Hyperpatone A, a polycyclic polyprenylated acylphloroglucinol with a rare 8/6/5/6/5 pentacyclic skeleton from Hypericum patulum†
Feng
Zhang
abc,
Jue
Yang
*abc,
Ping
Yi
abc,
Ya-Nan
Li
abc,
Xiao-Jiang
Hao
 *abcd and
Chun-Mao
Yuan
*abcd and
Chun-Mao
Yuan
 *abc
*abc
aState Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang 550014, People's Republic of China. E-mail: yaodadewo@163.com; haoxj@mail.kib.ac.cn; yuanchunmao01@126.com; Fax: +86 851 83804649; Tel: +86 851 83804649
bSchool of Pharmaceutical Sciences, Guizhou Medical University, Guiyang 550025, China
cThe Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, Guiyang 550014, China
dState Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China
First published on 28th November 2022
Abstract
Hyperpatone A (1), a highly oxidated polycyclic polyprenylated acylphloroglucinol (PPAP), along with a biosynthesized related PPAP (2) was isolated from Hypericum patulum under the guidance of LC-MS investigation. Architecturally, compound 1 represents the first PPAP with an unprecedented 8/6/5/6/5 pentacyclic skeleton and an intramolecular peroxy bridge, which might be derived from the [3.3.1]-type bicyclic polyprenylated acylphloroglucinol via the critical Baeyer–Villiger oxidation, decarboxylation, and intramolecular cyclization. The structures were established by extensive spectroscopic analysis, ACD software calculation, and quantum chemical computations. A plausible biogenetic pathway of 1 and 2 was also proposed. Importantly, both compounds exhibited moderate cytotoxic activities against the HEL cell line with the IC50 values ranging from 10.2 to 19.2 μM. Moreover, compound 1 showed an inhibitory effect on NO production in lipopolysaccharide-stimulated RAW264.7 cells at a lower concentration of 5 or 1 μM.
Introduction
The genus Hypericum (Garcinia) is widely distributed in the world and consists of more than 500 species.1 There are 64 species of Hypericum in southwest China and most of these plants are ethnomedicinal plants.2,3 In addition, a variety of chemical constituents, including polycyclic polyprenylated acylphloroglucinols (PPAPs), xanthones, flavonoids, and terpenoids, were reported from Hypericum.4–6 Among them, PPAPs with fascinating structures and good bioactivity have attracted continuing attention in the areas of natural product chemistry, synthetic chemistry, and pharmacology.7–9Hypericum patulum has been used as a traditional Chinese medicine for the treatment of hepatitis, amygdalitis, and cold.10 In particular, the Pingzhi capsule, made from the extract of H. patulum fruits, is used for internal hemorrhoids caused by the dampness and heat of the large intestine in the Chinese market.11
In recent years, ultraperformance liquid chromatography-electrospray ionization-mass spectrometry (UPLC-ESI-MS) has become a useful tool for detecting and isolating trace components in a complex mixture of plant extracts.12 Thus, in our systematic study of novel and bioactive PPAPs,13,14 a trace highly oxidated PPAP (1) as well as a biosynthesis-related PPAP (2) was isolated from H. patulum using UPLC-MS/MS guided isolation. Compound 1 represents the first PPAP with an unprecedented 8/6/5/6/5 pentacyclic skeleton and an intramolecular peroxy bridge, which might be derived from the [3.3.1]-type bicyclic polyprenylated acylphloroglucinol via the critical Baeyer–Villiger oxidation, decarboxylation, and intramolecular cyclization. Importantly, both compounds exhibited moderate cytotoxic activities against the HEL cell line with the IC50 values ranging from 10.2 to 19.2 μM. Moreover, compound 1 showed an inhibitory effect on NO production in lipopolysaccharide-stimulated RAW264.7 cells at a lower concentration of 5 or 1 μM. In this paper, we describe the LC-MS/MS guided isolation, structural elucidation, plausible biosynthesis pathway, and biological evaluation of the isolates (Fig. 1).
Results and discussion
UPLC-Q-Orbitrap-MS/MS was a pivotal tool to identify and analyse complex chemical constituents of plant extracts.12 A trace and interesting compound with a molecular formula of C34H52O7 was detected from the crude extract of H. patulum using the LC-MS/MS technique (ESI†). The crude extract was applied to UPLC-Q-Orbitrap-MS/MS and a trace compound with a molecular formula of C34H52O7 on the basis of HRESIMS data at m/z 595.3605 [M + Na]+ (calcd for C34H52O7Na, 595.3615) was observed (ESI†). After checking each fraction for this molecular formula during separation, hyperpatone A (1), a highly oxidated PPAP with a molecular formula of C34H52O7, along with a biosynthesis-related PPAP (2) was isolated from Fr. 2G.Hyperpatone A (1) was obtained as a colorless gum and its molecular formula was established as C34H52O7 on the basis of HRESIMS data at m/z 595.3605 [M + Na]+ (calcd for C34H52O7Na, 595.3615), with nine degrees of unsaturation. The IR spectrum clearly exhibited absorption bands of hydroxyl (3457 cm−1) and carbonyl (1764 and 1684 cm−1) groups. The 1H NMR spectrum of 1 (Table 1) showed the presence of eleven singlet methyl groups and two olefinic protons at δH 5.26 (1H, t, J = 7.3 Hz) and 5.08 (1H, t, J = 7.4 Hz). The 13C NMR data together with the HSQC spectrum (Table 1) showed the existence of 34 carbon signals, comprising eleven methyls, six methylenes, six methines (two olefinic carbons), and 11 quaternary carbons (two carbonyls and two olefinic carbons). Apart from four degrees of unsaturation accounted for two carbonyls and two double bonds, the remaining five degrees of unsaturation indicated that compound 1 possesses a pentacyclic ring system.
| Pos. | CDCl3 | DMSO-d6 | ||
|---|---|---|---|---|
| δ C | δ H (J in Hz) | δ C | δ H (J in Hz) | |
| 1 | 206.1 | 205.9 | ||
| 2 | 106.3 | 105.5 | ||
| 3 | 85.6 | 84.9 | ||
| 4 | 50.4 | 2.77 (m) | 49.8 | 2.59 (m) |
| 5 | 70.3 | 68.7 | ||
| 6 | 116.8 | 116.3 | ||
| 7 | 55.2 | 54.6 | ||
| 8α | 31.9 | 2.53 (m) | 30.4 | 2.23 (dd, 14.9, 5.1) |
| 8β | 1.39 (m) | 1.57 (m) | ||
| 9 | 40.5 | 1.40 (m) | 39.9 | 1.31 (m) |
| 10 | 54 | 53.8 | ||
| 11α | 33.3 | 1.94 (m) | 32.8 | 1.80 (m) |
| 11β | 1.71 (m) | 1.65 (m) | ||
| 12 | 26.7 | 1.76 (m) | 26.1 | 1.74 (m) |
| 13 | 218.2 | 217.5 | ||
| 14 | 41.3 | 3.92 (m) | 40.5 | 3.94 (m) |
| 15 | 21.6 | 1.09 (d, 7.1) | 21.1 | 0.99 (d, 6.5) |
| 16 | 21.1 | 1.21 (d, 7.1) | 20.9 | 1.09 (d, 6.5) |
| 17 | 20.5 | 0.97 (s) | 20.1 | 0.86 (s) |
| 18 | 30.1 | 1.20 (s) | 29.9 | 1.08 (s) |
| 19α | 34.4 | 2.70 (m) | 34.3 | 2.52 (m) |
| 19β | 2.70 (m) | 2.57 (m) | ||
| 20 | 116.3 | 5.26 (t, 6.0) | 116.3 | 5.26 (t, 7.4) |
| 21 | 137.1 | 136.0 | ||
| 22 | 18.0 | 1.64 (s) | 17.8 | 1.56 (s) |
| 23 | 26.0 | 1.69 (s) | 25.6 | 1.64 (s) |
| 24α | 40.7 | 2.13 (m) | 40.1 | 2.07 (m) |
| 24β | 1.88 (m) | 1.90 (m) | ||
| 25 | 83.7 | 4.13 (m) | 84.1 | 4.02 (m) |
| 26 | 70.5 | 69.6 | 4.71 (OH) | |
| 27 | 26.3 | 1.15 (s) | 27.1 | 1.24 (s) |
| 28 | 28.1 | 1.38 (s) | 27.4 | 1.05 (s) |
| 29α | 31.1 | 2.16 (m) | 30.7 | 2.09 (m) |
| 29β | 1.55 (m) | 1.53 (m) | ||
| 30 | 123.3 | 5.08 (t, 6.0) | 123.7 | 5.08 (t, 7.1) |
| 31 | 133.1 | 132.2 | ||
| 32 | 18.1 | 1.59 (s) | 17.7 | 1.56 (s) |
| 33 | 26.0 | 1.71 (s) | 25.8 | 1.69 (s) |
| 34 | 17.0 | 0.83 (s) | 16.2 | 0.82 (s) |
| OH-26 | 4.70 (s) | |||
Five fragments (a–e) with blue bold bonds were established from the 1H–1H COSY correlations of H-4/H2-12/H2-11, H2-19/H-20, H2-24/H-25, Me-15/H-14, and H2-8/H-9/H2-29/H-30 in Fig. 2. The connections for these fragments were established from the HMBC spectrum. In the HMBC spectrum, the key correlations from Me-34 (δH 0.83, 3H, s) to C-5 (δC 70.3), C-9 (δC 40.5), C-10 (δC 54.0), and C-11 (δC 33.3) implied the linkage of Me-34, C-11, C-9, and C-5 via C-10. Further HMBC cross-peaks from H-4 to C-3, C-5, C-6, and C-10 along with the above-established fragment a could easily construct a five-membered ring (ring A). An isobutyryl group and a 2-hydroxypropan-2-yl group were linked to C-5 and C-4 of ring A, respectively, implied by the HMBC correlations of Me-15/C-13, H-14/C-5, Me-18/C-3, and Me-18/C-4. Furthermore, HMBC correlations from H2-8 to C-6, C-7, and C-10 along with previously assigned fragments established ring B. A prenyl group was connected to ring B via C-9 by the above-mentioned fragment b and HMBC correlations of Me-33/C-31 and Me-33/C-30. Likewise, fragment c and a carbonyl (C-1) were connected to ring B through C-7 by the HMBC correlations of H2-24 to C-1, C-6, C-7, and C-8. Moreover, another 2-hydroxypropan-2-yl group was connected to fragment cvia C-25, by HMBC correlations from Me-27 to C-25, C-26, and C-28 and OH-26 to C-26. The connections of a prenyl group (fragment d) and C-1 via a characteristic hemiketal carbon (C-2) were inferred by HMBC correlations of H2-19 to C-1 and C-2, and Me-23 to C-21 and C-20. Thus, compound 1 with four unconnected carbons (C-2, C-3, C-6, and C-25) linked with oxygen atoms implied the existence of three oxygen rings to consume the remaining three degrees of unsaturation, as shown in Fig. 2.
Further comparing the structure of 1 with that of a known PPAP (hypericumoxide N),15 both compounds shared the same eight-membered ether ring between C-2 and C-3. The characteristic chemical shift of C-3 (δC 85.6) in 1 was almost the same as that of C-2 (δC 86.1) in hypericumoxide N, which could certify this deduction. The rest of the two degrees of unsaturation and three oxygen atoms implied the presence of two oxygen rings between C-2 and C-6, and between C-25 and C-6, including an oxygen ring and a peroxy ring. After carefully comparing the chemical shift of C-25 (δC 83.7) in 1 with the same carbon of the furan ring [C-8 (δC 83.8) in hyperattenin K;16 C-8 (δC 86.5) in hyperforcinol;17 C-8 (δC 86.1) in kiiacylphnol18] in the literature, a five-membered ether ring could be formed via C-25 and C-6. Finally, a peroxy bridge through C-2 and C-6 was constructed to consume the last degree of unsaturation, inferred from the characteristic peroxy chemical shifts (C-2, δC 106.3 and C-6, δC 116.8) and two unused oxygen atoms. Therefore, the planar structure of 1 with an unprecedented 8/6/5/6/5 pentacyclic skeleton was assigned.
Computer-assisted structure elucidation (CASE) is a technological development that has been used in recent years to elucidate complex structures.19–21 ACD software calculation has been widely accepted and used for the elucidation of uncertain complex structures with a lot of quaternary carbons.14,19–21 The 1D and 2D NMR data of 1 as well as its molecular formula were applied to the ACD/structure elucidator, which generated 100 molecules (ESI†). The top-hit molecular formula, with the highest R2 and lowest standard deviation values from Neural Net (dN) and HOSE (dA) methods (Fig. 3), is the best-matched structure.19–21 Moreover, all the possible oxygen rings were analyzed by DP4 analysis for Neural Net (dN) and HOSE (dA) methods. The results certified the correctness of compound 1 with a possibility of 97.9% and 99.0% for Neural Net (dN) and HOSE (dA) methods. Obviously, the complex structure of 1 was further confirmed by ACD software calculation.
 | ||
| Fig. 3 Top structure generated using Struc. Eluc. software and its linear regression plots through both HOSE and Neural Net methods of 13C chemical shift prediction. | ||
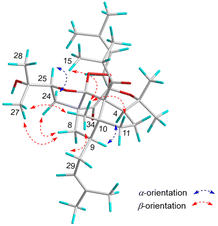
The relative configuration of 1 was assigned by the NOESY experiment (Fig. 4), in which the observed cross-peaks of Me-34 to Me-15, H-11β, H-29α, and Me-27, and of H-8β to Me-27 and H-24β demonstrated that these groups were cofacial and were randomly assigned as β-oriented. Thus, a 2-hydroxypropan-2-yl group at C-25, an isobutyryl group at C-5, a prenyl group at C-9, and a methyl group at C-10 were assigned as β-oriented. In contrast, the obvious NOESY correlations of H-25/H-24α, and H-4/H-9 indicated α-orientation of H-25, H-4, and H-9. Due to the rigid structure of this ring system, the rest of the chiral centers (C-2, C-6, and C-7) were fixed. Thus, the relative structure of 1 was elucidated. Moreover, the solvent of DMSO-d6 was also applied for the NMR test of compound 1, which further confirmed the relative configuration of this compound (Table 1).
To further confirm the correctness of compound 1, 13C NMR calculation was applied with the GIAO method at the B3LYP/6-31+g(d,p) level in chloroform.22–24 The calculated 13C NMR data matched well with the experimental data with a correlation coefficient (R2) of 0.998 from the linear regression analysis method in Fig. 5A. All in all, all the results undoubtedly confirm the correctness of compound 1.
 | ||
| Fig. 5 (A) Regression analysis of the experimental versus the calculated 13C NMR chemical shift of 1 in CDCl3. (B) Calculated and experimental ECD spectra of 1. | ||
Then, the absolute configuration of 1 was established by electric circular dichroism (ECD) analysis using the time-dependent density functional theory (TDDFT) methodology at the CAM-B3LYP/TZVP level in MeOH in Fig. 5B. The ECD spectra curves of two possible absolute configurations, (2R,4S,5R,6S,7S,9S,10R,25R)-1 and (2S,4R,5S,6R,7R,9R,10S,25S)-1, were calculated. Obviously, the absolute configuration of 1 was assigned as 2R, 4S, 5R, 6S, 7S, 9S, 10R, and 25R due to similar curves between its experimental and calculated ECD curve.25
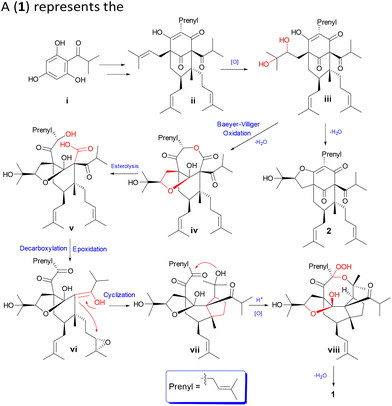
A plausible biogenetic pathway (Scheme 1) of 1 was proposed. A phloroglucinol derivative (i) could undergo a series of isopentenyl transferase and cyclization to give intermediate ii.26,27 Further oxidation of ii might give the common intermediate iii, which could undergo an intermolecular dehydration reaction between two hydroxyl groups to obtain compound 2. Baeyer–Villiger oxidation and nucleophilic reaction might happen for iii to give intermediate iv,28,29 which could be followed by esterolysis, decarboxylation, and epoxidation to obtain the key intermediate vi.30,31 Further intermolecular cyclization reaction might occur to give intermediate vii. Finally, the novel peroxy bridge of compound 1 could be obtained after the nucleophilic reaction, oxidation, and dehydration reaction of vii.32
The isolates were screened for cytotoxicity against the HEL (human erythroleukemia) cell line by an MTT method.19 Compounds 1 and 2 exhibited moderate cytotoxicity against the HEL cancer cell line, with IC50 values of 19.23 μM and 10.2 μM, respectively. Adriamycin was used as a positive control with an IC50 value of 0.17 μM. Given that compound 1 showed weaker cytotoxic activity than compound 2, compound 1 was evaluated for the inhibitory effects on LPS-stimulated NO production in RAW264.7 cells.30 As shown in Fig. 6, at a lower concentration of 5 or 1 μM,33 compound 1 showed inhibitory potency with inhibition rates of 26.5 and 21.8%, respectively.
Conclusions
In summary, hyperpatone A (1), a rare PPAP, as well as a biosynthesis-related PPAP (2) was isolated from Hypericum patulum under the guidance of LC-MS investigation. Compound 1 was elucidated by a series of methods, including 2D NMR spectroscopy, ACD software calculation, 13C NMR calculation, and ECD calculation. Architecturally, hyperpatone A (1) represents the first PPAP with a novel 8/6/5/6/5 pentacyclic skeleton and an intramolecular peroxy bridge, and the critical Baeyer–Villiger oxidation, decarboxylation, and intramolecular cyclization might be involved for its formation from the [3.3.1]-type bicyclic polyprenylated acylphloroglucinol (BPAP). Both isolates exhibited moderate cytotoxicity against the HEL cell line and compound 1 exhibited an inhibitory effect on NO production in lipopolysaccharide-stimulated RAW264.7 cells at a lower concentration of 5 or 1 μM. This fascinating structure and potential bioactivity not only enrich the diversity of PPAPs but also provide a lead compound for drug discovery.Experimental section
General experimental procedures
Optical rotations were applied on a JASCOP-1020 polarimeter. IR spectra were obtained on a Bruker FT-IR Tensor-27 and iCAN 9 infrared spectrophotometer with KBr pellets. UV spectra were tested on a Shimadzu UV-2401PC spectrometer. CD spectra were recorded with an Applied Photophysics Chirascan instrument. NMR spectra were performed on a Bruker Avance NEO 600 MHz spectrometer with TMS as the internal standard. HRESIMS data were acquired on a Thermo Q-Exactive Focus and Agilent 6500 QSTAR TOF time-of-flight mass spectrometer. Semi-preparative HPLC was performed on an instrument consisting of a Hanbon NP7005 controller, a Hanbon NP7005 pump, and a Hanbon NU3000c UV detector with a YMC-Triart-C18 column (250 × 10.0 mm, 5 μm). Column chromatography was performed using silica gel (40–80, 200–300, and 300–400 mesh, Qingdao Marine Chemical Co. Ltd, Qingdao, People's Republic of China), Sephadex LH-20 (40–70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden), and RP-C18 gel (40–63 μm, Merck, Darmstadt, Germany). Analytical-grade petroleum ether, ethyl acetate, and methanol were bought from General-Reagent Corporation (Shanghai, China). HPLC-grade methanol and water were purchased from Xinlanjing International Corporation (Pennsylvania, USA) and Hangzhou Wahaha Group Corporation (Hangzhou, China), respectively. We bought Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) from GIBCO Invitrogen Corporation (Carlsbad, USA). DMSO, streptomycin, penicillin, corticosterone (CORT), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were bought from Sigma-Aldrich (USA).Plant material
The aerial parts of H. patulum were collected in Guiyang, the Guizhou Province of China. The plant was identified by Mr Xiaoqi Hou. A voucher specimen (no. 20190826) was deposited in the Key Laboratory of Chemistry for the Natural Products of Guizhou Province and the Chinese Academy of Sciences.UPLC-Q-Orbitrap-MS/MS
Chromatographic analysis was performed on a Thermo Scientific Dionex (Sunnyvale, USA) Ultimate 3000 UHPLC system equipped with an Ultimate 3000 pump, an Ultimate 3000 degasser, an Ultimate 3000 RS autosampler and an Ultimate 3000 RS column compartment. The samples were separated using a Hypersil GOLDTM C18 column (3.0 μm, 2.1 × 150 mm; Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase consisted of (A) methanol and (B) water. The flow rate was 0.2 mL min−1, the column temperature was set at 40 °C, and the total run time was 30 min. An isocratic mode of 70% phase A was applied for 0–3 min, followed by a linear gradient from 70% to 100% phase A between 3–25 min, and 100% phase A was maintained over the last 5 min.Mass spectrometry with an electrospray ionization source (ESI) was conducted in the positive mode. High-resolution ESI-MS/MS experiments were performed on a Q-Exactive Orbitrap mass spectrometer equipped with an ESI source controlled by the Xcalibur 2.3 software (Thermo Fisher, Waltham, MA, USA). The mass resolution was 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 FWHM (full width at half maximum) at 200. Argon was used as the collision gas and high-purity nitrogen gas was used as the nebulizer and drying gas was set at a flow rate of 4 L min−1 and a pressure of 0.3 bar, respectively. The heated electrospray (HESI) ion source was used for ionization under the following interface conditions: spray voltage, 3.0 kV; capillary temperature, 320 °C; sheath gas flow rate, 35 units; auxiliary gas flow rate, 10 units; S lens RF level 50.
500 FWHM (full width at half maximum) at 200. Argon was used as the collision gas and high-purity nitrogen gas was used as the nebulizer and drying gas was set at a flow rate of 4 L min−1 and a pressure of 0.3 bar, respectively. The heated electrospray (HESI) ion source was used for ionization under the following interface conditions: spray voltage, 3.0 kV; capillary temperature, 320 °C; sheath gas flow rate, 35 units; auxiliary gas flow rate, 10 units; S lens RF level 50.
Isolation and purification
The air-dried and powdered leaves and twigs of H. patulum (46 kg) were percolated with MeOH at room temperature and filtered. MeOH was evaporated in vacuo to obtain the crude extract (6.4 kg). The crude extract was applied to UPLC-Q-Orbitrap-MS/MS and a trace compound with a molecular formula of C34H52O7 on the basis of HRESIMS data at m/z 595.3605 [M + Na]+ (calcd for C34H52O7Na, 595.3615) was observed (ESI†). Then, the crude extract was further purified by silica gel column chromatography and eluted with petroleum ether–ethyl acetate (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain seven fractions (Fr. 1–7). UPLC MS/MS detection for those fractions implied that this interesting compound was in Fr. 2. Therefore, Fr. 2 (111.2 g) was applied to an MCI-gel column washed with MeOH–H2O from 6
1) to obtain seven fractions (Fr. 1–7). UPLC MS/MS detection for those fractions implied that this interesting compound was in Fr. 2. Therefore, Fr. 2 (111.2 g) was applied to an MCI-gel column washed with MeOH–H2O from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 10
4 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to yield seven fractions (Fr. 2A–2G). Fr. 2G (683 mg) was eluted with petroleum ether–ethyl acetate (from 95
0 to yield seven fractions (Fr. 2A–2G). Fr. 2G (683 mg) was eluted with petroleum ether–ethyl acetate (from 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 50
5 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to yield eight fractions (Fr. 2G1–2G5). Compound 1 (7.2 mg, tR = 27 min) was obtained from Fr. 2G2 (57 mg) by semi-preparative HPLC (MeOH–H2O, 95
50) to yield eight fractions (Fr. 2G1–2G5). Compound 1 (7.2 mg, tR = 27 min) was obtained from Fr. 2G2 (57 mg) by semi-preparative HPLC (MeOH–H2O, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Fr. 2G5 (100 mg) was applied to a silica gel column eluted with petroleum ether–ethyl acetate (100
5). Fr. 2G5 (100 mg) was applied to a silica gel column eluted with petroleum ether–ethyl acetate (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 100
3 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) to yield compound 2 (100 mg).
15) to yield compound 2 (100 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 196 (3.75) nm; CD (MeOH) λmax (Δε) 198 (−4.75), 210 (38.16), 225 (−22.76), 268 (−0.26), 308 (−18.63), 331 (−3.81) nm; IR (KBr) νmax 3457, 2967, 2926, 2358, 2026, 1684, 1458, 1376, 1144, 1018 cm−1; 1H and 13C NMR data for CDCl3 and DMSO-d6, see Table 1; positive HRESIMS: m/z 595.3605 [M + Na]+ (calcd for C35H54O7Na, 595.3615).
ε) 196 (3.75) nm; CD (MeOH) λmax (Δε) 198 (−4.75), 210 (38.16), 225 (−22.76), 268 (−0.26), 308 (−18.63), 331 (−3.81) nm; IR (KBr) νmax 3457, 2967, 2926, 2358, 2026, 1684, 1458, 1376, 1144, 1018 cm−1; 1H and 13C NMR data for CDCl3 and DMSO-d6, see Table 1; positive HRESIMS: m/z 595.3605 [M + Na]+ (calcd for C35H54O7Na, 595.3615).
CASE analysis procedures
The commercially available ACD/Labs 2020 1.1 (File Version S15S41) was used for CASE analysis.18,19 All calculations were performed on a standard 2.90 GHz PC with 16 GB of RAM. The ACD/structure elucidator software was used and NMR data were extracted from the original NMR spectra of compound 1. Following the completion of the data import, the ACD/structure elucidator software generated an MCD automatically. Manual edits were carried out based on the common structural fragments. Subsequently, a table of probable structures ranked in the ascending order of smallest deviations is the output.Quantum chemical computations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 22–24.
Conformational searching of 1 was applied with the Spartan software using the Merck molecular force field (MMFF) with standard parameters. All conformers were subjected to density functional theory (DFT) geometry optimization at the B3LYP/6-31+G(d) level of theory in the gas phase using the Gaussian 16 package. Frequency analyses of all the optimized conformers were undertaken at the same level of theory to ensure that Boltzmann distribution ≥ 5% was chosen, and then they were initially optimized at B3LYP/6-31+G(d,p) levels in chloroform using the polarizable continuum model (PCM) solvent in the Gaussian 16 program package. The gauge-independent orbital (GIAO) shielding constants of these conformers were converted into unscaled chemical shifts (δu) by referencing to tetramethylsilane (TMS) (δu = σTMS − σCal), where σTMS was the shielding constant of TMS calculated at the same level. The scaled chemical shifts (δs) were computed using the formula δs = (δu − b)/m, where m and b are the slope and intercept, respectively. The linear correlation coefficient was calculated to evaluate deviations between the experimental and calculated results.
22–24.
Conformational searching of 1 was applied with the Spartan software using the Merck molecular force field (MMFF) with standard parameters. All conformers were subjected to density functional theory (DFT) geometry optimization at the B3LYP/6-31+G(d) level of theory in the gas phase using the Gaussian 16 package. Frequency analyses of all the optimized conformers were undertaken at the same level of theory to ensure that Boltzmann distribution ≥ 5% was chosen, and then they were initially optimized at B3LYP/6-31+G(d,p) levels in chloroform using the polarizable continuum model (PCM) solvent in the Gaussian 16 program package. The gauge-independent orbital (GIAO) shielding constants of these conformers were converted into unscaled chemical shifts (δu) by referencing to tetramethylsilane (TMS) (δu = σTMS − σCal), where σTMS was the shielding constant of TMS calculated at the same level. The scaled chemical shifts (δs) were computed using the formula δs = (δu − b)/m, where m and b are the slope and intercept, respectively. The linear correlation coefficient was calculated to evaluate deviations between the experimental and calculated results.
Cytotoxicity assay
About 5 × 103 cells per cell were seeded into 96-well plates for 24 h and treated with a gradient concentration of compounds for 48 h. Then, each well was added 20 μL of MTT solution (5 mg mL−1) (Solarbio, China) and incubated for another 4 h. After discarding the medium, 160 μL DMSO was added to each well for dissolving the formazan crystals, and the absorbance of the solution was determined at 570 nm using a multifunction microplate reader.17Inhibition of the nitric oxide production assay
Each compound was dissolved in DMSO and further diluted in medium to produce different concentrations. NO production in each well was assessed by adding 100 μL of Griess reagents A and B to 100 μL of each supernatant from LPS or the compound-treated cells in triplicate. After 5 min incubation, the absorbance was measured at 570 nm with a 2104 Envision multilabel plate reader (PerkinElmer Life Sciences, Inc., Boston, MA, USA). Cytotoxicity was determined using the MTT assay.19 PDTC was used as a positive control. The assay was performed as described previously.33Author contributions
Feng Zhang performed the isolation, structure deduction, and writing – original draft. Jue Yang tested the activity and wrote the original draft. Yanan Li contributed to writing – original draft. Ping Yi performed quantum chemical computations and the determination of the absolute configuration of the new compound. Xiaojiang Hao and Chunmao Yuan revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (32270413 and U1812403), the Science and Technology Department of Guizhou Province (QKHJC 2020-1Z076 and 2020-1Y400), the 13th Batch of Outstanding Young Scientific and Technological Talents in Guizhou Province (QKHPTRC [2021]5633), High-level Innovative Talents in Guizhou Province (Thousand Levels of Talent for Chunmao Yuan in 2018), and “Light of the West” Talent Cultivation Program of Chinese Academy of Sciences for Chunmao Yuan (RZ [2020]82).References
- N. K. B. Robson, Phytotaxa, 2016, 255, 181–198 CrossRef.
- Y. Y. Ji, A. Negrin, J. E. Kennelly and C. L. Long, Zhongguo Zhongyao Zazhi, 2018, 43, 3701–3707 Search PubMed.
- P. F. Stevens, Flowering Plants Eudicots, Springer, Berlin, Heidelberg, 2007, pp. 48–66 Search PubMed.
- H. Bridi, G. D. C. Meirelles and G. L. von Poser, Phytochemistry, 2018, 155, 203–232 CrossRef CAS PubMed.
- J. Zhao, W. Liu and J. C. Wang, Chem. Biodivers., 2015, 12, 309–349 CrossRef CAS.
- X. W. Yang, R. B. Grossman and G. Xu, Chem. Rev., 2018, 118, 3508–3558 CrossRef CAS PubMed.
- Y. P. Li, K. Hu, X. W. Yang and G. Xu, J. Nat. Prod., 2018, 81, 1098–1102 CrossRef CAS PubMed.
- K. Wiechmann, H. Müller, V. Huch, D. Hartmann, O. Werz and J. Jauch, Eur. J. Med. Chem., 2015, 101, 133–149 CrossRef CAS.
- X. W. Yang, Y. P. Li, J. Su, W. G. Ma and G. Xu, Org. Lett., 2016, 18, 1876–1879 CrossRef CAS.
- Editorial Board of “Chinese Materia Medica” of the State Administration of Traditional Chinese Medicine, Chinese Materia Medica, Shanghai:Shanghai Science and Technology Press, 1999 Search PubMed.
- Y. Z. Lan, A. M. Wang, Y. L. Wang, X. He, Y. J. Li and L. N. Liu, Determination of quercetin content in Pingzhi Capsules and fruits of Hypericum patulum Thunb by HPLC, J. Tradit. Chin. Med., 2003, 28, 35–37 Search PubMed.
- C. A. Geng, X. L. Chen, N. J. Zhou, H. Chen, Y. B. Ma, X. Y. Huang, X. M. Zhang and J. J. Chen, Org. Lett., 2014, 16, 370–373 CrossRef CAS.
- Y. R. Zeng, P. Yi, W. Gu, C. X. Xiao, L. J. Huang, D. S. Tian, H. Yan, D. Z. Chen, C. M. Yuan and X. J. Hao, Org. Biomol. Chem., 2018, 16, 4195–4198 RSC.
- Z. Z. Zhang, Y. R. Zeng, Y. N. Li, Z. X. Hu, L. J. Huang, W. Gu, X. J. Hao and C. M. Yuan, Org. Biomol. Chem., 2021, 19, 216–219 RSC.
- R. Liu, Y. Su, J. Yang and A. Wang, Phytochemistry, 2017, 142, 38–50 CrossRef CAS PubMed.
- D. Y. Li, G. Du, X. P. Gong, J. R. Guo, J. W. Zhang, C. M. Chen, Y. B. Xue, H. C. Zhu and Y. H. Zhang, Fitoterapia, 2018, 125, 130–134 CrossRef CAS.
- W. J. Lu, W. J. Xu, M. H. Zhang, Y. Q. Zhang, Y. R. Li, H. Zhang, J. Luo and L. Y. Kong, J. Nat. Prod., 2021, 84, 1135–1148 CrossRef CAS PubMed.
- Y. Duan, P. Hu, Y. Guo, P. Bu, Z. Shi, Y. Cao, Y. Zhang, H. Hu, Q. Tong, C. Qi and Y. Zhang, Phytochemistry, 2022, 199, 113166 CrossRef CAS.
- H. Y. Lou, Y. N. Li, P. Yi, J. Y. Jian, Z. X. Hu, W. Gu, L. J. Huang, Y. M. Li, C. M. Yuan and X. J. Hao, Org. Lett., 2020, 22, 6903–6906 CrossRef CAS PubMed.
- C. B. Naman, J. Li, A. Moser, J. M. Hendrycks, P. A. Benatrehina, H. Chai, C. Yuan, W. J. Keller and A. D. Kinghorn, Org. Lett., 2015, 17, 2988–2991 CrossRef CAS PubMed.
- M. Elyashberg, K. Blinov, S. Molodtsov and A. J. Williams, J. Nat. Prod., 2013, 76, 113–116 CrossRef CAS PubMed.
- Y. W. Li, W. J. Lu, X. Zhou, C. Zhang, X. Y. Li, P. F. Tang, L. Y. Kong and W. J. Xu, Bioorg. Chem., 2022, 127, 106005 CrossRef CAS.
- N. Grimblat, M. M. Zanardi and A. M. Sarotti, J. Org. Chem., 2015, 80, 12526–12534 CrossRef CAS PubMed.
- D. Xin, P. J. Jones and N. C. Gonnella, J. Org. Chem., 2018, 83, 5035–5043 CrossRef CAS.
- Z. Shi, H. Hu, Y. Guo, Y. Duan, Y. Zhang, B. Tao, P. Bu, W. Sun, C. Qi and Y. Zhang, Org. Biomol. Chem., 2022, 20, 1284–1291 RSC.
- M. Liu, C. C. Chen, L. Chen, X. Xiao, Y. Zheng, J. W. Huang, W. Liu, T. P. Ko, Y. S. Cheng, X. Feng, E. Oldfield, R. T. Guo and Y. Ma, Angew. Chem., Int. Ed., 2016, 55, 4721–4724 CrossRef CAS PubMed.
- R. Ciochina and R. B. Grossman, Chem. Rev., 2006, 106, 3963–3986 CrossRef CAS PubMed.
- L. Huang, Z. Z. Zhang, Y. N. Li, P. Yi, W. Gu, J. Yang, Y. M. Li, X. J. Hao and C. M. Yuan, Org. Lett., 2022, 24, 5967–5971 CrossRef CAS.
- L. Wang, X. Wang, G. Zhang, W. Fu, H. Zhang, H. Zhou, H. Xu and C. Zheng, Org. Chem. Front., 2021, 8, 2525–2531 RSC.
- W. J. Xu, P. F. Tang, W. J. Lu, Y. Q. Zhang, X. B. Wang, H. Zhang, J. Luo and L. Y. Kong, Org. Lett., 2019, 21, 8558–8562 CrossRef CAS.
- S. Xie, C. Qi, Y. Duan, Q. Xu, Y. Liu, Y. Huang, X. Yin, W. Sun, Y. Zhou and Y. Zhang, Org. Chem. Front., 2020, 7, 1349–1357 RSC.
- K. Mitsugi, T. Takabayashi, T. Ohyoshi and H. Kigoshi, Org. Lett., 2022, 24, 4635–4639 CrossRef CAS PubMed.
- Y. Duan, Y. Guo, Y. Deng, P. Bu, Z. Shi, Y. Cao, Y. Zhang, H. Hu, W. Sun, C. Qi and Y. Zhang, J. Org. Chem., 2022, 87, 6824–6831 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob01851a |
| This journal is © The Royal Society of Chemistry 2023 |