Graphene based magnetite carbon nanofiber composites as anodes for high-performance Li-ion batteries
Pitcheri
Rosaiah†
 *a,
Theophile
Niyitanga†
*a,
Theophile
Niyitanga†
 *b,
Sangaraju
Sambasivam
c and
Haekyoung
Kim
*b
*b,
Sangaraju
Sambasivam
c and
Haekyoung
Kim
*b
aDepartment of Physics, Paavai Engineering College, Namakkal, 637018, Tamilnadu, India. E-mail: dr.mosesrosaiah@gmail.com
bSchool of Materials Science and Engineering, Yeungnam University, 280 Daehak-Ro, Gyeongsan, 38541, South Korea. E-mail: hkkim@ynu.ac.kr
cNational Water and Energy Center, United Arab Emirates University, Al Ain – 15551, United Arab Emirates
First published on 7th December 2022
Abstract
For energy storage applications, highly flexible free-standing electrodes are ideal for the fabrication of electrochemical cells. In the present study, reduced graphene doped magnetite carbon nanofiber (rGO/Fe3O4 CNF) composite electrodes were prepared using a facile and eco-friendly electrospinning technique. X-ray diffraction (XRD), Raman spectroscopy, and X-ray phosphorescence spectroscopy (XPS) were used to investigate the structural properties and chemical state of the composites. The rGO/Fe3O4 CNF composites had a high specific surface area of 253.85 m2 g−1 and a pore volume of 0.243 cm3 g−1. The rGO/Fe3O4 CNF composites can be employed without any conductive agents as an anode for lithium-ion batteries (LIBs). As a freestanding electrode, the rGO/Fe3O4 CNF composite demonstrated a specific discharge capacity of 1514 mA h g−1 at 0.1 A g−1. Furthermore, it demonstrated excellent cycling performance, maintaining a capacity of 1126 mA h g−1 for 200 cycles despite a high current density of 1 A g−1. The enhanced electrochemical performance is ascribed to the flexible freestanding characteristics of the electrode and the synergistic effect between one dimensional (1D) CNFs, Fe3O4 nanoparticles (NP) and rGO. Therefore, freestanding rGO/Fe3O4 CNF electrodes can be used as potential candidates for practical applications in flexible electronics.
Introduction
Nowadays, energy consumption is soaring to unpredictably high levels, resulting in a substantial demand for alternate energy sources.1 Furthermore, the unprecedented exploitation of natural resources greatly affects the environment. Consequently, it is essential to focus on the development of efficient, environmentally-friendly, and sustainable energy storage systems to address this issue.2 Lithium-ion batteries (LIBs) and supercapacitors (SCs), which can power electric vehicles, mobile electronic devices, etc., have gained considerable attention recently.3–5 LIBs have been regarded as capable power sources in the scientific and industrial domains due to their outstanding qualities, such as their extended lifetime, high capacity, and high energy density.6,7It is well known that the electrode materials play a vital role in LIBs.8,9 Hence, there is a requirement to develop novel electrode materials with exceptional properties to achieve high-performances. Several metal oxides including Mn, Ni, Ti, V, Mo, Co, Fe oxides, etc., have been extensively investigated so far.10–12 Specifically, Fe oxide composites have found widespread application in energy storage because of their exceptional properties. Fe3O4 is unique among the Fe oxides (FeO, Fe2O3, and Fe3O4) since it shows both capacitive and battery characteristics. Moreover, Fe3O4 is a more desirable material for energy storage applications because of its exceptional properties, such as its high storage capacity (928 mA h g−1), accessibility, low-cost, and eco-friendliness.13–15 However, the electrochemical performance of Fe3O4 composites, especially their cyclic performance at high currents, has proven to be inadequate due to poor conductivity, severe constituent agglomeration, and structural uncertainty. Consequently, this deficiency can be solved by tailoring the surface architecture of Fe3O4 composites and incorporating conductive agents, such as graphene, activated carbon, carbon nanotubes, etc. into their structure.16
The use of carbonaceous materials (GO, RGO, CNFs and carbon nanotubes (CNT)) to enhance battery performance and stability has recently witnessed significant growth.17–19 Hence, these materials can be developed by tailoring their dimensions and doping them with suitable conductive agents. As one of the most practical and cost-efficient methods to develop one-dimensional (1D) electrode materials, electrospinning is considered to be one of the most important techniques. The electrospinning method is a versatile method for constructing hierarchical nanofibers with 1D structures that can be applied to electrochemical applications.20–22 In addition, it can be integrated with other synthesis approaches, such as hydrothermal and solvothermal, so as to further modify the surface properties.23–25 In general, it is well known that the performance of a battery will be affected by routine fabrication processes involving polymer binders and conductive carbons. These procedures not only increase the mass of the electrode but also need to follow several procedures and consume a lot of time. Moreover, device level applications require more mechanical strength and flexibility of the electrodes. In this regard, constructing self-supporting electrodes with good conductivity is more desirable.26,27
Herein, rGO/Fe3O4 CNF free-standing electrodes with a large surface area, flexibility and low dimensions are developed. With a high discharge capacity of 1514 mA h g−1 at a current density of 0.1 A g−1 and a stable cycle stability, these composites have also demonstrated an excellent electrochemical performance.
Experimental
Polyacrylonitrile (PAN, MW = 90k), polymethyl methacrylate (PMMA, MW = 120k), N,N-dimethylformamide (DMF), Fe acetate and graphene oxide were used without any refinement. Initially, 3.0 g Fe acetate and 0.33 g graphene oxide (GO) were dissolved in 25 mL of DMF. Next, 1.9 g PAN and 1.0 g PMMA were mixed with the above solution and stirred constantly at 75 °C for 6 h. The resultant blended solution was used for the electrospinning process, in which the voltage, flow rate and distance between the syringe and Al foil were 15 kV, 0.4 mL h−1 and 15 cm, respectively. The collected fibers were pre-oxidized in air at 240 °C for 5 h and were carbonized in a nitrogen atmosphere at various temperatures of 550–1000 °C for 5 h. The surface morphology was examined by a FESEM (Model: Hitachi:S-4700) and a TEM (Model: TechnaiF20ST). The phase purity and crystal structure were characterized by XRD (Model: PANalytikal). The vibrational characteristics were determined by Raman spectroscopy (Model: Explora-HORIBA). The oxidation states and stoichiometry were investigated by X-ray photoelectron spectroscopy (XPS) (Model: Thermo scientific). The surface area has been calculated using a surface analyzer (Model: 3Flex). All electrochemical experiments were performed on an electrochemical analyzer (Model: VERTEX). The obtained rGO/Fe3O4 CNF composites were punched into 12 mm diameter electrodes which were used as free-standing electrodes. The electrolyte was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v solution of LiPF6 in 1 M ethylene and dimethyl carbonates. The separator utilized was a Celgard-2400. The cells performed galvanostatic discharge/charge cycles over a potential range of 0.01 and 3.0 V on a battery tester.
1, v/v solution of LiPF6 in 1 M ethylene and dimethyl carbonates. The separator utilized was a Celgard-2400. The cells performed galvanostatic discharge/charge cycles over a potential range of 0.01 and 3.0 V on a battery tester.
Results and discussion
The synthesis protocol for the rGO/Fe3O4 CNF composites is presented schematically in Fig. 1. The surface morphology and structural integrity have been examined by FESEM and TEM. Fig. 2a–i shows the SEM images of the Fe3O4 CNF composites carbonized at different temperatures. The as-prepared NFs are very smooth and uniform composites with an average diameter of 350 nm (Fig. 2a). The surface architecture is virtually the same after carbonizing at different temperatures. Notably, the composite NFs maintained the surface structure even at 1000 °C, indicating the stabilization of the CNFs. | ||
| Fig. 2 FESEM images of Fe3O4 CNF composites carbonized at various temperatures (a) 0 °C, (b and c) 550 °C, (d and e) 650 °C, (f and g) 750 °C, (h and i) 1000 °C. | ||
Fig. 3a–c presents the SEM images of the rGO/Fe3O4 CNF composites. The as-prepared composite NFs are uniform and smooth (Fig. 3a). The roughness of the fibers has been increased as they are composed with Fe3O4 and rGO after carbonization. The TEM image of the rGO/Fe3O4 CNF composites (Fig. 3d) clearly shows the distribution of the MnO NPs on the CNFs. The lattice spaces that appear in HRTEM images clearly show high crystalline Fe3O4 NPs distributed on the CNFs (Fig. 3e). Moreover, rGO is covered on the surface of the Fe3O4 particles in the CNFs (Fig. 3e). The selected area electron diffraction (SAED) pattern shows diffraction rings and few spots indicating the distribution of polycrystalline Fe3O4 NPs on the CNFs (Fig. 3f). These results agree with the XRD data. Further, the EDS mapping images (Fig. 3g) show a uniform distribution of the Fe3O4 in the CNFs.
XRD, Raman spectroscopy, and XPS are used to examine the structural properties and chemical state of the synthesized composites. Fig. 4 depicts the XRD pattern of Fe3O4 CNF composites carbonized at various temperatures. At 550 °C, the Fe3O4 CNF composites only showed a few, very weak peaks. At, 650 °C, the XRD pattern of the rGO/Fe3O4 CNF composites showed high intense peaks corresponding to the Fe3O4 phase. These peaks are all in accordance with the JCPDS data (card number 1085-1436).28 Increasing the temperature up to 750 °C resulted in the formation of a mixed impurity phase. Therefore, the optimal carbonization temperature was fixed at 650 °C, and the rGO/Fe3O4 CNF composites were synthesized. As shown in Fig. 5a, the XRD pattern of the rGO/Fe3O4 CNF composites exhibited several broad peaks identical to the Fe3O4 CNF composites. All of the XRD patterns belong to the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group and have lattice parameters of 0.8395 nm, which are well matched with those of pure Fe3O4 CNF composites. Therefore, it can be seen that the incorporation of GO does not compromise the integrity of the composite structure. As demonstrated in Fig. 5b, Raman analyses further support the presence of Fe3O4 and carbon in the rGO/Fe3O4 CNF composite. The rGO/Fe3O4 CNF composite showed two peaks in the Raman spectrum, one at 1354 cm−1 (D-band) and the other at 1578 cm−1 (G-band). The ratio between these peaks (ID/IG) is 1.01, suggesting that there are more disorders and defects in the composite.29,30 In addition, the presence of Fe3O4 NPs in the composite was confirmed by the presence of weak peaks at 222, 404, and 663 cm−1, which correspond to the Eg, T2g, and A1g, respectively. The specific surface area is estimated using BET (Brunauer, Emmett, and Teller) experiments. N2 adsorption/desorption isotherms were carried out to examine the porosity of the composite. Fig. 5c displays the N2 adsorption/desorption isotherm profiles of the rGO/Fe3O4 CNF composite, which showed a type-IV hysteresis loop, confirming the mesoporous structure of the material.31 As shown in Fig. 5d, the majority of the pore distribution is seen within 6 nm. The rGO/Fe3O4 CNF composite had a high specific surface area of 253.85 m2 g−1 and a pore volume of 0.243 cm3 g−1. The rGO doped composites with high surface area and high porosity are indeed beneficial for electrochemical performance.32
m space group and have lattice parameters of 0.8395 nm, which are well matched with those of pure Fe3O4 CNF composites. Therefore, it can be seen that the incorporation of GO does not compromise the integrity of the composite structure. As demonstrated in Fig. 5b, Raman analyses further support the presence of Fe3O4 and carbon in the rGO/Fe3O4 CNF composite. The rGO/Fe3O4 CNF composite showed two peaks in the Raman spectrum, one at 1354 cm−1 (D-band) and the other at 1578 cm−1 (G-band). The ratio between these peaks (ID/IG) is 1.01, suggesting that there are more disorders and defects in the composite.29,30 In addition, the presence of Fe3O4 NPs in the composite was confirmed by the presence of weak peaks at 222, 404, and 663 cm−1, which correspond to the Eg, T2g, and A1g, respectively. The specific surface area is estimated using BET (Brunauer, Emmett, and Teller) experiments. N2 adsorption/desorption isotherms were carried out to examine the porosity of the composite. Fig. 5c displays the N2 adsorption/desorption isotherm profiles of the rGO/Fe3O4 CNF composite, which showed a type-IV hysteresis loop, confirming the mesoporous structure of the material.31 As shown in Fig. 5d, the majority of the pore distribution is seen within 6 nm. The rGO/Fe3O4 CNF composite had a high specific surface area of 253.85 m2 g−1 and a pore volume of 0.243 cm3 g−1. The rGO doped composites with high surface area and high porosity are indeed beneficial for electrochemical performance.32
 | ||
| Fig. 5 XRD pattern (a), Raman spectrum (b), nitrogen adsorption/desorption isotherms (c) and pore size distributions (d) of the rGO/Fe3O4 CNF composites. | ||
XPS is used to examine the chemical state of the rGO/Fe3O4 CNF composite. The XPS spectrum reveals the presence of Fe, C, O, and N components at their respective positions (Fig. 6a). Fig. 6d depicts two peaks associated with Fe 2p3/2 (711.60 eV) and Fe 2p1/2 (724.07 eV). By further deconvoluting Fe 2p3/2, the presence of Ni2+ and Ni3+ ions in Fe3O4 was confirmed by two peaks situated at 710.02 and 712.46 eV. The absence of typical Fe2O3 satellite peaks at 719 eV for Fe 2p3/2 and 729.5 eV for Fe 2p1/2 confirms the formation of Fe3O4. The high-resolution spectrum of C 1s (Fig. 6b) consists of three peaks corresponding to C–C, C–N/C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at binding energies of 284.75, 286.34 and 288.36 eV, respectively. The presence of N is also noticed in the spectrum (Fig. 6c) which can improve the active sites for Li+ intercalation, by helping with electronic conductivity of the carbon frame, and is favorable in promoting electron transfer and Li+ diffusion kinetics, thereby increasing the Li-ion storage.33,34 The absence of additional peaks in the XPS spectrum of the rGO/Fe3O4 CNF composites indicates the structural and chemical stability of the composites.
N at binding energies of 284.75, 286.34 and 288.36 eV, respectively. The presence of N is also noticed in the spectrum (Fig. 6c) which can improve the active sites for Li+ intercalation, by helping with electronic conductivity of the carbon frame, and is favorable in promoting electron transfer and Li+ diffusion kinetics, thereby increasing the Li-ion storage.33,34 The absence of additional peaks in the XPS spectrum of the rGO/Fe3O4 CNF composites indicates the structural and chemical stability of the composites.
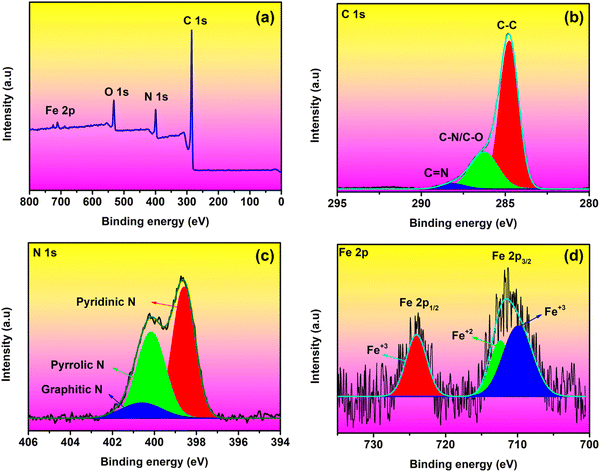 | ||
| Fig. 6 XPS spectra of the rGO/Fe3O4 CNF composites: (a) survey spectrum, (b) C 1s, (c) N 1s and (d) Fe 2p spectrum. | ||
The lithium storage performance of the rGO/Fe3O4 CNF composites has been thoroughly investigated. Fig. 7a shows the CV curves of the rGO/Fe3O4 CNF composites, which were measured at a scan rate of 0.1 mV s−1 over a voltage range of 0.01–3.0 V vs. Li+/Li. During the initial cathodic scan, two peaks at 0.62 and 0.91 V are observed, which are attributed to the conversion of Fe3O4 to Fe and the formation of an SEI film, and which disappear in subsequent cycles.35 Two peaks at 1.61 and 1.85 V, indicate the reversible oxidation of Fe0 to Fe2+/Fe3+ during the anodic scan. In subsequent cycles, a peak at 0.91 V vanished while another peak (0.62 V) shifted to 0.79 V, indicating the presence of an irreversible reaction and structural modification from the second cycle. CV profiles exhibit high reversibility and structural integrity throughout the electrochemical reaction after the initial cycle.36,37
The GCD curves of the rGO/Fe3O4 CNF composites recorded in the potential range of 0.01–3.0 V at a current density of 0.1 A g−1 are shown Fig. 7b. The initial curve demonstrates a voltage plateau at 0.79 V, which corresponds to the Fe3O4 + 8Li+ + 8e− → 3Fe0 + 4Li2O.38,39 The specific capacity of the rGO/Fe3O4 CNF composites was 1514 mA h g−1 at 0.1 A g−1. The enhanced electrochemical performance is ascribed to the flexible freestanding characteristics of the electrode and the synergistic effect between one dimensional (1D) CNFs, Fe3O4 nanoparticles (NP) and rGO. The specific capacity observed in this study is much higher than the specific capacities reported in the literature for the various morphologies of the rGO/Fe3O4 CNF composites. For instance, Yukun et al. synthesized a Fe3O4/SnO2/rGO ternary composite and reported the initial specific capacity of 1362 mA h g−1. Yand et al. prepared Fe3O4/porous carbon composite electrodes that showed a high specific capacity of 1375 mA h g−1.40
Table 1 provides a detailed comparison of the current work with other reported literature. Furthermore, the presence of N in the composite is advantageous because it can improve pore affinity to the aqueous electrolyte, increase the active sites for Li+ intercalation, and promote electron transfer and Li+ diffusion kinetics, thereby increasing Li-ion storage.48,49 The large capacity loss during the first cycle is a common phenomenon caused by electrolyte decomposition as well as the formation of SEI films on the active material, which becomes stable in subsequent cycles and promotes electrochemical activity.50,51
| S. no. | Materials | Initial capacity (mA h g−1) | Restored capacity (mA h g−1) | Rate (A g−1) | Ref. no. |
|---|---|---|---|---|---|
| 1 | rGO/Fe 3 O 4 CNF | 1514 | 1126 (200 cycles) | 0.1 | Present work |
| 2 | Fe3O4 embedded rGO | 909 | 802 (250 cycles) | 0.5 | 13 |
| 3 | 3-Fe/rGO | 1290 | 954 (100 cycles) | 0.1 | 19 |
| 4 | Fe3O4 porous nanofibers | 872 | 700 (80 cycles) | 0.1 | 41 |
| 5 | Fe3O4 graphene | 1073 | 637 (50 cycles) | 0.2 | 42 |
| 6 | C–Fe3O4–C | 1350 | 850 (100 cycles) | 0.1 | 43 |
| 7 | Fe3O4@MWCNTs/graphene | 872 | 720 (100 cycles) | 0.2 | 44 |
| 8 | Fe3O4@C yolk–shell | 1247 | 954 (200 cycles) | 0.5 | 45 |
| 9 | Hollow Fe3O4 nanoparticle | 900 | 832 (60 cycles) | 0.1 | 46 |
| 10 | Fe3O4@graphene aerogel | 941 | 848 (100 cycles) | 0.1 | 47 |
Fig. 7c depicts the rate behavior of both the pure Fe3O4 CNF and the rGO/Fe3O4 CNF composites. At current densities of 0.1, 0.3, 0.5, 1.0 and 2.0 A g−1, the final capacities of pure Fe3O4 CNF-650 and the rGO/Fe3O4 CNF composites were 899, 774, 655, 545, 467 mA h g−1 and 1001, 886, 772, 657, 579 mA h g−1, respectively. When the current density was decreased to 0.1 A g−1, the reversible capacity of the rGO/Fe3O4 CNF composites was 1033 mA h g−1. Due to the highly conductive rGO, high specific surface areas, and porous nature of the sample, the rGO/Fe3O4 CNF composites demonstrated a high specific capacity and a good rate performance compared to all samples of pure Fe3O4 CNF.52,53
EIS studies are used to look deeper into the electrochemical kinetics. Fig. 7d shows the Nyquist plots of pure Fe3O4 CNF and the rGO/Fe3O4 CNF composites, which are display as a semi-circle and line. The high-frequency semicircle represents Li ion migration resistance and charge transfer resistance (Rct), whereas the low-frequency line indicates Warburg impedance.55 Obviously, the Rct value of the rGO/Fe3O4 CNF composite is significantly lower than that of pure Fe3O4 CNF-650, indicating rapid Li-ions kinetics. These findings prove the efficient electrochemical kinetics of composites doped with rGO. In addition, the NFs skeleton allows for rapid electron transit and Li-ion migration, and it can accommodate the volume change that occurs during charging and discharging.56
Fig. 7e shows the cycling behavior of pure Fe3O4 CNF-650 and the rGO/Fe3O4 CNF composites tested for 200 cycles at a high current density (1 A g−1). After a few early cycles, the capacity was gradually raised and remained constant for 100 cycles for both samples. After 200 cycles, the specific capacity of pure Fe3O4 CNF-650 begins to decline slowly and reaches 934 mA h g−1, whereas the specific capacity of the rGO/Fe3O4 CNF composite remains stable at 1126 mA h g−1, which is noteworthy and demonstrates the excellent stability of the rGO/Fe3O4 CNF composites.54 Furthermore, investigations were carried out to comprehend the structural and morphological features of the composite after it had been used in 200 cycles. The XRD pattern and SEM images after 200 cycles are shown in Fig. 8a and b. Interestingly, the major peaks of the XRD confirmed that the rGO/Fe3O4 CNF composite maintained its structural integrity. Moreover, the surface architecture of the CNF was not significantly changed. It might be the reason for the exceptional cycling performance of the rGO/Fe3O4 CNF composite even after several cycles. Therefore, the rGO/Fe3O4 CNF composites have considerable potential as a sustainable material for LIBs.
Conclusions
The rGO/Fe3O4 CNF composites were successfully synthesized by a facile and eco-friendly electrospinning technique and were employed as a freestanding anode material for LIBs. rGO and iron oxide are successfully composited and encapsulated on CNFs during the synthesis. The rGO/Fe3O4 CNF composites had a high specific surface area of 253.85 m2 g−1 and a pore volume of 0.243 cm3 g−1. The electrode materials that were fabricated delivered a high specific discharge capacity of 1514 mA h g−1 at 0.1 A g−1. Moreover, even at a high current density of 1 A g−1, it maintained a capacity of 1126 mA h g−1 for 200 cycles, demonstrating good cycling performance. Hence, the most promising strategy for practical application in energy storage is the simple and eco-friendly method of synthesizing freestanding electrodes with great flexibility and good conductivity.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the DST-SERB, Govt. of India (No: EEQ/2019/000330). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) NRF-2022R1A2C1005585).References
- Q. Wang, C. Tang, D. Sun, A. Du, J. Z. Ou, M. Wu and H. Zhang, Chem. Eng. J., 2022, 427, 131652 CrossRef CAS.
- S. K. Behera, J. Power Sources, 2011, 196, 8669–8674 CrossRef CAS.
- C. Li, B. Liu, N. Jiang and Y. Ding, Nano Res. Energy, 2022, 1, 1–8 Search PubMed.
- X. Guo, C. Wang, W. Wang, Q. Zhou, W. Xu, P. Zhang, S. Wei, Y. Cao, K. Zhu, Z. Liu, X. Yang, Y. Wang, X. Wu, L. Song, S. Chen and X. Liu, Nano Res. Energy, 2022, 1, 1–8 Search PubMed.
- Q. Liu, Y. Hu, X. Yu, Y. Qin, T. Meng and X. Hu, Nano Research Energy, 2022, 1, 1–8 Search PubMed.
- P. Rosaiah, J. Zhu, O. M. Hussain and Y. Qiu, Ionics, 2019, 25, 655–664 CrossRef CAS.
- P. Rosaiah, J. Zhu, O. M. Hussain and Y. Qiu, Ceram. Int., 2018, 44, 3077–3084 CrossRef CAS.
- H. Liu, X. Liu, W. Li, X. Guo, Y. Wang, G. Wang and D. Zhao, Adv. Energy Mater., 2017, 7, 1–24 Search PubMed.
- H. Tian, H. Tian, W. Yang, F. Zhang, W. Yang, Q. Zhang, Y. Wang, J. Liu, S. R. P. Silva, H. Liu and G. Wang, Adv. Funct. Mater., 2021, 31, 1–14 Search PubMed.
- M. Dhananjaya, A. Lakshmi Narayana, N. Guru Prakash, P. Rosaiah and O. M. Hussain, Mater. Chem. Phys., 2019, 237, 121825 CrossRef CAS.
- N. G. Prakash, M. Dhananjaya, A. L. Narayana, D. P. Shaik, P. Rosaiah and O. M. Hussain, Ceram. Int., 2018, 44, 9967–9975 CrossRef CAS.
- D. Huang, Z. Lu, Q. Xu, X. Liu, W. Yi, J. Gao, Z. Chen, X. Wang and X. Fu, Electrochim. Acta, 2022, 407, 139866 CrossRef CAS.
- P. Rosaiah, J. Zhu, L. Zhang, O. M. Hussain and Y. Qiu, J. Electroanal. Chem., 2019, 834, 173–179 CrossRef CAS.
- J. Song, A. Y. Maulana, H. Kim, B. Yun, H. Gim, Y. Jeong, N. An, C. M. Futalan and J. Kim, J. Alloys Compd., 2022, 921, 166082 CrossRef CAS.
- X. Li, C. Shao, X. Wang, J. Wang, G. Liu, W. Yu, X. Dong and J. Wang, Chem. Eng. J., 2022, 446, 137267 CrossRef CAS.
- H. Duan, J. Gnanaraj and J. Liang, J. Power Sources, 2011, 196, 4779–4784 CrossRef CAS.
- P. Rosaiah, J. Zhu, O. M. Hussain and Y. Qiu, Appl. Phys. A, 2018, 124, 597 CrossRef.
- P. Rosaiah, J. Zhu, D. P. Shaik, O. M. Hussain., Y. Qiu and L. Zhao, J. Electroanal. Chem., 2017, 794, 78–85 CrossRef CAS.
- Q. Pan, J. Zhao, J. Zhang, Z. Chen, N. Li, L. Yao, J. song, B. Xing, S. Jiang, W. Qu and R. Liu, SSRN Electron. J., 2022, 605, 154798 CAS.
- M. Liu, P. Zhang, Z. Qu, Y. Yan, C. Lai, T. Liu and S. Zhang, Nat. Commun., 2019, 10, 3917 CrossRef PubMed.
- J. Tan, D. Li, Y. Liu, P. Zhang, Z. Qu, Y. Yan, H. Hu, H. Cheng, J. Zhang, M. Dong, C. Wang, J. Fan, Z. Li, Z. Guo and M. Liu, J. Mater. Chem. A, 2020, 8, 7980–7990 RSC.
- Z. Yang, P. Zhang, J. Wang, Y. Yan, Y. Yu, Q. Wang and M. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 37434–37444 CrossRef CAS.
- Y. Guo, J. Li, R. Pitcheri, J. Zhu, P. Wen and Y. Qiu, Chem. Eng. J., 2019, 355, 390–398 CrossRef CAS.
- J. Zhu, R. Pitcheri, T. Kang, C. Jiao, Y. Guo, J. Li and Y. Qiu, J. Electroanal. Chem., 2019, 833, 151–159 CrossRef CAS.
- Y. Lin, R. Pitcheri, J. Zhu, C. Jiao, Y. Guo, J. Li and Y. Qiu, J. Alloys Compd., 2019, 785, 627–633 CrossRef CAS.
- L. Zhang, F. Zhang, G. Huang, J. Wang, X. Du, Y. Qin and L. Wang, J. Power Sources, 2014, 261, 311–316 CrossRef CAS.
- E. Samuel, A. Aldalbahi, M. El-Newehy, H. El-Hamshary and S. S. Yoon, Ceram. Int., 2022, 48, 18374–18383 CrossRef CAS.
- M. Chen, X. Shen, K. Chen, Q. Wu, P. Zhang, X. Zhang and G. Diao, Electrochim. Acta, 2016, 195, 94–105 CrossRef CAS.
- Y. Xie, Y. Qiu, L. Tian, T. Liu and X. Su, J. Alloys Compd., 2022, 894, 162384 CrossRef CAS.
- M. Ershadi, M. Javanbakht, D. Brandell, S. Ahmad Mozaffari and A. Molaei Aghdam, Appl. Surf. Sci., 2022, 601, 154120 CrossRef CAS.
- Y. Yan, X. Lu, Y. Li, J. Song, Q. Tian, L. Yang and Z. Sui, J. Alloys Compd., 2022, 899, 163342 CrossRef CAS.
- Z. Hu, H. Cui, J. Li, G. Lei and Z. Li, Ceram. Int., 2020, 46, 18868–18877 CrossRef CAS.
- Q. Wu, R. Zhao, X. Zhang, W. Li, R. Xu, G. Diao and M. Chen, J. Power Sources, 2017, 359, 7–16 CrossRef CAS.
- L. Guo, H. Sun, C. Qin, W. Li, F. Wang, W. Song, J. Du, F. Zhong and Y. Ding, Appl. Surf. Sci., 2018, 459, 263–270 CrossRef CAS.
- Y. Zhao, J. Wang, C. Ma, L. Cao and Z. Shao, Chem. Eng. J., 2019, 370, 536–546 CrossRef CAS.
- T. Li, X. Bai, Y. X. Qi, N. Lun and Y. J. Bai, Electrochim. Acta, 2016, 222, 1562–1568 CrossRef CAS.
- L. Liu, H. Zhang, S. Liu, H. Yao, H. Hou and S. Chen, Electrochim. Acta, 2016, 219, 356–362 CrossRef CAS.
- K. Zhu, Y. Zhang, H. Qiu, Y. Meng, Y. Gao, X. Meng, Z. Gao, G. Chen and Y. Wei, J. Alloys Compd., 2016, 675, 399–406 CrossRef CAS.
- J. Du, Y. Ding, L. Guo, L. Wang, Z. Fu, C. Qin, F. Wang and X. Tao, Appl. Surf. Sci., 2017, 425, 164–169 CrossRef CAS.
- Y. Liu, P. Li, Y. Wang, J. Liu, Y. Wang, J. Zhang, M. Wu and J. Qiu, J. Alloys Compd., 2017, 695, 2612–2618 CrossRef CAS.
- J. He, S. Zhao, Y. Lian, M. Zhou, L. Wang, B. Ding and S. Cui, Electrochim. Acta, 2017, 229, 306–315 CrossRef CAS.
- J. Su, M. Cao, L. Ren and C. Hu, J. Phys. Chem. C, 2011, 115, 14469–14477 CrossRef CAS.
- L. Yang, J. Hu, A. Dong and D. Yang, Electrochim. Acta, 2014, 144, 235–242 CrossRef CAS.
- H. Tang, K. Huang, Y. Bao, Y. Li and J. Zhong, J. Alloys Compd., 2017, 699, 812–817 CrossRef.
- B. Wang, X. Zhang, X. Liu, G. Wang, H. Wang and J. Bai, J. Colloid Interface Sci., 2018, 528, 225–236 CrossRef CAS.
- D. Chen, G. Ji, Y. Ma, J. Y. Lee and J. Lu, ACS Appl. Mater. Interfaces, 2011, 3, 3078–3083 CrossRef CAS PubMed.
- Y. Suo, Q. Q. Zhao, J. K. Meng, J. Li, X. C. Zheng, X. X. Guan, Y. S. Liu and J. M. Zhang, Mater. Lett., 2016, 174, 36–39 CrossRef CAS.
- A. Watanabe, K. Yamamoto, T. Uchiyama, T. Matsunaga, A. Hayashi, K. Maeda, H. Kageyama and Y. Uchimoto, ACS Appl. Energy Mater., 2020, 3, 4162–4167 CrossRef CAS.
- C. C. Hsieh and W. R. Liu, J. Alloys Compd., 2019, 790, 829–836 CrossRef CAS.
- L. Zhang, Y. Qin, Y. Liu, Q. Liu, Y. Ren, A. N. Jansen and W. Lu, J. Electrochem. Soc., 2018, 165, A2102–A2107 CrossRef CAS.
- P. W. Chi, T. Paul, Y. H. Su, K. H. Su, C. Y. Su, P. M. Wu, S. F. Wang and M. K. Wu, Sci. Rep., 2022, 12, 1–15 CrossRef.
- S. Petnikota, S. K. Marka, A. Banerjee, M. V. Reddy, V. V. S. S. Srikanth and B. V. R. Chowdari, J. Power Sources, 2015, 293, 253–263 CrossRef CAS.
- S. Petnikota, H. Maseed, V. V. S. S. Srikanth, M. V. Reddy, S. Adams, M. Srinivasan and B. V. R. Chowdari, J. Phys. Chem. C, 2017, 121, 3778–3789 CrossRef CAS.
- H. Wang, J. Xie, M. Follette, T. C. Back and P. B. Amama, RSC Adv., 2016, 6, 83117–83125 RSC.
- B. Yang, J. Zhao, J. Chen, M. He and S. Xu, RSC Adv., 2015, 5, 57906–57911 RSC.
- Y. Wan, Z. Yang, G. Xiong, R. Guo, Z. Liu and H. Luo, J. Power Sources, 2015, 294, 414–419 CrossRef CAS.
Footnote |
| † Authors equally contributed. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |





