Selective oxidative β-C–H bond sulfenylation of tetrahydroisoquinolines with elemental sulfur†
Tao
Guo
 a,
Lei
Bi
a,
Lu
Shen
a,
Quanhong
Wei
a,
Congjun
Zhu
a,
Lei
Bi
a,
Lu
Shen
a,
Quanhong
Wei
a,
Congjun
Zhu
 *a,
Panke
Zhang
*a,
Panke
Zhang
 *b and
Yunhui
Zhao
*b and
Yunhui
Zhao
 *c
*c
aSchool of Chemistry and Chemical Engineering, Henan University of Technology, Zhengzhou, Henan 450001, P. R. China. E-mail: CongjunZhu@haut.edu.cn
bGreen Catalysis Center, College of Chemistry, Henan Advanced Institute of Technology, Zhengzhou University, Zhengzhou 450001, PR China. E-mail: pkzhang@zzu.edu.cn
cSchool of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan, Hunan 411201, P. R. China. E-mail: zhao_yunhui@163.com
First published on 6th December 2022
Abstract
In this article, a convenient and efficient KIO3-promoted oxidative sulfenylation at the β-position of tetrahydroisoquinolines and subsequent aromatization in the presence of elemental S8 is presented. The reaction proceeds with moderate to good yields via a double C–S formation process. A wide range of structurally diverse 4-sulfenylisoquinolines/3-sulfenylpiperidine were synthesized with excellent functional group tolerance and high efficiency.
Introduction
Organosulfur compounds are prevalent in the areas of organic functional materials, natural products and pharmaceuticals. They often display versatile fluorescence, biological and medicinal properties.1 As a result, continuous efforts have been applied to the synthesis of various organosulfur compounds, including thiophenes, thioamides, sulfides, disulfides and thiazoles using a variety of sulfenylating reagents.2 In recent years, elemental sulfur (S8) has gained more interest than other sulfur sources (such as RSSR, RSO2Cl, RSO2NHNH2, RSO2Na, SO2) because it is economical, non-toxic and easily handled as a powder.3,4 Generally, the metal-mediated S-arylation/heteroarylation of R–X/OH/OTf/B(OH)2 with S8 through direct C–H activation has emerged as a reliable and valuable method for the synthesis of organosulfur compounds (Scheme 1a).5 Nevertheless, the pre-functionalization steps required for the preparation of appropriate halo or boronic acid fragments limit the application of these methods. From an atom- and step-economy standpoint, direct oxidative dual C–H sulfenylation represents a sustainable and concise strategy for the synthesis of organosulfur compounds.6 For example, Tang and co-workers reported a DTBP-induced α-C–H S-heteroarylation of ethers/alkanes with imidazopiperidine in the presence of elemental S8 (Scheme 1b).7 Subsequently, our group described a DMSO-promoted Ar–H/HetAr–H S-heteroarylation of arylamines/arenols/indoles using elemental sulfur (Scheme 1c).8 However, more efficient synthetic methods involving elemental sulfur are still required.Because of their simplicity and commercial availability, 1,2,3,4-tetrahydroisoquinolines (THIQs) are commonly used in the synthesis of value-added compounds, often via their C(sp3)–H functionalization. Recently, various methods have been developed for the direct C–H functionalization at the C1(α) position of THIQs, such as N-arylation, α-sulfenylation, phosphonylation and annulation.9 Due to its lower activity, functionalization of the β-C–H bond of THIQs is more challenging, and therefore much less studied, than functionalization of the α-C–H bond.10 Organosulfur compounds that include an isoquinoline motif represent a diverse library of bioactivities and unique properties. These activities are critically dependent on the nature of the substituent at the 4-position;11 thus, several advancements in the β-sulfenylation of isoquinolines have been made in recent years. However, most of these studies involve multiple steps or require pre-functionalization reactions.12 Continuing our efforts in S8-mediated C–H sulfenylation for the construction of organosulfur compounds in medicinal chemistry,8,13 here we present a metal-free cascade method for the generation of 4-sulfenylisoquinolines and 3-sulfenylpyridines via the KIO3-promoted oxidative β-C–H bond sulfenylation and aromatization of THIQs/piperidines (Scheme 1d). This strategy presents the following benefits: (i) the β-C–H bond of the N-heterocycles is directly functionalized, and the ring is further aromatized, without the introduction of directing or protecting groups; (ii) a thioether is synthesized, which links the two subunits, using stable and odorless S8 as the sulfur source; (iii) the reaction is green and environmentally friendly through its use of a non-metallic oxidant.
Results and discussion
Because the imidazopyridine backbone is a core unit of many commercially available drugs, we surmised that imidazopyridine would be a good candidate for reaction development. Initially, THIQ (1a), 2-phenylimidazopyridine (2a) and elemental sulfur were selected as model substrates for optimization reactions with various oxidants, additives and solvents under air atmosphere (Table 1). In consideration of easy oxidation features14 and purity of THIQ (96%, Innochem), 4 equivalents of 1a was used. First, the role of the oxidant was examined through background experiments. No product was observed in the absence of oxidant (entry 1). The use of a metallic oxidant (Cu(OAc)2, Ag2CO3) also did not lead to the desired product (entries 2 and 3). Instead, metal-free oxidative reagents (KIO3) were found to promote the reaction; coupling product 3a was isolated in 27% yield (using DMF as a reaction solvent, entry 4). Only a trace amount of product was detected when a stronger oxidant was used (entry 5). Next, the investigation of different solvents (DMAc, xylene, NMP and DMSO), revealed that DMSO was the most favorable, affording 3a in 56% yield (entries 6–9). Increasing the reaction temperature to 110 °C (from 100 °C) resulted in an increased yield. However, when the reaction temperature was further increased (120 °C) or reduced (to 90 °C), lower yields were observed (entries 10–12). The amount of oxidant influenced the reaction efficiency; decreasing the amount of KIO3 from 2 equiv. to 1 equiv. lowered the yield of the desired product to 39% (entries 13 and 14). Changing the concentartion of solvent did not improve the yield of the reaction. Several different additives (I2, TBAI, FeCl3) were trailed; however, none of them promoted the formation of desired product 3a (entries 15–17). Furthermore, a longer reaction time did not increase the yield (entry 18). Control experiments revealed that a nitrogen atmosphere decreased the yield of 3a (entry 19). However, reaction using an oxygen balloon did not increase the yield (affording 75% yield, compared to 78% with an air atmosphere), suggesting that the use of an oxygen atmosphere was not necessary (entry 20). Consequently, the conditions in entry 11 were selected as optimized conditions.| Entrya | Oxidant (eq.) | Additive (eq.) | Solvent (0.1 M) | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.8 mmol), S8 (0.6 mmol), 2a (0.2 mmol), oxidant, additive, and solvent in air reacted for 10 h. b Isolated yield. c Reaction time: 24 h. d Under N2. e Under O2. | |||||
| 1 | –– | –– | DMF | 100 | ND |
| 2 | Cu(OAc)2 (2) | –– | DMF | 100 | ND |
| 3 | Ag2CO3 (2) | –– | DMF | 100 | ND |
| 4 | KIO3 (2) | –– | DMF | 100 | 27 |
| 5 | KIO4 (2) | –– | DMF | 100 | Trace |
| 6 | KIO3 (2) | –– | DMAc | 100 | Trace |
| 7 | KIO3 (2) | –– | Xylene | 100 | ND |
| 8 | KIO3 (2) | –– | NMP | 100 | Trace |
| 9 | KIO3 (2) | –– | DMSO | 100 | 56 |
| 10 | KIO3 (2) | –– | DMSO | 90 | 33 |
| 11 | KIO 3 (2) | –– | DMSO | 110 | 78 |
| 12 | KIO3 (2) | –– | DMSO | 120 | 62 |
| 13 | KIO3 (1) | –– | DMSO | 110 | 39 |
| 14 | KIO3 (3) | –– | DMSO | 110 | 73 |
| 15 | KIO3 (2) | I2 (0.5) | DMSO | 110 | 70 |
| 16 | KIO3 (2) | TBAI (0.5) | DMSO | 110 | 68 |
| 17 | KIO3 (2) | FeCl3 (0.2) | DMSO | 110 | 30 |
| 18c | KIO3 (2) | –– | DMSO | 110 | 76 |
| 19d | KIO3 (2) | –– | DMSO | 110 | 45 |
| 20e | KIO3 (2) | –– | DMSO | 110 | 75 |
To explore the substrate scope of the reaction, various substituted THIQs and imidazoheterocycles were tested (Table 2). Imidazoheterocycles containing electron-withdrawing (CN, CF3, NO2) or electron-donating (OMe, OH) substituents at various positions on the phenyl ring were tolerated; these compounds reacted with THIQ (1a) to afford the expected products 3b–3g in 34–80% yields. However, steric hindrance from the OH group at the ortho position negatively impacted the yield (3f). Furthermore, n-pentyl, naphthyl, and thienyl-substituted imidazopyridines worked well, indicating that a π–π interaction is not required for the transformation. Substrates with Br or Me substituents on the pyridine ring of the imidazopyridine were also applicable (3j–3k). A hetero-analog (3l) was obtained in 50% yield, by subjecting imidazo[2,1-b]thiazole to the optimized reaction conditions. The structure of 3l was further confirmed by X-ray crystallography (CCDC 2168420).†
Next, several THIQs were subjected to the reaction with S8 and 2a. THIQs functionalized with Me, OMe, Cl, NO2 and NH2 groups were tolerated. The electronic nature of the phenyl ring had a significant effect on the yield of the reaction; substrates bearing strong electron-donating groups (OMe, NH2) afforded lower yields than those bearing electron-withdrawing groups (Cl, NO2, CO2Me) (3n–pversus3q–s). Additionally, 3-methylpiperidine delivered the desired coupling product 3t in 49% yield. Notably, 3u was generated using 4-methylpiperidine as a substrate, albeit with definite steric hindrance. Most importantly, elemental S8 could be replaced with Se under similar conditions to afford 4-selanylisoquinoline 3v, highlighting the wide applicability of the method.
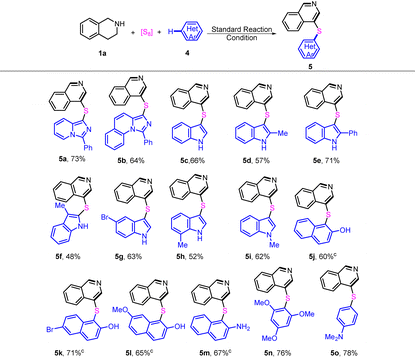
Next, we turned our attention to the variation of the coupling partners, the result shown in Table 3. Other heteroarene/arene substrates 4, such as imidazo[1,5-a]pyridines, indoles and 2-naphthols, were employed instead of 2 in the reaction. Imidazo[1,5-a]pyridine and imidazo[1,5-a]quinolone were first treated with THIQ and S8 under the standard conditions, giving 5a–5b in 73% and 64% yields, respectively. The reaction of indoles with Me, phenyl, or Br substituents at different positions afforded the corresponding products 5c–5h in 48–71% yields (a slight decrement in yield was observed in the case of 3-methylindole). The Br group, which may facilitate further synthetic transformations, was well-tolerated in the reaction. Gratifyingly, N-Me indole also reacted smoothly with THIQ and S8, affording the corresponding product 5i in 62% yield. Furthermore, 2-naphthol derivatives were suitable as substrates, affording 4-sulfenylisoquinolines 5j–5l in 60–71% yields. Expectedly, the reaction of 2-naphthylamine gave the desired product 5m in 67% yield. Similarly, replacing the imidazoheterocycle core with a different arene core (1,3,5-trimethoxybenzene or N,N-dimethylaniline) also proved possible, resulting in the formation of 5n and 5o in 76% and 78% yields, respectively.
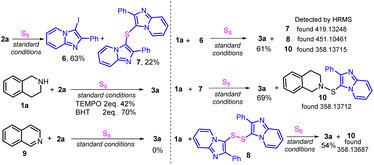
To probe the mechanism of the reaction, several control experiments were conducted (Scheme 2). First, treatment of 2a with S8 without THIQ afforded 3-iodo-2-phenylimidazopyridine (6, 63%) and 7 (22%). Next, with the addition of the radical inhibitor TEMPO (2,2,6,6-tetramethylpiperidine, 2.0 equiv.) or the radical scavenger BHT (butylated hydroxytoluene, 2.0 equiv.), 3a was isolated in 42% and 70% yields, respectively, indicating that the reaction probably did not proceed via a radical intermediate. Furthermore, sulfide 7, disulfide 8, and isoquinoline 9 were detected by mass spectrometry in the standard reaction (see ESI P97–99 for details†). Next, potential intermediates were examined. No desired product was obtained from the reaction of 9 with 2a, excluding the possibility of isoquinoline as the intermediate. Treatment of THIQ 1a with 6, 7, and 8 afforded the desired product in 61%, 69%, and 54% yields, respectively, suggesting that all of 6–8 may be intermediates. Notably, the crucial intermediate 10 was confirmed by HRMS.
In light of these experimental observations and reports in the literature,15 a feasible reaction mechanism is presented in Scheme 3. Initially, 6 is generated by the oxidation of 2a. This is followed by sulfenylation and dimerization to yield disulfide 8,15a–c which can be attacked by 2a to form sulfide 7.15d Next, the nitrogen atom of THIQ can attack 7 and 8 to form the symmetrical thioether species 10.15e,f Finally, 10 can undergo rearrangement11c and further proton abstraction to yield 4-sulfenylisoquinoline 3a.
Conclusions
In summary, a novel KIO3-promoted oxidative sulfenylation reaction of tetrahydroisoquinolines using elemental sulfur and air conditions has been developed. A variety of 4-sulfenylisoquinolines and 3-sulfenylpyridines were prepared from the double C–H sulfenylation of 1,2,3,4-tetrahydroisoquinolines/piperidines with (hetero)arenes with excellent functional group compatibility and wide substrate scope. Importantly, this research paves the way for applications of elemental sulfur in the β-C–H bond sulfenylation and aromatization of N-heterocycles in organic chemistry without the need for directing or protecting groups. The exploration of applications of the methods developed in this paper and the antitumor activities of the compounds is currently underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22202060), the Science and Technology Foundation of Henan Province (No. 212102210202), the Project of Youth Backbone Teachers of Henan University of Technology (21420072), the Science and Technology Cooperation Foundation of Zhengzhou City (21ZZXTCX16), the Innovative Funds Plan of Henan University of Technology (2021ZKCJ08), the Funding plan of key scientific research projects in colleges and universities of Henan Province (23A150029), the Ph.D. Foundation of Henan University of Technology (2021BS080), and Natural Science Foundation of Hunan Provincial (No. 2020JJ4028). The authors are also thankful to Xiaodi Yang, Shanghai University of Traditional Chinese Medicine, for her assistance with X-ray crystallography.References
- (a) C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC; (b) M. Mellah, A. Voituriez and E. J. C. r. Schulz, Chem. Rev., 2007, 107, 5133–5209 Search PubMed; (c) S. Otocka, M. Kwiatkowska, L. Madalinska and P. Kielbasinski, Chem. Rev., 2017, 117, 4147–4181 CrossRef CAS PubMed.
- (a) W. Cai and Z. Gu, Org. Lett., 2019, 21, 3204–3209 Search PubMed; (b) A. Ghosh, M. Lecomte, S. H. Kim-Lee and A. T. Radosevich, Angew. Chem., Int. Ed., 2019, 58, 2864–2869 CrossRef CAS PubMed; (c) Y. S. Kang, P. Zhang, M. Y. Li, Y. K. Chen, H. J. Xu, J. Zhao, W. Y. Sun, J. Q. Yu and Y. Lu, Angew. Chem., Int. Ed., 2019, 58, 9099–9103 CrossRef CAS PubMed; (d) S. Karreman, S. B. H. Karnbrock, S. Kolle, C. Golz and M. Alcarazo, Org. Lett., 2021, 23, 1991–1995 CrossRef CAS PubMed; (e) N. R. Koning, A. P. Sundin and D. Strand, J. Am. Chem. Soc., 2021, 143, 21218–21222 Search PubMed; (f) D. Liu, H. X. Ma, P. Fang and T. S. Mei, Angew. Chem., Int. Ed., 2019, 58, 5033–5037 Search PubMed; (g) C. J. Nalbandian, Z. E. Brown, E. Alvarez and J. L. Gustafson, Org. Lett., 2018, 20, 3211–3214 CrossRef CAS PubMed; (h) K. Sun, Y. Li, R. Feng, S. Mu, X. Wang and B. Zhang, J. Org. Chem., 2020, 85, 1001–1008 CrossRef CAS PubMed; (i) P. Sun, D. Yang, W. Wei, M. Jiang, Z. Wang, L. Zhang, H. Zhang, Z. Zhang, Y. Wang and H. Wang, Green Chem., 2017, 19, 4785–4791 RSC; (j) X. Yang, Y. Bao, Z. Dai, Q. Zhou and F. Yang, Green Chem., 2018, 20, 3727–3731 RSC; (k) C. Qu, R. Liu, Z. Wang, Y. Lv, H. Yue and W. Wei, Green Chem., 2022, 24, 4915–4920 RSC.
- For recent reviews on elemental sulfur, see: (a) H. Huang, G.-J. Deng and S. Liu, Synlett, 2020, 142–158 Search PubMed; (b) J. Lim, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249–3258 Search PubMed; (c) T. B. Nguyen, Adv. Synth. Catal., 2017, 359, 1066–1130 CrossRef CAS; (d) T. B. Nguyen, Adv. Synth. Catal., 2020, 362, 3448–3484 CrossRef CAS.
- For research articles on elemental sulfur, see: (a) T. B. Nguyen and P. Retailleau, Org. Lett., 2018, 20, 186–189 CrossRef CAS PubMed; (b) C. K. Ran, L. Song, Y. N. Niu, M. K. Wei, Z. Zhang, X. Y. Zhou and D. G. Yu, Green Chem., 2021, 23, 274–279 RSC; (c) M. Wu, Y. Jiang, Z. An, Z. Qi and R. Yan, Adv. Synth. Catal., 2018, 360, 4236–4240 CrossRef CAS; (d) H. Xie, G. Li, F. Zhang, F. Xiao and G. J. Deng, Green Chem., 2018, 20, 827–831 RSC; (e) Q. Xing, Y. Ma, H. Xie, F. Xiao, F. Zhang and G. J. Deng, J. Org. Chem., 2019, 84, 1238–1246 Search PubMed; (f) J. Zhang, C. Song, L. Sheng, P. Liu and P. Sun, J. Org. Chem., 2019, 84, 2191–2199 CrossRef CAS PubMed.
- (a) C. Chen, Y. Xie, L. Chu, R. W. Wang, X. Zhang and F. L. Qing, Angew. Chem., Int. Ed., 2012, 51, 2492–2495 CrossRef CAS PubMed; (b) J. C. Deng, Y. C. Gao, Z. Zhu, L. Xu, Z. D. Li and R. Y. Tang, Org. Lett., 2019, 21, 545–548 CrossRef CAS PubMed; (c) S. Jin, S. J. Li, X. Ma, J. Su, H. Chen, Y. Lan and Q. Song, Angew. Chem., Int. Ed., 2021, 60, 881–888 CrossRef CAS PubMed; (d) S. Murakami, T. Nanjo and Y. Takemoto, Org. Lett., 2021, 23, 7650–7655 CrossRef CAS PubMed; (e) C. Ravi, N. N. Reddy, V. Pappula, S. Samanta and S. Adimurthy, J. Org. Chem., 2016, 81, 9964–9972 Search PubMed; (f) M. Saito, S. Murakami, T. Nanjo, Y. Kobayashi and Y. Takemoto, J. Am. Chem. Soc., 2020, 142, 8130–8135 CrossRef CAS PubMed; (g) J. R. Zhang, L. Z. Zhan, L. Wei, Y. Y. Ning, X. L. Zhong, J. X. Lai, L. Xu and R. Y. Tang, Adv. Synth. Catal., 2018, 360, 533–543 CrossRef CAS; (h) N. R. Chaubey and A. R. Kapdi, Chem. Commun., 2021, 57, 8202–8205 RSC.
- (a) H. Huang, Q. Wang, Z. Xu and G. J. Deng, Adv. Synth. Catal., 2018, 361, 591–596 CrossRef; (b) S. Moon, Y. Nishii and M. Miura, Org. Lett., 2021, 23, 49–53 CrossRef CAS PubMed; (c) S. Shi, J. Chen, S. Zhuo, Z. a. Wu, M. Fang, G. Tang and Y. Zhao, Adv. Synth. Catal., 2019, 361, 3210–3216 CrossRef CAS; (d) B. Zhang, D. Liu, Y. Sun, Y. Zhang, J. Feng and F. Yu, Org. Lett., 2021, 23, 3076–3082 CrossRef CAS PubMed.
- J. R. Zhang, Y. Y. Liao, J. C. Deng, K. Y. Feng, M. Zhang, Y. Y. Ning, Z. W. Lin and R. Y. Tang, Chem. Commun., 2017, 53, 7784–7787 RSC.
- T. Guo, X. N. Wei, M. Zhang, Y. Liu, L. M. Zhu and Y. H. Zhao, Chem. Commun., 2020, 56, 5751–5754 RSC.
- (a) M. L. Deb, C. D. Pegu, P. J. Borpatra, P. J. Saikia and P. K. Baruah, Green Chem., 2017, 19, 4036–4042 Search PubMed; (b) M. Huang, J. Dai, X. Cheng and M. Ding, Org. Lett., 2019, 21, 7759–7762 CrossRef CAS PubMed; (c) P. Jha, S. Husen and R. Kumar, Green Chem., 2021, 23, 2950–2955 Search PubMed; (d) E. K. Kirkeby and A. G. Roberts, Chem. Commun., 2020, 56, 9118–9121 RSC; (e) W. Lin, T. Cao, W. Fan, Y. Han, J. Kuang, H. Luo, B. Miao, X. Tang, Q. Yu, W. Yuan, J. Zhang, C. Zhu and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 277–281 Search PubMed; (f) Z. Qu, F. Zhang, G. J. Deng and H. Huang, Org. Lett., 2019, 21, 8239–8243 CrossRef CAS PubMed; (g) M. T. Richers, M. Breugst, A. Y. Platonova, A. Ullrich, A. Dieckmann, K. N. Houk and D. Seidel, J. Am. Chem. Soc., 2014, 136, 6123–6135 CrossRef CAS PubMed.
- (a) K. N. Singh, P. Singh, P. Singh and Y. S. Deol, Org. Lett., 2012, 14, 2202–2205 CrossRef CAS PubMed; (b) B. Sundararaju, M. Achard, G. V. Sharma and C. Bruneau, J. Am. Chem. Soc., 2011, 133, 10340–10343 Search PubMed; (c) J. Zhang, S. Park and S. Chang, J. Am. Chem. Soc., 2018, 140, 13209–13213 Search PubMed.
- (a) S. H. Ahn, K. S. Hwang, D. S. Shin, S. S. Kim, J. Y. Yang, B. H. Lee, E. J. Bae, B. W. Choi, M. A. Bae and J. H. Ahn, Bioorg. Med. Chem. Lett., 2020, 30, 127201 Search PubMed; (b) M. Chrzanowska, A. Grajewska and M. D. Rozwadowska, Chem. Rev., 2016, 116, 12369–12465 CrossRef CAS PubMed; (c) H. Wang, Y. Li, Q. Lu, M. Yu, X. Bai, S. Wang, H. Cong, H. Zhang and A. Lei, ACS Catal., 2019, 9, 1888–1894 Search PubMed; (d) Y. Q. Zou, L. Q. Lu, L. Fu, N. J. Chang, J. Rong, J. R. Chen and W. J. Xiao, Angew. Chem., Int. Ed., 2011, 50, 7171–7175 Search PubMed.
- (a) M. Jouffroy, C. B. Kelly and G. A. Molander, Org. Lett., 2016, 18, 876–879 CrossRef CAS PubMed; (b) H. P. Zhang, X. H. Yang, P. Peng and J. H. Li, Synthesis, 2011, 8, 1219–1226 Search PubMed; (c) A. Nandy, I. Kazi, S. Guha and G. Sekar, J. Org. Chem., 2021, 86, 2570–2581 CrossRef CAS PubMed; (d) N. R. Vinueza, E. F. Archibold, B. J. Jankiewicz, V. A. Gallardo, S. C. Habicht, M. S. Aqueel, J. J. Nash and H. I. Kenttamaa, Chem. – Eur. J., 2012, 18, 8692–8698 CrossRef CAS PubMed.
- (a) T. Guo, X. N. Wei, H. Y. Wang, Y. L. Zhu, Y. H. Zhao and Y. C. Ma, Org. Biomol. Chem., 2017, 15, 9455–9464 RSC; (b) T. Guo, X. N. Wei, Y. Liu, P. K. Zhang and Y. H. Zhao, Org. Chem. Front., 2019, 6, 1414–1422 Search PubMed; (c) T. Guo, L. Bi, M. Zhang, C. J. Zhu, L. B. Yuan and Y. H. Zhao, J. Org. Chem., 2022 DOI:10.1021/acs.joc.2c02248.
- (a) R. Yang, S. Yue, W. Tan, Y. Xie and H. Cai, J. Org. Chem., 2021, 86, 2570–2581 CrossRef PubMed; (b) X. Tuo, S. Chen, P. Jiang, P. Ni, X. Wang and G. J. Deng, RSC Adv., 2020, 10, 8348–8351 Search PubMed.
- (a) C. Feng, Y. Peng, G. Ding, X. Li, C. Cui and Y. Yan, Chem. Commun., 2018, 54, 13367–13370 Search PubMed; (b) S. M. A. Shakoor, D. S. Agarwal, S. Khullar, S. K. Mandal and R. Sakhuja, Chem. - Asian J., 2017, 12, 3061–3068 CrossRef PubMed; (c) K. Ganesan, R. C. Malhotra and K. Sekhar, J. Sulfur Chem., 2006, 26, 353–357 CrossRef; (d) Z. Gan, X. Zhu, Q. Yan, X. Song and D. Yang, Chin. Chem. Lett., 2021, 32, 1705–1708 CrossRef CAS; (e) S. Torii, H. Tanaka and M. Ukida, J. Org. Chem., 2002, 43, 3223–3227 CrossRef; (f) Y. X. Wu, K. Peng, Z. C. Hu, Y. H. Fan, Z. Shi, E. J. Hao and Z. B. Dong, Eur. J. Org. Chem., 2021, 2021, 5889–5894 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob01976k |
| This journal is © The Royal Society of Chemistry 2023 |