpalladium Amides with Unsymmetrical Coordination Spheres, J. Am. Chem. Soc., 2003, 125, 16347–16360 CrossRef CAS PubMed.
- J.-C. Hierso, F. Lacassin, R. Broussier, R. Amardeil and P. Meunier, Synthesis and characterisation of a new class of phosphine-phosphonite ferrocenediyl dinuclear rhodium complexes, J. Organomet. Chem., 2004, 689, 766–769 CrossRef CAS.
- M. Laly, R. Broussier and B. Gautheron, Ferrocene-based phosphonite–phosphine ligands, Pd and Rh complexes, Tetrahedron Lett., 2000, 41, 1183–1185 CrossRef CAS.
- P. Štěpnička and I. Císařová, Synthesis of [1′-(diphenylthiophosphoryl)ferrocenyl]ethyne and alkyne-metal complexes thereof, J. Organomet. Chem., 2006, 691, 2863–2871 CrossRef.
- M. E. Wright, 1,1′-Bis(tri-n-butylstannyl)ferrocene: selective transmetalation applied to the synthesis of new ferrocenyl ligands, Organometallics, 1990, 9, 853–856 CrossRef CAS.
- J. Podlaha, P. Štěpnička, J. Ludvík and I. Císařová, 1′-(Diphenylphosphino)ferrocenecarboxylic Acid and Its P-Oxide and Methyl Ester: Synthesis, Characterization, Crystal Structure, and Electrochemistry, Organometallics, 1996, 15, 543–550 CrossRef CAS.
- L. Meca, D. Dvořák, J. Ludvík, I. Císařová and P. Štěpnička, Synthesis, Structures, and Electrochemistry of Group 6 Aminocarbenes with a P-Chelating 1′-(Diphenylphosphino)ferrocenyl Substituent, Organometallics, 2004, 23, 2541–2551 CrossRef CAS.
- I. R. Butler, S. J. Coles, M. B. Hursthouse, D. J. Roberts and N. Fujimoto, Ferrocene-based ligands in ruthenium alkylidene chemistry, Inorg. Chem. Commun., 2003, 6, 760–762 CrossRef CAS.
- K. Skoch, I. Cisarova, F. Uhlik and P. Stepnicka, Comparing the reactivity of isomeric phosphinoferrocene nitrile and isocyanide in Pd(ii) complexes: synthesis of simple coordination compounds vs. preparation of P-chelated insertion products and Fischer-type carbenes, Dalton Trans., 2018, 47, 16082–16101 RSC.
- V. C. Gibson, N. J. Long, A. J. P. White, C. K. Williams and D. J. Williams, The Synthesis and Metal Coordination Chemistry of a Novel Phosphine- and Thiolate-Substituted Ferrocenediyl Ligand, Organometallics, 2002, 21, 770–772 CrossRef CAS.
- W. Zhang, Y.-i. Yoneda, T. Kida, Y. Nakatsuji and I. Ikeda, Novel chiral P,N-ferrocene ligands in palladium-catalyzed asymmetric allylic alkylations[1], Tetrahedron: Asymmetry, 1998, 9, 3371–3380 CrossRef CAS.
- P. Štěpnička, I. Císařová and R. Gyepes, Synthesis and Structural Characterisation of Palladium and Group-12 Metal Complexes with a Hybrid Phosphanylphosphonate Ferrocene Ligand, Eur. J. Inorg. Chem., 2006, 2006, 926–938 CrossRef.
- P. Štěpnička and I. Císařová, Synthesis, characterization and structure of rhodium(I) carbonyl complexes with O,P-chelating 1′-(diphenylphosphino)ferrocenecarboxylate or P-monodentate 1′-(diphenylphosphino)ferrocenecarboxylic acid, J. Chem. Soc., Dalton Trans., 1998, 2807–2812 RSC.
- K. Mach, J. Kubista, I. Císařová and P. Štěpnička, [μ-1κ2O,O′:2(η5)-Cyclopentadienylcarboxylato][2(η5)-diphenylphosphinocyclopentadienyl]bis[1,1(η5)-tetramethylcyclopentadienyl]iron(II)titanium(III), Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2002, 58, m116–m118 CrossRef PubMed.
- R. C. J. Atkinson, V. C. Gibson, N. J. Long, A. J. P. White and D. J. Williams, Synthesis, Coordination Chemistry, and Catalytic Application of a Novel Unsymmetrical P/O Ferrocenediyl Ligand, Organometallics, 2004, 23, 2744–2751 CrossRef CAS.
- T. Yoshida, K. Tani, T. Yamagata, Y. Tatsuno and T. Saito, Preparation and structure of [Rh{(η5-C5H4(2-C5H4N))(η5-C5H4PPh2)}(cod)]PF6 and [Ir(H){Fe[η5-C5H3(2-C5H4N)](η5-C5H4PPh2)}(cod)]PF6; a Rh complex having a C–H⋯Rh interaction and a hydrido Ir complex (where cod = cyclo-octa-1,5-diene), J. Chem. Soc., Chem. Commun., 1990, 292–294 RSC.
- I. R. Butler, M. Kalaji, L. Nehrlich, M. Hursthouse, A. I. Karaulov and K. M. A. Malik, The palladium(II) coordination chemistry of ferrocenylphosphinobipyridines: a novel trans-chelating ligand system, J. Chem. Soc., Chem. Commun., 1995, 459–460 RSC.
- Z. Weng, S. Teo and T. S. A. Hor, Stabilization of Nickel(0) by Hemilabile P,N-Ferrocene Ligands and Their Ethylene Oligomerization Activities, Organometallics, 2006, 25, 4878–4882 CrossRef CAS.
- Z. Weng, S. Teo, L. L. Koh and T. S. A. Hor, A structurally characterized Ni–Al methyl-bridged complex with catalytic ethylene oligomerization activity, Chem. Commun., 2006, 1319–1321 RSC.
- M. Widhalm, U. Nettekoven and K. Mereiter, Chiral ferrocene derivatives containing a 2,2′-bridged binaphthyl moiety, Tetrahedron: Asymmetry, 1999, 10, 4369–4391 CrossRef CAS.
- O. Bárta, R. Gyepes, I. Císařová, A. Alemayehu and P. Štěpnička, Synthesis and study of Fe → Pd interactions in unsymmetric Pd(ii) complexes with phosphinoferrocene guanidine ligands, Dalton Trans., 2020, 49, 4225–4229 RSC.
- M. Navrátil, I. Císařová and P. Štěpnička, Synthesis and coordination of hybrid phosphinoferrocenes with extended donor pendants, Dalton Trans., 2022, 51, 14618–14629 RSC.
- Z. Weng, S. Teo, L. L. Koh and T. S. A. Hor, Ethylene Oligomerization at Coordinatively and Electronically Unsaturated Low-Valent Nickel, Angew. Chem., Int. Ed., 2005, 44, 7560–7564 CrossRef CAS PubMed.
- Z. Weng, S. Teo, L. L. Koh and T. S. A. Hor, Efficient Suzuki Coupling of Aryl Chlorides Catalyzed by Palladium(0) with a P,N Heteroligand and Isolation of Unsaturated Intermediates, Organometallics, 2004, 23, 4342–4345 CrossRef CAS.
- M. Abubekerov, S. I. Khan and P. L. Diaconescu, Ferrocene-bis(phosphinimine) Nickel(II) and Palladium(II) Alkyl Complexes: Influence of the Fe–M (M = Ni and Pd) Interaction on Redox Activity and Olefin Coordination, Organometallics, 2017, 36, 4394–4402 CrossRef CAS.
- K. Škoch, P. Vosáhlo, I. Císařová and P. Štěpnička, Synthesis and characterisation of Pd(ii) and Au(i) complexes with mesoionic carbene ligands bearing phosphinoferrocene substituents and isomeric carbene moieites, Dalton Trans., 2020, 49, 1011–1021 RSC.
- M. R. Ringenberg, Beyond Common Metal–Metal Bonds, κ3-Bis(donor)ferrocenyl → Transition-Metal Interactions, Chem. – Eur. J., 2019, 25, 2396–2406 CrossRef CAS PubMed.
- S. Dey, J. W. Quail and J. Müller, [2]Ferrocenophanes with Nitrogen in Bridging Positions, Organometallics, 2015, 34, 3039–3046 CrossRef CAS.
- I. R. Butler and S. C. Quayle, The synthesis and characterisation of heterosubstituted aminoferrocenes, J. Appl. Crystallogr., 1998, 552, 63–68 CAS.
- S. Dey, PhD Doctoral Dissertation, University of Saskatchewan, 2016.
- H. Bhattacharjee, S. Dey, J. Zhu, W. Sun and J. Muller, Strained azabora[2]ferrocenophanes, Chem. Commun., 2018, 54, 5562–5565 RSC.
- S. Dey, W. Sun and J. Muller, [n]Ferrocenophanes (n = 2, 3) with Nitrogen and Phosphorus in Bridging Positions, Inorg. Chem., 2016, 55, 3630–3639 CrossRef CAS PubMed.
- M. Kleoff, J. Schwan, L. Boeser, B. Hartmayer, M. Christmann, B. Sarkar and P. Heretsch, Scalable Synthesis of Functionalized Ferrocenyl Azides and Amines Enabled by Flow Chemistry, Org. Lett., 2020, 22, 902–907 CrossRef CAS PubMed.
- C. Metallinos, J. Zaifman, T. Dudding, L. Van Belle and K. Taban, Asymmetric Lithiation of Boron Trifluoride–Activated Aminoferrocenes: An Experimental and Computational Investigation, Adv. Synth. Catal., 2010, 352, 1967–1982 CrossRef CAS.
- C. Metallinos, J. Zaifman and L. Dodge, Aminoferrocene Lithiation by Boron Trifluoride Activation, Org. Lett., 2008, 10, 3527–3530 CrossRef CAS PubMed.
- M. B. Hursthouse, S. J. Coles and I. R. Butler, CSD Commun., 2003 DOI:10.5517/cc79s09.
- S. Dey, F. Roesler, M. V. Höfler, C. Bruhn, T. Gutmann and R. Pietschnig, Synthesis, Structure and Cu–Phenylacetylide Coordination of an Unsymmetrically Substituted Bulky dppf–Analog, Eur. J. Inorg. Chem., 2021, 2022, e202100939 Search PubMed.
- S. Dey, D. Buzsaki, C. Bruhn, Z. Kelemen and R. Pietschnig, Bulky 1,1′-bisphosphanoferrocenes and their coordination behaviour towards Cu(i), Dalton Trans., 2020, 49, 6668–6681 RSC.
- G. Pilloni, B. Longato, G. Bandoli and B. Corain, Bonding ability of 1,1′-bis(diphenylthiophosphoryl)ferrocene (dptpf) and its selenium analogue towards copper(I). Crystal structure of [Cu(dptpf)]BF4, J. Chem. Soc., Dalton Trans., 1997, 819–826 RSC.
- K. M. Gramigna, J. V. Oria, C. L. Mandell, M. A. Tiedemann, W. G. Dougherty, N. A. Piro, W. S. Kassel, B. C. Chan, P. L. Diaconescu and C. Nataro, Palladium(II) and Platinum(II) Compounds of 1,1′-Bis(phosphino)metallocene (M = Fe, Ru) Ligands with Metal–Metal Interactions, Organometallics, 2013, 32, 5966–5979 CrossRef CAS.
- K. D. Cabrera, A. T. Rowland, J. M. Szarko, P. L. Diaconescu, M. W. Bezpalko, W. S. Kassel and C. Nataro, Monodentate phosphine substitution in [Pd(κ3-dppf)(PR3)][BF4]2 (dppf = 1,1′-bis(diphenylphosphino)ferrocene) compounds, Dalton Trans., 2017, 46, 5702–5710 RSC.
- M. Sato, H. Shigeta, M. Sekino and S. Akabori, Synthesis, some reactions, and molecular structure of the Pd(BF4)2 complex of 1,1′-bis(diphenylphosphino)ferrocene, J. Organomet. Chem., 1993, 458, 199–204 CrossRef CAS.
- B. L. Blass, R. H. Sánchez, V. A. Decker, M. J. Robinson, N. A. Piro, W. S. Kassel, P. L. Diaconescu and C. Nataro, Structural, Computational, and Spectroscopic Investigation of [Pd(κ3-1,1′-bis(di-tert-butylphosphino)ferrocenediyl)X] + (X = Cl, Br, I) Compounds, Organometallics, 2016, 35, 462–470 CrossRef CAS.
- G. Mann, Q. Shelby, A. H. Roy and J. F. Hartwig, Electronic and Steric Effects on the Reductive Elimination of Diaryl Ethers from Palladium(II), Organometallics, 2003, 22, 2775–2789 CrossRef CAS.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, Mercury: visualization and analysis of crystal structures, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, Mercury CSD 2.0− new features for the visualization and investigation of crystal structures, J. Appl. Crystallogr., 2008, 41, 466–470 CrossRef CAS.
- K. Jess, D. Baabe, T. Bannenberg, K. Brandhorst, M. Freytag, P. G. Jones and M. Tamm, Ni–Fe and Pd–Fe Interactions in Nickel(II) and Palladium(II) Complexes of a Ferrocene-Bridged Bis(imidazolin-2-imine) Ligand, Inorg. Chem., 2015, 54, 12032–12045 CrossRef CAS PubMed.
- S. Weller, S. H. Schlindwein, C. M. Feil, Z. Kelemen, D. Buzsáki, L. Nyulászi, S. Isenberg, R. Pietschnig, M. Nieger and D. Gudat, A Ferrocenophane-Based Diaminophosphenium Ion, Organometallics, 2019, 38, 4717–4725 CrossRef CAS.
- D. Kargin, Z. Kelemen, K. Krekic, M. Maurer, C. Bruhn, L. Nyulaszi and R. Pietschnig, [3]Ferrocenophanes with the bisphosphanotetryl bridge: inorganic rings on the way to tetrylenes, Dalton Trans., 2016, 45, 2180–2189 RSC.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist, Organometallics, 2010, 29, 2176–2179 CrossRef CAS.
- G. M. Sheldrick, A short history of Shelx, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, Mercury CSD 2.0− new features for the visualization and investigation of crystal structures, J. Appl. Crystallogr., 2008, 41, 466 CrossRef CAS.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, Mercury: visualization and analysis of crystal structures, J. Appl. Crystallogr., 2006, 39, 453 CrossRef CAS.
- A. L. Spek, Platon Squeeze: a tool for the calculation of the disordered solvent contribution to the calculated structure factors, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9 CrossRef CAS PubMed.
- I. Noviandri, K. N. Brown, D. S. Fleming, P. T. Gulyas, P. A. Lay, A. F. Masters and L. Phillips, The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer, J. Phys. Chem. B, 1999, 103, 6713 CrossRef CAS.
- P. Vosáhlo, J. Schulz, K. Škoch, I. Císařová and P. Štěpnička, Synthesis and characterisation of palladium(II) complexes with hybrid phosphinoferrocene ligands bearing additional O-donor substituents, New J. Chem., 2019, 43, 4463–4470 RSC.
Footnotes |
| † Dedicated to Prof. Dr László Nyulászi on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Synthetic protocols, NMR spectra, crystal refinement data, CV and details of DFT calculations. CCDC 217930 and 2243451–2243462 for compounds 1, 3b, 4a, 7–15, respectively. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00576c |
| This journal is © the Partner Organisations 2023 |