DOI:
10.1039/D3NJ04872A
(Paper)
New J. Chem., 2024,
48, 171-181
Co–MnO/C nanoparticles derived from MOFs with improved conductivity and reduced volume change for lithium-ion batteries†
Received
20th October 2023
, Accepted 20th November 2023
First published on 21st November 2023
Abstract
MnO, used as an anode material for lithium-ion batteries (LIBs), suffers from severe volume changes, poor conductivity and limited cycle performance. Herein, we applied carbon materials derived from metal–organic frameworks (MOFs) to obtain a considerable specific surface area and fitting aperture, which can be used to physically fix MnO nanoparticles. The ultrafine Co particles further anchor MnO nanoparticles through chemical interaction. The combination of physical and chemical anchoring of MnO alleviated the problem of volume changes during the intercalation reaction of LIBs. The composite of carbon material and the doping of transition metal Co improved the conductivity of MnO. In addition, the convenient channel provided by the porous structure for the diffusion of ions, the slowing down of volume changes during the process of lithiation/delithiation and the improvement in conductivity are propitious to electrochemical performance of LIBs. Therefore, Co–MnO/C delivered an ultrahigh capacity of 1653 mA h g−1 at 100 mA g−1. The specific capacity tested at 100 and 1000 mA g−1 can still reach 1414 and 772 mA h g−1, respectively, after about 300 cycles. Mn-based materials with their outstanding electrochemical performance, environmental friendliness and convenient synthesis strategy demonstrate the feasibility of Co–MnO/C composite materials in practical applications of LIBs.
1. Introduction
It is well known that the rapid economic development has intensified the demand for non-renewable energy (coal, natural gas, fossil fuels, etc.). And with the improvement in people's awareness about environmental protection, it is urgent for scientists to develop new energy storage technologies.1–5 In the field of electric toothbrushes, mobile phones, electric vehicles, portable computers and other applications, lithium-ion batteries (LIBs) with good structural flexibility and low self-discharge have made a difference.6–9 At present, the theoretical specific capacity of commercial graphitic negative electrode materials for LIBs is only 372 mA h g−1, which makes it difficult to satisfy the demands for higher power and rate stability.10,11 Research aimed at modifying the anode to promote the electrochemical performance of LIBs has attracted attention.
Transition metal oxides (TMOs) exhibit excellent theoretical capacity, high energy density and safety and are considered as the most promising candidate materials for LIBs.12,13 For example, MnO,14–16 Fe3O4,17 TiO217 and SnOx18,19 present outstanding electrochemical performance during the charge–discharge process. A desirable anode material should show good structural stability, high theoretical capacity, low electromotive force and high density. The octahedral structure of MnO exhibits strong crystal field strength and good structural stability.20 Meanwhile, MnO offers a high theoretical capacity of 755.1 mA h g−1 (more than twice that of graphene), low electromotive force for high energy (1.032 V vs. Li+/Li) and cost-effectiveness.14,16 In addition, Mn is environmentally friendly and abundant in reserves, which makes MnO-based materials the most promising anode candidate materials. However, the severe volume change that occurs in MnO materials during the lithiation/delithiation process, the poor cyclic performance and conductivity have hindered their application in LIBs.
The following effective strategies are envisaged to solve the above problems: (1) varying the particle size of MnO to alleviate the volume change and improve the electrochemical performance. The smaller particle size shows a larger specific surface area, which can fully contact with electrolyte solution, shorten the diffusion path of Li+21 and effectively prevent volume expansion.16,22 The introduction of carbon materials could effectively disperse TMO particles, reduce mechanical stress caused by volume changes and improve conductivity.16 Typically, the carbon materials derived from MOFs have the stability of MOFs, and also have the advantages of small particle size, high surface area and ordered nanostructure,23–25 such as MOx/C.26 It has been shown that the transition metal ions used as the central ions of MOF precursors to prepare TMOs show excellent electrochemical performance. Guo et al.27 synthesized core–shell ZnO@C:N composites with excellent reversible capacity. Wang et al.28 prepared FeO/C materials and provided a reference for MOx/C as an anode electrode of LIBs. (2) In the field of chemicals and materials, the act of purposefully mixing other substances into a material or matrix in order to change the properties of the material or matrix is called adulteration. Typically, the content of doped substances is relatively limited. To achieve excellent conductivity and cycle stability, doping other transition metal elements is a viable strategy, such as Co, Cu, and Ag. Compared with pure anode materials, the electronic conductivity and cycle stability of MnO–X (X = Co, Cu, Ag, etc.) can be significantly improved.29,30 In all of the strategies mentioned above, the electrochemical performance of MnO-based electrode materials has been optimized. (3) Porous materials and doped transition metals anchor and adsorb MnO physically and chemically, respectively, thus alleviating the problem of volume change in intercalation reactions of LIBs.
Herein, we synthesized Co–MnO/C by using Co/Mn MOFs in a convenient and straightforward strategy. First, Co–Mn MOFs were obtained by the hydrothermal method. Second, Co–MnO/C was synthesized through further calcination of the Co–Mn MOF under an Ar/H2 (10%) atmosphere. The doping of Co makes the structural arrangement of carbon coated on MnO nanoparticles more regular, and the conductivity will also be improved.30 The porous carbon matrix and Co particles play an important role in firmly restricting MnO nanoparticles, greatly alleviating the volume change caused by Li+ intercalation and deintercalation during charging and discharging, showing good structural stability. In addition, the diffusion rate of ions is also greatly improved. This is attributed to the porous carbon matrix that facilitates ion transport and the doping of transition metals that can enhance transport rates.31 The unique structure ensures the ultrahigh electrochemical performance and rate performance of Co–MnO/C as an anode material for LIBs. The scheme illustration of the method for the synthesis of the Co–MnO/C anode is shown in Scheme S1 (ESI†).
2. Results and discussion
2.1. Sample characterization
Fig. 1 shows the X-ray diffraction (XRD) pattern of the materials to identify their composition and structure. The sample shows strong diffraction peaks at 2θ = 10, 15 and 30° in Fig. 1a, and there are no characteristic peaks of other impurities, which means that the diffraction peaks of the Co–Mn MOF matched well with those of the simulated Co-based MOF and Mn-based MOF. And it is consistent with previous reports, indicating that the Co–Mn MOF has been successfully synthesized.32Fig. 1b shows the XRD patterns of Co–MnO/C at different temperatures. When the calcination temperature is 400 °C, the Co–Mn MOF is partially converted into Co (JCPDS No. 15-0806) and MnO (JCPDS No. 07-0230), and the Co–Mn MOF is still retained. As the temperature reaches 450 °C and 500 °C, the characteristic diffraction peaks of the Co–Mn MOF disappear, transforming completely into Co and MnO, and the pyrolysis of MOFs as precursors under non-oxidizing conditions also leads to the ligand skeleton being retained as a carbon structure, indicating the formation of Co–MnO/C materials. When the calcination temperature is 500 °C, Co–MnO/C shows the best crystallinity and when the calcination temperature is 450 °C, Co–MnO/C shows poor crystallinity. According to previous research studies, fine particles with poor crystallinity may be beneficial for the improvement of electrochemical performance.33 Therefore, our study focused on Co–MnO/C-450 °C. The results of inductively coupled plasma spectroscopy (ICP) estimated that the content of Co accounts for about 30 wt% in the composite. Then, Fourier transformation infrared (FT-IR) spectroscopy was employed to characterize the structure of composites as shown in Fig. 2. The stretching vibration of the coordination group (–COO−) appears at 1553 cm−1 and 1387 cm−1, and the band at 3445 cm−1 is related to the stretching vibration of –OH. In addition, the lower peak intensity in the range of 750–1100 cm−1 can be assigned to the C–H stretching vibration bands and the peaks at 602 cm−1 and 510 cm−1 are attributed to the Co–O and Mn–O characteristic stretching vibration. However, the characteristic stretching vibration bands of –COO− and –OH groups gradually weaken or even disappear at 500 °C, which indicates that Co–Mn MOFs are completely reduced to Co and MnO by the hydrogen atmosphere. The existence of characteristic stretching vibration bands of –OH at 450 °C and 500 °C may be related to water in the air. Therefore, the XRD and FT-IR tests suggest that the Co–Mn MOF and Co–MnO/C have been successfully prepared.
 |
| | Fig. 1 XRD patterns of (a) Co–Mn MOF (b) Co–MnO/C-400 °C, Co–MnO/C-450 °C, and Co–MnO/C-500 °C. | |
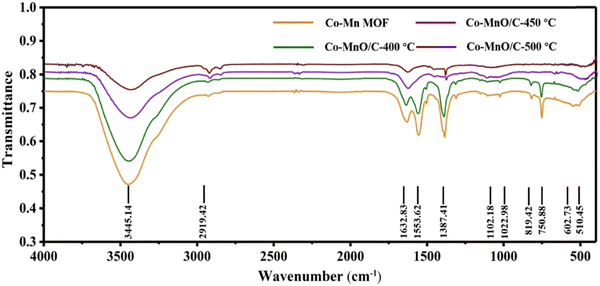 |
| | Fig. 2 The FT-IR spectra recorded for the Co–Mn MOF, Co–MnO/C-400 °C, Co–MnO/C-450 °C and Co–MnO/C-500 °C. | |
The N2 adsorption isotherm test characterization is shown in Fig. 3, where the Co–MnO/C nanoparticles show a specific surface area of 113.06 m2 g−1, which is higher than that reported previously,34,35 and the pore size distribution is less than 10 nm, indicating the existence of mesopores in Co–MnO/C nanoparticles. Mesopores contain abundant active sites, facilitating the intercalation chemistry of Li+.36,37 This mesoporous structure benefits the electrochemical performance of the anode material because it facilitates the transportation of electrolyte through the whole material.22 As shown in Fig. 4, the X-ray photoelectron spectroscopy (XPS) results of Co–MnO/C are collected to explore the chemical compositions and valence in the material. The full scan spectrum of the Co–MnO/C composite presented in Fig. 4a proves the existence of Co, Mn, O and C elements. The two main peaks in the high-resolution XPS spectrum shown in Fig. 4b are located at 641.02 eV and 652.7 eV, corresponding to Mn(II) 2p3/2 and Mn 2p1/2, respectively, which confirm the presence of MnO in the sample.38 The two characteristic peaks at 779.73 eV and 795.58 eV in the Co(0) XPS spectrum (Fig. 4c) could be ascribed to Co 2p3/2 and Co 2p1/2, respectively, which are consistent with the previous literature studies.39,40 As shown in Fig. 4d, the C–C type band corresponds to the main peak at 284.20 eV, and due to incomplete reduction/carbonization, the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O type bands exhibit two weak peaks at 285.17 eV and 288.60 eV.41
O type bands exhibit two weak peaks at 285.17 eV and 288.60 eV.41
 |
| | Fig. 3 Nitrogen adsorption/desorption isotherms (a) and the corresponding pore size distributions (b) of Co–MnO/C. | |
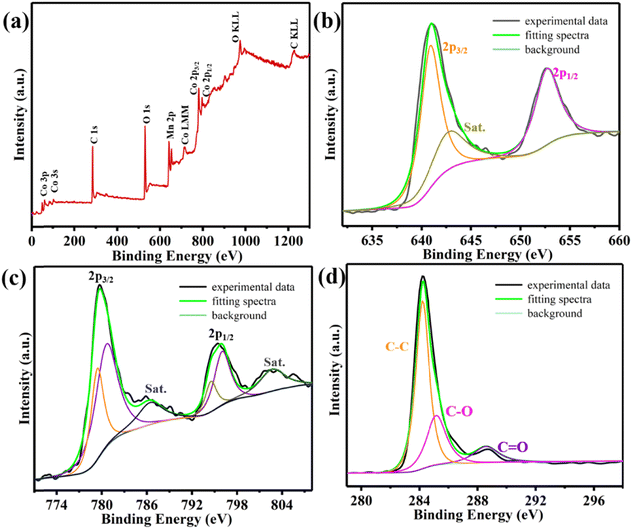 |
| | Fig. 4 Full scan XPS spectrum of (a) Co–MnO/C-450 °C and high-resolution XPS spectra of (b) Mn 2p, (c) Co 2p, and (d) C 1s of Co–MnO/C-450 °C composites. | |
The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) test results are shown in Fig. 5 to further observe the microstructure and mapping of Co–MnO/C nanoparticles. Fig. 5a shows the SEM images of MnO and Co nanoparticles stacked together, with an average composite diameter of 40–70 nm. The nano-sized particles can effectively reduce the diffusion distance of Li+ and mitigate the volume change.22,42 In addition, the MnO and Co nanoparticles are tightly combined within the carbon matrix, ensuring excellent structural flexibility and electronic conductivity. The TEM image is further analyzed to identify the detailed morphology and microstructure (Fig. 5b). From the high-resolution TEM (HRTEM) image observed in Fig. 5c, the d-spacing of 0.2821, 0.4678, 0.2249, and 0.3183 nm corresponds to (hkl) planes of (200), (311), (200), and (220), respectively. The elemental mapping images shown in Fig. 5d further confirm the distribution of Mn, Co, C and O on the Co–MnO/C composite.
 |
| | Fig. 5 (a) SEM images, (b) TEM image, and (c) HRTEM images of Co–MnO/C, and (d) the corresponding elemental mapping of Mn, Co, C and O. | |
2.2. Electrochemical properties of the Co–MnO/C composite
The electrochemical properties of the Co–MnO/C composite are evaluated as the anode electrode of LIBs. The first three cyclic voltammograms (CV) for the Co–MnO/C electrode are collected at room temperature to confirm the reaction mechanism. By measuring the CV curves of the Co–MnO/C electrode at scan rates of 0.1 mV s−1 (Fig. 6a) and 1.0 mV s−1 (Fig. S1, ESI†) from 0.01 V to 3 V, a slightly broad reduction peak at 0.45 V and 0.7 V in the first cathodic scan can be ascribed to the reduction of metal ions (Mn2+ → Mn(0)) and the formation of the solid electrolyte interface (SEI).43 And in the first anodic scan, the oxidation peaks are located at around 1.34 V and 2.0 V, which can be assigned to the de-alloying of Li–Mn alloy. The irreversible phase transformation caused by the formation of Li2O and metallic manganese14 matches the reduction peaks of 0.5 V in the following cycles. It is noteworthy that other characteristic peaks were observed in the composite materials, which are possibly related to the Co element. The CV curves in the second and third cycle almost overlap, suggesting the full activation process and good reversibility of Co–MnO/C electrode materials.15,44 The reaction mechanism of the working electrode during the process of lithiation and delithiation can be described using eqn (1)–(3):14| | | Li+ + e− + electrolyte → SEI(Li) | (1) |
| | | MnO + 2Li+ + 2e− → Mn + Li2O | (2) |
| | | Mn + Li2O → MnO + 2Li+ + 2e− | (3) |
 |
| | Fig. 6 (a) The first three consecutive CV curves. (b) and (c) EIS profiles of the Co–MnO/C electrode at different cycles. (d) The linear fitting of Warburg impedance of the Co–MnO/C electrode. (e) CV curves of the Co–MnO/C electrode at various sweep scans. (f) Corresponding log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i vs. log i vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) v plots in the oxidized and the reduced state of the Co–MnO/C electrode. (g) The capacitive and diffusion-controlled contribution to the overall charge storage of the Co–MnO/C electrode at a sweep rate of 1.0 mV s−1. (h) The contribution ratio of capacitive-controlled and diffusion-controlled at different scan rates. v plots in the oxidized and the reduced state of the Co–MnO/C electrode. (g) The capacitive and diffusion-controlled contribution to the overall charge storage of the Co–MnO/C electrode at a sweep rate of 1.0 mV s−1. (h) The contribution ratio of capacitive-controlled and diffusion-controlled at different scan rates. | |
To investigate the reason for the electrochemical characteristics of Co–MnO/C electrode materials, the electrochemical impedance spectroscopy (EIS) measurement was performed at room temperature. From Fig. 6b, the charge-transfer resistance (Rct) and the constant phase element of the interface of the electrode and electrolyte (CPE1) correspond to the medium frequency semicircle and RSEI is related to the resistance of the SEI surface at high frequency.45 There is an inclined line in the low frequency region, which can be ascribed to the Warburg impedance (Zw) owing to Li+ diffusion in the active materials.46
| |  | (4) |
The Rct of the pure MnO electrode is 305 Ω (Fig. S2, ESI†), much higher than that of the Co–MnO/C electrode (194 Ω), indicating that the introduction of porous carbon and Co could enhance the electronic conductivity and ion transfer of MnO composite materials. As depicted in Fig. 6c, the Rct of the Co–MnO/C electrode decreases from 194 Ω to 135 Ω after 30 cycles. In addition, the slope of Z′ vs. ω−1/2 represents Zw, and the Li+ diffusion coefficient (D) can be obtained according to the following eqn (5),47–49 where the Vm, F, S and (−dE/dx) are parameters. Therefore, the Li+ diffusion coefficient (DLi+) is proportional to (1/Zw)2 and the eqn (6) is obtained by transforming eqn (5).47
| | | DLi+ = 0.5[(Vm/FAZw)(−dE/dx)]2 | (5) |
| | | DLi+ = 0.5[(RT)/(n2F2ACZw)]2 | (6) |
where the gas constant
R is 8.314 J K
−1 mol
−1,
T is the absolute temperature (298 K),
A is the electrode surface area (about 1.1 cm
−3),
C can be preliminarily calculated from the density and the molecular weight of the electrode material (0.0292 mol cm
−3),
F equals to 96
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
485 C mol
−1, and n is the charge transfer number. Then, the calculated Li
+ diffusion coefficient of the fresh Co–MnO/C electrode, after the 1st cycle, and 30th cycle electrode is 2.06 × 10
−16, 2.2 × 10
−16, and 4.5 × 10
−16 cm
2 s
−1, respectively. The diffusion rate of Li
+ is increased due to the gradual penetration of electrolyte after the first cycle.
50
The CV curves of Co–MnO/C electrode materials tested at different sweep rates (0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mV s−1) are shown in Fig. 6e to thoroughly investigate the lithium storage performance. One reduction peak and two oxidation peaks appeared in each curve at different scanning rates. Analyzed from previous research studies, the lithium storage mechanism can be quantitatively summarized using eqn (7) and (8).51
where
a and
b are adjustable parameters,
i is the measured current from CV, and
v is the different sweep rates (0.1–1.0 mV s
−1). The
b-value can be determined from the linear relationship between log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i
i and log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v, which can be used to determine the type of charge storage kinetics.
51 When the value of
b approaches 0.5 or 1, it indicates that the capacity of LIBs is dominated by diffusion-controlled or capacity-controlled processes, respectively.
52 When the
b-value is between 0.5 and 1, the charge storage kinetics are contributed by two co-existing processes.
53 As can be seen from
Fig. 6f, the related calculated
b-values are evaluated as 0.78, 0.85, and 0.95, respectively, demonstrating that the charge–discharge process contains both diffusion-controlled and capacitive-controlled contributions. It could be inferred that the diffusion-controlled process is related to MnO and the capacitive controlled process is attributed to MOF-derived materials with hybrid nanoporous and large surface areas.
Eqn (9) can be used to calculate the normalized contribution fractions:
where
k1v and
k2v1/2 represent capacitive-controlled and diffusion-controlled contributions, respectively, and
i(v) represents the current value at a given voltage.
54Fig. 6g displays that the capacitive-controlled contribution of Co–MnO/C electrode materials reach 80% at a sweep rate of 1.0 mV s
−1. With the increase in sweep rates (
Fig. 6h), the capacitive-controlled contribution becomes 54%, 57%, 60%, 73%, and 80%, showing that the capacitive-controlled contribution dominates the lithium storage performance at high scan rates, which is beneficial for Li
+ transfer during the charge–discharge process.
The electrochemical performance of Co–MnO/C electrodes is investigated in the range of 0.01–3 V as shown in Fig. 7. The initial five charge–discharge cycling curves of Co–MnO/C and MnO electrodes at a current density of 100 mA g−1 are presented in Fig. 7a and b, respectively. The first charge capacity of the Co–MnO/C electrode is 1498 mA h g−1, which is much lower than the first discharge capacity (2357 mA h g−1), which can be attributed to irreversible processes including the electrolyte decomposition and the formation of SEI film.55 In comparison to MnO and Co–MnO/C electrodes during cycling, the MnO electrode exhibits lower discharge capacity (762 mA h g−1), indicating that the synergistic effect of cobalt doping and the carbon structure together enhance the conductivity as well as stability. It is worth mentioning that the carbonization of the Co–Mn MOF structure results in porous carbon, which improves the electron transport and rate capability. Furthermore, when the calcination temperature is 400 °C, the electrochemical performance of MnO is not obviously improved (Fig. S4a, ESI†), which may be attributed to incomplete conversion of MOFs. The increase in calcination temperature causes the samples to melt into large particles and reduces the pore size, preventing the transfer of Li+ ions (Fig. S4b, ESI†).33 Then, the charge/discharge curves at different current densities (ranging from 100 to 2000 mA g−1, Fig. S3, ESI†) exhibited a high discharge capacity of Co–MnO/C electrodes.
 |
| | Fig. 7 Galvanostatic charging/discharging curves of the Co–MnO/C (a) and MnO (b). (c) Rate performance of Co–MnO/C and MnO. (d) Change in the discharge specific capacity with current density of Co-doped MnO/C. (e) Cycle performance at various current densities of Co–MnO/C and MnO. | |
The rate performance of MnO and Co–MnO/C were tested and are shown in Fig. 7c. The Co–MnO/C electrode demonstrates average discharge capacities of 1653, 1485, 1215, 853 and 740 mA h g−1 at 100, 200, 500, 1000, and 2000 mA g−1, respectively, which are higher than those of the pure MnO electrode. At low current densities of 100–500 mA g−1, the electrochemical performance of Co–MnO/C can be maintained at a high level, reaching 1200 mA h g−1. Moreover, when the current density returned to 100 mA g−1, the discharge capacities of MnO electrode only reached 700 mA g−1. The low rate performance of the MnO electrode can be attributed to poor conductivity and the large volume change during the charge/discharge process. The change in discharge specific capacity with an increase in current density is presented in Fig. 7d.
In addition to the rate performance, the cycling performance is also an important index to measure the structural stabilization of Co–MnO/C during cycling. The reaction of the electrolyte with lithium metal forms the SEI film on the surface of the negative electrode, which is spatially and compositionally inhomogeneous and fragile. As a consequence, when subjected to volume expansion of the electrode material, the SEI film is easily disrupted, exposing “fresh lithium”, which continues to react with the electrolyte and consume the electrolyte, which explains the capacity degradation of the cycling performance as shown in Fig. 7e and Fig. S5 (ESI†).33,56 It is a common phenomenon for TMOs that the reversible capacity at different current density tends to increase after 100 cycles.14,33 Co–MnO/C exhibits a theoretical initial reversible capacity of 1441 mA h g−1 at 100 mA g−1; after cycling up to 300 times, the specific capacity has declined from 1441 mA h g−1 to 1414 mA h g−1. At high current density (1000 mA g−1), the discharge capacities of the Co–MnO/C electrode can reach 772 mA h g−1 (at the 300th cycles), and the Coulombic efficiency remains at about 99% during the whole cycle test. However, the Coulombic efficiency at 1000 mA g−1 is lower than that at 100 mA g−1 (as shown in Fig. 7e), which is because the SEI is more susceptible to damage under high currents, exposing “fresh lithium” to react with the electrolyte, and the depletion of the electrolyte leads to capacity degradation and a decrease in Coulombic efficiency. It can be found that the Co–MnO/C capacities are stable after the initial rapid decline at a current density of 2000 mA g−1 (Fig. S5, ESI†). By comparison with the Co–MnO/C and MnO electrode, the Co–MnO/C displays superior electrochemical stability and excellent lithium storage performance. For a comparison of the cycling performance of MnO under different methods, please refer to Table 1. In addition, in order to study the morphology evolution of the Co–MnO/C electrode material after charge/discharge cycles, Fig. 8a and b show the SEM images of this electrode. Due to the unique carbon skeleton structure of MOF derivatives, the MnO nanoparticles still maintain the structural integrity after more than 600 charge/discharge processes. Fig. 8e shows the photographs of the Co–MnO/C electrode during bending tests, demonstrating the excellent flexibility of the electrode material. There are obvious cracks on the surface of MnO electrode after cycle performance tests (Fig. 8g), which can explain why the capacity decays so fast. In contrast, the unique structure of MOF-derived materials can effectively alleviate the volume expansion during charge/discharge processes (Fig. 8f). These results confirm that Co–MnO/C is a potential anode material for LIBs.
Table 1 Comparison of electrochemical performance of the Co–MnO/C composite electrode with previously reported MnO-based materials
| Samples |
Synthetic method |
Cycling stability (mA h g−1) |
Current density (mA g−1) |
Ref. |
| MnO/CNFs |
Electrospinning process |
1082/100th |
100 |
57
|
| 575/200th |
1000 |
| MnO@C |
Two-step approach |
800/80th |
100 |
15
|
| MnO@carbon |
Hydrothermal method and calcination |
801/200th |
200 |
58
|
| MnO nanospheres |
Calcination treatment |
1096.6/100th |
100 |
14
|
| MnO/C–N |
MOF-derivation |
1085/100th |
100 |
16
|
| MnO@Mn3N2/C |
Calcination treatment |
600/10th |
100 |
59
|
| MnO microspheres |
MnCO3 precursor |
625/60th |
100 |
60
|
| MnO/C nanocomposite |
Manganese-salts/glycerol sol |
1280/50th |
100 |
61
|
| 725/400th |
1000 |
| MnO@3D N-PCF |
Freeze-drying-calcination |
1037/100th |
100 |
62
|
| MnO/SCPC |
Pre-carbonized-impregnation |
894/100 th |
100 |
63
|
| 584/1000th |
1000 |
| Co–MnO/C |
MOF-derivation |
1414/300th |
100 |
Present work |
| 772/300th |
1000 |
| 605/200th |
2000 |
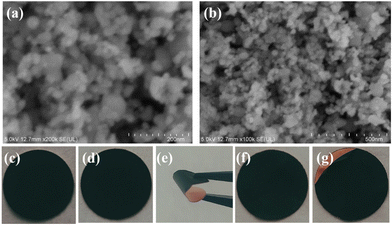 |
| | Fig. 8 (a) and (b) SEM images of the Co–MnO/C electrode after 630 charge/discharge cycles. (c) and (d) The digital photographs of Co–MnO/C (c) and MnO (d) electrode. (e) High bendable properties of the Co–MnO/C composite. The digital photographs of Co–MnO/C (f) and MnO (g) electrodes after 630 charge/discharge cycles. | |
3. Conclusions
In summary, we have described a convenient and straightforward strategy to synthesize Co–MnO/C as an anode material for LIBs by using Co/Mn-based MOFs and carrying out calcination at 450 °C under an Ar/H2 (10%) atmosphere. Compared to pure MnO, Co–MnO/C exhibits a series of advantages, such as superior cycling stability (1414 mA h g−1 after cycling 300 times at 100 mA g−1) and excellent Coulombic efficiency (about 99% after 300 cycles). Two reasons for the improvement in electrochemical properties of Co–MnO/C electrode materials are analyzed and summarized: (I) the nano-sized particles of MnO promote the diffusion of Li+ and the permeation of electrolytes. (II) The existence of carbon and metallic Co not only reduces mechanical stress caused by volume changes, but also improves the electronic conductivity and cycle stability. Based on the reasonable and efficient improvement of MnO, Co–MnO/C has the potential to become a new candidate for anode materials in LIBs.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52202178) and the Natural Science Foundation of Shanghai (22ZR1426300). We appreciate eceshi (http://www.eceshi.com) for the XPS analysis.
References
- L. Ji, Z. Lin, M. Alcoutlabi and X. Zhang, Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries, Energy Environ. Sci., 2011, 4(8), 2682–2699, 10.1039/C0EE00699H.
- M. Park, X. Zhang, M. Chung, G. B. Less and A. M. Sastry, A review of conduction phenomena in Li-ion batteries, J. Power Sources, 2010, 195(24), 7904–7929, DOI:10.1016/j.jpowsour.2010.06.060.
- Y. Wang and G. Cao, Developments in nanostructured cathode materials for high-performance lithium-ion batteries, Adv. Mater., 2008, 20(12), 2251–2269, DOI:10.1002/adma.200702242.
- Y. Yang, Y. Yue, L. Wang, X. Cheng, Y. Hu, Z.-Z. Yang, R. Zhang, B. Jin and R. Sun, Facile synthesis of mesoporous TiNb2O7/C microspheres as long-life and high-power anodes for lithium-ion batteries, Int. J. Hydrogen Energy, 2020, 45(22), 12583–12592, DOI:10.1016/j.ijhydene.2020.02.214.
- F. Yang, K. Zhang, W. Li and K. Xu, Structure-designed synthesis of hierarchical NiCo2O4@ NiO composites for high-performance supercapacitors, J. Colloid Interface Sci., 2019, 556, 386–391, DOI:10.1016/j.jcis.2019.08.078.
- M. M. Thackeray, S.-H. Kang, C. S. Johnson, J. T. Vaughey, R. Benedek and S. A. Hackney, Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries, J. Mater. Chem., 2007, 17(30), 3112–3125, 10.1039/b702425h.
- X. Zhou, L. J. Wan and Y. G. Guo, Binding SnO2 nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries, Adv. Mater., 2013, 25(15), 2152, DOI:10.1002/adma.201300071.
- Q. Wu, Y. Liu, H.-G. Wang, J. Hou, Y. Li and Q. Duan, Graphene encapsulated metallic state Ce2Sn2O7 as a novel anode material for superior lithium-ion batteries and capacitors, J. Mater. Chem. A, 2020, 8(11), 5517–5524, 10.1039/c9ta13086a.
- X. Fan, Y. Rui, X. Han, J. Yang, Y. Wang and Q. Zhang, Spray-coated monodispersed SnO2 microsphere films as scaffold layers for efficient mesoscopic perovskite solar cells, J. Power Sources, 2020, 448, DOI:10.1016/j.jpowsour.2019.227405.
- L. Chen, Y. Liu, Z. Deng, H. Jiang and C. Li, Edge-enriched MoS2@C/rGO film as self-standing anodes for high-capacity and long-life lithium-ion batteries, Sci. China Mater., 2021, 64, 96–104, DOI:10.1007/s40843-020-1348-y.
- X. Cao, Y. Shi, W. Shi, X. Rui, Q. Yan, J. Kong and H. Zhang, Preparation of MoS2-coated three-dimensional graphene networks for high-performance anode material in lithium-ion batteries, Small, 2013, 9(20), 3433, DOI:10.1002/smll.201202697.
- S. Fang, D. Bresser and S. Passerini, Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries, Adv. Energy Mater., 2020, 10(1), 1902485, DOI:10.1002/aenm.201902485.
- Y. Chu, B. Xi and S. Xiong, One-step construction of MoO2 uniform nanoparticles on graphene with enhanced lithium storage, Chin. Chem. Lett., 2021, 32(6), 1983–1987, DOI:10.1016/j.cclet.2020.10.024.
- L. Zhang, D. Ge, G. Qu, J. Zheng, X. Cao and H. Gu, Formation of porous nitrogen-doped carbon-coating MnO nanospheres for advanced reversible lithium storage, Nanoscale, 2017, 9(17), 5451–5457, 10.1039/c7nr01425b.
- T. Wang, Z. Peng, Y. Wang, J. Tang and G. Zheng, MnO nanoparticle@mesoporous carbon composites grown on conducting substrates featuring high-performance lithium-ion battery, supercapacitor and sensor, Sci. Rep., 2013, 3, 2693, DOI:10.1038/srep02693.
- J. L. Niu, G. X. Hao, J. Lin, X. B. He, P. Sathishkumar, X. M. Lin and Y. P. Cai, Mesoporous MnO/C-N Nanostructures Derived from a Metal-Organic Framework as High-Performance Anode for Lithium-Ion Battery, Inorg. Chem., 2017, 56(16), 9966–9972, DOI:10.1021/acs.inorgchem.7b01486.
- X. Wang, J. Wang, Z. Chen, K. Yang, Z. Zhang, Z. Shi, T. Mei, J. Qian, J. Li and X. Wang, Yolk-double shell Fe3O4@C@C composite as high-performance anode materials for lithium-ion batteries, J. Alloys Compd., 2020, 822, DOI:10.1016/j.jallcom.2020.153656.
- S. M. Jung, D. W. Kim and H. Y. Jung, Unconventional capacity increase kinetics of a chemically engineered SnO2 aerogel anode for long-term stable lithium-ion batteries, J. Mater. Chem. A, 2020, 8(17), 8244–8254, 10.1039/d0ta02188a.
- X. Li, J. Zheng, C. He, K. Wang, W. Chai, Y. Duan, B. Tang and Y. Rui, MOF-derived Cu–C loaded with SnOx as a superior anode material for lithium-ion batteries, Electrochim. Acta, 2019, 326, DOI:10.1016/j.electacta.2019.134960.
- L. Chen, X. Guo, W. Lu, M. Chen, Q. Li, H. Xue and H. Pang, Manganese monoxide-based materials for advanced batteries, Coord. Chem. Rev., 2018, 368, 13–34, DOI:10.1016/j.ccr.2018.04.015.
- Y. Chu and S. Xiong, Mixed transition-metal oxides@ carbon core-shell nanostructures derived from heterometallic clusters for enhanced lithium storage, Chin. Chem. Lett., 2022, 33(1), 486–490, DOI:10.1016/j.cclet.2021.06.074.
- W. Li, H. Li, F. Yang, Y. Rui and B. Tang, Facile preparation of four SnOx-C hybrids with superior electrochemical performance for lithium-ion batteries, Electrochim. Acta, 2018, 288, 20–30, DOI:10.1016/j.electacta.2018.08.073.
- K. Wang, W. Ye, W. Yin, W. Chai, B. Tang and Y. Rui, One-step synthesis of MOF-derived Ga/Ga2O3@C dodecahedra as an anode material for high-performance lithium-ion batteries, Dalton Trans., 2019, 48(33), 12386–12390, 10.1039/c9dt02651g.
- R. C. K. Reddy, X. Lin, A. Zeb and C.-Y. Su, Metal–organic frameworks and their derivatives as cathodes for lithium-ion battery applications: a review, Electrochem. Energy Rev., 2022, 1–36, DOI:10.1007/s41918-021-00101-x.
- J.-E. Zhou, J. Chen, Y. Peng, Y. Zheng, A. Zeb and X. Lin, Metal-organic framework-derived transition metal sulfides and their composites for alkali-ion batteries: A review, Coord. Chem. Rev., 2022, 472, 214781, DOI:10.1016/j.ccr.2022.214781.
- L. Wang, Y. Han, X. Feng, J. Zhou, P. Qi and B. Wang, Metal–organic frameworks for energy storage: Batteries and supercapacitors, Coord. Chem. Rev., 2016, 307, 361–381, DOI:10.1016/j.ccr.2015.09.002.
- Y. Guo, Z. Wang, X. Lu, J. Lu, K. Rabia, H. Chen, R. Hu, H. Tang, Q. Zhang and Z. Li, Core–shell ZnO@ C: N hybrids derived from MOFs as long-cycling anodes for lithium ion batteries, Chem. Commun., 2020, 56(13), 1980–1983, 10.1039/C9CC08478A.
- K. Wang, D. Ju, G. Xu, Y. Wang, S. Chen, J. Zhang, Y. Wu and W. Zhou, Study on electrochemical performances of composite carbon (FeO/C) materials fabricated by coal tar pitch and Fe3O4 particles, Int. J. Hydrogen Energy, 2019, 44(46), 25199–25206, DOI:10.1016/j.ijhydene.2019.04.148.
- B. Hu, L. Li, X. Xiong, L. Liu, C. Huang, D. Yu and C. Chen, High-performance of copper-doped vanadium pentoxide porous thin films cathode for lithium-ion batteries, J. Solid State Electrochem., 2019, 23(5), 1315–1324, DOI:10.1007/s10008-019-04220-w.
- H. Yue, Z. Shi, Q. Wang, Z. Cao, H. Dong, Y. Qiao, Y. Yin and S. Yang, MOF-derived cobalt-doped ZnO@C composites as a high-performance anode material for lithium-ion batteries, ACS Appl. Mater. Interfaces, 2014, 6(19), 17067, DOI:10.1021/am5046873.
- B. Zhang, L. Wang, H. Zhang, H. Xu and X. He, Revelation of the transition-metal doping mechanism in lithium manganese phosphate for high performance of lithium-ion batteries, Battery Energy, 2022, 1(4), 20220020, DOI:10.1002/bte2.20220020.
- S. H. Kazemi, B. Hosseinzadeh, H. Kazemi, M. A. Kiani and S. Hajati, Facile Synthesis of Mixed Metal-Organic Frameworks: Electrode Materials for Supercapacitors with Excellent Areal Capacitance and Operational Stability, ACS Appl. Mater. Interfaces, 2018, 10(27), 23063–23073, DOI:10.1021/acsami.8b04502.
- F. Yang, W. Li and B. Tang, Two-step method to synthesize spinel Co3O4-MnCo2O4 with excellent performance for lithium ion batteries, Chem. Eng. J., 2018, 334, 2021–2029, DOI:10.1016/j.cej.2017.11.157.
- B. Liu, X. Hu, H. Xu, W. Luo, Y. Sun and Y. Huang, Encapsulation of MnO nanocrystals in electrospun carbon nanofibers as high-performance anode materials for lithium-ion batteries, Sci. Rep., 2014, 4(1), 1–6 Search PubMed.
- J. Su, H. Liang, X.-N. Gong, X.-Y. Lv, Y.-F. Long and Y.-X. Wen, Fast preparation of porous MnO/C microspheres as anode materials for lithium-ion batteries, Nanomaterials, 2017, 7(6), 121, DOI:10.3390/nano7060121.
- B. Wang, X.-Y. Lu, C.-W. Tsang, Y. Wang, W. K. Au, H. Guo and Y. Tang, Charge-driven self-assembly synthesis of straw-sheaf-like Co3O4 with superior cyclability and rate capability for lithium-ion batteries, Chem. Eng. J., 2018, 338, 278–286, DOI:10.1016/j.cej.2017.12.124.
- F. Bozheyev, A. Zhexembekova, S. Zhumagali, A. Molkenova and Z. Bakenov, MoS2 nanopowder as anode material for lithium-ion batteries produced by self-propagating high-temperature synthesis, Mater. Today: Proc., 2017, 4(3), 4567–4571, DOI:10.1016/j.matpr.2017.04.031.
- C. Zhang, J.-G. Wang, D. Jin, K. Xie and B. Wei, Facile fabrication of MnO/C core–shell nanowires as an advanced anode material for lithium-ion batteries, Electrochim. Acta, 2015, 180, 990–997 CrossRef CAS.
- W. Liu, Y. Fu, Y. Li, S. Chen, Y. Song and L. Wang, Three-dimensional carbon foam surrounded by carbon nanotubes and Co-Co3O4 nanoparticles for stable lithium-ion batteries, Composites, Part B, 2019, 163, 464–470, DOI:10.1016/j.compositesb.2019.01.038.
- Z. Li, C. Li, X. Ge, J. Ma, Z. Zhang, Q. Li, C. Wang and L. Yin, Reduced graphene oxide wrapped MOFs-derived cobalt-doped porous carbon polyhedrons as sulfur immobilizers as cathodes for high performance lithium sulfur batteries, Nano Energy, 2016, 23, 15–26, DOI:10.1016/j.nanoen.2016.02.049.
- Y. Yu, L. Gu, C. Wang, A. Dhanabalan, P. A. Van Aken and J. Maier, Encapsulation of Sn@ carbon nanoparticles in bamboo-like hollow carbon nanofibers as an anode material in lithium-based batteries, Angew. Chem., 2009, 121(35), 6607–6611, DOI:10.1002/ange.200901723.
- Y. Liu, Y. Wang, Y. Zhang, S. Liang and A. Pan, Controllable preparation of V 2 O 5/graphene nanocomposites as cathode materials for lithium-ion batteries, Nanoscale Res. Lett., 2016, 11(1), 1–9, DOI:10.1186/s11671-016-1764-3.
- B. Liu, Z. Liu, D. Li, P. Guo, D. Liu, X. Shang, M. Lv and D. He, Nanoscale α-MnS crystallites grown on NS co-doped rGO as a long-life and high-capacity anode material
of Li-ion batteries, Appl. Surf. Sci., 2017, 416, 858–867, DOI:10.1016/j.apsusc.2017.04.230.
- Y. Liu, Y. Wang, Y. Zhang, S. Liang and A. Pan, Controllable Preparation of V2O5/Graphene Nanocomposites as Cathode Materials for Lithium-Ion Batteries, Nanoscale Res. Lett., 2016, 11(1), 549, DOI:10.1186/s11671-016-1764-3.
- B. Liu, Z. Liu, D. Li, P. Guo, D. Liu, X. Shang, M. Lv and D. He, Nanoscale α-MnS crystallites grown on N-S co-doped rGO as a long-life and high-capacity anode material of Li-ion batteries, Appl. Surf. Sci., 2017, 416, 858–867, DOI:10.1016/j.apsusc.2017.04.230.
- N. Yu, L. Zou, C. Li and K. Guo, In-situ growth of binder-free hierarchical carbon coated CoSe2 as a high performance lithium ion battery anode, Appl. Surf. Sci., 2019, 483, 85–90, DOI:10.1016/j.apsusc.2019.03.258.
- J. Bai, H. Wu, S. Wang, G. Zhang, C. Feng and H. Liu, Synthesis of CoSe2-SnSe2 nanocube-coated nitrogen-doped carbon (NC) as anode for lithium and sodium ion batteries, Appl. Surf. Sci., 2019, 488, 512–521, DOI:10.1016/j.apsusc.2019.05.096.
- X.-L. Wu, Y.-G. Guo, J. Su, J.-W. Xiong, Y.-L. Zhang and L.-J. Wan, Carbon-Nanotube-Decorated Nano-LiFePO4@C Cathode Material with Superior High-Rate and Low-Temperature Performances for Lithium-Ion Batteries, Adv. Energy Mater., 2013, 3(9), 1155–1160, DOI:10.1002/aenm.201300159.
- X. Wang, H. Hao, J. Liu, T. Huang and A. Yu, A novel method for preparation of macroposous lithium nickel manganese oxygen as cathode material for lithium ion batteries, Electrochim. Acta, 2011, 56(11), 4065–4069, DOI:10.1016/j.electacta.2010.12.108.
- W. Yin, W. Chai, K. Wang, W. Ye, Y. Rui and B. Tang, A highly Meso@Microporous carbon-supported Antimony sulfide nanoparticles coated by conductive polymer for high-performance lithium and sodium ion batteries, Electrochim. Acta, 2019, 321, DOI:10.1016/j.electacta.2019.134699.
- B. Li, R. Wang, Z. Chen, D. Sun, F. Fang and R. Wu, Embedding heterostructured MnS/Co1−xS nanoparticles in porous carbon/graphene for superior lithium storage, J. Mater. Chem. A, 2019, 7(3), 1260–1266, 10.1039/c8ta09740b.
- J. Yang, H. Gao, S. Men, Z. Shi, Z. Lin, X. Kang and S. Chen, CoSe2 Nanoparticles Encapsulated by N-Doped Carbon Framework Intertwined with Carbon Nanotubes: High-Performance Dual-Role Anode Materials for Both Li- and Na-Ion Batteries, Adv. Sci., 2018, 5(12), 1800763, DOI:10.1002/advs.201800763.
- Y. Zhang, Q. Ma, S. Wang, X. Liu and L. Li, Poly(vinyl alcohol)-Assisted Fabrication of Hollow Carbon Spheres/Reduced Graphene Oxide Nanocomposites for High-Performance Lithium-Ion Battery Anodes, ACS Nano, 2018, 12(5), 4824–4834, DOI:10.1021/acsnano.8b01549.
- T. Brezesinski, J. Wang, S. H. Tolbert and B. Dunn, Ordered mesoporous alpha-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors, Nat. Mater., 2010, 9(2), 146, DOI:10.1038/nmat2612.
- W. Sun, C. Cai, X. Tang, L.-P. Lv and Y. Wang, Carbon coated mixed-metal selenide microrod: Bimetal-organic-framework derivation approach and applications for lithium-ion batteries, Chem. Eng. J., 2018, 351, 169–176, DOI:10.1016/j.cej.2018.06.093.
- G. Li, L. Xu, Y. Zhai and Y. Hou, Fabrication of hierarchical porous MnCo2O4 and CoMn2O4 microspheres composed of polyhedral nanoparticles as promising anodes for long-life LIBs, J. Mater. Chem. A, 2015, 3(27), 14298–14306, 10.1039/c5ta03145a.
- B. Liu, X. Hu, H. Xu, W. Luo, Y. Sun and Y. Huang, Encapsulation of MnO Nanocrystals in Electrospun Carbon Nanofibers as High-Performance Anode Materials for Lithium-Ion Batteries, Sci. Rep., 2014, 4(1), 4229, DOI:10.1038/srep04229.
- X. Li, S. Xiong, J. Li, X. Liang, J. Wang, J. Bai and Y. Qian, MnO@carbon core-shell nanowires as stable high-performance anodes for lithium-ion batteries, Chemistry, 2013, 19(34), 11310, DOI:10.1002/chem.201203553.
- Y. Wu, M. Liu, H. Feng and J. Li, Carbon coated MnO@Mn3N2 core-shell composites for high performance lithium ion battery anodes, Nanoscale, 2014, 6(24), 14697, 10.1039/c4nr05043f.
- X. Wang, S. Qiu, G. Lu, C. He, J. Liu, L. Luan and W. Liu, Fabrication of porous MnO microspheres with carbon coating for lithium ion battery application, CrystEngComm, 2014, 16(9), 1802–1809, 10.1039/c3ce42247j.
- S. Ma, D. Chen and W. L. Wang, MnO nanoparticles embedded in a carbon matrix as high performance lithium-ion battery anodes: preparation, microstructure and electrochemistry, Phys. Chem. Chem. Phys., 2016, 18(28), 19130, 10.1039/c6cp01691j.
- C. He, J. Li, X. Zhao, X. Peng, X. Lin, Y. Ke, X. Xiao, X. Zuo and J. Nan, In situ anchoring of MnO nanoparticles into three-dimensional nitrogen-doped porous carbon framework as a stable anode for high-performance lithium storage, Appl. Surf. Sci., 2023, 614, 156217, DOI:10.1016/j.apsusc.2022.156217.
- X. Chen, Z. Zhang, R. Xu, X. Gao, D. Zhou, T. Yuan, Y. Chen and L. Cui, In-situ embedding of ultrasmall MnO nanoparticles into pollen biomass-derived hollow porous N heteroatoms enriched carbon microspheres as high-performance anode for lithium-ion batteries, Chem. Eng. J., 2023, 145117, DOI:10.1016/j.cej.2023.145117.
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  *
*

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O type bands exhibit two weak peaks at 285.17 eV and 288.60 eV.41
O type bands exhibit two weak peaks at 285.17 eV and 288.60 eV.41



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1, and n is the charge transfer number. Then, the calculated Li+ diffusion coefficient of the fresh Co–MnO/C electrode, after the 1st cycle, and 30th cycle electrode is 2.06 × 10−16, 2.2 × 10−16, and 4.5 × 10−16 cm2 s−1, respectively. The diffusion rate of Li+ is increased due to the gradual penetration of electrolyte after the first cycle.50
485 C mol−1, and n is the charge transfer number. Then, the calculated Li+ diffusion coefficient of the fresh Co–MnO/C electrode, after the 1st cycle, and 30th cycle electrode is 2.06 × 10−16, 2.2 × 10−16, and 4.5 × 10−16 cm2 s−1, respectively. The diffusion rate of Li+ is increased due to the gradual penetration of electrolyte after the first cycle.50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = b
i = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + log
v + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i and log
i and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v, which can be used to determine the type of charge storage kinetics.51 When the value of b approaches 0.5 or 1, it indicates that the capacity of LIBs is dominated by diffusion-controlled or capacity-controlled processes, respectively.52 When the b-value is between 0.5 and 1, the charge storage kinetics are contributed by two co-existing processes.53 As can be seen from Fig. 6f, the related calculated b-values are evaluated as 0.78, 0.85, and 0.95, respectively, demonstrating that the charge–discharge process contains both diffusion-controlled and capacitive-controlled contributions. It could be inferred that the diffusion-controlled process is related to MnO and the capacitive controlled process is attributed to MOF-derived materials with hybrid nanoporous and large surface areas.
v, which can be used to determine the type of charge storage kinetics.51 When the value of b approaches 0.5 or 1, it indicates that the capacity of LIBs is dominated by diffusion-controlled or capacity-controlled processes, respectively.52 When the b-value is between 0.5 and 1, the charge storage kinetics are contributed by two co-existing processes.53 As can be seen from Fig. 6f, the related calculated b-values are evaluated as 0.78, 0.85, and 0.95, respectively, demonstrating that the charge–discharge process contains both diffusion-controlled and capacitive-controlled contributions. It could be inferred that the diffusion-controlled process is related to MnO and the capacitive controlled process is attributed to MOF-derived materials with hybrid nanoporous and large surface areas.




