Catalytic synthesis of seven-membered carbocycles via ring expansion of cyclic β-ketoesters†
Li
Chen‡
a,
Huangjiang
Huang‡
a,
Benlong
Luo
*d,
Jinggong
Liu
 *c,
Shuang
Yang
*b and
Xinqiang
Fang
*c,
Shuang
Yang
*b and
Xinqiang
Fang
 *ab
*ab
aCollege of Chemistry and Materials Science, Fujian Normal University, Fuzhou 350007, China
bFujian Institute of Research on the Structure of Matter, University of Chinese Academy of Sciences, Fuzhou 350100, China. E-mail: yangshuang@fjirsm.ac.cn; xqfang@fjirsm.ac.cn
cOrthopedics Department, Guangdong Provincial Hospital of Traditional Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510120, China. E-mail: liujinggong001@163.com
dPingxiang University, Pingxiang 337055, China. E-mail: 20010060@pxu.edu.cn
First published on 22nd November 2022
Abstract
A catalytic method affording seven-membered carbon rings via two-carbon expansion of five-membered β-ketoesters has been developed. This method tolerates various alkynyl 1,2-diketones, common ynones, and alkynyl ketoesters, and affords the corresponding products with multiple functional groups under mild and environmentally benign conditions.
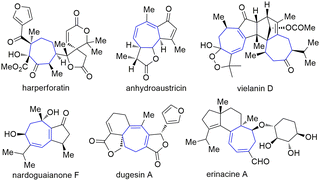
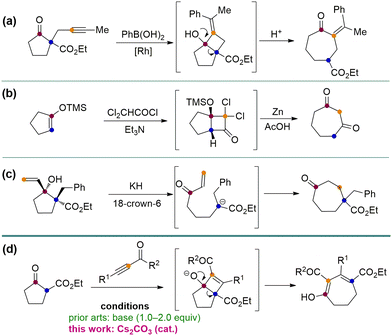
Seven-membered carbon rings are privileged scaffolds in a large number of natural products and bioactive substances (Fig. 1).1 However, the synthesis of such types of molecules bearing various functional groups is still a challenging research topic. Among the established methods of making seven-membered rings, the five-to-seven ring expansion method is particularly charming since it provides a versatile, reliable, and predictable strategy affording medium-sized rings from normal and easily available five-membered ring compounds.2 For instance, the Rh-catalyzed alkyne arylation–cyclization–ring opening sequence has been developed to make cycloheptanone (Scheme 1a).2a A [2+2] annulation-ring expansion method has been reported to afford cycloheptane-1,3-diones (Scheme 1b).2b,c Furthermore, a direct base-mediated ring-opening of vinyl cyclopentanols followed by Michael addition was developed to furnish γ-substituted cycloheptanones (Scheme 1c).2d,e Despite the robustness of these methods, there are still certain limitations such as using multistep operations and relatively tedious steps to synthesize the substrates. Here we report a convenient method employing easily available cyclic β-ketoesters and ynones to undergo facile five-to-seven ring expansion and afford highly functionalized seven-membered rings (Scheme 1d). This method is catalysed by the inexpensive base Cs2CO3 and uses an environmentally benign solvent iPrOH. Li, Wang and co-workers ever reported the ring expansion strategy to construct a wide range of seven-membered ring systems,3 but all of their works involve the use of stoichiometric amounts of bases to induce the reaction (Scheme 1d). Our method enhances the atom economy by using a catalytic amount of the base, and expands the scope to three types of ynones. Herein we report the results.
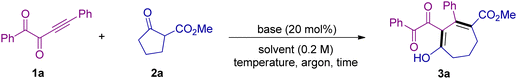
As shown in Table 1, we selected alkynyl 1,2-diketone 1a and cyclic β-ketoester 2a as the standard substrates to make a preliminary investigation of the reaction under base catalysis. We were glad to find that when Cs2CO3 was used as the catalyst, the reaction can proceed to deliver the seven-membered ring product 3a in 36% yield (Table 1, entry 1). The attempts of using other bases such as DBU and Et3N failed to produce any product (Table 1, entries 2 and 3). Then using Cs2CO3 as the base, we tested a series of other solvents (Table 1, entries 4–6). To our pleasure, when we used iPrOH as the solvent, the yield was further improved to 57% (Table 1, entry 5). Enhancing the amount of substrate 2a can increase the yield to 76% (Table 1, entries 7 and 8). But increasing the reaction temperature or lowering the dosage of 2a had no obvious positive effects on the reaction (Table 1, entries 9–11). Then we tried to change the reaction operation and found that under the conditions without quenching by NH4Cl, the yield can be further improved to 84% (Table 1, entries 12 and 13). Shortening the reaction time and reducing the amount of catalyst both lead to lower yields, indicating that the catalyst played a key role in the smooth occurrence of the reaction (Table 1, entries 14–16). Other bases such as K2CO3 and Na2CO3 have also been tested under the standard conditions, and moderate yields of 3a were obtained (Table 1, entries 17 and 18). Moreover, EtOH and MeOH were also evaluated, but both gave poor results (Table 1, entries 19 and 20).
| Entry | Equiv. of 2a | Base | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), catalyst (20 mol%), solvent (0.2 M), under argon protection, and quenched by saturated aqueous NH4Cl. b Isolated yields based on 1a. c Reactions were worked up directly without quenching. d Argon protection was not applied. e 10 mol% of the catalyst was used. | ||||||
| 1 | 1 | Cs2CO3 | CH2Cl2 | rt | 12 | 36 |
| 2 | 1 | DBU | CH2Cl2 | rt | 12 | — |
| 3 | 1 | Et3N | CH2Cl2 | rt | 12 | — |
| 4 | 1 | Cs2CO3 | THF | rt | 12 | 23 |
| 5 | 1 | Cs2CO3 | iPrOH | rt | 4 | 57 |
| 6 | 1 | Cs2CO3 | Toluene | rt | 12 | 20 |
| 7 | 1.2 | Cs2CO3 | iPrOH | rt | 4 | 67 |
| 8 | 2 | Cs2CO3 | iPrOH | rt | 4 | 76 |
| 9 | 1 | Cs2CO3 | iPrOH | 40 | 4 | 68 |
| 10 | 0.5 | Cs2CO3 | iPrOH | rt | 4 | 55 |
| 11 | 2 | Cs2CO3 | iPrOH | 40 | 4 | 76 |
| 12c | 2 | Cs2CO3 | iPrOH | rt | 4 | 79 |
| 13cd | 2 | Cs2CO3 | iPrOH | rt | 4 | 84 |
| 14c | 2 | Cs2CO3 | iPrOH | rt | 1.5 | 76 |
| 15ce | 2 | Cs2CO3 | iPrOH | rt | 4 | 79 |
| 16c | 2 | — | iPrOH | rt | 4 | — |
| 17 | 2 | K2CO3 | iPrOH | rt | 4 | 67 |
| 18 | 2 | Na2CO3 | iPrOH | rt | 4 | 67 |
| 19 | 2 | Cs2CO3 | MeOH | rt | 4 | 21 |
| 20 | 2 | Cs2CO3 | EtOH | rt | 4 | 43 |
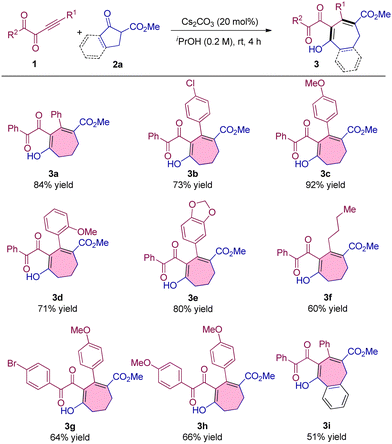
After the establishment of the optimal reaction conditions, we commenced investigating the generality and limitations of the reaction. First, we tested a series of alkynyl 1,2-diketones with different substituents and found that the introduction of electron-withdrawing and electron-donating groups into the phenyl ring of alkynyl moieties did not affect the reaction, producing 3b–3d in up to 92% yield (Table 2, 3b–3d). 1,3-Benzodioxole substituted alkynyl 1,2-diketones can also react with 2a smoothly to obtain the product in 80% yield (Table 2, 3e). The alkyl substituent was also proved to be compatible under the optimal conditions although the product was obtained with a little lower yield (Table 2, 3f). Furthermore, alkynyl 1,2-diketones with different substituents at the ketone moieties were also examined and no obvious influence on the results was observed (Table 2, 3g and 3h). Benzocyclic ketoester was also a suitable substrate for the seven-membered ring formation, delivering 3i in 51% yield (Table 2, 3i).
| a Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), Cs2CO3 (20 mol%), iPrOH (0.2 M), rt, 4 h; all yields were of isolated products. |
|---|
|
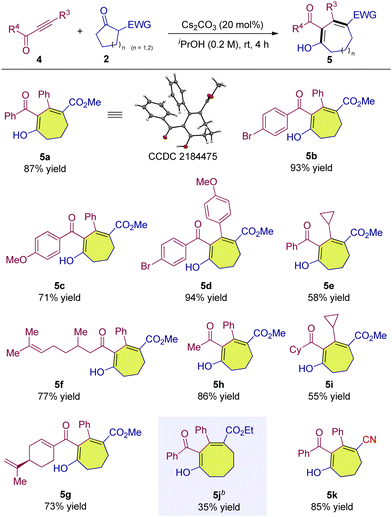
We continued to investigate the reaction of common ynones 4 with β-ketoesters. To our delight, similar to alkynyl 1,2-diketones, the reaction of common alkynyl ketone can also proceed smoothly and deliver the corresponding seven-membered ring product in 87% yield (Table 3, 5a). The variation of substituents on the phenyl rings of the ynones showed a limited impact on the outcomes, allowing access to 5b–5d in high yields (Table 3, 5b–5d). When the R3 of the ynone substrate was replaced by an alkyl group such as cyclopropyl, 5e was also obtained (Table 3, 5e). The use of aliphatic substituents as the R4 group in ynones also proved successful, and the yields were not affected significantly (Table 3, 5f–5h). The simultaneous variation of both R3 and R4 groups to alkyls also worked well, delivering 5i in a moderate yield (Table 3, 5i). The six-membered cyclic ketoester could also participate in the reaction to release the eight-membered product albeit in 35% yield (Table 3, 5j). The cyclic ketone with an α-cyano group also underwent a smooth conversion to provide 5k in a high yield (Table 3, 5k). The configuration of 5a was determined via single crystal X-ray structure analysis, and other products were assigned by analogy.
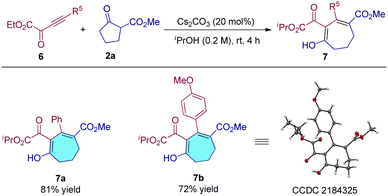
We also tested the reactivity of alkynyl α-ketoester substrates, and the results showed that this type of ynone is also tolerated under the optimal conditions, and products 7a and 7b were obtained in 81% and 72% yields, respectively (Table 4, 7a and 7b). The configuration of product 7b was also determined by single crystal X-ray structure analysis.
| a Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), Cs2CO3 (20 mol%), iPrOH (0.2 M), rt, 4 h; all yields were of isolated products. |
|---|
|
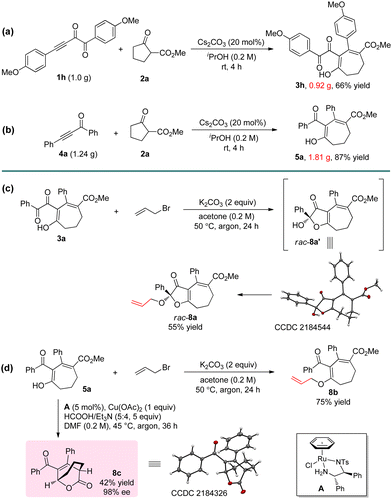
The reaction can be easily carried out on a large scale. By using 1 g of 1h and 1.24 g of 4a as the substrates, products 3h and 5a could be obtained without erosion of the yields (Scheme 2a and b). A series of transformations were achieved to further prove the practicability of the reaction (Scheme 2c and d). For example, the ketal product 8a and enol ether 8b can be successfully obtained by the reactions of 3a and 5a with allyl bromide. 8a could also be obtained using a one-pot method by the direct reaction of 1a, 2a, and allyl bromide, but the yield was only 10%. The Ru-catalyzed asymmetric transfer hydrogenation of 5a resulted in bridged ring product 8c in 42% yield with 98% ee. The configuration of product 8c was determined by single crystal X-ray structure analysis.
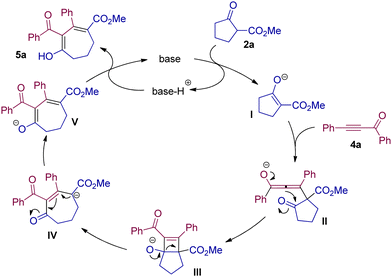
The postulated mechanism is demonstrated in Scheme 3. Substrate 2a is dehydrogenated by a base to produce the enolate intermediate I. Then I attacks ynone 4avia Michael addition to deliver the allenolate intermediate II. The intramolecular ring closure of intermediate II allows access to intermediate III with a cyclobutene structure, which undergoes further ring opening to produce the seven-membered intermediate IV. Then V is formed via isomerization of IV, and after protonation, 5a is obtained.
Conclusions
In summary, we have developed a catalytic method affording seven-membered carbon cycles via ring expansion of five-membered β-ketoesters. This method tolerates a series of alkynyl 1,2-diketones, common ynones, and alkynyl ketoesters, and affords the corresponding products with multiple functional groups. Gram-scale reactions are possible, and the synthetic applications of this work have been demonstrated in the alkylation and asymmetric transfer hydrogenation reactions. This work provides a concise method for producing medium rings under catalytic, mild, and environmentally benign conditions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support was provided by the NSFC (22071242 and 21871260), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), and the Fujian Natural Science Foundation (2018J05035 and 2021J01522).Notes and references
- (a) S. Choodej, D. Sommit and K. Pudhom, Rearranged Limonoids and Chromones from Harrisonia perforate and Their Anti-Inflammatory Activity, Bioorg. Med. Chem. Lett., 2013, 23, 3896–3900 CrossRef CAS; (b) C.-C. Chen, T.-T. Tzeng, C.-C. Chen, C.-L. Ni, L.-Y. Lee, W.-P. Chen, Y.-J. Shiao and C.-C. Shen, Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus, J. Nat. Prod., 2016, 79, 438–441 CrossRef CAS; (c) G. Xu, L. Peng, X. Niu, Q. Zhao, R. Li and H. Sun, Novel Diterpenoids from Salvia dugesii, Helv. Chim. Acta, 2004, 87, 949–955 CrossRef CAS; (d) I. M. Saitbaeva, A. Mallabaev and G. P. Sidyakin, Anhydroaustricin from Artemisia leucodes, Chem. Nat. Compd., 1983, 19, 373 CrossRef; (e) Y. Takaya, M. Akasaka, Y. Takeuji, M.-A. Tanitsu, M. Niwa and Y. Oshima, Novel Guaianoids, Nardoguaianone E–I, from Nardostachys chinensis Roots, Tetrahedron, 2000, 56, 7679–7683 CrossRef CAS; (f) C. Kamperdicka, N. M. Phuonga, G. Adam and T. Van Sung, Guaiane Dimers from Xylopia vielana, Phytochemistry, 2003, 64, 811–816 CrossRef; (g) K. H. Kim, H. J. Noh, S. U. Choi, K. M. Park, S.-J. Seok and K. R. Lee, Lactarane Sesquiterpenoids from Lactarius subvellereus and Their Cytotoxicity, Bioorg. Med. Chem. Lett., 2010, 20, 5385–5388 CrossRef CAS PubMed.
- (a) T. Miura, M. Shimada and M. Murakami, Acyl 1,3-Migration in Rhodium-Catalyzed Reactions of Acetylenic b-Ketoesters with Aryl Boronic Acids: Application to Two-Carbon-Atom Ring Expansions, Angew. Chem., Int. Ed., 2005, 44, 7598–7600 CrossRef CAS; (b) J. A. Ragan, T. W. Makowski, D. J. am Ende, P. J. Clifford, G. R. Young, A. K. Conrad and S. A. Eisenbeis, A Practical Synthesis of Cycloheptane-1,3-dione, Org. Process Res. Dev., 1998, 2, 379–381 CrossRef CAS; (c) J. A. Ragan, J. A. Murry, M. J. Castaldi, A. K. Conrad, B. P. Jones, B. Li, T. W. Makowski, R. McDermott, B. J. Sitter, T. D. White and G. R. Young, Investigation of Methods for Seven-Membered Ring Synthesis: A Practical Synthesis of 4-Oxo-5,6,7,8-tetrahydro-4H-cyclohepta[b]furan-3-carboxylic Acid, Org. Process Res. Dev., 2001, 5, 498–507 CrossRef CAS; (d) S. Kanaya, Y. Asaji, T. Yoshimura and J.-I. Matsuo, Two-Carbon Ring-Enlargement of Cyclic 1,3-Diketones to Cyclic 1,5-Diketones, Synlett, 2020, 1201–1204 CAS; (e) K.-S. Shia, N.-W. Jan, J.-L. Zhu, T. W. Ly and H.-J. Liu, A Facile Two-Carbon Ring Expansion Process Based on the 2-Cyano-1-Vinylcycloalkanol System, Tetrahedron Lett., 1999, 40, 6753–6756 CrossRef CAS.
- (a) Z. Xing, B. Fang, S. Luo, X. Xie and X. Wang, Generation of Fused Seven-Membered Polycyclic Systems via Ring Expansion and Application to the Total Synthesis of Sesquiterpenoids, Org. Lett., 2022, 24, 4034–4039 CrossRef CAS; (b) Y. Yuan, Z. Guo, Y. Mu, Y. Wang, M. Xu and Y. Li, Synthesis of Spiro[5.n (n = 6–8)]heterocycles Through Successive Ring-Expansion/Indole C–2 Functionalization, Adv. Synth. Catal., 2020, 362, 1298–1302 CrossRef CAS; (c) M. Wang, L. Kong, Y. Wang, B. Song, Y. Sun, R. Tang and Y. Li, Sequential C–C σ-Bond Cleavage/(sp2) C–O Bond Formation via C–H Functionalization toward Pyranoindolones Fused with Medium-Sized Rings, Org. Lett., 2018, 20, 6130–6134 CrossRef CAS; (d) M. Wang, Y. Yang, L. Yin, Y. Feng and Y. Li, Selective Synthesis of Pyrano[3,2-b]indoles or Cyclopenta[b]indoles Tethered with Medium-Sized Rings via Cascade C−C σ-Bond Cleavage and C−H Functionalization, J. Org. Chem., 2021, 86, 683–692 CrossRef CAS; (e) Y. Zhou, X. Tao, Q. Yao, Y. Zhao and Y. Li, Insertion of isolated alkynes into Carbon-Carbon-Bonds of Unstrained Cyclic-ketoesters via Transition-Metal Free Tandem Reactions: Synthesis of Medium-Sized Ring Compounds, Chem. – Eur. J., 2016, 22, 17936–17939 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2184475, 2184325, 2184544 and 2184326. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2nj03473e |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |