Dual-response thermosensitive microcapsules based on hydrogel†
Yibin
Liu‡
 a,
Chenyang
Liu‡
a,
Jianghao
Liu
*a,
Yun
Qiao
a,
Yuanyuan
Liu
a,
Chenyang
Liu‡
a,
Jianghao
Liu
*a,
Yun
Qiao
a,
Yuanyuan
Liu
 b,
Yang
Zhou
b,
Yang
Zhou
 b,
Gongming
Li
a,
Zhitong
Yang
a,
Zhenzhen
Li
a and
Zhicheng
Sun
b,
Gongming
Li
a,
Zhitong
Yang
a,
Zhenzhen
Li
a and
Zhicheng
Sun
 *a
*a
aBeijing Engineering Research Center of Printed Electronics, School of Printing and Packaging Engineering, Beijing Institute of Graphic Communication, Beijing, 102600, China. E-mail: arisliu@bigc.edu.cn; sunzhicheng@bigc.edu.cn
bKey Laboratory of Advanced Materials of Tropical Island Resources of Ministry of Education and School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan 570228, China
First published on 22nd November 2022
Abstract
Dual-response thermosensitive microcapsules based on hydrogel were prepared via environmentally friendly physical and chemical methods. They exhibit reversible discoloration and expansion (tactile) feedback at different temperatures.
Owing to the rapid industrialization of microcapsule technology in the 1930s, a new application platform of polymer materials has emerged. Since then, the development and application of functional microcapsules, such as thermal expansion microcapsules,1 phase change microcapsules,2 sustained-release microcapsules,3 and self-healing microcapsules,4 have drawn considerable attention. However, current single-function microcapsules cannot satisfy the rapidly increasing requirements and demands of various applications. Therefore, multifunctional microcapsules must be advanced to overcome the current limitations of single-function microcapsules.
To date, several multifunctional microcapsules have been reported in the literature, including dual-stimuli-responsive microcapsules for drug release in biomedicine,5 dual-stimuli-responsive microcapsules towards temperature and pH for environmental monitoring,6 and thermochromic and self-healing bifunctional microcapsules for coatings.7–10 Although these multifunctional microcapsules have been successfully applied in various fields, shortcomings that limit their manufacturing and further utilization include their complicated preparation process, high cost of fabrication, generation of VOCs, and instability in practical use. These issues substantially hinder the current development and further application of multifunctional microcapsules. Therefore, it is of great interest to develop novel fabrication methods and desired multifunctionalities for microcapsule applications.
Currently, functional hydrogels are widely used in various fields, including sustained-release drug delivery,11 efficient gel-electrolytes for solar energy conversion,12 and stretchable conductors for wearable electronic.13 Particularly, hydrogel combined with microcapsules has the advantages of simple preparation, environmental protection and safety, stable structure, etc.14,15 In addition, a hydrogel prepared by the sharp-hole coagulation bath method was employed as the core material of microcapsules, endowing them with the advantages of high encapsulation rate, few by-products, and rapid reaction. This can solve the problems of preparation technology, VOC emission, and application stability.16,17 Therefore, the development of functional microcapsules via hydrogel technology has great potential in multifunctional applications.
In this work, based on previously reported thermal expansion microcapsules,18–20 thermosensitive microcapsules (TSMs) with single-stimulus dual-response nature were prepared by incorporating colour-changing materials. TSMs are characterized by colour change and expansion under different temperatures. Stable core hydrogels and TSMs were successively obtained by two steps: sharp-hole coagulation bath and in situ polymerization. This method mainly adopted the reaction of biodegradable materials in a water-in-water system (W/W), replacing the organic solvent system to reduce harm to the environment. Our dual-response thermosensitive TSMs are expected to have great application value in the fields of anti-counterfeiting, monitoring, printing, and packaging.
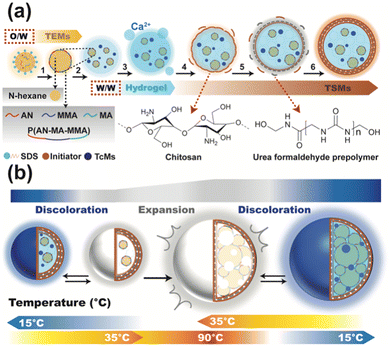
Fig. 1a shows the preparation of TSMs (details are included in the ESI†). The preparation of thermal expansion microcapsules (TEMs) is shown in step 1 of Fig. 1a. Three shell material monomers (acrylonitrile (AN), methyl acrylate (MA) and methyl methacrylate (MMA)) and the core material (N-hexane) were mixed to form an oil phase, and the surfactant sodium dodecyl sulfate (SDS) was used to form an oil-in-water system (O/W). Then, suspension in situ polymerization was carried out under the action of the initiator to produce the thermoplastic block copolymer shell material (P(AN-MA-MMA)) (Fig. S2, ESI†), and thermal expansion microcapsules were finally obtained. The functional thermochromic materials (TcMs) comprised crystal violet lactone (CVL), bisphenol A and polyol (dodecanol). The TEMs were obtained after filtration and washing, whereas the TcMs were obtained after high-speed shearing by mixing with a sodium alginate colloid (step 2 of Fig. 1a). The TEMs and TcMs were fixed in the “egg lattice” of the calcium alginate (Ca-ALG) hydrogel structure formed by a chelation reaction using the sharp-hole coagulation bath method (step 3 of Fig. 1a). To better seal TEMs and TcMs inside the core material, electrostatic interaction between chitosan (CS) and Ca-ALG was used as a surfactant. In addition, through the copolymerization of urea formaldehyde prepolymer (PUF) and CS in the W/W system, shell materials with a layer-by-layer stacking structure were formed on the surface of the hydrogel (Fig. 1a steps 4–6 and Fig. S1, ESI†), and single-stimulus dual-response thermosensitive microcapsules were finally obtained. It is worth mentioning that electrostatic interaction plays an important role in the W/W environmental protection reaction system. Specifically, owing to the existence of electrostatic interaction, the synthetic system can avoid the use of organic surfactants and drive the copolymerization of PUF and CS around the core materials.
 | ||
| Fig. 1 (a) Flow chart of thermosensitive microcapsule synthesis (steps 1–6). (b) Dual-response concept of thermosensitive microcapsules. | ||
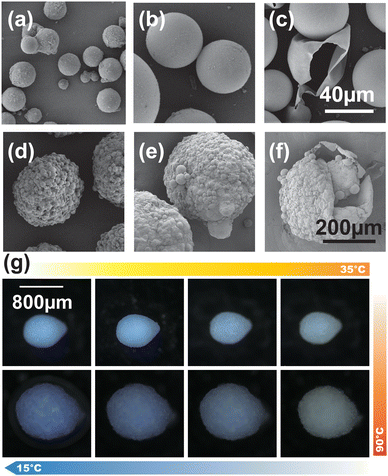
The surface morphology of the thermosensitive microcapsules was observed using scanning electron microscopy (Fig. 2a–f). Fig. 2a–c show the morphology of thermal expansion microcapsules in the TSMs. Particularly, the core material (N-hexane) was transformed into gas (liquid-to-gas) during the phase change at a certain temperature, and the corresponding pressure led to an expansion effect (Fig. 2a and b). Using the W/W reaction system, TSMs were observed with a large number of TEMs inside (Fig. 2d–f), affording an uneven surface. In addition, the expansion of TSMs was caused by the expansion and extrusion of several TEMs inside. In fact, TcMs presented in TSMs can play a significant and visible role before and after expansion, bringing about two kinds of changes in microcapsules under different temperatures. Further analysis of particle size (Fig. S4, ESI†) provided direct evidence that the particle size of the microcapsules before and after expansion gradually increased, confirming the expansion effect and reversible phenomenon of the discoloration of TSMs (Fig. 2g). Owing to the fluorescence characteristic of CVL, the fluorescence phenomenon before and after temperature change was observed under ultraviolet irradiation at 359–371 nm, which also show that TcMs are completely wrapped in TSMs (Fig. S5, ESI†).
The molecular weight change of the TSM shell material can be obtained from Table 1. Firstly, the oligomer (PUF) was formed by urea and formaldehyde under alkaline conditions, and the weight-average molecular weight of PUF was 980. After polymerization with CS under acidic conditions, it increased to 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 588, which confirmed that a layer of polymer shell was modified on the surface of the TSMs. To further verify the structural composition and thermal performance, the synthetic microcapsules were fully characterized. The infrared spectrum in Fig. S6 (ESI†) indicates the presence of functional groups in the microcapsules. The peaks in the infrared characteristic region (3000–3500 cm−1) can be attributed to the stretching vibration of –OH and –NH, suggesting that TSMs cover ALG, CS, and PUF. The peaks of the stretching vibration of C–H at 2923 and 2855 cm−1 verify that the TSMs contain TcMs, while the peak at 1720 cm−1 corresponding to C
588, which confirmed that a layer of polymer shell was modified on the surface of the TSMs. To further verify the structural composition and thermal performance, the synthetic microcapsules were fully characterized. The infrared spectrum in Fig. S6 (ESI†) indicates the presence of functional groups in the microcapsules. The peaks in the infrared characteristic region (3000–3500 cm−1) can be attributed to the stretching vibration of –OH and –NH, suggesting that TSMs cover ALG, CS, and PUF. The peaks of the stretching vibration of C–H at 2923 and 2855 cm−1 verify that the TSMs contain TcMs, while the peak at 1720 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the TEMs confirms the existence of TEMs in the TSMs. In addition, the peaks of TSMs in the infrared fingerprint regions of ∼1060 and ∼595 cm−1 further support the existence of TcMs and PUF in the TSMs. The full (Fig. S7, ESI†) and narrow XPS spectra (C 1s, Fig. S8 and N 1s, Fig. S9, ESI†) both show the presence of C, O, and N as well as a small amount of Ca and Cl. In addition to the organic components of the shell material, CaCl2 of the aqueous phase system (step 3 of Fig. 1a) was also attached to the surface of the microcapsules. By contrast, after C 1s peak splitting calibration, the order from right to left is C–C, C–N, C–O, and C
O of the TEMs confirms the existence of TEMs in the TSMs. In addition, the peaks of TSMs in the infrared fingerprint regions of ∼1060 and ∼595 cm−1 further support the existence of TcMs and PUF in the TSMs. The full (Fig. S7, ESI†) and narrow XPS spectra (C 1s, Fig. S8 and N 1s, Fig. S9, ESI†) both show the presence of C, O, and N as well as a small amount of Ca and Cl. In addition to the organic components of the shell material, CaCl2 of the aqueous phase system (step 3 of Fig. 1a) was also attached to the surface of the microcapsules. By contrast, after C 1s peak splitting calibration, the order from right to left is C–C, C–N, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the binding energy and carbon content correspond to 284.8, 285.36, 287.03, and 289.32 eV and 15%, 53%, 26%, and 6%, respectively. In the narrow spectrum of N 1s, the binding energies of the imino group (–NH–) and nitrogen cation (–N+) were 399.84 and 401.55 eV, respectively, and the ratio of nitrogen content was about 4
O, and the binding energy and carbon content correspond to 284.8, 285.36, 287.03, and 289.32 eV and 15%, 53%, 26%, and 6%, respectively. In the narrow spectrum of N 1s, the binding energies of the imino group (–NH–) and nitrogen cation (–N+) were 399.84 and 401.55 eV, respectively, and the ratio of nitrogen content was about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Specifically, the surface of the TSMs mainly comprised CS and PUF as well as a lot of C–N, N–H, C
1. Specifically, the surface of the TSMs mainly comprised CS and PUF as well as a lot of C–N, N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –N+, which represent the characteristic groups of electrostatic adsorption between ALG and CS. Furthermore, the thermal performance of the TSMs was evaluated using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (Fig. S10 and S11, ESI†). As per the DSC data, TEMs and TcMs exhibit endothermic phenomena at 93 °C and 25 °C, respectively, resulting from the evaporation of the core material of TEMs (N-hexane) and liquefaction of TcM solvent (dodecanol) during the phase change. This combined with the thermogravimetric results suggests that the gas broke through the shell material, causing the loss after the expansion of TEMs. TSMs and TcMs had similar weight loss trends at 150–200 °C, indicating that TcMs existed in the TSMs.
O and –N+, which represent the characteristic groups of electrostatic adsorption between ALG and CS. Furthermore, the thermal performance of the TSMs was evaluated using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (Fig. S10 and S11, ESI†). As per the DSC data, TEMs and TcMs exhibit endothermic phenomena at 93 °C and 25 °C, respectively, resulting from the evaporation of the core material of TEMs (N-hexane) and liquefaction of TcM solvent (dodecanol) during the phase change. This combined with the thermogravimetric results suggests that the gas broke through the shell material, causing the loss after the expansion of TEMs. TSMs and TcMs had similar weight loss trends at 150–200 °C, indicating that TcMs existed in the TSMs.
| Polymer | M w | M n | Polydispersity |
|---|---|---|---|
| M w and Mn are the weight-average molecular weight and number-average molecular weight, respectively. Polydispersity is the dispersity of a substance (Mw/Mn). | |||
| PUF | 980 | 608 | 1.61 |
| CS-PUF | 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 588 588 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434 |
4.13 |
Further, we explain the mechanism of the working process of the TSMs and the role of the individual components. When subjected to different temperatures, TSMs undergo two processes: discoloration and expansion. The discoloration process mainly involves an electron donor (CVL), electron acceptor (bisphenol A) and solvent compound (polyol). In addition, the redox potentials of the electron donor and acceptor are similar, but their relative changes are different. When the temperature drops to a certain level, bisphenol A releases protons. Thus, bisphenol A obtains CVL electrons at the hydroxyl group, promoting CVL ring opening (Fig. S2 and S3, ESI†), resulting in a rearrangement of the molecular structure. The conjugated double chains are connected and combined with bisphenol A ions, thus exhibiting colour. By contrast, the expansion mechanism is a physical change process. When the temperature rises, the thermoplastic shell material (P(AN-MA-MMA)) of TEMs begins to soften, and the inner core material (N-hexane) changes from liquid to gas. Thus, the microcapsule expands outward owing to the increase in internal pressure. Therefore, the two mechanisms are used to achieve visual and tactile feedback effects at different temperatures, satisfying a prerequisite for the realization of single-stimulus dual-response.
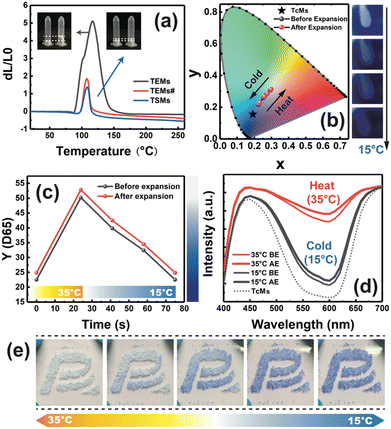
Moreover, the expansion and discoloration performance of the TSMs were explored (Fig. 3) and were applied to assess pattern practicality (Fig. 3e). Fig. 3a shows that the expansion ratio of TEMs was about 5 times and that of the TSMs wrapped with TEMs and TcMs was about 1.5 times (Video S1, ESI†). Additionally, we used the process of TSMs to wrap only TEMs to obtain TEMs#, and the expansion ratio was similar to that of the TSMs. Typically, calcium alginate solid scaffold forms a support for the inner core material and the hydrogel of calcium alginate contains a large number of gaps after drying. Thus, a large number of TEMs and TcMs attach to the scaffold. Therefore, during the expansion process of TEMs, the internal gaps of TSMs might be filled initially before expansion, which is likely to be the direct cause for the gap between the expansion ratio of the TSMs and TEMs. Fig. 3b and Fig. S12 (ESI†) present the CIE1931 colour coordinates of the TSMs and TcMs. TcMs with dark blue colour in TSMs were influenced by the white TEMs and gradually lead to fading of colour after TSM synthesis. Before and after the expansion, the heating (or cooling) of TSMs changed colour to the white (or blue) region (Fig. S13a, ESI†). In contrast, the difference in discoloration before and after expansion was not easy to observe. Therefore, the influence of cooling (15 °C) and heating (35 °C) on brightness (Y) was analysed under a D65 light source (6500 K). Interestingly, larger Y value leads to whiter colour, indicating less absorption from the light source and greater reflection. This method can not only directly impart sensitivity to the discoloration reaction but also indirectly reflect the colour-shift phenomenon before and after TSM expansion. Fig. 3c shows that the TSMs are very sensitive to heating (25 s) and cooling and took nearly double the time to restore discoloration (50 s). The TSMs turned slightly white after expansion compared to that before expansion, which is because the particle size of the white TEMs became larger and clung to the shell material, resulting in a slight colour shift. As shown in Fig. 3d, the discoloration process of the TSMs mainly depended on the wavelength range of 600 nm (non-blue range), and the absorption at 600 nm gradually decreased with increasing temperature. Fig. S13b and c (ESI†) show that the colour change trend of the TSMs before and after expansion was almost unchanged. After being wrapped by TSMs, the colour of TcMs also became lighter (Fig. 3d and Fig. S13, dotted line, ESI†), which was caused by the white TEMs. According to the above performance analysis of the TSMs, different temperatures showed significant influence on expansion and discoloration performance, and exhibit gradient feedback (Fig. S14, ESI†). In addition, the dual-response of the TSMs provides a possibility for multi-domain applications.
It is worth mentioning that TSMs will play a unique role in the fields of intelligent monitoring, interactive experience and anti-counterfeiting in the future by virtue of their advantages of simple and fast preparation, non-toxicity and environmental friendliness. Importantly, their single-stimulus dual-response function is highly attractive. In the field of anti-counterfeiting in particular,21,22 this microcapsule technology can be inserted into labels, bar codes, two-dimensional codes, and even currencies to induce a visual colour-change process via finger temperature (∼35 °C) and feel a tactile bump change under high temperature (Video S2, ESI†). This not only provides a good identification method to judge the authenticity of products but also increases the repeated preparation cost and imitation difficulty of fake and inferior products. Therefore, based on their multifunctional nature, TSMs provide a new scheme for the preparation and application of multifunctional microcapsules and will make important contributions to industrialization, intelligence and lightweight applications in the future.
To summarize, novel dual-response thermosensitive microcapsules (TSMs) were developed using an environmentally friendly and efficient physical and chemical synthesis method. In this study, TSMs were obtained via the copolymerization of CS and PUF to form a shell material. The core material was coated with solid TEMs and oil-liquid TcMs. The synthesis and encapsulation of the shell and core were confirmed by FTIR, XPS, DSC and TG. The thermal expansion and discoloration performance of the TSMs were observed at different temperatures. The results show that the TSMs can exhibit visual discoloration feedback and tactile expansion feedback at 25 °C and 90 °C, respectively. Their sensitivity and reversible discoloration are highlighted. Finally, we expect that TSMs will have great potential application value in the field of anti-counterfeiting and monitoring.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22278037, 21776021, 22007025) Key Scientific Research Project of Beijing Municipal Commission of Education (No. KZ201910015016), and Hainan Provincial Natural Science Foundation of China (220MS006, 220QN182, 222RC549).Notes and references
- X. B. Wang, Adv. Mater. Res., 2012, 496, 130–133 CAS.
- K. Wei-Dong, W. Xiu-Wen and Z. Jin-Lin, Colloids Surf., A, 2021, 627, 127124 CrossRef.
- B. AppaRao, M. R. Shivalingam, Y. V. K. Reddy, N. Sunitha, T. Jyothibasu and T. Shyam, Int. J. Pharm. Biomed. Res., 2010, 1, 90–93 Search PubMed.
- F. Li, S. Jiao, Z. Sun, Y. Liu, Q. Zhang, J. Wen and Y. Zhou, Green Chem., 2021, 23, 927–934 RSC.
- W. Xiao, X. Zeng, H. Lin, K. Han, H. Z. Jia and X. Z. Zhang, Chem. Commun., 2015, 51, 1475–1478 RSC.
- S. Wang, H. Liu, D. Wu and X. Wang, J. Colloid Interface Sci., 2021, 583, 470–486 CrossRef CAS PubMed.
- Y. Xiaoxing, Z. Wenting and W. Lin, Polymers, 2021, 13, 3109 CrossRef PubMed.
- Y. Liu, Q. Fan, G. Zhu, G. Shi, H. Ma, W. Li, T. Wu, J. Chen, Y. Yin and J. Guan, Mater. Horiz., 2021, 8, 2032–2040 RSC.
- F. Adeli, F. Abbasi, M. Babazadeh and S. Davaran, J. Nanobiotechnol., 2022, 20, 1–24 CrossRef.
- R. Platel, L. Vaure, E. Palleau, S. Raffy, F. Guerin, D. Lagarde, R. Cours, C. Marcelot, B. Warot-Fonrose and C. Nayral, J. Colloid Interface Sci., 2021, 582, 1243–1250 CrossRef CAS.
- B. Thu, P. Bruheim, T. Espevik, O. Smidsrød, P. Soon-Shiong and G. Skjåk-Bræk, Biomaterials, 1996, 17, 1069–1079 CrossRef CAS.
- J.-H. Kim, D.-H. Kim, J.-H. So and H.-J. Koo, Energies, 2021, 15, 219 CrossRef.
- J. Y. Sun, C. Keplinger, G. M. Whitesides and Z. Suo, Adv. Mater., 2014, 26, 7608–7614 CrossRef CAS PubMed.
- S. Yan, J. Zhu, Z. Wang, J. Yin, Y. Zheng and X. Chen, Eur. J. Pharm. Biopharm., 2010, 78, 336–345 CrossRef PubMed.
- R. Wang, H. Hu, X. He, W. Liu, H. Li, Q. Guo and L. Yuan, J. Appl. Polym. Sci., 2011, 121, 2202–2212 CrossRef CAS.
- R. Barbaz-Isfahani, S. Saber-Samandari and M. Salehi, Int. J. Biol. Macromol., 2022, 200, 532–542 CrossRef CAS.
- S.-Q. Li, R.-C. Tang and C.-B. Yu, Polym. Degrad. Stab., 2022, 196, 109826 CrossRef CAS.
- S. Jiao, Z. Sun, Y. Zhou, F. Li, J. Wen, Y. Chen, X. Du, L. Li and Y. Liu, Chem. – Asian J., 2019, 14, 4328–4336 CrossRef CAS PubMed.
- S.-Y. Chen, Z.-C. Sun, L.-H. Li, Y.-H. Xiao and Y.-M. Yu, Chin. Chem. Lett., 2017, 28, 658–662 CrossRef CAS.
- Y. Liu, Y. Qiao, Z. Sun, W. Zhang, C. Liu, J. Wen, Y. Liu, Q. Zhang, Y. Zhou and J. Chen, J. Mater. Chem. C, 2022, 10, 12221–12231 RSC.
- Q. Chen, H. Tang, J. Liu, R. Wang, J. Sun, J. Yao, Z. Shao and X. Chen, Chem. Eng. J., 2021, 422, 130091 CrossRef CAS.
- H. Jia, Y. Teng, N. Li, D. Li, Y. Dong, D. Zhang, Z. Liu, D. Zhao, X. Guo and W. Di, ACS Mater. Lett., 2022, 4, 1306–1313 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj05199k |
| ‡ Yibin Liu and Chenyang Liu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |